Abstract
OBJECTIVE
To examine the survival of infants with hypoplastic left heart syndrome (HLHS) and potential influence of demographic and clinical characteristics on survival using population-based data.
METHODS
Infants with nonsyndromic HLHS (n = 212) born between 1979 and 2005 were identified through the Metropolitan Atlanta Congenital Defects Program. Vital status was ascertained through 2009 based on linkage with vital records. We estimated Kaplan-Meier survival probabilities stratified by select demographic and clinical characteristics.
RESULTS
The overall survival probability to 2009 was 24% and significantly improved over time: from 0% in 1979–1984 to 42% in 1999–2005. Survival probability was 66% during the first week, 27% during the first year of life, and 24% during the first 10 years. Survival of very low and low birth weight or preterm infants and those born in high-poverty neighborhoods was significantly poorer. For children with information on surgical intervention (n = 88), the overall survival was 52%, and preterm infants had significantly poorer survival (31%) compared with term infants (56%). For children who survived to 1 year of age, long-term survival was ~90%.
CONCLUSIONS
Survival to adolescence of children with nonsyndromic HLHS born in metropolitan Atlanta has significantly improved in recent years, with those born full term, with normal birth weight, or in a low-poverty neighborhood having a higher survival probability. Survival beyond infancy to adolescence is high. A better understanding of the growing population of survivors with HLHS is needed to inform resource planning.
Hypoplastic left heart syndrome (HLHS) is a complex congenital heart defect (CHD) occurring in ~2 per 10 000 livebirths.1 It is fatal without surgical intervention and responsible for 25% to 40% of all neonatal cardiac mortality.2 Studies have shown 1-year survival for HLHS ranges from 20% to 60%,3–9 with relatively stable 5-year, 10-year, and 15-year survival of ~40%.4,6 The highest mortality for infants with HLHS undergoing surgical intervention is usually with stage 1 palliation and the interstage period between stage 1 and stage 2 surgery, both done in the first year of life.10
Survival estimates for HLHS have improved in the recent birth era,6 reflecting significant improvements in management of HLHS.11 In a national, population-based study using death certificate data from 1979 to 1997, there was no significant decrease in infant mortality attributed to HLHS after the mid-1980s.12 Another population-based study in Texas showed significantly lower mortality for HLHS in the period from 2001–2003 compared with the period from 1996–2000.6 A population-based study in New York State linking to in-state mortality records examined the survival of children born between 1983 and 2006 with selected congenital heart defects and found that children with HLHS born in later years had a significantly higher survival probablity.13 However, no other population-based study has examined survival of HLHS beyond childhood using multiple sources of mortality data, across several birth eras, and prognostic factors. In addition to improvements in medical care, other potential prognostic factors include infant characteristics such as low birth weight, prematurity, presence of other birth defects or chromosomal anomalies, race/ethnicity, and high poverty neighborhood.6,14,15 However, the extent to which these factors may influence survival is uncertain.
In this study, we examined changes over time in survival of children with HLHS using population-based birth defects surveillance data collected on children born over a period of 30 years in metropolitan Atlanta, Georgia. In addition, we evaluated clinical and demographic characteristics as potential prognostic factors for such a birth cohort of HLHS.
METHODS
Birth Cohort
We included children with HLHS ascertained by the Metropolitan Atlanta Congenital Defects Program (MACDP), an ongoing population-based birth defects surveillance system, for birth years 1979 to 2005. MACDP has actively monitored birth defects among live-born infants and fetal deaths of ≥20 weeks’ gestation or >500 g born to resident mothers of the 5-county metropolitan Atlanta area since 1968. MACDP operates in partnership with the Georgia Department of Public Health and has approval of the institutional review board of the Centers for Disease Control and Prevention. Trained abstractors collect demographic and clinical information of structural birth defects, chromosomal abnormalities, and genetic syndromes from several data sources. Additional information about MACDP has been published previously.16
The surveillance data for children with HLHS in MACDP were previously reviewed and classified by experts in pediatric cardiology using standard clinical nomenclature from the Society of Thoracic Surgeons and developmental schema.17 In our study, we included children with isolated HLHS (ie, no other major CHD) and those with associated major extracardiac defects. We defined associated defects as “major” if they had surgical, medical, or serious cosmetic importance.18 Children with chromosomal abnormalities or known syndromes (eg, trisomies or Noonan syndrome) were excluded.
Follow-up
We linked children with HLHS born during the period 1979 to 2005 to Georgia vital records to identify in-state deaths through 2009. We also linked these children with death records available through 2009 in the National Death Index (NDI), a centralized index of death record information compiled by the National Center for Health Statistics. NDI data files were unavailable beyond 2009; hence, we could not trace survival through multiple sources for more recent years. General information and details about the matching process have been described previously.19,20 We considered children without death records alive and censored their survival times at the end of the follow-up period on December 31, 2009.
Prognostic Factors
In our survival analysis, we considered several demographic and clinical characteristics as potential prognostic factors. Demographic variables included birth period (1979–1984, 1985–1991, 1992–1998, 1999–2005), race/ethnicity (non-Hispanic white, non-Hispanic black, other), infant gender (male, female), maternal age (<20, 20–34; ≥35 years of age), and neighborhood poverty (low, high). Poverty level was based on the percentage of people living in poverty during the closest US census data year (1980, 1990, or 2000) in the census tract linked to the geocoded maternal residence at the time of birth: (1) if <20% of people living in a given census tract were living below the federally defined poverty line,21 the area was considered as a low-poverty neighborhood; and (2) ≥20%, a high-poverty neighborhood. Using census tract data as proxies for socioeconomic status has been suggested and validated by previous studies.22 Clinical variables included birth weight (<1500, 1500–2499, ≥2500 g), gestational age (preterm or <37, term or ≥37 weeks), plurality (single, multiple), presence of other major extracardiac defects (absent, present), and occurrence of cardiac surgery shortly after birth documented in abstraction record (yes, no). Information on surgical interventions in abstraction records were scarce before 1992; hence, we only included this information on the birth cohorts from 1992 through 2005 and stratified analysis accordingly.
Statistical Analyses
We estimated the survival probability of children with HLHS by age and birth cohort using the Kaplan-Meier product-limit method.23 To calculate the variance of the estimated survival probability and their 95% confidence intervals (CIs), we applied Greenwood's method.24 We used the log-rank test to determine whether the survival probabilities were significantly different among different levels of the potential risk factors, both among all children with HLHS and among those who underwent surgical interventions. Variables associated with P values < .05 in the univariate analysis were included in the Cox proportional hazards regression models.25 Cox proportional hazard models were used to estimate unadjusted and adjusted hazard ratios (aHR) for possible prognostic factors. The assumption of proportionality for the hazards was checked by plotting estimated log-cumulative hazard versus log of survival time for different categories of the risk factors. aHRs were estimated from a multivariable proportional hazard model, with the birth periods collapsed to create a covariate designating eras without and with surgical information (1979–1991 and 1992–2005). All statistical analyses were performed by using SAS version 9.3 (SAS Institute Inc, Cary, NC).
RESULTS
Of 229 children with HLHS identified by MACDP born 1979–2005, 212 were classified as nonsyndromic (183 or 86% with no associated major defects) and 17 as having a known chromosomal defect or syndrome. Of the 212 nonsyndromic HLHS children who were the focus of this analysis, 160 died during the follow-up period (23 deaths found only in NDI). The overall survival probability of children with nonsyndromic HLHS was 24%, with most deaths occurring within the first year. The survival probability by age was 95% during the first day, 66% during the first week, 40% during the first month, and 27% during the first year of life, remaining relatively stable thereafter (Table 1). Among children who survived to 1 year of age (n = 58), 90% (n = 52) survived up to 18 years of age. The survival probability varied by birth period and age group (Table 1), being 0% in age groups beyond 1 year in the earliest birth cohort (1979–1984) and 48% among 1- to 5-year-olds for the more recent birth cohort (1999–2005).
TABLE 1.
Survival Probability by Birth Period and Age Interval Among Children With HLHS, Atlanta, Georgia
| Age Interval | All Births 1979–2005 |
Birth Period |
|||||
|---|---|---|---|---|---|---|---|
| No. at Risk | No. of Deaths | Survival % (95% CI) | 1979–1984 Survival % (95% CI) | 1985–1991 Survival % (95% CI) | 1992–1998 Survival % (95% CI) | 1999–2005 Survival % (95% CI) | |
| Birth–<1 d | 212 | 11 | 94.8 (90.8–97.1) | 90.2 (76.1–96.2) | 91.4 (80.5–96.3) | 98.1 (87.4–99.7) | 98.3 (88.8–99.8) |
| 1 d–<7 d | 201 | 62 | 65.6 (58.8–71.5) | 31.7 (18.3–46.0) | 53.5 (39.9–65.3) | 83.0 (69.9–90.8) | 85.0 (73.2–91.9) |
| 7 d–<28 d | 139 | 54 | 40.1 (33.5–46.6) | 12.2 (4.5–24.1) | 19.0 (10.1–29.9) | 60.4 (46.0–72.1) | 61.7 (48.2–72.6) |
| 28 d–<1 y | 85 | 27 | 27.4 (21.5–33.5) | 2.4 (0.2–11.0) | 10.3 (4.2–19.7) | 41.5 (28.2–54.3) | 48.3 (35.3–60.2) |
| 1 y–<5 y | 58 | 3 | 25.9 (20.3–32.0) | 0.0 | 6.9 (2.2–15.3) | 41.5 (28.2–54.3) | 48.3 (35.3–60.2) |
| 5 y–<10 y | 55 | 2 | 24.7 (19.1–30.8) | 0.0 | 6.9 (2.2–15.3) | 41.5 (28.2–54.3) | 42.5 (28.9–55.4) |
| 10 y–<30 y | 53 | 1 | 23.7 (18.0–29.8) | 0.0 | 6.9 (2.2–15.3) | 39.3 (26.2–52.2) | — |
| Overall (<30 y) | 212 | 160 | 23.7 (18.0–29.8) | 0.0 | 6.9 (2.2–15.3) | 39.3 (26.2–52.2) | 42.5 (28.9–55.4) |
Birth period: 1979–2005; follow-up: December 31, 2009; unadjusted. —, no data available beyond 10 years of age for this birth period.
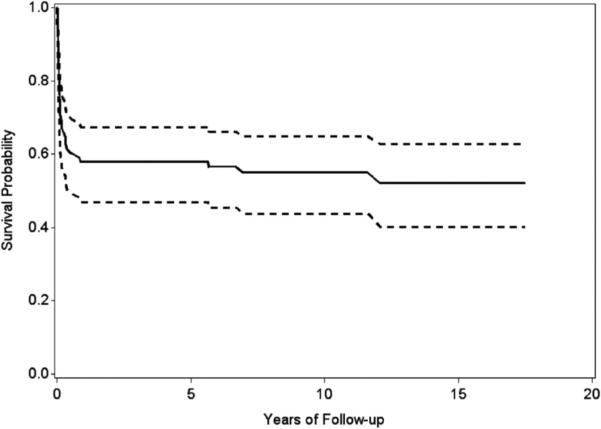
Table 2 shows unadjusted survival probabilities by select demographic and clinical characteristics. Survival significantly improved over time, from 0% in 1979–1984 to 42% in 1999–2005 (P < .001). Combining birth periods to 1979–1991 and 1992–2005 resulted in the same significant improvement in survival probability (P < .001; data not shown). The survival probability did not differ significantly by race/ethnicity, infant gender, maternal age, plurality, or presence of extracardiac defects. Survival probabilities of children with very low or low birth weight (0% and 16% respectively), those born preterm (12%), or those born in high-poverty neighborhoods (9%) were significantly lower than children of normal birth weight (26%), born at term (26%), or those born in low-poverty neighborhoods (25%; P = .01, .02, .01, respectively; Table 2). In the birth period (1992–2005) for which information was ascertained on cardiac surgery, 23 of 111 (21%) infants with HLHS did not have surgical intervention, and all died. Among those who underwent surgery, survival probability up to 18 years was 52% (Fig 1).
TABLE 2.
Survival Probability of Children With HLHS by Selected Demographic and Clinical Characteristics, Atlanta, Georgia
| Characteristics | No. of Births | No. of Deaths (%) | Survival % (95% CI) | P (Log-Rank Test) |
|---|---|---|---|---|
| Birth period | <.001 | |||
| 1979–1984 | 41 | 41 (100.0) | 0.0c | |
| 1985–1991 | 58 | 54 (93.1) | 6.9 (2.2–15.3) | |
| 1992–1998 | 53 | 32 (60.4) | 39.3 (26.2–52.2) | |
| 1999–2005 | 60 | 33 (55.0) | 42.5 (28.9–55.4) | |
| Race/ethnicitya | .45 | |||
| White (non-Hispanic) | 104 | 78 (75.0) | 23.9 (15.8–32.8) | |
| Black (non-Hispanic) | 83 | 66 (79.5) | 20.3 (12.4–29.6) | |
| Other | 23 | 16 (69.6) | 30.4 (13.5–49.3) | |
| Infant gender | .51 | |||
| Male | 118 | 92 (78.0) | 21.1 (14.0–29.3) | |
| Female | 94 | 68 (72.3) | 27.0 (18.3–36.4) | |
| Maternal age (y) | .17 | |||
| <20 | 28 | 23 (82.1) | 17.9 (6.5–33.7) | |
| 20–34 | 164 | 125 (76.2) | 22.7 (16.3–29.7) | |
| ≥35 | 20 | 12 (60.0) | 40.0 (19.3–60.0) | |
| Birth weight (g) | .01 | |||
| <1500 | 4 | 4 (100.0) | 0.0 | |
| 1500–2499 | 32 | 27 (84.4) | 15.6 (5.7–30.0) | |
| ≥2500 | 176 | 129 (73.3) | 25.6 (19.1–32.6) | |
| Gestational age (wk)a | .02 | |||
| Preterm (<37) | 34 | 30 (88.2) | 11.8 (3.7–24.9) | |
| Term (≥ 7) | 177 | 129 (72.9) | 26.0 (19.5–33.0) | |
| Plurality | .19 | |||
| Single | 202 | 151 (74.8) | 24.3 (18.4–30.7) | |
| Multiple | 10 | 9 (90.0) | 10.0 (0.6–35.8) | |
| Other major extracardiac defects | .38 | |||
| Absent | 183 | 139 (76.0) | 23.1 (17.1–29.8) | |
| Present | 29 | 21 (72.4) | 27.6 (13.1–44.3) | |
| Neighborhood povertya | ||||
| Low | 173 | 127 (73.4) | 25.4 (18.9–32.5) | .01 |
| High | 32 | 29 (90.6) | 9.4 (2.4–22.3) | |
| Cardiac surgeryb | <.001 | |||
| No | 23 | 23 (100.0) | 0.0 | |
| Yes | 88 | 40 (45.5) | 52.1 (40.1–62.8) |
Birth period: 1979–2005; follow-up: December 31, 2009; unadjusted. Low-poverty neighborhood: <20% of mothers living in a given census tract were living in poverty; high-poverty neighborhood: ≥20% of mothers living in a given census tract were living in poverty.
Subjects with unknown status were deleted.
1992–2005 births only; n = 111 with known surgery status.
No survivors with the characteristic on in the birth period.
FIGURE 1.
Overall survival with 95% confidence intervals among infants with hypoplastic left heart syndrome undergoing surgical intervention, Atlanta, Georgia.
Birth period: 1979–2005; follow-up: December 31, 2009; unadjusted.
In multivariable analysis adjusted for birth period, gestational age, and neighborhood poverty, children born in recent time period had a significantly better survival (aHR = 0.28; 95% CI: 0.20–0.40) but survival was poorer among preterm infants (aHR = 1.66; 95% CI: 1.11–2.48) (data not shown).
Survival by Surgical Intervention
Limiting our analysis to the 1992–2005 birth period for which there was more reliable information on cardiac surgery than in previous years, survival probability among infants undergoing surgical intervention (n = 88) was 95% during the first week, 78% during the first month, and 58% during the first year of life (Table 3). Among children undergoing cardiac surgery who survived to 1 year of age (n = 51), 94% (n = 48) survived up to 18 years of age. The analysis of the same individual demographic and clinical factors as for the larger group (data not shown) revealed that preterm infants had a significantly poorer overall survival (31%) compared with term infants (56%; P = .02). None of the other factors examined showed a significant effect on survival. Although children who underwent surgical intervention and had normal birth weight or were born in low-poverty neighborhood had a better survival (54% and 61%, respectively) than children with low birth weight or born in high-poverty neighborhood (38% and 47%, respectively), the differences were not statistically significant.
TABLE 3.
Survival Probability by Age Among Children With HLHS Undergoing Surgical Intervention, Atlanta, Georgia
| Age Interval | No. of Births | No. of Deaths | Survival (%) | 95% CI |
|---|---|---|---|---|
| Birth–<7 d | 88 | 4 | 95.5 | (88.3–98.3) |
| 7 d–<28 d | 84 | 15 | 78.4 | (68.3–85.6) |
| 28 d–<1 y | 69 | 18 | 58.0 | (47.0–67.5) |
| 1 y–<5 y | 51 | 0 | 58.0 | (47.0–67.5) |
| 5 y–<18 y | 51 | 3 | 52.1 | (40.1–62.8) |
| Overall (<18 y) | 88 | 40 | 52.1 | (40.1–62.8) |
Birth period: 1992–2005; follow-up: December 31, 2009; unadjusted.
DISCUSSION
This population-based survival study of children born with nonsyndromic HLHS over a 30-year period in metropolitan Atlanta shows significant improvement in survival over time. Although overall survival was only 24%, among children born during the more recent birth period of 1992–2005 who underwent surgery, the survival probability was 52%. The majority of mortality occurs in the first year of life, with relatively stable survival after 1 year of age. The survival probability of children with HLHS improved by birth period in all age groups. This finding is consistent with a study in metropolitan Atlanta showing improved 1-year survival over birth era (from 67% in 1979–1993% to 83% in 1994–2005) for infants with several types of critical CHDs.26 Factors found to impair survival included preterm birth, having a very low or low birth weight, or being born to mothers residing in high-poverty neighborhoods.
Our findings of a 1-year survival probability of 27% among all birth cohorts, which includes all children with HLHS regardless of their surgical intervention status, and of 58% among the birth cohort of 1992–2005 undergoing surgery fall within the wide range of infant survival probabilities of 20% to 60% published elsewhere.3–9,13,26 Variation in study survival rates, which we could not be assessed with our data, could be due to differences in operative techniques, different institution types where surgery is done, inclusion of birth eras with limited potential for surgical intervention, or inclusion of infants with chromosomal anomalies. However, although all studies note stabilization in survival beyond infancy, our study demonstrates relative stability through adolescence. We did not have data on longer term outcomes, such as potential heart failure in young adulthood.
Among children with HLHS who survived infancy, ~90% survived up to 18 years of age, a finding similar to the 10-year survival rate of 81% to 86% observed in a Texas study.7 This finding of a high survival rate beyond infancy indicates that the most critical and vulnerable period of mortality is the first year of life, and both health care professionals and parents face challenging treatment decisions.27–29 With more families opting for surgery,30 our finding of a high survival rate beyond infancy also suggests that a growing number of children with HLHS are likely to reach adolescence and adulthood. In a recent population-based study of pooled data on survival of children with several types of birth defects,9 children with HLHS had similar survival (50%) to 8 years of age as in our current findings, but much lower survival compared with other noncardiac defects, likely due to the severity of the HLHS malformation. However, CHDs as a group are relatively common birth defects, and it will be important to monitor this growing population of persons with HLHS for their long-term care needs and to better inform planning and allocation of resources.15,31,32
Our findings on prognostic factors are consistent with those of a population-based study in Michigan,14 which reported that infants with HLHS had significantly poorer survival at 1 year of age if they were born preterm, had low birth weight, and lived in an area with high poverty. Chromosomal anomalies such as trisomy 18 and trisomy 13 are also known to affect overall survival and mortality.14,15,33 In our study, we excluded children with HLHS and chromosomal anomalies so as to have a more homogenous cohort in which to examine nongenetic prognostic factors. The poorer survival of children born in high-poverty neighborhoods, a proxy for parental socioeconomic status, is also noteworthy and may suggest that parents may have had more limited access to care when their children were born. Some studies have suggested racial/ethnic disparities in survival for CHDs including HLHS,6,9,12–14 which needs to be looked at more closely in future studies. Possible reasons that we did not find these disparities could be small sample size, and inability to distinguish some racial/ethnic groups. For example, our data did not distinguish Hispanics until the 1990s, and these were often classified as white, black, or other.
Fixler and colleagues had slightly different findings from the current study,6 possibly due to differences in study design, population, and birth period. They studied mortality up to age 5 in children with single ventricle defects born 1996–2003, and reported that children with HLHS had 42% overall survival at 1 year of age and 38% overall survival at 5 years of age. The survival of infants with HLHS and extracardiac defects was lower at 1 and 5 years of age (27% and 21%) compared with those without extracardiac defects (44% and 41%, respectively). In our study, presence of other major extracardiac defects did not affect survival probabilities. Possible reasons for this finding are a smaller sample size and less severe extracardiac defects after exclusion of chromosomal disorders.
Strengths
We included all live births from a defined population captured by a well-established, population-based birth defects surveillance system, with active and multiple source ascertainment methods. Identification of children with HLHS did not rely solely on administrative coding but resulted from a clinically classified data set, which minimized the potential for misclassification of HLHS. We were able to estimate long-term survival probability based on up to 30 years of follow-up for the earliest birth cohorts, which is longer than has been previously reported. For ascertainment of vital status, we used multiple sources, which previously have been shown to be reliable in identifying a high proportion of deaths.34,35 Finally, we were able to examine a wide range of demographic and clinical characteristics as potential predictors of survival.
Limitations
First, we assumed that children with HLHS for whom no date of death was available were alive at the end of follow-up. Although it is possible that some deaths might have been missed because of missing data on a linkage variable, it is likely that the number of missed deaths was small, because we used multiple data sources and the NDI uses several factors for linkage.20 A related limitation is that our multisource follow-up was only through 2009. NDI data were not available to search for more recent deaths; further linkage to Georgia vital records through 2013 did not identify any additional in-state deaths. This information on follow-up beyond 2009 is not included in our results because only one data source was used. Second, statistical power to analyze some characteristics as prognostic factors (eg, older maternal age, presence of major extracardiac defects) may have been limited due to the small number of affected individuals with HLHS having such characteristics. Third, because we did not have information on direct indicators of individual socioeconomic status, we had to use a proxy for socioeconomic status, namely, neighborhood poverty indicator based on the mother's census tract at the time of delivery. We did not have data with which to assess the validity of this proxy as a measure of socioeconomic status. Furthermore, we lacked complete information on other possible demographic and clinical characteristics that could affect survival such as maternal and paternal education and occupation, insurance status, or access to care; therefore, we could not explore the potential impact of these factors on survival. Fourth, our data on surgical intervention was limited to the abstracted information; we did not review original medical records for specific surgical procedures or outcomes. Thus, our reported results may be subject to an underreporting of surgical information.
CONCLUSIONS
In this population-based study, survival to adolescence among children born from 1979 to 2005 with nonsyndromic HLHS in metropolitan Atlanta has improved significantly over time, with those born full-term or undergoing surgery having better survival. Of those who survive infancy, the long-term survival rate was ~90%. These findings support the concept that with improved treatment, there is a growing population of adults with CHDs, including HLHS. Understanding predictors of survival is one step toward caring for this new aging population and adds to the growing literature on resource use and outcomes for persons with CHDs. Better understanding of the growing population with HLHS and their health care needs can inform future resource planning and allocation. Further work is warranted to assess the potential effect of socioeconomic status and access to care factors on survival of children with HLHS, which may further inform strategies to improve health outcomes.
WHAT'S KNOWN ON THIS SUBJECT: Hypoplastic left heart syndrome (HLHS) is a critical congenital heart defect with high mortality. With advances in surgical intervention in recent years, survival of infants with HLHS has improved, but information on long-term survival using population-based data is limited.
WHAT THIS STUDY ADDS: In this population-based study, survival to adolescence of children with HLHS has significantly improved in recent years. Among infant survivors, >90% survived up to 18 years. Gestational age, birth weight, and neighborhood poverty may affect survival.
ACKNOWLEDGMENTS
The authors acknowledge the MACDP staff members for their conscientious and skilled data collection.
FUNDING: No external funding.
ABBREVIATIONS
- aHR
adjusted hazard ratio
- CHD
congenital heart defect
- CI
confidence interval
- HLHS
hypoplastic left heart syndrome
- MACDP
Metropolitan Atlanta Congenital Defects Program
- NDI
National Death Index
Footnotes
Dr Siffel conceptualized and designed the study, analyzed and interpreted data, drafted the manuscript, and revised the manuscript critically for important intellectual content; Drs Riehle-Colarusso, Oster, and Correa contributed substantially to the study concept and design and interpretation of data, and revised the manuscript critically for important intellectual content; and all authors approved the final manuscript as submitted.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
REFERENCES
- 1.Reller MD, Strickland MJ, Riehle-Colarusso T, Mahle WT, Correa A. Prevalence of congenital heart defects in metropolitan Atlanta, 1998–2005. J Pediatr. 2008;153(6):807–813. doi: 10.1016/j.jpeds.2008.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barron DJ, Kilby MD, Davies B, Wright JG, Jones TJ, Brawn WJ. Hypoplastic left heart syndrome. Lancet. 2009;374(9689):551–564. doi: 10.1016/S0140-6736(09)60563-8. [DOI] [PubMed] [Google Scholar]
- 3.Cleves MA, Ghaffar S, Zhao W, Mosley BS, Hobbs CA. First-year survival of infants born with congenital heart defects in Arkansas (1993–1998): a survival analysis using registry data. Birth Defects Res A Clin Mol Teratol. 2003;67(9):662–668. doi: 10.1002/bdra.10119. [DOI] [PubMed] [Google Scholar]
- 4.Mahle WT, Spray TL, Wernovsky G, Gaynor JW, Clark BJ., III Survival after reconstructive surgery for hypoplastic left heart syndrome: A 15-year experience from a single institution. Circulation. 2000;102(19 suppl 3):III136–III141. doi: 10.1161/01.cir.102.suppl_3.iii-136. [DOI] [PubMed] [Google Scholar]
- 5.Tweddell JS, Hoffman GM, Mussatto KA, et al. Improved survival of patients undergoing palliation of hypoplastic left heart syndrome: lessons learned from 115 consecutive patients. Circulation. 2002;106(12 suppl 1):I82–I89. [PubMed] [Google Scholar]
- 6.Fixler DE, Nembhard WN, Salemi JL, Ethen MK, Canfield MA. Mortality in first 5 years in infants with functional single ventricle born in Texas, 1996 to 2003. Circulation. 2010;121(5):644–650. doi: 10.1161/CIRCULATIONAHA.109.881904. [DOI] [PubMed] [Google Scholar]
- 7.Nembhard WN, Xu P, Ethen MK, Fixler DE, Salemi JL, Canfield MA. Racial/ethnic disparities in timing of death during childhood among children with congenital heart defects. Birth Defects Res A Clin Mol Teratol. 2013;97(10):628–640. doi: 10.1002/bdra.23169. [DOI] [PubMed] [Google Scholar]
- 8.McGuirk SP, Griselli M, Stumper OF, et al. Staged surgical management of hypoplastic left heart syndrome: a single institution 12 year experience. Heart. 2006;92(3):364–370. doi: 10.1136/hrt.2005.068684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Liu G, Canfield MA, et al. National Birth Defects Prevention Network. Racial/ethnic differences in survival of United States children with birth defects: a population-based study. J Pediatr. 2015;166(4):819–826. e1–e2. doi: 10.1016/j.jpeds.2014.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McHugh KE, Hillman DG, Gurka MJ, Gutgesell HP. Three-stage palliation of hypoplastic left heart syndrome in the University HealthSystem Consortium. Congenit Heart Dis. 2010;5(1):8–15. doi: 10.1111/j.1747-0803.2009.00367.x. [DOI] [PubMed] [Google Scholar]
- 11.Feinstein JA, Benson DW, Dubin AM, et al. Hypoplastic left heart syndrome: current considerations and expectations. J Am Coll Cardiol. 2012;59(suppl 1):S1–S42. doi: 10.1016/j.jacc.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boneva RS, Botto LD, Moore CA, Yang Q, Correa A, Erickson JD. Mortality associated with congenital heart defects in the United States: trends and racial disparities, 1979–1997. Circulation. 2001. 103(19):2376–2381. doi: 10.1161/01.cir.103.19.2376. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Liu G, Druschel CM, Kirby RS. Maternal race/ethnicity and survival experience of children with congenital heart disease. J Pediatr. 2013;163(5):1437–1442. e1–e2. doi: 10.1016/j.jpeds.2013.06.084. [DOI] [PubMed] [Google Scholar]
- 14.Hirsch JC, Copeland G, Donohue JE, Kirby RS, Grigorescu V, Gurney JG. Population-based analysis of survival for hypoplastic left heart syndrome. J Pediatr. 2011;159(1):57–63. doi: 10.1016/j.jpeds.2010.12.054. [DOI] [PubMed] [Google Scholar]
- 15.Menon SC, Keenan HT, Weng HY, et al. Outcome and resource utilization of infants born with hypoplastic left heart syndrome in the Intermountain West. Am J Cardiol. 2012;110(5):720–727. doi: 10.1016/j.amjcard.2012.04.050. [DOI] [PubMed] [Google Scholar]
- 16.Correa A, Cragan JD, Kucik JE, et al. Reporting birth defects surveillance data 1968–2003. Birth Defects Res A Clin Mol Teratol. 2007;79(2):65–186. doi: 10.1002/bdra.20350. [DOI] [PubMed] [Google Scholar]
- 17.Riehle-Colarusso T, Strickland MJ, Reller MD, et al. Improving the quality of surveillance data on congenital heart defects in the metropolitan Atlanta congenital defects program. Birth Defects Res A Clin Mol Teratol. 2007;79(11):743–753. doi: 10.1002/bdra.20412. [DOI] [PubMed] [Google Scholar]
- 18.Rasmussen SA, Olney RS, Holmes LB, Lin AE, Keppler-Noreuil KM, Moore CA, National Birth Defects Prevention Study Guidelines for case classification for the National Birth Defects Prevention Study. Birth Defects Res A Clin Mol Teratol. 2003;67(3):193–201. doi: 10.1002/bdra.10012. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention, National Center for Health Statistics . National Death Index User Manual. National Center for Health Statistics; Hyattsville, MD: 2000. [Google Scholar]
- 20.Wentworth DN, Neaton JD, Rasmussen WL. An evaluation of the Social Security Administration master beneficiary record file and the National Death Index in the ascertainment of vital status. Am J Public Health. 1983;73(11):1270–1274. doi: 10.2105/ajph.73.11.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.US Census Bureau . Census 2000 Summary File 2—Technical documentation, prepared by the U.S. Census Bureau; Department of Commerce; Washington, DC: 2001. [Google Scholar]
- 22.Krieger N, Chen JT, Waterman PD, Soobader MJ, Subramanian SV, Carson R. Choosing area based socioeconomic measures to monitor social inequalities in low birth weight and childhood lead poisoning: The Public Health Disparities Geocoding Project (US). J Epidemiol Community Health. 2003;57(3):186–199. doi: 10.1136/jech.57.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 24.Greenwood M. The natural duration of cancer. Rep Public Health Med Subj (Lond) 1926;33:1–26. [Google Scholar]
- 25.Cox DR, Oakes D. Analysis of Survival Data. Chapman & Hall; London: 1984. [Google Scholar]
- 26.Oster ME, Lee KA, Honein MA, Riehle-Colarusso T, Shin M, Correa A. Temporal trends in survival among infants with critical congenital heart defects. Pediatrics. 2013;131(5) doi: 10.1542/peds.2012-3435. Available at: www.pediatrics.org/cgi/content/full/131/5/e1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ellinger MK, Rempel GR. Parental decision making regarding treatment of hypoplastic left heart syndrome. Adv Neonatal Care. 2010;10(6):316–322. doi: 10.1097/ANC.0b013e3181fc7c5d. quiz 323–324. [DOI] [PubMed] [Google Scholar]
- 28.Karamlou T, Diggs BS, Ungerleider RM, Welke KF. Evolution of treatment options and outcomes for hypoplastic left heart syndrome over an 18-year period. J Thorac Cardiovasc Surg. 2010;139(1):119–126. doi: 10.1016/j.jtcvs.2009.04.061. discussion 126–127. [DOI] [PubMed] [Google Scholar]
- 29.Yates AR, Hoffman TM, Boettner B, Feltes TF, Cua CL. Initial counseling prior to palliation for hypoplastic left heart syndrome. Congenit Heart Dis. 2011;6(4):347–358. doi: 10.1111/j.1747-0803.2011.00525.x. [DOI] [PubMed] [Google Scholar]
- 30.Gordon BM, Rodriguez S, Lee M, Chang RK. Decreasing number of deaths of infants with hypoplastic left heart syndrome. J Pediatr. 2008;153(3):354–358. doi: 10.1016/j.jpeds.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 31.Boris JR. Primary care cardiology for patients with hypoplastic left heart syndrome. Cardiol Young. 2011;21(suppl 2):53–58. doi: 10.1017/S1047951111001594. [DOI] [PubMed] [Google Scholar]
- 32.Said SM, Dearani JA, Silversides CK, Martinez RM, Drajpuch DB. Longer-term issues for young adults with hypoplastic left heart syndrome: contraception, pregnancy, transition, transfer, counselling, and re-operation. Cardiol Young. 2011;21(suppl 2):93–100. doi: 10.1017/S1047951111001661. [DOI] [PubMed] [Google Scholar]
- 33.Jacobs JP, O'Brien SM, Chai PJ, Morell VO, Lindberg HL, Quintessenza JA. Management of 239 patients with hypoplastic left heart syndrome and related malformations from 1993 to 2007. Ann Thorac Surg. 2008;85(5):1691–1696. doi: 10.1016/j.athoracsur.2008.01.057. discussion 1697. [DOI] [PubMed] [Google Scholar]
- 34.Lash TL, Silliman RA. A comparison of the National Death Index and Social Security Administration databases to ascertain vital status. Epidemiology. 2001;12(2):259–261. doi: 10.1097/00001648-200103000-00021. [DOI] [PubMed] [Google Scholar]
- 35.Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the National Death Index and Equifax Nationwide Death Search. Am J Epidemiol. 1994;140(11):1016–1019. doi: 10.1093/oxfordjournals.aje.a117191. [DOI] [PubMed] [Google Scholar]