Abstract
Objective
Clinical studies suggest that platelet P2Y12 inhibitors reduce mortality from sepsis, although the underlying mechanisms have not been clearly defined in vivo. We hypothesized that P2Y12 inhibitors may improve survival from sepsis by suppressing systemic inflammation and its prothrombotic effects. We therefore determined whether clopidogrel and the novel, more potent P2Y12 inhibitor, ticagrelor, modify these responses in an experimental human model.
Approach and Results
We randomized 30 healthy volunteers to ticagrelor (n=10), clopidogrel (n=10) or no antiplatelet medication (controls; n=10). We examined the effect of P2Y12 inhibition on systemic inflammation, which was induced by intravenous injection of E.coli endotoxin. Both P2Y12 inhibitors significantly reduced platelet-monocyte aggregate formation and peak levels of major pro-inflammatory cytokines, including TNFα, IL-6 and CCL2. In contrast to clopidogrel, ticagrelor also significantly reduced peak levels of IL-8 and G-CSF and increased peak levels of the anti-inflammatory cytokine IL-10. In addition, ticagrelor altered leukocyte trafficking. Both P2Y12 inhibitors suppressed D-dimer generation and scanning electron microscopy revealed that ticagrelor also suppressed prothrombotic changes in fibrin clot ultrastructure.
Conclusions
Potent inhibition of multiple inflammatory and prothrombotic mechanisms by P2Y12 inhibitors demonstrates critical importance of platelets as central orchestrators of systemic inflammation induced by bacterial endotoxin. This provides novel mechanistic insight into the lower mortality associated with P2Y12 inhibitors in patients with sepsis in clinical studies.
Keywords: Platelets, P2Y12, antiplatelet therapy, systemic inflammation
Introduction
Sepsis is one of the most devastating clinical syndromes in medicine and severe sepsis still has a mortality rate of 20-30% and remains resistant to specific pharmacological therapy.1 Sepsis is characterized by dysregulated systemic inflammatory response to bacterial components, such as endotoxin (lipopolysaccharide; LPS).1 Excessive innate immune activation causes a pro-inflammatory cytokine storm, extravasation of activated neutrophils and disturbances of the coagulation system, leading to collateral host tissue damage and increased mortality.1,2 A number of pathological processes, such as sepsis, involve the formation of platelet-leukocyte aggregates.3 These platelet-leukocyte interactions have a potentially important role in the pathogenesis of inflammation as they augment leukocyte production of pro-inflammatory cytokines, leukocyte recruitment and activation of coagulation.3,4 However, the overall magnitude of the contribution of platelets to systemic inflammation and the pathophysiology of human sepsis is not well defined.
Platelet P2Y12 inhibitors, such as clopidogrel and ticagrelor, inhibit a central ADP-mediated amplification pathway and therefore blunt a broad spectrum of platelet functions.5 It is well established that the antithrombotic effect of this is beneficial for patients with atherothrombosis, which has led to P2Y12 medications becoming some of the most commonly prescribed medications worldwide. However, in addition, this inhibits the formation of platelet-leukocyte aggregates, which is primarily mediated by inhibition of platelet expression of the adhesion molecule P-selectin.3 A very recent cohort study of 683,421 patients with sepsis has shown that current use of antiplatelet therapy is independently associated with a significant reduction in mortality from sepsis (odds ratio [OR] 0.78; 95% confidence interval [CI] 0.76 – 0.79; p < 0.001).6 This also corresponds with previous observational studies that suggest that clopidogrel reduces mortality from sepsis.7,8 However, the mechanisms underpinning this reduction in mortality have not been clearly demonstrated in vivo, since animal models of sepsis conflict regarding the immunomodulatory effects of clopidogrel, which may be species dependent.9-12
Ticagrelor is a novel P2Y12 inhibitor that causes more potent and consistent P2Y12 inhibition than clopidogrel13 and also weakly inhibits cellular uptake of adenosine.14 In the PLATelet inhibition and patient Outcomes (PLATO) study of over 18,000 patients with acute coronary syndromes (ACS), ticagrelor reduced all-cause mortality compared to clopidogrel (HR 0.78; p<0.001), which was out of proportion to its incremental cardiovascular benefit.15 Intriguingly, ticagrelor was associated with lower mortality related to infection (HR 0.67; p<0.05)16,17 and fewer deaths following sepsis and pulmonary infections than clopidogrel.18
We therefore sought to determine the mechanistic impact of P2Y12 inhibitors on pathophysiological processes that are central to sepsis responses in humans. We hypothesized that P2Y12 inhibitors may reduce mortality from sepsis by suppressing systemic inflammation and its prothrombotic effects, mediated by inhibition of platelet-leukocyte interactions. We hypothesised that the more potent P2Y12 inhibitor, ticagrelor, suppresses these responses more potently than clopidogrel. To test these hypotheses in humans, we used a well-established model of systemic inflammation, which involves intravenous injection of E.coli endotoxin (lipopolysaccharide; LPS) into healthy volunteers.19 The particular strength of this unique model is that it allows direct assessment of dynamic cellular and molecular pathways that are also major mediators of the pathophysiology of sepsis in humans.
Materials and Methods
We randomized 30 healthy volunteers to ticagrelor (n=10), clopidogrel (n=10) or no antiplatelet medication (controls; n=10) to determine their effect on systemic inflammation, which was induced by intravenous injection of E.coli endotoxin (2 ng/kg lipopolysaccharide [LPS]) using a well-established method. Additional details on the Materials and Methods are available in the online-only Data Supplement.
Results
Baseline characteristics were comparable in all of the treatment groups (Table I in the online data supplement). Thirty healthy volunteers underwent LPS administration (see CONSORT flowchart [Fig I] in the online supplement). To avoid any possibility of administering intravenous E.coli LPS to a pregnant female, volunteers were only included if they were not of childbearing potential; no eligible female subjects volunteered and so all recruited volunteers were male. Compliance was assessed from a diary and pill-count and all subjects were >90% compliant. After LPS administration, all subjects developed anticipated flu-like symptoms and signs of sepsis that peaked at 90 - 180 minutes and resolved within 6 hours (Table II in the online data supplement). There were no unexpected adverse reactions.
Both Ticagrelor and Clopidogrel Reduce Peak Levels of IL-6, TNFα and CCL2, whilst Ticagrelor Additionally Reduces Peak Levels of IL-8 and G-CSF and Increases Peak Levels of IL-10
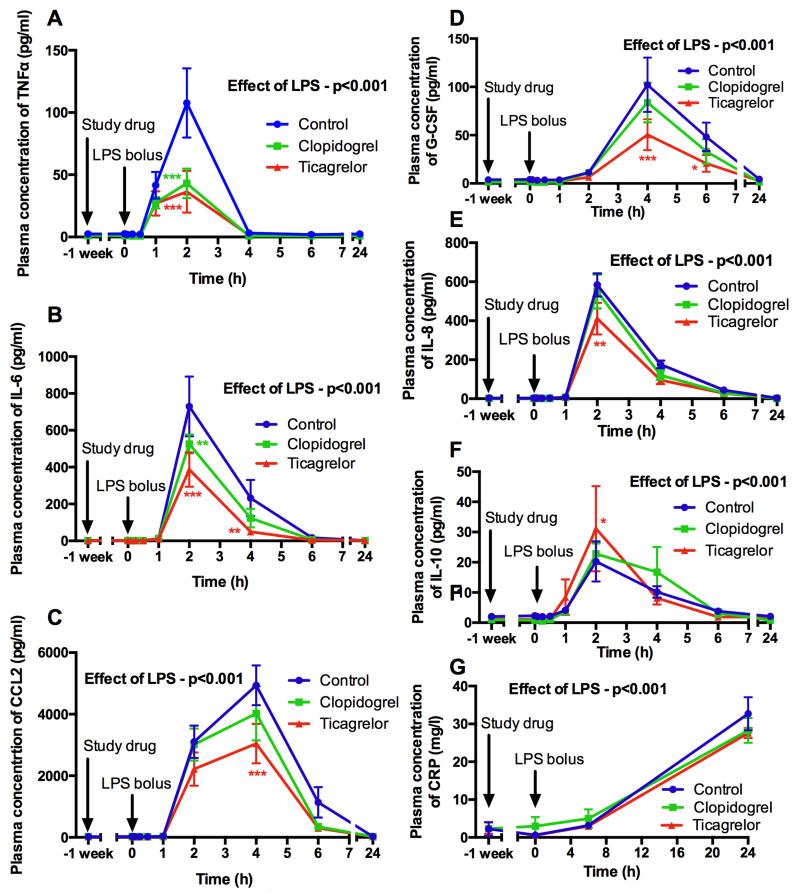
We assessed systemic inflammation in response to LPS administration by measuring the release of major pro-inflammatory cytokines and we determined the modulatory effect of P2Y12 inhibitors. Plasma levels of interleukin (IL)-6, TNFα, IL-8, chemokine (C-C motif) ligand (CCL)-2, growth colony stimulating factor (G-CSF) and high sensitivity C-reactive protein (hsCRP) significantly increased after LPS administration (all p<0.001) (Fig 1). Compared to control, both P2Y12 inhibitors had a marked effect on the pro-inflammatory cytokine response, reducing peak levels of TNFα (66% reduction [p<0.001] and 60% reduction [p<0.001] respectively; Fig 1A), IL-6 (47% reduction [p<0.001] and 28% reduction [p=0.001] respectively; Fig 1B), and CCL2 (38% reduction [p<0.001] and 19% reduction [p=0.049] respectively; Fig 1C). In addition, ticagrelor, but not clopidogrel, significantly reduced peak levels of G-CSF (51% reduction; p<0.001; Fig 1D) and IL-8 (29% reduction; p=0.001; Fig 1E) compared to control. Ticagrelor, but not clopidogrel, also significantly increased peak levels of the anti-inflammatory cytokine IL-10 compared to control (54% increase; p=0.02; Fig 1F). Neither drug significantly modified the hsCRP response (Fig 1G).
Figure 1. Levels of pro-inflammatory cytokines TNFα (A), IL-6 (B), CCL2 (C), G-CSF (D), IL-8 (E), IL-10 (F) and hsCRP (G) before and after 1 week of antiplatelet treatment and following LPS administration (t = 0 hours).
Data expressed as mean ± SEM (n=10 in each group). The overall effect of LPS and the effect of ticagrelor and clopidogrel (both compared to control at each time point) determined using 2-way ANOVA with Dunnett’s correction for multiple comparisons for the cytokines (*p<0.05, **p<0.01 and ***p<0.001). For hsCRP, the effect of ticagrelor and clopidogrel compared to control was determined using ANOVA of AUC.
Ticagrelor Inhibits LPS-induced Platelet-Monocyte Aggregate Formation
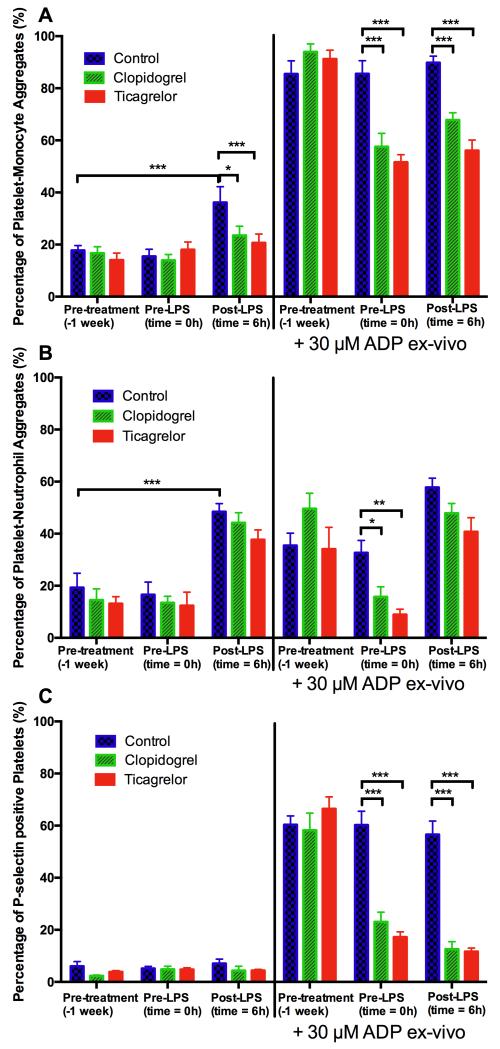
Formation of platelet-leukocyte aggregates (defined as leukocyte expression of the platelet marker CD42a) amplifies leukocyte release of pro-inflammatory cytokines.3 We therefore investigated whether this is a mechanism by which P2Y12 inhibitors reduce systemic inflammation. Ticagrelor significantly reduced formation of platelet-monocyte aggregates compared to control (21% vs 36%; p<0.001) that occurred 6 hours after LPS administration (Fig 2A). Clopidogrel also significantly reduced the formation of platelet-monocyte aggregates compared to control (23% vs 36%; p=0.04; Fig 2A). A similar pattern of effect of LPS and modulation by the antiplatelet medications was seen in platelet-neutrophil aggregate formation, but the effects on platelet-neutrophil aggregate formation were not statistically significant (Fig 2B). Platelet P-selectin expression did not significantly change after LPS administration (Fig 2C).
Figure 2. Platelet-monocyte (A) and platelet-neutrophil (B) aggregate formation and platelet P-selectin expression (C) at baseline, immediately before LPS administration and 6 hours after LPS administration, in unstimulated samples and samples stimulated by 30 μM ADP ex vivo.
Data expressed as mean ± SEM (n=10 in each group). The overall effect of LPS and the effect of ticagrelor and clopidogrel (both compared to control at each time point) were determined using 2-way ANOVA with Dunnett’s correction for multiple comparisons (*p<0.05, **p<0.01 and ***p<0.001).
Inhibition of platelet P2Y12 ADP receptors was also assessed by measuring platelet aggregation, platelet-leukocyte aggregate formation and platelet P-selectin expression in response to ADP added ex vivo. Ticagrelor and clopidogrel inhibited ADP-induced platelet aggregation, platelet-monocyte aggregate formation, platelet-neutrophil aggregate formation and platelet P-selectin expression compared to control at all time points (all p<0.001; Fig 2). After randomized treatment, platelet aggregation responses following 5 minutes exposure to ADP (final platelet aggregation response) were 2±1%, 14±6% and 70±11% in the ticagrelor, clopidogrel and control groups respectively (Fig II in the online supplement). Final platelet aggregation responses did not significantly change following LPS administration in any of the treatment groups (all p>0.05).
Ticagrelor Increases Neutrophil Counts and Alters Monocyte Dynamics During Systemic Inflammation
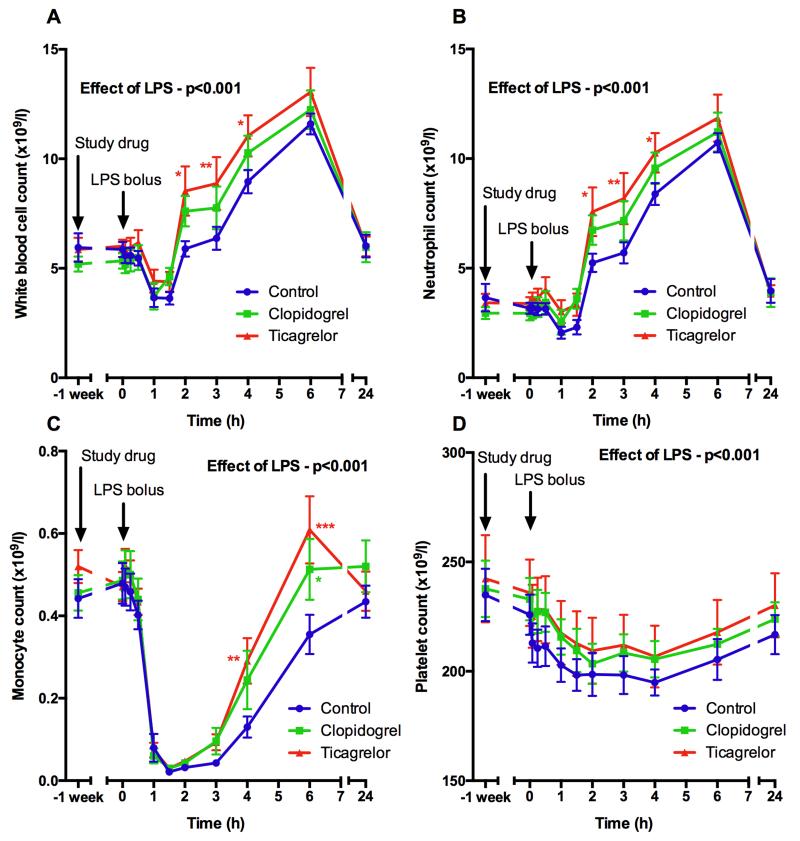
Since the formation of platelet-leukocyte aggregates facilitates adhesion of leukocytes to the endothelium and subsequent extravasation3 we investigated whether inhibition of these processes by P2Y12 inhibitors affects leukocyte trafficking. Ticagrelor potentiated the increase in neutrophil count, which was significantly higher than controls 2-4 hours after LPS administration (p<0.05; Fig 3B) and may have been due to inhibition of non-specific sequestration of neutrophils. Clopidogrel did not have a significant effect (Fig 3B). Similarly, subjects receiving ticagrelor showed altered monocyte dynamics. Transient monocyte sequestration was observed after LPS administration in all volunteers, but recovery from this was significantly greater in the ticagrelor and clopidogrel groups (Fig 3C). Neither P2Y12 inhibitor significantly affected the decrease in platelet count that occurred after LPS administration (Fig 3D).
Figure 3. Leukocyte (A), neutrophil (B), monocyte (C) and platelet (D) counts before and after 1 week of antiplatelet treatment and following LPS administration (t = 0 hours).
Data expressed as mean ± SEM (n=10 in each group). The overall effect of LPS and the effect of ticagrelor and clopidogrel (both compared to control at each time point) were determined using 2-way ANOVA with Dunnett’s correction for multiple comparisons (*p<0.05, **p<0.01 and ***p<0.001).
LPS Induces Prothrombotic Changes in the Fibrin Network that are Attenuated by Ticagrelor
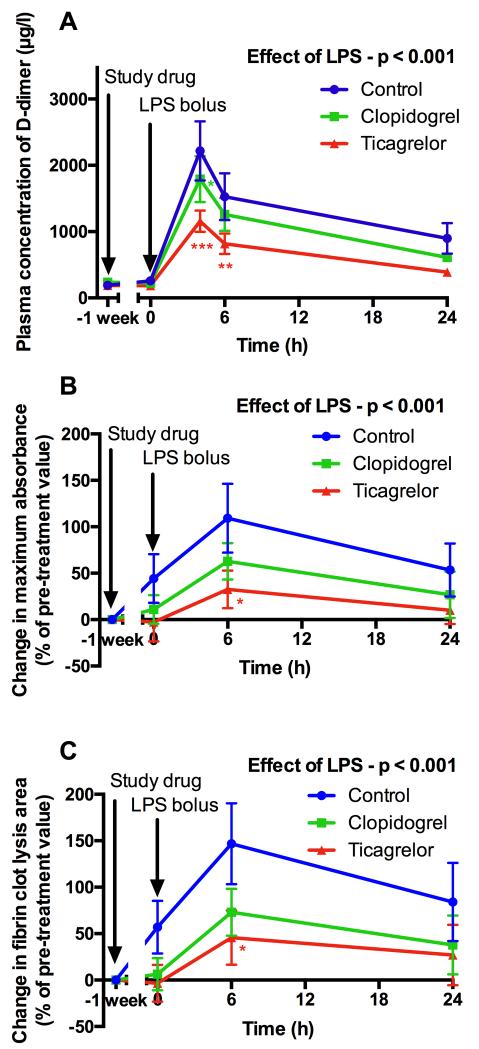
The development of a stable fibrin clot represents the critical final stage of thrombosis and has the potential to be modified by systemic inflammation20. Turbidimetric assays of individual samples showed that fibrin clot maximum absorbance (a measure of clot density) and lysis area (a complex measure that assesses both clot formation and lysis) increased after LPS administration (p<0.001) (Fig 4A and 4B). Ticagrelor significantly reduced the rise in maximum absorbance after LPS administration compared with control (percentage increase from baseline of 33% vs. 109%; p=0.02; Fig 4A). Similarly, ticagrelor also reduced the increase in lysis area after LPS administration compared with control (percentage increase from baseline of 46% vs. 147%; p=0.02; Fig 4B). Clopidogrel had a similar, less potent effect that was not statistically significant (Fig 4A and 4B).
Figure 4. Levels of D-dimer (A) and fibrin clot maximum absorbance (B) (a measure of clot density) and lysis area under the curve (C) (a complex measure that assesses both clot formation and lysis) determined by turbidimetry (expressed as percentage change from baseline value) following treatment and LPS administration.
Data expressed as mean ± SEM (n=10 in each group). The overall effect of LPS and the effect of ticagrelor and clopidogrel (both compared to control at each time point) were determined using 2-way ANOVA with Dunnett’s correction for multiple comparisons (*p<0.05, **p<0.01 and ***p<0.001).
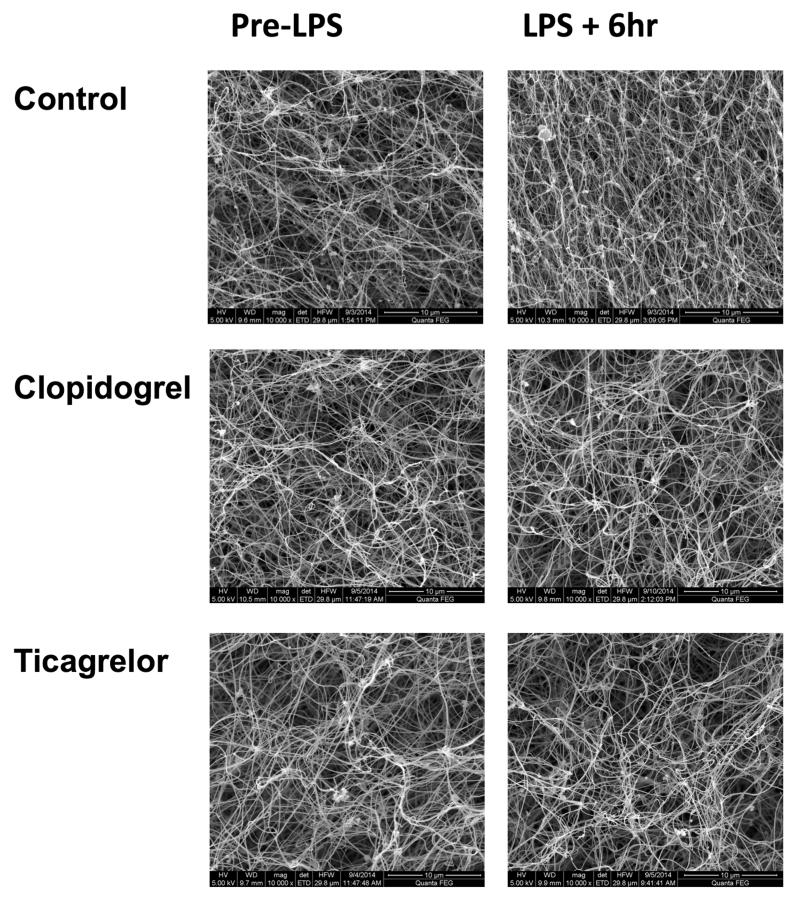
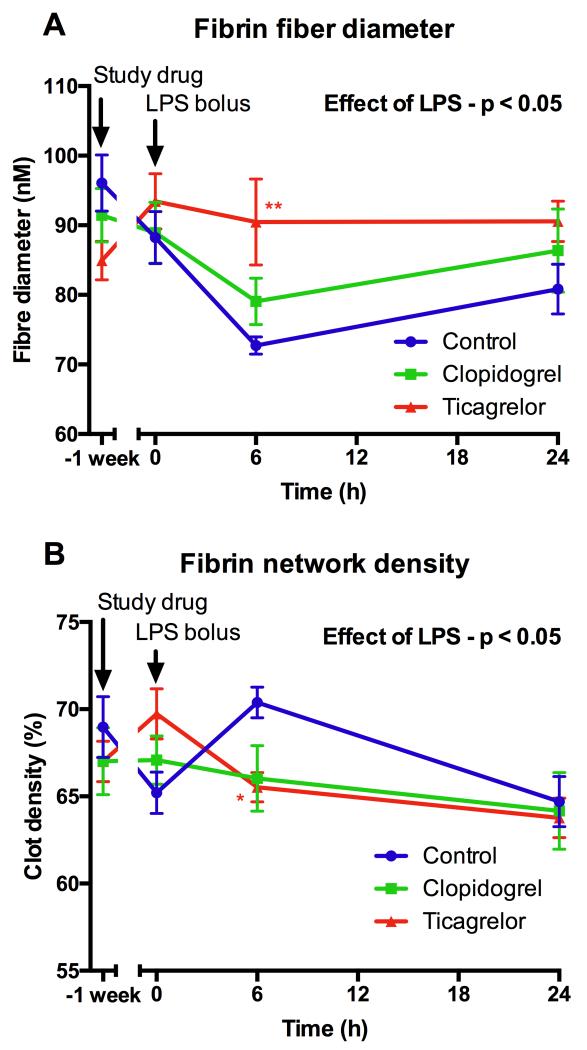
More detailed analysis of clot ultrastructure using scanning electron microscopy of pooled plasma samples demonstrated that LPS administration resulted in more compact clot formation (Fig 5), shown by a significant increase in fibrin clot density (p=0.02) and a decrease in fibrin fiber diameter (p=0.01) (Fig 5-6). These changes have been shown to increase clot stability and confer resistance to fibrinolysis, both of which contribute to a prothrombotic state20. Ticagrelor significantly reduced LPS-induced changes in fiber density and fiber diameter (Fig 6), whereas clopidogrel had a similar less potent effect that was not statistically significant. LPS induced a marked increase in D-dimer (Fig 4C), which peaked at 4 hours (p<0.001). Ticagrelor significantly reduced peak levels of D-dimer by 48% compared to control (p<0.001) and clopidogrel significantly inhibited peak levels by 19% compared to control (p=0.01).
Figure 5. Representative electron microscope images of fibrin clots formed from plasma ex vivo in each treatment group immediately before and 6 hours after LPS administration.
Clots were prepared in duplicate and 4 photographs were taken of each clot at each time point. In the control group, there is an increase in fibrin network density following LPS whereas in the clopidogrel and ticagrelor groups this is not apparent.
Figure 6. Fibrin fiber diameter (A) and fibrin network density (B) (determined by electron microscopy) following LPS administration.
Data expressed as mean ± SEM (n=8 in each group). The overall effect of LPS and the effect of ticagrelor and clopidogrel (both compared to control at each time point) were determined using 2-way ANOVA with Dunnett’s correction for multiple comparisons (*p<0.05, **p<0.01 and ***p<0.001).
Discussion
Sepsis is a devastating syndrome for which therapeutic options remain limited. In addition to the immediate collateral host tissue damage and mortality caused by sepsis, there is a 20-fold increase in risk of myocardial infarction and stroke following sepsis by mechanisms that are not fully understood.21 Data from clinical studies of platelet P2Y12 inhibitors suggest that their use improves mortality from sepsis.6-8 However, animal models of sepsis have conflicted regarding the immunomodulatory effect of platelet P2Y12 inhibition on sepsis responses9-12, which may be species dependent. We therefore sought to determine the mechanistic impact of P2Y12 inhibitors on key molecular and cellular pathways that are central to sepsis responses in humans. We investigated the effects of P2Y12 inhibitors on leukocyte responses, interactions with platelets that may govern such interactions, and with activation of the coagulation system. Our data point to a substantial modulatory effect of ticagrelor in particular and place the regulation of platelet activation at the heart of systemic inflammation induced by LPS in humans.
To our knowledge, this is the first study to demonstrate marked suppression of response to bacterial endotoxemia by platelet P2Y12 inhibitors. Both P2Y12 inhibitors potently reduced peak levels of D-dimer and major pro-inflammatory cytokines, including IL-6, TNFα, CCL2. In contrast to clopidogrel, ticagrelor also significantly reduced peak levels of IL-8 and G-CSF and increased the peak level of IL-10 compared to control. Additionally, ticagrelor reduced platelet-leukocyte aggregate formation, altered leukocyte trafficking and suppressed prothrombotic changes in fibrin clot ultrastructure. Since these changes in fibrin clot structure have been shown to shift the haemostatic balance towards thrombosis,20 this represents a novel mechanism by which ticagrelor inhibits the prothrombotic consequences of systemic inflammation.
Inhibition of platelet-monocyte aggregate formation demonstrates a mechanism by which platelet P2Y12 inhibition reduced systemic inflammation, as the formation of platelet-monocyte aggregates amplifies monocyte release of pro-inflammatory cytokines, including TNFα, CCL2 and IL-8.22,23 Levels of residual platelet P2Y12 reactivity before endotoxin administration significantly correlated with subsequent inflammatory and prothrombotic response suggesting that the responses were P2Y12-mediated. Ticagrelor and clopidogrel belong to different chemical classes, inhibit platelet P2Y12 receptors by different mechanisms, and do not have any structural similarities or shared metabolites. From a pharmacological perspective, shared non-P2Y12-mediated effects are therefore unlikely. P2Y12 receptors were originally identified to be almost exclusive to platelets, although they have now also been identified on a limited number of other cell types.24 The extent to which leukocytes express P2Y12 still remains unclear, particularly as platelets often contaminate isolated leukocyte preparations. In mice, dendritic cells express P2Y12, which appears to mediate the secretion of certain cytokines, such as IL-12.24 This offers an additional mechanism by which P2Y12 may mediate inflammatory responses, although it has not been established whether dendritic cells function in the same way in humans. Recent studies have demonstrated that vascular smooth muscle cells (VSMC) also express P2Y12, which mediates CCL2 release.25 This may have contributed to the modulatory effect of P2Y12 inhibition on inflammation, although it has been shown that clopidogrel does not inhibit VSMC P2Y12 in rats, possibly due to the potential for nucleated cells to regenerate P2Y12.26 Ticagrelor is also a weak inhibitor of cellular uptake of adenosine, which increases extracellular levels of adenosine.14 Since adenosine can increase macrophage production of IL-10, which is associated with a reduction in TNFα and IL-6, this offers a further mechanism by which ticagrelor may modify systemic inflammation.27
LPS administration induced platelet-leukocyte aggregate formation, which was inhibited by ticagrelor in particular. Platelet-monocyte aggregate formation potentiates monocyte release of pro-inflammatory cytokines, including TNFα, CCL2 and IL-8, mediated by NF-κB.22,23 Additionally, platelet-neutrophil aggregates have been implicated in the pathophysiology of acute lung injury.28 Therefore greater inhibition of the formation of platelet-leukocyte aggregates represents a mechanism by which ticagrelor may have conferred greater protection against excessive innate immune activation during sepsis compared to clopidogrel in the PLATO study. Our data show that LPS administration induced an initial neutropenia and monocytopenia, which is held to be due to rapid sequestration of leukocytes to the endothelium.29 This was then followed by a rapid increase in neutrophil count due to bone marrow mobilization. It is notable that neutrophil counts were consistently higher in the ticagrelor group compared to the control group, despite lower overall levels of pro-inflammatory cytokines. This was apparent from very early time points onwards, which suggests that this was due to inhibition of leukocyte sequestration, rather than increased bone marrow mobilisation, which would tend to have a more delayed effect. It is logical that inhibition of platelet-leukocyte aggregate formation reduces leukocyte sequestration, as formation of platelet-leukocyte aggregates upregulates leukocyte expression of adhesion molecules and facilitates adhesion to the endothelium.30
In our study, and in other studies, platelet expressed P-selectin was not increased at 30 minutes or 6 hours after LPS administration. This is likely to be due to the transient nature of platelet expression of P-selectin, which is shed by degranulated platelets, although they continue to function normally and aggregate.31 It has been asserted that the formation of platelet-monocyte aggregates is therefore a more reliable marker of platelet activation in vivo.32 The precise cause of thrombocytopenia related to systemic inflammation has still not been clarified and the relative contribution of platelet activation is unclear. Neither ticagrelor nor clopidogrel significantly attenuated the LPS-induced reduction in platelet count. The findings of our study therefore suggest that thrombocytopenia is not entirely mediated by platelet activation or the formation of platelet-monocyte aggregates, as P2Y12 inhibitors inhibit these processes. The formation of platelet-neutrophil aggregates, or other processes where platelet P2Y12 has a less prominent role, may therefore have a greater contribution towards thrombocytopenia.
Our study provides a number of novel insights into potential mechanisms for increased risk of atherothrombotic events following bacteremia and sepsis.21 Although a prothrombotic state is well recognized in sepsis,33 the underlying mechanisms are incompletely understood and the relative role of platelets has not been well defined. For the first time, this study demonstrates that exposure to bacterial LPS directly causes prothrombotic changes in the fibrin network that increase clot stability and confer resistance to fibrinolysis, both of which shift the haemostatic balance towards thrombosis 20. Platelet P2Y12 inhibitors inhibited these prothrombotic effects of LPS and our results suggest that this may have been due to a reduction in levels of pro-inflammatory cytokines, as TNFα in particular has been shown to be a potent activator of the coagulation system in vivo.34 The greater overall effect of ticagrelor on these pathways compared to clopidogrel suggests a mechanism by which ticagrelor reduced cardiovascular death following infection in the PLATO study. The combined effect of ticagrelor, in particular, on leukocyte production of cytokines, leukocyte sequestration, platelet-leukocyte aggregate formation and subsequent changes in fibrin clot ultrastructure point to a substantial role for platelets in orchestrating the innate immune response to LPS. This suggests potential for timed platelet P2Y12 inhibition in patients with infection to modify the risk of sepsis and associated thrombotic complications. In this study and in observational clinical studies, subjects were already taking P2Y12 inhibitors at the onset of systemic inflammation and sepsis respectively. However, no studies have established the effect of administration of P2Y12 inhibitors to patients with established sepsis. The greater infectious and inflammatory burden of established sepsis presents a different balance of risks and benefits and it is critical that any studies that investigate these medications in the context of sepsis carefully address the optimal timing of administration. In sepsis, excess fibrin deposition and impaired anticoagulant mechanisms lead to exhaustion of the coagulation cascade, causing coagulopathy and bleeding.1 Although antiplatelet medications normally exacerbate bleeding, this may therefore be counter-balanced by attenuating the prothrombotic state that drives the development of coagulopathy during sepsis. In support of this, antiplatelet medications are not associated with excess bleeding in patients with sepsis in observational studies.7 However, it is important to recognize that patients with established coagulopathy may have their antiplatelet medications discontinued in these studies and are less likely to benefit from these agents.
Ticagrelor and clopidogrel are some of the most commonly prescribed medications worldwide, due to their established benefit in the management of atherothrombosis. The results of this study therefore have potentially important clinical implications for millions of patients who are currently treated with these medications. In addition, there is great interest in the use of specific immunomodulatory therapy for the treatment of acute coronary syndromes.35 The results of our study elucidate the background anti-inflammatory effects of medications that are already used for acute coronary syndromes. This is crucial information for determining the most appropriate inflammatory targets in the design of novel treatment strategies.
A limitation of this study is that clopidogrel has a less potent inhibitory effect in patient populations compared to its effects in healthy volunteers.36 This is not the case for ticagrelor, which has a consistently potent effect in patient populations.37 Therefore the results of this study may actually underestimate the additional efficacy of ticagrelor compared to clopidogrel in suppressing systemic inflammation in patients. Whilst this study demonstrates the key cellular and molecular pathways by which platelet P2Y12 inhibitors could reduce mortality from sepsis, further randomized human studies are needed to determine whether this improves outcomes in patients.
In conclusion, this study demonstrates for the first time that clopidogrel and ticagrelor have a marked effect on multiple critical mechanisms involved in the pathophysiology of sepsis. This suggests a promising line of investigation for novel applications of P2Y12 inhibitors in a syndrome that has proved elusive to almost all previous pharmacological strategies. The greater overall effect of ticagrelor compared to clopidogrel also provides critical mechanistic insight into the lower mortality following sepsis observed in the ticagrelor group compared to the clopidogrel group in the PLATO study.
Supplementary Material
Significance.
The main findings of this study were that platelet P2Y12 inhibitors, such as ticagrelor and clopidogrel, potently suppress systemic inflammation and its prothrombotic effects in an experimental human model. This provides critical mechanistic insight into the findings of clinical studies that have shown that treatment with platelet P2Y12 inhibitors is associated with a reduced mortality from sepsis. This suggests the potential for novel strategies for the treatment of sepsis, which has a high mortality and has remained elusive to almost all other therapeutic approaches.
This is the first study to demonstrate that platelet P2Y12 inhibitors reduce systemic inflammation induced by bacterial endotoxin in vivo. Ticagrelor and clopidogrel are some of the most commonly prescribed medications worldwide, due to their established benefit in the treatment of acute coronary syndromes and atherothrombosis. The findings of this study are therefore important for cardiologists and the millions of patients worldwide who are already treated with these medications for atherothrombosis.
Acknowledgements
We thank the Sheffield Clinical Research Facility (funded by the National Institute for Health Research, UK) for hosting the study and providing administrative and nursing support. We are very grateful to Dr Anthony Suffredini at the National Institutes of Health (US) for kindly providing the endotoxin. The views expressed are those of the authors and not necessarily those of the MRC, the NHS, the NIHR or the Department of Health.
Source of Funding
This work was funded by the Medical Research Council UK (MRC Clinical Research Training Fellowship [R/136413-11-1] awarded to Dr Mark Thomas).
Abbreviations
- ACS
Acute coronary syndrome
- ADP
Adenosine diphosphate
- CCL2
Chemokine (C-C motif) ligand 2
- G-CSF
Growth colony stimulating factor
- hsCRP
high sensitivity C-reactive protein
- IL
Interleukin
- LPS
Lipopolysaccharide
- PLATO
PLATelet inhibition and patient Outcomes study
- TNFα
Tumor necrosis factor α
Footnotes
Disclosures
Dr Ramzi Ajjan reports research support from the NIHR, British Heart Foundation, Sir Jules Thorn Trust, Abbott, Bayer, Eli Lilly, LifeScan, NovoNordisk and Takeda. He also received Advisory Board and speaker's honoraria from Abbott, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Glaxo SmithKline, Merck Sharpe & Dohme, NovoNordisk, Roche and Takeda.
Prof David Dockrell reports research grants from GlaxoSmithKline and speaker fees/consultancy fees from GlaxoSmithKline, ViiV Healthcare, Gilead, MSD, Bristol-Myers Squibb and Janssen-Cilag to support meeting attendance.
Prof Robert Storey reports institutional grants from AstraZeneca and Merck; research support from Accumetrics; honoraria from AstraZeneca, Merck, Accumetrics, and Medscape; consultancy fees from AstraZeneca, Correvio, Merck, Novartis, Accumetrics, Sanofi-Aventis, Regeneron, Roche, The Medicines Company, Aspen, PlaqueTec and Thermo Fisher Scientific.
The other authors have no competing interests.
References
- 1.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369(9):840–851. doi: 10.1056/NEJMra1208623. doi:10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 2.de Stoppelaar SF, van 't Veer C, van der Poll T. The role of platelets in sepsis. Thromb Haemost. 2014;112(4):666–677. doi: 10.1160/TH14-02-0126. doi:10.1160/TH14-02-0126. [DOI] [PubMed] [Google Scholar]
- 3.Thomas MR, Storey RF. The role of platelets in inflammation. Thromb Haemost. 2015;114(3):449–458. doi: 10.1160/TH14-12-1067. doi:10.1160/TH14-12-1067. [DOI] [PubMed] [Google Scholar]
- 4.Semple JW, Italiano JE, Freedman J. Platelets and the immune continuum. Nat Rev Immunol. 2011;11(4):264–274. doi: 10.1038/nri2956. doi:10.1038/nri2956. [DOI] [PubMed] [Google Scholar]
- 5.Storey RF, Sanderson HM, White AE, May JA, Cameron KE, Heptinstall S. The central role of the P(2T) receptor in amplification of human platelet activation, aggregation, secretion and procoagulant activity. Br J Haematol. 2000;110(4):925–934. doi: 10.1046/j.1365-2141.2000.02208.x. [DOI] [PubMed] [Google Scholar]
- 6.Tsai MJ, Ou SM, Shih CJ, Chao P, Wang LF. Association of prior antiplatelet agents with mortality in sepsis patients: a nationwide population-based cohort study. Intensive Care Med. 2015;41(5):806–813. doi: 10.1007/s00134-015-3760-y. doi:10.1007/s00134-015-3760-y. [DOI] [PubMed] [Google Scholar]
- 7.Akinosoglou K, Alexopoulos D. Use of antiplatelet agents in sepsis: a glimpse into the future. Thromb Res. 2014;133(2):131–138. doi: 10.1016/j.thromres.2013.07.002. doi:10.1016/j.thromres.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Gross AK, Dunn SP, Feola DJ, Martin CA, Charnigo R, Li Z, Abdel-Latif A, Smyth SS. Clopidogrel treatment and the incidence and severity of community acquired pneumonia in a cohort study and meta-analysis of antiplatelet therapy in pneumonia and critical illness. J Thromb Thrombolysis. 2013;35(2):147–154. doi: 10.1007/s11239-012-0833-4. doi:10.1007/s11239-012-0833-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lipcsey M, Larsson A, Olovsson M, Sjölin J, Eriksson MB. Early endotoxin-mediated haemostatic and inflammatory responses in the clopidogrel-treated pig. Platelets. 2005;16(7):408–414. doi: 10.1080/09537100500163168. doi:10.1080/09537100500163168. [DOI] [PubMed] [Google Scholar]
- 10.Hagiwara S, Iwasaka H, Hasegawa A, Oyama M, Imatomi R, Uchida T, Noguchi T. Adenosine diphosphate receptor antagonist clopidogrel sulfate attenuates LPS-induced systemic inflammation in a rat model. Shock. 2011;35(3):289–292. doi: 10.1097/SHK.0b013e3181f48987. doi:10.1097/SHK.0b013e3181f48987. [DOI] [PubMed] [Google Scholar]
- 11.Winning J, Claus RA, Pletz MW, Bauer M, Lösche W. Adenosine Diphosphate Receptor Antagonist Clopidogrel Sulfate Attenuates LPS-Induced Systemic Inflammation in a Rat Model. Shock. 2011;36(3):317. doi: 10.1097/SHK.0b013e318224f66a. doi:10.1097/SHK.0b013e318224f66a. [DOI] [PubMed] [Google Scholar]
- 12.Liverani E, Rico MC, Yaratha L, Tsygankov AY, Kilpatrick LE, Kunapuli SP. LPS-induced systemic inflammation is more severe in P2Y12 null mice. J Leukoc Biol. 2014;95(2):313–323. doi: 10.1189/jlb.1012518. doi:10.1189/jlb.1012518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Storey RF, Angiolillo DJ, Patil SB, Desai B, Ecob R, Husted S, Emanuelsson H, Cannon CP, Becker RC, Wallentin L. Inhibitory effects of ticagrelor compared with clopidogrel on platelet function in patients with acute coronary syndromes: the PLATO (PLATelet inhibition and patient Outcomes) PLATELET substudy. J Am Coll Cardiol. 2010;56(18):1456–1462. doi: 10.1016/j.jacc.2010.03.100. doi:10.1016/j.jacc.2010.03.100. [DOI] [PubMed] [Google Scholar]
- 14.Bonello L, Laine M, Kipson N, Mancini J, Helal O, Fromonot J, Gariboldi V, Condo J, Thuny F, Frere C, Camoin-Jau L, Paganelli F, Dignat-George F, Guieu R. Ticagrelor increases adenosine plasma concentration in patients with an acute coronary syndrome. J Am Coll Cardiol. 2014;63(9):872–877. doi: 10.1016/j.jacc.2013.09.067. doi:10.1016/j.jacc.2013.09.067. [DOI] [PubMed] [Google Scholar]
- 15.Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045–1057. doi: 10.1056/NEJMoa0904327. doi:10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 16.Varenhorst C, Scirica BM, Hogue CW, Åsenblad N, Storey RF, Steg PG, Horrow J, Mahaffey KW, Becker RC, James S, Cannon CP, Brandrup-Wognsen G, Wallentin L, Held C. Factors contributing to the lower mortality with ticagrelor compared with clopidogrel in patients undergoing coronary artery bypass surgery. J Am Coll Cardiol. 2012;60(17):1623–1630. doi: 10.1016/j.jacc.2012.07.021. doi:10.1016/j.jacc.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 17.Varenhorst C, Alström U, Braun OO, Storey RF, Mahaffey KW, Bertilsson M, Cannon CP, Scirica BM, Himmelmann A, James SK, Wallentin L, Held C. Causes of mortality with ticagrelor compared with clopidogrel in acute coronary syndromes. Heart. 2014;100(22):1762–1769. doi: 10.1136/heartjnl-2014-305619. doi:10.1136/heartjnl-2014-305619. [DOI] [PubMed] [Google Scholar]
- 18.Storey RF, James SK, Siegbahn A, Varenhorst C, Held C, Ycas J, Husted SE, Cannon CP, Becker RC, Steg PG, Åsenblad N, Wallentin L. Lower mortality following pulmonary adverse events and sepsis with ticagrelor compared to clopidogrel in the PLATO study. Platelets. 2014;25(7):517–525. doi: 10.3109/09537104.2013.842965. doi:10.3109/09537104.2013.842965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suffredini AF, Suffredini AF, Hochstein HD, Hochstein HD, McMahon FG, McMahon FG. Dose-related inflammatory effects of intravenous endotoxin in humans: evaluation of a new clinical lot of Escherichia coli O:113 endotoxin. Journal of Infectious Diseases. 1999;179(5):1278–1282. doi: 10.1086/314717. doi:10.1086/314717. [DOI] [PubMed] [Google Scholar]
- 20.Undas A, Ariëns RAS. Fibrin clot structure and function: a role in the pathophysiology of arterial and venous thromboembolic diseases. Arterioscler Thromb Vasc Biol. 2011;31(12):e88–e99. doi: 10.1161/ATVBAHA.111.230631. doi:10.1161/ATVBAHA.111.230631. [DOI] [PubMed] [Google Scholar]
- 21.Dalager-Pedersen M, Søgaard M, Schønheyder HC, Nielsen H, Thomsen RW. Risk for myocardial infarction and stroke after community-acquired bacteremia: a 20-year population-based cohort study. Circulation. 2014;129(13):1387–1396. doi: 10.1161/CIRCULATIONAHA.113.006699. doi:10.1161/CIRCULATIONAHA.113.006699. [DOI] [PubMed] [Google Scholar]
- 22.Bournazos S, Rennie J, Hart SP, Fox KAA, Dransfield I. Monocyte functional responsiveness after PSGL-1-mediated platelet adhesion is dependent on platelet activation status. Arterioscler Thromb Vasc Biol. 2008;28(8):1491–1498. doi: 10.1161/ATVBAHA.108.167601. doi:10.1161/ATVBAHA.108.167601. [DOI] [PubMed] [Google Scholar]
- 23.Neumann FJ, Marx N, Gawaz M, Brand K, Ott I, Rokitta C, Sticherling C, Meinl C, May A, Schömig A. Induction of Cytokine Expression in Leukocytes by Binding of Thrombin-Stimulated Platelets. Circulation. 1997;95(10):2387–2394. doi: 10.1161/01.cir.95.10.2387. doi:10.1161/01.CIR.95.10.2387. [DOI] [PubMed] [Google Scholar]
- 24.Gachet C. P2Y(12) receptors in platelets and other hematopoietic and nonhematopoietic cells. Purinergic Signalling. 2012;8(3):609–619. doi: 10.1007/s11302-012-9303-x. doi:10.1007/s11302-012-9303-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Satonaka H, Nagata D, Takahashi M, Kiyosue A, Myojo M, Fujita D, Ishimitsu T, Nagano T, Nagai R, Hirata Y. Involvement of P2Y12 Receptor in Vascular Smooth Muscle Inflammatory Changes via MCP-1 Upregulation and Monocyte Adhesion. AJP: Heart and Circulatory Physiology. 2015;308(8):853–861. doi: 10.1152/ajpheart.00862.2013. doi:10.1152/ajpheart.00862.2013. [DOI] [PubMed] [Google Scholar]
- 26.Grzesk GG, Kozinski MM, Navarese EPE, Krzyzanowski MM, Grzesk EE, Kubica AA, Siller-Matula JMJ, Castriota FF, Kubica JJ. Ticagrelor, but not clopidogrel and prasugrel, prevents ADP-induced vascular smooth muscle cell contraction: a placebo-controlled study in rats. Thromb Res. 2012;130(1):65–69. doi: 10.1016/j.thromres.2011.12.029. doi:10.1016/j.thromres.2011.12.029. [DOI] [PubMed] [Google Scholar]
- 27.Haskó G, Pacher P. Regulation of Macrophage Function by Adenosine. Arterioscler Thromb Vasc Biol. 2012;32(4):865–869. doi: 10.1161/ATVBAHA.111.226852. doi:10.1161/ATVBAHA.111.226852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zarbock A, Singbartl K, Ley K. Complete reversal of acid-induced acute lung injury by blocking of platelet-neutrophil aggregation. J Clin Invest. 2006;116(12):3211–3219. doi: 10.1172/JCI29499. doi:10.1172/JCI29499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coughlan AF. P-selectin and platelet-activating factor mediate initial endotoxin-induced neutropenia. J Exp Med. 1994;179(1):329–334. doi: 10.1084/jem.179.1.329. doi:10.1084/jem.179.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.da Costa Martins PA, van Gils JM, Mol A, Hordijk PL, Zwaginga JJ. Platelet binding to monocytes increases the adhesive properties of monocytes by up-regulating the expression and functionality of beta1 and beta2 integrins. J Leukoc Biol. 2006;79(3):499–507. doi: 10.1189/jlb.0605318. doi:10.1189/jlb.0605318. [DOI] [PubMed] [Google Scholar]
- 31.Michelson AD, Barnard MR, Hechtman HB, MacGregor H, Connolly RJ, Loscalzo J, Valeri CR. In vivo tracking of platelets: circulating degranulated platelets rapidly lose surface P-selectin but continue to circulate and function. Proc. Natl. Acad. Sci. U.S.A. 1996;93(21):11877–11882. doi: 10.1073/pnas.93.21.11877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Michelson AD, Barnard MR, Krueger LA, Valeri CR, Furman MI. Circulating monocyte-platelet aggregates are a more sensitive marker of in vivo platelet activation than platelet surface P-selectin: studies in baboons, human coronary intervention, and human acute myocardial infarction. Circulation. 2001;104(13):1533–1537. doi: 10.1161/hc3801.095588. doi:10.1161/hc3801.095588. [DOI] [PubMed] [Google Scholar]
- 33.Donzé JD, Ridker PM, Finlayson SRG, Bates DW. Impact of sepsis on risk of postoperative arterial and venous thromboses: large prospective cohort study. BMJ. 2014;349:g5334–g5334. doi: 10.1136/bmj.g5334. doi:10.1136/bmj.g5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van der Poll T, Büller HR, Cate ten H, Wortel CH, Bauer KA, van Deventer SJ, Hack CE, Sauerwein HP, Rosenberg RD, Cate ten JW. Activation of coagulation after administration of tumor necrosis factor to normal subjects. N Engl J Med. 1990;322(23):1622–1627. doi: 10.1056/NEJM199006073222302. doi:10.1056/NEJM199006073222302. [DOI] [PubMed] [Google Scholar]
- 35.Ridker PM, Luscher TF. Anti-inflammatory therapies for cardiovascular disease. Eur Heart J. 2014;35(27):1782–1791. doi: 10.1093/eurheartj/ehu203. doi:10.1093/eurheartj/ehu203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Breet NJ, Van Werkum JW, Bouman HJ, Kelder JC, Ruven HJT, Bal ET, Deneer VH, Harmsze AM, van der Heyden JAS, Rensing BJWM, Suttorp MJ, Hackeng CM, Berg ten JM. Comparison of platelet function tests in predicting clinical outcome in patients undergoing coronary stent implantation. JAMA. 2010;303(8):754–762. doi: 10.1001/jama.2010.181. doi:10.1001/jama.2010.181. [DOI] [PubMed] [Google Scholar]
- 37.Gurbel PA, Bliden KP, Butler K, Tantry US, Gesheff T, Wei C, Teng R, Antonino MJ, Patil SB, Karunakaran A, Kereiakes DJ, Parris C, Purdy D, Wilson V, Ledley GS, Storey RF. Randomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: the ONSET/OFFSET study. Circulation. 2009;120(25):2577–2585. doi: 10.1161/CIRCULATIONAHA.109.912550. doi:10.1161/CIRCULATIONAHA.109.912550. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.