Abstract
Background:
Chronic obstructive pulmonary disease (COPD) was ranked the sixth-most common cause of death worldwide in 1990, but now it is the third-most common cause. The goal of the present study was to assess the prevalence and determine the causes and risk factors of COPD in Tehran.
Materials and Methods:
This study followed a stratified cluster sampling strategy with proportional allocation within strata. The target population was all non-institutionalized inhabitants, aged 18 to 40 in one group and over 40 in another who resided in Tehran in 2013. The core questionnaire was developed from pre-existing validated questionnaires that had already been used in multi-national studies. The single most important outcome measure obtained as part of this protocol was spirometry before and after the administration of 200 mg (two puffs) of salbutamol.
Results:
The most commonly reported respiratory symptoms were: sputum production in 291 patients (16.2%) [95% confidence interval (CI): 14.5-17.9%], chronic cough in 171 (9.5%) (95% CI: 8.2-10.9%), wheezing in 377 (21.0%) (95%CI: 19.1-22.9%) and dyspnea in 388 patients (21.6%) (95% CI: 19.7-23.5%). The overall COPD prevalence defined by the post-bronchodilator spirometric functional criteria was 9.2%. This value in men (10.1%) was higher than in women (8.5%); the prevalence was significantly higher in subjects aged over 55 years (P ≤ 0.002). The prevalence of COPD was strongly dependent on smoking status, especially in ex-smokers, and increased considerably with age. 69% of patients with COPD were non-smoker.
Conclusion:
The high prevalence of verified COPD, a great deal of which was undiagnosed before by a physician, highlights the necessity of raising awareness of this disease among health professionals, and use of spirometry in the primary care setting. A future cross-sectional and prospective cohort study should be performed to explore all risk factors and their impact on decline in lung function and worsening of respiratory symptoms especially in non-smokers.
KEY WORDS: Chronic obstructive pulmonary disease, Iran, prevalence
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) was ranked as the sixth-most common cause of death worldwide in 1990, but is now the third-most common cause;[1] it has been ranked fourth in developed countries, with approximately 2.75 million deaths per year, or 4.8% of all deaths in these countries.[2]
It has been estimated that the global burden of chronic respiratory diseases (CRDs) will increase notably in the future, even though a lot of preventable CRDs can be controlled with adequate management in both developed and developing countries.[3] COPD is characterized by chronic inflammation and non-fully reversible airflow obstruction, involving structural changes in the lungs that can be demonstrated by a postbronchodilator FEV1/FVC ratio of 70% (“fixed ratio”).[4]
Tobacco smoking has proven to be the most important risk factor for development of COPD;[5] however, not all patients with COPD have a history of smoking. It seems that about 50% of cases worldwide are related to smoking,[6] and an estimated 10%-12% of individuals with COPD have never smoked.[7] In other words, non-fully reversible airflow obstruction also occurs in nonsmokers. Iran is a developing country where the smoking rate (14.8%) is lower than that observed in many developed countries.[8]
National estimates of COPD prevalence are usually based on self-reported questionnaires without objective measurement of lung function by spirometry testing.
One survey of COPD in Isfahan estimated the prevalence of 5.7%.[9] Several investigations, using spirometry, conducted in different countries have demonstrated the under-diagnosis of COPD. The most extreme example was observed in Japan, where the results of the 2004 population-based prevalence of COPD survey contrasts with the estimates of the Japanese Ministry of Health (10.9% vs. 0.3%, respectively).[10] Similar rates of under diagnosis have been frequently reported.[11]
Considering the impact of COPD, it seems that this chronic disease fails to receive sufficient attention from the healthcare community and governments and is rather unknown among the public. A great problem seems to be the lack of information about the prevalence and risk factors of COPD, especially in developing countries.
The estimation of COPD prevalence undoubtedly depends on the characteristics of screened population and criteria used to diagnose COPD, with continuing debate over the fixed spirometry ratio of 0.7 or lower limit of normal.[12]
Research on different aspects of COPD is an important health priority of academic institutions and governments, both for its high prevalence in different regions[13] and because this disease has proven to be potentially preventable[14] by cessation of smoking and air pollution control.
Although COPD is one of the leading causes of mortality and morbidity, epidemiological data on COPD are very limited in the Eastern Mediterranean Region, including Iran. The international, population-based Burden of Obstructive Lung Disease (BOLD) initiative was designed a decade ago to develop robust models that can be used to estimate the prevalence and current and future economic burden of COPD.[15]
The goal of our study was to describe the prevalence and determine the causes and risk factors of COPD in Tehran.
MATERIALS AND METHODS
Population and sampling strategy
The sample in this study was the population of Tehran. It was considered to be 8.1 million.
Sample size
Considering a design effect of 1.5, prevalence rate of 11% in previous studies, and a response rate of 60%, total sample size was calculated to be 750.
Sampling plan
This study followed a stratified cluster sampling strategy with proportional allocation within strata. The target population was all non-institutionalized inhabitants, aged 18-40 in one group and over 40 in another, who resided in Tehran in 2013.
The stratification of the sample was based upon the 22 municipal districts of Tehran. Proportional to the number of households in the 22 districts, the appropriate number of clusters was weighted according to each district. The number of clusters was based on total sample size, mean household members, and logistical facilities for subject enumeration, transport, and examination.
For each cluster, a team of three members (one male and one female aged less than 28 as interviewers) approached the index household, specified through the aforementioned random selection of clusters, and continued the enumeration in 10 neighboring households in a systematic manner. In the indexed household if there was more than one person, interviewers were advised to use the Kish method to choose the right participant(s). This method is a table of numbers, and is named according to the statistician who invented it (the number of people in the household is discovered, and a random number is chosen to select a particular person).
Examination protocol
The examination protocol included a questionnaire covering respiratory symptoms, health status, activity limitation, and exposure to potential risk factors, such as tobacco smoke, occupational risk factors, and biomass exposure. Subjects also underwent pre- and post-bronchodilator spirometry tests. Spirometry records provide the 1-second and 6-second forced expiratory volumes (FEV1 and FEV6) and the forced vital capacity (FVC).
Questionnaires
The core questionnaire was developed from pre-existing validated questionnaires that had already been used in multi-national studies.[5] The questionnaire contained information about respiratory symptoms, exposure to potential risk factors such as smoking, occupation, respiratory diseases, co-morbidities, healthcare provisions, medication use, activity limitation, and health status.
Participants also completed an occupational questionnaire and a “stages of change” questionnaire assessed readiness of current cigarette smokers to quit smoking. There was also a questionnaire to assess exposure to biomass fuels used at home for either heating or cooking. All questionnaires were translated to Persian first and then back translated to English by a different translator. Trained certified staffs administered the questionnaires; self administration of questionnaires was not allowed.
Spirometry
The single most important outcome measure obtained as part of this protocol was spirometry results before and after the administration of 200 mg (2 puffs) of salbutamol. Our diagnosis of COPD was based on documentation of FEV1/FVC below the fixed ratio of 0.7. To optimize quality control in this study, all teams were required to use the 2120 In2itive Vitalograph Spirometer. This particular device was chosen because of its accuracy, robustness, portability, and ease of storage. Also, it can be used easily in the field and where there is no electric power. The 2120 In2itive Vitalograph Spirometer has been approved by the National Research Institute of Tuberculosis and Lung Disease to meet the predetermined performance criteria in terms of the reliability of measurement, suitability for field use, and easy access to data.
COPD was defined using the Global Initiative for Obstructive Lung Disease definition of post-bronchodilator FEV1/FVC of 0.7. Identification of symptomatic patients was based on clinical diagnosis of dyspnea, chronic cough or sputum production.[5]
Statistical analysis
In calculating standard errors and the 95% confidence interval for categorical and continuous variables, the cluster sampling design was taken into account and adjusted. In addition to descriptive analyses, odds ratios were calculated with multivariate logistic regression in order to control potential confounding variables, and account for cluster design effects.
RESULTS
Participants
A total of 1,798 individuals were visited in 22 districts of Tehran; 811 (45.1%) men and 987 (54.9%) women participated in the structured interviews. These districts were located in the north, south, west, east, and center of Tehran. Table 1 shows the demographic characteristics of subjects.
Table 1.
Demographic characteristics
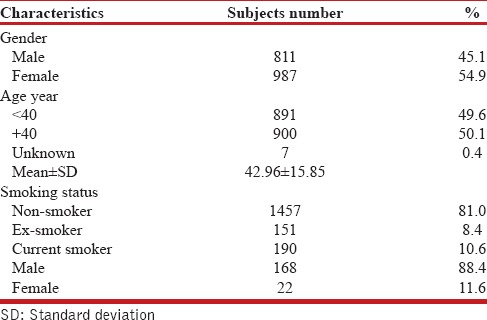
Smoking habits
A history of current or past smoking was more frequent in men than women. Of all participants, 190 (10.6%) were smokers; out of which, 168 (88.4%) were men and 22 (11.6%) were women; 151 (8.4%) were ex-smokers [Table 1].
Amongst subjects with COPD according to the spirometry tests, 9 (12.6%) were smokers, 13 (18.3%) were ex-smokers, and 49 (69%) were non-smokers.
Respiratory symptoms in subjects
The most commonly reported respiratory symptoms were: Sputum production in 291 patients (16.2%) (95% CI: 14.5-17.9%), chronic cough in 171 (9.5%) (95% CI: 8.2-10.9%), wheezing in 377 (21.0%) (95% CI: 19.1-22.9%) and dyspnea in 388 patients (21.6%) (95% CI: 19.7-23.5%). Of patients over 40 years, 337 men and 438 women underwent spirometry measurement and 125 participants did not meet spirometry criteria [Table 2]. The overall COPD prevalence defined by the post-bronchodilator spirometric functional criteria was 9.2%, higher in men (10.1%) than in women (8.5%); the prevalence was significantly higher in subjects aged over 55 years [P ≤ 0.002, Tables 2 and 3, Figure 1].
Table 2.
COPD prevalence according to age, gender, and COPD definition criteria*
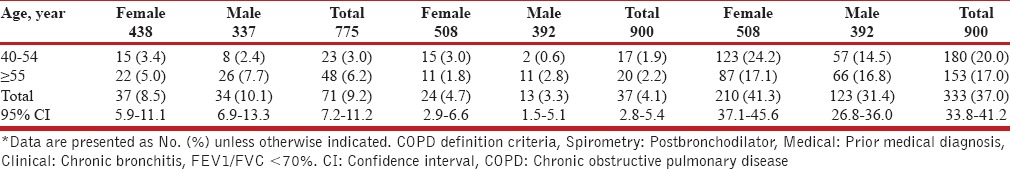
Table 3.
COPD distribution according to sociodemographic and disease characteristics
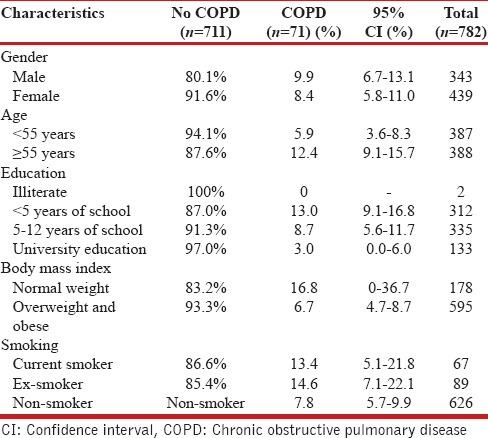
Figure 1.
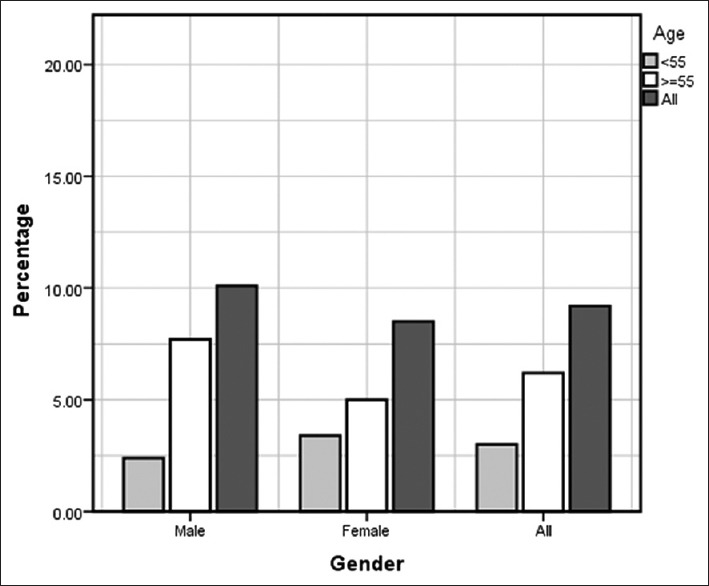
Chronic obstructive pulmonary disease (COPD) prevalence by gender
The prevalence of self-reported chronic bronchitis, emphysema or COPD in all participants over 40- years was 333 (37%). This value is four times more than the estimated prevalence of GOLD stage I or higher COPD in our study. The prevalence of prior medical diagnosis among all participants over 40-years was 37 (4.1%). In both cases, women were higher compared to men [Table 2].
Multivariate relationships
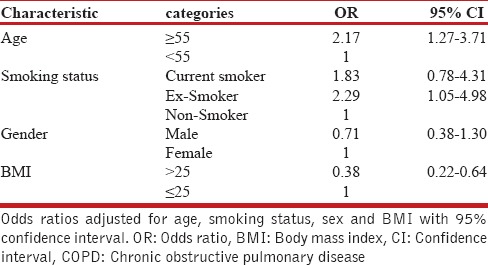
We performed univariate and multivariate logistic regression to assess the association of COPD and risk factors. In univariate logistic regression, the odds ratio (OR) for gender was 1.195 (95% CI: 0.733-1.949), and after including age, gender, smoking habits, family history of obstructive airway disease, and socio-economic group in the model, and after mutual adjustment for all these potential factors in the model, we found that age and smoking were the two major risk factors in our understudy population. The prevalence of COPD was strongly dependent on smoking status, especially in ex-smokers, and increased considerably with age [Table 4].
Table 4.
The odds ratio for having COPD (according to spirometry results)
DISCUSSION
In this study, we estimated the prevalence of COPD using questionnaires and spirometry of people aged 18 years and older living in Tehran. The sample in this study was representative of Tehran's adult population and the participation rates were exceptionally high for a comprehensive survey like this one. The key finding of this prevalence survey was that 9.2% of the residents of the capital of Iran, aged 40 years or older, had at least Stage I COPD, and this was more common in men than in women. This finding is similar to the results of studies in many other countries using the same BOLD methodology. COPD prevalence in Tehran is within the range found in other Asian countries like China (Guangzhou), and Philippines (Manila), but is lower than the observed prevalence in South Africa (Cape Town), Australia (Sydney), and Turkey (Adana).[16] These differences could be attributed to different rates of smoking habits in these regions, or possibly other risk factors, which have not been assessed yet and need further investigations (e.g., air pollution, district, biomass fuel, etc.). Moreover, similar findings were obtained in many other previous epidemiological studies.[17,18]
According to a study done in Isfahan, another mega city in Iran, the prevalence of airway limitation in the general population 40 years and older was 5.7%.[9] However, the above-mentioned studies had a different methodology and there seems to be a lack of relative longitudinal studies, conducted locally in Iran; whereas in other countries, researchers like Vasankari et al. compared the prevalence of COPD throughout a decade and found no increase in the prevalence trend in Finland.[19] Soriano et al. found an unexpected decrease in COPD prevalence in Spain.[20] The data from our present study will serve as a baseline for future studies on COPD.
The higher percentage of women in the study (54.9%) cannot be explained by the composition of the population in Iran, which is nearly equal between genders[21] but, mainly, by the cultural trait that women spend more time at home than men. In this study, the COPD prevalence in women was lower than that in men. This could be due to the fact that Iranian women are less likely to smoke. This situation proved to be contrary to some developed countries, where the prevalence of smoking in women is higher than men and the same for COPD.[17] There has been considerable controversy as to whether women are at equal or perhaps at greater risk than men given an equal exposure. This controversy has not been resolved, although there is increasing evidence that women may be more vulnerable.[22] In developing countries, the increase in smoking among women, that is likely to occur, will probably lead to a tidal wave of COPD as women have more exposure and live longer. Women are also more likely than men to be exposed to high indoor air pollution levels in developing countries. Fossil fuel pollution has been found to have a greater effect on women compared to men.[23]
It had been difficult for a long time to estimate the COPD prevalence,[5] and there has been an ongoing discussion about how to improve early detection of COPD. In this report, we found a low prevalence (4.1%) of previously diagnosed COPD, and a high prevalence of self-reporting. The stepwise and slow progressive nature of COPD means that most sufferers tend not to complain of respiratory symptoms and consequently the disease usually remains undetected by health providers for many years. Moreover, an insufficient distribution of spirometers in the clinics may influence the prevalence of undiagnosed COPD cases. Another reason for the high rate of undiagnosed patients seems to be physicians’ and patients’ attitudes. Studies have shown that under-diagnosis of COPD has been raised partly due to “physicians’ delay”, i.e., doctors who do not suspect underlying COPD[24] and partly because of “patients’ delay”, i.e., patients who self-report good health.[25] However, the early stages of COPD are difficult to diagnose without spirometry. Actually, spirometry is not widely used among Iranian general physicians. This study suggests that general clinics must be equipped with spirometers in order to reduce the number of undiagnosed COPD cases. This is particularly relevant when evaluating the respiratory function of individuals with clinical signs of airflow obstruction.
It seems that primary screening for airflow obstruction with a handheld spirometer prior to full spirometry testing, which is easy to carry-out in all settings, could be considered as an efficient way to screen or find new COPD cases.[26]
In this study, we found that the prevalence of airflow limitation was higher in individuals who had ever smoked, which is similar to previous reports.[27] The odds of COPD were higher for ex-smokers. These figures suggest that approximately 25% of never-in-life smokers may develop COPD; although this figure has been reported from 15% to 50% in the literature.[17,28] Early detection of COPD seems to be important for early smoking cessation intervention and the administration of beta-agonists and inhaled corticosteroids.
Since this was a cross-sectional study, we were not able to assess the outcomes of COPD. However, the prevalence of COPD in non-smokers was 7.8%, which is similar to other countries in similar studies[28,29] and suggests that factors other than smoking exposure might also be involved in COPD.
However, it is surprising that in this study, a majority of patients with COPD were never smoker. The first reason could be that the subjects in our study were from general population, and were likely to have better lung function and fewer symptoms than a hospitalized COPD population. The second reason could be explained by available epidemiologic data which demonstrate that domestic biomass, fuel smoke, indoor air pollution, poor economic and social status, and existence of asthma might be important causes of COPD in never smokers.[30,31]
A future cross-sectional and prospective cohort study should be performed to explore these risk factors and their impact on decline in lung function and worsening of respiratory symptoms especially in non-smokers.
The present study may be subject to several biases: Although a weighting procedure was performed to make the sample as representative as possible, there was still a possibility of a selection bias because there was no means to evaluate the reasons of rejection, which could have led to an overestimation or underestimation of COPD prevalence. The authors of this study guess that refusals were linked to cultural traits, lack of motivation, illiteracy, or maybe chronic conditions that individuals did not want to disclose (cancer or other diseases). There is also a possibility of information bias in this study; as in all studies requiring questionnaires, relying on individuals reporting for different variables may include a recall bias (previous exposure data for example) or subjectivity bias (report of weight, height and symptoms for example). The main strength of this survey was the use of BOLD protocol, with a precise methodology to attain the upmost accuracy of the survey and a high-quality post-bronchodilator spirometry. This methodology ensured that data were as easy to compare as possible with other BOLD studies.[15] However, the methods used in the study are the ones that are currently being used in a large number of countries all over the world for this kind of study. As far as we realize, PREPOCOL study is the first population-based study done in South America and PLATINO is the second in Latin America, which used a spirometric definition (post-bronchodilator FEV1/FVC ratio of 70%) as the primary standard for determining the COPD prevalence. Although the use of a fixed ratio may underestimate the prevalence of airway obstruction in young and overestimate it in older individuals, we decided on BOLD method to facilitate the comparison of the prevalence with that determined by other studies.[5,29]
CONCLUSIONS
The 9.2% prevalence of COPD indicates that this disease is common in Tehran. The high prevalence of verified COPD, a great deal of which was undiagnosed before by a physician, provokes the necessity of raising awareness of this disease among health professionals, and the necessity to use spirometry in the primary care setting.
Prevention programs including smoking cessation interventions must be implemented to reduce prevalence, morbidity, and mortality of COPD. Thus, it is crucial for physicians to identify the high-risk group and offer them spirometry testing for early detection of COPD, which may motivate them to quit smoking.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raherison C, Girodet PO. Epidemiology of COPD. Eur Respir Rev. 2009;18:213–21. doi: 10.1183/09059180.00003609. [DOI] [PubMed] [Google Scholar]
- 3.Haahtela T, Tuomisto LE, Pietinalho A, Klaukka T, Erhola M, Kaila M, et al. A 10 year asthma programme in Finland: Major change for the better. Thorax. 2006;61:663–70. doi: 10.1136/thx.2005.055699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balkissoon R, Lommatzsch S, Carolan B, Make B. Chronic obstructive pulmonary disease: A concise review. Med Clin North Am. 2011;95:1125–41. doi: 10.1016/j.mcna.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 5.Global Initiative for Chronic Obstructive Lung Disease Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease. 2013. [Last accessed on 2013 Jul 20]. Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2013_Feb20.pdf .
- 6.Eisner MD, Anthonisen N, Coultas D, Kuenzli N, Perez-Padilla R, Postma D, et al. ; Committee on Nonsmoking COPD, Environmental and Occupational Health Assembly. An official American Thoracic Society public policy statement: Novel risk factors and the global burden of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;182:693–718. doi: 10.1164/rccm.200811-1757ST. [DOI] [PubMed] [Google Scholar]
- 7.Lamprecht B, McBurnie MA, Vollmer WM, Gudmundsson G, Welte T, Nizankowska-Mogilnicka E, et al. ; BOLD Collaborative Research Group. COPD in never smokers: Results from the population-based burden of obstructive lung disease study. Chest. 2011;139:752–63. doi: 10.1378/chest.10-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hooman S, Zahra H, Safa M, Hassan FM, Reza MM. Association between cigarette smoking and suicide in psychiatric inpatients. Tob Induc Dis. 2013;11:5. doi: 10.1186/1617-9625-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amra B, Golshan M, Fietze I, Penzel T, Welte T. Correlation between chronic obstructive pulmonary disease and obstructive sleep apnea syndrome in a general population in Iran. J Res Med Sci. 2011;16:885–9. [PMC free article] [PubMed] [Google Scholar]
- 10.Fukuchi Y, Nishimura M, Ichinose M, Adachi M, Nagai A, Kuriyama T, et al. COPD in Japan: The Nippon COPD epidemiology study. Respirology. 2004;9:458–65. doi: 10.1111/j.1440-1843.2004.00637.x. [DOI] [PubMed] [Google Scholar]
- 11.Schirnhofer L, Lamprecht B, Vollmer WM, Allison MJ, Studnicka M, Jensen RL, et al. COPD prevalence in Salzburg, Austria: Results from the burden of obstructive lung disease (BOLD) study. Chest. 2007;131:29–36. doi: 10.1378/chest.06-0365. [DOI] [PubMed] [Google Scholar]
- 12.Vollmer WM, Gislason T, Burney P, Enright PL, Gulsvik A, Kocabas A, et al. Comparison of spirometry criteria for the diagnosis of COPD: Results from the BOLD study. Eur Respir J. 2009;34:588–97. doi: 10.1183/09031936.00164608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gingter C, Wilm S, Abholz HH. Is COPD a rare disease? Prevalence and identification rates in smokers aged 40 years and over within general practice in Germany. Fam Pract. 2009;26:3–9. doi: 10.1093/fampra/cmn084. [DOI] [PubMed] [Google Scholar]
- 14.Gershon AS, Wang C, Guan J, Vasilevska-Ristovska J, Cicutto L, To T. Identifying individuals with physcian diagnosed COPD in health administrative databases. COPD. 2009;6:388–94. doi: 10.1080/15412550903140865. [DOI] [PubMed] [Google Scholar]
- 15.Buist AS, Vollmer WM, Sullivan SD, Weiss KB, Lee TA, Menezes AM, et al. The burden of obstructive lung disease initiative (BOLD): Rationale and design. COPD. 2005;2:277–83. [PubMed] [Google Scholar]
- 16.Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, et al. ; BOLD Collaborative Research Group. International variation in the prevalence of COPD (The BOLD Study): A population-based prevalence study. Lancet. 2007;370:741–50. doi: 10.1016/S0140-6736(07)61377-4. [DOI] [PubMed] [Google Scholar]
- 17.Lundbäck B, Lindberg A, Lindström M, Rönmark E, Jonsson AC, Jönsson E, et al. Obstructive Lung Disease in Northern Sweden Studies? Not 15 but 50% of smokers develop COPD? - Report from the Obstructive Lung Disease in Northern Sweden Studies. Respir Med. 2003;97:115–22. doi: 10.1053/rmed.2003.1446. [DOI] [PubMed] [Google Scholar]
- 18.Tzanakis N, Anagnostopoulou U, Filaditaki V, Christaki P, Siafakas N COPD group of the Hellenic Thoracic Society. Prevalence of COPD in Greece. Chest. 2004;125:892–900. doi: 10.1378/chest.125.3.892. [DOI] [PubMed] [Google Scholar]
- 19.Vasankari TM, Impivaara O, Heliövaara M, Heistaro S, Liippo K, Puukka P, et al. No increase in the prevalence of COPD in two decades. Eur Respir J. 2010;36:766–73. doi: 10.1183/09031936.00178109. [DOI] [PubMed] [Google Scholar]
- 20.Soriano JB, Ancochea J, Miravitlles M, García-Río F, Duran-Tauleria E, Muñoz L, et al. Recent trends in COPD prevalence in Spain: A repeated cross-sectional survey 1997-2007. Eur Respir J. 2010;36:758–65. doi: 10.1183/09031936.00138409. [DOI] [PubMed] [Google Scholar]
- 21.The President's Office Deputy of Strategic Planning and Control Statistical Center of Iran. National Population and Housing Census 2011. Available from: http://iran.unfpa.org/Documents/Census2011/2011 CensusSelected Results - Eng.pdf .
- 22.Feenstra TL, van Genugten ML, Hoogenveen RT, Wouters EF, Rutten-van Mölken MP. The impact of aging and smoking on the future burden of chronic obstructive pulmonary disease: A model analysis in the Netherlands. Am J Respir Crit Care Med. 2001;164:590–6. doi: 10.1164/ajrccm.164.4.2003167. [DOI] [PubMed] [Google Scholar]
- 23.Varkey AB. Chronic obstructive pulmonary disease in women: Exploring gender differences. Curr Opin Pulm Med. 2004;10:98–103. doi: 10.1097/00063198-200403000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Albers M, Schermer T, Molema J, Kloek C, Akkermans R, Heijdra Y, et al. Do family physicians’ records fit guideline diagnosed COPD? Fam Pract. 2009;26:81–7. doi: 10.1093/fampra/cmp005. [DOI] [PubMed] [Google Scholar]
- 25.Hvidsten SC, Storesund L, Wentzel-Larsen T, Gulsvik A, Lehmann S. Prevalence and predictors of undiagnosed chronic obstructive pulmonary disease in a Norwegian adult general population. Clin Respir J. 2010;4:13–21. doi: 10.1111/j.1752-699X.2009.00137.x. [DOI] [PubMed] [Google Scholar]
- 26.Robalo Cordeiro C, Singh S, Herth FJ, Ley S, Chavannes NH, Clini E, et al. Selected clinical highlights from the 2010 ERS Congress in Barcelona. Eur Respir J. 2011;38:209–17. doi: 10.1183/09031936.00039011. [DOI] [PubMed] [Google Scholar]
- 27.Mannino DM, Gagnon RC, Petty TL, Lydick E. Obstructive lung disease and low lung function in adults in the United States: Data from the National Health and Nutrition Examination Survey, 1988-1994. Arch Intern Med. 2000;160:1683–9. doi: 10.1001/archinte.160.11.1683. [DOI] [PubMed] [Google Scholar]
- 28.Rennard SI, Vestbo J. Natural histories of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5:878–83. doi: 10.1513/pats.200804-035QC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferguson GT, Enright PL, Buist AS, Higgins MW. Office Spirometry for lung health assessment in adults: A consensus statement from the National Lung Health Education Program. Chest. 2000;117:1146–61. doi: 10.1378/chest.117.4.1146. [DOI] [PubMed] [Google Scholar]
- 30.Fang X, Wang X, Bai C. COPD in China: The burden and importance of proper management. Chest. 2011;139:920–9. doi: 10.1378/chest.10-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J, Ou JX, Bai CX. Tobacco smoking in China: Prevalence, disease burden, challenges and future strategies. Respirology. 2011;16:1165–72. doi: 10.1111/j.1440-1843.2011.02062.x. [DOI] [PubMed] [Google Scholar]


