Abstract
Background
In 2009, enhanced poliovirus surveillance was established in polio-endemic areas of Uttar Pradesh and Bihar, India, to assess poliovirus infection in older individuals.
Methods
In Uttar Pradesh, stool specimens from asymptomatic household and neighborhood contacts of patients with laboratory-confirmed polio were tested for polioviruses. In Bihar, in community-based surveillance, children and adults from 250 randomly selected households in the Kosi River area provided stool and pharyngeal swab samples that were tested for polioviruses. A descriptive analysis of surveillance data was performed.
Results
In Uttar Pradesh, 89 of 1842 healthy contacts of case patients with polio (4.8%) were shedding wild poliovirus (WPV); 54 of 85 (63.5%) were ≥5 years of age. Shedding was significantly higher in index households than in neighborhood households (P < .05). In Bihar, 11 of 451 healthy persons (2.4%) were shedding WPV in their stool; 6 of 11 (54.5%) were ≥5 years of age. Mean viral titer was similar in older and younger children.
Conclusions
A high proportion of persons ≥5 years of age were asymptomatically shedding polioviruses. These findings provide indirect evidence that older individuals could have contributed to community transmission of WPV in India. Polio vaccination campaigns generally target children <5 years of age. Expanding this target age group in polio-endemic areas could accelerate polio eradication.
Keywords: poliomyelitis, community transmission, India, enhanced surveillance
In 1988, the World Health Assembly resolved to eradicate polio worldwide [1]. In that year, the estimated number of paralytic cases was 350 000 in 125 countries. In 2009 there were <2000 cases of paralytic polio detected worldwide, and in 2012 only 217 cases were reported from the 3 remaining polio-endemic countries (Nigeria, Pakistan, and Afghanistan); an additional 5 cases were detected in Chad, the last remaining country with reestablished wild poliovirus (WPV) transmission, and 1 case, imported from Nigeria, was detected in Niger [2, 3]. WPV has 3 serotypes (1, 2, and 3). WPV2 has been successfully eradicated, with the last cases reported in 1999 [4]. In 2012, 88% of all reported cases of paralytic polio were due to WPV1 [2].
India was a polio-endemic country until 2011, with the last paralytic case of polio detected in January 2011 [2, 5]. This remarkable success is attributed to the Government of India and their local and international partners, who have been investing significant human and financial resources into polio eradication for many years. The main strategies of the India polio eradication program have been to focus on high-quality supplemental immunization activities (SIAs) targeting children from birth to 5 years of age with oral poliovirus vaccine (OPV), a highly developed social mobilization strategy and approach to reach millions of migrants with SIAs, a well-performing acute flaccid paralysis (AFP) surveillance system, and strengthening of routine immunization.
Prior to the successful elimination of the last foci of WPV transmission in 2011, India had prolonged low-level WPV circulation, as well as occasional outbreaks of both WPV1 and WPV3 concentrated mainly in the northern states of Uttar Pradesh and Bihar [6–12]. The introduction, in 2005, of more-efficient monovalent and, later, bivalent OPV vaccines into SIAs was considered to be the main innovation leading to successful eradication of WPV from India [13–15]. During 2005–2011, SIAs were conducted practically every month in the high-risk areas of western Uttar Pradesh and Bihar; despite the high quality of these SIAs, it took >6 years of intense efforts and enormous resources to rid India of WPV.
The strategy to target children <5 years of age in SIAs was first successfully implemented in the Americas in the 1980s and was later adopted in other regions [16]. This strategy is based on empirical data of the age distribution of paralytic cases and the assumption that older children and adults are mostly immune to infection. Older individuals who do get infected are mostly asymptomatic, and they are also assumed to have limited ability to transmit virus to others, because of behavioral factors, such as good personal hygiene, because of a shorter duration and lower load of poliovirus shedding in stool, compared with younger individuals [17–26]. The operating premise of the global polio eradication program is that interrupting transmission in young children is sufficient to achieve poliovirus eradication. While the obvious success of eliminating poliovirus circulation in India supports this strategy, it does not address whether alternative approaches would have achieved the same goal more quickly and, possibly, more cost-effectively [16].
In India in 2009, however, the assumption that immunizing only children <5 years of age would lead to polio eradication was questioned because disease due to WPV was being detected in older individuals (from 2004 to 2009, 4.2% of cases of paralytic poliomyelitis [127/3050] were reported in individuals ≥5 years of age) and because WPVs of Indian origin were exported to other countries on multiple occasions (ie, Angola in 2005 or Nepal in 2008), suggesting that adult travelers contributed to these outbreaks [27–29]. In addition, multiple serological studies from India showed that a very high proportion of young children were seropositive for poliovirus and therefore protected against polio, particularly against disease due to WPV1 (>98%), and yet low-level circulation of WPV1 continued [15].
Reinfection and shedding of infectious virus in immune individuals has been well documented, as has the fact that enteric mucosal immunity wanes with time [30, 31]. Although young children who are routinely targeted by SIAs have the opportunity to have their mucosal immunity boosted, those outside the target age group will lose mucosal immunity over time. Because these individuals represent the majority of the population, even small individual contributions to virus shedding could, in aggregate, contribute to virus circulation. The relative contribution of different age groups and different immunity status to virus circulation remains very difficult to measure.
In response to the persistence of WPV transmission in northern India, enhanced surveillance for WPV was temporarily set up in 2009 in the 2 Indian regions where the incidence of poliovirus infection was greatest, western Uttar Pradesh and the Kosi river area of Bihar, to assess the potential role of older individuals in WPV circulation by measuring the rate of WPV shedding in different target populations. The surveillance data from Uttar Pradesh were obtained from investigations of household and close neighborhood contacts of patients with confirmed poliomyelitis (Uttar Pradesh Contact Surveillance); the data from Bihar were obtained from community surveillance (Bihar Community Surveillance). This report summarizes the epidemiologic and virologic data from the enhanced surveillance.
METHODS
Uttar Pradesh Contact Surveillance
Stool specimens were collected from asymptomatic contacts of case patients with laboratory-confirmed polio (index cases) and tested for the presence of polioviruses in 18 high-risk districts in western Uttar Pradesh during the high transmission season of 2009 (1 June–31 October). The total population of these 18 districts is estimated to be 42 million.
A contact was defined as a person of any age without polio-related symptoms who resided in physical proximity to the index case. Two types of contacts were sampled: household contacts in the index households and neighborhood contacts from households near the index household (a typical settlement in rural western Uttar Pradesh consists of a cluster of houses surrounded by fields).
We defined a household as a group of people sharing the same kitchen area. Most families in rural and urban areas of western Uttar Pradesh live in walled compounds, often sharing a common space with other families but having their own food preparation area. An index household was defined as a household where a confirmed polio case patient resided. A neighborhood household was defined as a household within the same village as the index household; in some cases, this was within the same cluster of houses but did not involve sharing the same kitchen.
In index households, up to a maximum of 10 individuals irrespective of age were asked to provide 1 stool sample. In neighborhood households, a total of 28 persons were selected at random from multiple households and were also asked to provide 1 stool sample. The contacts from neighborhood households were selected on the basis of their age and included 4 children 0–4 years of age and 8 persons each from the following age groups: 5–9, 10–14, and ≥15 years. To increase variability, a maximum of 4 persons were selected from a single neighborhood household. The selection of neighborhood households was done in concentric circles around the index household until a sufficient number of contacts were identified. The distance in meters between the index and neighborhood households was estimated by enumerators, using subjective observation. A brief questionnaire on demographic characteristics and vaccination history was administered to all contacts in index and neighborhood households. The collection of contact samples was performed as soon as possible after confirmation of the index case.
Bihar Community Surveillance
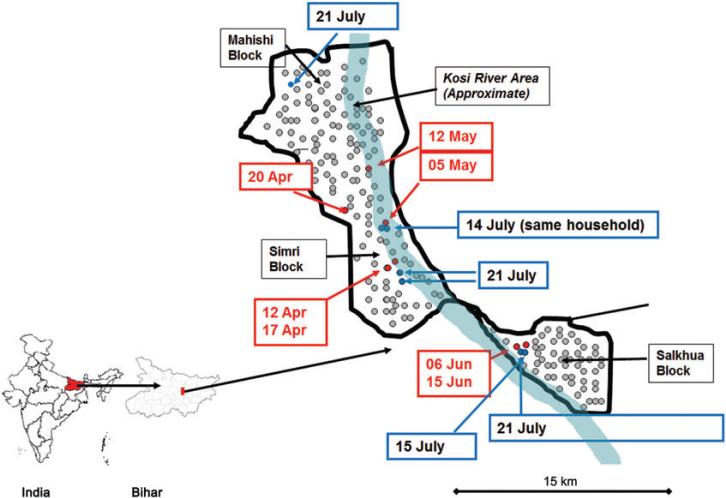
From 12 April to 15 June 2009, 7 WPV1-associated cases of paralytic polio were reported in young and fully immunized children from a small geographic area (referred to as the “Surveillance Zone;” Figure 1) spanning a radius of approximately 15 km within 3 blocks of Saharsa district (Mahishi, Salkhua, and Simri Bakht) inside or near the Kosi river area embankment (blocks in India denote subdivisions of districts). The Surveillance Zone is a rural flood plain area with a largely homogenous, well-defined village population that had limited mobility because of a lack of infrastructure, except during the annual flooding season, when a large proportion of this population migrates to higher ground. The total population of the Surveillance Zone is estimated to be 120 000.
Figure 1.
Geographic distribution of wild poliovirus type 1 (WPV1) cases detected from acute flaccid paralysis surveillance and asymptomatic WPV1 shedders identified by enhanced surveillance, April–July 2009, Bihar, India. The black line denotes the border of the surveillance zone; the red dots denote cases of paralytic polio due to WPV1 detected from 12 April to 15 June 2009 in Saharsa (dates of onset of paralysis are specified in red boxes), blue dots denote asymptomatic WPV1 shedders during contact screening (dates of stool collection are specified in blue boxes), and grey dots denote randomly selected households identified for contact screening in the surveillance zone.
By using simple random sampling, 250 households were selected within the Surveillance Zone. The list of households was available through polio campaign microplans prepared by the polio eradication program. These lists capture the vast majority of households and are updated monthly. In each household, 1 child ≤15 years of age and 1 adult >15 years of age were selected at random.
A brief questionnaire was administered that focused on demographic characteristics, self-reported vaccination history, and travel history. From each person, 2 collections of stool and pharyngeal swab samples were performed, and samples were tested for polioviruses during cycle I and cycle II with an interval of 1 week. Cycle I took place during 13–15 July 2009, and cycle II occurred during 20–22 July 2009, the end of season in which poliovirus transmission was in Bihar.
Laboratory Testing and Data Analysis
In both enhanced surveillance projects, descriptive analysis of the data obtained was performed. Stools and pharyngeal swab samples were tested for the presence of polioviruses in the poliovirus-testing-accredited reference laboratories in India, and, if positive, genetic sequencing was performed and viral titer was calculated in the polio reference laboratory in Mumbai [32, 33]. Excel and EPI Info software were used to analyze the data.
RESULTS
Uttar Pradesh Contact Surveillance
From 1 June to 31 October 2009, 21 WPV1-associated and 442 WPV3-associated paralytic polio cases were detected in 18 high-risk districts of Uttar Pradesh. Contact sampling was performed around 21/21 (100%) WPV1 cases and around 33 of 442 (7.5%) WPV3 cases. During these investigations, 2084 asymptomatic contacts living in 585 households were included (54 were index households and 531 were neighborhood households). The number of investigations around the WPV3 cases was limited because of concurrent resource mobilization needed for response to an ongoing WPV3 outbreak.
Among the 2084 asymptomatic contacts, 1842 (88.4%) provided adequate stool samples in good condition and were tested for the presence of WPV. The remaining 242 contacts did not provide stool samples in sufficient volume for testing.
In 54 index households, stool samples were collected from 368 contacts. An average of 6.8 samples were collected from each index household. The median age of contacts in the index households was 18 years, and the range was 2 months to 75 years; 50.8% were female (Table 1).
Table 1.
Findings of Enhanced Surveillance in Uttar Pradesh, India: Household Description
| Characteristic | Index Households, No. (%) | Neighborhood Households, No. (%) | Total, No. (%) |
|---|---|---|---|
| Households provided samples | 54 (9.2) | 531 (90.8) | 585 (100) |
| At least 1 sample positive for wild poliovirus | 21 (38.9) | 42 (7.9) | 63 (10.8) |
| >1 sample positive for poliovirus | 8 (14.8) | 8 (1.5) | 16 (2.7) |
In 531 neighborhood households, stool samples were collected from 1716 contacts. Samples were collected from an average of 3.2 contacts in each neighborhood household. The median age of contacts in neighborhood households was 11 years, and the range was 1 month to 90 years; 47% were female. (Table 1)
In 63 of 585 households (10.8%), at least 1 healthy person was shedding WPV. Households with at least 1 asymptomatic WPV shedder were significantly more prevalent among index households (38.9%) than among neighborhood households (7.9%; P < .05). This association remained significant when adjusted for the number of stool samples collected in each household (P < .05). In 16 of 585 households (2.7%), >1 person was found to be shedding WPV. The maximum number of WPV shedders per household was 5; the mean was 1.4.
In total, 89 of 1842 contacts (4.8%) had WPV-positive stool samples. Of these, 20 were WPV1 positive and collected around WPV1 index cases, 65 were WPV3 positive and collected around WPV3 index cases, and 4 were WPV3 positive and collected around WPV1 index cases. The latter 4 WPV3-positive samples were found in a single household adjacent to a WPV1 index case and were removed from further analysis because the link to the WPV1 index case was coincidental (Table 2).
Table 2.
Findings of Enhanced Surveillance for Wild Poliovirus in Stool Samples From Asymptomatic Contacts of Case Patients With Polio, Uttar Pradesh, India, 1 June–31 October 2009
| Variable | Contact Investigations Around WPV1 Index Cases, Proportion (%) | Contact Investigations Around WPV3 Index Cases, Proportion (%) | Total, Proportion (%) |
|---|---|---|---|
| Investigations with at least 1 WPV-positive contact/investigations completed | 10/21 (47.6) | 25/33(75.8) | 35/54 (64.8) |
| Contacts with WPV in stool/all contacts tested, by neighborhood type | |||
| All households | 20/638 (3.1) | 65/1204 (5.4) | 85/1842 ( 4.6) |
| Index households | 10/130 (7.7) | 24/208 (11.5) | 34/338 (10.1) |
| Neighborhood households | 10/508 (2.0) | 41/996 (4.1) | 51/1504 (3.4) |
| WPV-positive contacts/all contacts tested, by age | |||
| <5 y | 5/88 (5.7) | 26/177 (14.7%) | 31/265 (11.7) |
| 5–15 y | 13/323 (4.0) | 29/605 (4.8%) | 42/928 (4.5) |
| >15 y | 2/227 (0.9) | 10/422 (2.4%) | 12/649 (1.8) |
| WPV-positive contacts in index households/all contacts tested, byage | |||
| <5 y | 3/18 (16.7) | 9/35 (25.7) | 12/53 (22.6) |
| 5–15 y | 7/41 (17.0) | 9/62 (14.5) | 16/103 (15.5) |
| >15 y | 0/71 (0) | 6/111 (5.4) | 6/182 (3.3) |
| WPV-positive contacts in neighborhood households/all contacts tested, by age | |||
| <5 y | 2/70 (2.9) | 17/142 (12.0) | 19/212 (9.0) |
| 5–15 y | 6/282 (2.1) | 20/543 (3.7) | 26/825 (3.2) |
| >15 y | 2/156 (1.3) | 4/311 (1.3) | 6/467 (1.3) |
Abbreviation: WPV, wild poliovirus.
In our sample, 54 of 85 shedders (63.5%) were older than 5 years. However, the rate of shedding was highest in individuals <5 years old and decreased with increasing age for both WPV1 and WPV3 in both index and neighborhood households (Table 2). The median age of WPV shedders was 6 years and 2 months (interquartile range, 3–11 years). In index households contacts were selected at random, and in neighborhood households they were selected on the basis of their age. However, the proportion of shedders aged >5 years in index households (22/34; 64.7%) and neighborhood households (32/51; 62.7%) was similar.
We did not observe significant differences in vaccination history with OPV between those found to be shedding WPV and those who were not shedding WPV. Among those who knew their vaccination histories (863; 41.4%), the average number of self-reported doses of OPV received was 19, and the proportion of those reporting receiving >3 OPV doses was 96.8%.
The mean distance between index households and neighborhood households was 20 m for households where no shedders were found and 16 m for households where shedders were found; the difference in distance was not statistically significant (P > .05). The range of distance was 0–250 m.
The median number of days between onset of paralysis of index cases and stool collection in contacts was 21 (range, 1–48 days) for index households and 25 (range, 16–89 days) for neighborhood households. This period was not significantly associated with the probability of finding WPV shedders (P > .05).
Bihar Community Surveillance
Of the 250 randomly selected households in the Surveillance Zone of Saharsa district, 138 were in Mahishi block, 75 were in Salkhua, and 37 were in Simri Bakht. Ten of the 250 selected households were locked and excluded. In the remaining 240 households, 214 children ≤15 years of age and 237 adults >15 years of age were randomly selected. From these individuals, we collected 800 stool samples (414 in cycle I and 386 in cycle II) and 843 pharyngeal swab samples (434 in cycle I and 409 in cycle II). Of 800 stools collected, 799 were in good condition and were analyzed. Of 843 pharyngeal samples collected, 835 were in good condition and were analyzed (Table 3).
Table 3.
Results of Tests for Polioviruses (PVs) and Nonpoliovirus Enteroviruses in Stool and Pharyngeal Samples Obtained During Enhanced Community Surveillance in Bihar, India, July 2009
| Pathogen | Positive Stool Specimens, No. (%) | Positive Pharyngeal Swab Specimens, No. (%) |
|---|---|---|
| Poliovirus | ||
| Overall | 20 (2.5) | 1 (0.1) |
| WPV1 | 7 (0.9) | 0 (0) |
| WPV3 | 4 (0.5) | 0 (0) |
| PV1 SL | 3 (0.4) | 1 (0.1) |
| PV2 SL | 6 (0.8) | 0 (0) |
| Nonpoliovirus enterovirus | 100 (12.5) | 5 (0.6) |
| Total | 799 (100) | 835 (100) |
Abbreviations: SL, Sabin like; WPV, wild poliovirus.
The proportion of males in the adult group was 28.7%, compared with 53.7% among the children; a large proportion of the adult male population was seeking temporary work outside of the Kosi river area during the sampling period. Every fourth adult responded that they had slept outside of their village for at least 1 night in the past 30 days, compared with about 1 in 6 children. Most children <5 years of age (93.5%) reported receiving >3 doses of oral polio vaccine. Most of the children 5–14 years of age (76.9%) and adults (96.6%) had an unknown vaccination history. Hindu religion was reported by 83% of households and Muslim religion by 17% of households.
We found 11 WPVs (7 WPV1 and 4 WPV3) in the stool samples. No WPVs were found in pharyngeal samples. We found 9 vaccine (Sabin-like) polioviruses in stool samples (3 type 1 and 6 type 2) and 1 Sabin-like poliovirus type 1 in pharyngeal samples.
The age distribution of those shedding WPV in stool is shown in Table 4. In one household, WPV was found in a young child and in an older person. In all other cases, the WPV shedders lived in separate households. Six of 7 individuals shedding WPV1 resided within a 2-km radius of households with paralytic WPV1 cases reported during April–June 2009 (Figure 1). Mean viral titers were similar in younger and older age groups (range, 1.6–4.5 log10 50% cell culture infectious doses/0.1 mL). The sequencing of polioviruses showed close genetic proximity of the WPV found through enhanced surveillance to the previously detected isolates from patients in Saharsa with paralytic polio. No significant associations were found between reported vaccination history, travel history, sex, or religion and WPV shedding.
Table 4.
Age Distribution of Persons Observed to Be Shedding Wild Poliovirus (WPV) Types 1 and 3, Community Surveillance, Bihar, India, July 2009
| Age | WPV1 Positive, Proportion (%) | WPV3, Proportion (%) |
|---|---|---|
| <5 y | 3/162 (1.9) | 2/162 (1.2) |
| 5–15 y | 3/214 (1.4) | 2/214 (0.9) |
| >15 y | 1/411 (0.2) | 0/411 (0) |
DISCUSSION
The analysis of data from enhanced surveillance in Uttar Pradesh and Bihar demonstrated that a high proportion of persons ≥5 years of age were asymptomatically shedding WPV in stool. In Uttar Pradesh, the total number of WPV shedders ≥5 years of age was higher than the total number of shedders among children <5 years of age. We believe that older individuals shed WPV because their intestinal immunity to poliovirus waned while their humoral immunity persisted. In these persons, humoral immunity prevented development of paralytic disease but failed to prevent intestinal replication and shedding of the poliovirus [34–39].
We observed both household transmission of WPV (among contacts in index households) and community transmission of WPV (among contacts in neighborhood households) in the Uttar Pradesh contact surveillance investigation. It appears that transmission within households, as well as community transmission in all ages and over short distances, played an important role in WPV circulation in western Uttar Pradesh, where there is high population density, crowding, and poor hygiene.
Most of the asymptomatic persons shedding WPV in Bihar were found to reside in the same village as previously reported case patients with paralytic polio. The interval from onset of paralytic polio cases and collection of stool from an asymptomatic WPV shedder was 1–3 months; and up to 2 SIAs with OPV were performed during that interval. WPV in Bihar remained confined to a small geographical area for an extended period, which suggests that conducting SIAs around confirmed WPV cases, perhaps involving older children and young adults, might have interrupted WPV circulation faster.
SIAs with OPV vaccine were performed almost on a monthly basis in this part of Bihar in 2009. We found fewer people shedding vaccine-type polioviruses than WPVs. This finding probably reflects a higher force of infection of WPVs than of vaccine polioviruses, as well as high immunity to polio-virus in most of the population [40–42].
No difference in load of viral shedding in stools was detected between older and younger individuals. However, the ability to interpret data on duration of shedding and viral load was limited because only 1 stool specimen was collected from each person in Uttar Pradesh, and in Bihar, the number of WPV excretors was low. Pharyngeal shedding of WPV likely did not substantially contribute to WPV transmission in Bihar, because we did not find any WPV-positive pharyngeal samples; this is consistent with previous studies that documented pharyngeal shedding of polioviruses to be shorter and less frequent than shedding in stool [35, 43].
These findings provide indirect evidence that older individuals may have infected others and contributed to community transmission of poliovirus and were in part responsible for persisting WPV circulation in India. In recent years, vaccinating older individuals in outbreaks with epidemiologic evidence of cases in older age groups has become a norm and was applied for control of outbreaks in Tajikistan (2010), Republic of Congo (2010–2011), Democratic Republic of Congo (2010–2011), and Namibia (2006). Observations from these outbreaks suggest that including older children and adults in SIAs led to shortening the duration of outbreaks and reduced the number of SIAs needed to control the outbreak (World Health Organization, unpublished data, 2012). Expanding the target age groups for SIAs in polio-endemic areas with similar conditions as those found in northern India (ie, prolonged low-level transmission in highly immune populations), such as select areas of northern Nigeria and Pakistan, might accelerate achievement of global polio eradication.
Acknowledgments
We thank the staff of the National Polio Surveillance Project, for kindly sharing the data from the 2 surveillance projects with us and for their help with data analysis; and to staff of viral laboratories in Mumbai and Ahmadabad, for promptly analyzing laboratory samples.
Footnotes
Supplement sponsorship. This article is part of a supplement entitled “The Final Phase of Polio Eradication and Endgame Strategies for the Post-Eradication Era,” which was sponsored by the Centers for Disease Control and Prevention.
Potential conflict of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Ward NA, Milstien JB, Hull HF, Hull BP, Kim-Farley RJ. The WHOEPI initiative for the global eradication of poliomyelitis. Biologicals. 1993;21:327–33. doi: 10.1006/biol.1993.1092. [DOI] [PubMed] [Google Scholar]
- 2.Global Polio Eradication Initiative [25 February 2013];Cases of wild poliovirus by country and year. http://www.polioeradication.org/Dataandmonitoring/Polio thisweek/Wildpolioviruslist.aspx.
- 3.Global Polio Eradication Initiative [12 October 2011];History of polio. http://www.polio eradication.org/Polioandprevention/Historyofpolio.aspx.
- 4.Progress toward the global interruption of wild poliovirus type 2 transmission, 1999. MMWR Morb Mortal Wkly Rep. 1999;48:736–8. 47. [PubMed] [Google Scholar]
- 5.Centers for Disease C. Prevention. Progress toward poliomyelitis eradication–-India, January 2010–September 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1482–6. [PubMed] [Google Scholar]
- 6.Progress towards poliomyelitis eradication, India, 1998. Wkly Epidemiol Rec. 1998;73:297–300. [PubMed] [Google Scholar]
- 7.Progress towards poliomyelitis eradication in India, 2002. Wkly Epidemiol Rec. 2003;78:66–71. [PubMed] [Google Scholar]
- 8.Progress towards poliomyelitis eradication in India, 2003. Wkly Epidemiol Rec. 2004;79:121–5. [PubMed] [Google Scholar]
- 9.Progress towards poliomyelitis eradication in India, January 2004 to May 2005. Wkly Epidemiol Rec. 2005;80:235–9. [PubMed] [Google Scholar]
- 10.Progress towards poliomyelitis eradication in India, January 2005 to June 2006. Wkly Epidemiol Rec. 2006;81:286–91. [PubMed] [Google Scholar]
- 11.Progress towards poliomyelitis eradication: India, January 2006-September 2007. Wkly Epidemiol Rec. 2007;82:402–7. [PubMed] [Google Scholar]
- 12.Progress towards poliomyelitis eradication in India, January 2007-May 2009. Wkly Epidemiol Rec. 2009;84:281–7. [PubMed] [Google Scholar]
- 13.Grassly NC, Fraser C, Wenger J, et al. New strategies for the elimination of polio from India. Science. 2006;314:1150–3. doi: 10.1126/science.1130388. [DOI] [PubMed] [Google Scholar]
- 14.Grassly NC, Wenger J, Durrani S, et al. Protective efficacy of a monova-lent oral type 1 poliovirus vaccine: a case-control study. Lancet. 2007;369:1356–62. doi: 10.1016/S0140-6736(07)60531-5. [DOI] [PubMed] [Google Scholar]
- 15.Estivariz CF, Jafari H, Sutter RW, et al. Immunogenicity of supplemental doses of poliovirus vaccine for children aged 6–9 months in Moradabad, India: a community-based, randomised controlled trial. Lancet Infect Dis. 2012;12:128–35. doi: 10.1016/S1473-3099(11)70190-6. [DOI] [PubMed] [Google Scholar]
- 16.de Quadros CA, Andrus JK, Olive JM, Guerra de Macedo C, Henderson DA. Polio eradication from the Western Hemisphere. Annu Rev Public Health. 1992;13:239–52. doi: 10.1146/annurev.pu.13.050192.001323. [DOI] [PubMed] [Google Scholar]
- 17.Gelfand HM, Holguin AH. Enterovirous infections in healthy children. Study during 1960. Arch Environ Health. 1962;5:404–11. doi: 10.1080/00039896.1962.10663305. [DOI] [PubMed] [Google Scholar]
- 18.Gelfand HM, Holguin AH, Marchetti GE, Feorino PM. A continuing surveillance of enterovirus infections in healthy children in six United States Cities. I. Viruses isolated during 1960 and 1961. Am J Hyg. 1963;78:358–75. doi: 10.1093/oxfordjournals.aje.a120355. [DOI] [PubMed] [Google Scholar]
- 19.Froeschle JE, Feorino PM, Gelfand HM. A continuing surveillance of enterovirus infection in healthy children in six United States cities. II. Surveillance enterovirus isolates 1960–1963 and comparison with enterovirus isolates from cases of acute central nervous system disease. Am J Epidemiol. 1966;83:455–69. doi: 10.1093/oxfordjournals.aje.a120597. [DOI] [PubMed] [Google Scholar]
- 20.Melnick JL, Paul JR, Walton M. Serologic epidemiology of poliomyelitis. Am J Public Health Nations Health. 1955;45:429–37. doi: 10.2105/ajph.45.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fox JP, Gelfand HM, Leblanc DR, Conwell DP. Studies on the development of natural immunity to poliomyelitis in Louisiana. I. Over-all plan, methods and observations as to patterns of seroimmunity in the study group. Am J Hyg. 1957;65:344–66. doi: 10.1093/oxfordjournals.aje.a119874. [DOI] [PubMed] [Google Scholar]
- 22.Gelfand HM, Leblanc DR, Fox JP, Conwell DP. Studies on the development of natural immunity to poliomyelitis in Louisiana. II. Description and analysis of episodes of infection observed in study group households. Am J Hyg. 1957;65:367–85. doi: 10.1093/oxfordjournals.aje.a119876. [DOI] [PubMed] [Google Scholar]
- 23.Gelfand HM, Le BD, Fox JP, Potash L. Studies on the development of natural immunity to poliomyelitis in Louisiana. III. The serologic response to commercially produced “Salk vaccine” of children totally or partially susceptible to poliovirus infection. Am J Hyg. 1959;70:303–11. doi: 10.1093/oxfordjournals.aje.a120079. [DOI] [PubMed] [Google Scholar]
- 24.Gelfand HM, Le BD, Potash L, Fox JP. Studies on the development of natural immunity to poliomyelitis in Louisiana. IV. Natural infections with polioviruses following immunization with a formalin-inactivated vaccine. Am J Hyg. 1959;70:312–27. doi: 10.1093/oxfordjournals.aje.a120080. [DOI] [PubMed] [Google Scholar]
- 25.Gelfand HM, Fox JP, Leblanc DR, Elveback L. Studies on the development of natural immunity to poliomyelitis in Louisiana. V. Passive transfer of polioantibody from mother to fetus, and natural decline and disappearance of antibody in the infant. J Immunol. 1960;85:46–55. [PubMed] [Google Scholar]
- 26.Potash L, Gelfand HM, Fox JP. Studies on the development of natural immunity to poliomyelitis in Louisiana. VI. The incidence of poliovirus infections during 1958 as an indication of the effect of Salk-type vaccine on virus dissemination. Am J Hyg. 1960;71:418–26. [PubMed] [Google Scholar]
- 27.Kidd S, Goodson JL, Aramburu J, et al. Poliomyelitis outbreaks in Angola genetically linked to India: risk factors and implications for prevention of outbreaks due to wild poliovirus importations. Vaccine. 2011;29:3760–6. doi: 10.1016/j.vaccine.2011.03.034. [DOI] [PubMed] [Google Scholar]
- 28.Centers for Disease C. Prevention. Outbreaks following wild poliovirus importations—Europe, Africa, and Asia, January 2009-September 2010. MMWR Morb Mortal Wkly Rep. 2010;59:1393–9. [PubMed] [Google Scholar]
- 29.Macdonald N, Hebert PC. Polio outbreak in Tajikistan is cause for alarm. CMAJ. 2010;182:1013. doi: 10.1503/cmaj.100831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duintjer Tebbens RJ, Pallansch MA, Chumakov KM, et al. Review and assessment of poliovirus immunity and transmission: synthesis of knowledge gaps and identification of research needs. Risk Anal. 2013;33:606–46. doi: 10.1111/risa.12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duintjer Tebbens RJ, Pallansch MA, Chumakov KM, et al. Expert review on poliovirus immunity and transmission. Risk Anal. 2013;33:544–605. doi: 10.1111/j.1539-6924.2012.01864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kew OM, Nottay BK. Molecular epidemiology of polioviruses. Rev Infect Dis. 1984;6(Suppl 2):S499–504. doi: 10.1093/clinids/6.supplement_2.s499. [DOI] [PubMed] [Google Scholar]
- 33.Deshpande JM, Shetty SJ, Siddiqui ZA. Environmental surveillance system to track wild poliovirus transmission. Appl Environ Microbiol. 2003;69:2919–27. doi: 10.1128/AEM.69.5.2919-2927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Modlin JF, Halsey NA, Thoms ML, Meschievitz CK, Patriarca PA. Humoral and mucosal immunity in infants induced by three sequential inactivated poliovirus vaccine-live attenuated oral poliovirus vaccine immunization schedules. Baltimore Area Polio Vaccine Study Group. J Infect Dis. 1997;175(suppl 1):S228–34. doi: 10.1093/infdis/175.supplement_1.s228. [DOI] [PubMed] [Google Scholar]
- 35.Onorato IM, Modlin JF, McBean AM, Thoms ML, Losonsky GA, Bernier RH. Mucosal immunity induced by enhance-potency inactivated and oral polio vaccines. J Infect Dis. 1991;163:1–6. doi: 10.1093/infdis/163.1.1. [DOI] [PubMed] [Google Scholar]
- 36.Grassly NC, Jafari H, Bahl S, et al. Waning intestinal immunity after vaccination with oral poliovirus vaccines in India. J Infect Dis. 2012;205:1554–61. doi: 10.1093/infdis/jis241. [DOI] [PubMed] [Google Scholar]
- 37.Hird TR, Grassly NC. Systematic review of mucosal immunity induced by oral and inactivated poliovirus vaccines against virus shedding following oral poliovirus challenge. PLoS Pathog. 2012;8:e1002599. doi: 10.1371/journal.ppat.1002599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ogra PL, Okayasu H, Czerkinsky C, Sutter RW. Mucosal immunity to poliovirus. Expert Rev Vaccines. 2011;10:1389–92. doi: 10.1586/erv.11.106. [DOI] [PubMed] [Google Scholar]
- 39.Okayasu H, Sutter RW, Czerkinsky C, Ogra PL. Mucosal immunity and poliovirus vaccines: impact on wild poliovirus infection and transmission. Vaccine. 2011;29:8205–14. doi: 10.1016/j.vaccine.2011.08.059. [DOI] [PubMed] [Google Scholar]
- 40.Mas Lago P, Diaz J, Diaz Gonzalez M, et al. Isolates of poliovirus vaccine and immune response to different doses of oral polio vaccine. Rev Cubana Med Trop. 2005;57:111–9. [PubMed] [Google Scholar]
- 41.Mas Lago P, Gary HE, Jr, Perez LS, et al. Poliovirus detection in waste-water and stools following an immunization campaign in Havana, Cuba. Int J Epidemiol. 2003;32:772–7. doi: 10.1093/ije/dyg185. [DOI] [PubMed] [Google Scholar]
- 42.Fine PE, Carneiro IA. Transmissibility and persistence of oral polio vaccine viruses: implications for the global poliomyelitis eradication initiative. Am J Epidemiol. 1999;150:1001–21. doi: 10.1093/oxfordjournals.aje.a009924. [DOI] [PubMed] [Google Scholar]
- 43.Modlin JF. Mucosal immunity following oral poliovirus vaccine and enhanced potency inactivated poliovirus vaccine immunization. Pediatr Infect Dis J. 1991;10:976–8. doi: 10.1097/00006454-199112000-00031. [DOI] [PubMed] [Google Scholar]