ABSTRACT
Objective:
Changes in soluble intercellular adhesion molecule-1 (sICAM-1) and E-selectin levels as well as leukocyte count were examined in this study to explore the relationship between leukopenia and ICAMs in Graves' disease (GD).
Methods:
Fasting blood samples were obtained from 37 GD patients with normal leukocytes and 32 GD patients with leukopenia. Enzyme-linked immunosorbent assay (ELISA) was performed to determine serum sICAM-1 and E-selectin levels for comparison. The same analyses were repeated for the GD patients with leukopenia after glucocorticoid treatment (15 mg/day to 30 mg/day prednisone).
Results:
The ELISA results showed that E-selectin levels were higher in GD patients with leukopenia than those with normal leukocytes (p < 0.05), but these levels decreased after glucocorticoid (prednisone) treatment (p < 0.05). No significant change in sICAM-1 levels was observed (p = 0.12). Correlation analysis showed that leukocyte count and E-selectin were negatively correlated (r = −0.778; p < 0.05).
Conclusion:
E-selectin may have an important function in GD with leukopenia, and glucocorticoids (prednisone) could decrease E-selectin level, which may be a new therapy target for GD with leukopenia.
Keywords: Graves' disease, hyperthyroidism, intercellular adhesion molecule, leukopenia
RESUMEN
Objetivo:
En este estudio se examinaron los cambios en los niveles de la molécula-1 de adhesión intercelular soluble (sICAM-1) y E-selectina, con el fin explorar la relación entre la leucopenia y las ICAMs en la enfermedad de Graves (EG).
Métodos:
Se obtuvieron muestras de sangre en ayuno de 37 pacientes de EG con leucocitos normales, y 32 pacientes de EG con leucopenia. Se realizó un ‘ensayo por inmunoabsorción ligado a enzimas (ELISA) para determinar y comparar los niveles séricos de sICAM-1 y E-selectina. Los mismos análisis se repitieron para los pacientes de EG con leucopenia después de tratamiento con glucocorticoides (prednisona 15 mg/día a 30 mg/día).
Resultados:
Los resultados de ELISA mostraron que los niveles de E-selectina fueron más altos en los pacientes de EG con leucopenia que en aquellos con leucocitos normales (p < 0.05), pero estos niveles disminuyeron tras el tratamiento con glucocorticoides [prednisona] (p < 0.05). No se observó ningún cambio significativo en los niveles de sICAM-1 (p = 0.12). El análisis de correlación mostró que el conteo de leucocitos y la E-selectina se correlacionaban negativamente (r = −0.778; p < 0.05).
Conclusión:
La E-selectina puede tener una función importante en la EG con leucopenia, y los glucocorticoides (prednisona) podrían disminuir el nivel de E-selectina, que puede ser un nuevo objetivo de la terapia para la EG con leucopenia.
INTRODUCTION
Graves' disease (GD), an organ-specific autoimmune disease, is a common type of hyperthyroidism that occurs at any age, with a high incidence rate. Graves' disease is often associated with leukopenia, especially agranulocytosis, which often leads to a critical condition, as well as severe secondary inflammation. In GD treatment, antithyroid drugs (ATDs) are necessary for symptom control prior to surgery treatment or iodine-131 treatment. Otherwise, hyperthyroidism crisis may occur. However, the use of ATDs may cause leukopenia and influence patients' prognosis. Therefore, GD complicated by leukopenia has not been clearly elucidated in clinical practice (1, 2).
The improvement of leukocyte levels in patients with hyperthyroidism seems important. The specific mechanisms of leukopenia, such as marrow depression, anti-neutrophil antibodies, and so on, have been unclear and controversial (3–5), thereby complicating ATD treatment. In recent years, the understanding of cell adhesion molecules in the cells, among the cells, and between cell and matrix has been attracting attention. Except for inflammatory and autoimmune mechanisms, adhesion molecule changes may affect the distribution of granulocytes (6). In untreated GD, the high concentration of thyroxine affects adhesion molecules and promotes the adhesion and aggregation of leukocytes on the blood vessel walls at the edge of the pool (7). An in-depth study is needed to further elucidate the possible mechanism involved in GD with leukopenia.
In this study, leukocyte count and adhesion molecule (soluble intercellular adhesion molecule-1 (sICAM-1) and E-selectin) levels were examined in GD patients to understand the relationship between leukopenia and adhesion molecules as well as the possible mechanism involved in GD with leukopenia. Results were explored for use in clinical treatment, which could provide a theoretical basis for a new target therapy.
SUBJECTS AND METHODS
Patients with initial GD were selected from the Endocrine Department of the Second People's Hospital of Lianyungang between June 2009 and June 2010 according to the hyperthyroidism diagnostic criteria (5). These patients had no other immune-related diseases; those with hypersplenism, aplastic anaemia, other blood diseases, and any treatment (to rule out drug factors) were excluded from the study. This criterion was applied such that any leukocyte changes observed in the patients can be attributed to hyperthyroidism.
The selected patients were divided into two groups according to leukocyte count: the normal group [leukocyte count ≥ 4.0 × 109/L, neutrophil count ≥ 1.5 × 109 cells/L] and the leukopenia group [leukocyte count ≤ 4.0 × 109/L, neutrophil count ≤ 1.5 × 109/L] (8). The normal group consisted of 37 patients (seven males and 30 females; mean age 42 ± 13 years), whereas the leukopenia group included 32 patients (five males and 27 females; mean age 38 ± 15 years). Age and gender in both groups were matched. This study was conducted in accordance with the Declaration of Helsinki and with approval from the Ethics Committee of the Second People's Hospital of Lianyungang. Written informed consent was obtained from all participants.
Fasting blood samples were obtained to measure leukocytes, sICAM-1 and E-selectin by enzyme-linked immunosorbent assay (ELISA). Patients with Graves' disease and leukopenia were treated with glucocorticoids (15 mg/day to 30 mg/day prednisone) until the leukocyte and neutrophil counts were ≥ 4.0 × 109/L and ≥ 1.5 × 109/L, respectively. Venous blood sample extraction was then repeated to determine sICAM-1 and E-selectin levels after the treatment. The French DIOCLONG company provided the kits, the instructions of which were strictly followed.
SPSS 11.5 statistical software was used to analyse the data which were expressed as mean ± standard deviation (). T-test and Pearson correlation method were also performed, with p < 0.05 considered as statistically significant.
RESULTS
Comparison of sICAM-1 and E-selectin levels
The sICAM-1 levels of the GD patients with leukopenia were slightly higher than those of the normal serum group, but the difference was not statistically significant (p = 0.12). E-selectin levels in the leukopenia group were higher than those in the normal group, and the difference was significant (p < 0.05), as shown in the Table. Moreover, the Table also shows that serum sICAM-1 and E-selectin levels of GD patients with leukopenia decreased (p < 0.05) after glucocorticoid treatment.
Table. Leukocyte count, serum sICAM-1 and E-selectin levels changes ( ± s).
| Group | n | Leukocyte count (× 109) | sICAM-1 (μg/l) | E-selectin (μg/l) |
|---|---|---|---|---|
| Graves' disease with normal leukocytes | 37 | 5.34 ±1.20 | 1301.48 ±140.02 | 76.74 ±14.06 |
| Graves' disease with leukopenia | 32 | 3.33 ±0.45 | 1364.96 ±190.87 | 100 ±17.03* |
| The group after glucocorticoid treatment | 32 | 8.40 ±2.30 | 1296.5 ±160.57** | 79.80 ±18.10** |
Compared with Graves' disease with normal leukocytes,
p < 0.05; compared with Graves' disease with leukopenia,
p < 0.05.
Normal soluble intercellular adhesion molecule-1 (sICAM-1) is about 571 ± 168 ng/mL and normal E- selectin is about 51.99 ± 26.65 ng/mL
Correlation of leukopenia and serum E-selectin
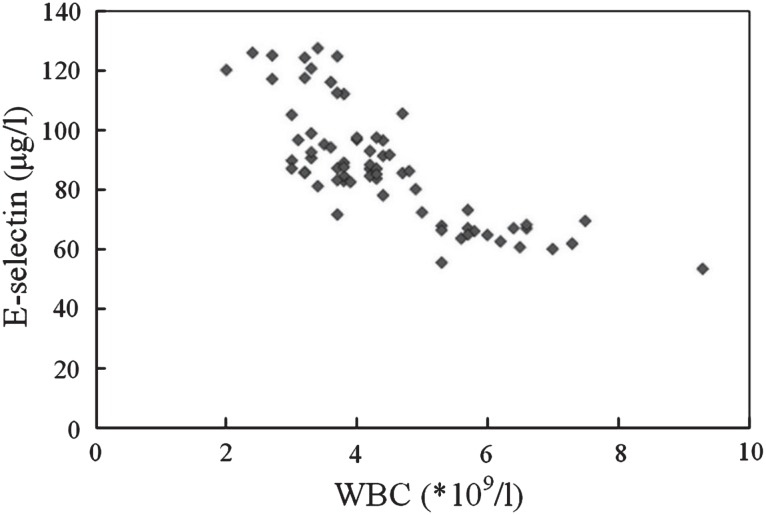
Correlation analysis showed that leukocyte count and E-selectin were negatively correlated [r = −0.778; p < 0.05] (Figure).
Figure. The correlation of leukocyte count and E-selectin.
WBC = white blood cells
DISCUSSION
Graves' disease with leukopenia involves complex factors (9, 10). In addition to ATD side effects, abnormal haematopoietic function or hyperthyroidism may be considered. We selected patients with recent diagnosis of GD who have not experienced any treatment, with no blood diseases, and other factors such that hyperthyroidism could be considered as the cause of leukopenia. As an autoimmune disease, the specific cause of hyperthyroidism with leukopenia is unclear. Previous studies suggested that GD patients with hyperthyroidism can produce antibodies against leukocyte and anti-neutrophil cytoplasmic antibody, which results in increased leukocyte destruction (11). Several studies considered the involvement of genetic predisposition in the pathogenesis (12), whereas others suggested that a high level of thyroid hormones causes abnormal leukocyte distribution, namely, leukocytes gather at the edge of the pool. The mechanism might involve the preferential main influence of thyroxine on myeloid migration and/or adhesion factors (7). Previous studies have also reported that no significant correlation was found between leukopenia and thyroxine hormone levels (13). In recent years, with the in-depth study and gradually increasing understanding of CAMs, many common features between cell-cell as well as cell-matrix and mechanical-chemical signal transfer mechanism have been found. These features were related with the adhesion system, which also had some common characteristics. Adhesion molecule levels may affect leukocyte distribution and influence leukocyte count (7).
The results of this study showed that the serum sICAM-1 levels in GD patients with leukopenia were slightly higher than those in the normal group, but the difference was not statistically significant (p = 0.12). The E-selectin levels of the leukopenia group were higher than the normal group (p < 0.05). Correlation analysis indicated that leukocyte count and E-selectin levels were negatively correlated (r = −0.778). Soluble intercellular adhesion molecule-1 is one of the first discovered immunoglobulin superfamily of adhesion molecules that is present in blood circulation in the soluble form. Soluble intercellular adhesion molecule-1 is expressed in a variety of cells, such as blood vessels, endothelial cells, dendritic cells, macrophages, fibroblasts, and so on [including the thyroid cells] (14). Moreover, sICAM-1 is a class of cells that can mediate mutual contact and binding among cells and between cells and interstitial molecules, mainly mediating interactions between lympho-cytes and target cells in effector organs to direct lymphocytes to the effective site for aggregation, positioning and antigen presentation (15). Previous studies suggested that in the pathogenesis of thyroid-associated ophthalmopathy, sICAM-1 has an important intermediary function (16). However, no noticeable change in sICAM-1 level was observed in this study. This finding is presumably correlated with the different effector cells it acted on. E-selectin, which is also an important member of the adhesion molecule selectin family, is also known as “leukocyte endothelial cell adhesion molecule-1 CD62E”. This induced adhesion molecule is secreted by activated vascular endothelial cells. E-selectin, which is expressed in vascular endothelial cells after certain stimulation, mediates leukocyte and endothelial cell adhesion (17, 18). Previous studies on asthma mechanisms indicated that E-selectin may be involved in the recruitment and aggregation of leukocytes in inflammatory response (19–21). Another study suggested that adhesion molecule gene defects reduce leukocyte-mediated inflammatory response (22).
Graves' disease with leukopenia is related with increased levels of adhesion molecules (E-selectin), promotion of leukocyte adhesion on vessel wall and leukocyte aggregation at the edge of the pool. After glucocorticoid treatment, serum sICAM-1 and E-selectin levels decreased in Grave's disease patients with leukopenia (p < 0.05). This result indicates that glucocorticoids may have a certain function in the anti-adhesion and regulation of adhesion molecules. Grave's disease with leukopenia was found to be associated with systemic immune disorders, and immunomodulatory therapy with glucocorticoids can affect the expression of ICAMs (23, 24). In this study, glucocorticoids may have affected leukocyte distribution through its effect on adhesion molecules. The anti-inflammatory agent dexamethasone activates endothelial cells. The covalent bond of dexamethasone links to an E-selectin antibody to form dexamethasone-e-selectin-antibody through E-selectin-targeted drug-antibody conjugation, thereby causing specific actions (25). The specific molecular biological mechanism needs further studies.
In summary, this study showed that GD with leukopenia was affected by the change in the level of adhesion molecules, and adhesion molecules affected leukocyte distribution. This new understanding is proposed to address the causes and treatment of hyperthyroidism with leukopenia to change leukocyte migration and adhesion factors such that the aggregation of leukocytes at the edge of the pool is reduced. This positive investigation on anti-adhesion is productive for the treatment of hyperthyroidism with leuko-penia, and further studies are needed on its specific mechanism.
REFERENCES
- 1.Lehtihet M, Zedenius J, Helldén A, Axelsson R, Calissendorff J. Antithyroid drug-induced agranulocytosis, a rare but dreaded condition. Lakartidningen. 2009;106:2607–8, 2610–1. [PubMed] [Google Scholar]
- 2.Andrès E, Zimmer J, Mecili M, Weitten T, Alt M, Maloisel F. Clinical presentation and management of drug-induced agranulocytosis. Expert Rev Hematol. 2011;4:143–151. doi: 10.1586/ehm.11.12. [DOI] [PubMed] [Google Scholar]
- 3.Oliver R, Nama VV, Howard RJ, Nikookam KH. Leucopenia and atrial fibrillation: rare presentations of thyrotoxicosis in the first trimester. J Obstet Gynaecol. 2007;27:521–523. doi: 10.1080/01443610701482126. [DOI] [PubMed] [Google Scholar]
- 4.Harper L, Chin L, Daykin J, Allahabadia A, Heward J, Gough SC, et al. Propylthiouracil and carbimazole associated-antineutrophil cytoplasmic antibodies (ANCA) in patients with Graves' disease. Clin Endocinol (Oxf) 2004;60:671–675. doi: 10.1111/j.1365-2265.2004.02029.x. [DOI] [PubMed] [Google Scholar]
- 5.Teng WP, Zeng ZP, Li GW. Chinese diagnostic and therapeutic guidance of thyroid disease. Chin J Intern Med. 2007;46:876–882. [Google Scholar]
- 6.Frommhold D, Kamphues A, Hepper I, Pruenster M, Lukic IK, Socher I, et al. RAGE and ICAM-1 cooperate in mediating leukocyte recruitment during acute inflammation in vivo. Blood. 2010;116:841–849. doi: 10.1182/blood-2009-09-244293. [DOI] [PubMed] [Google Scholar]
- 7.Tong J, Fu YS, Hao HJ, Guo TL, Han D, Wang Q, et al. Changes of granulocyte distribution induced by thyroxin and epinephrine: experiment with rabbits. Zhonghua Yi Xue Za Zhi. 2008;88:2826–2828. [PubMed] [Google Scholar]
- 8.Ponassi A, Morra L, Caristo G, Parodi GB, Biassoni P, Sacchetti C. Disorders of granulopoiesis in patients with untreated Graves' disease. Acta Haematol. 1983;70:19–23. doi: 10.1159/000206684. [DOI] [PubMed] [Google Scholar]
- 9.Sun MT, Tsai CH, Shih KC. Antithyroid drug-induced agranulocytosis. J Chin Med Assoc. 2009;72:438–441. doi: 10.1016/S1726-4901(09)70402-2. [DOI] [PubMed] [Google Scholar]
- 10.Tajiri J, Noquchi S. Antithyroid drug-induced agranulocytosis: special reference to no normal white blood cell count agranulocytosis. Thyroid. 2004;14:459–462. doi: 10.1089/105072504323150787. [DOI] [PubMed] [Google Scholar]
- 11.Tektonidou MG, Anapliotou M, Vlachoyiannopoulos P, Moutsopoulos HM. Presence of systemic auto immune disorders in patients with autoimmune thyroid diseases. Ann Rheum Dis. 2004;63:1159–1161. doi: 10.1136/ard.2004.022624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tamai H, Sudo T, Kimura A, Mukuta T, Matsubayashi S, Kuma K, et al. Association between the DRB1*08032 histocompatibility antigen and methimazole-induced agranulocytosis in Japanese patients with Graves' disease. Ann Intern Med. 1996;124:490–494. doi: 10.7326/0003-4819-124-5-199603010-00005. [DOI] [PubMed] [Google Scholar]
- 13.Ma ZS, Zhang P, Qiu MC. The exploration of the treatments for Graves' disease hyperthyroidism with leukopenia. Chin J Intern Med. 2004;43:781–782. [Google Scholar]
- 14.Arao T, Morimoto I, Kakinuma A, Ishida O, Zeki K, Tanaka Y, et al. Thyrocyte proliferation by cellular adhesion to infiltrating lymphocytes through the intercellular adhesion molecule-1/lymphocyte function-associated antigen-1 pathway in Graves' disease. J Clin Endocrinol Metab. 2000;85:382–389. doi: 10.1210/jcem.85.1.6320. [DOI] [PubMed] [Google Scholar]
- 15.Long EO. ICAM-1: getting a grip on leukocyte adhesion. J Immunol. 2011;186:5021–5023. doi: 10.4049/jimmunol.1100646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kulig G, Pilarska K, Kulig J, Krzyzanowska-Swiniarska B, Robaczyk M, Baraniak A. Usefulness of soluble ICAM-1 measurements for the evaluation of the disease activity and efficiency of therapy in patients with infiltrative Graves' ophthalmopathy. Pol Arch Med Wewn. 2002;108:1161–1169. [PubMed] [Google Scholar]
- 17.Cullere X, Shaw SK, Andersson L, Hirahashi J, Luscinskas FW, Mayadas T. Regulation of vascular endothelial barrier function by Epac, a cAMP-mediated exchange factor for Rap GTPase. Blood. 2005;105:1950–1955. doi: 10.1182/blood-2004-05-1987. [DOI] [PubMed] [Google Scholar]
- 18.Carlos TM, Harlan JM. Leukocyte-endothelial adhesion molecules. Blood. 1994;4:2068–2101. [PubMed] [Google Scholar]
- 19.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 20.Bijanzadeh M, Ramachandra NB, Mahesh PA, Savitha MR, Vijayakumar GS, Kumar P, et al. Soluble intercellular adhesion molecule-1 and E-selectin in patients with asthma exacerbation. Lung. 2009;187:315–320. doi: 10.1007/s00408-009-9171-5. [DOI] [PubMed] [Google Scholar]
- 21.Stadtmann A, Brinkhaus L, Mueller H, Rossaint J, Bolomini-Vittori M, Bergmeier W, et al. Rap1a activation by CalDAG-GEF1 and p38 MAPK is involved in E-selectin-dependent slow leukocyte rolling. Eur J Immunol. 2011;41:2074–2085. doi: 10.1002/eji.201041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kakkar AK, Lefer DJ. Leukocyte and endothelial adhesion molecule studies in knockout mice. Curr Opin Pharmacol. 2004;4:154–158. doi: 10.1016/j.coph.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 23.He WM, Luo QL, Zeng JH. Effect of dexamethasone on expression of intercellular adhesion molecule-1 in orbital fibroblasts from thyroid associated ophthalmopathy. Sichuan Da Xue Xue Bao Yi Xue Ban. 2007;38:113–115. [PubMed] [Google Scholar]
- 24.Wehling-Henricks M, Lee JJ, Tidball JG. Prednisolone decreases cellular adhesion molecules required for inflammatory cell infiltration in dystrophin-deficient skeletal muscle. Neuromuscul Disord. 2004;14:483–490. doi: 10.1016/j.nmd.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 25.Everts M, Kok RJ, Asgeirsdóttir SA, Melgert BN, Moolenaar TJ, Koning GA, et al. Selective intracellular delivery of dexamethasone into activated endothelial cells using an E-selectin-directed immunoconjugate. J Immunol. 2002;168:883–889. doi: 10.4049/jimmunol.168.2.883. [DOI] [PubMed] [Google Scholar]