Abstract
Background
Rapid but simple diagnostic tool for detecting drug resistant (DR) tuberculosis (TB) has been acknowledged as important for effective management and control of DR-TB. Our objective was to establish a molecular line-probe assay (GenoType® MTBDRplus) for detecting DR-TB in Ghana.
Method
We first screened 113 Mycobacterium tuberculosis isolates by indirect proportion method and MTBDRplus. For isolates found resistant either by phenotypic DST and/or MTBDRplus, the rpoB, and katG genes as well as the promoter regions of oxyR-ahpC and inhA were sequenced to identify mutations. We then analyzed an additional 412 isolates by MTBDRplus only.
Results
Forty-three (8.2%) and 8 (1.5%) isolates were resistant to isoniazid (INH) and rifampicin (RIF), respectively, and 8 (1.5%) were multidrug-resistant. Among these resistant isolates, mutations in codon 450 of rpoB and codon 315 of katG conferring resistance to RIF and INH, respectively, dominated. We found two RIF resistant isolates with S450L substitution each harboring an additional mutation at S388L and Q409R, respectively. Using the phenotypic testing as gold standard, the MTBDRplus assays showed a sensitivity/specificity for the detection of RIF and INH resistance and MDR of 100%/100%, 83.3%/100% and 100%/100%, respectively.
Conclusion
The high sensitivity makes MTBDRplus a valuable addition to the conventional TB diagnostic algorithm in Ghana.
Introduction
Tuberculosis (TB) continues to be a major public health problem globally, with an annual incidence of 9 million new cases, killing more than 1.5 million people annually, most of which occurs in low resource countries.1 One of the main challenges in TB control is the emergence and spread of drug resistance (DR).2, 3 Even though TB is a treatable disease, if DR is not controlled, it may eventually result in TB becoming untreatable. Multidrug resistance (MDR) is defined as resistance to at least isoniazid (INH) and rifampicin (RIF).3 According to 2014 WHO Global Tuberculosis Control Report, there were about nine million TB cases and among these, close to 480,000 were MDR cases.1 In 2005, the global Technical and Advisory Group on TB approved a new Stop TB Strategy and indicated in addition to many other things, for effective control of TB, DR-TB must be well managed.4
Drug resistance arises due to improper use of antibiotics in chemotherapy such as inadequate treatment regimens, and failure to ensure that patients complete the whole course of treatment.5 When a patient is infected with a drug-susceptible strain of Mycobacterium tuberculosis complex (MTBC), poor adherence to treatment will lead to a drug resistant form of the disease; this type of drug resistance is termed acquired drug-resistance. Individuals who develop active disease with a drug-resistant MTBC strain can transmit this form of TB to other individuals, if not detected early and treated appropriately. New TB patients initially infected with a drug-resistant form are termed primary resistant cases.6 To reduce the emergence and subsequent spread of drug-resistant TB, there is the need for early diagnosis so as to put patients on appropriate drugs as soon as possible.6,7.
The conventional methods for drug susceptibility testing (DST) are labour intensive, involving sequential procedures for isolation of mycobacteria from clinical specimen in liquid or solid media, identification of MTBC, and in vitro testing of susceptibility to anti-TB drugs. At the same time, MTBC is a slow growing organism taking several weeks for macroscopic growth and requiring biosafety level 3 containment. Thus standardized and optimised MTBC culture and DST procedures require well equipped and safe laboratories, as well as trained personnel operating under quality assured protocols. Because of these factors, it takes several weeks to months for laboratory results to become available, and during this time, patients may be prescribed inadequate treatment, thus fuelling the development and/or spread of drug resistance. Moreover, mycobacterial culture and DST capabilities are severely limited in resource-poor countries.
Resistance to anti-TB drugs is caused by chromosomal mutations in genes encoding drug targets, in regulatory regions of the target gene and in drug-activating genes. Several molecular diagnostic methods have been developed recently for rapid identification of MDR-TB, some of which are also suitable for resource-poor countries.6,8,9,10 In this study, we established the line probe assay (LPA) known as MTBDRplus in Ghana, and compared the results to the standard phenotypic DST using the indirect proportion method.11
Materials and Methods
Mycobacterial Isolates
This was a cross-sectional analytical study in which, all consecutive individuals, diagnosed with smear-positive pulmonary TB cases attending six TB diagnostic health facilities in three regions of Ghana were enrolled between October 2007 and July 2009. Isolates used in this study were cultivated in a previous study that aimed to genotype isolates from Ghana for phylogenetic and molecular epidemiological analysis.12 The procedures used for sample collection, diagnosis and treatment of TB was as routinely employed by the National Tuberculosis Programme (NTP); however the protocol was reviewed by the institutional review board of the Noguchi Memorial Institute for Medical Research (NMIMR), with federal-wide assurance number FWA00001824. The isolates which were previously stored at −80 °C were sub-cultured on Lowenstein-Jensen media slants, incubated at 37 °C until confluent growth was observed. After harvest, the pellet was heat inactivated at 95 °C in nuclease free water for 60 min and allowed to cool under room temperature. The heat-inactivated cells in 1.5 mL microfuge tubes were centrifuged at 14,000 rpm to pellet cells for DNA extraction.
Isolation of Genomic DNA
After harvest, the pellet was heat inactivated at 95 °C in nuclease free water for 60 min and allowed to cool under room temperature. The heat-inactivated cells in 1.5 mL microfuge tubes were centrifuged at 14,000 rpm to pellet cells for DNA extraction. Genomic DNA was extracted according to the protocol outlined by van Soolingen et al., 1993.13 Briefly, the mycobacterial cell wall was disrupted by adding lysozyme (50 μL lysozyme of 10 mg/mL) vortexed and incubated overnight, followed by addition of 75 μL of 10% SDS, 10 μL proteinase K (20 mg/mL), vortexed softly and incubated 15 min at 65 °C. After, we added 100 μL of 5M NaCl followed by 100 μL CTAB/NaCl which was pre-warmed at 65 °C. After vortexing, the extracted DNA was purified by chloroform/ isoamyl alcohol extraction. The DNA contained in the upper phase was precipitated with isopropanol and washed with ethanol. The dried DNA was then re-suspended in 100 mL of water.
Anti-TB Drug Susceptibility Testing
Phenotypic Drug Susceptibility Testing
The indirect proportion method with LJ slants using critical concentrations of INH (Sigma, I3377) (0.2 μg/mL) and RIF (Sigma, R3501) (40 μg/mL) was used to screen 113 isolates. Drug resistance was expressed as the proportion of colonies that grew on drug containing medium to drug-free medium and the critical proportion for resistance was 1%.11
Molecular Drug Susceptibility Testing by Line Probe Assay
Clinical MTBC isolates were screened for their susceptibility to INH and RIF using the Genotype MTBDRplus (Hain lifescience), according to the manufacturer’s protocol10. Drug resistance was expressed as the absence of wild-type band, presence of mutation band or both.
Mutation Analysis of Drug Targets
The isolates diagnosed as drug-resistant either by phenotypic or LPA were used for targeted DNA sequence analyses. Four resistance genes, rpoB (RIF), katG and promoter regions of inhA and oxyR-ahpC (INH), were amplified by PCR for direct DNA sequencing. The PCR reaction in all instances contained 3 μL of 10X buffer, 1.8 μL of 15 mM MgCl2, 3 μL of Q solution, 0.6 μL of 10 mM dNTP mix, 1.8 μL of each primer, 0.2 μL of Hot-start Taq polymerase from Qiagen, 14.8 μL of nuclease-free water and 3 μL of template DNA. Cycling conditions were: initial denaturation at 95 °C for 5 min and 35 cycles of denaturation at 96 °C for 1 min, annealing at primer-specific Tm (Table 1) for 1 min, extension at 68 °C for 1 min and final extension at 72 °C for 10 minutes and the obtained amplicons were sequenced by outsourcing.
Table 1. The primers used for the DNA sequencing assay.
| Gene | Primer Name | Primer sequence (5′-3′) | Amplicon size | Tm |
|---|---|---|---|---|
| inhApro | Ko3 | GGCACGTACACGTCTTTATGTA | 478 bp | 65 °C |
| Ko4 | GGTGCTCTTCTACCGCCGTGAA | |||
| katG | Ko11 | CCAGCGGCCCAAGGTATC | 850 bp | 66 °C |
| Ko12 | GCTGTGGCCGGTCAAGAAGAAGT | |||
| rpoB | Ko1 | GTAGTCCACGCCGTAAACGG | 601 bp | 65 °C |
| Ko2 | ACGTCCATGTAGTCCACCTCAG | |||
| oxyR-ahpC | Ko56 | ACCACTGCTTTGCCGCCACC | 236 bp | 70 °C |
| Ko57 | CCGATGAGAGCGGTGAGCTG |
Data Analysis
Data obtained from the various tests were double entered and validated to remove duplicates and data entry inconsistencies. The DNA sequence reads were screened for possible mutations by comparing the gene sequences with corresponding sequences from H37Rv genome downloaded from the Tuberculist database using the Staden software.14 DNA sequencing was repeated for all isolates with un-reported mutation(s) for verification. The result of the phenotypic DST assay was used as the gold standard to calculate the sensitivity and specificity for detecting INH and RIF resistance by LPA.
Results
Phenotypic Susceptibility test, GenoType® MTBDRplus and Mutations in Drug Resistance Genes
We determined resistance profiles of 113 isolates phenotypically using the indirect proportion method. These 113 isolates form a subset of the total 525 isolates used in this study and was consecutively selected. Comparative analysis demonstrated good overall agreement between the LPA and phenotypic DST results. Ten out of the 12 (83.3%) phenotypically INH mono-resistant isolates were also found resistant by MTBDRplus assay (Table 2). From the DNA sequencing analyses, all the 10 INH mono-resistant isolates identified by MTBDRplus showed katG substitution S315T. The remaining 2 isolates phenotypically resistant to INH had no mutation in any of the target genes we sequenced. Both RIF mono resistant and both MDR isolates, diagnosed resistant by phenotypic DST were confirmed by MTBDRplus. DNA sequencing showed that both RIF mono-resistant isolates(Table 2) had H445Y rpoB amino acid substitution whereas one MDR isolate had katG S315T with rpoB S450L and the other katG S315T with rpoB D435V (Table 2).
Table 2. Correlation between phenotypic DST, MTBDRplus assay and target sequencing analyses.
| Isolate | INH | RIF | ||||
|---|---|---|---|---|---|---|
| Phenotype | MTBDRplus | Mutation | Phenotype | MTBDRplus | Mutation | |
| TBNM008 | R | R | KatG S315T | S | S | - |
| TBNM016 | R | R | KatG S315T | S | S | - |
| TBNM022 | R | R | KatG S315T | S | S | - |
| TBNM059 | R | R | KatG S315T | S | S | - |
| TBNM072 | S | S | - | R | R | rpoB H445Y |
| TBNM078 | R | R | KatG S315T | R | R | rpoB S450L |
| TBNM082 | R | R | KatG S315T | S | S | - |
| TBNM086 | S | S | - | R | R | rpoB H445Y |
| TBNM114 | R | R | KatG S315T | S | S | - |
| TBNM117 | R | R | KatG S315T | S | S | - |
| TBNM139 | R | S | - | S | S | - |
| TBNM147 | R | R | KatG S315T | R | R | rpoB D435V |
| TBNM148 | R | R | KatG S315T | S | S | - |
| TBNM155 | R | S | - | S | S | - |
| TBNM169 | R | R | KatG S315T | S | S | - |
| TBNM171 | R | R | KatG S315T | S | S | - |
Drug Susceptibility Testing with GenoType® MTBDRplus
Overall, 525 isolates were analysed by GenoType® MTBDRplus in this study. These came from a retrospective collection and have all been confirmed using IS6110-PCR and LSP as members of the MTBC.12 As summarised in Table 3, MTBDRplus identified 43/525 (8.2%), 8/525 (1.5%) and 8/525 (1.5%) of the isolates as INH-mono-resistant, RIF mono-resistant and MDR, respectively, and 59 (11.2%) harboured at least one drug resistance mutation.
Table 3. Summary of MTBDRplus Assay Results of 525 MTBC Isolates.
| Resistance | Isolates | Locus | WT Band | MT Band | WT and MT bands |
|---|---|---|---|---|---|
| INH Only | 43 (8.2%) | katG (37) | 2 | Mt1 (23) Mt2 (1) |
WT / Mt1 (9) WT / Mt2 (2) |
| inhApro (4) | - | - | WT1 / Mt1 (3) WT2 / Mt3A (1) |
||
| Both (2) | - | katG Mt1/ inhApro Mt1 (2) | - | ||
| RIF Only | 8 (1.5%) | RRDR (8) | - | Mt2A / 2B (1) Mt2B (1) Mt3 (2) |
WT7 / Mt2A (2) WT8 / Mt3 (2) |
| MDR | 8 (1.5%) |
rpoB / katG.(6) rpoB/ katG /inhApro (2) |
- |
katG Mt1 / rpoB Mt1 (1) rpoB Mt2A / katG Mt1 (1) rpoB Mt3 / katG Mt1 (2) katG Mt1/ inhApro Mt3A / rpoB Mt2A (1) |
katG WT / rpoB Mt3 (1) rpoB WT7 / katG WT /inhApro WT/ rpoB Mt2A/katG Mt1/inhApro Mt3A (1) rpoB WT7/katG WT/rpoB Mt2A/ katG Mt1 (1) |
| ANY | 59 (11.2%) | - | - | - |
NB: INH Only: - Isolates that had mutation (s) in the inhApro region and or in the katG gene without any mutation in the rpoB gene.
RIF Only: - Isolates with mutation(s) in the rpoB gene without any in the inhApro or the katG gene.
MDR: - Isolates with mutations in rpoB gene and inhApro and/or katG gene.
ANY: - Total number of isolates with at least one mutation.
R RRDR: - Rifampicin Resistance Determining Region of the rpoB gene
WT:-Wild-type band absent
MT: - Mutation band present
Among the INH-mono resistant strains, 37/43 (86.0%) had mutation(s) within the katG target only; and of these, 23/37 (62.2%) and 1/37 (2.7%) had katG Mt1 and katG Mt2 mutation bands, respectively. Nine out of the 37 (24.3%) isolates had both presence of katG Mt1 band and absence of a wild type band, while 2 (5.4%) had both katG Mt2 band present and absence of a wild type band. Four out of the 43 INH resistant isolates (9.3%) had mutation(s) within the inhA promoter region alone; of these, 3/4 (75%) had both inhA Mt1 band present and absence of inhA WT1 band and the remaining 1/4 (25%) isolate had inhA Mt3A present and WT2 band absent. The remaining 2 of the 43 (4.7%) INH resistant isolates had both KatG Mt1 and inhA Mt1 bands (Table 3).
Four of the 8 (50.0%) RIF mono-resistant isolates were identified by the presence of mutation bands only; 2 had rpoB Mt3, 1 each had rpoB Mt2B and both rpoB Mt2A and rpoB Mt2B bands respectively. Of the 4 (50.0%) remaining RIF resistant isolates, 2 had rpoB Mt3 band present as well as absence of rpoB WT8 band, while the other 2, had rpoB Mt2A band and absence of rpoB WT7 band (Table 3). The mutations associated with the MDRs are also indicated in Table 3.
Frequency of Mutations in Isoniazid and Rifampicin Resistance Associated Targets
Based on the GenoType® MTBDRplus results, out of the 51 INH resistant isolates, 16 (31.4%) had mutations in the promoter region of inhA (Table 4; Figure 1A). Moreover, 42/51 (82.4%) isolates had the S315T katG mutation which is generally the most prominent INH resistance associated mutation found in clinical isolates.15 In addition to the above mentioned non-synonymous SNPs, we found several synonymous mutations (Table 4; Figure 1).
Table 4. Mutations identified from the DNA sequencing of INH and RIF resistance associated loci.
| Gene (Number of Isolates Screened) | Mutation | Effect of Mutation | Number of isolates with specific SNP |
|---|---|---|---|
| inhApro (51) | −8T/C | - | 2 (3.9%) |
| −15C/T | - | 4 (7.8%) | |
| −47G/C | - | 5 (9.8%) | |
| −102G/C | - | 5 (9.8%) | |
|
|
|||
| katG (51) | G944C & C723G | S315T & P241P | 1 (1.9%) |
| G944C | S315T | 39 (76.5%) | |
| G944A, A949G & C723G | S315N, I317V & P241P | 1 (1.9%) | |
| G(C)944(5)C(T) | S315T | 1 (1.9%) | |
| G944C & C1132T | S315T & L378L | 1 (1.9%) | |
|
|
|||
| rpoB (16) | C1163T & C1349T | S388L* & S450L | 1 (6.3%) |
| A1226G & C1349T | Q409R@ & S450L | 1 (6.3%) | |
| C1349T | S450L | 5 (31.3%) | |
| C1333T | H445Y | 5 (31.3%) | |
| C1333G | H445D | 2 (12.5%) | |
| A1334G | H445R | 1 (6.3%) | |
| A1304T | D435V | 1 (6.3%) | |
NB: The reference gene (rpoB) used here is the MTBC (H37Rv) and not the E. coli variant.
-compensatory rpoB mutation identified by whole genome sequencing of rifampicin resistant M. tuberculosis isolates (Comas et al., 2012).
- a novel mutation which may be a compensatory rpoB mutation.
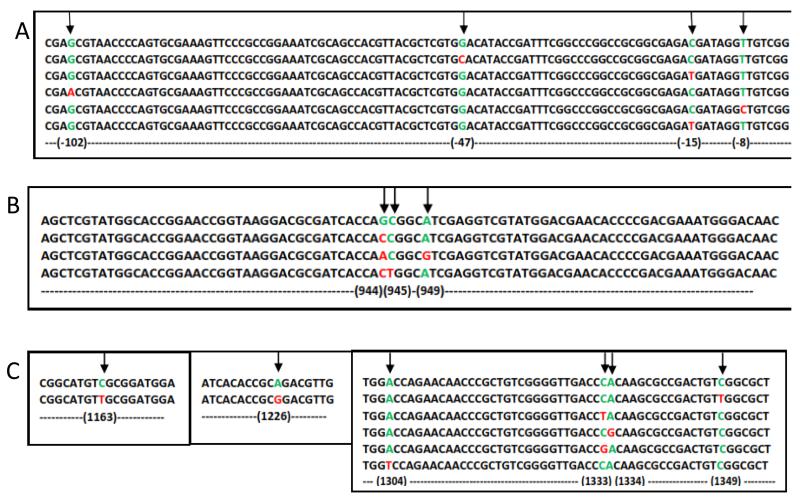
Figure 1.
Alignment of sequenced genes with corresponding genes of H37Rv. A is the alignment of inhApro sequence of some INH resistant isolates with that of H37Rv, B is the alignment of katG gene sequence of the INH resistant isolate with that of H37Rv and C is the alignment of rpoB gene sequence of the RIF resistant isolates with that of H37Rv. At locus of mutation, wild-type nucleotides are shown in green and mutants red.
All the 16 RIF resistant isolates had at least one mutation within the resistance-determining region (RRDR) of the rpoB gene (Table 4; Figure 1). Five isolates (31.25%) each had the SNPs C1349T and C1333T translated as S450L and H445Y, respectively; 2 isolates with C1333G translated as H445D, 1 isolate each with SNP A1334G and A1304T translated as H445R and D435V, respectively, and lastly, 1 isolate each with double SNPs C1163T/C1349T and A1226G/C1349T, respectively, translated as S388L/S450L and Q409R/S450L (Table 4).
Discussion
We analysed 525 MTBC isolates from patients with pulmonary TB for drug resistance by the genotype MTBDRplus assay and identified RIF mono-resistance in 8 (1.5%), INH mono-resistance in 43 (8.2%) and MDR in 8 (1.5%) of the isolates. The level of INH resistance and MDR cases found in this study as compared to our previous study15 was not significantly different- INH (p=0.117) and MDR (p=0.257)- respectively. However we observed a significant reduction in RIF mono-resistance (p=0.005). The observed reduction in the level of drug resistance could be due to the intensification of control activities by the National Tuberculosis Control Program under the global fund to improve compliance and access to quality care. In all, 59 (11.2%) isolates showed any form of resistance. The proportion of INH resistance as measured by the MTBDRplus was found to be significantly higher than that for RIF among our clinical isolates from Ghana (p <0.001); this supports our earlier findings using the proportion method.15 The observed proportion of MDR is similar as reported by Homolka et al. between 2001 and 200416 and comparable to the 1.9% reported by the National Control Programme in 20131. These findings indicate that the MDR rate in Ghana is low and has been stable for about a decade.
It has been shown that association of RIF resistance with mutations within the RRDR varies from 78% to 100% in different countries17, 18. Among the isolates that we worked on, all phenotypically RIF resistant strains were also detected by the MTBDRplus. Thus, we sequenced the RRDR of all isolates that had some form of RIF resistance and found that all the 16 RIF resistant isolates had at least one non synonymous mutation within the RRDR. The role of the new mutation Q409R we detected from the sequencing cannot at the moment be inferred from the available findings. Overall, our results strongly support the use of diagnostics that target mutations within the RRDR of the MTBC as a rapid laboratory DST to support patients care in Ghana.
Contrary to RIF resistance, MTBC acquires isoniazid (INH) resistance through mutations in multiple genes such as those involved in mycolic acid biosynthesis and cellular response to oxidative stress.19, 20, 21. Similar to other settings, 43/51 (84.3%) of INH resistant isolates had mutations within katG with 42 isolates having the katG mutation S315T and the remaining isolate harbouring S315N, one novel amino acid substitution I317V and an additional synonymous mutation 723C/G. Two out of the 42 katG S315T mutant isolates in addition had the additional silent mutations at nucleotide position 723 (C/G) and 1132 (C/T), respectively. In total, 16/51 (31.4%) of the INH resistant isolates were found to have mutations within the inhA promoter region; 10/16 (62.5%) of the inhApro mutant isolates also had the S315T katG mutation. Four and two out of the six inhApro mutant isolates without the S315T katG mutation respectively were −15C/T and −102G/A. These findings compare with other reports as it is known that mutations in katG are responsible for 50% to 95% of INH resistant strains and inhA promoter mutations in 10-30% of strains22,23,24 The role of the new mutations identified in this work were not studied further here but are worth pursuing. Within the isolates that were analysed, we did not find mutations within the promoter region of the oxyR-ahpC contrary to an earlier work done on some Ghanaian MTBC isolates.16
In summary, we found a good correlation between phenotypic RIF resistance and mutation within resistant conferring targets, making rapid diagnostic test (MTBDRplus line-probe assay) that explore these mutations a good tool for detection of RIF mono-resistant and MDR cases in Ghana. Currently the algorithm for laboratory support for TB case management has been established which includes line probe assay in Ghana; cases that do not convert after 2 months intensive anti-tuberculosis drugs are rapidly tested for INH and RIF resistance. Nevertheless, misdiagnosis of approximately 14.3% of INH resistant isolates (from 113) as susceptible by MTBDRplus line-probe assay is worrying. Considering that 14.3% INH resistant isolates and close to 2% of all isolates are MDR means 10% MDR cases may be misdiagnosed as RIF mono-resistant leading to continual transmission of MDR-TB.
Acknowledgement
The authors express their gratitude to all laboratory staff of the various health facilities from which samples were collected. In addition we thank staff of the Bacteriology Department of the Noguchi Memorial Institute for medical Research. This work was supported by the Leverhulme-Royal Society Africa Award (award number AA080019), the Wellcome Trust (097134/Z/11), and the Swiss National Science Foundation (PP00P3_150750).
References
- 1.World Health Organization (WHO) Global tuberculosis report. 2014.
- 2.Ormerod LP. Multidrug-resistant tuberculosis (MDR-TB): Epidemiology, prevention and treatment. British Medical Bulletin. 2005;73:74–17. doi: 10.1093/bmb/ldh047. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization (WHO) Global report on multidrug and extensively drug-resistant TB (M/XDR-TB) Geneva: 2010. [Google Scholar]
- 4.World Health Organization (WHO) Fifth Meeting. Strategic and Technical Advisory Group for Tuberculosis; 20-22 June 2005; World Health Organization; [Google Scholar]
- 5.World Health Organization (WHO) Drug- and multidrug resistant tuberculosis (D/MDR-TB) Geneva: 2010. [Google Scholar]
- 6.Banerjee R, Schecter GF, Flood J, et al. Extensively drug-resistant tuberculosis: new strain, new challenges. Expert Review of Anti-Infective Therapy. 2008;6(5):713–724. doi: 10.1586/14787210.6.5.713. [DOI] [PubMed] [Google Scholar]
- 7.Blower S, Supervie V. Predicting the future of XDR tuberculosis. Lancet Infect Dis. 2007;7(7):443. doi: 10.1016/S1473-3099(07)70143-3. [DOI] [PubMed] [Google Scholar]
- 8.Drobniewski FA, Hoffner S, Rusch-Gerdes S, et al. WHO European Laboratory Strengthening Task Force. Recommended standards for modern tuberculosis laboratory services in Europe. Eur Respir J. 2006;28:903–909. doi: 10.1183/09031936.06.00084906. [DOI] [PubMed] [Google Scholar]
- 9.GenoType MTBDRplus: Version 1.0 [product insert] [Internet] Hain Lifescience, GmbH; Nehren, Germany: [updated 2007 Apr 3; accessed 2007 May 9]. Feb, 2007. [Google Scholar]
- 10.Marinus B, Heidi A, Gerrit C, et al. Rapid Molecular Screening for Multidrug-Resistant Tuberculosis in a High-Volume Public Health Laboratory in South Africa. Am J Respir Crit Care Med. 2008;177:787–792. doi: 10.1164/rccm.200709-1436OC. [DOI] [PubMed] [Google Scholar]
- 11.Canetti G, Fox W, Khomenko A, et al. Advances in techniques of testing mycobacterial in tuberculosis control programmes. Bull. WHO. 1969;41:21–43. [PMC free article] [PubMed] [Google Scholar]
- 12.Asante-Poku A, Yeboah-Manu D, Otchere ID, et al. Mycobacterium africanum Is Associated with Patient Ethnicity in Ghana. PLoS Negl Trop Dis. 2015;9(1):e3370. doi: 10.1371/journal.pntd.0003370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Soolingen D, de Hass EW, Hermans PW, et al. Comparison of various repetitive DNA elements as genetic markers for strain differentiation and epidemiology of Mycobacterium tuberculosis. J Clin Microbiol. 1993;31:1987. doi: 10.1128/jcm.31.8.1987-1995.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Staden R, Beal KF, Bonfield JK. The STADEN Package. In: Misener S, Krawetz SA, editors. Bioinformatics Methods and Protocols. Volume 132. The Humana Press Inc.; Totow, NJ: 1998. pp. 115–130. (Computer Methods in Molecular Biology, Volume 132). [Google Scholar]
- 15.Yeboah-Manu D, Asante-Poku A, Bodmer T, et al. Genotypic Diversity and Drug Susceptibility Patterns among M. tuberculosis Complex Isolates from South-Western Ghana. PLoS ONE. 2011;6(7):e21906. doi: 10.1371/journal.pone.0021906. doi:10.1371/journal.pone.0021906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Homolka S, Meyer CG, Hillemann D, et al. Unequal distribution of resistance-conferring mutations among Mycobacterium tuberculosis and Mycobacterium africanum strains from Ghana. Intl J Med Microbiol. 2010;300(7):489–495. doi: 10.1016/j.ijmm.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 17.Telenti A, Imboden P, Marchesi F, et al. Detection of rifampicin-resistance mutations in Mycobacterium tuberculosis. Lancet. 1993;341:647–650. doi: 10.1016/0140-6736(93)90417-f. [DOI] [PubMed] [Google Scholar]
- 18.Hillemann D, Weizenegger M, Kubica T, et al. Use of the genotype MTBDR assay for rapid detection of rifampin and isoniazid resistance in Mycobacterium tuberculosis complex isolates. J Clin Microbiol. 2005;43:699–703. doi: 10.1128/JCM.43.8.3699-3703.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zyang Y, Yew WW. Mechanisms of drug resistance in Mycobacterium tuberculosis. Int J Tuberc Lung Dis. 2009;13(11):1320–1330. [PubMed] [Google Scholar]
- 20.Ozturk CE, Sanic A, Kaya D, et al. Molecular Analysis of Isoniazid, Rifampin and Streptomycin Resistance in Mycobacterium tuberculosis Isolates from Patients with Tuberculosis in Duzce, Turkey. Japan J Infect Dis. 2005;58(5):309–312. [PubMed] [Google Scholar]
- 21.Costa ERD, Ribeiro MO, Silva MSN, et al. Correlations of Mutations in katG, oxyR-ahpC and inhA Genes and in Vitro Susceptibility in Mycobacterium tuberculosis Clinical Strains Segregated by Spoligotype Families from Tuberculosis Prevalent Countries in South America. BMC Microbiol. 2009;9:39. doi: 10.1186/1471-2180-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poudel A, Nakajima C, Fukushima Y, et al. Molecular characterization of multidrug-resistant Mycobacterium tuberculosis isolated in Nepal. Antimicrob Agents Chemother. 2012;56(6):2831–2836. doi: 10.1128/AAC.06418-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramaswami S, Musser JM. Molecular genetic basis of antimicrobial agent resistance in Mycobacterium tuberculosis. Tuberc Lung Dis. 1998;79:3–29. doi: 10.1054/tuld.1998.0002. [DOI] [PubMed] [Google Scholar]
- 24.Slayden RA, Barry CE. The genetics and biochemistry of isoniazid resistance in Mycobacterium tuberculosis. Microb Infect. 2000;2:659–669. doi: 10.1016/s1286-4579(00)00359-2. [DOI] [PubMed] [Google Scholar]