Abstract
Foodborne botulism is a severe, paralytic illness caused by ingestion of preformed neurotoxins produced by Clostridium botulinum. In 2003, we conducted a population-based household survey of home canning practices to explore marked regional variations in botulism incidence in the Republic of Georgia (ROG). We designed a cluster sampling scheme and subdivided each of the 10 regions of the ROG into a variable number of strata. Households were selected from each stratum using a two-step cluster sampling methodology. We administered a questionnaire about home canning practices to household members responsible for food preparation. Using multivariate logistic regression analysis, we modeled high (eastern ROG) against low (western ROG) incidence areas. Overall, we surveyed 2,742 households nationwide. Home canning with a capping device hermetically sealing the lid covering the jar was practiced by 1,909 households (65.9%; 95% confidence interval [CI]: 59.8 to 72.1%). Canning was more prevalent in regions of low botulism incidence (34 versus 32%; P < 0.01). When compared with low-botulism areas, the following practices were associated with an increased risk in high-botulism areas: ≥6 months between canning vegetables and consuming them (adjusted odds ratio [aOR] = 2.1; 95% CI: 1.3 to 3.5) and adding any of the following ingredients to the jar at time of preparation: >1 tablespoon of salt per liter (aOR = 5.1; 95% CI: 1.2 to 22.6); vinegar (aOR = 2.2; 95% CI: 1.3 to 3.7), and greens (aOR = 5.6; 95% CI: 1.7 to 18.2). The following practices were associated with a decreased risk in high-botulism areas: >57 jars canned per household annually (aOR = 0.5; 95% CI: 0.3 to 0.9), covering or immersing vegetables in boiling water before placing them into the jar (aOR = 0.3 95% CI: 0.2 to 0.6), covering or immersing vegetables in boiling water after placing them into the jar (aOR = 0.4; 95% CI: 0.2 to 0.9), or adding garlic (aOR = 0.2; 95% CI: 0.1 to 0.5) or aspirin (aOR = 0.1; 95% CI: 0.1 to 0.2) to the jar at the time of preparation.
Foodborne botulism is a severe, paralytic illness caused by ingestion of preformed neurotoxins produced by Clostridium botulinum. The acute illness is well described, with cranial nerve dysfunction and symmetric descending flaccid paralysis, potentially progressing to respiratory failure, which in the absence of treatment can lead to death (11). C. botulinum spores are ubiquitous in the environment but germinate under a confluence of circumstances that include anaerobic milieu, low acidity, low salinity, low water activity, and temperature between 3 and 37°C (7). Foodborne botulism is caused by ingestion of foods contaminated with botulinum toxin, which is produced by the growth of C. botulinum or related species under such conditions (2). In several countries, improperly home-canned foods have been identified as important sources of foodborne botulism. The Republic of Georgia (ROG), a former Soviet republic of 4.4 million people, reported one of the highest incidences of foodborne botulism in the world, with home-canned vegetables implicated in 80% of botulism events (12) and a cumulative annual incidence of 5.1 cases per 100,000 individuals in 2004 through 2011 (9). Home canning is extensively practiced in ROG. Botulism events are usually associated with vegetables conserved using a capping device that hermetically seals the lid covering the jar, commonly referred to as a modern method of canning. The traditional method of canning using high concentrations of salt and vinegar is also practiced in ROG, but because this method does not include a capping device that creates anaerobic conditions inside a jar, the traditional method has not been associated with botulism cases.
In a previous article presenting national ROG botulism surveillance data, we reported marked regional variations in incidence. In particular, the western part of the country had a significantly lower incidence than did the eastern part (12). This finding prompted us to undertake the present study, in which we conducted a population-based household survey of home canning practices. We then compared home canning practices in regions of high botulism incidence to those in regions of low botulism incidence to identify canning practices that might represent a risk for this disease. This study was conducted in 2003, but the results remain highly relevant today because the incidence of botulism has remained high. Although public health interventions were implemented in response to the original study, the causes of botulism likely remain the same.
MATERIALS AND METHODS
A botulism event was defined as an outbreak (two or more cases epidemiologically linked to each other) or a sporadic case (individual case not associated with other cases) of botulism. Outbreaks and sporadic cases were each counted as one event (12). We used the incidence of botulism events by region of residence per 100,000 persons in the ROG from 1980 through 2002. We calculated the cumulative incidence for each region and defined regions as having high or low incidence based on whether the regional cumulative incidence was greater or less than the median cumulative incidence for all regions.
To randomly sample households nationwide, we designed a cluster sampling scheme based on 2002 census data. Regions of political instability (Abkhazia and South Ossetia) were not included in the sampling design. The ROG is subdivided into 10 geographic regions along extant administrative borders. In the ROG, region is a traditional administrative-historical unit with unique subethnic features, including food preparation practices (Table 1). These regions were used in the sampling process to reflect areas of high incidence and low incidence of botulism and to allow direct comparison between regions. We used the square root of the number of households within each region as a base sample size.
TABLE 1.
Regions of ROG with number of households sampled and cumulative incidence of botulism events, 1980 through 2002
| Region | No. of households sampled | Cumulative incidence of botulism events per 100,000 personsa |
|---|---|---|
| Western ROG | 1,365 | |
| Guria-Ajara | 296 | 1.9 |
| Imereti | 381 | 1.9 |
| Racha-Lechkhumi | 195 | 0.0 |
| Samegrelo-Poti | 283 | 0.0 |
| Samtskhe-Javakheti | 210 | 0.4 |
| Eastern ROG | 1,377 | |
| Kakheti | 260 | 6.3 |
| Kvemo Kartli | 272 | 10.0 |
| Mtskheta-Mtianeti | 196 | 8.2 |
| Shida Kartli | 215 | 3.4 |
| Tbilisi | 434 | 14.6 |
| Total | 2,742 |
Cumulative incidence of botulism events for 1980 through 2002 were previously reported (12).
To adequately reflect diversity within each region, we subdivided each of the 10 regions into a variable number of strata. A stratum is defined as a geographical subunit of a region that reflects certain characteristics of a subsection of the resident population. Criteria for identifying strata include a predominantly urban or rural nature, the size of the settlements (cities, towns, or villages), elevation above sea level, and topography (mountainous or valley settlements). Strata were created to assure a representative population sample from each region and to reflect the number of households within a region. For example, in Tbilisi, a populous urban region, a total of 10 strata were designated; in less populous regions, only two were designated. Each stratum was represented in the survey in proportion to the square root of the number of households within each stratum.
Households were selected from each stratum using a two-step cluster sampling methodology. Each stratum comprised administrative units, called rayons. On average, 58 households per rayon were selected. Rayons are divided into villages in rural areas and into census units in urban areas. We randomly selected rayons within each stratum according to a probability proportional to size methodology. We then randomly selected villages or census units from each selected rayon, also according to this methodology.
Within each designated census unit or village, a randomly generated start address and the total number of households sampled was specified based on 2002 national census data. We calculated a sampling interval based on the total number of households and the estimated population. Subsequent households were identified based on a “random walk” sampling algorithm. We administered a standardized and validated questionnaire to the household member responsible for food preparation, which included questions about basic household characteristics, home canning practices, frequency of vegetable canning, and storage of home-canned food.
We tested the pH of the contents of home-canned vegetable jars using pH test strips (Sigma, St. Louis, MO) from a convenience sample of households.
Data analysis was conducted using SAS (version 9.3, SAS Institute Inc., Cary, NC). Univariate analysis was conducted to explore how each potential risk factor was associated with the high incidence of botulism. Significance was defined at P < 0.05 and was determined using χ2 tests. In multivariate logistic regression, we included variables that were significantly associated with high botulism incidence in the univariate analysis and those believed a priori to be associated with botulinum intoxication. Potential risk factors independently associated with high risk areas were investigated by binary logistic regression using the SURVEYLOGISTIC procedure in SAS, where 30 of the 864 variables from the questionnaire were included. In the final model, we compared high-incidence areas (eastern ROG) with low-incidence areas (western ROG).
The human subjects protection staff of the Centers for Disease Control and Prevention (Atlanta, GA) determined that this project did not constitute research but rather was a nonresearch response to an ongoing public health threat and therefore did not require Institutional Review Board oversight. The human subjects review board at the National Center for Disease Control (Tbilisi, ROG) also approved the study.
RESULTS
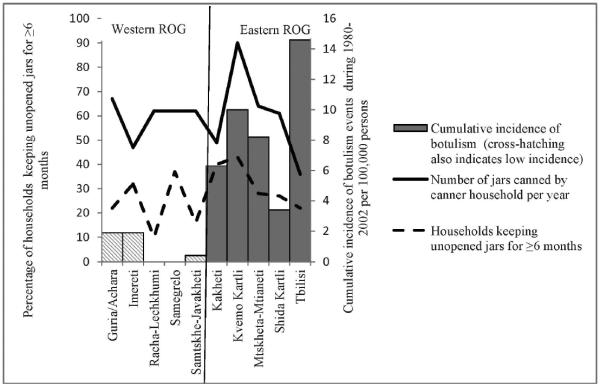
The median cumulative incidence of botulism for all regions for 1980 through 2002 was 2.65 events per 100,000 population (12). Regions with higher than the median cumulative incidence (2.7 events per 100,000 persons) were considered high-incidence areas: Shida Kartli, Kvemo Kartli, Tbilisi, Mtskheta-Mtianeti, and Kakheti, all located in eastern ROG. All regions for which the number of cumulative botulism events was below the median were located in western ROG (Figs. 1 and 2).
FIGURE 1.
Percentage of households engaged in home canning using a capping device (modern method) and using certain ingredients by region, ROG, 2003.
FIGURE 2.
Number of jars canned per year per canner household and percentage of households keeping unopened jars ≥6 months by region, ROG, 2003.
Between 1 October 2003 and 22 December 2003, we surveyed 2,742 households nationwide (100% response rate) selected within 144 villages and census areas and 47 strata. Among these 2,742 households, 1,377 (56%) were in high-incidence areas and 1,365 (44%) were in low-incidence areas. Overall, the median age of respondents responsible for food preparation in their households was 48 years (range, 18 to 94 years), and 2,715 (99%) were female. Home canning using the modern method (with a capping device that hermetically seals the lid covering the jar) was practiced by 1,909 households (65.9%; 95% confidence interval [CI]: 59.8 to 72.1%), and its prevalence differed significantly between high- and low-incidence areas. Canning was more prevalent in low-incidence areas (34 versus 32%; P < 0.01). The most frequently cited reason for home canning was the high cost of fresh vegetables during the winter (57.9%; 95% CI: 51.0 to 64.8%). A total of 542 households (30%; 95% CI: 24.8 to 35.3%) reported storing home-canned vegetable jars for ≥6 months on average before consumption. The mean reported number of jars of home-canned vegetables made per year was 37.7 (range, 0 to 500 jars). During the most recent canning season (fall 2002), an average of 35.1 jars (range, 0 to 550 jars) were made per household.
Tomatoes were reported as the most frequently canned vegetable, cited by 26.8% of respondents (95% CI: 20.8 to 32.8%), followed by eggplants (14.7%; 95% CI: 11.0 to 18.4%) and cucumbers (12.3%; 95% CI: 10.0 to 14.6%). Frequent spoilage of home-canned vegetables was reported by 0.5% of households (95% CI: 0.3 to 0.8%).
The home-canned vegetables most commonly consumed without cooking were cucumbers (45.1%; 95% CI: 38.4 to 51.7%) and whole tomatoes (36.6%; 95% CI: 30.8 to 42.3%). This practice did not differ significantly between high- and low-botulism areas for whole tomatoes (18.1 versus 18.4%; P = 0.08) but did differ significantly for cucumbers (18.1 versus 27.0%; P < 0.01).
The average pH of the contents of 155 jars collected during the survey was 4.8 (range, 3.1 to 7.0). Storing jars of canned food above the ground level was reported by 60% of respondents (95% CI: 55.7 to 64.1%), and this practice did not differ between the regions (P = 0.2).
In logistic regression analysis, the final multivariate models included 30 variables. The variables significantly associated with outcomes are summarized in Table 2. The following practices were associated with a decreased risk in high-botulism areas: >57 jars canned per household annually, covering or immersing vegetables in boiling water before placing them into the jar, covering or immersing vegetables in boiling water after placing them into the jar, presence of garlic, and adding aspirin to the jar at the time of preparation. Adding greens, salt (>1 tablespoon per liter jar), or vinegar and the amount of time vegetables were usually kept in jars before being eaten (≥6 versus <6 months) were associated with increased risk.
TABLE 2.
Multivariate logistic regression analysis modeling high-incidence (eastern) area of the ROG, 2003
| Characteristics and home canning practices of householdsa | Adjusted odds ratio | 95% CI | P value |
|---|---|---|---|
| Adding greens | 5.6 | 1.7–18.2 | <0.01 |
| Adding salt, >1 tablespoon per liter jar | 5.1 | 1.2–22.6 | 0.03 |
| Adding vinegar | 2.2 | 1.3–3.7 | <0.01 |
| Time vegetables are usually kept in jars before they are eaten (≥6 vs <6 mo) | 2.1 | 1.3–3.5 | <0.01 |
| Average no. of jars typically canned per household (>57 vs ≤57) | 0.5 | 0.3–0.9 | 0.02 |
| Vegetables covered in boiling water after being put into jar | 0.4 | 0.2–0.9 | 0.02 |
| Vegetables immersed or covered in boiling water before being put into jar | 0.3 | 0.2–0.6 | <0.01 |
| Adding garlic | 0.2 | 0.1–0.5 | <0.01 |
| Adding aspirin | 0.1 | 0.1–0.2 | <0.01 |
Significant variables only.
DISCUSSION
The ROG persistently has a high incidence of botulism, one of the highest in the world, and home-canned vegetables have long been recognized as the principal source of botulinum toxin exposure (12). The eastern part of the country has traditionally experienced higher rates of botulism than has the western part (12). We conducted a national population-based survey of home-canning practices to compare the high-botulism regions of eastern ROG and the low-botulism regions of western ROG. Home canning is very popular in both areas of the country. In areas of low botulism incidence, a higher number of households engaged in home canning and ate home-canned vegetables without thermal processing. We identified practices in eastern ROG that are likely to increase the potential for botulism and that may be useful intervention targets for prevention. Although the data from our national population-based survey of home canning practices in the ROG are from 2003, the findings remain relevant because the sources of botulism in the ROG are likely unchanged since the time of the original study.
Home canning in the ROG is a complex process involving multiple steps. Home-canned naturally acidic vegetables, such as tomatoes, might be considered safe. Tomatoes were the most frequently canned vegetable reported by our respondents but were implicated as a vehicle in only 15% of botulism cases (12). Use of vinegar in preparation of home-canned vegetables would be expected to decrease the risk of botulism because of the acidifying effect, yet vinegar use was reported more frequently in the high-botulism regions of eastern ROG. However, vinegar use is highly correlated with ethnicity, with households of Azeri ethnicity, which have a very high incidence of botulism (12), reporting very low use of vinegar (data not shown). We therefore believe that a true association exists between low vinegar use and high botulism incidence in Azeri households.
Home-canned products in the ROG sometimes are kept more than 2 years. The prevalence of keeping unopened home-canned vegetables for ≥6 months was higher in households of eastern ROG than in households in western ROG. Long incubation times (2 to 3 months) are required for C. botulinum toxin production (6). Initially low pH levels of highly acidic products may increase over time due to the activity of mold and other organisms in jars, creating favorable conditions for C. botulinum spore germination and toxin production (4, 10). The lowest percentage (10%) of households keeping jars for ≥6 months was found in Racha-Lechkhumi (Fig. 2). This region is the only one in western ROG where use of aspirin and/or vinegar in home-canned products was rarely reported, yet no cases of botulism were reported from this region (Fig. 1).
Immersion of vegetables in boiling water before or after being put into the jar was protective based on the logistic regression analysis. This process will not eliminate C. botulinum spores but may reduce the number of other viable organisms capable of raising the pH several months after canning and eliminate oxygen needed for subsequent vegetation and growth (3, 5).
Addition of aspirin was protective for all vegetable recipes in the study. In previous studies, antimicrobial properties of aspirin have included eradicating biofilms of Escherichia coli, Candida albicans, and Pseudomonas aeruginosa (1). Aspirin, especially in high concentrations, delays the growth of and toxin production by C. botulinum spores (8). The concentration of aspirin used in home-canned vegetables in the ROG is not known but likely is >0.49 mg/ml, based on the typical locally available tablet and the volume of commonly employed jars. The mechanism of action of aspirin in home-canned vegetables is unknown. We speculate that the protective effect may be attributed to two actions: maintenance of a low pH level for several months by reducing the number of viable aerobic and facultative anaerobic organisms and directly retarding the growth of and toxin production by C. botulinum spores by some unknown mechanism. Aspirin is most widely used by ethnic Georgians, especially those living in western ROG. The lowest frequency of aspirin use was detected among ethnic Azeris (data not shown), who have the highest incidence of botulism in the ROG (12).
Our study has several limitations. First, in this ecological study we assessed regional practices rather than risk factors in botulism patients. Canning practices correlated with regional customs may have little effect on the risk of botulism but may nonetheless have been statistically significant. Second, the data are nearly 10 years old. However, botulism incidence in the ROG remains high, and its causes and associated food preparation methods probably have not changed. Therefore, the data remain relevant and offer important and practicable guidance for botulism prevention.
In conclusion, we identified specific practices of home vegetable preservation in the ROG that we believe constitute potential risk factors for botulism. These findings should be used as the basis for public health interventions to reduce the occurrence of botulism in the ROG. Our conclusions are supported by several lines of reasoning. The implicated practices are significantly associated with the high-botulism incidence region of eastern ROG, though this association is subject to the limitations of ecological studies. All the practices associated with the high-risk region are biologically plausible for supporting germination and toxin production of C. botulinum; the one apparent exception, the prevalence of vinegar use in the high-risk eastern ROG region, is likely explained by the fact that this finding camouflages the lack of vinegar use by the Azeri population of eastern ROG, which has the highest incidence of botulism in the ROG (12). The implicated factors are consistent with the known microbiology of C. botulinum.
Botulism can be prevented by using a pressure cooker, sterilizing equipment, and taking other precautions. The challenges to adapting these technologies are affordability and cultural acceptability. Further evaluation of approaches already used in other regions of the ROG should be conducted to find an affordable and culturally accepted approach.
ACKNOWLEDGMENT
This study was funded by the U.S. Department of Health and Human Services, Biotechnology Engagement Program. The funding source played no role in designing the study; collecting, analyzing, and interpreting the data; writing the report; or deciding to submit the results for publication. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Al-Bakri AG, Othman G, Bustanji Y. The assessment of the antibacterial and antifungal activities of aspirin, EDTA and aspirin-EDTA combination and their effectiveness as antibiofilm agents. J. Appl. Microbiol. 2009;107:280–286. doi: 10.1111/j.1365-2672.2009.04205.x. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention [Accessed 21 October 2013];Botulism in the United States, 1899–1996. Handbook for epidemiologists, clinicians and laboratory workers. 1998 Available at: http://www.cdc.gov/ncidod/dbmd/diseaseinfo/files/botulism.pdf.
- 3.Gangneux J-P, Noussair L, Bouakline A, Roux N, Lacroix C, Derouin F. Experimental assessment of disinfection procedures for eradication of Aspergillus fumigatus in food. Blood. 2004;104:2000–2002. doi: 10.1182/blood-2003-09-3176. [DOI] [PubMed] [Google Scholar]
- 4.Huhtanen CN, Naghski J, Custer CS, Russell RW. Growth and toxin production by Clostridium botulinum in moldy tomato juice. Appl. Environ. Microbiol. 1976;32:711–715. doi: 10.1128/aem.32.5.711-715.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johanningsmeier SD, Franco W, Perez-Diaz I, McFeeters RF. Influence of sodium chloride, pH, and lactic acid bacteria on anaerobic lactic acid utilization during fermented cucumber spoilage. J. Food Sci. 2012;77:M397–M404. doi: 10.1111/j.1750-3841.2012.02780.x. [DOI] [PubMed] [Google Scholar]
- 6.Kimura B, Kimura R, Fukaya T, Sakuma K, Miya S, Fujii T. Growth and toxin production of proteolytic Clostridium botulinum in aseptically steamed rice products at pH 4.6 to 6.8, packed under modified atmosphere, using a deoxidant pack. J. Food Prot. 2008;71:468–472. doi: 10.4315/0362-028x-71.3.468. [DOI] [PubMed] [Google Scholar]
- 7.Lindström M, Kiviniemi K, Korkeala H. Hazard and control of group II (non-proteolytic) Clostridium botulinum in modern food processing. Int. J. Food Microbiol. 2006;108:92–104. doi: 10.1016/j.ijfoodmicro.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Ma L, Zhang G, Sobel J, Doyle MP. Evaluation of the effect of acetylsalicylic acid on Clostridium botulinum growth and toxin production. J. Food Prot. 2007;70:2860–2863. doi: 10.4315/0362-028x-70.12.2860. [DOI] [PubMed] [Google Scholar]
- 9.National Center for Disease Control and Health Statistics, Republic of Georgia. Unpublished data. [Google Scholar]
- 10.Odlaug TE, Pflug IJ. Clostridium botulinum growth and toxin production in tomato juice containing Aspergillus gracilis. Appl. Environ. Microbiol. 1979;37:496–504. doi: 10.1128/aem.37.3.496-504.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sobel J. Botulism. Clin. Infect. Dis. 2005;41:1167–1173. doi: 10.1086/444507. [DOI] [PubMed] [Google Scholar]
- 12.Varma JK, Katsitadze G, Moiscrafishvili M, Zardiashvili T, Chokheli M, Tarkashvili N, Jhorjholiani E, Chubinidze M, Kukhalashvili T, Khmaladze I, Chakvetadze N, Imnadze P, Sobel J. Foodborne botulism in the Republic of Georgia. Emerg. Infect. Dis. 2004;10:1601–1605. doi: 10.3201/eid1009.030806. [DOI] [PMC free article] [PubMed] [Google Scholar]