Abstract
Rituximab is a chimeric monoclonal antibody that targets CD-20 antigen expressed in more than 90% of all B cell non-Hodgkin's lymphoma (NHL). We report a case of 33-year-old female without any comorbidities, newly diagnosed with stage IIIB follicular lymphoma treated with rituximab-based chemotherapy. Patient developed exertional dyspnea and dry cough after the fourth cycle of rituximab-based chemotherapy. Diagnostic high-resolution computed tomography (HRCT) of the lungs revealed bilateral patchy ground glass opacities suggestive of interstitial lung disease (ILD). It was managed successfully with supplemental oxygen and corticosteroids with discontinuation of the Rituximab. Extensive review of the literature did not reveal ample of material on rituximab-induced ILD (RTX-ILD).
KEY WORDS: Follicular lymphoma, interstitial lung disease, non-Hodgkin's lymphoma, rituximab
INTRODUCTION
Rituximab is a chimeric monoclonal antibody that targets CD-20 antigen expressed in more than 90% of all B cell NHL. It is approved by the US Food and Drug Administration for the treatment of CD20 positive low-grade NHL including follicular lymphomas (FL). The use of rituximab-based chemotherapy regimens in previously untreated advanced stage FL is associated with increased overall response rate (ORR), response duration, progression-free survival (PFS) and overall survival (OS).[1] Most common adverse reactions seen with use of rituximab are infusion reactions, tumor lysis syndrome, and adverse skin reactions. A spectrum of extremely rare but potentially lethal rituximab-induced lung diseases including acute respiratory distress syndrome, cryptogenic organizing pneumonia, interstitial lung disease, alveolar hemorrhage has been described in the recent literature mostly in case reports.[2] Herein, we report a case of RTX-ILD in a young female patient with FL. This is one of the very few cases reported from India.
CASE REPORT
A 33-year-old non-smoker female patient presented with generalized lymphadenopathy involving neck, axilla, abdomen and inguinal region. She had a history suggestive of B symptoms but there was no prior history of diabetes mellitus, dyslipidemia or occupational exposure to any kind of dusts. Her height, weight, and body surface area (BSA) were 170 cm, 67 kg, and 1.78, respectively. Routine blood tests including complete blood count, renal and liver function tests were within normal limits. Excisional lymph node biopsy showed infiltration by small, medium-sized lymphoid cells which appear to be forming follicles. Immunohistochemistry markers were positive for CD-10, CD-20, Bcl-2, Bcl-6 and Ki-67 proliferation index was 22%. Bone marrow was uninvolved. These findings were consistent with the diagnosis of stage IIIB FL. She was treated with R-CHOP 21 regimen consisting of three weekly rituximab (375 mg/m2 of BSA), cyclophosphamide (750 mg/m2 of BSA), doxorubicin (50 mg/m2 of BSA), vincristine (1.4 mg/m2 of BSA), and prednisolone (100 mg daily). After the fourth R-CHOP chemotherapy, she was admitted with the chief complaints of exertional dyspnea and dry cough. There was no history of fever. Physical examination showed tachypnea and bilateral basal crackles. Arterial blood gas analysis revealed hypoxemia. Her routine blood counts, liver function test, renal function test, urine routine/microscopy and D-dimer were normal. The ECG showed sinus tachycardia. A chest X-ray showed bilateral reticulo-nodular infiltrates [Figure 1] and HRCT scan of the lung revealed bilateral patchy ground-glass opacities, suggestive of interstitial lung disease (ILD) [Figure 2]. The pulmonary function testing (PFT) showed a restrictive pattern [Table 1]. As it is quite difficult to rule out other causes of ILD in cancer patients, various microbiological tests were carried out to rule out respiratory tract infections. Sputum examination for Gram stain, Zn stain for acid-fast bacilli as well as for fungi and Pneumocystis carinii did not reveal any abnormality. Routine bacterial, mycobacterial and fungal cultures were sterile. Antinuclear antibody (ANA) and antineutrophil cytoplasmic antibody (ANCA) were negative. BAL fluid cytology was negative for malignant cells. Pulmonary eosinophilia was excluded based on symptomatology and laboratory diagnosis. Patient did not have symptoms like persistent or recurrent cough aggravated at night, weight loss, low grade fever; she was a resident of non-endemic area for filariasis, there was no peripheral eosinophilia, and peripheral blood did not show any presence of abnormal organism. Hence we did not consider lung biopsy for the same.
Figure 1.
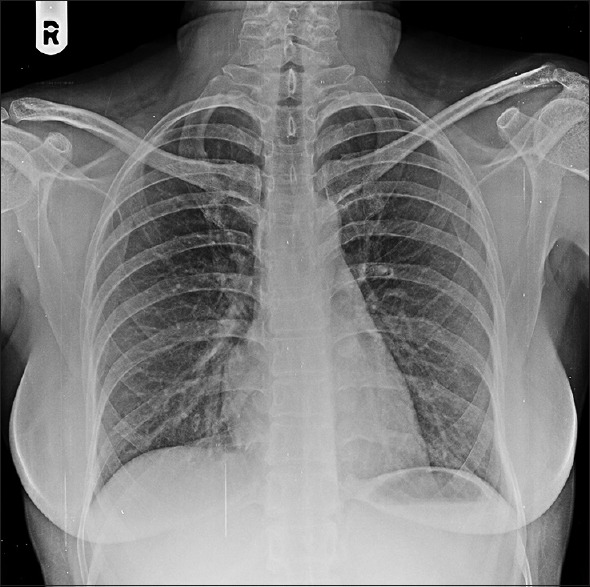
Chest X-ray PA view showing bilateral reticulo-nodular infiltrates
Figure 2.
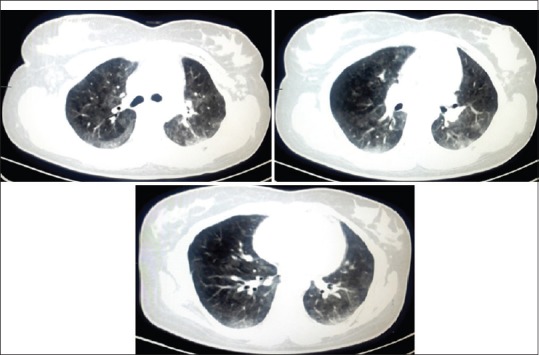
High-resolution computed tomography (HRCT) scan of the lung: On axial lung window images, there are patchy ground glass opacities noted in bilateral lung fields, more in upper lobes
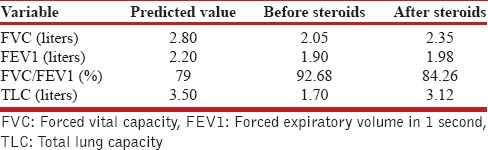
Table 1.
PFT results before and after steroid treatment
Extrapulmonary symptoms like the skin, joint and eye findings were absent. There was no bilateral hilar adenopathy on chest radiography. There was no anemia, elevated liver enzymes, hypercalciuria. There were no granulomatous lesions on imaging. Thus sarcoidosis was ruled out.
Echocardiography was normal including the ventricular functions. Depending on the development of new and progressive pulmonary infiltrates in a previously normal lung a diagnosis of R-ILD was made.
Being hypoxemic at rest, patient was immediately treated with moist O2 inhalation along with intravenous methyl prednisolone (1 g daily for 3 days) in an intensive care unit. She had dramatic symptomatic improvement, so was switched to oral prednisolone at a dose of 1 mg/kg body weight for the first 2 weeks followed by gradual taper by 0.25 mg/kg body weight every two weekly. On follow-up, chest was clear on auscultation, imaging modalities showed near total resolution of ground glass opacities [Figure 3] and pulmonary function tests were within normal limits. Because of limited safety data and concern regarding possibility of severe second reaction as compared to first one, our patient was not re-challenged with rituximab. She was further treated with two cycles of CHOP regimen without any evidence of further lung injury during the treatment as well as follow-up. Currently the patient is asymptomatic for her FL, ILD and is on regular follow-up.
Figure 3.
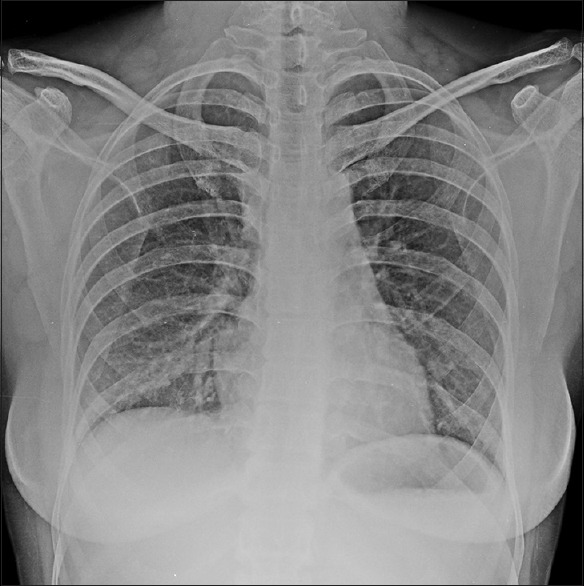
Chest X-ray PA view after treatment with steroids showing near normal resolution of reticulo-nodular infiltrates
DISCUSSION
Rituximab is a chimeric monoclonal antibody that targets CD-20 antigen that is expressed on more than 90% of all B cell NHL but not expressed on early pre-B cells, plasma cells, and normal bone marrow stem cells. Rituximab, the very treatment evolution of hematological malignancies was approved by the US Food and Drug Administration in 1997 for the treatment of low-grade non-Hodgkin's lymphoma expressing CD20, follicular lymphomas and chronic lymphocytic leukemia, and subsequently diffuse large B-cell NHL. Besides it is also used in other non-hematological and autoimmune conditions like steroid-resistant ITP, rheumatoid arthritis and SLE.
FL is the most common subtype of indolent NHL, and accounts for about 22% of all newly diagnosed cases of NHL. A multicentre phase II trial demonstrated the safety and efficacy of R-CHOP chemotherapy in relapsed and newly diagnosed indolent NHL. The superiority of R-CHOP to CHOP as first line therapy was established in a prospective randomized phase III study conducted by German Low Grade Lymphoma Study Group (GLSG) in previously untreated advanced stage FL patients. The addition of rituximab to combination chemotherapy regimens has been associated with increased overall response rate (ORR), response duration, progression-free survival (PFS) and overall survival (OS) benefit.[1] Single agent rituximab in treatment-naive patients with FL has yielded an ORR of 72-73%, with a median time to disease progression around 2 years. PRIMA study,[3] and several other studies supports the use of maintenance Rituximab, with an improvement in PFS and an OS in a pooled analysis.[4] Besides an effective therapy, rituximab has a favorable toxicity profile even in elderly patients.
Most common adverse reactions noted with rituximab are infusion-related symptoms, including fever, chills, urticaria, flushing, headache, bronchospasm, dyspnea, angioedema, hypotension usually occurring within 30 minutes to 2 hours after the start of the infusion that usually resolves upon slowing or interrupting the infusion and incidence decreases with subsequent infusion. Other adverse reactions include tumor lysis syndrome; skin reactions like pemphigus, Stevens Johnson syndrome; infusional arrhythmias and chest pain; rarely myelosuppression.
Rare respiratory side effects, mostly described in recent literature include transient respiratory symptoms as part of an infusion-related reaction, hypersensitivity pneumonitis, interstitial pneumonitis, organizing pneumonia, pulmonary fibrosis, and alveolar hemorrhage.[5]
The incidence of rituximab-induced lung injury is currently less than 0.03 percent.[6] Median age of diagnosis RTX-ILD is 65 years (range from 43 to 80 years) with male to female ratio of 2:1 and the elderly individuals appear to be at increased risk. Case fatality rate is around 20%, more in extremes of age. Need for mechanical ventilation is associated with poor prognosis. Pathogenesis of RTX-ILD is less completely understood. Few theories that have been proposed are Nod-like receptor pyrin domain-containing protein 3 (NLRP3) inflammasome-activated lung injury and dysregulated cellular cytotoxicity. RTX-ILD is a diagnosis of exclusion. Important differential diagnosis includes infection and disease progression where bronchoscopy-guided BAL fluid cytology and/or lung biopsy is necessary. If the clinical, radiological or pulmonary function test findings are equivocal, a lung biopsy can be helpful to verify the diagnosis. As the patient was hypoxemic at rest, she was immediately shifted to an intensive care unit. Worsened symptomatology and hemodynamic instability did not allow us to attempt a lung biopsy. Like our patients scenario, diagnosis can reasonably be based upon compatible clinical features and typical HRCT appearances and, in this context; a surgical biopsy adds no useful information. This was another reason for not considering lung biopsy. BAL was done which turned out to be negative.
18FDG-PET appears to be a sensitive imaging modality to detect RTX-ILD at an early stage. Time interval between diagnosis by 18FDG-PET and first appearance of symptom of RTX-ILD may be up to 3 months. The limitation is that it has no specificity for RTX-ILD.[7]
Modified ATS/ERS criteria[8] was used for diagnosis in the absence of surgical lung biopsy. Our patient meets all the major criteria like exclusion of known causes, restrictive pulmonary function tests, typical HRCT findings, negative BAL to exclude alternative diagnosis and most of the minor criteria as well such as bibasal velcro crepitations, insidious onset of symptoms.
In an attempt to prevent RTX-ILD, patient is premedicated with acetaminophen (paracetamol) 1 g, an antihistamine ranitidine 50 mg and methylprednisolone 100 mg. Rituximab infusion should be started 30 min after the end of the methylprednisolone infusion. Risk of the rituximab-induced lung injury is greatest on day 15 and around cycle 4.
In a study by Liote et al.,[9] mean time from the first rituximab infusion to onset of the respiratory manifestations was 3 months, with a peak at the fourth cycle and a mean cumulative dosage of 1,600 mg/m2. Our patient developed RTX-ILD after completion of 4th cycle of rituximab, result similar to study done by Andreas et al.[7] in which median duration was four cycles of rituximab and average cumulative dose before the onset of symptoms was 1500 mg/m2.
After the clinical and radiological resolution, rituximab may be rechallenged under the cover of prophylactic steroids (0.5 mg/kg body weight) to be continued for at least 4 weeks after the rituximab infusion. Although cyclophosphamide and vincristine may cause drug-induced lung injury, our patient was treated with only CHOP regimen afterwards without any additional lung injury which is an indirect evidence of rechallenge with these two drugs that turned to be negative. In addition, Liu et al. reported that among 107 patients treated with R-CHOP regimen, nine patients developed ILD when compared to none out of the 66 patients treated with CHOP alone.[10] So, we may infer that ILD was attributed to use of rituximab.
The following recommendations were put forward by Liote et al.[9] in order to prevent and/or manage RTX-ILD:
Patients detailed counseling regarding the risk of respiratory symptoms that may develop, being greatest 15 days after the last infusion and around the fourth cycle
Monitoring of respiratory symptoms during and after each infusion
Immediate assessment by chest radiography how mildly symptomatic the patient may be
Prompt initiation of steroid therapy with the first suspicion of rituximab-induced lung disease.
CONCLUSIONS
To conclude, despite being difficult to diagnose, prompt recognition and treatment of a rare but potentially fatal rituximab-induced interstitial lung disease in a patient presenting with respiratory symptoms and/or new onset pulmonary infiltrates is of utmost importance due to increasing use of the drug for treatment of variety of hematological and non-hematological disorders.
ACKNOWLEDGEMENTS
I would like to acknowledge the contribution of Dr. Malay Sarkar (Department of Pulmonary Medicine, Indira Gandhi Medical College, Shimla, Himachal Pradesh, India) and Dr. Irappa Madabhavi (Department of Medical and Pediatric Oncology, Gujarat Cancer Research Institute, Ahmedabad, Gujarat) for there continuous, relentless support in preparation of this manuscript.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Hiddemann W, Kneba M, Dreyling M, Schmitz N, Lengfelder E, Schmits R, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: Results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005;106:3725–32. doi: 10.1182/blood-2005-01-0016. [DOI] [PubMed] [Google Scholar]
- 2.González V, Salgueiro E, Jimeno FJ, Hidalgo A, Rubio T, Manso G. Post-marketing safety of antineoplasic monoclonal antibodies: Rituximab and trastuzumab. Pharmacoepidemiol Drug Saf. 2008;17:714–21. doi: 10.1002/pds.1587. [DOI] [PubMed] [Google Scholar]
- 3.Salles G, Seymour JF, Offner F, López-Guillermo A, Belada D, Xerri L, et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): A phase 3, randomized controlled trial. Lancet. 2011;377:42–51. doi: 10.1016/S0140-6736(10)62175-7. [DOI] [PubMed] [Google Scholar]
- 4.Vidal L, Gafter-Gvili A, Leibovici L, Dreyling M, Ghielmini M, Hsu Schmitz SF, et al. Rituximab maintenance for the treatment of patients with follicular lymphoma: Systematic review and meta-analysis of randomized trials. J Natl Cancer Inst. 2009;101:248–55. doi: 10.1093/jnci/djn478. [DOI] [PubMed] [Google Scholar]
- 5.Krishnaswamy UM, Maka VV, Subramanian M, Chitrapur R, Kilara N. Rituximab induced interstitial lung disease in patients with non-hodgkin's lymphoma: A clinical study of six cases and review of the literature. Lymphoma. 2014;2014:1–6. [Google Scholar]
- 6.Burton C, Kaczmarski R, Jan-Mohamed R. Interstitial pneumonitis related to rituximab therapy. N Engl J Med. 2003;348:2690–1. doi: 10.1056/NEJM200306263482619. [DOI] [PubMed] [Google Scholar]
- 7.Hadjinicolaou AV, Nisar MK, Parfrey H, Chilvers ER, Ostör AJ. Non-infectious pulmonary toxicity of rituximab: A systematic review. Rheumatology (Oxford) 2012;51:653–62. doi: 10.1093/rheumatology/ker290. [DOI] [PubMed] [Google Scholar]
- 8.Bradley B, Branley HM, Egan JJ, Greaves MS, Hansell DM, Harrison NK, et al. ; British Thoracic Society Interstitial Lung Disease Guideline Group, British Thoracic Society Standards of Care Committee; Thoracic Societyof Australia; New Zealand Thoracic Society; Irish Thoracic Society. Interstitial lung disease guideline: The British Thoracic Society in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic Society. Thorax. 2008;63(Suppl 5):v1–58. doi: 10.1136/thx.2008.101691. [DOI] [PubMed] [Google Scholar]
- 9.Lioté H, Lioté F, Séroussi B, Mayaud C, Cadranel J. Rituximab induced lung disease: A systematic literature review. Eur Respir J. 2010;35:681–7. doi: 10.1183/09031936.00080209. [DOI] [PubMed] [Google Scholar]
- 10.Liu X, Hong XN, Gu YJ, Wang BY, Luo ZG, Cao J. Interstitial pneumonitis during rituximab-containing chemotherapy for non-Hodgkin lymphoma. Leuk Lymphoma. 2008;49:1778–83. doi: 10.1080/10428190802270886. [DOI] [PubMed] [Google Scholar]


