Abstract
Background:
Epidermal growth factor receptor (EGFR) mutation analysis has become an important part of the initial workup of non-squamous non-small cell lung cancer (NS-NSCLC) patients as it is now recognized both as a prognostic and predictive marker to therapy with EGFR tyrosine kinase inhibitors (TKI).
Aim:
In this retrospective study conducted at a University hospital, we evaluated the prevalence of EGFR mutations in patients with NS-NSCLC, clinico-pathological correlation and outcome to treatment with EGFR TKIs.
Materials and Methods:
Case records of 147 patients of NS-NSCLC in whom EGFR mutation status was tested were screened. EGFR mutation analysis was done using DNA sequencing by real time polymerase chain reaction method from tissue and cell blocks prepared from core biopsy, fine needle aspiration cytology and pleural fluid specimens.
Results:
EGFR mutations were seen in 30.6% of the 111 evaluable specimens, with a significantly higher rate in females (44% vs 19.6% P = 0.0072) as compared to men and non-smokers (41% vs 12% P = 0.0013) as against smokers. Most common mutations were observed in exons 19 (71%) and 21 (25%). The estimated median progression free survival for patients with and without mutations when treated with upfront TKIs was 12 months and 3 months respectively and the estimated median overall survival for patients with and without mutations was 20 and 9 months respectively.
Conclusion:
This study from India, further establishes the importance of upfront EGFR mutation testing in all NS-NSCLC patients, not only to prognosticate, but also to identify that subset of patients who could benefit from EGFR TKI therapy, early in the course of their disease.
KEY WORDS: Epidermal growth factor receptor mutations, non-squamous non-small cell lung cancer, tyrosine-kinase inhibitor
INTRODUCTION
Lung cancer has been the most common cancer in the world for several decades. According to the Global Burden of Cancer Study (GLOBOCAN) 2012 report, there are estimated to be 1.8 million new cases in 2012 (12.9% of the total), 58% of which occurred in the less developed regions. India had 53,728 new lung cancer cases in males and 16,547 new lung cancer cases in females, with the corresponding mortality rates of 13.7 and 4.6%, respectively.[1] More than three quarters of the lung cancer patients present in an advanced stage (stage IIIB and IV) at diagnosis, resulting in higher mortality rates.[2]
The two major forms of lung cancer are non-small cell lung cancer (NSCLC), accounting for about 85% of all lung cancers and small-cell lung cancer (SCLC).[3] NSCLC patients can be further categorized broadly into squamous and non-squamous (adenocarcinoma, large cell carcinoma) histologies. Recent advances in our understanding of lung cancer biology have led to personalized therapies based on the molecular characteristics of the tumor, targeting specific genes and pathways. One such pathway that is deregulated in some NSCLC patients, particularly nonsmokers, is the epidermal growth factor receptor (EGFR) signaling pathway. The EGFR is a receptor tyrosine kinase of the v-erb-b2 erythroblastic leukemia viral oncogene homolog 2 (ErbB) family, which includes ErbB1 (or HER-1 or EGFR), ErbB2 (or HER-2/neu), ErbB3 (or HER-3), and ErbB4 (or HER-4).[4] Autophosphorylation and transphosphorylation of the receptors through their tyrosine kinase domains lead to the recruitment of downstream effectors and the activation of several signaling pathways, including Ras-Raf-Mek and the pathway consisting of phosphoinositide 3-kinase (PI3K), Akt, and the mammalian target of rapamycin (mTOR), which in turn may have an effect on the proliferation, survival, invasiveness, metastatic spread, and tumor angiogenesis, through pathways that are either dependent on or independent of the hypoxia inducible factor (HIF).[3,5]
Several investigators across the world independently identified activating somatic mutations in the kinase domain of EGFR in NSCLC patients, ranging from 10% in the United States to 30–50% of the specimens from patients in Asia. The mutations occur with a significantly higher frequency in women, nonsmokers, and those with adenocarcinoma. EGFR positive lesions show exquisite sensitivity to EGFR tyrosine kinase inhibitors like gefitinib and erlotinib, and thus appear to explain most of the dramatic responses to these agents. The most common activating mutations in the kinase domain of EGFR are exon 19 deletions that eliminate a leucine–arginine–glutamate–alanine motif and point to mutations at codon 858 in exon 21, resulting in the substitution of arginine for leucine (L858R). Tumors with activating mutations in the EGFR are highly dependent on continued EGFR signaling for proliferation and survival, which is also precisely the reason for their exquisite sensitivity to EGFR TKIs. EGFR mutations are associated with an improved prognosis in NSCLC, even when treated with chemotherapy. The Iressa Pan-Asia Study (IPASS) in Asia[6] and other randomized controlled trials in advanced NSCLC[7,8,9] have confirmed EGFR-activating mutations as the main predictor of the clinical outcome of TKI therapy with Geftinib or Erlotinib, for NSCLC. We carried out this study to determine the prevalence of EGFR mutations in non-squamous non-small cell lung cancer (NS-NSCLC) and their correlation to TKI therapy in the Indian subpopulation.
MATERIALS AND METHODS
This is a retrospective analysis of all NS-NSCLC patients, who were treated in the Department of Medical Oncology at our institute for a three-year period, between January 2011 and December 2013. The demographic, clinical, and treatment details were retrieved from the patients’ case records from the Tumor Registry. Deoxyribonucleic acid (DNA) sequencing for activating EGFR mutations in exons 18 through 21 was performed on paraffin-embedded tissue/cell blocks prepared from biopsy, fine needle aspiration cytology (FNAC), and pleural fluid cytology specimens of patients with a pathological diagnosis of NS-NSCLC, as part of the initial workup. The EGFR mutational status was assessed and correlated with the clinical and pathological parameters, including age, gender, smoking status, histology, stage of disease, and treatment. All the patients were staged according to the American Joint Committee on Cancer (AJCC) TNM staging manual, Seventh edition.[10] Response to therapy, adverse effects recorded during the follow up visits during treatment, and after completion of therapy were obtained from the case records. This protocol was approved by the Institutional Ethics Committee (IEC).
Statistical analysis
Graph pad prism version 6 and Microsoft office 2010 were used for statistical analysis. The association between EGFR mutations, sex, smoking status, histology, and stage of the disease were evaluated using the Chi-square test. The differences in progression free survival (PFS) and overall survival (OS) between the two groups were compared using the Kaplan-Meier curves and log-rank tests.
RESULTS
Baseline characteristics of the patients are represented in Table 1. A total of 147 NS-NSCLC patients were screened for the presence of activating EGFR mutations during the three-year period. The median age of the study cohort was 56 (range 30–80) years, with more than half (58%) of the patients in the 40 to 60 years age group [Figure 1]. Males constituted about 60%, whereas, females constituted 40%. Most of the patients (95%) presented in an advanced stage of the disease, with adenocarcinoma being the predominant histology.
Table 1.
Demographic, clinical, and EGFR mutation status
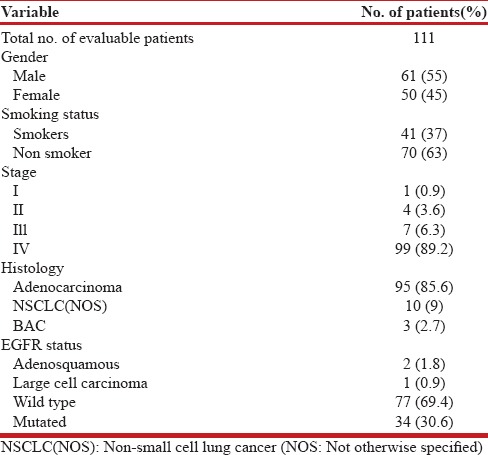
Figure 1.
Age and sex distribution
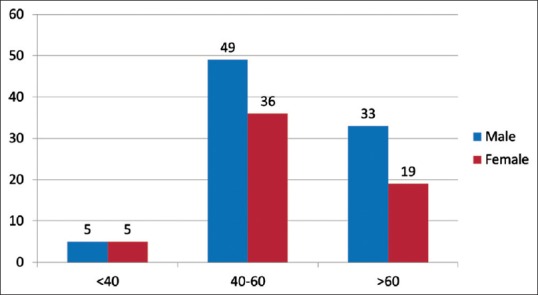
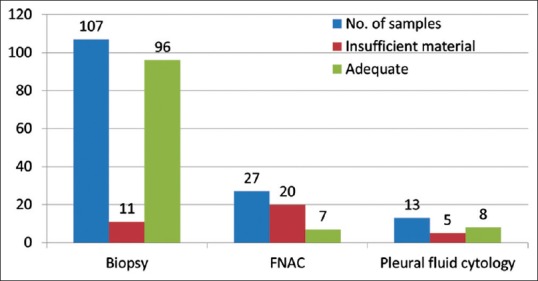
Out of the 147 samples sent for EGFR mutational analysis, 36 specimens (biopsy specimens - 11 and cell blocks prepared from FNAC and pleural fluid - 20 and 5, respectively) had insufficient tumor tissue for the test to be performed [Figure 2]. Of the evaluable 111 patients, any one of the activating EGFR mutation was detected with a frequency of 30.6% [Figure 3]. The most common EGFR activating mutations observed were in frame deletions in exon 19 (71%) and a missense mutation L858R in exon 21 (25%). Overall the EGFR mutations were significantly higher in females as compared to males (44% vs. 19.6%, Fisher's exact two-tailed test, P = 0.0072). Nonsmokers had significantly higher mutations than smokers (41% vs. 12%, Fisher's exact two-tailed test, P = 0.0013). The EGFR mutations did not correlate with the histology and stage of the disease [Table 2].
Figure 2.
Diagnostic method used
Figure 3.
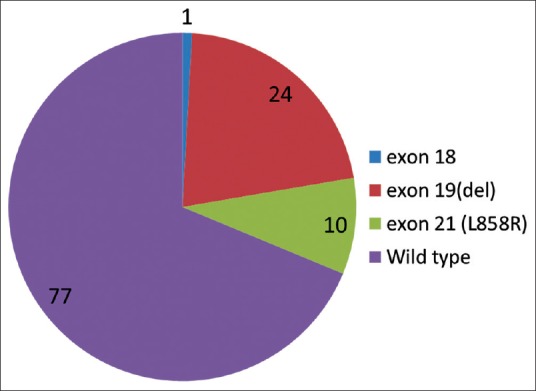
EGFR mutation status
Table 2.
EGFR mutation and clinical correlates
The estimated progression-free survival (PFS) of the patients harboring activated EGFR mutations treated with first-line TKIs was 12 months, whereas, in those patients with wild-type EGFR it was three months, P < 0.0001 by log rank test [Figure 4]. Similarly the estimated overall survival [Figure 5] was also significantly higher for patients with EGFR mutations as compared to those without (20 months vs. 9 months, P = 0.0002 by the log rank test). Skin rash (52%) and diarrhea (38%) were the most frequently observed toxicities in our study.
Figure 4.
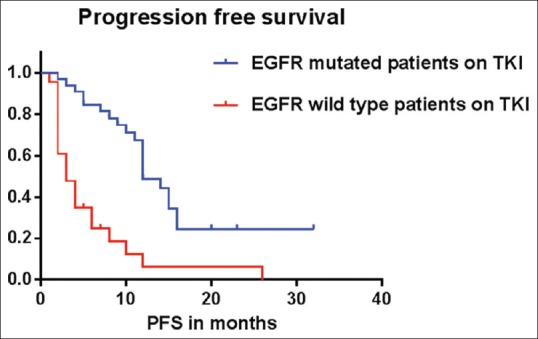
Estimated median PFS for the EGFR mutant patients was 10 months, while the estimated median PFS for EGFR mutation negative patients was three months, P < 0.0001 by log rank test (Mantel Cox)
Figure 5.
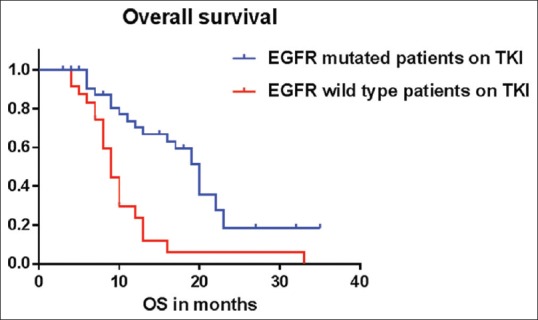
Estimated median OS for EGFR mutant patients was 20 months, while the estimated median OS for EGFR mutation negative patients was nine months, P = 0.0002 by the log rank test (Mantel Cox)
DISCUSSION
The discovery of EGFR mutations in NSCLC and the dramatic response to TKIs is a major breakthrough achieved in the management of NSCLC patients in the last decade. Multiple prospective studies have convincingly demonstrated that activating mutations in the EGFR tyrosine kinase domain, especially in the frame deletions in exon 19 and a missense mutation, L858R, in exon 21, are the best predictors of the response and survival benefit with EGFR TKIs.[6,7,8] Many centers across the world and in India have now incorporated evaluation of the EGFR mutation status in the initial management algorithm of NS-NSCLC patients. The prevalence rates of EGFR mutations in NS-NSCLC vary widely across different ethnicities. It has been reported to be in the rate of 10 – 15% in North Americans and Europeans, 26 – 30% in various East Asian series, including Chinese, Koreans, and Japanese.[11,12,13,14,15,16] We report a prevalence rate of 30%, whereas, few studies from India have reported mutation rates varying from 35 to 51.8%.[17,18,19,20,21,22] Mutations have been found to be highly co-existent with adenocarcinoma histology, women, and nonsmokers. Mutations in EGFR, in our study, are found to be higher in patients with adenocarcinoma histology, but it has not reached a statistical significance. However, females (44%) and nonsmokers (41%) have significantly higher mutation rates than men and smokers, respectively. These findings are in concordance with the results world over. Out of all the patients with an activating EGFR mutation, females account for about two-thirds (64%) and nonsmokers account for 84%.
According to a study done by Sandra P. D’ Angelo, 31% percent of all EGFR mutations would be missed if testing were restricted to women, 40% would be missed if testing were restricted to persons who had never smoked, and 57% would be missed if testing were restricted to women who never smoked cigarettes.[23] In the present study, about 36% of all EGFR mutations that would benefit from TKI therapy could have been missed if testing was restricted to females and about 16% could have been missed if the test was confined to nonsmokers, thus supporting the growing consensus all over the world that all patients with a pathological diagnosis of NS-NSCLC should undergo mutation testing at diagnosis, if the tissue is available.[23] With regard to the frequencies of the type of EGFR mutations observed in our study, 71% of the patients had an in-frame deletion in exon 19, 25% had the L858R missense mutation in exon 21, and only 4% patients had the G719C point mutation in exon 18. According to the published literature, approximately 45 to 54% of EGFR mutations are in-frame deletions in exon 19 and 40% of the EGFR mutations are missense mutations in L858R, in exon 21, and between 4 to 9% of the mutations are reported in exon 20.[6]
EGFR TKIs are now accepted worldwide as a standard first-line therapy for patients with activating EGFR mutations.[24,25] Regarding the survival analysis, in our study, the estimated PFS for patients with EGFR mutation treated with TKIs was significantly longer at 12 months, as compared to three months for EGFR-negative patients treated with first-line TKIs, P < 0.0001 by the log rank test. The longest PFS for an EGFR wild-type patient noted in our study was 26 months, whereas, for mutated patients, it was 32 months. The estimated median overall survival (OS) of patients on first-line TKI with EGFR activating mutations was significantly longer at 20 months, as compared to an estimated median OS of nine months for EGFR-negative patients, P = 0.0002 by the log rank test. Our results were comparable to the recently published data by Noronha et al., who reported a median PFS of four and ten months, respectively, for the EGFR wild-type and EGFR mutated patients on TKIs. The median OS reported in that study was 21 and 10 months, respectively, for the patients with mutated and wild-type EGFR.[18] Bhatt et al. reported a PFS of 11 months in EGFR mutated patients treated with first-line TKIs.[22] Maemondo et al. reported a PFS of 10.8 months and an OS of 30.5 months following Gefitinib therapy.[7] The reason for the varying survival rates to TKI therapy among patients who do not harbor an EGFR activating mutation, may be due to the lack of availability of highly sensitive methods to detect the EGFR mutation. It is very likely that few patients who are labeled as EGFR wild-type might in reality harbor an activating mutation, which is not detected by the available methods. The toxicity profile in our patients was similar to that described in the literature. Skin rash was the major toxicity noted, which occurred in 52% of the patients, followed by diarrhea in 38% of the study subjects. The landmark study IPASS, reported skin rash and GI toxicity in 66 and 47% of the patients on Geftinib, respectively.[6] Ours being a retrospective analysis, a lesser number of side effects have been noted, as compared to other published literature.
To conclude, the prevalence of activating EGFR mutations and their clinical correlations in our study are comparable to those previously published and Indian patients with EGFR mutations have a significantly better response rate, progression free survival, and overall survival when treated with EGFR-targeted therapies. These data dictate that EGFR mutation analysis should be incorporated in the early management algorithm of NS-NSCLC patients. More sensitive techniques are needed to identify this subset of patients, who show dramatic responses with a tolerable side effect profile to oral TKI therapy.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. Lyon, France: International Agency for Research on Cancer; 2013. [Last accessed on 2014 Mar 21]. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11. Available from: http://www.globocan.iarc.fr . [Google Scholar]
- 2.Noronha V, Dikshit R, Raut N, Joshi A, Pramesh CS, George K, et al. Epidemiology of lung cancer in India: Focus on the differences between non-smokers and smokers: A single-centre experience. Indian J Cancer. 2012;49:74–81. doi: 10.4103/0019-509X.98925. [DOI] [PubMed] [Google Scholar]
- 3.Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359:1367–80. doi: 10.1056/NEJMra0802714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jänne PA, Engelman JA, Johnson BE. Epidermal growth factor receptor mutations innon-small-cell lung cancer: Implications for treatment and tumor biology. J Clin Oncol. 2005;23:3227–34. doi: 10.1200/JCO.2005.09.985. [DOI] [PubMed] [Google Scholar]
- 5.Jorissen RN, Walker F, Pouliot N, Garrett TP, Ward CW, Burgess AW. Epidermal growth factor receptor: Mechanisms of activation and signalling. Exp Cell Res. 2003;284:31–53. doi: 10.1016/s0014-4827(02)00098-8. [DOI] [PubMed] [Google Scholar]
- 6.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–57. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 7.Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. North-East Japan Study Group. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–8. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 8.Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. West Japan Oncology Group. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouringmutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–8. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 9.Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Spanish Lung Cancer Group in collaboration with Groupe Français de Pneumo-Cancérologie and AssociazioneItaliana Oncologia Toracica. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): Amulticentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–46. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 10.Gallbladder . In: AJCC Cancer Staging Manual. 7th ed. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. New York, NY: Springer; 2010. pp. 211–7. [Google Scholar]
- 11.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 12.Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 13.Dogan S, Shen R, Ang DC, Johnson ML, D’Angelo SP, Paik PK, et al. Molecular epidemiology of EGFR and KRAS mutations in 3,026 lung adenocarcinomas: Higher susceptibility of women to smoking-related KRAS-mutant cancers. Clin Cancer Res. 2012;18:6169–77. doi: 10.1158/1078-0432.CCR-11-3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu YL, Zhong WZ, Li LY, Zhang XT, Zhang L, Zhou CC, et al. Epidermal growth factor receptor mutations and their correlation with gefitinib therapy in patients with non-small cell lung cancer: A meta-analysis based on updated individual patient data from six medical centers in mainland China. J Thorac Oncol. 2007;2:430–9. doi: 10.1097/01.JTO.0000268677.87496.4c. [DOI] [PubMed] [Google Scholar]
- 15.Kim HJ, Oh SY, Kim WS, Kim SJ, Yoo GH, Kim WD, et al. Clinical investigation of EGFR mutation detection by pyrosequencing in lung cancer patients. Oncol Lett. 2013;5:271–6. doi: 10.3892/ol.2012.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang PC, ShiY, Au JS, Srinivasan S, Cornelio GH, Tsai CM, et al. Molecular epidemiological prospective study of EGFR mutations from Asian patients with advanced lung adenocarcinoma. J Clin Oncol. 2012;30(No 15_suppl (May 20 Supplement) 2012):1534. [Google Scholar]
- 17.Sahoo R, Harini VV, Babu VC, PatilOkaly GV, Rao S, Nargund A, et al. Screening for EGFR mutations inlung cancer, a report from India. Lung Cancer. 2011;73:316–9. doi: 10.1016/j.lungcan.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 18.Noronha V, Prabhash K, Thavamani A, Chougule A, Purandare N, Joshi A, et al. EGFR mutations in indian lung cancer patients: Clinical correlation and outcome to EGFR targeted therapy. PLoS One. 2013;8:e61561. doi: 10.1371/journal.pone.0061561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehta J. Molecular epidemiology of epidermal growth factor receptor mutations in lung cancers in Indian population. Indian J Cancer. 2013;50:102–6. doi: 10.4103/0019-509X.117019. [DOI] [PubMed] [Google Scholar]
- 20.Veldore VH, Rao RM, Kakara S, Pattanayak S, Tejaswi R, Sahoo R, et al. Epidermal growth factor receptor mutation in non-small-cell lung carcinomas: A retrospective analysis of 1036 lung cancer specimens from a network of tertiary cancer care centers in India. Indian J Cancer. 2013;50:87–93. doi: 10.4103/0019-509X.117013. [DOI] [PubMed] [Google Scholar]
- 21.Choughule A, Noronha V, Joshi A, Desai S, Jambhekar N, Utture S, et al. Epidermal growth factor receptor mutation subtypes and geographical distribution among Indian non-small cell lung cancer patients. Indian J Cancer. 2013;50:107–11. doi: 10.4103/0019-509X.117023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhatt AD, Pai R, Rebekah G, Nehru GA, Dhananjayan S, Samuel A, et al. Clinicopathologic features of non-small cell lung cancer in India and correlation with epidermal growth factor receptor mutational status. Indian J Cancer. 2013;50:94–101. doi: 10.4103/0019-509X.117016. [DOI] [PubMed] [Google Scholar]
- 23.D’Angelo SP, Pietanza MC, Johnson ML, Riely GJ, Miller VA, Sima CS, et al. Incidence of EGFR exon 19 deletions and L858R in tumor specimens from men and cigarette smokers with lung adenocarcinomas. J Clin Oncol. 2011;29:2066–70. doi: 10.1200/JCO.2010.32.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ettinger DS, Akerley W, Bepler G, Blum MG, Chang A, Cheney RT, et al. Non-small cell lung cancer. J Natl Compr Canc Netw. 2010;8:740–801. doi: 10.6004/jnccn.2010.0056. [DOI] [PubMed] [Google Scholar]
- 25.Azzoli CG, Temin S, Aliff T, Baker S, Jr, Brahmer J, Johnson DH, et al. American Society of Clinical Oncology.2011 Focused Update of 2009 American Society of Clinical Oncology Clinical Practice Guideline Update on Chemotherapy for Stage IV Non-Small-Cell Lung Cancer. J Clin Oncol. 2011;29:3825–31. doi: 10.1200/JCO.2010.34.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]


