Abstract
Age-related changes in cognitive abilities are well-documented, and a very important indicator of health, functioning, and decline in later life. However, less is known about the course of cognitive functioning before and after retirement and specifically whether job characteristics during one's time of employment (i.e., higher vs. lower levels of mental work demands) moderate how cognition changes both before and after the transition to retirement. We used data from n = 4,182 (50% women) individuals in the Health and Retirement Study, a nationally representative panel study in the United States, across an 18 year time span (1992–2010). Data were linked to the O'NET occupation codes to gather information about mental job demands to examine whether job characteristics during one's time of employment moderates level and rate of change in cognitive functioning (episodic memory and mental status) both before and after retirement. Results indicated that working in an occupation characterized by higher levels of mental demands was associated with higher levels of cognitive functioning before retirement, and a slower rate of cognitive decline after retirement. We controlled for a number of important covariates, including socioeconomic (education and income), demographic, and health variables. Our discussion focuses on pathways through which job characteristics may be associated with the course of cognitive functioning in relation to the important transition of retirement. Implications for job design as well as retirement are offered.
Keywords: cognitive aging, cognitive functioning, mental demands, job complexity, retirement, Health and Retirement Study (HRS)
One of the hallmarks of the aging process is a decline in fluid cognitive abilities, including working memory, episodic memory, processing speed, and reasoning as people age (e.g., Park, 2000; Salthouse, 2012; Salthouse, Atkinson, & Berish, 2003; Schaie, 2005; Verhaeghen & Salthouse, 1997). Cognitive impairment is a highly prevalent, deleterious, and costly problem related to functional impairment and disability that affects more than one third of adults over age 70 in the United States (Hurd, Martorell, Delavande, Mullen, & Langa, 2013; Plassman et al., 2008; 2007). Furthermore, cognitive functioning is related to multiple domains of health (Salthouse, 2012), including depression (González, Bowen, & Fisher, 2008), physical health (Der et al., 2009; Infurna, Gerstorf, Ryan, & Smith, 2011), and mortality (Batty, Deary, & Gottfredson, 2007). Marked declines in cognitive functioning indicative of mild cognitive impairment or dementia have been studied extensively in the epidemiology and medical literatures as important health outcomes (Hurd et al., 2013; Plassman et al., 2007). As individuals are living longer and the proportion of older adults in the population grows, identifying factors related to cognitive status and the maintenance of cognitive functioning in later life is becoming increasingly important. This topic is clearly relevant to occupational health psychology to the extent that the nature of work activities may be related to cognitive functioning both during one's time of employment and later in life when an individual is no longer engaged in gainful employment.
Occupational characteristics, such as the level of mental processing involved in one's job, may moderate how cognition changes in adulthood and old age. Although prior research has examined the relation between mental work demands and cognitive functioning over age, the purpose of the present study was to examine whether cognitive job complexity or mental work demands are related to cognitive functioning in relation to retirement. We define mental work demands as the cognitive or mental processing activities one performs as part of his or her job. We sought to examine the relation between mental work demands and intraindividual changes in cognitive functioning in relation to retirement by examining trajectories of change both before and after retirement.
Theoretical Framework
Three theoretical approaches described in the cognitive aging literature are relevant to understanding the role of work activities in relation to cognitive functioning in older age: 1) the “use-it-or-lose-it” hypothesis (Hultsch, Hertzog, Small, & Dixon, 1999; Salthouse, 1991, 2006), 2) the brain or cognitive reserve hypothesis (Fratiglioni & Wang, 2007; Mortimer & Graves, 1993; Stern, 2002), and 3) Schooler's (1984, 1990) theory of the mechanisms in the environment that influence cognitive functioning.
The “use it or lose it” hypothesis dates back almost 100 years (e.g., Foster & Taylor, 1920; Thorndike, Tilton, & Woodyard, 1928). This hypothesis is based on the idea that cognitive performance on complex tasks is moderated by a myriad of contextual variables, experiences, and environments throughout life. Changes in intellectual activity during different stages of life may impact cognitive functioning in two ways: 1) the amount of mental activity during earlier life leads to the level of mental activity in later life, or 2) the amount of mental activity is determined by both one's prior and current level of mental ability (Salthouse, 1991). This led Salthouse (2006) to describe patterns of cognitive functioning in terms of differential preservation (i.e., how much cognitive functioning is preserved as age increases depends on one's current mental activity), and preserved differentiation, which refers to the notion that those who are more mentally active are those who have had higher levels of cognitive functioning throughout their lives. In the context of trying to improve our understanding of the role of cognitive activities involved in one's occupation, preserved differentiation addresses the problem of selection bias: individuals with higher levels of cognitive functioning in earlier life pursue more education and also choose occupations with higher levels of mental demands. Although in the early 1990s Salthouse was a strong advocate for the “use it or lose it” hypothesis, his more recent work has cast doubt on the existence of empirical evidence to support the phenomenon of differential preservation (Salthouse, 2006; Salthouse, Berish, & Miles, 2002). This issue has been debated in the literature, as Schooler and others have found empirical results in support of differential preservation (e.g., Bielak, Anstey, Christensen, & Windsor, 2012; Potter, Helms, & Plassman, 2008; Schooler, 2007). We describe such findings later on in this article.
The neurology and neuroepidemiology research literature (e.g., Fratiglioni & Wang, 2007; González, Tarraf, Bowen, Johnson-Jennings, & Fisher, 2013; Mortimer & Graves, 1993; Stern, 2002) describes the notion of a brain or cognitive reserve, such that engagement in mentally stimulating environments is associated with increased neuronal development. The premise of the cognitive reserve hypothesis is that mental stimulation yields additional neuronal resources that are believed to increase one's threshold for neuronal insults that may serve to stave off detectable cognitive decline. Though prior empirical evidence has been mixed, some research has suggested that educational and occupational achievement are associated with less cognitive decline (e.g., Potter, Plassman, Helms, Foster, & Edwards, 2006).
Schooler's (1984, 1990) work has focused the psychological mechanisms underlying how the environment influences intellectual functioning across life. Specifically, this theory suggests that the complexity of a person's environment is comprised of both stimulus and demand characteristics. As the environmental stimuli increase in diversity, people are required to make more decisions. There are more considerations for such decisions and contradictions, which create a more complex environment. In environments where cognitive effort is rewarded, people should be motivated to not only develop their intellect, but to generalize the processes across different situations. However, if the environment changes and such high functioning are unnecessary, it is unlikely to be maintained, leading to decreases in cognitive functioning.
Some researchers have found empirical support for the notion that a person's current cognitive status is related to later cognitive functioning. For example, Wilson, Barnes, Krueger, Hoganson, Bienias, and Bennett (2005) surveyed a cohort of older people's activity levels at points throughout their lives (participants were asked to recall activity from childhood to current life stage) and found that higher levels of cognitive activity at one point in time were associated with better cognitive functioning in subsequent periods including later stages of life, even when controlling for past cognitive activity. Newson and Kemps (2005) also suggested that participating in general lifestyle activities (i.e., household maintenance, domestic chores, social activities, and service to others) may facilitate successful cognitive aging (see Infurna & Gerstorf, in press; Small, Dixon, McArdle, & Grimm, 2012). This effect might be strongest as people approach retirement age, as Schooler, Multau, and Oates (1999, 2004) point to the continued benefit of mentally stimulating work on the intellectual flexibility of older workers.
The Role of Job Characteristics and Work Design
It is possible that characteristics of work itself also contribute to cognitive functioning later in life. For example, Kanfer and Ackerman (2004) postulated that older workers compensate for declines in fluid abilities through their ability to see problems from a larger perspective, and utilize problem solving skills. Morgeson and colleagues (e.g., Morgeson & Humphrey, 2006; Morgeson, Medsker, & Campion, 2008), in their model of job design, describe task characteristics emerging from the work itself, social characteristics resulting from working with others, and contextual factors that arise from the physical and organizational environment.
Our focus here is on work activities or task characteristics that require mental processes (e.g., work requiring concentration, attention, or memory; Morgeson & Humphrey, 2006; Morgeson et al., 2008). Consistent with this idea, Kohn and Schooler (1973) defined “substantively complex work” as work that by its substance necessitates thought and independent judgment. Some studies have used other terms to refer to the degree to which one's job involves cognitive activities, such as job complexity (i.e., “the extent to which the tasks on a job are complex and difficult to perform”; Morgeson & Humphrey, 2006, p. 1323), and intellectual flexibility (i.e., flexibility in coping with the intellectual demands of a complex situation; Kohn & Schooler, 1983), among others. Although the specific measures of these constructs have varied to some degree from one study to the next, the various terms generally refer to the extent to which one's job requires high levels of cognitive processing.
Prior empirical work has provided support for the notion that working in complex jobs is related to better cognitive functioning among older adults. For example, Schooler et al. (1999) investigated the relation between intellectually demanding work and intellectual flexibility throughout a person's work life. They hypothesized that complex environments would have a positive effect on the intellectual flexibility of older workers, while simple environments would have a negative effect. Their results indicated that as workers aged, the level of complexity of their jobs was associated with their intellectual flexibility, such that individuals with more complex jobs (defined as work that requires thought and independent decisions) experienced an increase in their intellectual flexibility, whereas those individuals with less complex jobs experienced a decrease in their intellectual flexibility.
Bosma, van Boxtel, Ponds, Houx, and Jolles (2003) found that those with fewer mental demands at work showed more rapid declines in processing speed, memory, and general cognitive status 3 years later. Their results support the theoretical proposition that being engaged in more mental processes at work may be related to slower declines in cognitive functioning, at least up to retirement. In addition, their results indicated that the difference in cognitive decline may be reduced with an increase in mentally stimulating work for those with lower levels of education.
Andel and colleagues (2005) studied the relationship between the cognitive complexity of occupations and risk of dementia among Swedish twins. Three dimensions of cognitive complexity were assessed, including cognitive complexity of work data, people, and things. Andel et al.'s results indicated that one dimension of work complexity, complexity of work with people (i.e., listed from highest to lowest level of complexity, activities in this job complexity classification include: mentoring, negotiating, instructing, supervising, amusing others, persuading, speaking/signaling, serving, and taking instructions/helping) was associated with a lower risk of Alzheimer's disease. In other words, those whose work required higher mental demands in the forms of organizing tasks, supervising, and negotiating were less likely to develop Alzheimer's disease later in life. Similarly, Potter and colleagues studied World War II veterans and found that work in more intellectually demanding jobs was associated with higher levels of cognitive functioning in later life when controlling for age, education, and general intelligence in early adulthood (Potter et al., 2008). Their study was the first to examine the relationship between mental work demands and later life cognitive status while controlling for cognitive ability in early adulthood. Potter et al. (2008) reported an interaction of earlier life general cognitive ability such that individuals with lower intelligence earlier on seemed to benefit more from doing work that was characterized as intellectually demanding compared with individuals with higher ability levels earlier in life.
Although these studies provided some empirical evidence to indicate that the types of activities required on one's job have a long-term impact on level of cognitive functioning, much less is known regarding how cognitive job complexity or mental work demands are related to cognitive functioning in relation to retirement. In other words, what is the course of change in cognitive functioning in relation to retirement?
Two studies to date have examined levels of cognitive functioning in relation to retirement. Rohwedder and Willis (2010) used cross-sectional data from the United States, United Kingdom, and 11 other European nations to determine whether average age at retirement was related to aggregate levels of cognitive functioning. By examining comparable measures of episodic memory in relation to labor force participation among people in their 50s and 60s cross-nationally at a country-aggregate level, they concluded that early retirement has a significant negative impact on cognitive functioning. However, their study did not examine intraindividual change in cognitive functioning in relation to retirement. The only study to date to examine this issue at the individual level was conducted by Finkel et al. (2009). In a sample of 462 Swedish individuals from twin pairs, Finkel et al. (2009) examined changes in cognitive scores and found that those in jobs characterized by being more complex demonstrated an increase in verbal skills up until retirement, at which point they showed a sharper decline than those with less complex jobs.
Based on Salthouse's use it or lose it hypothesis, the cognitive reserve hypothesis, and Schooler's work regarding environmental influences on cognitive functioning as well as prior research that has found a relationship between mental demands at work and cognitive functioning, we hypothesize:
H1: Individuals in jobs with higher mental demands have higher levels of cognitive functioning before retirement compared with those in jobs with lower levels of mental work demands.
H2: Individuals in jobs with higher mental demands experience less steep declines (i.e., a slower rate of cognitive decline) before retirement compared with those in jobs with less mental work demands.
Furthermore, we sought to determine whether levels and rates of change in cognitive functioning differ after retirement, as compared with the years leading up to retirement. Based on Schooler's model and the “use it or lose it” hypothesis, individuals who are no longer engaged in cognitively stimulating activities will be more likely to experience cognitive decline. The cognitive reserve hypothesis suggests that individuals who were engaged in more mentally demanding work will not experience cognitive decline or will experience slower cognitive decline relative to those who were engaged in less mentally demanding work. Therefore, we hypothesize the following:
H3: Individuals in jobs with higher mental demands have higher levels of cognitive functioning after retirement compared with those in jobs with lower levels of mental work demands.
H4: Individuals in jobs with higher mental demands experience less steep declines (i.e., a slower rate of cognitive decline) after retirement compared with those in jobs with less mental work demands.
To date little research has been conducted to specifically categorize jobs in terms of overall mental work demands in relation to retirement. No research been conducted using a sample from the United States to examine intraindividual change in cognition before and after retirement. Public policy and norms regarding retirement vary from country to country (Rohwedder & Willis, 2010), resulting in differences in age of retirement. For example, the United States has experienced a shift in retirement policy such that the normal age of eligibility for full Social Security retirement benefits is increasing and the proportion of defined contribution pension plans relative to defined benefit plans has also increased since the 1990s, resulting in more variability in retirement age compared with other countries. These differences highlight the need for additional research, and particularly research in the United States, to examine the role of mental work demands on cognitive functioning not only before retirement, but after retirement as well. Although there are potential sources of cognitively demanding activities outside of work, our focus is on the nature of mental work activities and cognitive functioning.
We extend prior research by examining trajectories of cognitive functioning during adulthood and old age by focusing on change both before and after retirement using data from a large, nationally representative sample of older adults in the United States gathered over 18 years (1992–2010). Although direct measures of earlier life cognitive functioning were not available for these respondents, we controlled for educational attainment and income, both of which are highly correlated with cognitive ability (Deary, Strand, Smith, & Fernandes, 2007). We also controlled for health status because prior research has shown that health is related to cognitive functioning (Hultsch, Hammer, & Small, 1993).
Method
Participants and Procedure
Participants were 4,182 respondents (50% men, 50% women, 81% White) in the Health and Retirement Study (HRS) who retired over the course of the study. The HRS is a longitudinal study conducted in the United States among a large, nationally representative sample of older adults. The HRS is a cooperative agreement between the National Institute of Aging (grant number NIA U01AG009740) and the University of Michigan; data were collected by the University of Michigan Institute for Social Research. The HRS began in 1992 with a sample of N = 12,652 individuals age 51–61 (Juster & Suzman, 1995) who have been reinterviewed biennially since then with data being jointly collected with the AHEAD study beginning in 1993 and refresher cohorts being included in 1998, 2004, and 2010. Letters with a prepaid incentive were mailed to respondents inviting them to participate. Participants were interviewed either face-to-face or over the telephone by a trained interviewer used by the Survey Research Center at the University of Michigan. Baseline interviews were primarily conducted face-to-face and follow-up interviews were primarily conducted via telephone. The response rate for the baseline interview was 81.7%, and reinterview response rates at subsequent waves ranged from 84.0% to 89.1% (HRS, 2011).
The sample analyzed in this study included individuals who were between the ages of 51–61 in either the 1992 (initial) or the 1998 wave (i.e., when the sample of individuals age 51–56 was replenished). They were working for pay in an occupation they held for at least 10 years at the time of retirement, participated in at least two waves of the HRS, and retired between the ages of 50 and 71. In total, 5,094 participants met these criteria. The HRS sample consisted of age eligible individuals and their spouse regardless of age. Therefore, among households with two age-eligible respondents, only one was randomly selected for inclusion in the study to not violate the statistical assumption of independence by including individuals nested within households. Participants in the study completed an average of 8.29 interviews between 1992 and 2010 (SD = 2.11, range 21 to 10), of which 16,001 took place before retirement and 18,663 occurred after retirement. On average, participants were 54.81 years of age at their first wave of assessment and age 62.24 (SD = 3.79, range 50 to 70) at retirement. Participants worked in a wide variety of jobs and had been doing the same type of work for M = 26.61 years (SD = 10.93).
Measures
Mental work demands
Mental work demands were measured using 10 items from the Occupational Information Network (O*NET) database regarding work activities, and the level of various mental processes required by one's job.2 Items assessed work activities such as analyzing data or information, developing objectives and strategies, making decisions and solving problems, evaluating information, and thinking creatively, among others. Each respondent's occupation at each wave of HRS (coded using U.S. Census codes) was linked to the O*NET database (via a crosswalk from U.S. Census codes to standard occupation classification [SOC] codes to O*NET occupation codes) to obtain external occupational-level ratings of job characteristics pertaining to occupation at each wave. The 10 items were measured on a behaviorally anchored rating scale specific to each time from 1 (a very low level) through 7 (the highest level). The 10 items were averaged to create a composite score across the 10 items (α > .94).
Cognitive functioning
Cognitive functioning was assessed in each wave of the HRS using two dimensions that have been used extensively as indicators of cognition and screening for cognitive impairment: episodic memory and mental status. The first measure, episodic memory, was comprised of scores on an immediate and delayed word recall task (McArdle, Fisher, & Kadlec, 2007; Ofstedal, Fisher, & Herzog, 2005). During the first two waves, a 20-noun list was used. Later waves (starting in 1996) used a 10-noun list. To compare scores across waves and simplify measures for analyses, we rescaled each immediate and delayed word recall variable to a percentage score, consistent with the scoring method used by McArdle et al. (2007). The cognitive functioning score included in the models was a sum of immediate and delayed recall items correctly recalled divided by the maximum score and multiplied by 100. Episodic memory is a leading indicator of cognitive impairment, important for day-to-day functioning, and sensitive to change over time. Episodic memory was assessed seven or more times for 81% of participants. Only 35 respondents had as few as two data points. Episodic memory was standardized to a T score, which is a standardized score (similar to z-scores) that was done to improve the interpretability of the results. The mean was centered at 50, and 1 SD was indicated by 10 T score units.
The second dimension, mental status, was comprised of items in the Telephone Interview of Cognitive Status (Brandt, Spencer, & Folstein, 1988), many of which were similar to items in the Mini Mental State Exam, a common tool used in clinical practice to screen for cognitive impairment (Potter et al., 2008). This mental status dimension is comprised of 10 items, including a 5-item Serials 7 task in which respondents counted backward from 100 by 7 for a total of five trials, counting backward from 20, and eight Mini-Mental State Exam-type items that assess orientation. Seventy-two percent of respondents provided three or more data points regarding mental status. Prior research by McArdle et al. (2007) demonstrated the factor structure of episodic memory and mental status as two unique dimensions of cognitive functioning. Crimmins, Kim, Langa, and Weir (2011) demonstrated the validity of these measures for assessing cognitive impairment and dementia in the HRS.
Because missing data on cognitive measures are often not missing at random, we used a publicly available version of the HRS cognitive measures in which missing cognitive scores were imputed using a regression-based multiple imputation procedure implemented using IVEware (Fisher, Hassan, Rodgers, & Weir, 2013). This method minimized missing data for respondents who participated in a given wave but did not complete the cognitive tests. It did not impute missing data because of sample attrition over time. Details about the imputation method and rates of missing data are described elsewhere (Fisher et al., 2013).
Practice effects
Prior research examining cognitive measures in the HRS has demonstrated practice effects between the first and second administration of the cognitive measures such that respondents performed slightly better the second time they completed the cognitive performance tasks relative to the first time (McArdle et al., 2007; Rodgers, Ofstedal, & Herzog, 2003). Therefore, we sought to control for practice effects in this study. We constructed a dummy-coded variable that was coded as a 0 for each individual's first memory observation and a 1 for all subsequent observations (0 = baseline measure, 1 = all assessments following baseline) to control for practice effects in the analysis. The manner in which practice effects were incorporated in the data analysis is described in the next section regarding statistical analysis.
Health status
Overall self-rated health status was asked using a single item, “Would you say your health is excellent, very good, good, fair, or poor?” This same question was asked at every wave and we included participants' self-rated health at their first wave in the HRS. Presence of cardiovascular disease was a sum index of whether participants reported self-reported physician-diagnosed medical conditions, including heart problems (includes heart attack, coronary heart disease, or congestive heart failure) and stroke at their first wave in the HRS. Health status was included as a covariate in data analyses based on previous research that has demonstrated a relationship between health and cognitive functioning (Anstey & Christensen, 2000; Hultsch et al., 1993).
Depressive symptoms
Depressive symptoms were included in the study as a time-invariant covariate by including participants' scores at their first wave in the HRS. Based on prior research that has demonstrated an association between depression and cognitive status (e.g., González, Bowen, & Fisher, 2008; Steffens, Fisher, Langa, Potter, & Plassman, 2009). Depressive symptoms were measured using eight items from the Center for Epidemiology Scale of Depression (CES-D; Steffick, 2000). Participants answered each of the items using a “yes” or “no” response scale to indicate whether they felt a particular way during the last week. A summary variable was constructed by counting the number of “yes” responses after reverse coding the two positive items.
Demographic characteristics
Demographic characteristics, including age, gender, and years of formal education were obtained at the time of the baseline interview. Age at retirement was calculated based on available birth date and date of retirement information. Age, gender, and education were included as time-invariant covariates in the models.
Retirement
Respondents reported their current work status at each wave of the HRS. If they indicated that they were retired and no longer working, they were then asked to indicate the month and year when they stopped working. We used this information to realign their individual time series in relation to the date of retirement.
Statistical Analysis
To examine change in cognitive functioning in relation to retirement, we used a latent growth curve modeling approach. This approach is related to latent curve analysis, multilevel models, and hierarchical linear modeling, depending on the specific model(s) applied and software used (Raudenbush, 2001). This is a longitudinal data analysis technique that examines repeated measures of dependent measures as a function of time and other measures (Meredith & Tisak, 1990). In particular, it permits the examination of an individual's relative standing as a function of effects that vary randomly across individuals (i.e., random effects) as well as systematic intra- and interindividuals differences in change (fixed effects).
We used this technique to estimate change (growth or decline) over time in cognitive functioning in relation to the event of retirement. Two-phase latent growth curve models were fit to the data to examine time-related changes in episodic memory and mental status in relation to retirement (assessed biennially up to 10 times between 1992 and 2010; see Ram & Grimm, 2007; Singer & Willet, 2003). The first step was to define a time-varying dummy-coded variable that would designate when individuals were involved in gainful employment and transitioned to retirement (i.e., the pivot point). This method is consistent with what has been previously done in other studies (e.g., Fauth, Gerstorf, Ram, & Malmberg, 2012; Infurna, Gerstorf, & Zarit, 2013). In this case, the onset of retirement serves as an index for evaluating changes in participants' episodic memory and indicates a shift in respondents' employment status. The model was specified as
where person i's level of episodic memory at time t, episodic memoryti, is a function of an individual-specific intercept parameter, β0i, that represents memory levels before retirement; an within-person slope parameter, β1i, that captures the rate of linear memory change over time (i.e., before and after retirement); an individual-specific parameter, β2i, that represents the change in episodic memory with retirement onset; an individual-specific interaction between linear rate of memory change and retirement period, β3i, that examines whether the rate of change in episodic memory differs pre- and postretirement; an individual-specific practice effect, β4i, that examines whether participant memory improved in the observations after their first assessment; an individual-specific interaction between linear rate of memory change and practice effects, β5i, that examines whether participants reported differential rates of memory decline after their first assessments; and residual error, eti. Although practice effects may take many different forms, accounting for practice or retest effects even in this simple way helps avoid underestimation of changes in memory (Ferrer, Salthouse, Stewart, & Schwartz, 2004). Individual-specific intercepts and slopes (βs from the Level 1 model in Equation 1) were modeled as:
(i.e., Level 2 model), where γ00, γ10, γ20, γ30, γ40, and γ50 are the sample means and between-person differences, and u0i, u1i, u12i, and u3i are assumed to be normally distributed, correlated with each other, and uncorrelated with the residual errors, eti.
Subsequently, covariates (sociodemographic, depressive symptoms, and cardiovascular disease) and mental demands were included as predictors at the between-person level (Level 2), predicting β0i, β1i, and β3i. Of particular interest was whether these variables were related to individuals' change before (β1i) and after (β1i + β3i) retirement. We fit mixed models without adjustment using sample weights using standard mixed modeling software (i.e., SAS PROC MIXED; Littell et al., 2006), with SAS 9.2 (SAS Institute Inc., Cary, NC). In general, the analytic approach used here was based on the methods used by McArdle et al. (2007) and Infurna et al. (2013). Missing data were treated as missing at random at the within-person level, and to retain longitudinal data, missing completely at random at the between-person level (Little & Rubin, 1987).
Results
In a first step, we checked the relative proportion of between-and within-person variation in the repeated measures of episodic memory. The intraclass correlation was .40, indicating that 40% of the total variance in episodic memory was between-person variance and 60% was within-person variance. Thus, the data appeared to contain both substantial amounts of between-person differences and within-person variation over time.
Table 1 presents descriptive statistics and correlations among study variables. Results from the multiphase growth curve model, examining how episodic memory changes in relation to retirement and whether the level of mental demands in one's job moderates such changes, is shown in Table 2. First, the pattern of change in memory in relation to retirement was not characterized by a multiphase pattern. Participants, on average, exhibited declines in memory in the years leading up to retirement (γ10 = −0.46 T score units per year or 0.46 SD per 10 years of time) and average levels of memory before retirement (γ00 = 50.52 T score units). The transition to retirement did not result in declines in memory in the surrounding retirement years (γ20 = −0.08 T score units). The rate of change in memory after retirement was slightly less steep compared with the years leading up to retirement (γ30 = −0.46 + 0.06 = −0.40 T score units per year or −0.40 SD per 10 years). This difference is small, but not likely because of chance.
Table 1. Means, SDs, and Correlations Among Study Variables.
| M | SD | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age at time of retirement | 62.25 | 3.79 | — | ||||||||
| 2. Women | 0.51 | 0.50 | −.02 | — | |||||||
| 3. Education | 12.67 | 2.92 | −.08* | .05* | — | ||||||
| 4. Job length | 26.46 | 10.94 | .08* | −.22* | .06* | — | |||||
| 5. Depressive symptoms | 0.69 | 1.25 | −.05* | .05* | —.11* | −.06* | — | ||||
| 6. Cardiovascular disease | 0.11 | 0.32 | .002 | −.07* | .01 | −.01 | .04* | — | |||
| 7. Self-rated health | 3.68 | 1.03 | .01 | .002 | .25* | .03 | −.22* | −.21* | — | ||
| 8. Log income | 7.44 | 1.63 | −.01 | .19* | .17* | −.01 | .07 | −.02 | .03* | — | |
| 9. Mental job demands | 50.18 | 10.76 | −.06* | −.09* | .55* | .13* | −.11* | .001 | .21* | .08* | — |
Note. N = 4,182. Age and job length measurements were taken at the assessment period at the time of retirement. Depressive symptoms, cardiovascular disease illness, self-rated health, and log income was taken at the first assessment period.
p < .05.
Table 2. Fixed and Random Effects for Change In Episodic Memory to or From Retirement.
| Estimate | SE | |
|---|---|---|
| Fixed effects | ||
| Level, γ00 | 50.52* | 0.14 |
| Age, γ01 | −0.34* | 0.03 |
| Women, γ02 | 2.76* | 0.26 |
| Education, γ03 | 0.66* | 0.05 |
| Job length, γ04 | −0.001 | 0.01 |
| Depressive symptoms, γ05 | −0.57* | 0.10 |
| Cardiovascular disease, γ06 | 0.46 | 0.40 |
| Self-rated health, γ07 | 0.20 | 0.13 |
| Log income, γ08 | 0.02 | 0.08 |
| Mental demands, γ09 | 0.06* | 0.01 |
| Time-to or from-retirement, γ10 | −0.46* | 0.02 |
| Age, γ11 | −0.01 | 0.005 |
| Women, γ12 | −0.06 | 0.03 |
| Education, γ13 | 0.01 | 0.01 |
| Job length, γ14 | 0.002 | 0.002 |
| Depressive symptoms, γ15 | −0.01 | 0.01 |
| Cardiovascular disease, γ16 | 0.02 | 0.06 |
| Self-rated health, γ17 | −0.03 | 0.02 |
| Log income, γ18 | 0.01 | 0.01 |
| Mental demands, γ19 | −0.005* | 0.002 |
| Retirement onset, γ20 | −0.08 | 0.14 |
| Age, γ21 | 0.08* | 0.03 |
| Women, γ22 | 0.26 | 0.26 |
| Education, γ23 | −0.01 | 0.05 |
| Job length, γ24 | −0.001 | 0.01 |
| Depressive symptoms, γ25 | 0.28* | 0.10 |
| Cardiovascular disease, γ26 | −0.21 | 0.40 |
| Self-rated health, γ27 | 0.21 | 0.13 |
| Log income, γ28 | −0.08 | 0.08 |
| Mental demands, γ29 | 0.02 | 0.01 |
| Differences between pre- and postretirement change, γ30 | 0.06* | 0.03 |
| Age, γ31 | −0.01 | 0.01 |
| Women, γ32 | 0.06 | 0.04 |
| Education, γ33 | −0.02* | 0.01 |
| Job length, γ34 | −0.001 | 0.002 |
| Depressive symptoms, γ35 | 0.01 | 0.02 |
| Cardiovascular disease, γ36 | −0.06 | 0.07 |
| Self-rated health, γ37 | 0.05* | 0.02 |
| Log income, γ38 | −0.003 | 0.01 |
| Mental demands, γ39 | 0.01* | 0.002 |
| Practice effects, γ40 | −0.06 | 0.25 |
| Practice effects × time-to-retirement, γ50 | −0.04 | 0.03 |
| Random effects | ||
| Variance intercept, σ2u0 | 26.38* | 1.41 |
| Variance time-to-retirement, σ2u1 | 0.14* | 0.02 |
| Variance retirement onset, σ2u2 | 4.92* | 1.40 |
| Variance postretirement change, σ2u3 | 0.14* | 0.04 |
| Covariance, σu0u1 | 0.57* | 0.16 |
| Covariance, σu0u2 | −5.81* | 1.19 |
| Covariance, σu0u3 | −1.07* | 0.19 |
| Covariance, σu1u2 | −0.43* | 0.16 |
| Covariance, σu1u3 | −0.12* | 0.03 |
| Covariance, σu2u3 | 0.55* | 0.16 |
| Residual, σ2e1 | 33.71* | 0.32 |
| Psuedo R2 | .220 | |
| Goodness-of-fit | ||
| −2 LL | 229,333 | |
| BIC | 229,775 | |
Note. N = 4,182. Number of observations: 34,664. Intraclass Correlation (ICC) = .397.
p < .05.
Focusing on mental job demands, we found support for Hypotheses 1–4. Figure 1 illustrates how mental job demands moderated changes in episodic memory in relation to retirement. Individuals who were engaged in occupations with greater mental demands (1 SD mental demands; black line) were more likely to exhibit better episodic memory before retirement and slower decline in the years after retirement (−1 SD mental demands; dashed line) than individuals who were engaged in occupations with fewer mental demands. These results are depicted by the small but statistically significant difference in slope before and after retirement. Specifically, we observed that individuals who were in occupations characterized by more mental activities (e.g., analyzing data or information, making decisions and solving problems, evaluating information, and thinking creatively) were more likely to exhibit better memory (γ09 = 0.06, p < .05) and less steep declines in memory in the years after retirement (γ39 = 0.01, p < .05). Additional factors associated with higher levels of episodic memory before retirement included younger age, being a woman, higher education, and reporting fewer depressive symptoms; we controlled for these factors in the analysis to examine unique variance related to work demands. Results support our hypotheses that individuals in jobs involving more mental demands functioned at higher levels both before and after retirement, and the rate of cognitive change after retirement was slower compared with individuals with lower levels of mental activities. Based on the parameter estimates in Table 2, the difference in memory for individuals with high (1 SD) and low (−1 SD) mental work demands at the time of retirement (year 0) was 1.72 T score units. Fifteen years after retirement, the difference goes up to 3.34 T scores. Therefore, the difference between high and low in job complexity doubles over 15 years and is about .34 SD 15 years after retirement, compared with .17 SD at the time of retirement.
Figure 1.
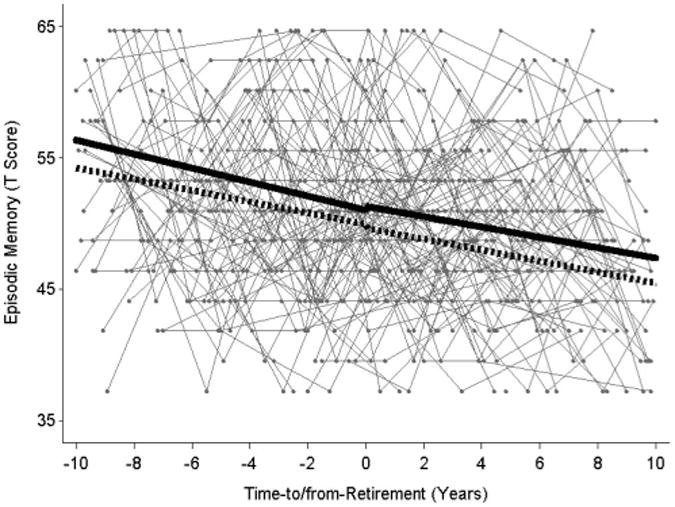
Mental job demands moderate changes in episodic memory in relation to retirement. Note. Gray lines are raw data for a subsample of 100 participants. Individuals who were engaged in occupations characterized by more mental demands (1 SD black line) were more likely to exhibit better episodic memory during employment and less declines after retirement, as compared with individuals who were engaged in occupations characterized by fewer mental demands (−1 SD dotted line).
We conducted a second set of analyses to examine a second dimension of cognitive functioning, mental status (McArdle et al., 2007). Specifically, we examined whether level of mental work demands moderated changes in another measure of cognition, namely mental status, after retirement. We only included postretirement observations of mental status because it was only assessed in every wave for respondents age 65 and older (Ofstedal et al., 2005). Results from Table 3 show that mental status showed slight declines after retirement (γ10 = −0.27 T score units per year or −0.27 SD per 10 years of time). Table 3 shows that there were between-person differences in levels of mental status at the time of retirement (σ2u0 = 39.36, p < .05) and change in the years thereafter (σ2u1 = 0.17, p < .05). Similar to our analyses focusing on verbal memory, we found evidence to support Hypotheses 3–4; that individuals in occupations characterized by higher levels of mental job demands were more likely to exhibit higher levels of mental status at the time of retirement (Hypothesis 3; γ09 = 0.11, p < .05) and less steep declines in mental status after retirement (Hypothesis 4; γ19 = 0.004, p < .05) compared with individuals in occupations with lower levels of job demands. Additional factors associated with higher levels of mental status at the time of retirement included younger age, being a man, higher years of education, more years in the same occupation, fewer depressive symptoms, and better self-rated health. Additional factors associated with less steep rates of decline in mental status after retirement included younger age. Once again, these results supported our hypotheses. Based on the parameters from Table 3, the difference between low and high job complexity is 2.37 T score units at the time of retirement (year 0) and grows to 3.66 T-score units at 15 years after retirement. Again, at the time of retirement the differences may not be large, but they grow over time.
Table 3. Fixed and Random Effects for Change in Mental Status After Retirement.
| Estimate | SE | |
|---|---|---|
| Fixed effects | ||
| Level, γ00 | 49.76* | 0.13 |
| Age, γ01 | −0.16* | 0.02 |
| Women, γ02 | −0.96* | 0.03 |
| Education, γ03 | 0.96* | 0.25 |
| Job length, γ04 | 0.03* | 0.05 |
| Depressive symptoms, γ05 | −0.32* | 0.01 |
| Cardiovascular disease, γ06 | 0.49 | 0.11 |
| Self-rated health, γ07 | 0.90* | 0.37 |
| Log income, γ08 | −0.02 | 0.17 |
| Mental demands, γ09 | 0.11* | 0.08 |
| Postretirement change, γ10 | −0.27* | 0.02 |
| Age, γ11 | −0.01* | 0.005 |
| Women, γ12 | 0.01 | 0.04 |
| Education, γ13 | 0.003 | 0.01 |
| Job length, γ14 | 0.002 | 0.002 |
| Depressive symptoms, γ15 | −0.02 | 0.02 |
| Cardiovascular disease, γ16 | −0.01 | 0.05 |
| Self-rated health, γ17 | −0.02 | 0.02 |
| Log income, γ18 | 0.02 | 0.01 |
| Mental demands, γ19 | 0.004* | 0.002 |
| Random effects | ||
| Variance intercept, σ2u0 | 39.36* | 1.23 |
| Variance postretirement change, σ2u1 | 0.17* | 0.02 |
| Covariance, σu0u1 | 0.85* | 0.12 |
| Residual, σ2e1 | 28.23* | 0.46 |
| Psuedo R2 | .115 | |
| Goodness-of-fit | ||
| −2 LL | 88,951 | |
| BIC | 89,149 | |
Note. N = 3,700. Number of observations: 13,294. Intraclass Correlation (ICC) = .630.
p < .05.
Discussion
The purpose of this article was to extend prior work on the relationship between mental work demands and cognitive functioning during the latter part of life to distinguish intraindividual trajectories before and after retirement. Only one study to date has taken retirement into account when studying the role of occupational characteristics and intraindividual differences in cognition (Finkel et al., 2009). Our study not only served an important purpose in seeking to cross-validate findings of a single study to date, but did so using a much larger, more heterogenous sample (>4,000 workers compared with 462) and was the first study of this sort using a U.S. sample. We analyzed data that were collected in a large, nationally representative sample over an 18 year time period with up to 10 occasions per respondent. In summary, results supported Hypotheses 1–2, that mental job demands were related to better cognitive functioning during one's time of employment (i.e., both level and rate of cognitive change before retirement), and Hypotheses 3–4, that mental demands were related to cognitive functioning (both level and rate of cognitive change) after retirement. In other words, individuals with higher levels of mental job demands had higher levels of cognitive functioning and experienced less steep declines in cognitive functioning in the years preceding and after retirement compared with individuals in jobs with lower levels of mental job demands. More specifically, individuals engaged in occupations characterized by higher levels of mental job demands were more likely to exhibit higher levels of cognitive functioning before retirement and were protected to a small degree against declines in cognition in the years after retirement. We tested these hypotheses by examining intraindividual differences in episodic memory before and after retirement, and mental status in the years after retirement. Although mental status was only available postretirement, it was included to broaden the domain of cognitive functioning assessed in the study, and because mental status is an important indicator of cognitive impairment (Crimmins et al., 2011). Results regarding mental status were consistent with results regarding episodic memory, demonstrating consistency in the relations between mental job demands and cognition after retirement.
One of the strengths of this study is that we did not measure workers' perceptions about their mental job demands, but used O*NET ratings to indicate workers' level of mental job demands. A second strength is that we examined patterns of normal cognitive aging among a national, heterogeneous community-based sample, rather than focusing on processes of dementia. However, it is challenging to accurately identify whether cognitive decline is part of normal cognitive aging or pathological dementia processes (Fisher, Plassman, Heeringa, & Langa, 2008). This is because of the fact that assessment methods and diagnostic criteria lack the sensitivity and specificity to distinguish between those who will progress to dementia and those who will live the remainder of their lives with “normal” cognition.
Our results support the notion of differential preservation (Salthouse, 2006) and are consistent with prior empirical work that has demonstrated long-term cognitive benefits to being involved in work with high levels of mental demands (e.g., Andel et al., 2005; Finkel et al., 2009; Potter et al., 2008; Schooler, 2007). These results are also consistent with Schooler's theory that environmental mechanisms influence cognitive functioning, as well as prior empirical results by Schooler et al. (1999, 2004). We specifically examined retirement as a pivot-point in investigating whether involvement of work characterized by high levels of job demands is protective beyond one's time of employment. More specifically, individuals who were engaged in work characterized by greater mental demands exhibited slightly less steep declines in cognitive functioning in the years after retirement. However, although the results were statistically significant, the differences in intraindividual rates of change before versus after retirement were quite small. In other words, our results provide initial evidence that working in a job with higher levels of mental demands may be protective against cognitive decline, but only to a small degree.
This was the first study to consider this issue directly in the occupational health psychology literature and as such, our results have implications for job or vocational choice as well as job design or redesign. In other words, although the effect sizes found in our study were relatively small, these results suggest that choosing an occupation requiring a variety of mental processes or redesigning less cognitively complex jobs to be more cognitively complex (e.g., include more thinking, reasoning, decision making, etc.) may be beneficial to employees. Research by Finkel et al. (2009) demonstrated that complexity of work with people was beneficial with regard to trajectories of verbal functioning. This is consistent with other research that has suggested cognitive benefits to social engagement (e.g., Bielak, 2010; Bielak et al., 2012; Hultsch et al., 1999).
It may be of practical benefit to provide people with enriched work environments not only to enhance job satisfaction and involvement (Humphrey, Nahrgang, & Morgeson, 2007), but also cognitive functioning. In fact, our results pose the likelihood that being exposed to new experiences (e.g., mentoring) or more cognitively complex job duties may be beneficial not only to newer workers, but to more seasoned employees as well. Employers should strive to improve mental demands at work, and if possible, outside of work as well, such as by emphasizing lifelong learning activities. This is consistent with the literature on aging in general, such that cognitive stimulation and novelty have shown positive benefits with cognitive aging (Greenwood & Parasuraman, 2012; Hertzog, Kramer, Wilson, & Lindenberger, 2008). Job redesign efforts to increase the amount of mental work activities involved in work characterized by lower mental demands may be beneficial for workers' cognitive functioning in later life. However, additional research is needed because it is possible that adding to the mental processing one does on his or her job may also be a potential stressor. In addition, results imply that cognitive interventions may be best to target those in jobs that are characterized by fewer mental demands. If people in jobs with more mental demands have higher levels and more positive changes in cognitive functioning, then they may benefit less than those in jobs with fewer mental demands.
Limitations and Future Directions
As with all studies, there were methodological limitations that merit further discussion. Our study does not facilitate interpretation of the causal relations between mental work demands and cognitive change, as it does not permit an empirical investigation of reverse causality. That is, our findings could be explained by the notion that individuals with higher levels of cognitive ability may select more cognitively complex work. Potter et al. (2008) conducted the first study to control for earlier life cognitive ability and obtained results consistent with ours here, although their study focused on older men in a sample of World War II veterans and did not examine cognitive level or rate of change before and after retirement. Our study did control for formal educational attainment and income, as education and socioeconomic status are highly correlated with occupational complexity as well as job level. In our model, education was related to post-, but not preretirement, change in cognitive ability. We added these control variables to adjust for other variables related to selection into occupations with higher levels of mental demands. However, inclusion of these control variables in our analysis may have also reduced the size of the effects in examining levels of mental demands at work in relation to both levels and rate of change in cognitive functioning.
Second, relations studied here may be attenuated because of missing data related to sample attrition as well as range restriction on measures of cognitive functioning, particularly on the mental status dimension. Our measures included episodic memory and mental status (McArdle et al., 2007), both of which are recognized as important indicators of cognitive impairment in later life (Ofstedal et al., 2005). Additional research should examine other dimensions of cognitive abilities to determine whether mental job demands are more strongly related to other cognitive domains. Although the HRS has recently added additional measures of fluid abilities, including quantitative reasoning, verbal reasoning, and verbal fluency, these measures were only added recently in the 2010 wave and longitudinal data are not yet available. The cognitive measures we did study, however, are both important and strong indicators of cognitive impairment and functioning in later life (Crimmins et al., 2011; Hurd et al., 2013; Potter et al., 2008). Given the high prevalence, problems, and costs associated with cognitive impairment, episodic memory and mental status are important domains of cognitive functioning to consider, particularly in later life.
Another limitation is that this study did not take into account the mental demands of what individuals do outside the workplace. For example, some workers may be very active in cognitively complex activities, and others may not. Our research here did not include nonwork activities because measures were not available in the HRS. Although this was a limitation in the present study, one constraint of using archival data is being limited by measures that are available (Fisher & Barnes-Farrell, 2013). The strengths of the research seem to outweigh this limitation. Future research ought to consider nonwork activities as well.
Although the current investigation does contribute to the literature on understanding the cognitive functioning of aging workers both before and after they retire, future research ought to examine the role of cognition in relation to retirement decisions. In other words, are workers more likely to retire sooner if they experience noticeable declines in cognitive ability? If so, do job characteristics moderate this relationship such that workers in more mentally demanding jobs are more likely to retire sooner? How is cognitive ability related to postretirement adjustment? Retirement is considered to be a process (Adams & Beehr, 2003; Fisher & Willis, 2013; Wang & Shultz, 2010), and workers may take different paths to retirement, such as bridge employment or unretirement (e.g., retiring, returning to work, and retiring again; Maestas, 2005). Additional research should examine the relationship between cognitive functioning and alternative patterns of retirement. In other words, is returning to work after retirement or engaging in bridge employment, even if it means working in a less demanding job compared to one's career job, better for workers' cognitive functioning than retiring and no longer working? Future research should also examine the extent to which there are cohort differences in the results obtained here. As such, future research should be conducted using a large, heterogenous sample similar to the HRS data presented here to make comparisons across different groups, and using longitudinal data to examine changes in cognition over time rather than at one point in time. One possibility is to examine subgroups of individuals within the population, such as those who report chronic diseases before retirement. This may be particularly important because cognitive ability is also related to health (Salthouse, 2012) and common chronic health conditions (e.g., heart disease, diabetes) are a risk factor for cognitive impairment and dementia (Llewellyn et al., 2010).
This study was similar to a previous study by Finkel et al. (2009), which was conducted using data from a Swedish sample. We found similar results despite possible differences in culture. For example, jobs that are characterized as having high mental work demands may be different in Sweden compared to jobs in the United States. Sweden differs from the United States to the extent that age 67 is currently the mandatory retirement age, whereas in the United States, very few occupations (e.g., airline pilots, federal law enforcement personnel) have a mandatory retirement age. Rohwedder and Willis (2010) examined the impact of different nations' retirement policies on cognitive functioning at the nationwide aggregate level, though additional cross-cultural research is needed. Future research should consider examining cross-cultural differences in work demands as well as trajectories of cognitive functioning to better understand these relationships on a more global level.
Another avenue for future research is to study the extent to which declines in work ability, defined as an individual's job-related functional capacity or his or her ability to meet the current demands of their job, are related to declines in workers' cognitive abilities. Furthermore, are health-related departures from the workforce related to cognitive decline? These issues are likely to become increasingly important in light of changes to economic policy that are designed to encourage older workers to remain in the workforce until later ages. A clearer understanding of the role of cognitive ability and cognitive declines in relation to how workers plan for retirement and make decisions about how and when to retire would be useful. Answers to such questions about the retirement process are important for understanding what individuals and organizations can do to facilitate the retention of older workers in the workforce and anticipate when workers may retire.
In summary, this was the first study to examine the association of mental job demands and pre- versus postretirement intraindividual trajectories of cognitive functioning in a large, heterogeneous U.S. sample. Our results, based on data spanning 18 years, suggest benefits to being engaged in cognitively complex work not only in the years approaching retirement, but beyond. This study, which is the first of its kind to raise this issue in the occupational health literature, highlights important implications for the design of work activities to enhance and protect workers' cognitive functioning in later life.
Acknowledgments
GGF was supported by Grants R01 AG027010 and R37 AG007137 from the National Institute on Aging. JDF was supported by U01AG009740 from the National Institute on Aging. Funding for the data linkage of HRS and O'NET was provided by the National Institute for Occupational Safety and Health. We thank Lisa Marchiondo for her work in conducting the O'NET linkage and Ross Andel and Jack McArdle for their helpful comments on this article.
Footnotes
Only 35 respondents completed only two waves. Results did not differ when these 35 respondents were excluded.
The O'NET database was developed by the U.S. Department of Labor to assess a variety of work and worker characteristics across many different occupations. We incorporated data from O'NET to assess occupational characteristics because the HRS data do not contain extensive measures of job characteristics nor are the limited measures assessed at every wave.
The findings and conclusions in this article are those of the authors and do not necessarily reflect the official position of the Centers for Disease Control and Prevention.
Contributor Information
Gwenith G. Fisher, Department of Psychology, Colorado State University
Alicia Stachowski, Department of Psychology, University of Wisconsin–Stout.
Frank J. Infurna, Department of Psychology, Arizona State University
Jessica D. Faul, Institute Social Research Survey Research Center, University of Michigan
James Grosch, National Institute for Occupational Safety and Health, U.S. Centers for Disease Control and Prevention.
Lois E. Tetrick, Department of Psychology, George Mason University
References
- Adams GA, Beehr TA, editors. Retirement: Reasons, processes, and results. New York, NY: Springer; 2003. [Google Scholar]
- Andel R, Crowe M, Pedersen N, Mortimer J, Crimmins E, Johansson B, Gatz M. Complexity of work and risk of Alzheimer's disease: A population-based study of Swedish twins. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2005;60:P251–P258. doi: 10.1093/geronb/60.5.P251. [DOI] [PubMed] [Google Scholar]
- Anstey K, Christensen H. Education, activity, health, blood pressure and apolipoprotein E as predictors of cognitive change in old age: A review. Gerontology. 2000;46:163–177. doi: 10.1159/000022153. [DOI] [PubMed] [Google Scholar]
- Batty GD, Deary IJ, Gottfredson LS. Premorbid (early life) IQ and later mortality risk: Systematic review. Annals of Epidemiology. 2007;17:278–288. doi: 10.1016/j.annepidem.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Bielak AA. How can we not “lose it” if we still don't understand how to “use it”? Unanswered questions about the influence of activity participation on cognitive performance in older age–a mini-review. Gerontology. 2010;56:507–519. doi: 10.1159/000264918. [DOI] [PubMed] [Google Scholar]
- Bielak AAM, Anstey KJ, Christensen H, Windsor TD. Activity engagement is related to level, but not change in cognitive ability across adulthood. Psychology and Aging. 2012;27:219–228. doi: 10.1037/a0024667. [DOI] [PubMed] [Google Scholar]
- Bosma H, van Boxtel MPJ, Ponds RWHM, Houx PJH, Jolles J. Education and age-related cognitive decline: The contribution of mental workload. Educational Gerontology. 2003;29:165–173. doi: 10.1080/10715769800300191. [DOI] [Google Scholar]
- Brandt J, Spencer M, Folstein M. The telephone interview for cognitive status. Neuroepidemiology. 1988;1:111–117. doi: 10.1159/000264678. [DOI] [Google Scholar]
- Crimmins EM, Kim JK, Langa KM, Weir DR. Assessment of cognition using surveys and neuropsychological assessment: The Health and Retirement Study and the Aging, Demographics, and Memory Study. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2011;66B(Suppl 1):i162–i171. doi: 10.1093/geronb/gbr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary IJ, Strand S, Smith P, Fernandes C. Intelligence and educational achievement. Intelligence. 2007;35:13–21. doi: 10.1016/j.intell.2006.02.001. [DOI] [Google Scholar]
- Der G, Batty GD, Deary IJ. The association between IQ in adolescence and a range of health outcomes at 40 in the 1979 US National Longitudinal Study of Youth. Intelligence. 2009;37:573–580. doi: 10.1016/j.intell.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauth EB, Gerstorf D, Ram N, Malmberg B. Changes in depressive symptoms in the context of disablement processes: Role of demographic characteristics, cognitive function, health, and social support. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2012;67B:167–177. doi: 10.1093/geronb/gbr078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer E, Salthouse TA, Stewart WF, Schwartz BS. Modeling age and retest processes in longitudinal studies of cognitive abilities. Psychology and Aging. 2004;19:243–259. doi: 10.1037/0882-7974.19.2.243. [DOI] [PubMed] [Google Scholar]
- Finkel D, Andel R, Gatz M, Pedersen NL. The role of occupational complexity in trajectories of cognitive aging before and after retirement. Psychology and Aging. 2009;24:563–573. doi: 10.1037/a0015511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher GG, Barnes-Farrell JL. The use of archival data in OHP research. In: Wang M, Sinclair RR, Tetrick LE, editors. Research methods in occupational health psychology: State of the art in measurement, design, and data analysis. New York, NY: Routledge; 2013. pp. 290–322. [Google Scholar]
- Fisher GG, Hassan H, Rodgers WL, Weir DR. HRS/AHEAD Documentation Report. Ann Arbor, MI: Survey Research Center, Institute for Social Research, University of Michigan; 2013. Health and retirement study imputation of cognitive functioning measures: 1992–2010. Retrieved from the Health and Retirement Study website. ( http://hrsonline.isr.umich.edu/modules/meta/xyear/cogimp/desc/COGIMPdd.pdf) [Google Scholar]
- Fisher GG, Plassman BL, Heeringa SG, Langa KM. Assessing the relationship of cognitive aging and processes of dementia. In: Hofer SM, Alwin DF, editors. The handbook of cognitive aging: Interdisciplinary perspectives. Thousand Oaks, CA: Sage; 2008. pp. 340–350. [DOI] [Google Scholar]
- Fisher GG, Willis RJ. Research methods in retirement research. In: Wang M, editor. The Oxford handbook of retirement. New York, NY: Oxford University Press; 2013. pp. 177–201. [Google Scholar]
- Foster JC, Taylor GA. The applicability of mental tests to persons over fifty years of age. Journal of Applied Psychology. 1920;4:39–58. doi: 10.1037/h0071260. [DOI] [Google Scholar]
- Fratiglioni L, Wang HX. Brain reserve hypothesis in dementia. Journal of Alzheimer's Disease. 2007;12:11–22. doi: 10.3233/jad-2007-12103. [DOI] [PubMed] [Google Scholar]
- González HM, Bowen ME, Fisher GG. Memory decline and depressive symptoms in a nationally representative sample of older adults: The Health and Retirement Study (1998–2004) Dementia and Geriatric Cognitive Disorders. 2008;25:266–271. doi: 10.1159/000115976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González HM, Tarraf W, Bowen ME, Johnson-Jennings MD, Fisher GG. What do parents have to do with my cognitive reserve life course perspectives on twelve-year cognitive decline. Neuroepidemiology. 2013;41:101–109. doi: 10.1159/000350723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood PM, Parasuraman R. Nurturing the older brain and mind. Cambridge, MA: The MIT Press; 2012. [DOI] [Google Scholar]
- Hertzog C, Kramer AF, Wilson RS, Lindenberger U. Enrichment effects on adult cognitive development: Can the functional capacity of older adults be preserved and enhanced. Psychological Science in the Public Interest. 2008;9:1–65. doi: 10.1111/j.1539-6053.2009.01034.x. [DOI] [PubMed] [Google Scholar]
- HRS. Health and Retirement Study: Sample sizes and response rates. 2011 Retrieved from http://hrsonline.isr.umich.edu/sitedocs/sampleresponse.pdf.
- Hultsch DF, Hammer M, Small BJ. Age differences in cognitive performance in later life: Relationships to self-reported health and activity life style. The Journal of Gerontology. 1993;48:P1–P11. doi: 10.1093/geronj/48.1.P1. [DOI] [PubMed] [Google Scholar]
- Hultsch DF, Hertzog C, Small BJ, Dixon RA. Use it or lose it: Engaged lifestyle as a buffer of cognitive decline in aging? Psychology and Aging. 1999;14:245–263. doi: 10.1037/0882-7974.14.2.245. [DOI] [PubMed] [Google Scholar]
- Humphrey SE, Nahrgang JD, Morgeson FP. Integrating motivational, social, and contextual work design features: A metaanalytic summary and theoretical extension of the work design literature. Journal of Applied Psychology. 2007;92:1332–1356. doi: 10.1037/0021-9010.92.5.1332. [DOI] [PubMed] [Google Scholar]
- Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM. Monetary costs of dementia in the United States. The New England Journal of Medicine. 2013;368:1326–1334. doi: 10.1056/NEJMsa1204629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infurna FJ, Gerstorf D. Linking perceived control, physical activity, and biological health to memory change. Psychology and Aging. 28:1147–1163. doi: 10.1037/a0033327. in press. [DOI] [PubMed] [Google Scholar]
- Infurna FJ, Gerstorf D, Ryan LH, Smith J. Dynamic links between memory and functional limitations in old age: Longitudinal evidence for age-based structural dynamics from the AHEAD Study. Psychology and Aging. 2011;26:546–558. doi: 10.1037/a0023023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infurna FJ, Gerstorf D, Zarit SH. Substantial changes in mastery perceptions of dementia caregivers with the placement of a care recipient. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2013;68:202–214. doi: 10.1093/geronb/gbs063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juster F, Suzman R. An overview of the health and retirement study. Journal of Human Resources. 1995;30(Suppl):S7–S56. doi: 10.2307/146277. [DOI] [Google Scholar]
- Kanfer R, Ackerman PL. Aging, adult development, and work motivation. The Academy of Management Review. 2004;29:440–458. doi: 10.2307/20159053. [DOI] [Google Scholar]
- Kohn ML, Schooler C. Occupation and psychological functioning. American Sociological Review. 1973;38:97–118. doi: 10.2307/2094334. [DOI] [Google Scholar]
- Kohn ML, Schooler C. Work and personality: An inquiry into the impact of social stratification. Norwood, NJ: Ablex; 1983. [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O. SAS system for mixed models. 2nd. Cary, NC: SAS Institute; 2006. [Google Scholar]
- Little RJA, Rubin DB. Statistical analysis with missing data. New York, NY: Wiley; 1987. [Google Scholar]
- Llewellyn DJ, Lang IA, Matthews FE, Plassman BL, Rogers MAM, Morgenstern LB, et al. Langa KM. Vascular health, diabetes, APOE and dementia: The aging, demographics, and memory study. Alzheimer's Research and Therapy. 2010;2:19–26. doi: 10.1186/alzrt43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestas N. Back to work: Expectations and realizations of work after retirement. RAND Labor and Population Working Paper No WR. 2005:196–1. doi: 10.1353/jhr.2010.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle JJ, Fisher GG, Kadlec KM. A latent growth curve analysis of age trends in tests of cognitive ability in the elderly U.S. population, 1992–2004. Psychology and Aging. 2007;22:525–545. doi: 10.1037/0882-7974.22.3.525. [DOI] [PubMed] [Google Scholar]
- Meredith W, Tisak J. Latent curve analysis. Psychometrika. 1990;55:107–122. doi: 10.1007/BF02294746. [DOI] [Google Scholar]
- Morgeson FP, Humphrey SE. The work design questionnaire (WDQ): Developing and validating a comprehensive measure for assessing job design and the nature of work. Journal of Applied Psychology. 2006;91:1321–1339. doi: 10.1037/0021-9010.91.6.1321. [DOI] [PubMed] [Google Scholar]
- Morgeson FP, Medsker GJ, Campion MA. Job and team design. In: Salvendy G, editor. Handbook of human factors and ergonomics. 3rd. Hoboken, NJ: Wiley; 2008. pp. 428–457. [Google Scholar]
- Mortimer JA, Graves AB. Education and other socioeconomic determinants of dementia and Alzheimer's disease. Neurology. 1993;43:S39–S44. [Google Scholar]
- Newson RS, Kemps EB. General lifestyle activities as a predictor of current cognition and cognitive change in older adults: A cross-sectional and longitudinal examination. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2005;60B:P113–P120. doi: 10.1093/geronb/60.3.P113. [DOI] [PubMed] [Google Scholar]
- Ofstedal MB, Fisher GG, Herzog AR. HRS/AHEAD Documentation Report DR-006. Ann Arbor, MI: Institute for Social Research, University of Michigan; 2005. Documentation of cognitive functioning measures in the health and retirement study. [Google Scholar]
- Park DC. The basic mechanisms accounting for age-related decline in cognitive function. In: Park DC, Schwarz N, editors. Cognitive aging: A primer. New York, NY: Routledge; 2000. pp. 3–21. [Google Scholar]
- Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, et al. Wallace RB. Prevalence of cognitive impairment without dementia in the United States. Annals of Internal Medicine. 2008;148:427–434. doi: 10.7326/0003-4819-148-6-200803180-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassman BL, Langa K, Fisher GG, Heeringa S, Weir DR, Ofstedal MB, et al. Wallace RB. Prevalence of dementia in a community sample of adults age 70 and older. Neuroepidemiology. 2007;29:125–132. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter GG, Helms MJ, Plassman BL. Associations of job demands and intelligence with cognitive performance among men in late life. Neurology. 2008;70:1803–1808. doi: 10.1212/01.wnl.0000295506.58497.7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter GG, Plassman BL, Helms MJ, Foster SM, Edwards NW. Occupational characteristics and cognitive performance among elderly male twins. Neurology. 2006;67:1377–1382. doi: 10.1212/01.wnl.0000240061.51215.ed. [DOI] [PubMed] [Google Scholar]
- Ram N, Grimm K. Using simple and complex growth models to articulate developmental change: Matching theory to method. International Journal of Behavioral Development. 2007;31:303–316. doi: 10.1177/0165025407077751. [DOI] [Google Scholar]
- Raudenbush SW. Toward a coherent framework for comparing trajectories of individual change. In: Collins LM, Sayer AG, editors. New methods for the analysis of change. Washington, DC: American Psychological Association; 2001. pp. 33–64. [DOI] [Google Scholar]
- Rodgers WL, Ofstedal MB, Herzog AR. Trends in scores on tests of cognitive ability in the elderly US population, 1993–2000. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2003;58:S338–S346. doi: 10.1093/geronb/58.6.S338. [DOI] [PubMed] [Google Scholar]
- Rohwedder S, Willis RJ. Mental retirement. Journal of Economic Perspectives. 2010;24:119–138. doi: 10.1257/jep.24.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Theoretical perspectives on cognitive aging. Hillsdale, NJ: Erlbaum; 1991. [Google Scholar]
- Salthouse TA. Mental exercise and mental aging: Evaluating the validity of the “use it or lose it” hypothesis. Perspectives on Psychological Science. 2006;1:68–87. doi: 10.1111/j.1745-6916.2006.00005.x. [DOI] [PubMed] [Google Scholar]
- Salthouse T. Consequences of age-related cognitive declines. Annual Review of Psychology. 2012;63:201–226. doi: 10.1146/annurev-psych-120710-100328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA, Atkinson TM, Berish DE. Executive functioning as a potential mediator of age-related cognitive decline in normal adults. Journal of Experimental Psychology: General. 2003;132:566–594. doi: 10.1037/0096-3445.132.4.566. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Berish DE, Miles JD. The role of cognitive stimulation on the relations between age and cognitive functioning. Psychology and Aging. 2002;17:548–557. doi: 10.1037/0882-7974.17.4.548. [DOI] [PubMed] [Google Scholar]
- Schaie KW. Developmental influences on adult intelligence: The Seattle Longitudinal Study. New York, NY: Oxford University Press; 2005. [DOI] [Google Scholar]
- Schooler C. Psychological effects of complex environments during the life span: A review and theory. Intelligence. 1984;8:259–281. doi: 10.1016/0160-2896(84)90011-4. [DOI] [Google Scholar]
- Schooler C. Psychological factors and effective cognitive functioning through the life span. In: Birren JE, Schaie KW, editors. Handbook of the psychology of aging. Orlando, FL: Academic Press; 1990. pp. 347–358. [DOI] [Google Scholar]
- Schooler C. Use it–and keep it, longer, probably. A reply to Salthouse (2006) Perspectives on Psychological Science. 2007;2:24–29. doi: 10.1111/j.1745-6916.2007.00026.x. [DOI] [PubMed] [Google Scholar]
- Schooler C, Mulatu MS, Oates G. The continuing effects of substantively complex work on the intellectual functioning of older workers. Psychology and Aging. 1999;14:483–506. doi: 10.1037/0882-7974.14.3.483. [DOI] [PubMed] [Google Scholar]
- Schooler C, Mulatu MS, Oates G. Occupational self-direction, intellectual functioning, and self-directed orientation in older workers: Findings and implications for individuals and societies. American Journal of Sociology. 2004;110:161–197. doi: 10.1086/385430. [DOI] [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. New York, NY: Oxford University Press; 2003. [DOI] [Google Scholar]
- Small BJ, Dixon RA, McArdle JJ, Grimm KJ. Do changes in lifestyle engagement moderate cognitive decline in normal aging? Evidence from the Victoria Longitudinal Study. Neuropsychology. 2012;26:144–155. doi: 10.1037/a0026579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens DC, Fisher GG, Langa KM, Potter GG, Plassman BL. Prevalence of depression among older Americans: The Aging, Demographics, and Memory Study. International Psychogeriatrics. 2009;21:879–888. doi: 10.1017/S1041610209990044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffick D. HRS Documentation Report DR-005. Survey Research Center, Institute for Social Research, University of Michigan; Ann Arbor, MI: 2000. Documentation of Affective functioning measures in the Health and Retirement Study. [Google Scholar]
- Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. Journal of the International Neuropsychological Society. 2002;8:448–460. doi: 10.1017/S1355617702813248. [DOI] [PubMed] [Google Scholar]
- Thorndike EL, Tilton JW, Woodyard E. Adult learning. New York, NY: MacMillan; 1928. [Google Scholar]
- Verhaeghen P, Salthouse TA. Meta-analyses of age-cognition relations in adulthood: Estimates of linear and nonlinear age effects and structural models. Psychological Bulletin. 1997;122:231–249. doi: 10.1037/0033-2909.122.3.231. [DOI] [PubMed] [Google Scholar]
- Wang M, Shultz KS. Employee retirement: A review and recommendations for future investigation. Journal of Management. 2010;36:172–206. doi: 10.1177/0149206309347957. [DOI] [Google Scholar]
- Wilson RS, Barnes LL, Krueger KR, Hoganson G, Bienias JL, Bennett DA. Early and late life cognitive activity and cognitive systems in old age. Journal of International Neuropsychological Society. 2005;11:400–407. doi: 10.1017/S1355617705050459. [DOI] [PubMed] [Google Scholar]


