Abstract
Background
Hepatitis B virus (HBV) is highly endemic in Cambodia with an estimated pre-vaccine hepatitis B surface antigen (HBsAg) prevalence of 9%. By 2005, a hepatitis B vaccination program was implemented to decrease infection rates in children. We conducted a serosurvey to evaluate the impact of the vaccination program in 2011.
Methods
A cross-sectional two-stage cluster survey was conducted to estimate HBsAg prevalence among children born from 2006 to 2007 in three provinces: Phnom Penh (urban), Kratie (rural), and Ratanakiri (remote). Demographic data, as well as written or oral vaccination history were collected. Children were tested for HBsAg. Factors associated with undervaccination and HBsAg positivity were modeled.
Results
Coverage of timely hepatitis B vaccine birth dose (administered at ≤24 hours) was 55% in Phnom Penh, 36% in Kratie, and 22% in Ratanakiri. Coverage with ≥3 hepatitis B vaccine doses (HepB3) was 91% in Phnom Penh, 82% in Kratie, and 64% in Ratanakiri. When compared with children who were born in health facilities with a skilled birth attendant (SBA), children born at home without a SBA were more likely not to have received a timely BD (adjusted relative risk [aRR]=1.94; 95% CI=1.75–2.15) as were children born at home with an SBA (aRR=1.54; 95% CI=1.32–1.80). The proportion of children who tested positive for HBsAg was 0.33% in Phnom Penh, 1.41% in Kratie, and 3.45% in Ratanakiri. In all three provinces, children who received their first dose after seven days of life and children who never received hepatitis B vaccine had the highest HBsAg prevalence.
Conclusions
Progress has been made in Cambodia in decreasing the burden of chronic HBV infection among children. Improvements in vaccination coverage will further decrease the burden disease.
Keywords: hepatitis B virus, Cambodia, seroprevalence, vaccine
INTRODUCTION
Chronic hepatitis B virus (HBV) infection is a global public health problem that affects an estimated 240 million people, with the greatest burden of disease among Asians, most of whom acquire the infection from perinatal or horizontal transmission occurring during the first five years of life [1, 2]. Cambodia is considered highly endemic for chronic HBV infection, with an estimate by the World Health Organization (WHO) Regional Office for the Western Pacific of 9% hepatitis B surface antigen (HBsAg) seroprevalence in the general population [3], ranging from 7.7%–13% in various subpopulations of adults based on convenience sample data [4–9].
In response to the large burden of disease in Cambodia, the government introduced hepatitis B vaccine for all infants in a geographically phased manner starting in 2001, with a nationwide program in place by 2005. Since 2005, the schedule has been a birth dose (BD) within 24 hours of birth, and doses at 6, 10, and 14 weeks of age.
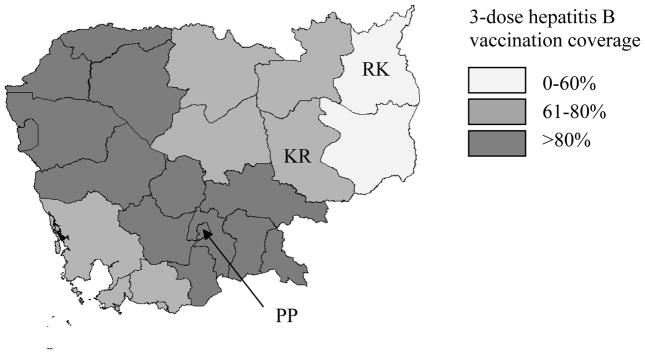
National BD and 3-dose hepatitis B vaccination coverage (HepB3) has been rising yearly since introduction from 25% in 2007 to 68% in 2011 for birth dose coverage and from 82% in 2006 to 94% in 2011 for HepB3 coverage [10]; however, coverage varied by province from 39%–97% for BD and 71%–159% for HepB3 in 2011 (unpublished official Ministry of Health immunization coverage data 2011, Figure 1). The range in provincial HepB3 coverage signifies variable access to routine immunization services, while BD coverage variability is largely attributed to access to health facilities and/or skilled birth attendants (SBAs) during delivery, with persons in urban areas having greater access to both. In 2010, 54% of all deliveries were in health facilities with provincial variation of 21%–93%; factoring in SBAs providing home care, 71% of deliveries were attended by an SBA, with provincial variation of 28%–99% [11]. Difficulties in knowing the true number of children eligible for vaccination also accounts for some of the variability in BD and HepB3 coverage by province, including coverage exceeding 100%.
Figure 1. 3-dose hepatitis B vaccination coverage among 1 year-olds by province.a.
Provinces selected for the study are noted (PP=Phnom Penh, KR=Kratie, RK=Ratanakiri).
aCambodia 2010 Demographic and Health Survey [11]
In 2006, a nationally representative seroprevalence study conducted in Cambodia among five-year-old children born before vaccine introduction using a rapid point-of-care test found a HBsAg seroprevalence of 3.5% (95% Confidence Interval [CI] 2.4–4.8%) [12]. However, HBsAg seroprevalence ranged from 8.5% (95% CI 6–11.8%) in least developed provinces to 3.2% (95% CI 1.7–5.7%) in most developed provinces. Reasons for the lower HBsAg prevalence in the 2006 study compared with the 9% national HBsAg estimate of the Regional Office for the Western Pacific in the general population have not been studied.
To assess the progress of the national immunization program in decreasing the burden of disease, we conducted serosurveys in three provinces to ascertain the hepatitis B vaccination coverage and HBsAg seroprevalence among children born after the national implementation of the hepatitis B vaccination program.
METHODS
In October 2011, we conducted three cross-sectional two-stage cluster surveys among children born from January 1, 2006 to December 31, 2007 in Phnom Penh, Kratie, and Ratanakiri provinces, which were selected to represent different regions of Cambodia with respect to population density (urban/rural/remote) and varying hepatitis B vaccination coverage (Figure 1). Phnom Penh is an urban province with urban poor, close quarters, good access to healthcare facilities, and 92% BD/90% HepB3 coverage in 2010. Kratie is a rural province, with 58% BD/74% HepB3 coverage in 2010. Ratanakiri is a remote sparsely populated province with few health facilities, and 47% BD/40% HepB3 coverage in 2010 [11]. Because of differences in coverage, the survey was designed to estimate province-specific HBsAg prevalence.
Sample size and sampling
Reported vaccination coverage was used to model expected province-specific HBsAg prevalence for sample size calculation [13]. The sample size of 1211 children in Phnom Penh, 563 children in Kratie, and 626 children in Ratanakiri was calculated based on the expected HBsAg seroprevalence and desired precision of 4.5%±1.5% in Phnom Penh, 5.9%±2.5% in Kratie, and 6.6 %±2.5% in Ratanakiri with 95% probability. Less precision for the rural/remote provinces was considered acceptable considering the difficult field logistics and that higher precision would not likely change the outcome and program guidance. The design effect for the cluster sampling design was assumed to be 1.5 with a 10% non-response rate, based on the design effect found in the 2006 national hepatitis B seroprevalence study which used a similar sampling design and testing methodology [12].
Sampling within provinces was done in two stages. In the first stage, 40 villages were chosen in Phnom Penh, 30 in Kratie, and 40 in Ratanakiri based on probability proportional to size sampling methodology. In the second stage, a fixed number of children born during 2006 and 2007 were selected within each village. In order to find these children, one team started in the center, and one team started at the periphery of the village. Each team chose a random direction and visited houses sequentially searching for children within the target age group until the desired number were enrolled. Only one child was selected in each household. In the few instances where a selected village did not have enough children to meet the required sample size, the remainder was randomly selected from a neighboring village.
Data Collection
If consent was obtained, a brief questionnaire was administered to the caregiver. The questionnaire collected demographic data, potential risk factors for infection and vaccination history. If written vaccination history was not available, vaccination history based on caregiver recall was obtained.
Specimen Collection and HBsAg Testing
Approximately 50uL of blood was collected from each child by finger prick and was tested in the field using the Alere Determine™ HBsAg point-of-care test strip (reported sensitivity: 95%–100%; reported specificity: 96%–100%) [14–16].
Data management and analysis
The data were double entered and stored in Epidata v3.5.3 (Odense, Denmark). Data were analyzed in SAS v9.3 (Cary, NC, USA) and SUDAAN v10 (Research Triangle Park, NC, USA). Provinces were analyzed separately; analyses accounted for the two-stage cluster design. Wilson CIs are given for proportions that are outside the range of 20%–80%, otherwise Wald CIs are reported.
For each province, model-adjusted relative risks were calculated using a multivariable logistic model to summarize variables independently associated with not receiving a timely BD and not receiving at least three doses of hepatitis B vaccine. We defined “timely BD” as a dose of hepatitis B vaccine given within 24 hours of birth. We defined “≤7 day BD” as a dose of hepatitis B vaccine given within seven days of birth. The models included all variables with epidemiologic plausibility as well as those having a p-value <0.1 on individual variable analysis. The adjusted relative risks (aRR) from the three provincial models were similar in magnitude and direction for all variables; therefore the data were combined into a single overall model.
Human Subjects Rights and Ethics
Informed consent was obtained from the caregivers of all participants. The study protocol was approved by the Cambodia National Ethics Committee for Health Research and the Ethics Review Committee at the WHO Regional Office for the Western Pacific.
RESULTS
In Phnom Penh, 1240 eligible children were identified, of whom 1199 participated, and 1196 were analyzed. In Kratie, 570 eligible children were identified, of whom 569 participated and were analyzed. In Ratanakiri, 640 eligible children were identified, of whom 640 participated and 637 were analyzed. Two participating children were found to be born outside of the age group targeted and were excluded from analysis; an additional three children were excluded from the study analysis because they refused the blood test. One child was excluded because the test was done incorrectly and was not able to be repeated.
Children in the three provinces were different with respect ethnicity, maternal education, location of birth and delivery attendant, school attendance, and vaccine card availability (Table 1), which reflect the variation in the underlying populations of the three provinces.
Table 1.
Characteristics of enrolled children, Phnom Penh, Kratie, and Ratanakiri provinces, Cambodia, 2011.
| Phnom Penh | Kratie | Ratanakiri | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | # | (%) | Total | # | (%) | Total | # | (%) | |
| Female | 1196 | 553 | (46) | 569 | 271 | (48) | 637 | 334 | (52) |
| Khmer ethnicity | 1196 | 1164 | (97) | 569 | 511 | (90) | 637 | 223 | (35) |
| Maternal education | |||||||||
| None | 1186 | 113 | (10) | 562 | 101 | (18) | 630 | 395 | (63) |
| Primary school | 1186 | 508 | (43) | 562 | 309 | (55) | 630 | 186 | (30) |
| Secondary school/University | 1186 | 565 | (48) | 562 | 152 | (27) | 630 | 47 | (7) |
| Location of birth and delivery attendant | |||||||||
| Born at home without an SBAa | 1189 | 37 | (3) | 562 | 180 | (32) | 628 | 295 | (47) |
| Born at home with an SBA | 1189 | 80 | (7) | 562 | 245 | (44) | 628 | 145 | (23) |
| Born in a health facility (with an SBA) | 1189 | 1072 | (90) | 562 | 137 | (24) | 628 | 188 | (30) |
| Attends school | 1196 | 679 | (57) | 569 | 271 | (48) | 637 | 194 | (30) |
| Vaccine card available for review | 1196 | 825 | (69) | 569 | 204 | (36) | 637 | 95 | (15) |
SBA=skilled birth attendant
Timely BD coverage, ≤7 day coverage, and HepB3 coverage were higher in Phnom Penh (55%, 77%, 91%), when compared with Kratie (36%, 53%, 82%), and Ratanakiri (22%, 30%, 64%) (Table 2).
Table 2.
Vaccination historya, Phnom Penh, Kratie, and Ratanakiri provinces, Cambodia, 2011.
| Phnom Penh | Kratie | Ratanakiri | ||||
|---|---|---|---|---|---|---|
| # (%) | 95% CIb | # (%) | 95% CI | # (%) | 95% CI | |
| Time to first dose of hepatitis B vaccine | Total=1070 | Total=502 | Total=491 | |||
|
| ||||||
| ≤24 hours (timely BDb) | 586 (55) | 51–58 | 182 (36) | 26–46 | 110 (22) | 14–31 |
|
| ||||||
| 2–7 days | 237 (22) | 20–25 | 84 (17) | 11–22 | 39 (8) | 5–11 |
|
| ||||||
| >7 days | 206 (19) | 16–23 | 187 (37) | 28–46 | 196 (40) | 31–49 |
|
| ||||||
| None | 41 (4) | 2–5 | 49 (10) | 6–14 | 146 (30) | 20–40 |
|
| ||||||
| Received at least 3 doses of hepatitis B vaccine | Total=1081 | Total=511 | Total=545 | |||
| 981 (91) | 88–93 | 418 (82) | 77–87 | 347 (64) | 55–72 | |
|
| ||||||
| Received at least 3 doses of hepatitis B vaccine including a BD | Total=1052 | Total=495 | Total=488 | |||
|
| ||||||
| Timely BD+ 2+ doses | 552 (53) | 49–56 | 170 (34) | 24–44 | 102 (21) | 13–29 |
|
| ||||||
| ≤7 day BD + 2+ doses | 772 (73) | 70–77 | 248 (50) | 40–60 | 138 (28) | 19–38 |
Vaccination history was collected from immunizations cards or recall when cards were not available.
CI=Confidence Interval, BD=birth dose.
Design effect (DE): Phnom Penh DE=0.94; Kratie DE=1.26; Ratanakiri DE= 2.08.
Estimated intra-class correlation (ICC) = (DE−1)/(b−1) where b is the average number of responses per cluster [35]. Phnom Penh ICC = −0.002; Kratie ICC= 0.014; Ratanakiri ICC= 0.073.
Caregivers had increased awareness about hepatitis B in Phnom Penh (88%) and Kratie (86%), compared with Ratanakiri (61%). Caregivers reported several reasons why their child had not received at least three doses of hepatitis B vaccination. Of 198 caregivers in Phnom Penh, 39 (20%) reported they were too busy, 26 (13%) reported they did not know where to go for vaccination, 24 (12%) were not in the area when vaccine was due, and 21 (11%) forgot it was time. Of 147 caregivers in Kratie, 64 (44%) reported being too busy, 41 (28%) were not in the area when the vaccine was due, 32 (22%) did not know where to go for vaccination, and 26 (18%) forgot it was time. Of 287 caregivers in Ratanakiri, 86 (30%) did not know where to go for vaccination, 77 (27%) reported being too busy, 54 (19%) were not in the area when the vaccine was due, and 31 (11%) reported thinking vaccination was unimportant.
Factors Associated with Under-Vaccination
When compared with children who were born in health facilities with an SBA, children born at home without a SBA were more likely not to have received a timely BD (aRR=1.94; 95% CI=1.75–2.15) (Table 3). The risk of not receiving a timely BD was also greater among children born at home with an SBA when compared with children born in a health facility with an SBA (aRR=1.54; 95% CI=1.32–1.80). Children with mothers who had received at least a primary or secondary school education were approximately 30% less likely to be unvaccinated with a timely BD compared with those with mothers having no schooling (Table 3). In the provincial-specific models, Khmer ethnicity was significantly associated with the receipt of birth dose only in Kratie (aRR=0.8 95% CI=0.6–0.9); ethnicity was not significant when the data were pooled from all three provinces.
Table 3.
Adjusted relative riska (aRR) for not having received a timely hepatitis B birth dose (≤24 hours) and for not having received at least three doses of hepatitis B vaccine in multivariable regression models for Phnom Penh, Kratie, and Ratanakiri provinces, Cambodia, 2011. Bolded numbers indicate statistical significance.
| aRR for Not Receiving a Timely Birth Dose (≤24 hours) (95% CIb) | aRR for Not Receiving 3 Doses of Hepatitis B Vaccine (95% CI) | |
|---|---|---|
| Ethnicity (reference: non-Khmer) | ||
| Khmer | 0.93 (0.69–1.26) | 0.54 (0.39–0.76) |
| Maternal Education (reference: no schooling) | ||
| Primary school | 0.74 (0.64–0.86) | 0.73 (0.55–0.97) |
| Secondary school/University | 0.72 (0.61–0.85) | 0.52 (0.35–0.78) |
| Material used for home (reference: other materials) | ||
| Wooden home | 1.04 (0.92–1.17) | 1.10 (0.83–1.44) |
| Caregiver knowledge of hepatitis B (reference: not heard of hepatitis B) | ||
| Caregiver has heard of hepatitis B | 0.88 (0.76–1.01) | 0.55 (0.42–0.73) |
| School Attendance (reference: does not attend school) | ||
| Child attends school | 0.88 (0.78–1.00) | 0.65 (0.52–0.80) |
| Location of birth and delivery assistance (reference: health facility with an SBA) | ||
| Born at home without SBA | 1.94 (1.75–2.15) | 2.55 (1.84–3.55) |
| Born at home with an SBA | 1.54 (1.32–1.80) | 1.11 (0.78–1.58) |
| Moved since birth (reference: not moved since birth) | ||
| Has moved to a different province since birth | 1.12 (0.97–1.29) | 1.46 (1.00–2.14) |
Model-adjusted relative risks were obtained from the marginal predictions (Rlogist procedure, SUDAAN v. 10)
CI=Confidence Interval
A different set of risk and protective factors were seen for under-vaccination with three doses of hepatitis B vaccine. A risk factor for under-vaccination was being born at home without an SBA (aRR=2.55, 95% CI=1.84–3.55), while being born at home with an SBA was not significantly different than being born in a health facility (aRR=1.11; 95% CI=0.78–1.58). Protective factors included being of Khmer ethnicity, maternal primary or secondary education, caregiver knowledge of hepatitis B, and attendance at school by the child (Table 3).
HBsAg Prevalence and Factors Associated with HBsAg Seropositivity
HBsAg was detected among 4 of 1196 (0.33%, 95% CI 0.1–0.9%) children in Phnom Penh; 8 of 569 (1.41%, 95% CI 0.6–3.1%) children in Kratie, and 22 of 637 (3.45%, 95% CI 1.9–6.3%) children in Ratanakiri.
In all three provinces, children who received their first dose after seven days of life and children who never received hepatitis B vaccine had the highest HBsAg prevalence (Table 4). Two children who received a timely BD were seropositive; one child had received only one subsequent dose approximately 10 weeks after BD administration; the other child only had caregiver-reported vaccination data provided, so dates of administration are unverified and based on recall.
Table 4.
Hepatitis B surface antigen (HBsAg) prevalence by interval from date of birth to first dose of hepatitis B vaccine, Phnom Penh, Kratie, and Ratanakiri provinces, Cambodia, 2011.
| Phnom Penh | Kratie | Ratanakiri | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Interval from birth to 1st dose | HBsAg positive | HBsAg positive | HBsAg positive | ||||||
| Total | # (%) | 95% CIa | Total | # (%) | 95% CIa | Total | # (%) | 95% CIa | |
| ≤ 24 hours | 586 | 1 (0.2) | 0.0–1.0 | 182 | 1 (0.5) | 0.1–3.2 | 110 | 0 (0) | |
| 2–7 days | 237 | 0 (0) | 84 | 0 (0) | 39 | 0 (0) | |||
| ≥8 days | 206 | 2 (1.0) | 0.3–3.5 | 187 | 5 (2.7) | 1.2–6.4 | 196 | 4 (2.0) | 0.8–5.1 |
| No vaccine given | 41 | 1 (2.4) | 0.4–12.6 | 49 | 1 (2.0) | 0.3–11.4 | 146 | 15 (10.3) | 4.9–20.2 |
CI=Confidence Interval
In Phnom Penh, HBsAg prevalence was 0.2% (2/981) among children with vaccination data either by card or recall who received ≥3 doses, compared with 2% (2/100) among children who received <3 doses (RR=0.1, 95% CI 0.01–0.7). In Kratie, HBsAg prevalence was 1.7% (7/418) among children with vaccination data either by card or recall who received ≥3 doses, compared with 1.1% (1/93) among children who received <3 doses, (RR=1.6, 95% CI 0.2–11.6). In Ratanakiri, HBsAg prevalence was 0.9% (3/347) among children with vaccination data either by card or recall who received ≥3 doses, compared with 8.6% (17/198) among children who received <3 doses (RR=0.1, 95% CI 0.03–0.4).
Discussion
The primary objectives of this evaluation were to assess vaccination coverage and burden of chronic HBV infection among vaccine-eligible children in three provinces. HBsAg seroprevalence was 0.33% among 4- to 5-year-old children in Phnom Penh, an urban province, 1.41% in Kratie, a rural province, and 3.45% in Ratanakiri, a remote province. These provincial estimates are considerably lower than the national pre-vaccine HBsAg prevalence estimate of 9% [3] and in two provinces were lower than the national prevalence estimate of 3.5% reported from the 2006 serosurvey [12]. However, these results cannot confirm a decrease in HBsAg prevalence nationally since heterogeneity in HBsAg prevalence exists throughout the country. What is notable is that the lowest seroprevalence estimates were in those areas with the highest vaccination coverage for timely BD coverage, ≤7 day BD coverage, and HepB3 coverage. Much room for improvement exists, especially with regards to timely BD in all three provinces, as well as 3-dose coverage, especially in Ratanakiri. Increasing coverage can prevent further transmission.
We evaluated the impact of the timing of BD administration on the risk of HBsAg seropositivity, although this analysis was limited due to the low number of seropositive children. Children who did not receive any vaccine or received the first dose after seven days of life were more likely to be HBsAg seropositive, which is consistent with other published literature [17, 18]. However, we were unable to differentiate the impact of receiving a hepatitis B vaccine dose ≤24 hours compared to 2–7 days after birth though the study was not powered for this analysis. In China, one study found no difference in seroprevalence between children who received a dose within ≤24 hours compared to 2–7 days, while a second study found that those who received a BD within 2–7 days were three times more likely to be HBsAg positive compared to children who received a BD within ≤24 hours [19, 20]. Further research is needed to guide future policy recommendations on timing of the birth dose. This should not preclude countries from striving to achieve high coverage of a timely BD as recommended by WHO [21].
We found that children were approximately two times more likely to miss a timely BD if they were born at home without an SBA when compared with children born in health facilities. Timely BD for a baby delivered without an SBA at home requires the mother to visit a health facility within 24 hours of delivery which can be challenging. Furthermore, in the remote province of Ratanakiri, where there are few health facilities that are far apart, the risk of not receiving a timely BD is greater. While less likely than home births without an SBA, children born at home with an SBA were 1.5 times more likely to not receive a timely BD when compared with children born in health facilities. This is an opportunity for improving timely BD administration, as all SBAs are trained and able to administer an injection. The ability of SBAs to provide timely BD can be strengthened by providing high-quality training and ensuring that all SBAs have a supply of hepatitis B vaccine.
Concerted efforts have been made in Cambodia to increase facility-based deliveries. Hospital deliveries accounted for 22% of all births in 2005 and 54% of all births in 2010; a concomitant rise nationally in timely BD coverage has been observed from 25% in 2006 to 57% in 2010 [10, 11, 22]. Until every child is delivered in a health facility, the Cambodian Ministry of Health is attempting to reach infants born at home via outreach services. In other countries with a high number of unattended home births, successful strategies, such as the use of simple injection devices (e.g. Uniject) and the use of the vaccine in a controlled-temperature chain have been implemented to reach these infants [23–26].
We found that children born in health facilities were more likely to receive at least three doses of hepatitis B vaccine compared with children born at home without an SBA. In Indonesia, children delivered by an SBA were more likely to have received measles vaccine; in South Africa, children delivered at home were less likely to have received measles vaccine [27, 28]. We hypothesize that delivery by an SBA and delivery in a health facility could be surrogate markers for increased access to immunization services. Additionally, improving facility delivery rates could help improve both BD vaccination coverage and coverage for other routine vaccines by providing an opportunity to educate mothers of the need for vaccines.
This study had several limitations. First, the rapid test used in this study has a reported sensitivity of 95%; therefore the HBsAg prevalence in this study might be lower than the true seroprevalence [15]. Vaccination data collected by recall are not as reliable as vaccination card data, especially since we asked questions to the parents about an event that happened four to five years ago. We assessed only a few risk factors for low vaccine coverage and for acquiring chronic HBV infection. Other important factors such as low socioeconomic status and household crowding were not assessed [29–33]. Additionally, small numbers of HBsAg positive children limited stratification by receiving a birth dose and limited evaluation of the impact of ≥3 dose hepatitis B coverage.
Within a decade, high 3-dose hepatitis B vaccination coverage has been achieved as well as a steadily improving timely BD coverage. Applying the provincial estimates from this study to the proportion of the country’s population living in urban (37%), rural (58%), and remote (6%) provinces, and assuming that heterogeneity in baseline seroprevalence and BD and 3-dose coverage can be explained by this geographical classification, we hypothesize that the WHO Western Pacific Region’s hepatitis B control milestone to reduce chronic HBV infection prevalence to <2% in children aged <5 years by 2012 might have been achieved in Cambodia.
The Cambodia Ministry of Health along with all other countries in the Western Pacific Region has adopted a goal to reduce HBsAg prevalence to <1%. To reach this goal, continued improvements in 3-dose hepatitis B vaccination coverage and timely BD coverage are needed, especially in rural and remote areas. Improvements can be made in timely BD coverage by continuing efforts to increase facility births, by ensuring that national policies are implemented in all health facilities to administer a BD to all infants within 24 hours of delivery, and for SBAs who attend home deliveries to administer a timely BD vaccine as part of newborn care services [34].
Acknowledgments
Funding: This work was supported by the World Health Organization’s Regional Office for the Western Pacific.
The authors wish to thank Theng Van Piseth, Thiep Chanthan, Saphonn Vonthanak, Mey Vannareth and Aim Sothea, as well as our field coordinators, supervisors and data collectors for their efforts and commitment to facilitate and gather high-quality data. Additionally, we would like to thank the provincial health department officers, operational health district officers and families participating in this evaluation.
Abbreviations
- aRR
adjusted Relative Risk
- BD
Hepatitis B birth dose
- CI
Confidence Interval
- DE
Design Effect
- HBsAg
hepatitis B surface antigen
- HBV
Hepatitis B virus
- HepB3
3-dose hepatitis B vaccination coverage
- ICC
Intra-class correlation
- RR
Relative Risk
- SBA
Skilled Birth Attendant
- WHO
World Health Organization
Contributor Information
Mao Bunsoth, Email: maobunsoth888@yahoo.com.
Minal K. Patel, Email: hgo9@cdc.gov.
Karen Hennessey, Email: hennesseyk@wpro.who.int.
Richard J. W. Duncan, Email: duncanr@wpro.who.int.
Kathleen Wannemuehler, Email: kpw9@cdc.gov.
Sann Chan Soeung, Email: workmoh@gmail.com.
References
- 1.Ott JJ, Stevens GA, Groeger J, Wiersma ST. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine. 2012 Mar 9;30(12):2212–9. doi: 10.1016/j.vaccine.2011.12.116. [DOI] [PubMed] [Google Scholar]
- 2.Clements CJ, Baoping Y, Crouch A, Hipgrave D, Mansoor O, Nelson CB, et al. Progress in the control of hepatitis B infection in the Western Pacific Region. Vaccine. 2006 Mar 15;24(12):1975–82. doi: 10.1016/j.vaccine.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization Regional Office for the Western Pacific. Western Pacific Regional Plan for Hepatitis B Control through Immunization. Manila, Philippines: World Health Organization; 2007. [Google Scholar]
- 4.Snitbhan R, Scott RM, Bancroft WH, Top FH, Jr, Chiewsilp D. Subtypes of hepatitis B surface antigen in Southeast Asia. J Infect Dis. 1975 Jun;131(6):708–11. doi: 10.1093/infdis/131.6.708. [DOI] [PubMed] [Google Scholar]
- 5.Thuring EG, Joller-Jemelka HI, Sareth H, Sokhan U, Reth C, Grob P. Prevalence of markers of hepatitis viruses A, B, C and of HIV in healthy individuals and patients of a Cambodian province. Southeast Asian J Trop Med Public Health. 1993 Jun;24(2):239–49. [PubMed] [Google Scholar]
- 6.Ohshige K, Morio S, Mizushima S, Kitamura K, Tajima K, Ito A, et al. Cross-sectional study on risk factors of HIV among female commercial sex workers in Cambodia. Epidemiol Infect. 2000 Feb;124(1):143–52. doi: 10.1017/s0950268899003428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.PATH. Hep B Sero-Prevalence Study Rural Cambodia, 2001 2001.
- 8.Ngoan LT. International epidemiological collaborative surveillance, epidemiology and prevention of HBV, HCV, HIV and rabies at the greater Mekong sub-region. Hanoi, Vietnam: Hanoi Medical University; 2010. [Google Scholar]
- 9.Ol HS, Bjoerkvoll B, Sothy S, Van Heng Y, Hoel H, Husebekk A, et al. Prevalence of hepatitis B and hepatitis C virus infections in potential blood donors in rural Cambodia. Southeast Asian J Trop Med Public Health. 2009 Sep;40(5):963–71. [PubMed] [Google Scholar]
- 10.World Health Organization. Immunization Profile - Cambodia. 2011 [cited August 22, 2012]; Available from: http://apps.who.int/immunization_monitoring/en/globalsummary/countryprofileresult.cfm.
- 11.National Institute of Statistics, Directorate General for Health, ICF Macro. Cambodia Demographic and Health Survey. Phnom Penh, Cambodia and Calverton, Maryland, USA: National Institute of Statistics, Directorate General for Health, and ICF Macro; 2011. [Google Scholar]
- 12.Soeung SC, Rani M, Huong V, Sarath S, Kimly C, Kohei T. Results from nationwide hepatitis B serosurvey in Cambodia using simple and rapid laboratory test: implications for National Immunization Program. Am J Trop Med Hyg. 2009 Aug;81(2):252–7. [PubMed] [Google Scholar]
- 13.Goldstein ST, Zhou F, Hadler SC, Bell BP, Mast EE, Margolis HS. A mathematical model to estimate global hepatitis B disease burden and vaccination impact. Int J Epidemiol. 2005 Dec;34(6):1329–39. doi: 10.1093/ije/dyi206. [DOI] [PubMed] [Google Scholar]
- 14.Lien TX, Tien NT, Chanpong GF, Cuc CT, Yen VT, Soderquist R, et al. Evaluation of rapid diagnostic tests for the detection of human immunodeficiency virus types 1 and 2, hepatitis B surface antigen, and syphilis in Ho Chi Minh City, Vietnam. Am J Trop Med Hyg. 2000 Feb;62(2):301–9. doi: 10.4269/ajtmh.2000.62.301. [DOI] [PubMed] [Google Scholar]
- 15.Alere. Alere Determine HBsAg Package Insert. 2011 http://www.alere.com/EN_ZA/products/alere-determine-hbsag/.Chiba,Japan.
- 16.World Health Organization. Hepatitis B Surface Antigen Assays: Operational Characteristics (Phase 1) Report 1. Geneva: World Health Organization; 2001. [Google Scholar]
- 17.Ruff TA, Gertig DM, Otto BF, Gust ID, Sutanto A, Soewarso TI, et al. Lombok Hepatitis B Model Immunization Project: toward universal infant hepatitis B immunization in Indonesia. J Infect Dis. 1995 Feb;171(2):290–6. doi: 10.1093/infdis/171.2.290. [DOI] [PubMed] [Google Scholar]
- 18.Marion SA, Tomm Pastore M, Pi DW, Mathias RG. Long-term follow-up of hepatitis B vaccine in infants of carrier mothers. American journal of epidemiology. 1994 Oct 15;140(8):734–46. doi: 10.1093/oxfordjournals.aje.a117321. [DOI] [PubMed] [Google Scholar]
- 19.Cui F, Li L, Hadler SC, Wang F, Zheng H, Chen Y, et al. Factors associated with effectiveness of the first dose of hepatitis B vaccine in China: 1992–2005. Vaccine. 2010 Aug 23;28(37):5973–8. doi: 10.1016/j.vaccine.2010.06.111. [DOI] [PubMed] [Google Scholar]
- 20.Shen LP, Zhang Y, Wang F, Zhang S, Yang JY, Fang KX, et al. Epidemiological changes in hepatitis B prevalence in an entire population after 20 years of the universal HBV vaccination programme. Epidemiol Infect. 2011 Aug;139(8):1159–65. doi: 10.1017/S0950268810002827. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. Hepatitis B vaccines. Wkly Epidemiol Rec. 2009 Oct 1;84(40):405–19. [PubMed] [Google Scholar]
- 22.National Institute of Public Health, National Institute of Statistics [Cambodia] Macro O. Cambodia Demographic and Health Survey 2005. Phnom Penh; Cambodia and Calverton, Maryland, USA: 2006. [Google Scholar]
- 23.Sutanto A, Suarnawa IM, Nelson CM, Stewart T, Soewarso TI. Home delivery of heat-stable vaccines in Indonesia: outreach immunization with a prefilled, single-use injection device. Bull World Health Organ. 1999;77(2):119–26. [PMC free article] [PubMed] [Google Scholar]
- 24.Otto BF, Suarnawa IM, Stewart T, Nelson C, Ruff TA, Widjaya A, et al. At-birth immunisation against hepatitis B using a novel pre-filled immunisation device stored outside the cold chain. Vaccine. 1999 Oct 14;18(5–6):498–502. doi: 10.1016/s0264-410x(99)00242-x. [DOI] [PubMed] [Google Scholar]
- 25.Wang L, Li J, Chen H, Li F, Armstrong GL, Nelson C, et al. Hepatitis B vaccination of newborn infants in rural China: evaluation of a village-based, out-of-cold-chain delivery strategy. Bull World Health Organ. 2007 Sep;85(9):688–94. doi: 10.2471/BLT.06.037002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hipgrave DB, Tran TN, Huong VM, Dat DT, Nga NT, Long HT, et al. Immunogenicity of a locally produced hepatitis B vaccine with the birth dose stored outside the cold chain in rural Vietnam. Am J Trop Med Hyg. 2006 Feb;74(2):255–60. [PubMed] [Google Scholar]
- 27.Fernandez R, Rammohan A, Awofeso N. Correlates of first dose of measles vaccination delivery and uptake in Indonesia. Asian Pac J Trop Med. Feb;4(2):140–5. doi: 10.1016/S1995-7645(11)60055-2. [DOI] [PubMed] [Google Scholar]
- 28.Coetzee N, Yach D, Blignaut R, Fisher SA. Measles vaccination coverage and its determinants in a rapidly growing peri-urban area. S Afr Med J. 1990 Dec 15;78(12):733–7. [PubMed] [Google Scholar]
- 29.Szmuness W. Recent advances in the study of the epidemiology of hepatitis B. The American journal of pathology. 1975 Dec;81(3):629–50. [PMC free article] [PubMed] [Google Scholar]
- 30.Kruszon-Moran D, McQuillan GM. Seroprevalence of six infectious diseases among adults in the United States by race/ethnicity: data from the third national health and nutrition examination survey, 1988–94. Advance data. 2005 Mar;9(352):1–9. [PubMed] [Google Scholar]
- 31.Pasquini P, Kahn HA, Pileggi D, Pana A, Terzi J, Guzzanti E. Prevalence of hepatitis B markers in Italy. American journal of epidemiology. 1983 Nov;118(5):699–709. doi: 10.1093/oxfordjournals.aje.a113680. [DOI] [PubMed] [Google Scholar]
- 32.Toukan AU, Sharaiha ZK, Abu-el-Rub OA, Hmoud MK, Dahbour SS, Abu-Hassan H, et al. The epidemiology of hepatitis B virus among family members in the Middle East. American journal of epidemiology. 1990 Aug;132(2):220–32. doi: 10.1093/oxfordjournals.aje.a115651. [DOI] [PubMed] [Google Scholar]
- 33.Stuver SO, Boschi-Pinto C, Trichopoulos D. Infection with hepatitis B and C viruses, social class and cancer. IARC scientific publications. 1997;(138):319–24. [PubMed] [Google Scholar]
- 34.Cambodian Ministry of Health. National Policy on Immunization Programme. Cambodia: 2010. [Google Scholar]
- 35.Bennett S, Woods T, Liyanage WM, Smith DL. A simplified general method for cluster-sample surveys of health in developing countries. World health statistics quarterly. Rapport trimestriel de statistiques sanitaires mondiales. 1991;44(3):98–106. [PubMed] [Google Scholar]