Abstract
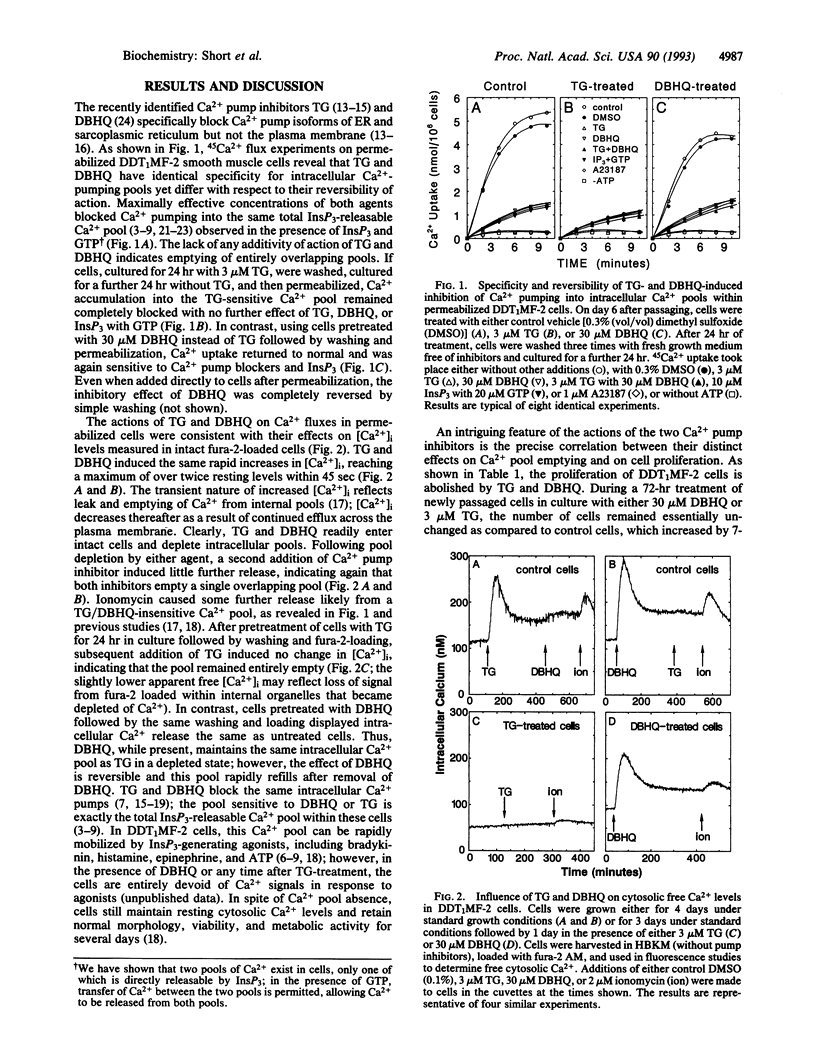
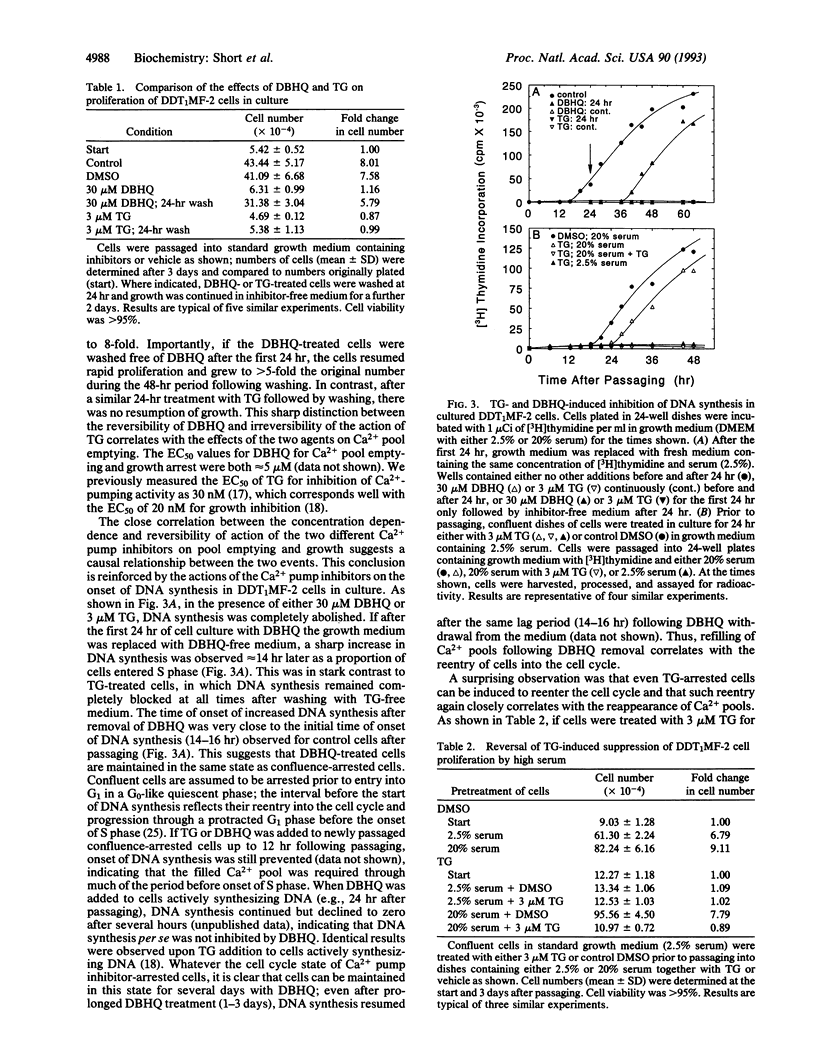
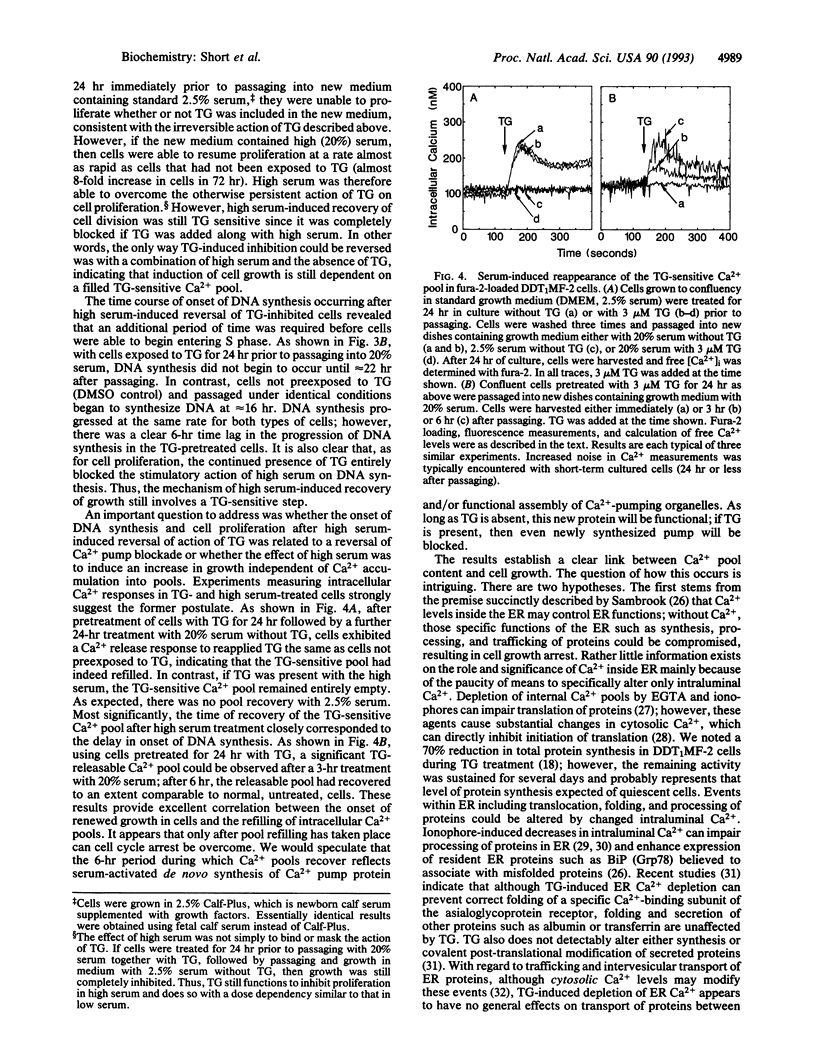
A close correlation was observed between intracellular Ca2+ pool depletion and refilling and the onset of DNA synthesis and proliferation of DDT1MF-2 smooth muscle cells. The intracellular Ca2+ pump inhibitors 2,5-di-tert-butyl-hydroquinone (DBHQ) and thapsigargin (TG) specifically emptied identical inositol 1,4,5-trisphosphate (InsP3)-sensitive Ca2+ pools and both arrested cell growth at concentrations corresponding to Ca2+ pump blockade. However, an important distinction was observed between the two inhibitors with respect to their reversibility of action. Upon removal of DBHQ from DBHQ-arrested cells, Ca2+ pools immediately refilled, and 14 hr later cells entered S phase followed by normal cell proliferation; the time for entry into S phase was identical to that for cells released from confluence arrest. Although TG irreversibly blocked Ca2+ pumping and emptied Ca2+ pools, high serum treatment of TG-arrested cells induced recovery of functional Ca2+ pools in 6 hr (via probable synthesis of new pump); thereafter cells proceeded to S phase and normal cell proliferation within the same time period (14 hr) as that following release of DBHQ-arrested cells. The precise relationship between Ca2+ pump blockade and growth arrest indicates that Ca2+ pool emptying maintains cells in a G0-like quiescent state; upon refilling of pools, normal progression into the cell cycle is resumed. It is possible that a specific cell cycle event necessary for G0 to G1 transition depends upon signals generated from the InsP3-sensitive Ca2+ pool.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckers C. J., Balch W. E. Calcium and GTP: essential components in vesicular trafficking between the endoplasmic reticulum and Golgi apparatus. J Cell Biol. 1989 Apr;108(4):1245–1256. doi: 10.1083/jcb.108.4.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Bian J. H., Ghosh T. K., Wang J. C., Gill D. L. Identification of intracellular calcium pools. Selective modification by thapsigargin. J Biol Chem. 1991 May 15;266(14):8801–8806. [PubMed] [Google Scholar]
- Brostrom C. O., Brostrom M. A. Calcium-dependent regulation of protein synthesis in intact mammalian cells. Annu Rev Physiol. 1990;52:577–590. doi: 10.1146/annurev.ph.52.030190.003045. [DOI] [PubMed] [Google Scholar]
- Chueh S. H., Gill D. L. Inositol 1,4,5-trisphosphate and guanine nucleotides activate calcium release from endoplasmic reticulum via distinct mechanisms. J Biol Chem. 1986 Oct 25;261(30):13883–13886. [PubMed] [Google Scholar]
- Chueh S. H., Mullaney J. M., Ghosh T. K., Zachary A. L., Gill D. L. GTP- and inositol 1,4,5-trisphosphate-activated intracellular calcium movements in neuronal and smooth muscle cell lines. J Biol Chem. 1987 Oct 5;262(28):13857–13864. [PubMed] [Google Scholar]
- Furuichi T., Yoshikawa S., Miyawaki A., Wada K., Maeda N., Mikoshiba K. Primary structure and functional expression of the inositol 1,4,5-trisphosphate-binding protein P400. Nature. 1989 Nov 2;342(6245):32–38. doi: 10.1038/342032a0. [DOI] [PubMed] [Google Scholar]
- Ghosh T. K., Bian J. H., Short A. D., Rybak S. L., Gill D. L. Persistent intracellular calcium pool depletion by thapsigargin and its influence on cell growth. J Biol Chem. 1991 Dec 25;266(36):24690–24697. [PubMed] [Google Scholar]
- Ghosh T. K., Bian J., Gill D. L. Intracellular calcium release mediated by sphingosine derivatives generated in cells. Science. 1990 Jun 29;248(4963):1653–1656. doi: 10.1126/science.2163543. [DOI] [PubMed] [Google Scholar]
- Ghosh T. K., Eis P. S., Mullaney J. M., Ebert C. L., Gill D. L. Competitive, reversible, and potent antagonism of inositol 1,4,5-trisphosphate-activated calcium release by heparin. J Biol Chem. 1988 Aug 15;263(23):11075–11079. [PubMed] [Google Scholar]
- Ghosh T. K., Mullaney J. M., Tarazi F. I., Gill D. L. GTP-activated communication between distinct inositol 1,4,5-trisphosphate-sensitive and -insensitive calcium pools. Nature. 1989 Jul 20;340(6230):236–239. doi: 10.1038/340236a0. [DOI] [PubMed] [Google Scholar]
- Gill D. L. Calcium signalling: receptor kinships revealed. Nature. 1989 Nov 2;342(6245):16–18. doi: 10.1038/342016a0. [DOI] [PubMed] [Google Scholar]
- Gill D. L., Chueh S. H. An intracellular (ATP + Mg2+)-dependent calcium pump within the N1E-115 neuronal cell line. J Biol Chem. 1985 Aug 5;260(16):9289–9297. [PubMed] [Google Scholar]
- Gill D. L., Ghosh T. K., Bian J., Short A. D., Waldron R. T., Rybak S. L. Function and organization of the inositol 1,4,5-trisphosphate-sensitive calcium pool. Adv Second Messenger Phosphoprotein Res. 1992;26:265–308. [PubMed] [Google Scholar]
- Gill D. L., Ueda T., Chueh S. H., Noel M. W. Ca2+ release from endoplasmic reticulum is mediated by a guanine nucleotide regulatory mechanism. Nature. 1986 Apr 3;320(6061):461–464. doi: 10.1038/320461a0. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Han J. K., Fukami K., Nuccitelli R. Reducing inositol lipid hydrolysis, Ins(1,4,5)P3 receptor availability, or Ca2+ gradients lengthens the duration of the cell cycle in Xenopus laevis blastomeres. J Cell Biol. 1992 Jan;116(1):147–156. doi: 10.1083/jcb.116.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao J. P., Alderton J. M., Tsien R. Y., Steinhardt R. A. Active involvement of Ca2+ in mitotic progression of Swiss 3T3 fibroblasts. J Cell Biol. 1990 Jul;111(1):183–196. doi: 10.1083/jcb.111.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R. V., Wolfman A., Panniers R., Henshaw E. C. Mechanism of inhibition of polypeptide chain initiation in calcium-depleted Ehrlich ascites tumor cells. J Cell Biol. 1989 Jun;108(6):2107–2115. doi: 10.1083/jcb.108.6.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsov G., Brostrom M. A., Brostrom C. O. Demonstration of a calcium requirement for secretory protein processing and export. Differential effects of calcium and dithiothreitol. J Biol Chem. 1992 Feb 25;267(6):3932–3939. [PubMed] [Google Scholar]
- Lodish H. F., Kong N. Perturbation of cellular calcium blocks exit of secretory proteins from the rough endoplasmic reticulum. J Biol Chem. 1990 Jul 5;265(19):10893–10899. [PubMed] [Google Scholar]
- Lodish H. F., Kong N., Wikström L. Calcium is required for folding of newly made subunits of the asialoglycoprotein receptor within the endoplasmic reticulum. J Biol Chem. 1992 Jun 25;267(18):12753–12760. [PubMed] [Google Scholar]
- Moore G. A., McConkey D. J., Kass G. E., O'Brien P. J., Orrenius S. 2,5-Di(tert-butyl)-1,4-benzohydroquinone--a novel inhibitor of liver microsomal Ca2+ sequestration. FEBS Lett. 1987 Nov 30;224(2):331–336. doi: 10.1016/0014-5793(87)80479-9. [DOI] [PubMed] [Google Scholar]
- Mullaney J. M., Chueh S. H., Ghosh T. K., Gill D. L. Intracellular calcium uptake activated by GTP. Evidence for a possible guanine nucleotide-induced transmembrane conveyance of intracellular calcium. J Biol Chem. 1987 Oct 5;262(28):13865–13872. [PubMed] [Google Scholar]
- Mullaney J. M., Yu M., Ghosh T. K., Gill D. L. Calcium entry into the inositol 1,4,5-trisphosphate-releasable calcium pool is mediated by a GTP-regulatory mechanism. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2499–2503. doi: 10.1073/pnas.85.8.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee A. B. G1 events and regulation of cell proliferation. Science. 1989 Nov 3;246(4930):603–608. doi: 10.1126/science.2683075. [DOI] [PubMed] [Google Scholar]
- Ross C. A., Meldolesi J., Milner T. A., Satoh T., Supattapone S., Snyder S. H. Inositol 1,4,5-trisphosphate receptor localized to endoplasmic reticulum in cerebellar Purkinje neurons. Nature. 1989 Jun 8;339(6224):468–470. doi: 10.1038/339468a0. [DOI] [PubMed] [Google Scholar]
- Rozengurt E. Early signals in the mitogenic response. Science. 1986 Oct 10;234(4773):161–166. doi: 10.1126/science.3018928. [DOI] [PubMed] [Google Scholar]
- Sagara Y., Inesi G. Inhibition of the sarcoplasmic reticulum Ca2+ transport ATPase by thapsigargin at subnanomolar concentrations. J Biol Chem. 1991 Jul 25;266(21):13503–13506. [PubMed] [Google Scholar]
- Sambrook J. F. The involvement of calcium in transport of secretory proteins from the endoplasmic reticulum. Cell. 1990 Apr 20;61(2):197–199. doi: 10.1016/0092-8674(90)90798-j. [DOI] [PubMed] [Google Scholar]
- Steinhardt R. A., Alderton J. Intracellular free calcium rise triggers nuclear envelope breakdown in the sea urchin embryo. Nature. 1988 Mar 24;332(6162):364–366. doi: 10.1038/332364a0. [DOI] [PubMed] [Google Scholar]
- Takemura H., Hughes A. R., Thastrup O., Putney J. W., Jr Activation of calcium entry by the tumor promoter thapsigargin in parotid acinar cells. Evidence that an intracellular calcium pool and not an inositol phosphate regulates calcium fluxes at the plasma membrane. J Biol Chem. 1989 Jul 25;264(21):12266–12271. [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thastrup O., Dawson A. P., Scharff O., Foder B., Cullen P. J., Drøbak B. K., Bjerrum P. J., Christensen S. B., Hanley M. R. Thapsigargin, a novel molecular probe for studying intracellular calcium release and storage. Agents Actions. 1989 Apr;27(1-2):17–23. doi: 10.1007/BF02222186. [DOI] [PubMed] [Google Scholar]
- Ueda T., Chueh S. H., Noel M. W., Gill D. L. Influence of inositol 1,4,5-trisphosphate and guanine nucleotides on intracellular calcium release within the N1E-115 neuronal cell line. J Biol Chem. 1986 Mar 5;261(7):3184–3192. [PubMed] [Google Scholar]
- Whitaker M., Patel R. Calcium and cell cycle control. Development. 1990 Apr;108(4):525–542. doi: 10.1242/dev.108.4.525. [DOI] [PubMed] [Google Scholar]



