Abstract
Inflammatory signals generated within the brain and peripheral nervous system (PNS) direct diverse biological processes. Key amongst the inflammatory molecules is tumor necrosis factor-alpha (TNF-α), a potent pro-inflammatory cytokine that, via binding to its associated receptors, is considered to be a master regulator of cellular cascades that control a number of diverse processes coupled to cell viability, gene expression, synaptic integrity and ion homeostasis. Whereas a self-limiting neuroinflammatory response generally results in the resolution of an intrinsically or extrinsically triggered insult by the elimination of toxic material or injured tissue to restore brain homeostasis and function, in the event of an unregulated reaction, where the immune response persists, inappropriate chronic neuroinflammation can ensue. Uncontrolled neuroinflammatory activity can induce cellular dysfunction and demise, and lead to a self-propagating cascade of harmful pathogenic events. Such chronic neuroinflammation is a typical feature across a range of debilitating common neurodegenerative diseases, epitomized by Alzheimer’s disease, Parkinson’s disease and amyotrophic lateral sclerosis, in which TNF-α expression appears to be upregulated and may represent a valuable target for intervention. Elaboration of the protective homeostasis signaling cascades from the harmful pathogenic ones that likely drive disease onset and progression could aid in the clinical translation of approaches to lower brain and PNS TNF-α levels and amelioration of inappropriate neuroinflammation.
Keywords: TNF-α, neuroinflammation, cytokine, chemokine, neurodegeneration, Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, Japanese encephalitis, dementia, thalidomide, revlimid
Introduction
Inflammation and inflammation related illness is not a new concept by any means, typically when one thinks of inflammation related illnesses one immediately considers the classical examples of rheumatoid arthritis, asthma, psoriasis or Crohn’s Disease, all of which are caused by inappropriate immune responses in peripheral tissues. However, perhaps a newer set of inflammatory disorders specific to peripheral and central nervous system (CNS) neuronal tissues are worthy of discussion. The response of living tissue to injury is inflammation, and by extension in neuronal tissues neuroinflammation, this term refers to the collective response of microglia and astrocyte actions in the CNS, or microglia and other immune cell actions in peripheral nervous tissues. Neuroinflammation incorporates a wide spectrum of complex cellular responses that often contribute to the pathogenesis and progression of a wide variety of neurological disorders [1,2]. Neuroinflammation related disorders are fast becoming some of the most common causes of mortality and morbidity in industrialized Western societies. The majority of CNS inflammatory diseases, such as Alzheimer’s disease (AD) and Parkinson’s disease (PD), which are neurodegenerative in nature, primarily tend to occur in older or aging populations and tend to be chronic in nature. Additional forms of chronic neuroinflammation can be seen in diverse illnesses such as sporadic and genetic forms of Lewy body associated dementia, ALS and in HIV-1-associated dementia.
The relevance of chronic neuroinflammation becomes apparent when one considers that the human population is generally living longer which means that there will be a far higher occurrence of age related illnesses, as exemplified by senile dementia. Thus as our populations age, the impact of old age illnesses on social and economic institutions will correspondingly become far greater. In 2009 the numbers of American’s with diagnosed AD was 5.3 million, while future estimates are that in the year 2050 there will be over 13 million people with AD in the United States alone [3].
Yet again there is an alternative manifestation of neuroinflammation that can be described as acute in nature. This form can occur in a person of any age. Typically acute neuroinflammation is related to brain injury typified by stroke, head trauma or infections, where the inflammation is induced by an acute insult which more often than not, can result in a progressive transition to a debilitating chronic form of inflammation. In either chronic or acute neuroinflammation, a key cellular player is the brain resident macrophage like cell - the microglial cell. One of the critical cellular factors synthesized by microglial cells following activation within the brain is the potent pro-inflammatory cytokine tumor necrosis-alpha (TNF-α). TNF-α is a potent activator of the immune system and can induce the activation of additional resting microglial cells or astrocytes located in the adjacent brain microenvironment. If this occurs it can result in an unregulated inflammatory CNS response. This review will attempt to address the involvement of the cytokine TNF-α in several neuroinflammation related human illnesses.
Microglial cell role in brain
Microglial cells are the macrophages of the central nervous system. Typically, microglia provide a protective role to neurons. Under normal physiological situations microglia can regulate the environment of the brain through their ability to rapidly respond to external signals such as invading pathogens, endogenous cellular signals such as membrane components, or cytoplasmic proteins from damaged cells, or cytokines from other immune cells. Microglia have been shown to be involved in neuronal tissue structural modifications via the physical removal of or the initiation of apoptosis in cells via the synthesis and release of cytokines and reactive oxygen species. Through these means of regulation of the brain cellular parenchyma, the microglia may play an important role in tissue remodeling after ischemic events [4,5]; or such as guidance of axons in white matter tracts and pruning of projections. While these events primarily occur during CNS development, there are lines of evidence that propose a role for microglia in neurogenesis, synaptogenesis, and synapse elimination, which are phenomena that continue through adulthood. Thus normally functioning microglia, play dynamic roles in neuronal communication throughout an organism’s life, in addition to and in conjunction with their maintenance of the brain microenvironment [6,7].
While programmed cell death is an essential process for normal development of the CNS and the maintenance of tissue homeostasis, unregulated neuroinflammation of an acute or chronic nature is associated with neuronal cell death (Figure 1). In these situations unregulated neuroinflammation often further exacerbates the conditions that initiated the signaling neuroinflammatory cascades, which in turn cause additional injury to neural tissues [8,9]. In the instance of neuroinflammation accompanying chronic conditions, discussion exists such that microglial cells undergo a type of cellular senescence that may contribute to the development of age-related brain dysfunction, specifically in the cases of neurodegenerative diseases such as AD and PD [10,11]. However, this cellular senescence is not to be confused with chronic neuroinflammation, although microglia undergoing cellular senescence may exhibit some features of activated microglia. Generally, activated microglia show a tendency to remain activated for a considerable length of time after the disruption of homeostasis occurred, even if the initiating factor(s) have been normalized. This unnecessarily long period of cellular activation can have adverse affects on the surrounding neurons, and other glial cells as pro-inflammatory cytokines are secreted on a mass scale and often lead to downstream cascades that initiate cell death [10].
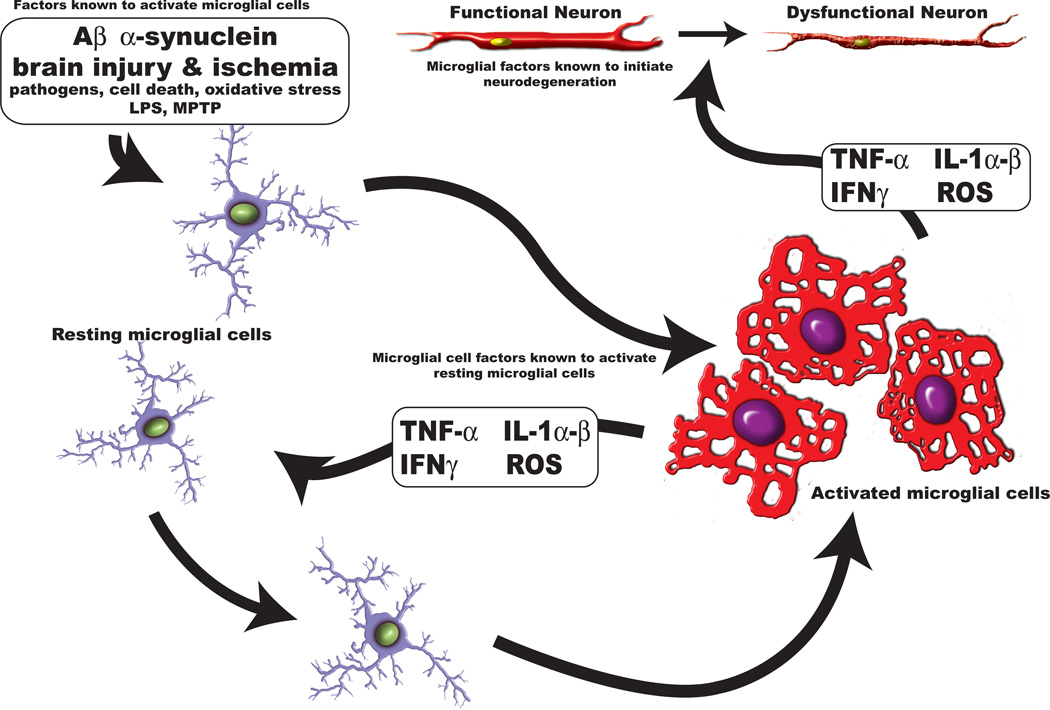
Figure 1. Microglial Cells.
Microglia are the resident macrophage-like cell in brain. They respond to various stimuli in their microenvironment, such as Aβ, α-synuclein, oxidative stress and pathogens, by undergoing morphological changes from a ramified – resting state to an ameboid – activated state. When microglia become activated they respond by synthesizing an array of soluble factors (TNF-α, IL-1 α/β, INFγ and reactive oxygen species). These factors can (1) induce resting cells to become activated cells or (2) they may damage functional neurons leading to the generation of dysfunctional neurons. In several central nervous system diseases conditions exist that allow for the formation of a TNF-α-mediated feed back loop of self-activating neuroinflammation.
Neuroinflammation in Alzheimer’s Disease
As previously discussed, certain neurodegenerative disorders, epitomized by AD, are occurring in alarmingly high numbers mainly due to our newly aged and aging populations, not surprisingly the high numbers of patients diagnosed with AD is having a significant impact on our societies. One of the hallmark biochemical features of the AD brain is the presence of amyloid-β (Aβ) plaques which are formed by various forms of insoluble Aβ that derive from amyloid precursor protein (APP) processing via the action of γ- and β-secretase activities. Postmortem histological evidence has highlighted a high association between Aβ plaques and activated microglial cells in the immediate surroundings of the plaque [12,13]. Importantly, the synthesis and release of IL-1β and TNF-α [12] by microglia and astrocytes, has been shown to stimulate an enhanced glial cellular expression of APP [14–16], in addition the actions of the cytokines has been reported to up regulate the conversion of APP into the pathological forms of Aβ peptides [17–19]. Furthermore, microglia and astrocytes can become activated by the presence of amyloid plaques [20,21] as well as by fibrillar Aβ, through a cell surface receptor complex, through which the microglial cells further increase their production of TNF-α and IL-1β [22,23] (Figure 2). Indeed, these cytokines are potent stimulators of γ-secretase, resulting in the increased production of Aβ [24]. This, thereby, creates the potential for a self-propagating cycle of APP induction, elevated Aβ generation and further neuroinflammation that may drive disease progression. Hence in brain, activated microglia and astrocytes are additional non-neuronal sources of APP, and they can play a role in promoting the occurrence of an unregulated neuroinnflammatory response [20,21]. It is noteworthy to consider that memory impairments were exhibited in an animal study in which Aβ peptides were injected into the brains of the mice, suggesting a strong relationship between elevated Aβ peptides and neuronal dysfunction [25]. Furthermore the use of thalidomide to inhibit the synthesis of TNF-α protein, in these mice was shown to be beneficial by preventing the memory impairments. Although early onset familial AD has been directly linked to mutations in the amyloid-β precursor protein gene, as well as to presenilin-1 and presenilin-2 [26], late-onset Alzheimer’s has been linked to chromosome 1p and chromosome 12p regions, which also contain TNF receptors 1 and 2 [27].
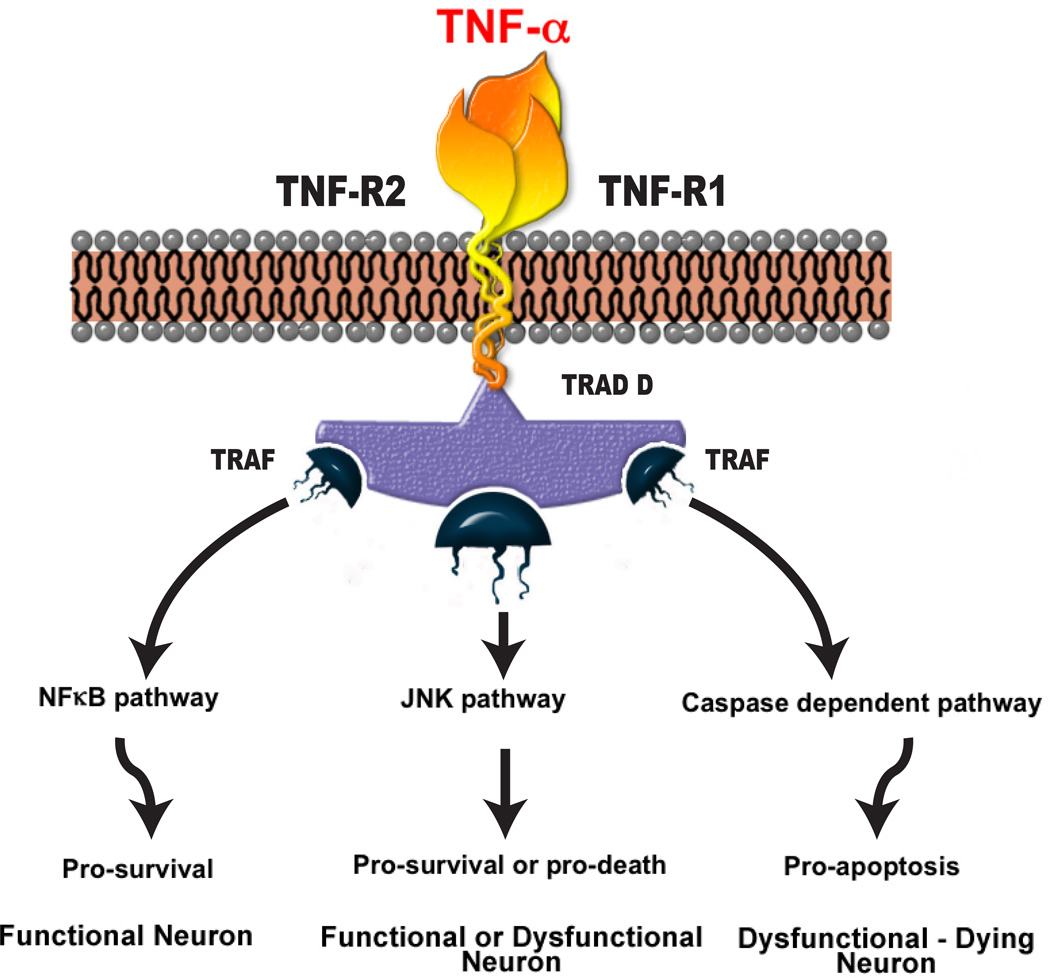
Figure 2. TNF-α signaling.
TNF-α can bind with either of its receptors and initiate several pathways: activation of NFκB dependent signaling can lead to beneficial effects [138, 139]. Activation of c-Jun N-terminal kinase (JNK) pathway may lead to either beneficial or harmful effects [170]. The activation of caspase dependent signaling typically leads to apoptotic mediated cell death in neurons [171].
In AD the impact of neuroinflammation is now well accepted and as such anti-inflammatory therapies may yield a promising line of therapeutic intervention [8,28]. A clear choice of target in the inflammatory cascades within the brain is lacking; however, there have been several studies that utilize animal models of various aspects of AD to assess novel treatment modalities. The potential that COX-1 and –2 inhibitors have demonstrated in cell culture and animal models has, unfortunately, not been shown to be reproducible in clinical AD [29]. However, a lower risk of development of the disease has been associated with the use of non-steroidal anti-inflammatory agents [30,31].
In 2003 Oddo and coworkers [32,33] described the generation of a novel triple transgenic mouse model of AD (3xTg AD). This model incorporated three genes (human Swedish mutation APP, human four-repeat tau P301L, also a knock-in mutation of presenilin 1 PS1M146V) which, when combined, allowed the mice to develop hallmark features of AD in an age-dependent manner; namely, Aβ plaques followed by tau protein tangle pathology, in brain regions relevant to human AD. Through the use of these mice, inflammatory changes that may underlie the development of AD have been explored [34]; as early as 2 months of age elevated expression of transcripts for TNF-α and monocyte chemoattractant protein-1 were detected in the brains of the mice, while they were absent in non-transgenic control animals. At 6 months of age the 3xTg AD mice displayed markedly significant elevations in these proteins. Additionally it was later determined that neurons from these mice were also able to express TNF-α gene transcripts in cells from the entorhinal cortex [35]. McAlpine and coworkers elegantly showed that inhibition of soluble TNF-α signaling can prevent lipopolysaccharide–induced Aβ plaque deposition in the triple transgenic AD mouse model [36]. Recently it was shown that by antagonizing the receptors of a protein involved in the complement cascade (C5a), it is possible to reduce the deposition of Aβ peptides and reduce the numbers of activated glial cells in two transgenic models of AD Tg2576 (human mutation of APP, KM670/671NL) and the 3xTg AD mouse model [37]. The actions of the C5a receptor antagonist were also associated with an improved behavioral task in a Tg2576 mouse model and the levels of hyperphosphorylated tau protein were reduced in the 3xTg AD mice. These studies point towards a clear role of neuroinflammation in the setting of models of AD and additionally suggest that interventions that limit the formation of critical inflammatory proteins (i.e. TNF-α) will be beneficial in the human disease.
This hypothesis is further bolstered by findings from studies taking an anti-TNF-α approach in AD patients where the brain TNF-α protein was sequestered by use of an agent that binds to and effectively removes the pro-inflammarory cytokine from the patient’s brain parenchyma [38–40]. Initially a 6 month pilot study indicated improvements in measures of cognition, later studies showed that patients displayed rapid cognitive and verbal fluency improvements which were observed following administration of the agent (Etanercept). These human studies validate TNF-α as an appropriate drug target in human AD.
Neuroinflammation in Parkinson’s Diseases
PD involves the progressive degeneration of dopaminergic motor neurons in the substantia nigra and projecting tracts, ultimately leading to tremors, rigidity, slow movement, stooped posture, and other motor and non-motor symptoms [41,42]. A significant similarity between AD and PD is the detection of abnormally high levels of TNF-α protein in the CNS of PD patients. In a study comparing PD patients to healthy volunteers, significantly higher plasma levels of TNF-α protein were found in PD patients, though the mechanism for the potentially causal role of TNF-α in exacerbation of PD symptoms is currently unknown [43]. In PD, inflammatory markers in the substantia nigra were reported long ago [9] and in addition to increased plasma TNF-α, there is a consistent finding of elevated CNS TNF-α protein detection where elevated levels of the cytokine have been found in the striatum and also in ventricular and lumbar cerebrospinal fluid of PD patients [44,45]. Additionally, the substantia nigra is known to be the brain area with the greatest abundance of microglial cells [46] which may heighten the sensitivity of this region to the adverse effects of unregulated neuroinflammation. In PD it has been established that the most likely source of TNF-α is from activated glial cells located in the substantia nigra [47]. Furthermore, higher levels of the TNF-α receptor (type 1 or p55TNFR), which is associated with programmed cell death through caspase-1 and –3 dependent pathways, have been found on dopaminergic neurons in PD patients [45,48].
Though recent review of literature [42,49] points to a genetic basis for early onset PD (e.g., PINK1, Parkin, DJ-1, ATP13A2 and other gene mutations), the lack of a firm genetic link enforced by the random constituency of people who develop PD, implies that the onset of PD can be brought about by primarily environmental factors or gene-environmental factor interactions [6,41]. Recreational drug users inadvertently exposed to a substance known as MPTP (1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine), rapidly developed symptoms that simulated the underling motor deficits seen in PD. In postmortem brain of such patients, structures resembling Lewy bodies, and the destruction of the substantia nigra cells were observed [50], as were activated microglial cells – indicative of neuroinflammation [50]. The mechanism of the MPTP-induced toxicity involves the two-step conversion of MPTP to MPP+, which is selectively taken up by dopamine neurons through the dopamine transporter, which ultimately inhibits the mitochondrial protein complex I that is a key component of the respiratory chain [42,51]. This agent has been used as a tool to further study possible mechanism underpinning PD. As an example, in a study by McGeer and colleagues [52] in rhesus monkeys, reactive microglia were identified in the SN of monkeys up to 14 years after administration of MPTP, and were in accord with findings from human MPTP cases [50]. This raises the concept that PD may, in certain circumstances, involve potential exposure to one of a variety of transient but watershed insults that disappear after initiating a long-lasting neuroinflammatory change [52,53]. Such an insult might be environmental, viral or possibly traumatic. Evidence to support each of these as initiating causes is available, although controversial. Viral parkinsonism has been described after influenza exposure [54], stemming from an outbreak of encephalitic lethargica, additionally known as von Economo’s disease, and the ensuing postencephalic parkinsonism that occurred following the 1918 pandemic influenza outbreak involving a type A H1N1 influenza virus [54]. Some cases were reported as having onset as late as 20 years after recovery from the acute infection [55]. Likewise, PD after head trauma is exhibited in numerous cases of dementia pugilistica [56–58]. Finally, environmental toxins, epitomized by the insecticide rotenone, the herbicide paraquat and the fungicide maneb, have long been linked to but have not specifically been shown to establish PD [49,59]. Several of the above insults have been utilized to model PD in animals, and whereas none can be considered to directly induce PD, they may act as the first in a two ‘hit’ hypothesis [53], to sensitize the brain through sustained inflammation to a later insult that might not have been pathogenic in the absence of an already primed system.
Animal models such as MPTP, rotenone and others also have proved of value to identify targets and candidate anti-PD therapeutic agents. The importance of TNF-α and the signaling events initiated by the cytokine in the development of PD are strengthened by animal models, where elevated levels of TNF-α are seen in a manner consistent to that of clinical PD [45,60–63]. A recent study employing the use of virally induced chronic expression and synthesis of soluble mouse TNF-α in rat substantia nigra, strongly supports an important role of TNF-α mediated signaling in the degeneration of tyrosine hydroxalyase containing cells [64].
Agents that interfere with the pro-inflammatory events connected to TNF-α synthesis and release can be predicted to be protective in the setting of experimental models of PD. Studies utilizing acute and subacute MPTP treatment on wild-type, TNF-α protein knock out (−/−) and wild-type mice treated with pharmacological agents, show this to be the case. Genetic ablation of TNF-α or treatment with a TNF-α synthesis inhibitor (thalidomide) can partly prevent MPTP-induced dopamine depletion and reduced striatal tyrosine hydroxylase fiber density [41]. These data implicate a role of TNF-α signaling events in the MPTP model of PD. The use of thalidomide, has been shown to afford partial protection to striatal dopaminergic neurons in a MPTP model of PD [41,65]. In accord with this, in transgenic mouse studies where the gene for TNF-α was functionally deleted, dopaminergic neurons in the striatum of mutant mice exhibited a greater degree of protection than that seen in wild type littermates when challenged with MPTP [41]. However, in other mouse models employing gene targeting events on either one or both of the TNF-α receptors (p55TNFR or p75TNFR); several studies have provided conflicting findings regarding the protective effects of TNF-α receptor deletion in toxin-induced PD [65– 68].
Dementia with Lewy bodies is considered to be the second most frequent dementing neurodegenerative condition after AD. It is associated with the accumulation of intraneuronal deposits of α-synuclein found in various regions of the brain. Lewy bodies are commonly associated with PD and can be considered as a hallmark feature of the disease. In postmortem studies it has been determined that activated microglial cells are located in the vicinity of Lewy bodies and a weak association with TNF-α and TNF-α activated signaling has been described [69,70]. Certain studies indicate that α-synuclein is capable of activating microglial cells and thus is another line of evidence supporting the role of a potentially unregulated inflammatory response in the progression of PD. [71,72]. Interestingly in clinical studies, an over expression of Il-1α, iNOS and TNF-α was associated in the most vulnerable brain regions of patients with Lewy body dementia, namely the amygdala, hippocampus, entorhinal and insular cortices [73], the presence of activated microglia and astrocytes were also found to be associated with neuritic plaques and extracellular neurofibrillary tangles. Overall, data obtained from clinical and basic research indicates a strong involvement of TNF-α and TNF-α signaling events in PD and Lewy body dementia.
Neuroinflammation in Amyotrophic Lateral Sclerosis
ALS is a progressive motor neuron degenerative disease, and is either sporadic or familial in nature (around 5 to 10% of cases are thought to be due to genetic mutations), however, both forms end in rapid premature death. For the familial form of ALS ~20% of the cases are associated with mutations in the SOD1 gene. Similar to clinical observations, in a transgenic mouse model of familial ALS (the Cu, Zn-superoxide dismutase, G93A–SOD1 model [74]) the life span of these mice is markedly shortened when compared to wild-type mice. In mice, the disease progression is accompanied by marked elevations in TNF-α mRNA transcripts and protein detected in reactive microglia found in the spinal cord. However, with this particular model of ALS, unlike clinical ALS, no evidence of increased TNF-α activity was observed in brain indicating that elevated TNF-α occurs locally at the spinal cord. There is a large body of evidence emphasizing a relationship between abnormal communications between microglial cells and neuronal cells specifically associated with the SOD1 mutation. Cell lines expressing the mutant form of SOD1, show an enhanced level of mitochondrial toxicity induced by inflammatory cytokines, primarily TNF-α and IFNγ, with an apparent synergism if both cytokines are utilized simultaneously [75]. Additionally, others report that activated microglial cells (BV-2 cells) transfected with mutant forms of SOD1 synthesize and release more TNF-α compared to microglial cell transfected with wild-type SOD1 or mock transfected cells [76]. Furthermore, purinergic signaling events between neurons and microglia are altered in SOD1 mutant mice, such that there is an enhancement of the release of microglial-neurotoxic factors namely, TNF-α and activation of cyclooxygenase-2 [77]. These lines of evidence shed further light on the probable cause of neuroinflammation seen with clinical ALS. Additionally, clearly documented deficits in motor function as well as protein oxidative damage have been reported in these mice [78–81]. Elevated levels of TNF-α have been observed in several different motor neuropathies in addition to ALS, when compared to healthy individuals [82].
In terms of possible therapies to slow down the disease progression, there have been several studies where various pharmacological interventions have increased the life span in these mice. Successful interventions have included the use of caspase inhibitors to block the later stages of apoptosis [83]. More recently an alternative approach has been to limit the amount of TNF-α signaling at the pre-symptomatic stage of the disease development by application of thalidomide and one of its analogs, Lenalidomide (Revlimid®) [84], both of which destabilize the mRNA of TNF-α and thereby reduce the abundance of circulating protein. More recently again, Lenalidomide when given at the onset of ALS symptoms in the ALS SOD1 (G93A) mouse extended the survival time of mice by ~45% and allowed for improvements in mouse functional behavior [85] strongly indicating, a detrimental role of TNF-α in the disease progression, and anti-TNF-α therapies with potentially benefits in human ALS disease.
Yet, there is a degree of confliction regarding the role of TNF-α in the disease in clinical ALS. There are studies where elevations in circulating TNF-α pathway proteins have been identified [86], however, there have also been cases where there were no elevations is TNF-α protein [87]. Recently, in a small scale human ALS phase II open label clinical trial, it was determined that thalidomide did not appear to modulate disease progression [88]. After 9 months of treatment with thalidomide the patients failed to display any benefits in basic ALS functional measures, or shifts seen in plasma cytokine profiles. It is possible that larger human clinical trials with thalidomide or analogs, such as Revlimid and more potent TNF-α lowering agents, may be required to fully address the potential benefits of this line of therapy in the setting of ALS. Additionally as the patients involved in this study were exhibiting symptoms prior to the use of thalidomide, it may be that thalidomide or analogs of thalidomide may provide benefits in patients if administered prior to the onset on the disease symptoms. This approach may only be considered where an individual with a clear genetic mutation predisposing to ALS has been identified. Also the identification of novel thalidomide analogs with more potent anti-TNF-α properties will be necessary for this to be fully assessed.
Neuroinflammation in infection related dementia
HIV-1-associated dementia is widely considered to be related to the presence of TNF-α released by activated microglia in the CNS [89]. In a study from postmortem human brain tissues where patients had exhibited symptoms of HIV-1 encephalitis there was evidence of diffuse microglial cell activation and astrocyte gliosis, while in nodular microglial infiltrates the microglia expressed IL-β1 and TNF-α [90,91]. The authors describe similar finding in a macaque model of acquired immunodeficiency syndrome dementia complex [92]. Also soluble factors released from Simian immunodeficiency virus infected microglia are capable of disrupting the neuronal autophagy in primary culture, leading to a reduced neuronal survival [93]. TNF-α and glutamate had similar effects to that of infected microglial factors on reducing neuronal autophagy, these findings point towards the role of microglial factors in the exacerbation of neurodegenerative processes in HIV infection. Williams and coworkers [94] have shown that TNF-α and INFγ are able to synergistically enhance the astrocyte production of a neurotoxic chemokine (CXCL10) in the setting of HIV-1 infection. HIV-1 viral proteins, gp120 and Tat are thought to cause the activation of the microglia that, in turn, generate and secrete proinflammatory cytokines and reactive oxygen species leading to cellular apoptosis. In HIV-1 clade C infected individuals in South India, it was determined that there was a strong correlation between HIV related neurological disease and CSF levels of high viral counts, low CD4 counts and higher cytokines that included TNF-α [95]. Furthermore, the use of a cytokine array detection system with CSF samples from HIV infected patients was able to identify highly elevated levels of INFγ, IL-1α, IL-15 and TNF-α in those patients selectively exhibiting symptoms of HIV-1 associated dementia [96]. Additionally, it has been demonstrated that in cases of HIV-associated dementia, TNF-α can be expressed in neurons in addition to microglia [97]. In vitro studies where cytokine production was suppressed by pharmacological intervention showed considerable benefits in experimental models of HIV-1 gp120 in combination with TNF-α induced cell death [98]. However, in a study looking at the acute effects of gp120 on neurobehavioral measures, viral protein infection-induced behavioral changes were not associated with an involvement of TNF-α [99], thereby suggesting a stronger role of TNF-α in a chronic setting.
Japanese encephalitis, which is caused by the Japanese encephalitis virus (JEV) is transmitted by a mosquito, and is associated with a high mortality rate. Infection of mice with JEV causes wide spread activation of microglial cells in a region specific pattern, with the highest levels of activated cells found in the hippocampus [100]. Infection of neuronal glial cultures with JEV caused neuronal death and microglial cell activation, with elevations in a number of cytokines, including TNF-α. Antibody neutralization studies indicated that the neuronal toxicity observed was mainly due to IL-1β and TNF-α [101]. JEV infection of neuronal cell lines induced apoptosis via a mitochondrial dependent mechanism that was not dependent upon functional Fas-associated death domain signaling [102]. Interestingly, in in vitro studies a strong dependence of TNFR-associated-death domain (TRADD) mediated signaling was observed for JEV mediated neuronal apoptosis to occur [103,104]. In clinical cases the levels of serum cytochrome c and various cytokines, including TNF-α, prove to be reliable predictors of the outcomes of the acute encephalopathy in children [105]. While other clinical related studies show a clear correlation between the occurrence of encephalitis and the detection of Il-6, RANTES and IL-8, yet not with TNF-α or IL-1β and several others proteins in CSF [106]. In Japan and East Asia, cases of influenza infection in children have been associated with CNS complications causing influenza-associated acute encephalopathy. High levels of child mortality have been identified with this condition. In children suffering from this form of encephalopathy elevated levels of RNA transcripts, serum and or cerebrospinal fluid protein for cytochrome c, IL-6 and TNF-α were consistently described [107–109]. Indeed, the detection of these proteins provided the only reliable markers to indicate the severity of the condition. On the whole, these data implicate the activation of TNF pathways in the severe pathology of this condition [110], further consolidating the detrimental role of TNF-α in neurological disorders of varying etiology.
Neuroinflammation in traumatic brain injury
Traumatic brain injury (TBI) represents a major public health concern and is the most common cause of mortality and disability in young adults. In addition, that associated with battlefield injury, blast-TBI, is currently particularly concerning. At present, no effective pharmaceutical therapies are available for TBI and existing treatment primarily involves optimized intensive care management following the injury [111,112]. The pathology of head injury is becoming increasingly better understood. Mechanical forces produce shearing and compression of neuronal and vascular tissue at the time of impact. A cascade of pathological events may then follow that lead to further brain injury. This ensuing secondary injury may be amenable to intervention and is worsened by secondary physiological insults. Specific risk factors for poor outcome after TBI have been recognized. Some of these are established at the time of injury, such as age, gender, mechanism of injury, and presenting signs, whereas others, such as hypoxia, hypotension and hyperglycemia, are potential areas for medical intervention [112]. Recent studies suggest a commonality between the biochemical cascades resulting in neuronal cell death from TBI and neurodegenerative diseases, and provide the opportunity that mechanism-based treatments for the latter could possibly provide cross-over potential, but this is an area requiring considerable further research.
With increasing characterization of the biochemical and pathological changes induced after TBI, it is increasingly evident that brain trauma represents a complex neurodegenerative disease, encompassing numerous molecular and cellular pathways, including inflammation [113,114]. The inflammatory cascades activated following TBI are mediated by the release of both pro- and anti-inflammatory cytokines that, all be they barely detectable in healthy brain, are swiftly upregulated in response to TBI. An early transient elevation in the mRNA expression of TNF-α, in particular, but also of IL-1β, and IL-6 has been reported in rodent following closed head injury, and preceded the appearance of ensuing cytokine activity and leukocyte recruitment, [115–117]. Numerous studies of TBI models have reported TNF-α protein upregulation in brain, in accord with that described for its RNA expression, within a few hours of trauma [118–120] and, likewise, elevated levels of IL-1β and IL-6 have been detected in the CSF and brain parenchyma within hours of brain injury in both humans and rodents [121,122]. Inhibition of TNF-α, utilizing both pentoxifylline that has been reported to attenuate TNF-α gene transcription, and with TNF-α binding protein, a physiological inhibitor of TNF-α that provides a false target to compete with the cell surface TNF-α receptor, has been reported to ameliorate closed head injury over the initial 24 hr [116], when administered directly after trauma. However, studies utilizing TNF-α protein and receptor deficient, knockout (KO), mice have been less easy to interpret.
In TNF-α protein KO mice, significantly reduced deficits in both memory function (assessed at 1 week) and neuromotor function (assessed at 48 h) were evident during the acute posttraumatic period after a controlled cortical impact, compared to normal, wild type (wt) mice [123]. However, injured wt mice recovered motor function within 2–3 weeks, whereas TNF-α mice showed no improvement over a post-injury 4 week period, and appeared to suffer greater cortical tissue loss [123]. These results are indicative of TNF-α playing a deleterious role during the acute phase of the traumatized brain, but providing beneficial effects in the delayed, chronic phase, and are in accord with results of greater deleterious effects in TNF-α receptor KO mice following TBI [124,125].
The mechanisms via which TNF-α can impact TBI acute and chronic outcomes are becoming elucidated. Acutely, depending on the severity of TBI, changes in blood–brain barrier permeability as well as glutamate-mediated toxicity may ensue. TNF-α has been implicated in blood–brain barrier breakdown after TBI [116] and altered permeability [126]. Additionally, TNF-α has been demonstrated to potentiate glutamate neurotoxicity and to inhibit glutamate uptake leading to its extracellular accumulation [127]. In cell culture, TNF-α has been described to be toxic to neuronal cells inducing both apoptotic and necrotic cell death. Additionally, dose-dependent administration of exogenous TNF-α has been described to intensify focal ischemic when provided acutely after the event [128]. Taken together with TNF-α inhibitor studies in TBI [116], the above results suggest that TNF-α may provide a useful target for therapeutic intervention in the acute posttraumatic period of TBI to diminish secondary brain injury.
Contrasting with its pathologic role in the acute posttraumatic period, TNF-α likely provides a beneficial role during the chronic period following TBI, where low concentrations may be neuroprotective. Hence timing and TNF-α concentration likely represent key factors to allow differentiation of the toxic versus neuroprotective effects of TNF-α. Mechanism whereby endogenous TNF-α protects neurons against ischemic and excitotoxic insults may involve the induction of antioxidant pathways. Low and more chronic TNF-α levels have been associated with induction of the expression of anti-inflammatory cytokines, typified by IL-10 and TGF-β [129] that may promote reparative mechanisms, and have actions on synaptic plasticity and ion homeostasis [130] depending on the pathway(s) via which actions are mediated.
In synopsis, the mechanisms underlying the acute pathology and behavioral deficits after TBI have yet to be fully elucidated. But current knowledge, nevertheless suggests that TNF-α represents a therapeutically interesting target to pharmacologically manipulate within a critical therapeutic time window to reduce traumatic brain damage and behavioral dysfunction.
TNF-α Synthesis and Signaling
As has been discussed, TNF-α is a potent pro-inflammatory cytokine implicated in several peripheral inflammatory human diseases such as asthma, rheumatoid arthritis, cancer, liver cirrhosis, and neurodegenerative disorders [60,131,132]. TNF-α stimulates the acute phase reaction in inflammation (Figure 2) where the downstream pathological cascades are also known to contribute to symptoms of chronic inflammatory diseases, particularly rheumatoid arthritis and Crohn’s Disease. Anti-TNF-α approaches adopted in these medical conditions have been shown to alleviate the disease symptoms [133], validating anti-TNF-α medications for use in the clinic. In the CNS TNF-α can be synthesized and released by astrocytes and microglia and other types of invading immune cells, and in certain conditions even neurons [133]. TNF-α protein is first synthesized as a monomeric type 2 II transmembrane protein (tmTNF-α), which is inserted into the membrane as a homotrimer. Thereafter, it is cleaved by TNF-alpha converting enzyme (TACE; ADAM17) a matrix metalloprotease, into a 51 kDa soluble circulating trimer [131,134,135] to form soluble TNF-α (solTNF-α). Both the soluble and membrane bound form of TNF-α can be synthesized in the CNS by microglia, astrocytes, and some populations of neurons [133,136,137]. Cellular signaling mechanisms exist by which TNF-α can exert neuroprotective actions, often through NF-κB activation [138,139]. In order to define possible treatment strategies for neuroinflammation, it is vital to gain a clear understanding that there is a complicated feedback relationship between NF-κB and JNK signaling events in addition to a caspase-mediated apoptotic pathway. Thus activation of TNF-α receptors can generally result in activation of two death-promoting pathways or one neuroprotective pathway [140]. Knowledge of these pathways allows one to see the potential benefit of down regulating TNF-α protein in patients with conditions characterized by chronic unregulated neuroinflammation. TNF-α receptor 1 (TNFR1) is expressed in most cell types, and preferentially binds solTNF-α, though it can also be activated by tmTNF-α [141,142]. The mechanism of TNFR1 activation is thought to begin with the dissociation of silencer of death domains (SODD) that permits the assembly of the TNFR1 signaling complex, allowing TNF-α receptor associated death domain (TRADD) activity [143,144]. Like other members of the TNF-α superfamily, TNFR1 contains an intracellular death domain, [131,145]. TNF-α receptor 2 (TNFR2) is preferentially activated by tmTNF, and expressed primarily by endothelial and immune cells, including microglia [141,142]. TNFR1 boasts responsibility for initiation of some of the cell-proliferating cascades that may occur upon TNF-α/receptor interaction, such as NF-κB activation, while simultaneously initiating other pro-apoptotic pathways, such as JNK [140]. In summary, TNF-α signaling events, similar to those initiated by other cytokines, are diverse and rather complicated when considered at an in vivo level [146], none-the-less a great deal about TNF-α signaling pathways have been elucidated by the use of important in vitro, cellular studies which will benefit human clinical disease management.
TNF-α Manipulation by pharmacological intervention
Theoretically, TNF-α signaling can be affected by several means, one could be to block the receptors, another could be to remove the TNF-α protein from the target tissue / organ extracellular environment, yet another could be to interfere with the secondary messenger systems associated with TNF-α signaling. Whereas these methods are excellent ways of limiting TNF-α signaling, they are somewhat hindered, by issues of classical pharmacological receptor subtype selectivity, a limited bioavailability of large proteins in the target area, i.e. typically proteins of the size of TNF-α will not readily cross the blood-brain barrier and enter into the brain; and the commonality of intermediates in second messenger pathways makes identifying selective targets difficult. However, one possible method, which is both feasible and of growing interest, is to limit the biosynthesis of the TNF-α with novel analogs of thalidomide. In 1993 Moreira and coworkers [147] determined that thalidomide was a TNF-α synthesis inhibitor, which worked destabilizing TNF-α mRNA and, thereby shortening its half-life and reducing the amount of generated TNF-α protein [147]. The use of thalidomide as a means of manipulating TNF-α protein levels has become increasingly popular in recent years. Although thalidomide is a controversial drug, several research groups are utilizing the backbone of thalidomide to generate analogs for study in several areas to medical research [148–151].
Thalidomide was first developed as an anti-epileptic in the 1950s and, with limited success in treating seizures, prescribed as a sedative and an anti-emetic. Due to teratogenicity (thalidomide caused limb bud malformations in offspring of pregnant women who were taking the drug whilst pregnant), thalidomide was removed from clinical use in the late 1950s to early 1960s [150,152]. This adverse effect was most likely related to an anti-angiogenic effect of thalidomide on the developing fetus. Secretion of vascular endothelial growth factor (VEGF) and beta fibroblast growth factor (bFGF) from bone marrow stromal cells is suppressed after exposure to thalidomide and other IMiDs [153]. Multiple myeloma cell lines proliferate in response to VEGF and [153,154]; as a consequence, the potential utility of thalidomide and an analog in the treatment of certain cancers such as multiple myeloma, myeloid cell disorders [155,156] has been more recently examined and found to be effective in a combination therapy with dexamethasone.
Thalidomide and its analogs have been shown to have regulatory effects on a number of immune markers [149]. Interestingly, the time-dependence of beneficial versus detrimental effects of these markers, such as TNF-α, further complicates our understanding of when down-regulation is most beneficial, and allows such IMiDs to be the best candidates for control of mass cytokine release and, consequently, inflammation [150].
Generally, two strategies have been adopted in the synthesis of thalidomide analogs; the first is to develop the structure based on target molecules to which thalidomide or its metabolites directly bind. The second strategy, to develop the structure of thalidomide based on hypothetical target molecules and biological responses, has led to the creation of compounds such as androgen and progesterone antagonists, cell differentiation inducers, and nuclear liver X receptors [157]. The chemical modification of the structural backbone of thalidomide has brought about second-generation immunomodulatory drugs (IMiDs) with the potential to be more selectively potent regarding anti-TNF-α activity and, simultaneously, less neurotoxic which is associated with the use of high doses of thalidomide [150].
Thalidomide’s effects in vivo have been demonstrated to be quite robust compared to its relatively weak effects on TNF-α and angiogenesis in cell culture models. This difference in activity implies that, in part, the in vivo utility of thalidomide may be driven by the generation of active metabolites [155]. For neurological use, as in neuroinflammation, modifications of thalidomide have been undertaken in our laboratory with the intent to provide a balanced aqueous/lipid solubility to combine a reasonable solubility in the aqueous phase of plasma with an ability of the compound to readily penetrate biological membranes, such as the blood-brain barrier and gastrointestinal tract, to achieve high target concentrations in brain and then to combine this with improved TNF-α inhibitory potency compared to thalidomide. Development of the novel thiothalidomide analogs resulted, as very little research had previously been conducted on the contribution of the four amide carbonyl groups of thalidomide to its biological activity. Replacement of a carbonyl group by a thiocarbonyl group resulted in generation of mono-thiothalidomide, di- and tri-thiothalidomides [155,158–160], each with improved potency in cellular and animal models. Currently our research on thiothalidomides continues, with the aim of identifying novel potent anti-TNF-α agents for assessment cellular and animal models of clinically relevant diseases, such AD and PD.
Summary
Clear proof exists indicating a role of inappropriate microglia physiological responses in the CNS with a striking association between glial related neuroinflammatory events and diseases, such as AD and PD. In addition, there is growing evidence of immune dysregulation, marked by increased cytokines - particularly TNF-α and IL-6, in psychiatric conditions, epitomized by major depression [161,162], that can occur alone and commonly accompany a neurodegenerative condition. Clearly in these conditions, a disruption to normal glial cell function has initiated a prolonged unregulated cytotoxic cellular response and dysregulation of numerous biochemical cascades able to induce pathological and mood changes, with downstream actions on neurogenesis and differentiation [163–168] as well as synaptogensis [165,169]. Pharmacological interventions that are able to reset this lost balance between protective and harmful glial cell actions should be of significant benefit in the clinic. TNF-α protein is a key candidate therapeutic target, as based on data from many clinical studies, cell biology and animal studies, abnormal regulation of this protein is strongly associated with unregulated glial cell activity. The identification of novel agents that can restore the normal function of activated CNS glial cells through means of reducing the proinflammatory effects of TNF-α protein in brain will be an essential step in human disease management. We believe that the exploration of available immunomodulatory agents, epitomized by thalidomide, and the development of newer more potent anti-TNF-α analogs in models of CNS neuroinflammation will provide vital insights into the role of TNF-α in neurological disorders, as well as badly needed therapies for human medicine.
Acknowledgements
This work was supported by the Intramural Research Program of the NIH, National Institute on Aging.
References
- 1.Frank-Cannon TC, Alto LT, McAlpine FE, Tansey MG. Does neuroinflammation fan the flame in neurodegenerative diseases? Mol Neurodegen. 2009;16:47. doi: 10.1186/1750-1326-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tansey MG, Goldberg MS. Neuroinflammation in Parkinson’s disease: its role in neuronal death and implications for therapeutic intervention. Neurobiol Dis. 2010;37:510–518. doi: 10.1016/j.nbd.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alzheimer’s Disease Facts and Figures. Alzheimer’s & Dementia. 2009;5:234–270. doi: 10.1016/j.jalz.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Yan YP, Lang BT, Vemuganti R, Dempsey RJ. Galectin-3 mediates post-ischemic tissue remodeling. Brain Res. 2009;1288:116–124. doi: 10.1016/j.brainres.2009.06.073. [DOI] [PubMed] [Google Scholar]
- 5.Lecca D, Trincavelli ML, Gelosa P, Sironi L, Ciana P, Fumagalli M, et al. The recently identified P2Y–like receptor GPR17 is a sensor of brain damage and a new target for brain repair. PLoS One. 2008;3(10):e3579. doi: 10.1371/journal.pone.0003579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bessis A, Béchade C, Bernard D, Roumier A. Microglial control of neuronal death and synaptic properties. Glia. 2007;55:233–238. doi: 10.1002/glia.20459. [DOI] [PubMed] [Google Scholar]
- 7.Streit WJ. Microglia and macrophages in the developing CNS. Neurotoxicol. 2001;22:619–624. doi: 10.1016/s0161-813x(01)00033-x. [DOI] [PubMed] [Google Scholar]
- 8.Liu B, Hong J-S. Role of microglia in inflammation-mediated neurodegenerative diseases: mechanisms and strategies for therapeutic intervention. J Pharmacol Exp Ther. 2003;304:1–7. doi: 10.1124/jpet.102.035048. [DOI] [PubMed] [Google Scholar]
- 9.McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology. 1988;38:1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- 10.Streit WJ, Xue Q-J. Life and death of microglia. J Neuroimmune Pharmacol. 2009;4:371–379. doi: 10.1007/s11481-009-9163-5. [DOI] [PubMed] [Google Scholar]
- 11.von Bernhardi R, Tichauer JE, Eugenín J. Aging-dependent changes of microglial cells and their relevance for neurodegenerative disorders. J Neurochem. 2010;112:1099–1114. doi: 10.1111/j.1471-4159.2009.06537.x. [DOI] [PubMed] [Google Scholar]
- 12.Das S, Potter H. Expression of the Alzheimer amyloid-promoting factor antichymotrypsin is induced in human astrocytes by IL-1. Neuron. 1995;4:447–456. doi: 10.1016/0896-6273(95)90300-3. [DOI] [PubMed] [Google Scholar]
- 13.Griffin WS, Stanley LC, Ling C, White L, MacLeod V, Perrot LJ, et al. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc Natl Acad Sci USA. 1989;86:7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rogers JT, Leiter LM, McPhee J, Cahill CM, Zhan SS, Potter H, Nilsson LN. Translation of the alzheimer amyloid precursor protein mRNA is up-regulated by interleukin-1 through 5’-untranslated region sequences. J Biol Chem. 1999;274:6421–6431. doi: 10.1074/jbc.274.10.6421. [DOI] [PubMed] [Google Scholar]
- 15.Rogers JT, Randall JD, Cahill CM, Eder PS, Huang X, Gunshin H, et al. An iron-responsive element type II in the 5’-untranslated region of the Alzheimer’s amyloid precursor protein transcript. J Biol Chem. 2002;277:45518–45528. doi: 10.1074/jbc.M207435200. [DOI] [PubMed] [Google Scholar]
- 16.Lahiri DK, Chen D, Vivien D, Ge YW, Greig NH, Rogers JT. Role of cytokines in the gene expression of amyloid β-protein precursor: Identification of a 5’-UTR-Binding nuclear factor and its implications in Alzheimer’s disease. J Alzheimer’s Disease. 2003a;5:81–90. doi: 10.3233/jad-2003-5203. [DOI] [PubMed] [Google Scholar]
- 17.Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 18.Avramovich Y, Amit T, Youdim MBH. Non-steroidal anti-inflammatory drugs stimulate secretion of non-amyloidogenic precursor protein. J Biol Chem. 2002;277:31466–31473. doi: 10.1074/jbc.M201308200. [DOI] [PubMed] [Google Scholar]
- 19.Lahiri DK, Farlow MR, Sambamurti K, Grieg NH, Giacobini E, Schneider LS. A critical analysis of new molecular targets and strategies for drug developments in Alzheimer’s disease. Curr Drug Targets. 2003b;4:97–112. doi: 10.2174/1389450033346957. [DOI] [PubMed] [Google Scholar]
- 20.Funato H, Yoshimura M, Yamazaki T, Saido TC, Ito Y, Yokofujita J, et al. Astrocytes containing amyloid beta-protein (Abeta)-positive granules are associated with Abeta40-positive diffuse plaques in the aged human brain. Am J Pathol. 1998;152:983–992. [PMC free article] [PubMed] [Google Scholar]
- 21.Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, et al. Inflammation and Alzheimer’s disease. Neurobiology of Aging. 2002;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bamberger ME, Harris ME, McDonald DR, Husemann J, Landreth GE. A cell surface receptor complex for fibrillar beta-amyloid mediates microglial activation. J Neurosci. 2003;23:2665–2674. doi: 10.1523/JNEUROSCI.23-07-02665.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Combs CK, Karlo JC, Kao SC, Landreth GE. Beta-Amyloid stimulation of microglia and monocytes results in TNF alpha-dependent expression of inducible nitric oxide synthase and neuronal apoptosis. J Neurosci. 2001;21:1179–1188. doi: 10.1523/JNEUROSCI.21-04-01179.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liao YF, Wang BJ, Cheng HT, Kuo LH, Wolfe MS. Tumor necrosis factor-alpha, interleukin-1beta, and interferon-gamma stimulate gamma-secretase-mediated cleavage of amyloid precursor protein through a JNK-dependent MAPK pathway. J Biol Chem. 2004;279:49523–49532. doi: 10.1074/jbc.M402034200. [DOI] [PubMed] [Google Scholar]
- 25.Alkam T, Nitta A, Mizoguchi H, Saito K, Seshima M, Itoh A, et al. Restraining tumor necrosis factor-alpha by thalidomide prevents the amyloid beta-induced impairment of recognition memory in mice. Behav Brain Res. 2008;189:100–106. doi: 10.1016/j.bbr.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 26.Zhang C, Browne A, Kim DY, Tanzi RE. Familial Alzheimer’s disease mutations in presenilin 1 do not alter levels of the secreted amyloid-beta protein precursor generated by beta-secretase cleavage. Curr Alzheimer Res. 2010;7:21–26. doi: 10.2174/156720510790274428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perry RT, Collins JS, Wiener H, Acton R, Go RC. The role of TNF and its receptors in Alzheimer’s disease. Neurobiol Aging. 2001;22:873–883. doi: 10.1016/s0197-4580(01)00291-3. [DOI] [PubMed] [Google Scholar]
- 28.Gao H-M, Liu B, Zhang W, Hong J-S. Novel anti-inflammatory therapy for Parkinson’s disease. Trends Pharmacol Sci. 2003;24:395–401. doi: 10.1016/S0165-6147(03)00176-7. [DOI] [PubMed] [Google Scholar]
- 29.Aisen PS. Anti-inflammatory therapy for Alzheimer’s disease: Implications of the prednisone trial. Acta Neurol Scand. 2000;176:85–89. doi: 10.1034/j.1600-0404.2000.00312.x. [DOI] [PubMed] [Google Scholar]
- 30.Hirsch EC, Hunot S, Damier P, Faucheux B. Glial cells and inflammation in Parkinson’s disease: a role in neurodegeneration? Ann Neurol. 1998;44:S115–S120. doi: 10.1002/ana.410440717. [DOI] [PubMed] [Google Scholar]
- 31.Stewart WF, Kawas C, Corrada M, Metter EJ. Risk of Alzheimer’s disease and duration of NSAID use. Neurology. 1997;48:626–632. doi: 10.1212/wnl.48.3.626. [DOI] [PubMed] [Google Scholar]
- 32.Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, et al. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 33.Oddo S, Caccamo A, Kitazawa M, Tseng BP, LaFerla FM. Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer’s disease. Neurobiol Aging. 2003;(8):1063–1070. doi: 10.1016/j.neurobiolaging.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 34.Janelsins MC, Mastrangelo MA, Oddo S, LaFerla FM, Federoff HJ, Bowers WJ. Early correlation of microglial activation with enhanced tumor necrosis factor-alpha and monocyte chemoattractant protein-1 expression specifically within the entorhinal cortex of triple transgenic Alzheimer’s disease mice. J Neuroinflammation. 2005;2:23. doi: 10.1186/1742-2094-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Janelsins MC, Mastrangelo MA, Park KM, Sudol KL, Narrow WC, Oddo S, et al. Chronic neuron-specific tumor necrosis factor-alpha expression enhances the local inflammatory environment ultimately leading to neuronal death in 3xTg-AD mice. Am J Pathol. 2008;173:1768–1782. doi: 10.2353/ajpath.2008.080528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McAlpine FE, Lee JK, Harms AS, Ruhn KA, Blurton-Jones M, Hong J, et al. Inhibition of soluble TNF signaling in a mouse model of Alzheimer’s disease prevents pre-plaque amyloid-associated neuropathology. Neurobiol Dis. 2009;34:163–177. doi: 10.1016/j.nbd.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fonseca MI, Ager RR, Chu SH, Yazan O, Sanderson SD, LaFerla FM, et al. Treatment with a C5aR antagonist decreases pathology and enhances behavioral performance in murine models of Alzheimer’s disease. J Immunology. 2009;183(2):1375–1383. doi: 10.4049/jimmunol.0901005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tobinick E, Gross H, Weinberger A, Cohen H. TNF-alpha modulation for treatment of Alzheimer’s disease: a 6-month pilot study. MedGenMed. 2006;8:25. [PMC free article] [PubMed] [Google Scholar]
- 39.Tobinick EL, Gross H. Rapid cognitive improvement in Alzheimer’s disease following perispinal etanercept administration. J Neuroinflammation. 2008;5:2. doi: 10.1186/1742-2094-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tobinick EL, Gross H. Rapid improvement in verbal fluency and aphasia following perispinal etanercept in Alzheimer’s disease. BMC Neurol. 2008;8:27. doi: 10.1186/1471-2377-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferger B, Leng A, Mura A, Hengerer B, Feldon J. Genetic ablation of tumor necrosis factor-alpha (TNF-α) and pharmacological inhibition of TNF-synthesis attenuates MPTP toxicity in mouse striatum. J Neurochem. 2004;89:822–833. doi: 10.1111/j.1471-4159.2004.02399.x. [DOI] [PubMed] [Google Scholar]
- 42.Westerlund M, Hoffer B, Olson L. Parkinson’s disease: Exit toxins, enter genetics. Prog Neurobiol. 2010;90:146–156. doi: 10.1016/j.pneurobio.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Varani K, Vincenzi F, Tosi A, Gessi S, Casetta I, Granieri G, et al. A2A adenosine receptor overexpression and functionality, as well as TNF-alpha levels, correlate with motor symptoms in Parkinson’s disease. FASEB J. 2010;24:587–598. doi: 10.1096/fj.09-141044. [DOI] [PubMed] [Google Scholar]
- 44.Mogi M, Harada M, Riederer P, Narabayashi H, Fujita K, Nagatsu T. Tumor necrosis factor-alpha (TNF-alpha) increases both in the brain and in the cerebrospinal fluid from parkinsonian patients. Neurosci Lett. 1994;165:208–210. doi: 10.1016/0304-3940(94)90746-3. [DOI] [PubMed] [Google Scholar]
- 45.Nagatsu T, Mogi M, Ichinose H, Togari A. Changes in cytokines and neurotrophins in Parkinson’s disease. J Neural Transm. 2000;60:277–290. doi: 10.1007/978-3-7091-6301-6_19. [DOI] [PubMed] [Google Scholar]
- 46.Lawson LJ, Perry VH, Dri P, Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39:151–170. doi: 10.1016/0306-4522(90)90229-w. [DOI] [PubMed] [Google Scholar]
- 47.Boka G, Anglade P, Wallach D, Javoy-Agid F, Agid Y, Hirsch EC. Immunocytochemical analysis of tumor necrosis factor and its receptors in Parkinson’s disease. Neurosci Lett. 1994;172:151–154. doi: 10.1016/0304-3940(94)90684-x. [DOI] [PubMed] [Google Scholar]
- 48.Mogi M, Togari A, Kondo T, Mizuno T, Komure O, Kuno S, et al. Caspase activities and tumor necrosis factor receptor R1 (p55) level are elevated in the substantia nigra from Parkinsonian brain. J Neural Transm. 2000;107:335–341. doi: 10.1007/s007020050028. [DOI] [PubMed] [Google Scholar]
- 49.Westerlund M, Hoffer B, Olson L. Parkinson’s disease: Exit toxins, enter genetics. Prog Neurobiol. 2010;90:146–156. doi: 10.1016/j.pneurobio.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219(4587):979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- 51.Nicklas WJ, Youngster SK, Kindt MV, Heikkila RE. MPTP, MPP+ and mitochondrial function. Life Sci. 1987;40:721–729. doi: 10.1016/0024-3205(87)90299-2. [DOI] [PubMed] [Google Scholar]
- 52.McGeer PL, Schwab C, Parent A, Doudet D. Presence of reactive microglia in monkey substantia nigra years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine administration. Ann Neurol. 2003;54:599–604. doi: 10.1002/ana.10728. [DOI] [PubMed] [Google Scholar]
- 53.Lahiri DK, Maloney B, Zawia NH. The LEARn model: an epigenetic explanation for idiopathic neurobiological diseases. Mol Psychiatry. 2009;14:992–1003. doi: 10.1038/mp.2009.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jang H, Boltz DA, Webster RG, Smeyne RJ. Viral parkinsonism. Biochim Biophys Acta. 2009;1792:714–721. doi: 10.1016/j.bbadis.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Calne DB, Lees AJ. Late progression of post-encephalitic Parkinson’s syndrome. Can J Neurol Sci. 1988;15:135–138. doi: 10.1017/s0317167100027499. [DOI] [PubMed] [Google Scholar]
- 56.Mawdsley C, Ferguson FR. Neurological disease in boxers. Lancet. 1963;2:795–801. doi: 10.1016/s0140-6736(63)90498-7. [DOI] [PubMed] [Google Scholar]
- 57.Bostantjopoulou S, Katsarou Z, Michael M, Petridis A. Reversible parkinsonism due to chronic bilateral subdural hematomas. J Clin Neurosci. 2009;16:458–460. doi: 10.1016/j.jocn.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 58.Rugbjerg K, Ritz B, Korbo L, Martinussen N, Olsen JH. Risk of Parkinson’s disease after hospital contact for head injury: population based case-control study. BMJ. 2008;337:a2494. doi: 10.1136/bmj.a2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cicchetti F, Drouin-Ouellet J, Gross RE. Environmental toxins and Parkinson’s disease: what have we learned from pesticide-induced animal models? Trends Pharmacol Sci. 2009;30:475–483. doi: 10.1016/j.tips.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 60.Tweedie D, Sambamurti K, Greig NH. TNF-alpha inhibition as a treatment strategy for neurodegenerative disorders: new drug candidates and targets. Curr Alzheimer Res. 2007;4:378–385. doi: 10.2174/156720507781788873. [DOI] [PubMed] [Google Scholar]
- 61.Ciesielska A, Joinec I, Przybylkowski A, Gromadzka G, Kurkowska-Jastrzebska I, Czlonkowska A, et al. Dynamics of expression of the mRNA for cytokines and inducible nitric synthase in a murine model of the Parkinson’s disease. Acta Neurobiol Exp (Wars) 2003;63:117–126. doi: 10.55782/ane-2003-1461. [DOI] [PubMed] [Google Scholar]
- 62.Hebert G, Arsaut J, Dantzer R, Demontes-Mainard J. Time-course of the expression of inflammatory cytokines and matrix metalloproteinases in the striatum and mesencephalon of mice injected with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, a dopaminergic neurotoxin. Neurosci Lett. 2003;349:191–195. doi: 10.1016/s0304-3940(03)00832-2. [DOI] [PubMed] [Google Scholar]
- 63.Mladenovic A, Perovic M, Raicevic N, Kanazir S, Rakic L, Ruzdijic S. 6-Hydroxydopamine increases the level of TNFα and bax mRNA in the striatum and induces apoptosis of dopaminergic neurons in hemiparkinsonian rats. Brain Res. 2004;996:237–345. doi: 10.1016/j.brainres.2003.10.035. [DOI] [PubMed] [Google Scholar]
- 64.De Lella Ezcurra AL, Chertoff M, Ferrari C, Graciarena M, Pitossi F. Chronic expression of low levels of tumor necrosis factor-alpha in the substantia nigra elicits progressive neurodegeneration, delayed motor symptoms and microglia/macrophage activation. Neurobiol Dis. 2010;37:630–640. doi: 10.1016/j.nbd.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 65.Boireau A, Bordier F, Dubedat P, Peny C, Imperato A. Thalidomide reduces MPTP-induced decrease in striatal dopamine levels in mice. Neurosci Lett. 1997;234:123–126. doi: 10.1016/s0304-3940(97)00685-x. [DOI] [PubMed] [Google Scholar]
- 66.Sriram K, Matheson JM, Benkovic SA, Miller DB, Luster MI, O’Callaghan JP. Mice deficient in TNF receptors are protected against dopaminergic neurotoxicity: implications for Parkinson’s disease. FASEB J. 2002;16:1474–1476. doi: 10.1096/fj.02-0216fje. [DOI] [PubMed] [Google Scholar]
- 67.Rousselet E, Callebert J, Parain K, Joubert C, Hunot S, Hartmann A, et al. Role of TNF-α receptors in mice intoxicated with the Parkinsonian toxin MPTP. Exper Neurology. 2002;177:183–192. doi: 10.1006/exnr.2002.7960. [DOI] [PubMed] [Google Scholar]
- 68.Leng A, Mura A, Feldon J, Ferger B. Tumor necrosis factor-alpha receptor ablation in a chronic MPTP mouse model of Parkinson’s disease. Neurosci Lett. 2005;375:107–111. doi: 10.1016/j.neulet.2004.10.077. [DOI] [PubMed] [Google Scholar]
- 69.Togo T, Iseki E, Marui W, Akiyama H, Ueda K, Kosaka K. Glial involvement in the degeneration process of Lewy body-bearing neurons and the degradation process of Lewy bodies in brains of dementia with Lewy bodies. J Neurological Sci. 2001;184:71–75. doi: 10.1016/s0022-510x(00)00498-6. [DOI] [PubMed] [Google Scholar]
- 70.Imamura K, Hishikawa N, Ono K, Suzuki H, Sawada M, Nagatsu T, et al. Cytokine production of activated microglia and decrease in neurotrophic factors of neurns in the hippocampus of Lewy body disease brains. Acta Neuropathol. 2005;109:141–150. doi: 10.1007/s00401-004-0919-y. [DOI] [PubMed] [Google Scholar]
- 71.Zhang W, Wang T, Pei Z, Miller DS, Wu X, Block ML, et al. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. FASEB J. 2005;19:533–542. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]
- 72.Su X, Maguire-Zeiss KA, Giuliano R, Prifti L, Venkatesh K, Federoff HJ. Synuclein activates microglia in a model of Parkinson’s disease. Neurobiol Aging. 2008;29:1690–1701. doi: 10.1016/j.neurobiolaging.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Katsuse O, Iseki E, Kosaka K. Immunohistochemical study of the expression of cytokines and nitric oxide synthases in brains of patients with dementia with Lewy bodies. Neuropathology. 2003;23:9–15. doi: 10.1046/j.1440-1789.2003.00483.x. [DOI] [PubMed] [Google Scholar]
- 74.Gurney ME, Pu Haifeng, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1172–1175. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 75.Ferri A, Nencini M, Cozzolino M, Carrara P, Moreno S, Carrì MT. Inflammatory cytokines increase mitochondrial damage in motoneuronal cells expressing mutant SOD1. Neurobiol Dis. 2008;32:454–460. doi: 10.1016/j.nbd.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 76.Liu Y, Hao W, Dawson A, Liu S, Fassbender K. Expression of amyotrophic lateral sclerosis-linked SOD1 mutant increases the neurotoxic potential of microglia via TLR2. J Biol Chem. 2009;284:3691–3699. doi: 10.1074/jbc.M804446200. [DOI] [PubMed] [Google Scholar]
- 77.D’Ambrosi N, Finocchi P, Apolloni S, Cozzolino M, Ferri A, Padovano V, et al. The proinflammatory action of microglial P2 receptors is enhanced in SOD1 models for amyotrophic lateral sclerosis. J Immunol. 2009;183:4648–4656. doi: 10.4049/jimmunol.0901212. [DOI] [PubMed] [Google Scholar]
- 78.Elliot JL. Cytokine upregulation in a murine model of familial amyotrophic lateral sclerosis. Mol Brain Res. 2001;95:172–178. doi: 10.1016/s0169-328x(01)00242-x. [DOI] [PubMed] [Google Scholar]
- 79.Hensley K, Floyd RA, Gordon B, Mou S, Pye QN, Stewart C, et al. Temporal patterns of cytokine and apoptosis-related gene expression in spinal cords of the G93A–SOD1 mouse model of amyotrophic lateral sclerosis. J Neurochem. 2002;82:365–374. doi: 10.1046/j.1471-4159.2002.00968.x. [DOI] [PubMed] [Google Scholar]
- 80.Hensley K, Fedynyshyn J, Ferrell S, Floyd RA, Gordon B, Grammas P, et al. Message and protein-level elevation of tumor necrosis factor alpha (TNF alpha) and TNF alpha-modulating cytokines in spinal cords of the G93A–SOD1 mouse model for amyotrophic lateral sclerosis. Neurobiol Disease. 2003;14:74–80. doi: 10.1016/s0969-9961(03)00087-1. [DOI] [PubMed] [Google Scholar]
- 81.Yoshihara T, Ishigaki S, Yamamoto M, Liang Y, Niwa J-I, Takeuchi H, et al. Differential expression of inflammation- and apoptosis- related genes in spinal cords of a mutant SOD1 transgenic mouse model of familial amyotrophic lateral sclerosis. J Neurochem. 2002;80:158–167. doi: 10.1046/j.0022-3042.2001.00683.x. [DOI] [PubMed] [Google Scholar]
- 82.Terenghi F, Allaria S, Nobile-Orazio E. Circulating levels of cytokines and their modulation by intravenous immunoglobulin in multifocal motor neuropathy. J Periph Nervous Sys. 2006;11:67–71. doi: 10.1111/j.1085-9489.2006.00065.x. [DOI] [PubMed] [Google Scholar]
- 83.Li M, Ona VO, Guegan C, Chen M, Jackson-Lewis V, Andrews LJ, et al. Functional role of caspase-1 and caspase-3 in an ALS transgenic mouse model. Science. 2000;288:335–339. doi: 10.1126/science.288.5464.335. [DOI] [PubMed] [Google Scholar]
- 84.Kiaei M, Petri S, Kipiani K, Gardian G, Choi D-K, chen J, et al. Thalidomide and lenalidomide extend survival in a transgenic mouse model of amyotrophic lateral sclerosis. Neurobiol Disease. 2006;26:2467–2473. doi: 10.1523/JNEUROSCI.5253-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Neymotin A, Petri S, Calingasan NY, Wille E, Schafer P, Stewart C, et al. Lenalidomide (Revlimid) administration at symptom onset is neuroprotective in a mouse model of amyotrophic lateral sclerosis. Exp Neurol. 2009;220:191–197. doi: 10.1016/j.expneurol.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Poloni M, Facchetti D, Mai R, Micheli A, Agnoletti L, francolini G, et al. Circulating levels of tumor necrosis factor-α and its soluble receptors are increased in the blood of patients with amyotrophic lateral sclerosis. Neurosci Letts. 2000;287:211–214. doi: 10.1016/s0304-3940(00)01177-0. [DOI] [PubMed] [Google Scholar]
- 87.Moreau C, Devos D, Brunaud-Danel V, Defebvre L, Perez T, Destee A, et al. Elevated IL-6 and TNF-α levels in patients with ALS: Inflammation or hypoxia? Neurology. 2005;65:1958–1960. doi: 10.1212/01.wnl.0000188907.97339.76. [DOI] [PubMed] [Google Scholar]
- 88.Stommel EW, Cohen JA, Fadul CE, Cogbill CH, Graber DJ, Kingman L, et al. Efficacy of thalidomide for the treatment of amyotrophic lateral sclerosis: a phase II open label clinical trial. Amyotroph Lateral Sclerosis. 2009;10:393–404. doi: 10.3109/17482960802709416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang JY, Peruzzi F, Lassak A, Del Valle L, Radhakrishnan S, Rappaport J, et al. Neuroprotective effects of IGF-1 against TNFα-induced neuronal damage in HIV-associated dementia. Virology. 2003;305:66–76. doi: 10.1006/viro.2002.1690. [DOI] [PubMed] [Google Scholar]
- 90.Xing HQ, Hayakawa H, Izumo K, Kubota R, Gelpi E, Budka H, et al. In vivo expression of proinflammatory cytokines in HIV encephalitis: an analysis of 11 autopsy cases. Neuropathology. 2009;29:433–442. doi: 10.1111/j.1440-1789.2008.00996.x. [DOI] [PubMed] [Google Scholar]
- 91.Xing HQ, Hayakawa H, Gelpi E, Kubota R, Budka H, Izumo S. Reduced expression of excitatory amino acid transporter 2 and diffuse microglial activation in the cerebral cortex in AIDS cases with or without HIV encephalitis. J Neuropathol Exp Neurol. 2009;68:199–209. doi: 10.1097/NEN.0b013e31819715df. [DOI] [PubMed] [Google Scholar]
- 92.Xing HQ, Moritoyo T, Mori K, Sugimoto C, Ono F, Izumo S. Expression of proinflammatory cytokines and its relationship with virus infection in the brain of macaques inoculated with macrophage-tropic simian immunodeficiency virus. Neuropathology. 2009;29:13–19. doi: 10.1111/j.1440-1789.2008.00929.x. [DOI] [PubMed] [Google Scholar]
- 93.Alirezaei M, Kiosses WB, Flynn CT, Brady NR, Fox HS. Disruption of neuronal autophagy by infected microglia results in neurodegeneration. PLoS One. 2008 Aug 6;3(8):e2906. doi: 10.1371/journal.pone.0002906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Williams R, Dhillon NK, Hegde ST, Yao H, Peng F, Callen S, et al. Proinflammatory cytokines and HIV-1 synergistically enhance CXCL10 expression in human astrocytes. Glia. 2009;57:734–743. doi: 10.1002/glia.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kamat A, Ravi V, Desai A, Satishchandra P, Satish KS, Kumar M. Estimation of virological and immunological parameters in subjects from South India infected with human immunodeficiency virus type 1 clade C and correlation of findings with occurrence of neurological disease. J Neurovirol. 2009;15:25–35. doi: 10.1080/13550280802338652. [DOI] [PubMed] [Google Scholar]
- 96.Nolting T, Lindecke A, Koutsilieri E, Maschke M, Husstedt IW, Sopper S, et al. Competence Network HIV/AIDS. Measurement of soluble inflammatory mediators in cerebrospinal fluid of human immunodeficiency virus-positive patients at distinct stages of infection by solid-phase protein array. J Neurovirol. 2009;15:390–400. doi: 10.3109/13550280903350192. [DOI] [PubMed] [Google Scholar]
- 97.Rostasy K, Monti L, Lipton SA, Hedreen JC, Gonzalez RG, Navia BA. HIV leucoencephalopathy and TNFα expression in neurons. J Neurol Neurosurg Psychiatry. 2005;76:960–964. doi: 10.1136/jnnp.2004.036889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Okamoto M, Ono M, Baba M. Suppression of cytokine production and neural cell death by the anti-inflammatory alkaloid cepharanthine: a potent agent against HIV-1 encephalopathy. Biochem Pharmacol. 2001;62:747–753. doi: 10.1016/s0006-2952(01)00692-x. [DOI] [PubMed] [Google Scholar]
- 99.Barak O, Goshen I, Ben-Hur T, Weidenfeld J, Taylor AN, Yirmiya R. Involvement of brain cytokines in the neurobehavioral disturbances induced by HIV-1 glycoprotein 120. Brain Res. 2002;933:98–108. doi: 10.1016/s0006-8993(02)02280-1. [DOI] [PubMed] [Google Scholar]
- 100.Ghoshal A, Das S, Ghosh S, Mishra MK, Sharma V, Koli P, et al. Proinflammatory mediators released by activated microglia induces neuronal death in Japanese encephalitis. Glia. 2007;55:483–496. doi: 10.1002/glia.20474. [DOI] [PubMed] [Google Scholar]
- 101.Chen CJ, Ou YC, Lin SY, Raung SL, Liao SL, Lai CY, et al. Glial activation involvement in neuronal death by Japanese encephalitis virus infection. J Gen Virol. 2010;91:1028–1037. doi: 10.1099/vir.0.013565-0. [DOI] [PubMed] [Google Scholar]
- 102.Tsao CH, Su HL, Lin YL, Yu HP, Kuo SM, Shen CI, et al. Japanese encephalitis virus infection activates caspase-8 and −9 in a FADD-independent and mitochondrion-dependent manner. J Gen Virol. 2008;89:1930–1941. doi: 10.1099/vir.0.2008/000182-0. [DOI] [PubMed] [Google Scholar]
- 103.Swarup V, Das S, Ghosh S, Basu A. Tumor necrosis factor receptor-1-induced neuronal death by TRADD contributes to the pathogenesis of Japanese encephalitis. J Neurochem. 2007;103:771–783. doi: 10.1111/j.1471-4159.2007.04790.x. [DOI] [PubMed] [Google Scholar]
- 104.Swarup V, Ghosh J, Das S, Basu A. Tumor necrosis factor receptor-associated death domain mediated neuronal death contributes to the glial activation and subsequent neuroinflammation in Japanese encephalitis. Neurochem Int. 2008;52:1310–1321. doi: 10.1016/j.neuint.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 105.Hosoya M, Kawasaki Y, Katayose M, Sakuma H, Watanabe M, Igarashi E, et al. Prognostic predictive values of serum cytochrome c, cytokines, and other laboratory measurements in acute encephalopathy with multiple organ failure. Arch Dis Child. 2006;91:469–472. doi: 10.1136/adc.2005.078436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kalita J, Srivastava R, Mishra MK, Basu A, Misra UK. Cytokines and chemokines in viral encephalitis: a clinicoradiological correlation. Neurosci Lett. 2010;473:48–51. doi: 10.1016/j.neulet.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 107.Kawada J-I, Kimura H, Ito Y, Hara S, Iriyama M, Yoshikawa T, et al. Systemic cytokine responses in patients with influenza-associated encephalopathy. J Infectious Diseases. 2003;188:690–698. doi: 10.1086/377101. [DOI] [PubMed] [Google Scholar]
- 108.Nunoi H, Mercado MR, Mizukami T, Okajima K, Morishima T, Sakata H, et al. Apoptosis under hypercytokinemia is a possible pathogenesis in influenza-associated encephalopathy. Pediatrics Int. 2005;47:175–179. doi: 10.1111/j.1442-200x.2005.02042.x. [DOI] [PubMed] [Google Scholar]
- 109.Hosoya M, Nunoi H, Aoyama M, Kawasaki Y, Suzuki H. Cytochrome c and tumor necrosis factor-α values in serum and cerebrospinal fluid of patients with influenza-associated encephalopathy. Ped Infectious Disease J. 2005;24:467–470. doi: 10.1097/01.inf.0000160995.07461.b8. [DOI] [PubMed] [Google Scholar]
- 110.Babu GN, Kalita J, Misira UK. Inflammatory markers in the patients of Japanese encephalitis. Neurological Res. 2006;28:190–192. doi: 10.1179/016164106X98062. [DOI] [PubMed] [Google Scholar]
- 111.Garner J, Brett SJ. Mechanisms of Injury by Explosive Devices. Anesthesiology Clin. 2007;25:147–160. doi: 10.1016/j.anclin.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 112.Moppett IK. Traumatic brain injury: assessment, resuscitation and early management. Br J Anaesth. 2007;99:18–31. doi: 10.1093/bja/aem128. [DOI] [PubMed] [Google Scholar]
- 113.Raghupathi R. Cell death mechanisms following traumatic brain injury. Brain Pathol. 2004;14:215–222. doi: 10.1111/j.1750-3639.2004.tb00056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lu J, Goh SJ, Tng PY, Deng YY, Ling EA, Moochhala S. Systemic inflammatory response following acute traumatic brain injury. Front Biosci. 2009;14:3795–813. doi: 10.2741/3489. [DOI] [PubMed] [Google Scholar]
- 115.Yang J, You Z, Kim HH, Hwang SK, Khuman J, Guo S, Lo EH, Whalen MJ. Genetic analysis of the role of tumor necrosis factor receptors in functional outcome after traumatic brain injury in mice. J Neurotrauma. 2010;27:1037–1046. doi: 10.1089/neu.2009.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shohami E, Gallily R, Mechoulam R, Bass R, Ben-Hur T. Cytokine production in the brain following closed head injury: dexanabinol (HU-211) is a novel TNF-α inhibitor and an effective neuroprotectant. J Neuroimmunol. 1997;72:169–177. doi: 10.1016/s0165-5728(96)00181-6. [DOI] [PubMed] [Google Scholar]
- 117.Israelsson C, Wang Y, Kylberg A, Pick CG, Hoffer BJ, Ebendal T. Closed head injury in a mouse model results in molecular changes indicating inflammatory responses. J Neurotrauma. 2009;26:1307–1314. doi: 10.1089/neu.2008.0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Taupin V, Toulmond S, Serrano A, Benavides J, Zavala F. Increase in IL-6, IL-1 and TNF levels in rat brain following traumatic lesion: influence of pre- and post-traumatic treatment with Ro5 4864, a peripheral-type (p site) benzodiazepine ligand. J Neuroimmunol. 1993;42:177–185. doi: 10.1016/0165-5728(93)90008-m. [DOI] [PubMed] [Google Scholar]
- 119.Shohami E, Novikov M, Bass R, Yamin A, Gallily R. Closed head injury triggers early production of TNFα and IL-6 by brain tissue. J Cereb Blood Flow Metab. 1994;14:615–619. doi: 10.1038/jcbfm.1994.76. [DOI] [PubMed] [Google Scholar]
- 120.Knoblach SM, Fan L, Faden AI. Early neuronal expression of tumor necrosis factor-α after experimental brain injury contributes to neurological impairment. J Neuroimmunol. 1999;95:115–125. doi: 10.1016/s0165-5728(98)00273-2. [DOI] [PubMed] [Google Scholar]
- 121.Winter CD, Iannotti F, Pringle AK, Trikkas C, Clough GF, Church MK. A microdialysis method for the recovery of IL-1β, IL-6 and nerve growth factor from human brain in vivo. J Neurosci Methods. 2002;119:45–50. doi: 10.1016/s0165-0270(02)00153-x. [DOI] [PubMed] [Google Scholar]
- 122.Woodroofe MN, Sarna GS, Wadhwa M, Hayes GM, Loughlin AJ, Tinker A, Cuzner ML. Detection of interleukin-1 and interleukin-6 in adult rat brain, following mechanical injury, by in vivo microdialysis: evidence of a role for microglia in cytokine production. J Neuroimmunol. 1991;33:227–236. doi: 10.1016/0165-5728(91)90110-s. [DOI] [PubMed] [Google Scholar]
- 123.Scherbel U, Raghupathi R, Nakamura M, Saatman KE, Trojanowski JQ, Neugebauer E, Marino MW, McIntosh TK. Differential acute and chronic responses of tumor necrosis factor-deficient mice to experimental brain injury. Proc Natl Acad Sci U S A. 1999;96:8721–8726. doi: 10.1073/pnas.96.15.8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Stahel PF, Shohami E, Younis FM, Kariya K, Otto VI, Lenzlinger PM, Grosjean MB, Eugster HP, Trentz O, Kossmann T, Morganti-Kossmann MC. Experimental closed head injury: analysis of neurological outcome, blood-brain barrier dysfunction, intracranial neutrophil infiltration, and neuronal cell death in mice deficient in genes for pro-inflammatory cytokines. J Cereb Blood Flow Metab. 2000;20:369–380. doi: 10.1097/00004647-200002000-00019. [DOI] [PubMed] [Google Scholar]
- 125.Sullivan PG, Bruce-Keller AJ, Rabchevsky AG, Christakos S, Clair DK, Mattson MP, Scheff SW. Exacerbation of damage and altered NF-κB activation in mice lacking tumor necrosis factor receptors after traumatic brain injury. J Neurosci. 1999;19:6248–6256. doi: 10.1523/JNEUROSCI.19-15-06248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rosenberg GA, Estrada EY, Dencoff JE, Stetler-Stevenson WG. Tumor necrosis factor-alpha-induced gelatinase B causes delayed opening of the blood-brain barrier: An expanded therapeutic window. Brain Res. 1995;703:151–155. doi: 10.1016/0006-8993(95)01089-0. [DOI] [PubMed] [Google Scholar]
- 127.Ye ZC, Sontheimer H. Cytokine modulation of glial glutamate uptake: a possible involvement of nitric oxide. NeuroReport. 1996;7:2181–2185. doi: 10.1097/00001756-199609020-00025. [DOI] [PubMed] [Google Scholar]
- 128.Barone FC, Arvin B, White RF, Miller A, Webb CL, Willete RN, Lysko PG, Feuerstein GZ. Tumor necrosis factor-alpha. A mediator of focal ischemic brain injury Stroke. 1998;28:1233–1244. doi: 10.1161/01.str.28.6.1233. [DOI] [PubMed] [Google Scholar]
- 129.Delannoy I, Lekeux P, Miossec P. Cytokine and anti-cytokine strategies in inflammatory reaction modulation. Vet Res. 1993;24:449–467. [PubMed] [Google Scholar]
- 130.Park KM, Bowers WJ. Tumor necrosis factor-alpha mediated signaling in neuronal homeostasis and dysfunction. Cell Signal. 2010;22:977–983. doi: 10.1016/j.cellsig.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chadwick W, Magnus T, Martin B, Keselman A, Mattson MP, Maudsley S. Targeting TNF-α receptors for neurotherapeutics. Trends Neurosci. 2008;311(10):504–511. doi: 10.1016/j.tins.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shiraki M, Terakura Y, Iwasa J, Shimizu M, Miwa Y, Murakami N, et al. Elevated serum tumor necrosis factor-alpha and soluble tumor necrosis factor receptors correlate with aberrant energy metabolism in liver cirrhosis. Nutrition. 2010;26:269–275. doi: 10.1016/j.nut.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 133.Clark IA. How TNF was recognized as a key mechanism of disease. Cytokine Growth Factor Rev. 2007;18:335–343. doi: 10.1016/j.cytogfr.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 134.Idriss HT, Naismith JH. TNF alpha and the TNF receptor superfamily: structure-function relationship(s) Microsc Res Tech. 2000;50:184–195. doi: 10.1002/1097-0029(20000801)50:3<184::AID-JEMT2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 135.MacEwan DJ. TNF receptor subtype signaling: differences and cellular consequences. Cell Signal. 2002;14:414–434. doi: 10.1016/s0898-6568(01)00262-5. [DOI] [PubMed] [Google Scholar]
- 136.Lieberman AP, Pitha PM, Shin HS, Shin ML. Production of tumor necrosis factor and other cytokines by astrocytes stimulated with lipopolysaccharide or a neurotropic virus. Proc Natl Acad Sci USA. 1989;86:6348–6352. doi: 10.1073/pnas.86.16.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Morganti-Kossman MC, Lenzlinger PM, Hans V, Stahel P, Csuka E, Ammann E, et al. Production of cytokines following brain injury: beneficial and deleterious for the damaged tissue. Mol Psychiatry. 1997;2:133–136. doi: 10.1038/sj.mp.4000227. [DOI] [PubMed] [Google Scholar]
- 138.Barger SW, Hörster D, Furukawa K, Goodman Y, Krieglstein J, Mattson MP. Tumor necrosis factors alpha and beta protect neurons against amyloid beta-peptide toxicity: evidence for involvement of a kappa B-binding factor and attenuation of peroxide and Ca2+ accumulation. Proc Natl Acad Sci USA. 1995;92:9328–9332. doi: 10.1073/pnas.92.20.9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Marchetti L, Klein M, Schlett K, Pfizenmaier K, Eisel UL. Tumor necrosis factor (TNF)-mediated neuroprotection against glutamate-induced excitotoxicity is enhanced by N-methyl-Daspartate receptor activation. Essential role of a TNF receptor 2-mediated phosphatidylinositol 3-kinase-dependent NF-kappa B pathway. J Biol Chem. 2004;279:32869–32881. doi: 10.1074/jbc.M311766200. [DOI] [PubMed] [Google Scholar]
- 140.Chen G, Goeddel DV. TNF-R1 Signaling: A Beautiful Pathway. Science. 2002;296:1634–1635. doi: 10.1126/science.1071924. [DOI] [PubMed] [Google Scholar]
- 141.Grell M. Tumor necrosis factor (TNF) receptors in cellular signaling of soluble and membrane-expressed TNF. J Inflamm. 1995;47:8–17. [PubMed] [Google Scholar]
- 142.Grell M, Wajant H, Zimmermann G, Scheurich P. The type 1 receptor (CD120a) is the high-affinity receptor for soluble tumor necrosis factor. Proc Natl Acad Sci USA. 1998;95:570–575. doi: 10.1073/pnas.95.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tartaglia LA, Ayres TM, Wong GH, Goeddel DV. A novel domain within the 55 kd TNF receptor signals cell death. Cell. 1993;74:845–853. doi: 10.1016/0092-8674(93)90464-2. [DOI] [PubMed] [Google Scholar]
- 144.Ware CF, VanArsdale S, VanArsdale TL. Apoptosis mediated by the TNF-related cytokine and receptor families. J Cell Biochem. 1996;60:47–55. doi: 10.1002/(SICI)1097-4644(19960101)60:1%3C47::AID-JCB8%3E3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 145.Lorz C, Mehmet H. The role of death receptors in neural injury. Front Biosci. 2009;14:583–595. doi: 10.2741/3265. [DOI] [PubMed] [Google Scholar]
- 146.Reale M, Greig NH, Kamal MA. Peripheral chemo-cytokine profiles in Alzheimer’s and Parkinson’s diseases. Mini Rev Med Chem. 2009;9:1229–1241. doi: 10.2174/138955709789055199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Moreira AL, Sampaio EP, Zmuidzinas A, Frindt P, Smith K, Kaplan G. Thalidomide exerts its inhibitor action on tumor necrosis factor alpha by enhancing mRNA degradation. J Exp Med. 1993;177:1675–1680. doi: 10.1084/jem.177.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Aragon-Ching JB, Li H, Gardner ER, Figg WD. Thalidomide analogues as anticancer drugs. Recent Pat Anticancer Drug Discov. 2007;2:167–174. doi: 10.2174/157489207780832478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Knight R. IMiDs: A novel class of immunomodulators. Sem Oncol. 2005;32(suppl 5):S24–S30. doi: 10.1053/j.seminoncol.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 150.Melchert M, List A. The thalidomide saga. Int J Biochem Cell Biol. 2007;39:1489–1499. doi: 10.1016/j.biocel.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 151.Wines NY, Cooper AJ, Wines MP. Thalidomide in dermatology. Australas J Dermatol. 2002;43:229–240. doi: 10.1046/j.1440-0960.2002.00608.x. [DOI] [PubMed] [Google Scholar]
- 152.Randall T. Thalidomide has 37-year history. JAMA. 1990;263(11):1474. doi: 10.1001/jama.1990.03440110028006. [DOI] [PubMed] [Google Scholar]
- 153.Bellamy W, Richter L, Sirjani D, Roxas C, Glinsmann-Gibson B, Frutiger Y, et al. Vascular endothelial growth factor is an autocrine promoter of abnormal localized immature myeloid precursors and leukemia progenitor formation in myelodysplastic syndromes. Blood. 2001;97:1427–1434. doi: 10.1182/blood.v97.5.1427. [DOI] [PubMed] [Google Scholar]
- 154.Gupta D, Treon SP, Shima Y, Hideshima T, Podar K, Tai YT, et al. Adherence of multiple myeloma cells to bone marrow stromal cells upregulates vascular endothelial growth factor secretion: therapeutic applications. Leukemia. 2001;15:1950–1961. doi: 10.1038/sj.leu.2402295. [DOI] [PubMed] [Google Scholar]
- 155.Zhu X, Giordano T, Yu QS, Holloway HW, Perry TA, Lahiri DK, et al. Thiothalidomides: novel isosteric analogues of thalidomide with enhanced TNF-alpha inhibitory activity. J Med Chem. 2003;46:5222–5229. doi: 10.1021/jm030152f. [DOI] [PubMed] [Google Scholar]
- 156.D’Amato R, Loughnan MS, Flynn E, Folkman J. Thalidomide is an inhibitor of angiogenesis. Proc. Natl. Acad. Sci. USA. 1994;91:4082–4085. doi: 10.1073/pnas.91.9.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hashimoto Y. Thalidomide as a multi-template for development of biologically active compounds. Arch Pharm Chem Life Sci. 2008;341:536–547. doi: 10.1002/ardp.200700217. [DOI] [PubMed] [Google Scholar]
- 158.Greig NH, Giordano T, Zhu X, Yu QS, Perry T, Holloway HW, et al. Thalidomide-based TNF-α inhibitors for neurodegenerative disease. Acta Neorobiol Exp. 2004;64:1–9. doi: 10.55782/ane-2004-1486. [DOI] [PubMed] [Google Scholar]
- 159.Luo W, Yu QS, Tweedie D, Deschamps J, Parrish D, Holloway HW, Li Y, Brossi A, Greig NH. Syntheses of aromatic substituted 6’-thiothalidomides. Synthesis. 2008;21:3415–3422. [Google Scholar]
- 160.Tweedie D, Luo W, Short RG, Brossi A, Holloway HW, Li Y, et al. A cellular model of inflammation for identifying TNF-alpha synthesis inhibitors. J Neurosci Methods. 2009;183:182–187. doi: 10.1016/j.jneumeth.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctôt KL. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 162.Schroeter ML, Abdul-Khaliq H, Krebs M, Diefenbacher A, Blasig IE. Serum markers support disease-specific glial pathology in major depression. J Affect Disord. 2008;111:271–280. doi: 10.1016/j.jad.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 163.Ekdahl CT, Claasen HH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci U S A. 2003;100:13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Iosif RE, Ekdahl CT, Ahlenius H, et al. Tumor necrosis factor receptor 1 is a negative regulator of progenitor proliferation in adult hippocampal neurogenesis. J Neurosci. 2006;26:9703–9712. doi: 10.1523/JNEUROSCI.2723-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bilbo SD, Schwarz JM. Early-life programming of later-life brain and behavior: a critical role for the immune system. Front Behav Neurosci. 2009;3:14. doi: 10.3389/neuro.08.014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Keohane A, Ryan S, Maloney E, Sullivan AM, Nolan YM. Tumour necrosis factor-alpha impairs neuronal differentiation but not proliferation of hippocampal neural precursor cells: Role of Hes1. Mol Cell Neurosci. 2010;43:127–135. doi: 10.1016/j.mcn.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 167.Liu JL, Tian DS, Li ZW, Qu WS, Zhan Y, Xie MJ, Yu ZY, Wang W, Wu G. Tamoxifen alleviates irradiation-induced brain injury by attenuating microglial inflammatory response in vitro and in vivo. Brain Res. 2010;1316:101–111. doi: 10.1016/j.brainres.2009.12.055. [DOI] [PubMed] [Google Scholar]
- 168.Iosif RE, Ahlenius H, Ekdahl CT, Darsalia V, Thored P, Jovinge S, Kokaia Z, Lindvall O. Suppression of stroke-induced progenitor proliferation in adult subventricular zone by tumor necrosis factor receptor 1. J Cereb Blood Flow Metab. 2008;28:1574–1587. doi: 10.1038/jcbfm.2008.47. [DOI] [PubMed] [Google Scholar]
- 169.Dziegielewska KM, Møller JE, Potter AM, Ek J, Lane MA, Saunders NR. Acute-phase cytokines IL-1beta and TNF-alpha in brain development. Cell Tissue Res. 2000;299:335–345. doi: 10.1007/s004419900157. [DOI] [PubMed] [Google Scholar]
- 170.Benasciutti E, Pagès G, Kenzior O, Folk W, Blasi F, Crippa MP. MAPK and JNK transduction pathways can phosphorylate Sp1 to activate the uPA minimal promoter element and endogenous gene transcription. Blood. 2004;104:256–262. doi: 10.1182/blood-2003-08-2661. [DOI] [PubMed] [Google Scholar]
- 171.Schneider-Brachert W, Tchikov V, Neumeyer J, Jakob M, Winoto-Morbach S, Held-Feindt J, Heinrich M, Merkel O, Ehrenschwender M, Adam D, Mentlein R, Kabelitz D, Schütze S. Compartmentalization of TNF receptor 1 signaling: internalized TNF receptosomes as death signaling vesicles. Immunity. 2004;21:415–428. doi: 10.1016/j.immuni.2004.08.017. [DOI] [PubMed] [Google Scholar]