Abstract
A patient diagnosed with metastatic melanoma developed the paraneoplastic syndrome of humoral hypercalcemia of malignancy and cachexia after receiving ipilumumab. The cause of the hypercalcemia was thought to be secondary to parathyroid hormone related peptide (PTHrP) as plasma levels were found to be elevated. The patient underwent two tumor biopsies: at diagnosis (when calcium levels were normal) and upon development of hypercalcemia and cachexia. PTHrP expression was higher in melanoma cells when hypercalcemia had occurred than prior to its onset. Metabolic characterization of melanoma cells revealed that, with development of hypercalcemia, there was high expression of monocarboxylate transporter 1 (MCT1) which is the main importer of lactate and ketone bodies into cells. MCT1 is associated with high mitochondrial metabolism. Beta-galactosidase (β-GAL), a marker of senescence had reduced expression in melanoma cells upon development of hypercalcemia compared to pre-hypercalcemia.
In conclusion, PTHrP expression in melanoma is associated with cachexia, increased cancer cell lactate and ketone body import, high mitochondrial metabolism and reduced senescence. Further studies are required to determine if PTHrP regulates cachexia, lactate and ketone body import, mitochondrial metabolism and senescence in cancer cells.
Keywords: Hypercalcemia, melanoma, lactate, ketone bodies, oxidative phosphorylation, senescence
Case Report
A 49-year-old female initially presented with multiple tender subcutaneous masses. Biopsies of subcutaneous masses on her back revealed metastatic melanoma from an unknown primary site. Her calcium level at diagnosis was 8.7mg/dL (normal value: 8.5-10.5 mg/dL). Fluoro 2-Deoxy-Glucose (FDG) PET/CT revealed multiple hypermetabolic subcutaneous and intramuscular nodules as well as lymph node enlargement in the supraclavicular, axillary, inguinal and iliac regions. The highest standardized uptake value (SUV) was 15.8 in the right gluteal region. A hypermetabolic focus was noted in the musculature of the right calf with an SUV of 15.07. The patient was treated with ipilimumab, and three months after starting treatment she had a partial response with reduced activity of the right gluteal nodule with an SUV of 6.49 and reduced activity in the right calf with an SUV of 4.35. She had resolution of several subcutaneous masses and the right supraclavicular lymphadenopathy. There was also reduced activity of the right axillary lymphadenopathy.
Four months after starting treatment, the patient developed left leg pain, swelling and erythema. The subcutaneous nodules had progressed on examination and on imaging studies. A biopsy of a subcutaneous nodule ultimately confirmed melanoma and recurrent progressive disease. At the time of disease progression she also developed cachexia and had an elevated calcium level of 14 mg/dL (see table 1 for details on laboratory measurements). A bone scan was unremarkable. A PTHrP serum level was elevated at 273 pcg/mL (normal range: 14-17 pcg/mL). The patient was aggressively hydrated and she received zoledronic acid with normalization of calcium levels. However, her calcium level once again became elevated and she was refractory to treatment with zoledronic acid, denosumab and calcitonin. She received palliative radiation therapy to several subcutaneous nodules. However, her cancer continued to progress and she died shortly thereafter.
Table 1.
Laboratory values at diagnosis and with development of hypercalcemia.
| Laboratory Parameter | 1st time-point (Diagnosis) | 2nd time-point (Hypercalcemia) |
|---|---|---|
| Calcium [8.5-10.5 mg/dL] | 8.7mg/dL | 14 mg/dL |
| Albumin [3.2-4.9 mg/dL] | 4mg/dL | 3.7 mg/dL |
| PTHrP [14-17 pg/mL] | Not measured | 273 pg/mL |
| Intact PTH [11-67 pg/mL] | Not measured | 3 pg/mL |
| Vit D-25OH total [ng/mL] | Not measured | 24.8 ng/mL |
| Vit D-1,25OH2 total [18-72 ng/mL] | Not measured | 27 ng/mL |
| Vit D3-1,25OH2 total [18-72 ng/mL] | Not measured | 27 ng/mL |
| Vit D2-1,25OH total [18-72 ng/mL] | Not measured | <8 ng/mL |
| TSH [0.3-5 IU/mL] | Not measured | 1.85 IU/mL |
| ACTH [9-46 pg/mL] | Not measured | <9 pg/mL |
Materials and Methods
The study was approved by the Institutional Review Board at Thomas Jefferson University. Pathological analyses were performed on skin, esophagus, non-small cell lung cancer (NSCLC) and diffuse large B cell lymphoma (DLBCL) samples. The samples had been fixed in 10% neutral buffered formalin and embedded in paraffin for hematoxylin and eosin (H&E) and immunohistochemistry (IHC) staining. The streptavidin–horse radish peroxidase method was used for IHC staining as previously described1. Primary antibodies used were PTHrP(Santa Cruz, sc-20728), and beta galactosidase (Abcam, Cambridge, MA, ab96239). MCT1 antibody was kindly provided by Dr Nancy Philp and has been extensively characterized1.
Metabolic characterization of melanoma
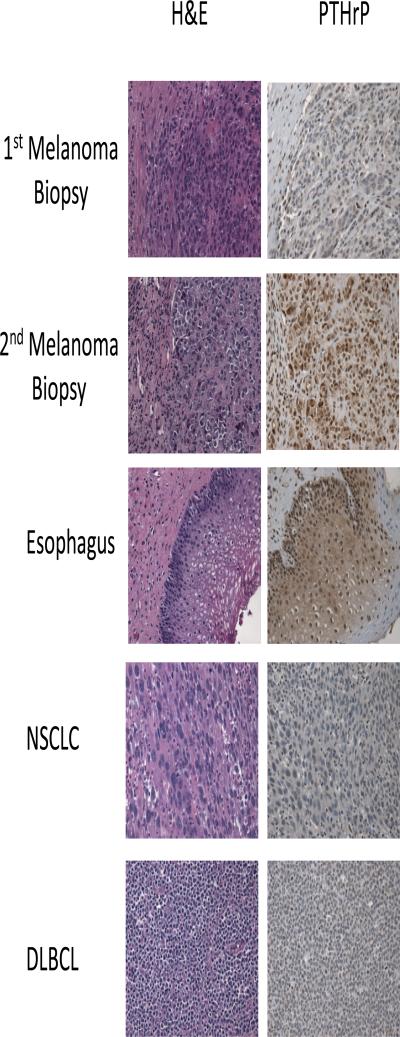
Two tumor biopsies were performed to assess this patient's melanoma. The first biopsy allowed for the diagnosis of metastatic melanoma to be made. This biopsy was conducted when calcium levels in the plasma were normal. The second biopsy was performed when the patient had developed hypercalcemia. These two samples were stained by immunohistochemistry for PTHrP at the same time. There was high PTHrP expression in melanoma cells only when hypercalcemia had occurred (Figure 1). This indicates a temporal relationship between the production of PTHrP by the melanoma cells and the development of hypercalcemia.
Figure 1. Hematoxylin and eosin (H&E) and Parathyroid hormone related peptide (PTHrP) staining.
H&E and PTHrP staining by immunohistochemistry were performed on melanoma samples from a patient who developed hypercalcemia. The first biopsy was obtained prior to developing hypercalcemia while as the second biopsy was obtained after developing hypercalcemia. Esophageal epithelia, non-small cell lung cancer (NSCLC) and diffuse large B cell lymphoma samples were stained for PTHrP as a positive control (esophageal epithelia) and negative controls (NSCLC and DLBCL). Note that high nuclear and cytoplasmic expression of PTHrP is found in the melanoma sample after development of hypercalcemia and in esophageal epithelia. NSCLC and DLBCL lack PTHrP expression.
As a positive control for the staining of PTHrP, an esophageal tissue sample was chosen, as PTHrP is know to be present in esophageal squamous epithelial cells2. Staining was positive for PTHrP in the nuclei of the esophageal surface squamous epithelium as expected (Figure 1). PTHrP staining was negative in samples of diffuse B cell lymphoma and squamous cell carcinoma of the lung, which are known to lack expression of PTHrP (Figure 1). The staining patterns of the positive control (esophageal epithelium) and negative controls (squamous cell lung cancer and diffuse large B cell lymphoma) confirm the specificity of the PTHrP antibody staining in the melanoma sample when the patient had developed hypercalcemia (Figure 1).
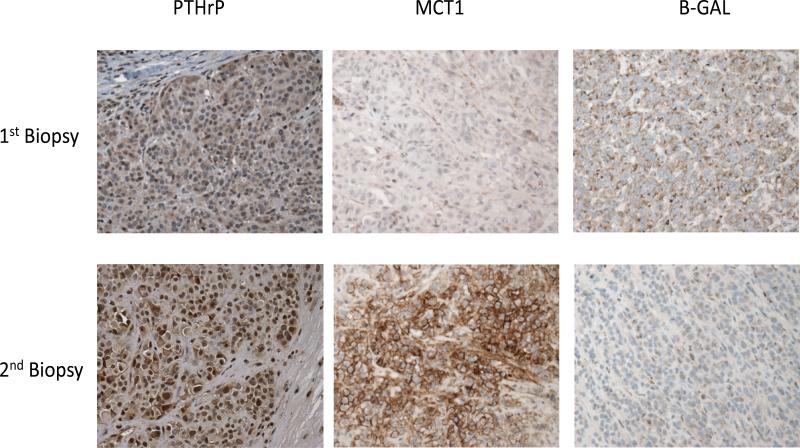
At the same time that PTHrP expression in melanoma cells increased there was increased expression of monocarboxylate transporter 1 (MCT1) (Figure 2). The monocarboxylates lactate and ketone bodies are mainly imported into cancer cells by MCT1 and it is a marker of mitochondrial oxidative phosphorylation and beta oxidation which convert acetyl-CoA or free fatty acids to carbon dioxide, water and ATP respectively. The expression of the senescence marker beta-galactosidase (β-GAL) was reduced with development of hypercalcemia and elevated PTHrP expression (Figure 2). In sum, hypercalcemia and elevated PTHrP levels were associated with increased lactate and ketone body uptake, increased mitochondrial metabolism and reduced senescence in human melanoma.
Figure 2. PTHrP, MCT1 and β-GAL staining.
PTHrP, MCT1 and β-GAL staining by immunohistochemistry of melanoma samples from a patient who developed hypercalcemia. The first biopsy was obtained prior to developing hypercalcemia while as the second biopsy was obtained after developing hypercalcemia. Note that MCT1 expression is upregulated while as β-GAL staining is downregulated when PTHrP expression increases in the second melanoma biopsy compared to the first biopsy, which was performed prior to development of hypercalcemia.
Discussion
MCT1 expression is upregulated in cancer cells when PTHrP expression increases. MCT1 is the main importer of lactate and ketone bodies into cancer cells3. Lactate and ketone bodies are catabolites that fuel cancer cell growth in a similar fashion to glucose and glutamine4. The tumor also had high uptake of deoxyglucose as measured by FDG-PET uptake. However, FDG-PET does not provide information as to the allocation of glucose carbon into any particular downstream pathway such as mitochondrial metabolism5. MCT1 is also a marker of mitochondrial metabolism via oxidative phosphorylation and beta oxidation since lactate and ketone bodies are utilized to generate ATP 3,6. Hence, PTHrP upregulation is associated with ketone body and lactate utilization in tumors. Future work will need to determine if PTHrP regulates MCT1 expression.
β-GAL is a marker of senescence and this patient's cancer cells had reduced expression of β-GAL when PTHrP was upregulated. Senescence represents a potent tumor suppressor mechanism7. It is induced by nutrient starvation, shortening of telomeres or alterations in oncogenes and tumor suppressors8. Nutrient starvation leads to cellular self-digestion or autophagy and ultimately can cause senescence9. Upregulation of MCT1 in this patient's cancer cells may have induced tumor progression by increasing their metabolic efficiency, which reduced nutrient starvation and thus senescence. As the cancer cells imported more catabolites, nutrient starvation was reduced in the malignant cells but the patient became cachectic. This highlights the parasitic relationship between cancer cells and the host whereby the host becomes nutrient deplete when cancer cells have enhanced nutrient uptake10. Modulation of β-GAL and MCT1 may be novel mechanisms by which PTHrP induces cachexia. The effects of PTHrP and MCT1 on telomere shortening and the balance between other oncogenes and tumor suppressors will need to be investigated further. The reason why hypercalcemia is associated with high mortality in cancer is poorly understood but altered metabolism and senescence may be contributing factors.
A paraneoplastic syndrome is defined as a sign or symptom due to cancer but not attributable to direct tumor invasion or compression11. Approximately 10% of patients with cancer have a paraneoplastic syndrome11. Hypercalcemia is one of the most common paraneoplastic syndromes and most cases of hypercalcemia in cancer are due to a paraneoplastic syndrome12. Reported incidence of hypercalcemia in patients with metastatic melanoma ranges from 1.1 to 4.9%13. The mortality of hypercalcemia in cancer is extremely high with approximately 50% mortality at 30 days14. Our patient died shortly after developing hypercalcemia, consistent with epidemiologic observations.
The most common cause of hypercalcemia in cancer is secretion of parathyroid hormone (PTH)-related protein (PTHrP) by tumor cells—known as humoral hypercalcemia of malignancy, which accounts for 70% of cases. Osteolytic activity at sites of skeletal metastases accounts for another 20% of cases and much less commonly hypercalcemia may result from tumor secretion of vitamin D or from ectopic tumor secretion of PTH15-17. Hypercalcemia of malignancy can be caused by other cytokines that stimulate bone resorption such as interleukin-6 (IL-6), IL1, prostaglandins, tumor necrosis factor alpha (TNF-α) and transforming growth factors alpha and beta18. Cancer cells create a cycle by which the PTHrP they generate stimulates osteoclastic resorption with subsequent release of bone-derived growth factors such as TGF-β and IL-6 which further stimulates tumor growth and PTHrP expression by tumor cells19. Other causes of hypercalcemia include hyperthyroidism, adrenal insufficiency and medication effects such as the use of calcium supplements, calcium antacids, vitamin D supplements, vitamin A intoxication, hydrochlorothiazide and lithium20, 21. Our patient had hypercalcemia of malignancy due to a paraneoplastic syndrome mediated by PTHrP. Alternative causes of hypercalcemia were evaluated and ruled out in our patient (Table 1).
Several case reports have described the presence of both hypercalcemia and malignant melanoma, although none have examined the interactions between metabolism and calcium homeostasis. Matsui et al. found the first instance of increased serum level of PTHrP in malignant melanoma22. Yeung et al. showed in a patient with hypercalcemia and melanoma that the cancer cells expressed PTHrP23. El Abdaimi et al. demonstrated in vitro a stepwise increase in PTHrP expression when cells progressed from normal to malignant melanocytes24. However, Kageshita et al. showed that there was high expression of PTHrP in both non-transformed and transformed melanocytic cells25. Hence, the relationship between PTHrP and melanoma aggressiveness is poorly understood.
PTHrP was discovered in the late 1980s as a protein that shared homology with intact PTH and that like PTH increases plasma calcium levels26. The Parathyroid Hormone-Like Hormone (PTHLH) gene, which is located on the short arm of chromosome 12 encodes PTHrP. Alternative splicing generates three separate isoforms of PTHrP of 139, 141, or 173 amino acids but the biological differences between the isoforms are unclear. PTHLH and PTH genes are closely related since the exon/intron organization of both genes encoding the pre-pro sequences and the initial portion of the mature peptides are identical. Furthermore, the amino-termini of secreted PTH and PTHrP are highly homologous, such that the peptides share eight of the first 13 amino acids and a similar secondary structure over the next 21 amino acids27. PTHrP is thought to have arisen after gene duplication of PTH, after which both gene products developed independently as two molecules with different structural complexities and mechanisms of control19.
Osteoblast, osteoclast and chondrocyte differentiation are regulated by PTHrP and it is responsible for normal endochondral bone formation28,28. PTHrP is highly expressed in the placenta and the breast during lactation to transfer calcium to the fetus or child27, 29. Development of the brain, hematopoiesis, vascular smooth muscle, skin, hair follicles and teeth is also regulated by PTHrP and it is highly expressed in Purkinje cells in the cerebellum and esophageal epithelium 30,27, 31. Loss of PTHrP nuclear expression is associated with senescence of neurons and hematopoietic cells31. However, the current description of cancer cells with reduced senescence upon PTHrP upregulation is the first report to our knowledge that it is associated with senescence in cancer cells.
PTHrP induces cachexia32-34. Adipose tissue browning is one of the mechanisms by which PTHrP induces cachexia35. Brown adipose tissue is metabolically inefficient since it catabolizes fatty acids and glucose at high rates to generate heat with little ATP production36. Browning of adipose tissue leads to cachexia because it induces depletion of white adipose tissue with catabolite release such as release of fatty acids36. PTHrP induces depletion of white adipose tissue31. MCT1 upregulation with increased catabolite uptake by cancer cells may also contribute to cancer cachexia when PTHrP expression increases and will need to be investigated further.
This patient had received ipilimumab prior to developing hypercalcemia. Ipilimumab is an immune checkpoint inhibitor that has demonstrated efficacy in the treatment of metastatic melanoma in a number of early and late phase trials37, 38, 39. Ipilimumab's mechanism of action involves the blocking of the immune checkpoint, CTLA-4, which in turn allows for enhanced T-cell activation and proliferation. This unleashing of the T-cell immune response can result in a potent anti-tumor response. Coupled with the desired anti-tumor response is the possibility of serious autoimmune consequences. Clinical trials investigating ipilimumab have showed the incidence of grade ≥3 immune related adverse event (irAE) to be anywhere from 10-50%. While the most common irAEs are noted to be dermatitis/pruritus and diarrhea/colitis, immune related endocrinopathies are not infrequent 38, 39, 40. Though there are numerous reports of likely drug related hypothyroidism, thyroiditis, hypophysitis, hypopituitarism, and adrenal insufficiency, hypercalcemia as a suspected irAE has rarely been mentioned. In a retrospective review of endocrine related adverse events following ipilimumab published by Ryder et al., they document two cases of incidentally detected hypercalcemia40. One patient had spontaneous resolution of the electrolyte abnormality and the other responded to one dose of bisphosphonate. They were unable to demonstrate a possible mechanism for this finding. In the patient presented here it is important to highlight that the onset of hypercalcemia occurred in the expected time frame for irAE from ipilimumab. However, the elevation in calcium coincided with the development of cachexia and disease progression. Future studies will need to characterize in detail if autoimmunity induces hypercalcemia in melanoma patients treated with immune checkpoint inhibitors and if autoimmunity induces cachexia and altered tumor metabolism.
In conclusion, we describe a patient who developed cachexia with hypercalcemia due to PTHrP expression with increased lactate and ketone body uptake and reduced senescence in cancer cells. Future studies are required to determine if PTHrP regulates metabolism of lactate and ketone bodies and senescence.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Martinez-Outschoorn UE, et al. Reverse Warburg effect in a patient with aggressive B-cell lymphoma: is lactic acidosis a paraneoplastic syndrome? Semin Oncol. 2013;40:403–18. doi: 10.1053/j.seminoncol.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 2.Lagarde SM, ten Kate FJ, Richel DJ, Offerhaus GJ, van Lanschot JJ. Molecular prognostic factors in adenocarcinoma of the esophagus and gastroesophageal junction. Ann Surg Oncol. 2007;14:977–91. doi: 10.1245/s10434-006-9262-y. [DOI] [PubMed] [Google Scholar]
- 3.Doherty JR, Cleveland JL. Targeting lactate metabolism for cancer therapeutics. J Clin Invest. 2013;123:3685–92. doi: 10.1172/JCI69741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sonveaux P, et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest. 2008;118:3930–42. doi: 10.1172/JCI36843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boroughs LK, DeBerardinis RJ. Metabolic pathways promoting cancer cell survival and growth. Nat Cell Biol. 2015;17:351–9. doi: 10.1038/ncb3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinez-Outschoorn UE, Sotgia F, Lisanti MP. Caveolae and signalling in cancer. Nat Rev Cancer. 2015;15:225–37. doi: 10.1038/nrc3915. [DOI] [PubMed] [Google Scholar]
- 7.Campisi J. Cellular senescence as a tumor-suppressor mechanism. Trends Cell Biol. 2001;11:S27–31. doi: 10.1016/s0962-8924(01)02151-1. [DOI] [PubMed] [Google Scholar]
- 8.Falandry C, Bonnefoy M, Freyer G, Gilson E. Biology of cancer and aging: a complex association with cellular senescence. J Clin Oncol. 2014;32:2604–10. doi: 10.1200/JCO.2014.55.1432. [DOI] [PubMed] [Google Scholar]
- 9.Perez-Mancera PA, Young AR, Narita M. Inside and out: the activities of senescence in cancer. Nat Rev Cancer. 2014;14:547–58. doi: 10.1038/nrc3773. [DOI] [PubMed] [Google Scholar]
- 10.Martinez-Outschoorn UE, Lisanti MP, Sotgia F. Catabolic cancer-associated fibroblasts transfer energy and biomass to anabolic cancer cells, fueling tumor growth. Semin Cancer Biol. 2014;25:47–60. doi: 10.1016/j.semcancer.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Pelosof LC, Gerber DE. Paraneoplastic syndromes: an approach to diagnosis and treatment. Mayo Clin Proc. 2010;85:838–54. doi: 10.4065/mcp.2010.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minisola S, Pepe J, Piemonte S, Cipriani C. The diagnosis and management of hypercalcaemia. BMJ. 2015;350:h2723. doi: 10.1136/bmj.h2723. [DOI] [PubMed] [Google Scholar]
- 13.des Grottes JM, Dumon JC, Body JJ. Hypercalcaemia of melanoma: incidence, pathogenesis and therapy with bisphosphonates. Melanoma Res. 2001;11:477–82. doi: 10.1097/00008390-200110000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Ralston SH, Gallacher SJ, Patel U, Campbell J, Boyle IT. Cancer-associated hypercalcemia: morbidity and mortality. Clinical experience in 126 treated patients. Ann Intern Med. 1990;112:499–504. doi: 10.7326/0003-4819-112-7-499. [DOI] [PubMed] [Google Scholar]
- 15.Clines GA. Mechanisms and treatment of hypercalcemia of malignancy. Curr Opin Endocrinol Diabetes Obes. 2011;18:339–46. doi: 10.1097/MED.0b013e32834b4401. [DOI] [PubMed] [Google Scholar]
- 16.Guise TA, et al. Evidence for a causal role of parathyroid hormone-related protein in the pathogenesis of human breast cancer-mediated osteolysis. J Clin Invest. 1996;98:1544–9. doi: 10.1172/JCI118947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kao PC, Klee GG, Taylor RL, Heath H., 3rd Parathyroid hormone-related peptide in plasma of patients with hypercalcemia and malignant lesions. Mayo Clin Proc. 1990;65:1399–407. doi: 10.1016/s0025-6196(12)62163-6. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi S, et al. Elevation of circulating plasma cytokines in cancer patients with high plasma parathyroid hormone-related protein levels. Endocr Relat Cancer. 2003;10:403–7. doi: 10.1677/erc.0.0100403. [DOI] [PubMed] [Google Scholar]
- 19.Mundy GR, Edwards JR. PTH-related peptide (PTHrP) in hypercalcemia. J Am Soc Nephrol. 2008;19:672–5. doi: 10.1681/ASN.2007090981. [DOI] [PubMed] [Google Scholar]
- 20.Rizzoli R, Pache JC, Didierjean L, Burger A, Bonjour JP. A thymoma as a cause of true ectopic hyperparathyroidism. J Clin Endocrinol Metab. 1994;79:912–5. doi: 10.1210/jcem.79.3.8077382. [DOI] [PubMed] [Google Scholar]
- 21.Rosner MH, Dalkin AC. Onco-nephrology: the pathophysiology and treatment of malignancy-associated hypercalcemia. Clin J Am Soc Nephrol. 2012;7:1722–9. doi: 10.2215/CJN.02470312. [DOI] [PubMed] [Google Scholar]
- 22.Matsui T, et al. Hypercalcemia in a patient with malignant melanoma arising in congenital giant pigmented nevus. A case of increased serum level of parathyroid hormone-related protein. Dermatology. 1998;197:65–8. doi: 10.1159/000017960. [DOI] [PubMed] [Google Scholar]
- 23.Yeung SC, et al. Hypercalcemia due to parathyroid hormone-related protein secretion by melanoma. Horm Res. 1998;49:288–91. doi: 10.1159/000023188. [DOI] [PubMed] [Google Scholar]
- 24.El Abdaimi K, Papavasiliou V, Goltzman D, Kremer R. Expression and regulation of parathyroid hormone-related peptide in normal and malignant melanocytes. Am J Physiol Cell Physiol. 2000;279:C1230–8. doi: 10.1152/ajpcell.2000.279.4.C1230. [DOI] [PubMed] [Google Scholar]
- 25.Kageshita T, et al. Widespread expression of parathyroid hormone-related peptide in melanocytic cells. Br J Dermatol. 2003;148:533–8. doi: 10.1046/j.1365-2133.2003.05171.x. [DOI] [PubMed] [Google Scholar]
- 26.Suva LJ, et al. A parathyroid hormone-related protein implicated in malignant hypercalcemia: cloning and expression. Science. 1987;237:893–6. doi: 10.1126/science.3616618. [DOI] [PubMed] [Google Scholar]
- 27.Wysolmerski JJ. Parathyroid hormone-related protein: an update. J Clin Endocrinol Metab. 2012;97:2947–56. doi: 10.1210/jc.2012-2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lanske B, et al. PTH/PTHrP receptor in early development and Indian hedgehog-regulated bone growth. Science. 1996;273:663–6. doi: 10.1126/science.273.5275.663. [DOI] [PubMed] [Google Scholar]
- 29.Philbrick WM, et al. Defining the roles of parathyroid hormone-related protein in normal physiology. Physiol Rev. 1996;76:127–73. doi: 10.1152/physrev.1996.76.1.127. [DOI] [PubMed] [Google Scholar]
- 30.Miao D, et al. Severe growth retardation and early lethality in mice lacking the nuclear localization sequence and C-terminus of PTH-related protein. Proc Natl Acad Sci U S A. 2008;105:20309–14. doi: 10.1073/pnas.0805690105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toribio RE, et al. The midregion, nuclear localization sequence, and C terminus of PTHrP regulate skeletal development, hematopoiesis, and survival in mice. FASEB J. 2010;24:1947–57. doi: 10.1096/fj.09-147033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iguchi H, Onuma E, Sato K, Sato K, Ogata E. Involvement of parathyroid hormone-related protein in experimental cachexia induced by a human lung cancer-derived cell line established from a bone metastasis specimen. Int J Cancer. 2001;94:24–7. doi: 10.1002/ijc.1425. [DOI] [PubMed] [Google Scholar]
- 33.Onuma E, et al. Generation of a humanized monoclonal antibody against human parathyroid hormone-related protein and its efficacy against humoral hypercalcemia of malignancy. Anticancer Res. 2004;24:2665–73. [PubMed] [Google Scholar]
- 34.Hashimoto H, et al. Parathyroid hormone-related protein induces cachectic syndromes without directly modulating the expression of hypothalamic feeding-regulating peptides. Clin Cancer Res. 2007;13:292–8. doi: 10.1158/1078-0432.CCR-06-1487. [DOI] [PubMed] [Google Scholar]
- 35.Kir S, et al. Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature. 2014;513:100–4. doi: 10.1038/nature13528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petruzzelli M, et al. A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab. 2014;20:433–47. doi: 10.1016/j.cmet.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 37.Sanderson K, et al. Autoimmunity in a phase I trial of a fully human anti-cytotoxic T-lymphocyte antigen-4 monoclonal antibody with multiple melanoma peptides and Montanide ISA 51 for patients with resected stages III and IV melanoma. J Clin Oncol. 2005;23:741–50. doi: 10.1200/JCO.2005.01.128. [DOI] [PubMed] [Google Scholar]
- 38.Wolchok JD, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155–64. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 39.Hodi FS, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ryder M, Callahan M, Postow MA, Wolchok J, Fagin JA. Endocrine-related adverse events following ipilimumab in patients with advanced melanoma: a comprehensive retrospective review from a single institution. Endocr Relat Cancer. 2014;21:371–81. doi: 10.1530/ERC-13-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]