SYNOPSIS
There has been significant progress made in 3D carotid plaque magnetic resonance imaging techniques in recent years. 3D plaque imaging clearly represents the future in clinical use. With effective flow suppression techniques, choices of different contrast weighting acquisitions, and time-efficient imaging approaches, 3D plaque imaging offers flexible imaging plane and view angle analysis, large coverage, multi-vascular beds capability, and even can be used in fast screening.
Keywords: 3D MRI, Vessel Wall Imaging, vulnerable plaque, atherosclerosis imaging, 3D MRA, 3D Vessel Wall Imaging
Introduction
Carotid atherosclerosis, especially atherosclerotic plaques at the carotid bifurcation, is a significant cause of ischemic stroke, a leading cause of death and disability worldwide. A primary goal of imaging of carotid atherosclerosis is, therefore, to identify lesions that will lead to ischemic stroke. Carotid plaques typically become flow-limiting as they progress. The simple assessment of luminal narrowing (stenosis), however, has proven to be of limited value in predicting the risk of ischemic stroke. Furthermore, atherosclerosis is a systemic condition that affects large- and medium-sized arteries, especially the coronary artery, so information on the condition of carotid atherosclerosis may provide a useful window to understanding system-wide disease burden and assess overall cardiovascular risk. Thus, carotid plaque imaging has two primary goals: one is to identify high risk plaques that can cause stroke or other ischemic events, the other is to screen for the presence of plaques for overall cardiovascular evaluation.
High risk plaques are defined as plaques that are likely to rupture leading to thrombosis in situ and/or downstream and potentially ischemic events. Based on histological examination of coronary atherosclerosis, key features of high risk plaques include a large lipid rich necrotic core (LRNC) with an overlying thin (or ruptured) fibrous cap (FC), active inflammation, plaque fissures, calcium nodules on luminal surface, and the presence of intraplaque hemorrhage (IPH) (1). These are mainly plaque compositional features. There have been few histological definitions of high risk plaques of the carotid arteries, perhaps due to limited access to intact carotid atherosclerosis specimens (1). Interestingly, recent literature has pointed to features that can be identified by MR carotid plaque imaging in prospective studies, such as the presence of LRNC, IPH and luminal surface disruption, as those at high risk for rupture or subsequent events (2) (3).
Ultrasound (US), especially intima media thickness (IMT) measurement, has been used extensively as a screening tool to use carotid artery condition to assess cardiovascular risk (4). Recent development in US, however, suggest that 2D and 3D B-mode US capable of visualizing the presence of plaques at the carotid region may provide improved predictive power of future CV risk (5, 6) over IMT. This leads to an interesting question on whether MRI can also play a role in screening, with its apparent advantages in soft tissue contrast and 3D capabilities.
This chapter is focused on MRI imaging techniques for carotid arteries, with a major emphasis on 3D techniques which are considered the future of carotid plaque imaging applications.
Technical considerations and existing 2D imaging techniques
Essential technical considerations for carotid plaque imaging includes the following: hardware considerations such as field strength and specialized radiofrequency (RF) coils, imaging factors such as spatial resolution and signal-to-noise ratio (SNR), and scan time, and tissue-specific factors including tissue contrast, contrast-to-noise (CNR) for specific vessel wall and atherosclerotic plaque tissue identification, and motion artifact suppression. Most of all, vessel wall visualization and the identification of components of atherosclerosis require effective suppression of signals from circulating blood. The carotid bifurcation, the most frequent site of carotid atherosclerosis, exhibits complex flow pattern that includes flow recirculation. Thus, complete flow suppression requires special consideration of the flow conditions at the carotid bifurcation.
Over the years, there have been extensive technical developments and validation for 2D based carotid plaque imaging. The developments can be summarized as follows: a) initial development of carotid imaging was on 1.5T scanners with extensive validation conducted using histology of carotid plaque specimens as the gold standard (7) (8). Studies then showed 3T clinical scanners offer clear SNR advantages (9) for carotid imaging. With application of specific RF coils and optimization of sequences, plaque composition can be identified with similar repeatability at 3T and 1.5T (10). However, quantification of some components, such as calcification and intraplaque hemorrhage, may differ due to higher susceptibility effects at 3T (11). Additionally, the specific absorption rate (SAR) is higher at 3T and should be accounted for in protocol optimization. b) Phased array surface coils with a small diameter and applied close to the neck provide improved SNR and are often used for vessel wall MRI (12, 13). In general, a spatial resolution of 0.5–0.6 mm in plane and 2 mm slice thickness has been used in many studies within clinical acceptable imaging time (8). Most commonly reported longitudinal coverage has been around 3 cm centered at the carotid bifurcation. c) There are many options for flow suppression with double inversion recovery (14) and motion sensitization (13,14) as the two most popular and effective techniques. d) For full characterization of carotid plaque, from lesion burden to tissue composition to luminal surface condition, a multi-contrast imaging approach is highly desired which includes both bright and black blood techniques, T1 and T2 or the application of contrast enhancement (15).
This 2D multi-contrast approach, though well accepted, has a number of shortcomings. It can be complicated to acquire multiple sequences with registered slice locations, the scan time tends to be long, the voxel size is not isotropic such that spatial information about plaque composition may be missed, and the longitudinal coverage is rather limited.
3D carotid plaque MRI – established techniques
Three-dimensional (3D) MRI acquisition, in general, enjoys improved spatial resolution and SNR compared to 2D imaging and can provide increased resolution in the slice-select direction. Isotropic voxels can potentially improve measurement accuracy and reproducibility of carotid MRI by minimizing slice-positioning error allowing registration of images in serial studies (16–18). But 3D imaging also faces several challenges including relative long scan time in which any motion may corrupt the entire data set as opposed to a sub set of data in 2D acquisition. Furthermore, the in-flow based flow suppression techniques used widely in 2D acquisition maybe less effective as the acquisition volume in 3D tend to be much larger. A desirable 3D sequence should have the following features: a) time efficient acquisition, b) effective flow suppression, c) large coverage and isotropic voxel acquisition, and 4) desirable contrast weighting.
1. 3D motion-sensitized driven equilibrium prepared rapid gradient echo (MERGE)
Dr. Balu et al. introduced the 3D MERGE technique in 2011 (19). It is a combination of low flip angle gradient echo acquisition with a flow suppression preparation module named motion-sensitized driven equilibrium (MSDE) (20). This motion suppression technique is based on dephasing flowing blood signals and is relatively independent of imaging acquisition plane. With 3D MERGE, it allows isotropic acquisition (examples of 0.7 x 0.7 x 0.7mm3) with much larger longitudinal coverage than 3 cm, and effective flow suppression with an acquisition time of 2 minutes. As a gradient echo based acquisition, this technique offered a mixed T1 and T2 contrast weighting and has been shown to be able to identify the presence of vessel wall lesions and quantify plaque burdens.
In a recently published clinical validation study, Zhao H et al, assessed 3D MERGE’s ability to measure stenosis using digital subtraction angiography (DSA) as a gold standard (Figure 1) (21). In this study, an improved MSDE 3D sequence (22) was used with 0.8 mm isotropic resolution and 25 cm longitudinal coverage in 2 minutes 42 seconds acquisition time. The researchers showed, by comparing results of 52 patients, that 3D MERGE is accurate to quantify moderate to severe carotid stenosis (Figure 1). As demonstrated in this figure, one can appreciate both the stenotic site and the complex lesion that caused the narrowing and its distribution. Interestingly because of the coverage, effective flow suppression, and the ability to visualize a large segment of the vessel wall, their study demonstrated the presences of lesions there were undetected by DSA. With the short 2–3 minutes acquisition time for bilateral carotid artery imaging, 3DMERGE may be a powerful screening tool for high risk population and the application of 3D MERGE may extend beyond the carotid artery. In a separate study, Makhijani and colleagues used compressed sensing with a hidden Markov tree model to accelerate 3D MERGE acquisition and showed that a factor of 4.5 acceleration was possible without visible degradation of diagnostic quality and quantitative measurements (23).
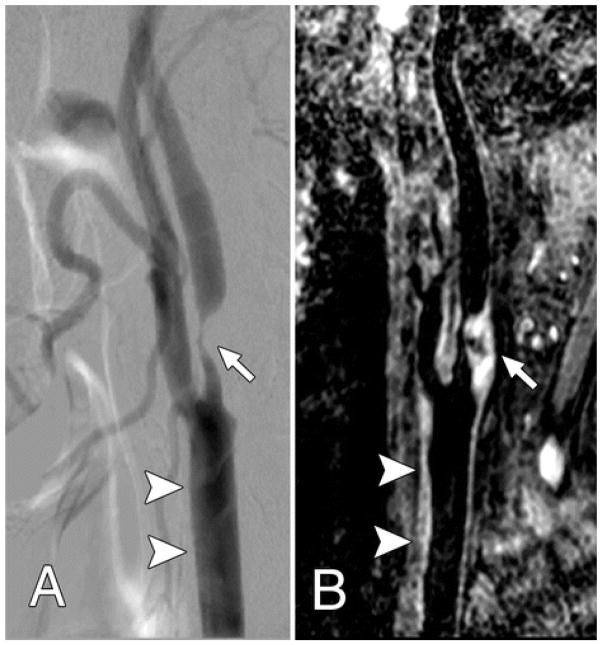
Figure 1.
Both, A, Lateral projection DSA image and, B, oblique 3D BB MR image, show advanced atherosclerotic disease (arrow), which caused severe internal carotid artery stenosis. The common carotid artery wall thickening (arrowheads) was visualized on, B, the 3D BB MR image, but presented as normal on, A, the DSA image.
From Zhao H, Wang J, Liu X, Zhao X, Hippe DS, Cao Y, Wan J, Yuan C, Xu J. Assessment of carotid artery atherosclerotic disease by using three-dimensional fast black-blood MR imaging: comparison with DSA. Radiology. 2015 Feb;274(2):508–16; with permission.
This technique, however, can lead to signal loss due to tissue water diffusion during the flow dephasing gradients and the effects of eddy currents caused by the rapid gradient switching. Since the original introduction, several techniques were introduced to reduce this signal loss and explore its use in multiple contrast weighting and in other vascular applications (22, 24–26). Zhu, et al (23) recently published a study focused on the optimization of improved MSDE (iMSDE) module for both 2D and 3D blood suppression in T1W and T2W spin echo acquisition and demonstrated the feasibility. This study showed iMSDE achieved better flow suppressed than double inversion recovery but with reduced vessel wall CNR efficiency in both T1W and T2W images.
2. 3D MPRAGE (27)(27)(27)(27)
Moody et al. was the first to introduce the use of heavily T1W 3D imaging technique to identify a marker of complicated plaque (27). He named this approach direct thrombus imaging (DTI) initially and the technique was based on 3D magnetization prepared rapid acquisition gradient echo (MPRAGE). With a 3.5 minutes acquisition time and a spatial resolution of roughly 1x2x1 mm3, his group showed that detection of high signals within plaques is prevalent in the ipsilateral carotid arteries of patients with recent cerebral ischemic events (28). This high signal feature was later shown to be from IPH. MPRAGE offers a simple, straightforward approach to identify IPH in plaques and was later shown to have higher diagnostic capability for the detection and quantification of IPH compared to standard T1W and TOF approaches (11).
Because of the heavy T1 weighting, MPRAGE was also found to be unable to identify other plaque tissues, especially co-existing calcification. Further, because MPRAGE is not a black blood technique, blood signals may sometimes be confused as IPH signals. It is worth noting, however, that since Moody’s’ introduction in 2003, this technique has been used in many studies that helped to establish the importance that detection of IPH as a high risk feature of carotid plaque (2, 3). (Figure 2.)
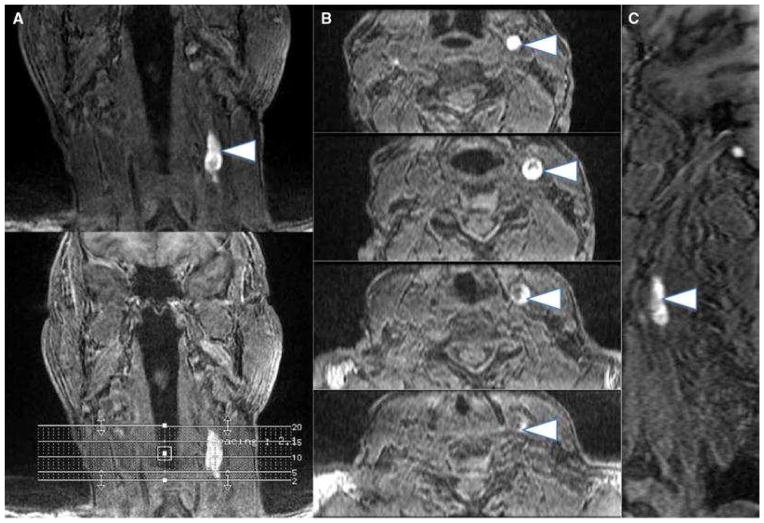
Figure 2.
Multiplanar images (a coronal, b axial, c sagittal) of carotid intraplaque hemorrhage (arrowheads) within the wall of the left carotid artery. Intraplaque hemorrhage is detected using a 3D-T1-weighted fat-suppressed fast field echo sequence by exploiting the T1 shortening effects of methemoglobin and the technique has been histologically validated
From Singh N, Moody AR, Rochon-Terry G, Kiss A, Zavodni A. Identifying a high risk cardiovascular phenotype by carotid MRI-depicted intraplaque hemorrhage. Int J Cardiovasc Imaging. 2013 Oct;29(7):1477–83; with permission.
3. 3D fast (turbo) spin echo (FSE) based approach
3D FSE is an effective spin echo acquisition technique that is able to generate T1, proton density, and T2W images. Recently, a black blood version combined with variable flip angle acquisition to reduce acquisition time was developed, named 3DSPACE, for vessel wall imaging (29). The advantage of this technique is its spin echo based acquisition with better control over tissue contrast selection and it has natural flow suppression capability due to the application of series spin echo trains. The initial technique was used to generate T2W images to study femoral vessel wall with a spatial resolution of 0.72x0.72x0.72 mm3 and 11 minutes acquisition. Carotid vessel wall imaging with improved flow suppression through flow phase dephasing (FSD) was then introduced (30). In this study, Fan et al, demonstrated the effectiveness of the FSD magnetization preparation in improving blood signal suppression of SPACE for isotropic 3D acquisition at 0.63x0.63x0.63mm3 and 6.4 minutes. The longitudinal coverage (Figure 3) almost doubled the 3 cm coverage by 2D technique. In another implementation of a 3D FSE technique, Zhu et al, compared the performance of pre- and post-contrast 3D CUBE to identify non-enhancing lipid-rich-necrotic core (LRNC) and showed good results (26).
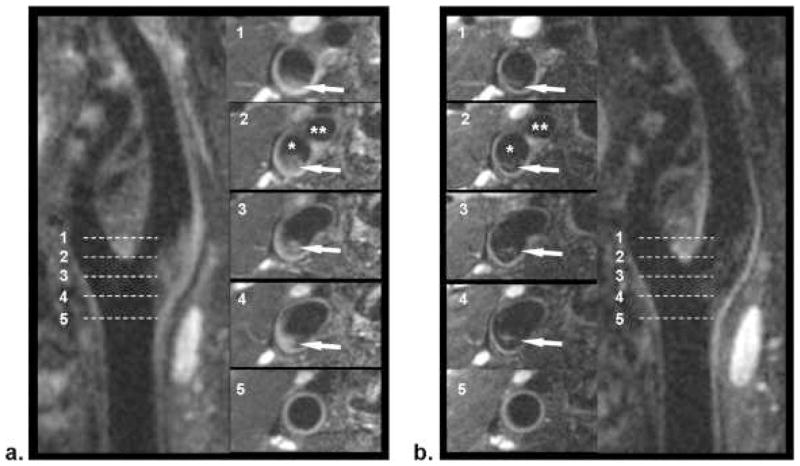
Figure 3.
Comparison of location-matched SPACE (a) and FSD-SPACE (b) images acquired from a healthy volunteer. Using multiplanar reconstruction (MPR), both longitudinal and cross-sectional views of the carotid artery can be visualized. The FSD preparation can dramatically suppress the rb signal (arrows) shown on SPACE images, thus resulting in larger apparent lumen and higher wall-lumen contrast. The locations of the five cross-sectional images (No. 1–5) are indicated by the dashed line on the longitudinal images. *Internal carotid lumen; **external carotid lumen.
From Fan Z, Zhang Z, Chung YC, et al. Carotid arterial wall MRI at 3T using 3D variable-flip-angle turbo spin-echo (TSE) with flow-sensitive dephasing (FSD). J Magn Reson Imaging. 2010 Mar;31(3):645–54. doi: 10.1002/jmri.22058; with permission.
4. 3D time of flight (TOF)
3D TOF has been used for carotid imaging clinically for over 20 years (31). A commonly used approach to screen luminal stenosis is to evaluate the maximum intensity projection (MIP) from different angles. Recently Yim et al and Yoshimura et al found that MIP can also be used to identify intraplaque hemorrhage (IPH) because IPH appears to have high intensity signal (HIS) (32, 33). This is not surprising because most TOF techniques have T1 weighting and IPH is hyperintensive on T1W images (34–36). But this is very interesting because the presence of IPH is considered as one of the important features of high risk carotid plaques (2, 37) and also because 3D TOF is a routinely used in clinical setting and simple to implement. Gupta et al recently expanded this concept to evaluate HIS’ association with symptomatic status (stroke and TIA) and found that HIS detection within carotid plaques is strongly associated with ipsilateral ischemic events (3). Gupta’s study further confirmed the usefulness of 3D TOF in plaque imaging and their results on IPH’s relationship with prior symptomatic status is similar to previous reports based on more comprehensive and sensitive plaque imaging techniques (27, 38).
A word of caution, however, was raised in another study led by Yamada, et al (39). In a study of seventy six patients with a suspected carotid artery stenosis or carotid plaque by ultrasonography underwent multicontrast carotid CMR. HIS presence and volume were measured from 3D TOF-MRA MIP images while IPH and lipid rich necrotic core (LRNC) volumes were separately measured from multicontrast CMR. The authors found that HIS on MIP images are associated with an increased size of IPH and LRNC, particularly in those with moderate to severe stenosis. But since IPH was frequently detected in arteries with low grade stenosis, where TOF-MRA MIP images were found to have low sensitivity, methods with higher sensitivity for IPH while preserving the desirable capability of visualizing the lumen in 3D are needed to better evaluate this subpopulation.
In summary, 3D MERGE, MPRAGE, TOF, FSE (with its various versions) are the main techniques for carotid plaque imaging – individually and combined, these sequences offer isotropic resolution, bright and black blood, large coverage acquisition. Collectively, they can replace 2D multiple-contrast to fully characterize carotid plaques from burden, to composition, and to fibrous cap conditions.
3D carotid plaque imaging - Novel techniques
Recently, some very exciting concepts and techniques have begun to emerge in 3D carotid plaque imaging. These methods are intended to streamline the image acquisition and improve acquisition efficiency by obtaining multiple tissue contrasts in one single acquisition. With detecting IPH becoming a major interest, these techniques tend to all have an option to acquire heavily T1W black blood images and combined with other tissue or lumen contrasts. Another concept is to imbed carotid plaque imaging in an angiographic technique as such that it can be easily implemented in clinical setting when MRA is ordered.
1. Simutaneous non-contrast angiography and intraplaque hemorrhage (SNAP) imaging
Wang J, et al. introduced this exciting new technique in 2013 (40). The SNAP sequence utilized a phase sensitive acquisition, and was designed to provide positive signals corresponding to intraplaque hemorrhage (IPH) and negative signals corresponding to blood signal in the vessel lumen. The technique was designed to have better IPH contrast as compared to MPRAGE because it used phase sensitive image reconstruction. For the same reason, flowing blood signals can be either suppressed as in black blood and or constructed as angiographic images. It takes 5 minutes to acquire a SNAP data set with 0.8x0.8x0.8 mm3 isotropic resolution and a longitudinal coverage of 16 cm. SNAP shows great promise for imaging both lumen size and carotid intraplaque hemorrhage with a single scan. With its large coverage, high resolution and simultaneous MRA/IPH images, SNAP has the potential to become the first- line imaging method for atherosclerosis patients in a clinical environment. (Figure 4)
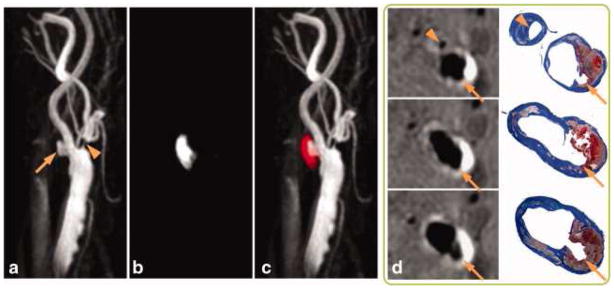
Figure 4.
SNAP with histology confirmation. 3D MIP images of the MRA-portion (a), IPH-portion (b) and color-coded joint view (c) of the SNAP images. Both IPH and luminal MRA were nicely delineated throughout the 160 mm coverage of bilateral carotid arteries. Even small branches of the carotid artery, high-risk features like ulceration (arrows) and high-level stenosis (arrowheads) were visualized. On cross-sectional reformatted images (d), both IPH and luminal shapes were confirmed by the matched Mallory's trichrome histology slides.
From Wang J, Börnert P, Zhao H, et al. Simultaneous noncontrast angiography and intraplaque hemorrhage (SNAP) imaging for carotid atherosclerotic disease evaluation. Magn Reson Med. 2013 Feb;69(2):337–45. doi: 10.1002/mrm.24254. Epub 2012 Mar 22; with permission.
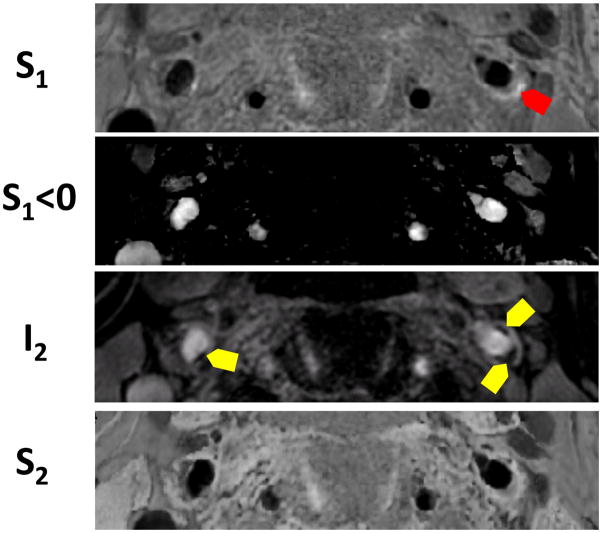
Dr. Balu and colleagues, later on, introduced a dual contrast vessel wall MRI concept based on the phase sensitive polarity maps which is the core of SNAP technology (41). By adjusting the imaging parameters in SNAP sequence and adding new image reconstruction scheme to combine phase and magnitude signal together, they are able to acquire four sets of images from one single acquisition that provide information on a) plaque burden, b) luminal stenosis, c) IPH, and d) juxtaluminal calcification. (Figure 5) This concept expands the utility of SNAP to multiple-contrast.
Figure 5.
High risk plaque identification using four images reconstructed from a single sequence in a patient. Red arrows show IPH and yellow areas show juxta-luminal calcification.
2. Multi-contrast atherosclerosis characterization (MATCH) of carotid plaque
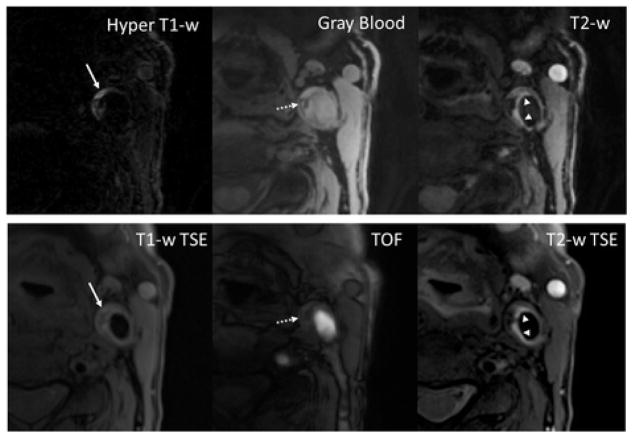
Fan Z, et al. recently introduced another exciting 3D based technique which is intended to generate multi-contrast, co-registered images with hyper T1W, gray blood, and T2-weighted images (42). MATCH, which is based on 3D spoiled segmented fast flow angle shot (FLASH) and flow-sensitive dephasing preparation for blood suppression, it is a gradient echo based acquisition with in plane resolution of 0.55 x 0.55 mm2, 2 mm slice thickness, longitudinal coverage of about 3 cm, and acquisition time of 4 min and 45 seconds. In a pilot study, Fan and his co-researchers found that MATCH was able to obtain a comparable ability to detect carotid plaque components such as LRNC, IPH, CA, and loose matrix with no problems of mis-registration compared with the conventional 2D multi-contrast protocol. This technique, however, does not provide luminal narrowing information and the acquisition time will need to be further reduced in order to have larger longitudinal coverage. (Figure 6)
Figure 6. A slice with co-existent plaque components including acute intra-plaque hemorrhage (solid arrows), calcification (dashed arrows), and loose matrix (arrowheads).
With the MATCH protocol, the unique contrast weightings and spatial coregistration facilitate easier identification of co-existent components and better appreciation of their spatial relations. The loose matrix is also hyper-intense on T1-w TSE, mimicking hemorrhage. However, it is not as hyper-intense as hemorrhage on TOF.
From Fan Z, Yu W, Xie Y, Dong L, et al. J Multi-contrast atherosclerosis characterization (MATCH) of carotid plaque with a single 5-min scan: technical development and clinical feasibility. Cardiovasc Magn Reson. 2014 Jul 25;16:53. doi: 10.1186/s12968-014-0053-5; with permission.
3. 3D SHINE
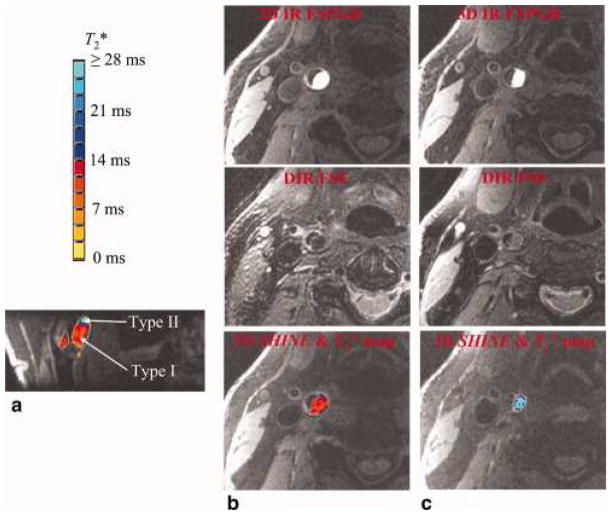
Zhu D. et al. in 2010 introduced one of the earliest multi-contrast 3D acquisition technique named 3D SHINE (43). Similar to MATCH, this is also a 3D gradient echo based approach (spoiled gradient recalled echo pulse) with multiple echo acquisition. This technique is intended to acquire black blood images with high-resolution volumetric coverage, multiple tissue contrasts, high signal-to-noise and contrast-to- noise ratios, and similar sensitivity and specificity in detecting whether intraplaque hemorrhage was present on an artery. The multiple echoes acquired with 3D SHINE allowed the estimation of intraplaque hemorrhage T2* and then the subsequent characterization of intraplaque hemorrhage (T2* for type I < 14 msec, and for type II > 14 msec). (Figure 7)
Figure 7.
An intraplaque hemorrhage with both type I and type II hemorrhagic regions is shown. The high-contrast hemorrhagic region is clearly depicted by 3D IR FSPGR or 3D SHINE. The T*2 value is color coded with the bar at the upper-left corner. The type I hemorrhage tends to have a T*2 value below 14 msec, and type II hemorrhage tends to have a T*2 value above 14 msec. The two hemorrhage types are shown clearly in the reformatted coronal view (a). Consistent with the results from 3D SHINE, (b) type I hemorrhage (shown are axial slices at predominately type I region) appears to be hypointense in DIR FSE, and (c) type II hemorrhage (shown are axial slices at predominately type II region) appears to be iso-intense in DIR FSE.
From Zhu DC1, Vu AT, Ota H, DeMarco JK. An optimized 3D spoiled gradient recalled echo pulse sequence for hemorrhage assessment using inversion recovery and multiple echoes (3D SHINE) for carotid plaque imaging. Magn Reson Med. 2010 Nov;64(5):1341–51. doi: 10.1002/mrm.22517; with permission.
4. 3D Delay alternating with nutation for tailored excitation (DANTE) –(44)
Delay alternating with nutation for tailored excitation (DANTE) pulse trains have been used as frequency-selective excitation methods in Fourier transform MRI and for spatial tagging in MRI. Recently, Li et al. demonstrated that nonselective DANTE pulse trains can be used in combination with gradient pulses and short repetition times to eliminate signal from moving fluid and structures (45). While the longitudinal magnetization of static tissue is mostly preserved, the magnetization from flowing spins fails to establish transverse steady state is almost completely attenuated due to the spoiling effect caused by flow along the applied gradient. The attenuation of flowing spins is effectively insensitive to spin velocity (above a very low threshold) and can be approximately quantified with a simple T1 longitudinal magnetization decay model. Based on this contrast mechanism, the DANTE preparation was applied to black blood vessel imaging and demonstrated excellent blood signal suppression and static tissue signal preservation.
5. CINE retrospective ordering and compressed sensing (ciné-ROCS) Turbo Spin echo (TSE), MERGE and MPRAGE
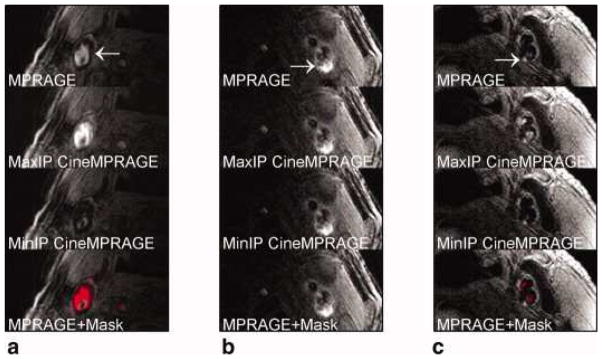
Mendes et al. investigated the effects of periodic motion in the neck on 2D and 3D carotid images. In conventional 2D and 3D TSE acquisitions, periodic motion of blood, arterial wall, and surrounding tissue with the cardiac or respiratory cycle can cause image degradation. Mendes et al. demonstrated that the measurement data acquired with conventional 2D and 3DTSE can be phase-ordered with the subjects cardiac signal (ECG or pressure pulse data) and the resulting sparse series of phase-ordered bins can be reconstructed using compressed sensing methods as a temporal sequence of 2D and 3D images (46). Despite the increased number of images (and resulting interpretation load), this technique appears to help in the discrimination of some artifacts. For TSE, ciné-ROCS reconstruction requires: 1) two or more measurement averages, and 2) a TR that spreads the measurements uniformly into the times of multiple selected cycle phases. (Figure 8)
Figure 8.
Identification of potential hemorrhage and hemorrhage mimicking flow artifacts using CineMPRAGE image reconstruction. A patient with flow artifact is shown in (a), whereas patients with potential IPH are shown in (b) and (c). The top row is the MPRAGE images reconstructed using the standard MPRAGE technique. In all cases, there are pixels with signal intensities above 1.5× sternocleidomastoid muscle. The maximum and minimum intensity projections (taken along the temporal direction) of the CineMPRAGE reconstruction are shown in the second and third rows, respectively. Although the potential hemorrhage signal is constant, the flow artifact varies across the cardiac cycle as evidenced by the difference in the maximum and minimum intensity projections. Color maps corresponding to the CineMPRAGE signal variation are overlaid on the conventional MPRAGE images on the bottom row.
From Mendes J1, Parker DL, Kim SE, et al. Reduced blood flow artifact in intraplaque hemorrhage imaging using Cine MPRAGE. Magn Reson Med. 2013 May;69(5):1276–84; with permission.
5. 3D for joint intra- and extracranial vessel wall imaging
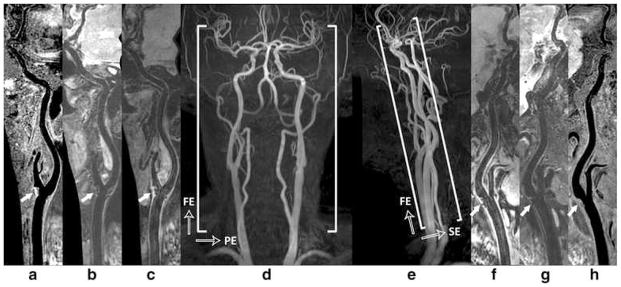
With 3D’s large coverage, and the importance of stroke caused by either intra and extracranial artery atherosclerosis, it is imperative that vessel wall imaging be extended from carotid to intracranial space. Zhou, et al. recently evaluated the performance of a novel 3D multi-contrast approach for this purpose (47). Added with a custom designed 36 channel neurovascular coil on a 3T scanner, they acquired images with 3D MERGE, SNAP, and VISTA (which is a different version of 3D FSE) sequences with T1, T2 and heavy T1 weighting covering the entire intra- and extracranial vasculature. The 3D multi-contrast images were acquired within 15mins allowed the vessel wall visualization with 0.8 mm isotropic spatial resolution covering intra- and extracranial segments (25 cm head-to-toe direction). Quantitative wall and lumen SNR measurements for each sequence showed effective blood suppression at all selected locations (P < 0.0001). Although the wall-lumen CNR varied across measured locations, each sequence provided good or adequate image quality in both intra- and extracranial segments. (Figure 9) This study shows the feasibility of a time efficient complete workup of intra-and extra- cranial vascular evaluation to identify high risk plaques.
Figure 9.
Illustration of 3D multi-contrast imaging coverage. Three stations of 3D time-of-flight coronal maximum intensity projection (MIP) fusion (d) and sagittal MIP fusion (e) are illustrated here only for a clear definition of imaging FOV. The curved multi-planar reconstruction (MPR) examples show the right (a - c) and left (f – h) sides arteries ranging from CCA through ICA to MCA, where (c, f), (b, g), and (a, h) correspond to the results of 3D-MERGE, T2-weighted VISTA and SNAP respectively. Note the plaques were detected at bilateral carotid bifurcations on all different contrast weighted images as shown by solid arrows
From Zhou Z, Li R, Zhao X. Evaluation of 3D multi-contrast joint intra- and extracranial vessel wall cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2015 May 27;17:41; with permission.
In summary, there has been significant progress made in 3D carotid plaque imaging techniques in recent years. 3D plaque imaging clearly represents the future in clinical use. With effective flow suppression techniques, choices of different contrast weighting acquisitions, and time-efficient imaging approaches, 3D plaque imaging offers flexible imaging plane and view angle analysis, large coverage, multi-vascular beds capability, and even can be used in fast screening.
KEY POINTS.
Significant progress made in 3D carotid plaque imaging techniques in recent years
Due to fast imaging times and results comparable to many clinical 3D vessel wall imaging by MRI may be able to improve on current clinical assessments
Our ability to predict clinical outcomes is limited by our knowledge of atherosclerosis etiology and progression—3D techniques may enable us to study understand the origin and outcomes of carotid atherosclerosis.
Information on the condition of carotid atherosclerosis may provide a useful window to understanding system-wide disease burden and assess overall cardiovascular risk.
Footnotes
DISCLOSURE STATEMENT
Dr. Yuan holds grants from the NIH and Philips Medical. He also serves as a Member of Radiology Advisory Network, Philips
Dr. Parker has nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Chun Yuan, Professor of Radiology, Dept. of Radiology, University of Washington. Director, Bio-Molecular Imaging Center, Co-Director, Vascular Imaging Lab.
Dennis L. Parker, Professor of Radiology, Director of the Utah Center for Advanced Imaging Research.
References
- 1.Virmani R, Kolodgie FD, Burke AP, Finn AV, Gold HK, Tulenko TN, Wrenn SP, Narula J. Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol. 2005;25(10):2054–61. doi: 10.1161/01.ATV.0000178991.71605.18. [DOI] [PubMed] [Google Scholar]
- 2.Saam T, Hetterich H, Hoffmann V, Yuan C, Dichgans M, Poppert H, Koeppel T, Hoffmann U, Reiser MF, Bamberg F. Meta-analysis and systematic review of the predictive value of carotid plaque hemorrhage on cerebrovascular events by magnetic resonance imaging. J Am Coll Cardiol. 2013;62(12):1081–91. doi: 10.1016/j.jacc.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 3.Gupta A, Baradaran H, Schweitzer AD, Kamel H, Pandya A, Delgado D, Dunning A, Mushlin AI, Sanelli PC. Carotid plaque MRI and stroke risk: a systematic review and meta-analysis. Stroke. 2013;44(11):3071–7. doi: 10.1161/STROKEAHA.113.002551. [DOI] [PubMed] [Google Scholar]
- 4.O'Leary DH, Polak JF. Intima-media thickness: a tool for atherosclerosis imaging and event prediction. Am J Cardiol. 2002;90(10C):18L–21L. doi: 10.1016/s0002-9149(02)02957-0. [DOI] [PubMed] [Google Scholar]
- 5.Sillesen H. Carotid intima-media thickness and/or carotid plaque: what is relevant? Eur J Vasc Endovasc Surg. 2014;48(2):115–7. doi: 10.1016/j.ejvs.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 6.Spence JD. Carotid plaque measurement is superior to IMT Invited editorial comment on: carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: a meta-analysis-Yoichi Inaba, M.D., Jennifer A. Chen M.D., Steven R. Bergmann M.D., Ph.D. Atherosclerosis. 2012;220(1):34–5. doi: 10.1016/j.atherosclerosis.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Cai JM, Hatsukami TS, Ferguson MS, Small R, Polissar NL, Yuan C. Classification of human carotid atherosclerotic lesions with in vivo multicontrast magnetic resonance imaging. Circulation. 2002;106(11):1368–73. doi: 10.1161/01.cir.0000028591.44554.f9. [DOI] [PubMed] [Google Scholar]
- 8.Yuan C, Kerwin WS. MRI of atherosclerosis. J Magn Reson Imaging. 2004;19(6):710–9. doi: 10.1002/jmri.20070. [DOI] [PubMed] [Google Scholar]
- 9.Yarnykh VL, Terashima M, Hayes CE, Shimakawa A, Takaya N, Nguyen PK, Brittain JH, McConnell MV, Yuan C. Multicontrast black-blood MRI of carotid arteries: comparison between 1.5 and 3 tesla magnetic field strengths. JMagn ResonImaging. 2006;23(5):691–8. doi: 10.1002/jmri.20562. [DOI] [PubMed] [Google Scholar]
- 10.Kerwin WS, Liu F, Yarnykh V, Underhill H, Oikawa M, Yu W, Hatsukami TS, Yuan C. Signal features of the atherosclerotic plaque at 3.0 Tesla versus 1.5 Tesla: Impact on automatic classification. Journal of Magnetic Resonance Imaging. 2008;28(4):987–95. doi: 10.1002/jmri.21529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ota H, Yarnykh VL, Ferguson MS, Underhill HR, Demarco JK, Zhu DC, Oikawa M, Dong L, Zhao X, Collar A, Hatsukami TS, Yuan C. Carotid intraplaque hemorrhage imaging at 3.0-T MR imaging: comparison of the diagnostic performance of three T1-weighted sequences. Radiology. 2010;254(2):551–63. doi: 10.1148/radiol.09090535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayes CE, Mathis CM, Yuan C. Surface coil phased arrays for high-resolution imaging of the carotid arteries. J Magn Reson Imaging. 1996;6(1):109–12. doi: 10.1002/jmri.1880060121. [DOI] [PubMed] [Google Scholar]
- 13.Tate Q, Kim SE, Treiman G, Parker DL, Hadley JR. Increased vessel depiction of the carotid bifurcation with a specialized 16-channel phased array coil at 3T. Magn Reson Med. 2013;69(5):1486–93. doi: 10.1002/mrm.24380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edelman RR, Chien D, Kim D. Fast selective black blood MR imaging. Radiology. 1991;181(3):655–60. doi: 10.1148/radiology.181.3.1947077. [DOI] [PubMed] [Google Scholar]
- 15.Yuan C, Kerwin WS, Ferguson MS, Polissar N, Zhang S, Cai J, Hatsukami TS. Contrast-enhanced high resolution MRI for atherosclerotic carotid artery tissue characterization. J Magn Reson Imaging. 2002;15(1):62–7. doi: 10.1002/jmri.10030. [DOI] [PubMed] [Google Scholar]
- 16.Antiga L, Wasserman BA, Steinman DA. On the overestimation of early wall thickening at the carotid bulb by black blood MRI, with implications for coronary and vulnerable plaque imaging. Magn Reson Med. 2008;60(5):1020–8. doi: 10.1002/mrm.21758. [DOI] [PubMed] [Google Scholar]
- 17.Balu N, Chu B, Hatsukami TS, Yuan C, Yarnykh VL. Comparison between 2D and 3D high-resolution black-blood techniques for carotid artery wall imaging in clinically significant atherosclerosis. J Magn Reson Imaging. 2008;27(4):918–24. doi: 10.1002/jmri.21282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balu N, Kerwin WS, Chu B, Liu F, Yuan C. Serial MRI of carotid plaque burden: influence of subject repositioning on measurement precision. Magn ResonMed. 2007;57(3):592–9. doi: 10.1002/mrm.21160. [DOI] [PubMed] [Google Scholar]
- 19.Balu N, Yarnykh V, Chu B, Wang J, Yuan C, editors. Carotid Plaque Assessment using Fast 3D Isotropic-Resolution Black-Blood MRI. International Society of Magnetic Resonance in Medicine; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J, Yarnykh VL, Hatsukami T, Chu B, Balu N, Yuan C. Improved suppression of plaque-mimicking artifacts in black-blood carotid atherosclerosis imaging using a multislice motion-sensitized driven-equilibrium (MSDE) turbo spin-echo (TSE) sequence. Magn ResonMed. 2007;58(5):973–81. doi: 10.1002/mrm.21385. [DOI] [PubMed] [Google Scholar]
- 21.Zhao H, Wang J, Liu X, Zhao X, Hippe DS, Cao Y, Wan J, Yuan C, Xu J. Assessment of carotid artery atherosclerotic disease by using three-dimensional fast black-blood MR imaging: comparison with DSA. Radiology. 2015;274(2):508–16. doi: 10.1148/radiol.14132687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J, Yarnykh VL, Yuan C. Enhanced image quality in black-blood MRI using the improved motion-sensitized driven-equilibrium (iMSDE) sequence. J Magn Reson Imaging. 2010;31(5):1256–63. doi: 10.1002/jmri.22149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Makhijani MK, Balu N, Yamada K, Yuan C, Nayak KS. Accelerated 3D MERGE carotid imaging using compressed sensing with a hidden Markov tree model. J Magn Reson Imaging. 2012;36(5):1194–202. doi: 10.1002/jmri.23755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J, Gerretsen SC, Maki JH, Jaarsma C, Kooi ME, Herzka D, Chu B, Yarnykh VL, Yuan C, Leiner T. Time-efficient black blood RCA wall imaging at 3T using improved motion sensitized driven equilibrium (iMSDE): feasibility and reproducibility. PLoS One. 2011;6(10):e26567. doi: 10.1371/journal.pone.0026567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Obara M, Kuroda K, Wang J, Honda M, Yoneyama M, Imai Y, Van Cauteren M. Comparison between two types of improved motion-sensitized driven-equilibrium (iMSDE) for intracranial black-blood imaging at 3.0 tesla. J Magn Reson Imaging. 2014;40(4):824–31. doi: 10.1002/jmri.24430. [DOI] [PubMed] [Google Scholar]
- 26.Zhu C, Graves MJ, Yuan J, Sadat U, Gillard JH, Patterson AJ. Optimization of improved motion-sensitized driven-equilibrium (iMSDE) blood suppression for carotid artery wall imaging. J Cardiovasc Magn Reson. 2014;16:61. doi: 10.1186/s12968-014-0061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moody AR, Murphy RE, Morgan PS, Martel AL, Delay GS, Allder S, MacSweeney ST, Tennant WG, Gladman J, Lowe J, Hunt BJ. Characterization of complicated carotid plaque with magnetic resonance direct thrombus imaging in patients with cerebral ischemia. Circulation. 2003;107(24):3047–52. doi: 10.1161/01.CIR.0000074222.61572.44. [DOI] [PubMed] [Google Scholar]
- 28.Murphy RE, Moody AR, Morgan PS, Martel AL, Delay GS, Allder S, MacSweeney ST, Tennant WG, Gladman J, Lowe J, Hunt BJ. Prevalence of complicated carotid atheroma as detected by magnetic resonance direct thrombus imaging in patients with suspected carotid artery stenosis and previous acute cerebral ischemia. Circulation. 2003;107(24):3053–8. doi: 10.1161/01.CIR.0000074204.92443.37. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Z, Fan Z, Carroll TJ, Chung Y, Weale P, Jerecic R, Li D. Three-dimensional T2-weighted MRI of the human femoral arterial vessel wall at 3.0 Tesla. Invest Radiol. 2009;44(9):619–26. doi: 10.1097/RLI.0b013e3181b4c218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fan Z, Zhang Z, Chung YC, Weale P, Zuehlsdorff S, Carr J, Li D. Carotid arterial wall MRI at 3T using 3D variable-flip-angle turbo spin-echo (TSE) with flow-sensitive dephasing (FSD) J Magn Reson Imaging. 2010;31(3):645–54. doi: 10.1002/jmri.22058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parker DL, Yuan C, Blatter DD. MR angiography by multiple thin slab 3D acquisition. Magn Reson Med. 1991;17(2):434–51. doi: 10.1002/mrm.1910170215. [DOI] [PubMed] [Google Scholar]
- 32.Yim YJ, Choe YH, Ko Y, Kim ST, Kim KH, Jeon P, Byun HS, Kim DI. High signal intensity halo around the carotid artery on maximum intensity projection images of time-of-flight MR angiography: a new sign for intraplaque hemorrhage. J Magn Reson Imaging. 2008;27(6):1341–6. doi: 10.1002/jmri.21284. [DOI] [PubMed] [Google Scholar]
- 33.Yoshimura S, Yamada K, Kawasaki M, Asano T, Kanematsu M, Takamatsu M, Hara A, Iwama T. High-intensity signal on time-of-flight magnetic resonance angiography indicates carotid plaques at high risk for cerebral embolism during stenting. Stroke. 2011;42(11):3132–7. doi: 10.1161/STROKEAHA.111.615708. [DOI] [PubMed] [Google Scholar]
- 34.Yuan C, Mitsumori LM, Ferguson MS, Polissar NL, Echelard D, Ortiz G, Small R, Davies JW, Kerwin WS, Hatsukami TS. In vivo accuracy of multispectral magnetic resonance imaging for identifying lipid-rich necrotic cores and intraplaque hemorrhage in advanced human carotid plaques. Circulation. 2001;104(17):2051–6. doi: 10.1161/hc4201.097839. [DOI] [PubMed] [Google Scholar]
- 35.Chu B, Kampschulte A, Ferguson MS, Kerwin WS, Yarnykh VL, O'Brien KD, Polissar NL, Hatsukami TS, Yuan C. Hemorrhage in the atherosclerotic carotid plaque: a high-resolution MRI study. Stroke. 2004;35(5):1079–84. doi: 10.1161/01.STR.0000125856.25309.86. [DOI] [PubMed] [Google Scholar]
- 36.Kampschulte A, Ferguson MS, Kerwin WS, Polissar NL, Chu B, Saam T, Hatsukami TS, Yuan C. Differentiation of intraplaque versus juxtaluminal hemorrhage/thrombus in advanced human carotid atherosclerotic lesions by in vivo magnetic resonance imaging. Circulation. 2004;110(20):3239–44. doi: 10.1161/01.CIR.0000147287.23741.9A. [DOI] [PubMed] [Google Scholar]
- 37.Takaya N, Yuan C, Chu BC, Saam T, Polissar NL, Jarvick G, Isaac C, McDonough J, Natiello C, Small R, Ferguson MS, Hatsukami TS. Presence of Intraplaque Hemorrhage Stimulates Progression of Carotid Atherosclerotic Plaques: A High-Resolution MRI study. Circulation. 2005;111:2768–75. doi: 10.1161/CIRCULATIONAHA.104.504167. [DOI] [PubMed] [Google Scholar]
- 38.Yuan C, Zhang S, Polissar NL, Echelard D, Ortiz G, Davis JW, Ellington E, Hatsukami TS. Identification of fibrous cap rupture with magnetic resonance imaging is highly associated with recent TIA or stroke. Circulation. 2002;105(2):181–5. doi: 10.1161/hc0202.102121. [DOI] [PubMed] [Google Scholar]
- 39.Yamada K, Song Y, Hippe DS, Sun J, Dong L, Xu D, Ferguson MS, Chu B, Hatsukami TS, Chen M, Zhou C, Yuan C. Quantitative evaluation of high intensity signal on MIP images of carotid atherosclerotic plaques from routine TOF-MRA reveals elevated volumes of intraplaque hemorrhage and lipid rich necrotic core. J Cardiovasc Magn Reson. 2012;14:81. doi: 10.1186/1532-429X-14-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang J, Börnert P, Zhao H, Hippe DS, Zhao X, Balu N, Ferguson MS, Hatsukami TS, Xu J, Yuan C, Kerwin WS. Simultaneous noncontrast angiography and intraplaque hemorrhage (SNAP) imaging for carotid atherosclerotic disease evaluation. Magn Reson Med. 2013;69(2):337–45. doi: 10.1002/mrm.24254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Balu NLH, Chen S, Chen H, Wang J, Zhou Z, Yuan C. Dual Contrast Vessel Wall MRI Using Phase Sensitive Polarity Maps. Proceedings of the 22st Scientific Meeting and Exhibition, International Society of Magnetic Resonance in Medicine; Milan, Italy. 2014. [Google Scholar]
- 42.Fan Z, Yu W, Xie Y, Dong L, Yang L, Wang Z, Conte AH, Bi X, An J, Zhang T, Laub G, Shah PK, Zhang Z, Li D. Multi-contrast atherosclerosis characterization (MATCH) of carotid plaque with a single 5-min scan: technical development and clinical feasibility. J Cardiovasc Magn Reson. 2014;16:53. doi: 10.1186/s12968-014-0053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu DC, Vu AT, Ota H, DeMarco JK. An optimized 3D spoiled gradient recalled echo pulse sequence for hemorrhage assessment using inversion recovery and multiple echoes (3D SHINE) for carotid plaque imaging. Magn Reson Med. 2010;64(5):1341–51. doi: 10.1002/mrm.22517. [DOI] [PubMed] [Google Scholar]
- 44.Li L, Chai JT, Biasiolli L, Robson MD, Choudhury RP, Handa AI, Near J, Jezzard P. Black-blood multicontrast imaging of carotid arteries with DANTE-prepared 2D and 3D MR imaging. Radiology. 2014;273(2):560–9. doi: 10.1148/radiol.14131717. [DOI] [PubMed] [Google Scholar]
- 45.Li L, Miller KL, Jezzard P. DANTE-prepared pulse trains: a novel approach to motion-sensitized and motion-suppressed quantitative magnetic resonance imaging. Magn Reson Med. 2012;68(5):1423–38. doi: 10.1002/mrm.24142. [DOI] [PubMed] [Google Scholar]
- 46.Mendes J, Parker DL, Kim SE, Treiman GS. Reduced blood flow artifact in intraplaque hemorrhage imaging using CineMPRAGE. Magn Reson Med. 2013;69(5):1276–84. doi: 10.1002/mrm.24354. Epub 2012/07/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou Z, Li R, Zhao X, He L, Wang X, Wang J, Balu N, Yuan C. Evaluation of 3D multi-contrast joint intra- and extracranial vessel wall cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2015;17:41. doi: 10.1186/s12968-015-0143-z. [DOI] [PMC free article] [PubMed] [Google Scholar]