Abstract
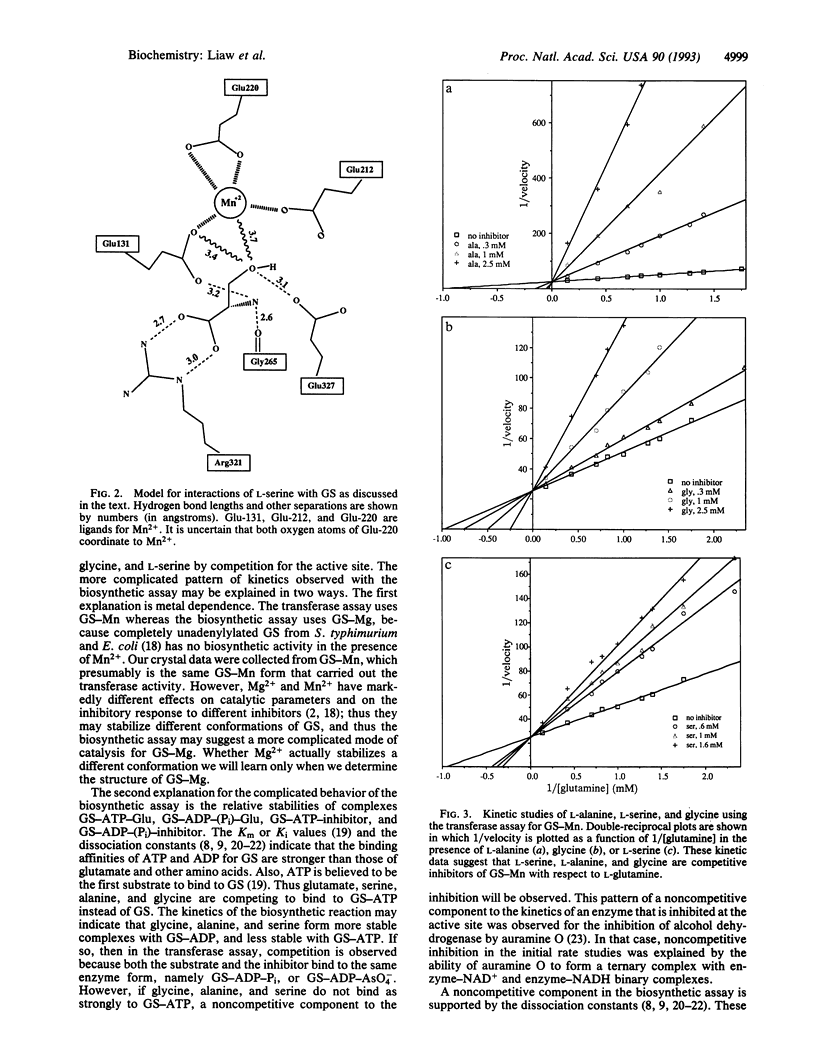
Bacterial glutamine synthetase (GS; EC 6.3.1.2) was previously shown to be inhibited by nine end products of glutamine metabolism. Here we present four crystal structures of GS, complexed with the substrate Glu and with each of three feedback inhibitors. The GS of the present study is from Salmonella typhimurium, with Mn2+ ions bound, and is fully unadenylylated. From Fourier difference maps, we find that L-serine, L-alanine, and glycine bind at the site of the substrate L-glutamate. In our model, these four amino acids bind with the atoms they share in common (the "main chain" +NH3-CH-COO-) in the same positions. Thus on the basis of our x-ray work, glycine, alanine, and serine appear to inhibit GS-Mn by competing with the substrate glutamate for the active site.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almassy R. J., Janson C. A., Hamlin R., Xuong N. H., Eisenberg D. Novel subunit-subunit interactions in the structure of glutamine synthetase. 1986 Sep 25-Oct 1Nature. 323(6086):304–309. doi: 10.1038/323304a0. [DOI] [PubMed] [Google Scholar]
- BOYER P. D., MILLS R. C., FROMM H. J. Hypotheses for and some kinetic studies with glutamine synthetase and acetate thiokinase. Arch Biochem Biophys. 1959 Mar;81(1):249–263. doi: 10.1016/0003-9861(59)90194-8. [DOI] [PubMed] [Google Scholar]
- Dahlquist F. W., Purich D. L. Regulation of Escherichia coli glutamine synthetase. Evidence for the action of some feedback modifiers at the active site of the unadenylylated enzyme. Biochemistry. 1975 May 6;14(9):1980–1989. doi: 10.1021/bi00680a028. [DOI] [PubMed] [Google Scholar]
- Ginsburg A. Conformational changes in glutamine synthetase from Escherichia coli. II. Some characteristics of the equilibrium binding of feedback inhibitors to the enzyme. Biochemistry. 1969 Apr;8(4):1726–1740. doi: 10.1021/bi00832a056. [DOI] [PubMed] [Google Scholar]
- Ginsburg A., Gorman E. G., Neece S. H., Blackburn M. B. Thermodynamics of active-site ligand binding to Escherichia coli glutamine synthetase. Biochemistry. 1987 Sep 22;26(19):5989–5996. doi: 10.1021/bi00393a007. [DOI] [PubMed] [Google Scholar]
- Janson C. A., Almassy R. J., Westbrook E. M., Eisenberg D. Isolation and crystallization of unadenylylated glutamine synthetase from Salmonella typhimurium. Arch Biochem Biophys. 1984 Feb 1;228(2):512–518. doi: 10.1016/0003-9861(84)90017-1. [DOI] [PubMed] [Google Scholar]
- LEVINTOW L., MEISTER A. Reversibility of the enzymatic synthesis of glutamine. J Biol Chem. 1954 Jul;209(1):265–280. [PubMed] [Google Scholar]
- Lovejoy B., Le T. C., Lüthy R., Cascio D., O'Neil K. T., DeGrado W. F., Eisenberg D. X-ray grade crystals of a designed alpha-helical coiled coil. Protein Sci. 1992 Jul;1(7):956–957. doi: 10.1002/pro.5560010714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek T. D., Villafranca J. J. Kinetic mechanism of Escherichia coli glutamine synthetase. Biochemistry. 1980 Nov 25;19(24):5513–5519. doi: 10.1021/bi00565a008. [DOI] [PubMed] [Google Scholar]
- Rhee S. G., Chock P. B. Mechanistic studies of glutamine synthetase from Escherichia coli: kinetics of ADP and orthophosphate binding to the unadenylylated enzyme. Biochemistry. 1976 Apr 20;15(8):1755–1760. doi: 10.1021/bi00653a025. [DOI] [PubMed] [Google Scholar]
- Rhee S. G., Villafranca J. J., Chock P. B., Stadtman E. R. Direct evidence for separate binding sites for L-Glu and amino acid feedback inhibitors on unadenylylated glutamine synthetase from E. coli. Biochem Biophys Res Commun. 1977 Sep 9;78(1):244–250. doi: 10.1016/0006-291x(77)91246-3. [DOI] [PubMed] [Google Scholar]
- Ross P. D., Ginsburg A. A calorimetric study of the binding of two feedback inhibitors to the glutamine synthetase from Escherichia coli. Biochemistry. 1969 Dec;8(12):4690–4695. doi: 10.1021/bi00840a005. [DOI] [PubMed] [Google Scholar]
- Shrake A., Park R., Ginsburg A. Calorimetric studies on the binding of substrates and the inhibitor L-alanine to unadenylylated glutamine synthetase of Escherichia coli. Biochemistry. 1978 Feb 21;17(4):658–664. doi: 10.1021/bi00597a015. [DOI] [PubMed] [Google Scholar]
- Sigman D. S., Glazer A. N. The site of auramine O binding to horse liver alcohol dehydrogenase. J Biol Chem. 1972 Jan 25;247(2):334–341. [PubMed] [Google Scholar]
- Timmons R. B., Rhee S. G., Luterman D. L., Chock P. B. Mechanistic studies of glutamine synthetase from Escherichia coli. Fluorometric identification of a reactive intermediate in the biosynthetic reaction. Biochemistry. 1974 Oct 22;13(22):4479–4485. doi: 10.1021/bi00719a002. [DOI] [PubMed] [Google Scholar]
- Woolfolk C. A., Shapiro B., Stadtman E. R. Regulation of glutamine synthetase. I. Purification and properties of glutamine synthetase from Escherichia coli. Arch Biochem Biophys. 1966 Sep 26;116(1):177–192. doi: 10.1016/0003-9861(66)90026-9. [DOI] [PubMed] [Google Scholar]
- Woolfolk C. A., Stadtman E. R. Regulation of glutamine synthetase. 3. Cumulative feedback inhibition of glutamine synthetase from Escherichia coli. Arch Biochem Biophys. 1967 Mar 20;118(3):736–755. doi: 10.1016/0003-9861(67)90412-2. [DOI] [PubMed] [Google Scholar]
- Yamashita M. M., Almassy R. J., Janson C. A., Cascio D., Eisenberg D. Refined atomic model of glutamine synthetase at 3.5 A resolution. J Biol Chem. 1989 Oct 25;264(30):17681–17690. doi: 10.2210/pdb2gls/pdb. [DOI] [PubMed] [Google Scholar]




