Abstract
Staphylococcus aureus is a part of the microbiota flora in many animal species. The clonal spread of S. aureus among animals and personnel in a Zoological Park was investigated. Samples were collected from colonized and infected sites among 32 mammals, 11 birds and eight humans. The genes mecA, mecC, lukF/lukS-PV (encoding Panton-Valentine leukocidin, PVL) and tst (toxic shock syndrome toxin-1) were investigated by PCR. Clones were defined by Multilocus Sequence Typing (MLST), spa type and Pulsed-Field Gel Electrophoresis (PFGE). Seven S. aureus isolates were recovered from four animals and one from an employee. All were mecA, mecC and tst–negative, whereas, one carried the PVL genes and was isolated from an infected Squirrel monkey. Clonal analysis revealed the occurrence of seven STs, eight PFGE and five spa types including ones of human origin. Even though a variety of genotypes were identified among S. aureus strains colonizing zoo park residents, our results indicate that colonization with human lineages has indeed occurred.
Keywords: Carriers, Clones, Humans, Staphylococcus aureus, Zoological Park
Introduction
Mammalians are colonized by a vast number of microorganisms which constitute the host’s microbiota. Staphylococcus aureus is common within the bacterial community which inhabits the skin, mucous membranes and anterior nares in humans, as well as various animal species, birds, and reptiles (Walther et al., 2008; Hasman et al., 2010; Espinosa-Gongora et al., 2012). Methicillin-resistant S. aureus strains (MRSA) pose a huge risk when they spread among animals of zoological parks, therefore treatment administration must be carefully determined for proper dosing, however, on several occasions the antimicrobials’ administration is based upon estimated and not precise body weights (Espinosa-Gongora et al., 2012; Vercammen et al., 2012). Zoological parks provide an easily accessible and highly diversified habitat to study the distribution of S. aureus in different animal species, some of them being exotic. In a study from the Royal Zoological Society of Antwerp, in Belgium, no MRSA was identified, but the authors did not investigate S. aureus prevalence in the tested population (Vercammen et al., 2012). In another study conducted in the Small Animal Clinic of the Veterinary Faculty at the Free University Berlin among small and exotic animals, 44.3% MRSA infections were identified (Walther et al., 2008).
Greece is a country with relatively high prevalence of MRSA in human population (>25%), as already published (Maltezou et al., 2009; Drougka et al., 2014). Moreover, in the literature it has been shown that strains of human origin have been identified to be transmitted to animals causing infections, whereas, environmental factors were also found to be responsible (Walther et al., 2008). S. aureus was characterized as a pathogen that has the ability to switch hosts, establishing its significance in the human and veterinary medicine (Espinosa-Gongora et al., 2012).
The aim of this study was to investigate the occurrence and distribution of S. aureus and MRSA among humans and other animal species in contact with humans and birds at the Attica Zoological Park in Greece.
Materials and Methods
Sample collection and S. aureus identification
This research was conducted during 2012 among 32 mammals belonging to 16 species, 11 birds belonging to seven species and eight humans from the staff of the Attica Zoological Park (Table 1). The Park, located in Spata, is a self-funded creation, in an area of 20 hectares hosting 50 species of land mammals, 180 bird species, 30 reptile species and two species of marine mammals. As a member of the European Association of Zoo and Aquaria and the only zoological park in Greece, it receives 300 000-400 000 visitors of all ages annually. In an effort to preserve endangered species, about 60% of the animals are rare. It participates in 35 European Studbooks and 29 European Endangered species Programs, whereas, in collaboration with Veterinary, Biology and Agriculture University Schools it participates in students’ educational projects and practice, scientific studies and research programs. Moreover, educational and entertainment projects for visitors and pupils are consistently organized.
Table 1.
Animal species included in this study for S. aureus carriage identification.
| Studied population | Scientific names | Sampling site | Number of samples per animal | Number of samples | Number of S. aureus isolates |
|---|---|---|---|---|---|
| Mammals (n= 40) | |||||
| Human (n=8) | Homo sapiens | Nostrils | 1 | 8 | 1 |
| Baringo giraffe (n=4) | Giraffa camelopardalis | Pile | 1 | 4 | 0 |
| Black and white ruffed Lemur (n=1) | Varecia variegata | Nostrils Pile | 2 | 1 | 0 |
| Pile | 1 | 0 | |||
| Black capped capuchin (n=1) | Cebus apella | Pile | 1 | 1 | 0 |
| Brown bear (n=4) | Ursus arctos | Nostrils | 2 | 4 | 0 |
| Pile | 4 | 0 | |||
| Bottle- nosed dolphin (n=2) | Tursiops truncatus | Nostrils | 1 | 2 | 0 |
| Cretan wild goat (n=2) | Capra aegagrus cretica | Nostrils | 2 | 2 | 1 |
| Pile | 2 | 0 | |||
| Grant’s zebra (n=2) | Equus quagga boehmi | Pile | 1 | 2 | 0 |
| Pygmy hippopotamus (n=2) | Choeropsis liberiensis | Nostrils | 2/1 | 2 | 0 |
| Pile | 1 | 0 | |||
| Ring tailed lemur (n=3) | Lemur catta | Nostrils | 3/1 | 1 | 0 |
| Pile | 2 | 0 | |||
| Skin lesion | 2 | 0 | |||
| Shetland pony (n=3) | Equus ferus caballus | Nostrils | 2 | 3 | 1 |
| Pile | 3 | 1 | |||
| Skyrian horse (n=2) | Equus cabalus Skyros Poni | Nostrils | 2 | 2 | 0 |
| Pile | 2 | 0 | |||
| Squirrel monkey (n=1) | Saimiri sciureus | Nostrils | 2 | 1 | 1 |
| Skin lesion | 1 | 1 | |||
| De Brazza’s monkey (n=1) | Cercopithecus neglectus | Nostrils | 2 | 1 | 0 |
| Pile | 1 | 0 | |||
| Javan langur (n=1) | Trachypithecus auratus | Nostrils | 2 | 1 | 0 |
| Pile | 1 | 0 | |||
| Cow (n=1) | Bos taurus | Nostrils | 2 | 1 | 0 |
| Pile | 1 | 0 | |||
| Rabbit (n=2) | Oryctolagus cuniculus | Nostrils | 2 | 2 | 1 |
| Pile | 2 | 1 | |||
| Birds (n= 11) | |||||
| Alexandrian parrot (n=2) | Psittacula eupatria | Skin | 1 | 2 | 0 |
| Blue and gold macaw (n=2) | Ara ararauna | Skin | 1 | 2 | 0 |
| Scarlet macaw (n=2) | Ara macao | Skin | 1 | 2 | 0 |
| Salmon- crested cockatoo (n=1) | Cacatua moluccensis | Skin | 1 | 1 | 0 |
| Military macaw (n=2) | Ara militaris | Skin | 1 | 2 | 0 |
| African grey parrot (n=1) | Psittacus erithacus | Skin | 1 | 1 | 0 |
| Eclectus parrot (n=1) | Eclectus roratus | Skin | 1 | 1 | 0 |
| Total (n=51) | 72 | 8 | |||
The study was approved by the Bioethics Committee of the University of Patras (No EB25). A written informed consent was obtained from the human individuals, who are working in the Park between two and 15 years. They all had companion animals such as dogs, cats and birds, but none was hospitalized during the last year. All eight humans are working as animal caregivers without any rotation; three with big mammals, three with birds and another two with monkeys and felines.
The study did not involve any experimentation, but was based on swabs collected by a veterinarian under routine diagnostic procedures from infected animals. Colonization samples were collected randomly among healthy animals at the Attica Zoological Park. S. aureus screening samples were obtained from the nostrils (n=23) and pile (n=27) or skin surfaces (n=11). Any sites of infection in animals were also sampled (n=3). Swabs were inoculated into 3 mL Trypticase Soy Broth (TSB) transport medium with 6% NaCl (Difco, Sparks, MD, USA), and transferred to the National Reference Laboratory for Staphylococci. One loop from each sample was inoculated into fresh TSB for enrichment, plated onto Trypticase Soy Agar with 5% Sheep Blood (Becton Dickinson, Le Pont de Claix, France) and incubated at 37°C for 48 h. Isolates were identified on the basis of colony morphology, Gram stain, catalase production and coagulase test (Slidex Staph Plus, bioMérieux, Marcy l’ Etoile, France), whereas, verification was carried out by PCR for nuc gene (Boye et al., 2014).
Antimicrobial susceptibility testing
Susceptibility testing was performed according to EUCAST guidelines by the disc diffusion method for cefoxitin, tetracycline, gentamicin, rifampicin, kanamycin, erythromycin, clindamycin, fusidic acid and sulfamethoxazole-trimethoprim (SXT), (SirScan, i2a, Park Eureka, Montpellier, France) (The EUCAST, 2012). MICs of oxacillin, vancomycin, teicoplanin, daptomycin and linezolid were determined by a gradient method (Etest, bioMérieux) (The EUCAST, 2012).
Molecular analysis
All isolates were tested by PCR for the presence of mecA, mecC (Stegger et al., 2012), the genes encoding Panton-Valentine leukocidin (PVL) (lukF/lukS-PV) and toxic shock syndrome toxin-1 (TSST-1, tst) (Jarraud et al., 2002), using the reference strains ATCC49775, LGA251, ATCC49775 and Fri913 as positive controls, respectively. DNA extraction was performed into agarose disks, whereas pulsed-field gel electrophoresis (PFGE) of SmaI DNA digests was carried out using a CHEF DR III apparatus (Bio-Rad Laboratories, Richmond, CA, USA). Visual interpretation of PFGE banding patterns and assignments of types were performed according to the published criteria (Tenover et al., 1995). PFGE types were named by capital letters and compared to previously identified pulsotypes among humans. A dendrogram was computed comparing molecular weights of strains’ of DNA fragments by using FPQuest software (Bio-Rad, Cat.Num:1709300). Clustering was based on ≥ 80% similarity (Fig. 1). Multi Locus Sequence Typing (MLST) was performed according to published protocols (http://saureus.mlst.net/misc/info.asp) (Enright et al., 2000). The polymorphic X-region of protein A gene (spa) was analyzed (Stegger et al., 2012) and the resulting spa types and clonal clusters were assigned using the software Ridom StaphType (Ridom GmbH, Wurzburg, Germany).
Fig. 1.
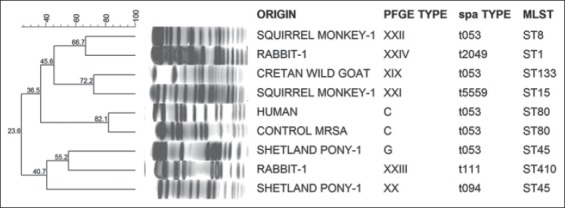
Dendogram of PFGE banding patterns of the isolated S. aureus strains and the respective MLST and spa types. One methicillin-resistant S. aureus strain (MRSA) of human origin, ST80-t053 and PFGE type. C is included as control.
Results
A total of seven S. aureus were recovered from four animals among 43 tested (prevalence 9.3%) and one isolate among the personnel (12.5%), (Table 1). Twelve mammalian species and all birds were negative for S. aureus carriage or infection (Table 1). The MIC of oxacillin was ≤ 1.5 mg/L, whereas the MICs of vancomycin ranged from 0.5 mg/L to 1 mg/L, teicoplanin from 1.5 mg/L to 3 mg/L and linezolid from 1 mg/L to 2 mg/L. Only two out of eight isolates showed resistance to fusidic acid and one to tetracycline (Table 2). All isolates were negative for mecA, mecC and tst genes, whereas, one carried the PVL genes, recovered from a skin infection of a Squirrel monkey (Saimiri sciureus).
Table 2.
Phenotypic and molecular characteristics among S. aureus isolates from four animals and an employee at the Attica Zoological Park.
| Species | MLST ST | MLST group | spa type | spa CC | PFGE type | PVL toxin | Antimicrobial resistance patterns | Colonized/infected (C/I) |
|---|---|---|---|---|---|---|---|---|
| Cretan wild goat | 133 | 10 | t053 | S | XIX | N | Fusidic acid | C |
| Shetland pony-1 | 45 | 3 | t053 | S | G | N | Susceptible | C |
| Shetland pony-1 | 45 | 3 | t094 | S | XX | N | Susceptible | C |
| Squirrel monkey-1 | 15 | 1 | t5559 | S | XXI | N | Tetracycline, fusidic acid | C |
| Squirrel monkey-1 | 8 | 1 | t053 | S | XXII | P | Susceptible | I |
| Rabbit -1 | 1 | 1 | t2049 | Common | XXIV | N | Susceptible | C |
| Rabbit -1 | 410 | New | t111 | CC | XXIII | N | Susceptible | C |
| Human | 80 | 14 | t053 | S | C | N | Susceptible | C |
ST: Sequence types assigned by multi-locus sequence typing (MLST), MLST Groups: Classified according to sequence types by eBURST (http://saureus.mlst.net/misc/info.asp). CC: Clusters assigned according to spa types by BURP clustering with Ridom StaphType (Ridom GmbH, Wurzburg, Germany): t111 and t2049 are assigned to a common CC, PFGE: Pulsed-field gel electrophoresis types, PVL: Panton-valentine leukocidin genes detected by PCR, S: Singleton, N: negative, P: positive. Antimicrobials tested: cefoxitin, tetracycline, gentamicin, rifampicin, kanamycin, erythromycin, clindamycin, fusidic acid, sulfamethoxazole-trimethoprim, oxacillin, vancomycin, teicoplanin, daptomycin and linezolid.
The human S. aureus strain belonged to ST80 clone spa type t053, PFGE type C and negative for PVL genes (Table 2, Fig. 1). Among the strains of animal origin, MLST revealed the occurrence of six STs (133, 45, 15, 8, 1 and 410), which were classified into three major MLST Groups (10, 3 and 1), whereas ST410 was new in our setting (eBURST, http://saureus.mlst.net/misc/info.asp), (Table 2). Five spa types were detected; t053 predominated (three strains), followed by t094, t111, t5559, and t2049 (one strain each). Seven PFGE types were identified, including type G that was previously characterized in humans (Drougka et al., 2014). The remaining five pulsotypes were detected in our setting for the first time in our present study (Fig. 1, Table 2).
Discussion
The prevalence of S. aureus carriage in animals and personnel of the zoo were 9.3% and 12.5%, respectively. This is much lower compared to published colonization rates of people in Greece (16.7% among hospital residents and 33.3% in health care workers) (Maltezou et al., 2009). Espinosa-Gongora et al. (2012) identified 35% S. aureus carriage among zoo animals that was comparable to their human population results. However, the aforementioned result can be explained by the low number of human participants that constitutes a limitation of our study. All S. aureus were methicillin-susceptible in this setting. The absence of MRSA has previously been detected among mammals of the zoological park in Belgium (Vercammen et al., 2012). Resistance to fusidic acid was detected in two isolates and to tetracycline in one; both are antimicrobials that are rarely used in the Zoological Park for local therapy only, whereas, this resistance phenotype is correlated with the human MRSA ST80 clone spread in the country (Drougka et al., 2014).
Molecular analysis classified the human S. aureus isolate as ST80, t053, PFGE type C and this isolate was PVL-negative. The ST80 spa t053 clone is the predominant clone among MRSA in Greece, it was first identified as a hospital-associated pathogen, and it is mainly associated with skin and soft tissue infections in the community, but has also infiltrated the healthcare system causing nosocomial infections (Aires de Sousa et al., 2003; Drougka et al., 2014). Among the isolates of animal origin, ST45 has been identified in two strains, both originating from the Shetland pony (Equus ferus caballus) and was classified into different PFGE and spa types (t053 and t094). A search of the MLST database showed that ST45 has been found among human nasal carriers and is associated with hospital infections. Both MRSA and MSSA ST45 strains have been reported from several European countries (www.saureus.mlst.net/sql/all_allelicprofileresults.asp). Espinosa-Gongora et al. (2012) identified ST45 strains in horses at the Copenhagen zoo. The identification of the human pulsotype G in the ST45 clone (Drougka et al., 2014), suggests that these strains may be of human origin and transmission of this clone might be associated with the close contact these animals have with personnel and visitors of the park.
Strains characterized as ST8 and ST15 clones were assigned into MLST Group1 and were isolated from the Squirrel monkey. These STs are mainly associated with human infections (Tavares et al., 2014), reinforcing the aspect that these strains might have been recently introduced to animals by humans and have not yet evolved for eliciting host specific traits. In Portugal, 4.7% of MSSA strains were classified to ST8-t008 clone (Tavares et al., 2014). In the present study, the strain characterized as ST8 belonged to spa type t053, a human lineage in Greece, previously identified in MRSA (ST8-SCCmec-IV), carrying also PVL genes (Drougka et al., 2014). Moreover, the ST8-t053 strain originating from the skin infection of a Squirrel monkey was PVL-positive, reinforcing the aspect of human to animal transfer and the importance of surveillance for S. aureus and not only for MRSA transmission.
ST133 isolated from a goat has been previously identified in cows, goats and sheep (Smyth et al., 2009). Two STs were identified from the rabbit: ST1, which was originally a human clone but has also colonized some other animal species (Gómez-Sanz et al., 2013), and ST410, a clone rarely reported in the literature (Smyth et al., 2009).
The predominant spa type t053 has been identified among strains of human origin and more specifically associated with the ST80 clone that is common in our country (Table 2) (Drougka et al., 2014), whereas worldwide, its frequency is very low (0.02%). It has previously been detected among ST5 strains in France, Germany, Romania and Spain (http://spa.ridom.de/frequencies.shtml). Moreover, in the present study, t053 was characterized among strains of different STs (Table 2), suggesting that zoo park animals are colonized by S. aureus clonal lineages of human origin.
Conclusions
Worldwide, studies investigating the distribution of S. aureus in zoo park population have been very scarce and this is the first study conducted in Greece. A variety of STs were identified among S. aureus colonizing the zoological park’s inhabitants, interpreted by the fact that in most cases they do not interact with each other, thus having no chance to transmit bacteria between different species. However, our results indicate that some animals are colonized with lineages of human origin. No transmission of infection among animals and personnel has been detected. Further studies should be carried out in order to provide additional information of S. aureus ecology in the animal kingdom.
Acknowledgements
We would like to thank, Prof. Jerome Etienne and Prof. Frederic Laurent, National Reference Laboratory for Staphylococci, Lyon, France, for kindly providing the reference S. aureus strains used in our experiments; Prof. Dr Alexander Friedrich and Prof. Dr Hajo Grundmann, Faculty of Medical Sciences, Medical Microbiology, Groningen, The Netherlands, for kindly providing the Ridom StaphType software; Mr Jean-Jacques Lesueur, founder of Attica Zoological Park, Athens, Greece, for kindly allowing us to conduct this study. This work was supported by funding from the National Staphylococcal Reference Laboratory, Greece, under the scientific responsibility of I. S. and E.D.A. (grant C954, Hellenic Centre for Disease Control and Prevention, HCDCP/KEELPNO).
Conflict of Interest
Authors E.D. and A.F. have received funding from the National Staphylococcal Reference Laboratory.
References
- Aires de Sousa Μ, Bartzavali C, Spiliopoulou I, Santos Sanches I, Crisostomo M.I, de Lencastre H. Two international methicillin-resistant Staphylococcus aureus clones endemic in a University Hospital in Patras, Greece. J. Clin. Microbiol. 2003;41:2027–2032. doi: 10.1128/JCM.41.5.2027-2032.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boye K, Schønning K, Westh H, Lisby G. Fast screening for MRSA by quadriplex real-time PCR. In the Proceedings of the 24th European Congress of Clinical Microbiology and Infectious Diseases; Barcelona, Spain, Poster Number: 1534. 2014 [Google Scholar]
- Drougka E, Foka A, Liakopoulos A, Doudoulakakis A, Jelastopulu E, Chini V, Spiliopoulou A, Levidiotou S, Panagea T, Vogiatzi A, Lebessi E, Petinaki E, Spiliopoulou I. A 12-year survey of methicillin-resistant Staphylococcus aureus infections in Greece: ST80-IV epidemic? Clin. Microbiol. Infect. 2014;20:796–803. doi: 10.1111/1469-0691.12624. [DOI] [PubMed] [Google Scholar]
- Enright M.C, Day N.P, Davies C.E, Peacock S.J, Spratt B.G. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J. Clin. Microbiol. 2000;38:1008–1015. doi: 10.1128/jcm.38.3.1008-1015.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa-Gongora C, Chrobak D, Moodley A, Bertelsen M.F, Guardabassi L. Occurrence and distribution of Staphylococcus aureus lineages among zoo animals. Vet. Microbiol. 2012;158:228–231. doi: 10.1016/j.vetmic.2012.01.027. [DOI] [PubMed] [Google Scholar]
- Gómez-Sanz E, Torres C, Benito D, Lozano C, Zarazaga M. Animal and human Staphylococcus aureus associated clonal lineages and high rate of Staphylococcus pseudintermedius novel lineages in Spanish kennel dogs: predominance of S. aureus ST398. Vet. Microbiol. 2013;166:580–589. doi: 10.1016/j.vetmic.2013.07.014. [DOI] [PubMed] [Google Scholar]
- Hasman H, Moodley A, Guardabassi L, Stegger M, Skov R.L, Aarestrup F.M. Spa type distribution in Staphylococcus aureus originating from pigs, cattle and poultry. Vet. Microbiol. 2010;141:326–331. doi: 10.1016/j.vetmic.2009.09.025. [DOI] [PubMed] [Google Scholar]
- Jarraud S, Mougel C, Thioulouse J, Lina G, Meugnier H, Forey F, Nesme X, Etienne J, Vandenesch F. Relationships between Staphylococcus aureus genetic background, virulence factors, agr groups (alleles), and human disease. Infect. Immun. 2002;70:631–641. doi: 10.1128/IAI.70.2.631-641.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltezou H.C, Vourli S, Katerelos P, Maragos A, Kotsalidou S, Remoudaki E, Papadimitriou T, Vatopoulos A.C. Panton-Valentine leukocidin-positive methicillin-resistant Staphylococcus aureus outbreak among healthcare workers in a long-term care facility. Int. J. Infect. Dis. 2009;13:401–406. doi: 10.1016/j.ijid.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Smyth D.S, Feil E.J, Meaney W.J, Hartigan P.J, Tollersrud T, Fitzgerald J.R, Enright M.C, Smyth C.J. Molecular genetic typing reveals further insights into the diversity of animal-associated Staphylococcus aureus. J. Med. Microbiol. 2009;58:1343–1353. doi: 10.1099/jmm.0.009837-0. [DOI] [PubMed] [Google Scholar]
- Stegger M, Andersen P.S, Kearns A, Pichon B, Holmes M.A, Edwards G, Laurent F, Teale C, Skov R, Larsen A.R. Rapid detection, differentiation and typing of methicillin-resistant Staphylococcus aureus harbouring either mecA or the new mecA homologue mecA (LGA251) Clin. Microbiol. Infect. 2012;18:395–400. doi: 10.1111/j.1469-0691.2011.03715.x. [DOI] [PubMed] [Google Scholar]
- Tavares A, Faria N.A, de Lencastre H, Miragaia M. Population structure of methicillin-susceptible Staphylococcus aureus (MSSA) in Portugal over a 19-year period (1992-2011) Eur. J. Clin. Microbiol. Infect. Dis. 2014;33:423–432. doi: 10.1007/s10096-013-1972-z. [DOI] [PubMed] [Google Scholar]
- Tenover F.C, Arbeit R.D, Goering R.V, Mickelsen P.A, Murray B.E, Persing D.H, Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis:criteria for bacterial strain typing. J. Clin. Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The European Committee on Antimicrobial Susceptibility Testing (EUCAST) Breakpoint tables for interpretations of MICs and zone diameters. Version 2.0, valid from 2012-01-01. [accessed on 30 October 2014]. Available at: [http://http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Disk_test_documents/EUCAST_breakpoints_v2.0_120101.pdf]
- Walther B, Wieler L.H, Friedrich A.W, Hanssen A.M, Kohn B, Brunnberg L, Lübke-Becker A. Methicillin-resistant Staphylococcus aureus (MRSA) isolated from small and exotic animals at a university hospital during routine microbiological examinations. Vet. Microbiol. 2008;127:171–178. doi: 10.1016/j.vetmic.2007.07.018. [DOI] [PubMed] [Google Scholar]
- Vercammen F, Bauwens L, De Deken R, Brandt J. Prevalence of methicillin-resistant Staphylococcus aureus in mammals of the Royal Zoological Society of Antwerp, Belgium. J. Zoo Wildl. Med. 2012;43:159–161. doi: 10.1638/2010-0107.1. [DOI] [PubMed] [Google Scholar]


