Abstract
Background
Elevated body mass index (BMI) is a risk factor for cardiovascular disease, diabetes, cancer, and other diseases. Inflammation or oxidative stress induced by high BMI may explain some of these effects. Millions of people drink arsenic-contaminated water worldwide, and ingested arsenic has also been associated with inflammation, oxidative stress, and cancer.
Objectives
To assess the unique situation of people living in northern Chile exposed to high arsenic concentrations in drinking water and investigate interactions between arsenic and BMI, and associations with lung and bladder cancer risks.
Methods
Information on self-reported body mass index (BMI) at various life stages, smoking, diet, and lifetime arsenic exposure was collected from 532 cancer cases and 634 population-based controls.
Results
In subjects with BMIs <90th percentile in early adulthood (27.7 and 28.6 kg/m2 in males and females, respectively), odds ratios (OR) for lung and bladder cancer combined for arsenic concentrations of <100, 100–800 and >800 μg/L were 1.00, 1.64 (95% CI, 1.19–2.27), and 3.12 (2.30–4.22). In subjects with BMIs ≥90th percentile in early adulthood, the corresponding ORs were higher: 1.00, 1.84 (0.75–4.52), and 9.37 (2.88–30.53), respectively (synergy index=4.05, 95% CI, 1.27–12.88). Arsenic-related cancer ORs >20 were seen in those with elevated BMIs in both early adulthood and in later life. Adjustments for smoking, diet, and other factors had little impact.
Conclusion
These findings provide novel preliminary evidence supporting the notion that environmentally-related cancer risks may be markedly increased in people with elevated BMIs, especially in those with an elevated BMI in early-life.
INTRODUCTION
Tens of millions of people worldwide are exposed to arsenic-contaminated drinking water. Ingested arsenic is an established cause of skin, bladder, and lung cancer, and has been linked to diabetes, and cardiovascular and lung disease (IARC 2012). Cancer risk from arsenic is very high, and the National Research Council has estimated that the cancer risk associated with the US standard for arsenic in drinking water of 10 μg/L may be as high as 1 in 300 (NRC 2001). This is more than an order of magnitude higher than the risks estimated for any other regulated drinking water carcinogen (Smith et al. 2002). Susceptibility to arsenic varies widely from person to person, and risks may be even higher in certain susceptible sub-populations. Inter-individual differences in diet, arsenic metabolism, co-exposures, and genetics have all been linked to increased risks, but to date most of the variability in susceptibility remains unexplained (National Research Council 2014).
The primary toxic mechanism of arsenic is unknown, but may involve inflammation or oxidative stress. Arsenic has been linked to biomarkers of oxidative stress and inflammation, alterations in immune status, and inflammatory and infectious diseases such as bronchiectasis, respiratory infections, and tuberculosis (Ahmed et al. 2011; Guha Mazumder et al. 2005; Ramsey et al. 2013; Smith et al. 2011). Elevated body mass indices (BMI) also increase oxidative stress and inflammation and has been linked to some, albeit not all, cancer types (De Pergola and Silvestris 2013; Marseglia et al. 2015). Given the common link to oxidative stress, inflammation, and cancer, we hypothesized that arsenic and elevated BMI may interact, to increase cancer risk. We evaluated this hypothesis using data from a cancer case-control conducted in high arsenic-exposed populations of the Atacama Desert area of northern Chile (Steinmaus et al. 2013). Previously, we and others have identified high risks of lung cancer, bladder cancer, cardiovascular disease, and other health outcomes in this area (Smith et al. 2012). The present study is to our knowledge the first to evaluate the potential impact of elevated BMI on arsenic-related cancer risk.
METHODS
Study area
Study design details are published elsewhere (Steinmaus et al. 2013). Briefly, the study area comprises two contiguous regions (Regions I, II) in northern Chile. This area lies within the Atacama Desert, one of the driest places on earth. Because of its dryness, most people live in one of the cities or small towns and receive drinking water from municipal supplies. Records of past arsenic concentrations in the water supplies are available for several past decades, and have ranged from <10 ug/L to >800 μg/L. The largest city in the area, Antofagasta, had a period of very high exposure starting in 1958 when drinking water for the city was sourced from two rivers with high arsenic concentrations. The high exposures ended in the 1970s when arsenic treatment plants were installed.
Subject ascertainment
Cases included people who: 1. Had primary lung or bladder cancer first diagnosed between October 2007 and December 2010; 2. Lived in the study area when diagnosed; 3. Were over age 25 years when diagnosed; and 4. Were able to provide interview data or had a close relative who could. Cases were ascertained from pathologists, hospitals, and radiologists in the study area. The majority of cases were histologically confirmed (98% for bladder cancer and 72% for lung cancer), the remaining diagnoses were based on radiologic and physician’s clinical assessments. Controls without lung or bladder cancer were randomly selected from the Chilean Voter Registry for the study area for the years 2007–2009, frequency matched to cases by gender and five-year age group. Our analysis of this registry showed that it contained >95% of people over age 40 years when compared to the Chile national census.
Of the 370 lung and 289 bladder cancer cases obtained from local pathologists, radiologists, or hospitals, 46 lung and 23 bladder cancer cases were ineligible based on age or residence criteria. Of the remaining, 4 lung (1.2%) and 12 (4.5%) bladder cancer cases (or their next of kin) could not be located, had moved outside the study area, or provided insufficient information. Of the remaining, 14 lung (4.4%) and 22 (8.7%) bladder cancer cases or their next of kin declined participation. Among 872 controls randomly selected from the Electoral Registry with viable addresses, 78 (8.9%) no longer lived at the address and could not be located, were ineligible due to illness, or gave insufficient information. Of the remaining, 154 (19.4%) declined to participate. Controls who did not participate were younger (63.7 vs. 66.3 years, respectively) and more likely to be male (72.5 vs. 67.3%) than participants, but overall inclusion rates among controls were similar among major arsenic exposure areas: 75.5% in highly exposed Antofagasta, 71.3% in moderately exposed Iquique and Calama, and 74.5% in low exposure Arica. One bladder and five lung cancer cases and six controls did not provide BMI information.
Participant interviews
All participants who gave informed consent were interviewed in person using a standardized questionnaire. Interviewers were not blinded to case status but were not made aware of hypotheses regarding excess BMI. For deceased subjects, we interviewed the next of kin (proxy). The proportions of proxy interviews were 8.7% for controls, 19.9% for bladder cancer, and 45.5% for lung cancer. Participants were asked to provide all residences at which the subject lived and all jobs held for six months or longer. Particular attention was given to mining work, a common occupation in northern Chile. Subjects were also asked about occupational exposures to specific chemicals linked to lung or bladder cancer, including silica, asbestos, and arsenic. Questions regarding tobacco smoke covered age when smoking began, periods quit, years smoked, number of cigarettes smoked per day, and childhood or adult secondhand smoke exposure. Subjects were also asked to provide information regarding their typical drinking water intake; however, this was not used here since it had only small impacts on categorizing exposure. When asked for information from the distant past, subjects were provided reminders of important events or jobs held during the corresponding time period.
Exposure indices
For each subject, each city or town of residence in Chile in which they lived was linked to a water arsenic measurement for that city or town so that an arsenic concentration could be assigned to each year of each subject’s life within Chile. Drinking water arsenic concentrations were collected from government agencies, published research studies, and water suppliers. The data were available for >94% of all drinking water sources in the study area (Ferreccio et al. 2000). Arsenic measurements were also available for all large cities in Chile outside the study area, and these were also linked to residences (most were <10 μg/L). Until recently, few people drank bottled water or used water filters. Annual arsenic concentration values were then used to calculate arsenic exposure indices, including the highest single-year of exposure in a subject’s lifetime, cumulative exposure (calculated by summing the yearly concentrations), and average lifetime exposure (cumulative exposure divided by the age at cancer diagnosis or study enrollment). Lag periods of 5, 10, 20, and 40 years were used to assess the effect of timing and dose of exposure on time of cancer appearance. Subjects were then categorized based on the arsenic concentrations in the largest cities in the study area (Arica and Iquique, <100 μg/L; Calama, 100–800 μg/L; and Antofagasta, >800 μg/L). Urinary arsenic metabolites were collected in 559 subjects at the time of interview, these were used to assess the relationship between BMI and arsenic methylation. Details of this collection are provided elsewhere (Melak et al. 2014).
Body mass index (BMI)
Subjects and proxy respondents were also asked to provide the subjects’ adult height, their typical weight at ages 20 and 40, and their typical weight in the ten years preceding the interview. Body mass index (BMI) at each period was then calculated as weight (kg) / height (m)2. The World Health Organization (WHO) definition for obesity is 30 mg/kg2, however since few subjects had a BMI >30 kg/m2 at age 20 we chose the sex-specific 90th percentiles to define high and low BMIs. Other cutoff points were considered, including ≥25 mg/kg2 which corresponds to WHO’s definition of overweight. For the cutoff point of ≥30 kg/m2 for BMI at age 20, there were 15 cases but no controls in the upper arsenic exposure category (>800 μg/L), so the odds ratio was estimated by adding 0.5 to each cell (Haldane 1956). Subjects reporting physician-diagnosed hypertension or anti-hypertension medication use were considered to be hypertensive. Subjects reporting physician-diagnosed diabetes or use of hypoglycemic medication were considered to be diabetics.
Statistical analysis
Odds ratios (OR) were calculated using unconditional logistic regression by categories of arsenic exposure. In some analyses, lung and bladder cancers were combined to increase statistical power. No heterogeneity in results was observed by sex, thus males and females were combined. Potential biologic interactions between arsenic and increased BMI were assessed by calculating cancer odds ratios for various levels of arsenic exposure in analyses stratified by high and low BMI, and using the Rothman Synergy Index (Rothman 1976). Logistic regression equations using arsenic water concentration as a continuous variable were also calculated and plotted for the following groups: 1. Those with BMIs < 90th percentile at all ages; 2. Those with a BMI ≥ 90th percentile at age 40 or during the 10 years preceding the interview but not at age 20; 3. Those with BMIs ≥90th percentile at age 20 but not at age 40 or at time of interview; and 4. Those with BMIs ≥90th percentile at age 20 and at age 40 or preceding the interview.
To evaluate arsenic exposures earlier and later in life, cancer ORs were calculated comparing subjects with highest known exposures >800 μg/L to subjects with highest known exposures <100 μg/L in analyses stratified by both BMI and the age at which they were first highly exposed (>800 μg/L). Antofagasta was the only area with exposures >800 μg/L, and these began in 1958. Thus, for this analysis subjects were stratified into whether they were born after 1938 (and thus had been highly exposed before age 20) and those born in 1938 or earlier (and thus would have been highly exposed only after age 20 or later).
Potential confounding variables entered into final logistic regression models included sex, age (ten-year groups), and smoking (ever vs. never). Entering mining work, exposure to a known lung or bladder carcinogen at work, typical daily fruit and vegetable consumption, second hand smoke exposure, drinking water intake, and socioeconomic status (SES) had little impact on results. Entering age or smoking as continuous variables (e.g., average number of cigarettes per week) had limited impact on results but led to unstable results in some analyses with small sample sizes.
Analyses were conducted using SAS version 9.2 (SAS Institute Inc., Cary NC) and all p-values are two-sided. Analysis of trends in odds ratios were assessed using the Cochrane-Armitage test for linear trend.
RESULTS
Cancer cases tended to be heavier smokers and had a greater arsenic exposure than controls but were not markedly different in terms of race (after arsenic adjustment), mining work, and SES (Table 1). Drinking water intake and fruit and vegetable consumption were higher in cases but differences were small. For BMI at age 20, the 90th percentile was 27.7 kg/m2 in men and 28.6 kg/m2 in women. BMI ranges below and above the 90th percentile at age 20 were 14.5–27.7 and 27.7–51.1 kg/m2 for men, and 16.2–28.4 and 28.8–48.6 kg/m2 for women. Subjects with a BMI ≥90th percentile at age 20 were older than subjects with lower BMIs (69.0 vs. 65.9 years old, p=0.002) but they were similar in terms of gender, smoking, race, occupational exposures, urinary arsenic metabolite proportions, and SES (Table S1). High BMI subjects had higher drinking water intake but again, the difference was small (2.00 vs. 1.85 L/day; p=0.45). Spearman correlation coefficients between BMI at age 20 and BMI at age 40 and preceding interview were 0.47 and 0.33, respectively (p-values <0.001).
Table 1.
Sociodemographic characteristics in cancer cases and controls
| Controls
|
Lung cancer
|
Bladder cancer
|
|||||
|---|---|---|---|---|---|---|---|
| N | N | OR | 95% CI | N | OR | 95% CI | |
| Sexa | |||||||
| Male | 427 | 210 | 169 | ||||
| Female | 207 | 91 | 62 | ||||
| Smoking | |||||||
| Never smoker | 237 | 57 | 1.00 | Ref | 64 | 1.00 | Ref |
| Ever smoker | 397 | 244 | 2.56 | 1.84–3.56 | 167 | 1.56 | 1.12–2.17 |
| Heavy smokerb | 30 | 52 | 7.21 | 4.22–12.30 | 18 | 2.22 | 1.16–4.24 |
| Secondhand smokec | |||||||
| No | 131 | 31 | 1.00 | Ref | 36 | 1.00 | Ref |
| Yes | 106 | 26 | 1.04 | 0.58–1.85 | 28 | 0.96 | 0.55–1.68 |
| Race | |||||||
| Other | 190 | 66 | 1.00 | Ref | 34 | 1.00 | Ref |
| Europeand | 444 | 235 | 1.52 | 1.10–2.10 | 197 | 2.48 | 1.66–3.71 |
| Mining work | |||||||
| No | 494 | 236 | 1.00 | Ref | 172 | 1.00 | Ref |
| Yes | 140 | 65 | 0.97 | 0.70–1.36 | 59 | 1.21 | 0.85–1.72 |
| Work lung carcinogene | |||||||
| No | 377 | 91 | 1.00 | Ref | 108 | 1.00 | Ref |
| Yes | 202 | 73 | 1.50 | 1.05–2.13 | 77 | 1.33 | 0.95–1.87 |
| Controls
|
Lung cancer
|
Bladder cancer
|
|||
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | p-value | Mean (SD) | p-value | |
| Age (years)a | 66.3 (11.3) | 65.9 (10.2) | 66.3 (11.9) | ||
| SES score (0–12) | 8.65 (2.81) | 8.10 (2.94) | 0.009 | 9.10 (2.73) | 0.03 |
| BMI (kg/m2) | |||||
| At age 20 | 23.14 (3.60) | 23.80 (3.79) | 0.006 | 23.46 (3.84) | 0.31 |
| At age 40 | 25.43 (4.81) | 25.15 (4.34) | 0.51 | 26.97 (4.48) | 0.05 |
| Preceding interview | 26.73 (4.32) | 25.20 (4.69) | <0.001 | 26.20 (4.82) | 0.14 |
| Fruit vegetable intakee,f | 1.96 (1.42) | 2.19 (1.20) | 0.002 | 2.18 (1.23) | 0.002 |
| Drinking water intakee | |||||
| Current (L/d) | 1.67 (0.91) | 1.84 (1.02) | <0.001 | 2.04 (1.14) | <0.001 |
| 20 years ago (L/d) | 1.80 (1.14) | 1.94 (1.05) | <0.001 | 1.97 (1.22) | 0.008 |
| Water arsenic (μg/L) | |||||
| Highest-lag 5 yrsg | 321.7 (341.4) | 468.8 (367.9) | <0.001 | 556.8 (345.3) | <0.001 |
| Highest-lag 40 yrsg | 285.6 (347.8) | 444.9 (380.1) | <0.001 | 511.1 (367.1) | <0.001 |
BMI, body mass index; CI, confidence interval; OR, odds ratio; SD, standard deviation; SES, socioeconomic factor score
Odds ratios or p-values not provided since cases and controls were frequency matched on age and sex
Average of ≥20 cigarettes per day when smoking. Odds ratios compared to never smokers
Only includes never smokers
Lung and bladder cancer ORs adjusted for arsenic water concentrations are 1.08 (0.76–1.54) and 1.47 (0.96–2.28), respectively
Only includes data from non-proxy subjects
Typical daily servings of fruit or vegetables 20 years before cancer diagnosis (cases) or interview (controls)
Excludes arsenic exposures in 5 or 40 years preceding cancer diagnosis (cases) or interview (controls)
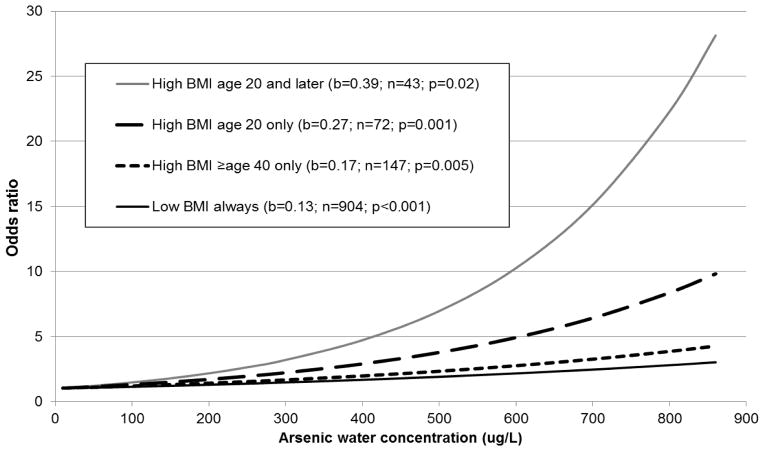
ORs for lung and bladder cancer combined in subjects with BMIs at age 20 below the 90th percentile for highest single year arsenic water concentrations of <100, 100–800 and >800 μg/L were 1.00, 1.64 (95% CI, 1.19–2.27), and 3.12 (2.30–4.22) (Table 2). In subjects with BMIs at age 20 ≥90th percentile, the corresponding ORs were higher: 1.00, 1.84 (0.75–4.52), and 9.37 (2.88–30.53) (synergy index=4.05; 95% CI, 1.27–12.88; p=0.016) (Table 3). Similar patterns were seen for lung and bladder cancer separately (Table 2), for different arsenic metrics (Figure S1), different BMI cutoff points (Figure S2), and after excluding proxy subjects (Table S2). Results were also similar when various lag periods (e.g. 5 to 40 years) were used (data not shown). The synergy index was greatest in those with higher BMIs who were born before 1938 (synergy index=38.64; 95% CI, 2.06–723.37) (Table 4). Figure 2 shows the plotted linear regression equation for cancer ORs in subjects with elevated BMIs at various ages. The highest ORs were in those with higher BMIs at age 20 and later (Table S6).
Table 2.
Lung and bladder cancer odds ratios by categories of arsenic water concentrations, stratified by BMI at age 20 above (“High”) or below (“Low”) the 90th percentilea
| Arsenic (μg/L) | Cases | Cont | Unadjusted
|
Adjustedb
|
|||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | ||||
| Low BMI | |||||||
| Lung cancer | <100 | 79 | 254 | 1.00 | Ref | 1.00 | Ref |
| 100–800 | 64 | 166 | 1.24 | 0.84–1.82 | 1.17 | 0.79–1.73 | |
| >800 | 122 | 159 | 2.47 | 1.75–3.49 | 2.31 | 1.63–3.29 | |
| p-trend | <0.001 | <0.001 | |||||
| Bladder cancer | <100 | 35 | 254 | 1.00 | Ref | 1.00 | Ref |
| 100–800 | 62 | 166 | 2.71 | 1.71–4.29 | 2.69 | 1.70–4.27 | |
| >800 | 110 | 159 | 5.02 | 3.27–7.71 | 4.97 | 3.22–7.67 | |
| p-trend | <0.001 | <0.001 | |||||
| Combined cancers | <100 | 114 | 254 | 1.00 | Ref | 1.00 | Ref |
| 100–800 | 126 | 166 | 1.69 | 1.23–2.33 | 1.64 | 1.19–2.27 | |
| >800 | 232 | 159 | 3.25 | 2.41–4.39 | 3.12 | 2.30–4.22 | |
| p-trend | <0.001 | <0.001 | |||||
| High BMI | |||||||
| Lung cancer | <100 | 14 | 34 | 1.00 | Ref | 1.00 | Ref |
| 100–800 | 10 | 16 | 1.52 | 0.56–4.15 | 1.48 | 0.53–4.14 | |
| >800 | 12 | 5 | 5.83 | 1.73–19.64 | 6.98 | 1.84–26.56 | |
| p-trend | 0.003 | 0.004 | |||||
| Bladder cancer | <100 | 5 | 34 | 1.00 | Ref | 1.00 | Ref |
| 100–800 | 7 | 16 | 2.98 | 0.82–10.83 | 2.80 | 0.74–10.6 | |
| >800 | 12 | 5 | 16.32 | 4.01–66.41 | 23.15 | 4.28–124.50 | |
| p-trend | <0.001 | <0.001 | |||||
| Combined cancers | <100 | 19 | 34 | 1.00 | Ref | 1.00 | Ref |
| 100–800 | 17 | 16 | 1.90 | 0.79–4.60 | 1.84 | 0.75–4.52 | |
| >800 | 24 | 5 | 8.59 | 2.82–26.20 | 9.37 | 2.88–30.53 | |
| p-trend | <0.001 | <0.001 | |||||
Abbreviations: BMI, body mass index; CI, confidence interval; cont, controls; OR, odds ratio; ref, reference group
The 90th percentiles are 27.7 kg/m2 for men and 28.6 kg/m2 for women.
Adjusted for age, sex, and smoking
Table 3.
Cancer odds ratios and synergy indices comparing subjects with BMIs at age 20 above (“High”) and percentile and arsenic water concentrations above 800 or below 100 μg/L
| Arsenic (μg/L) | BMI | Controls
|
Lung cancer
|
Bladder cancer
|
||||
|---|---|---|---|---|---|---|---|---|
| N | N | ORa | 95% CI | N | ORa | 95% CI | ||
| <100 | Low | 254 | 79 | 1.00 | Ref | 35 | 1.00 | Ref |
| <100 | High | 34 | 14 | 1.40 | 0.70–2.79 | 5 | 1.12 | 0.41–3.08 |
| >800 | Low | 159 | 122 | 2.31 | 1.62–3.28 | 110 | 4.97 | 3.22–7.68 |
| >800 | High | 5 | 12 | 7.97 | 2.65–24.02 | 12 | 17.63 | 5.78–53.73 |
| Synergy Index | 4.08 | 1.01–16.46 | 4.06 | 1.23–13.41 | ||||
Abbreviations: BMI, body mass index; CI, confidence interval; OR, odds ratio; ref, reference group
Odds ratios are adjusted for age, sex, and smoking
Table 4.
Odds ratios for lung and bladder cancer combined comparing subjects with BMIs at age 20 above (“High”) and below (“Low”) the 90th percentile and arsenic water concentrations above 800 or below 100 μg/L, in analyses stratified by the year of birth
| Birth year | Arsenic (μg/L) | BMI | Cases | Controls | Unadjusted
|
Adjusteda
|
||
|---|---|---|---|---|---|---|---|---|
| ORa | 95% CI | OR | 95% CI | |||||
| 1938 or later | <100 | Low | 72 | 175 | 1.00 | Ref | 1.00 | Ref |
| <100 | High | 11 | 12 | 2.23 | 0.94–5.28 | 2.16 | 0.90–5.14 | |
| >800 | Low | 166 | 96 | 4.20 | 2.90–6.10 | 4.08 | 2.80–5.95 | |
| >800 | High | 13 | 4 | 7.90 | 2.49–25.04 | 7.99 | 2.45–26.02 | |
| Synergy Index | 1.65 | 0.41–6.67 | ||||||
| Before 1938 | <100 | Low | 42 | 79 | 1.00 | Ref | 1.00 | Ref |
| <100 | High | 8 | 22 | 0.68 | 0.28–1.67 | 0.72 | 0.29–1.79 | |
| >800 | Low | 66 | 63 | 1.97 | 1.18–3.28 | 1.88 | 1.11–3.17 | |
| >800 | High | 11 | 1 | 20.69 | 2.58–165.79 | 23.92 | 2.92–196.32 | |
| Synergy Index | 38.64 | 2.06–723.37 | ||||||
Abbreviations: BMI, body mass index; CI, confidence interval; OR, odds ratio; ref, reference group
Odds ratios are adjusted for age, sex, and smoking
Figure 2.
Plots of lung and bladder cancer combined odds ratios based on logistic regression equations by arsenic water concentrations in subjects with BMIs ≥90th percentile at various life stagesa
Abbreviations: b, regression coefficient for the increase in log odds ratio of cancer for each 100 μg/L increase in arsenic water concentration
aOdds ratios are adjusted for age, sex, and smoking
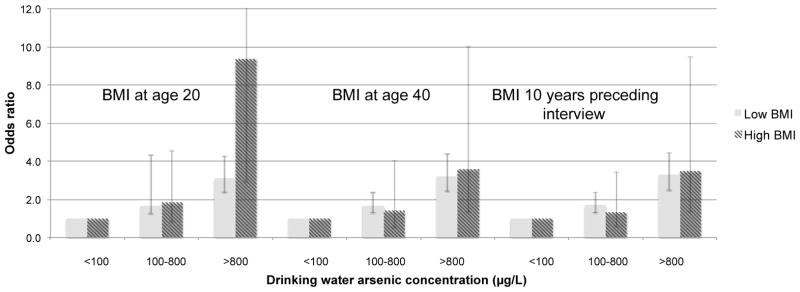
Clear evidence for synergy was not seen for elevated BMIs after age 20 (Figure 1 and Tables S3–S4) or for a gain in BMI from age 20 to later life (Table S5). For bladder cancer, ORs were higher in those with a higher BMI at age 40 and in the 10 years preceding interview (e.g. ORs of 5.24 (3.38–8.12) and 10.91 (2.63–45.1) for arsenic exposure >800 μg/L for a low and high BMI, respectively, preceding interview) (Table S4). However, the bladder cancer synergy indices (S) were not statistically significant (S=1.71 (0.72–4.09) for BMI at age 40 and S=1.48 (0.50–4.34) for BMI in the ten years preceding interview).
Figure 1.
Odds ratios of lung and bladder cancer combined for various categories of highest known arsenic drinking water concentrations stratified by BMIs above (“High”) and below (“Low”) the 90th percentile at age 20, at age 40, and in the 10 years preceding interviewa
aThose with a highest known arsenic water concentrations <100 μg/L are used as the reference category. ORs are adjusted for age, sex, and smoking.
DISCUSSION
Our findings of higher arsenic-related cancer ORs in subjects with higher BMIs, with an over 4-fold increase in the Rothman Synergy Index, provide evidence that arsenic and excess BMI interact in a greater than additive manner to increase arsenic-related cancer. The low p-values and confidence intervals with lower bounds >1.0 are evidence that these findings are unlikely due to chance. The fairly consistent findings for both bladder and lung cancer, across different measures of arsenic exposure, even after adjusting for multiple factors and using various BMI cutoff points, provides evidence that these findings represent true associations. A recent study from Taiwan reported evidence of synergy between obesity and alcohol consumption for hepatocellular cancer, with hazard ratios up to 5.16 (95%CI, 2.34–11.39), supporting the plausibility that excess weight can lead to synergistic cancer effects (Loomba et al. 2013). Although our findings are preliminary, we identified ORs >10 in some analyses. We are not aware of any previous study where excess BMI has been associated with cancer risks of this magnitude or where elevated BMI has been linked to synergistic cancer impacts with an environmental chemical exposure as widely present as arsenic.
The biologic mechanism by which excess BMI may increase arsenic-associated cancer risks is unknown but several possibilities exist. Although excess BMI has not been clearly linked to lung or bladder cancer (Henley et al. 2002), it has been linked to esophagus, thyroid, colon, rectum, kidney, endometrium, and other cancer types (Renehan et al. 2008). In fact, it has been estimated that 15–20% of all cancer deaths in US non-smokers are related to excess weight (Calle et al. 2003). Proposed mechanisms of obesity-related cancer include insulin resistance, oxidative stress, altered secretion of adipokines, increase in circulating estrogen-like compounds and chronic inflammation (van Kruijsdijk et al. 2009). Elevated BMI is associated with a low level chronic inflammatory state and multiple studies have shown increases in inflammatory markers, including TNF-alpha, interleukin-6 (IL-6), and C-reactive protein in people with higher BMIs (van Kruijsdijk et al. 2009). Inflammation has been proposed as an underlying mechanism for cancer, cardiovascular disease, diabetes, hypertension and several of the other diseases that have been linked to both elevated BMI and to arsenic (Manabe 2011). Increases in inflammatory markers, including IL-6, TNF-alpha, and others have been seen in people highly exposed to arsenic, as well as to arsenic-related malignant transformation, tumor promotion, and increased cancer risks (Karim et al. 2013; Liu et al. 2014; Qi et al. 2014; van Kruijsdijk et al. 2009; Xu et al. 2013). For example, laboratory studies have shown that malignant transformation of human bronchial epithelial cells by arsenic is much greater in the presence of IL-6 (Qi et al. 2014; Xu et al. 2013). The fact that both arsenic and elevated BMI have these common inflammatory effects suggests that the synergistic effects seen in our study might be due to some common inflammatory response. Insulin resistance has also been linked to some cancers, and both arsenic and elevated BMI have been associated with insulin resistance (Wang et al. 2014), but we did not see strong evidence of an interaction between diabetes and arsenic (data not shown) in our study. Inter-individual differences in arsenic metabolism have been linked to increased cancer risks, but associations between BMI and urinary arsenic metabolite proportions were not seen here. Although inflammation seems plausible, the true mechanism remains unknown and further research is required to better understand the underlying biology of the synergistic effects identified in this study.
WHO defines obesity based on a BMI ≥30 kg/m2; given our limited sample size, our analyses of BMIs using this cutoff point were limited. However, as shown in Figure S2, we found evidence of higher arsenic-associated cancer risks in the upper BMI category using a variety of BMI cutoff points, including a BMI of 30 kg/m2. In addition, above a certain level (about 25 kg/m2), the impacts of BMI on health appear to gradually increase as BMI increases, without an obvious threshold at 30 kg/m2 (Berrington de Gonzalez et al. 2010). As such, the specific cutoff point of 30 is somewhat arbitrary and our lack of control subjects with BMIs above 30 kg/m2 had little impact on our conclusion that excess BMI and arsenic may have synergistic effects.
Interestingly, we found the highest arsenic-related cancer ORs in those who had an elevated BMI in early adulthood (age 20) and whose highest arsenic exposures began near or after this age (i.e., those born well before the 1958–70 high exposure period) (Table 4). This suggests that the greatest impact of this synergy is with either concurrent exposures or when arsenic exposure occurs after BMI has increased beyond normal. However, we must accept that our data are limited and do not allow us to establish which of these was more likely. Earlier studies by ourselves and others provide evidence that in utero or early-life exposure to arsenic, followed by another carcinogen exposure (such as tobacco) later in life can lead to high cancer risks (Ferreccio et al. 2013; Waalkes et al. 2004). However, a number of animal studies have shown that arsenic can increase carcinogenicity when given concurrently or after exposure to another carcinogenic agent (Tokar et al. 2010), findings that agree with what we report here.
Although we found high cancer risks in all subjects who had elevated BMIs in early adulthood, by far the highest risks were seen in those whose BMIs exceeded the norm early and remained high into later life (Figure 2). In contrast, arsenic-cancer risks were markedly lower in those who had BMIs ≥90th percentile in early adulthood but not later. Most high arsenic exposures in Chile ceased by the 1970s, 30–35 years before the cancers in our study were diagnosed. As such, these findings may be an indication that people can reduce their arsenic-related cancer risks by lowering their BMIs, even many years after arsenic exposure ceased. If true, these findings could have important implications in parts of the USA, Chile, and elsewhere where high exposures have ended but elevated cancer risks will likely remain for years to come (Marshall et al. 2007; Steinmaus et al. 2013).
The BMIs used in this study were based on self-reports and so some misclassification is likely. When asking people about their past weight, we asked them to relate this to important milestones such as marriage, child birth, or changes in occupation. Research shows that men tend to overestimate and women tend to underestimate their past weights (Perry et al. 1995). Importantly though, despite these individual-level errors, self-reports seem to be fairly accurate for classifying subjects relative to one another. For example, in a study of 6,101 subjects in the National Health and Nutrition Examination Survey, measured weights from 10–20 years prior were recalled incorrectly by an average of 3.9 lbs (Kovalchik 2009). However, the correlation coefficient between measured and recalled weights was 0.96. This high correlation suggests that despite widespread under- or overestimation, recalled weight can be used to fairly accurately place subjects into low and high categories, like those used in our study. Despite this, some misclassification likely remained. However, since past weight was collected from all subjects using the same methods, most of this is probably non-differential and most likely biased our results towards the null. Misclassification of BMI or other factors like diet may be even greater for proxy subjects, although the synergistic effects we identified remained when these subjects were excluded.
Misclassification of arsenic exposure could have resulted from missing exposure data, inaccurate residential history, or arsenic from non-water sources. Because exposure was assessed similarly in cases and controls, most of this was likely non-differential and biased ORs towards the null. And, because exposure was determined mostly by the cities in which the subjects lived, and errors in recalling residential history are likely minimal, this bias was probably small. Arsenic may come from food, air, or work, although adjustments for occupational exposure had little impact. And, because the area is so dry, most food comes from outside the region and is not affected by local water contamination. An analysis of arsenic levels in air and food in this area showed that levels were relatively low (0.025–0.129 μg/m3 in air and about 13 μg/day from food) and were far outweighed by exposures from water (Ferreccio and Sancha 2006).
It’s possible that some confounding factor associated with elevated BMI is causing the synergistic associations seen here. Given the large magnitude of the synergy indices, that factor would have to be quite prevalent and very strongly associated with excess BMI as well as both lung and bladder cancer to cause the associations seen here (Axelson 1978). There is no obvious factor meeting these criteria in this population, including diet, occupation, and smoking, none of which were strongly associated with BMI in this study. We did not see similar synergistic relationships when we evaluated diabetes, and adjustments for diabetes, occupational carcinogen exposures, fruit and vegetable intake, direct smoking, and second hand smoke exposure had only small impacts on our results. Confounding by some dietary factor not strongly associated with fruit and vegetable intake is possible, but given the large synergy seen, no obvious factors are known. Overall, while confounding by some unknown factor cannot be ruled out, confounding seems an unlikely cause of the associations we report here.
CONCLUSIONS
These findings are the first evidence that excess BMI may be associated with large increases in the cancer risks related to a common environmental chemical exposure. Given the worsening epidemic of obesity and elevated BMI in many countries, and the very widespread nature of arsenic exposure, these findings could have important public health implications. Importantly though, our results are quite novel and sample sizes were small in some analyses. As such, these findings are preliminary and need confirmation. Future research with more detailed data on the relative timing of arsenic exposure and increases in BMI and on possible mechanisms would also provide useful information for interpreting the large synergistic associations we report here.
Supplementary Material
highlights.
A synergistic relationship was seen between elevated BMI and cancer.
Adjustments for smoking, diet, occupation and other factors had little impact.
Associations were greatest in subjects with excess BMI throughout adulthood.
Acknowledgments
This research was supported by US National Institute of Environmental Health Sciences grants 5R01ES014032 and P42ES04705
Footnotes
Disclaimer: The views in this paper do not necessarily reflect those of the Office of Environmental Health Hazard Assessment or the California Environmental Protection Agency.
Competing financial interests: Dr. Steinmaus has done consulting work on arsenic toxicity for both industry and environmental groups. The other authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed S, Mahabbat-e Khoda S, Rekha RS, Gardner RM, Ameer SS, Moore S, et al. Arsenic-associated oxidative stress, inflammation, and immune disruption in human placenta and cord blood. Environ Health Perspect. 2011;119:258–264. doi: 10.1289/ehp.1002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelson O. Aspects on confounding in occupational health epidemiology. Scand J Work Environ Health. 1978;4:85–89. doi: 10.5271/sjweh.2720. [DOI] [PubMed] [Google Scholar]
- Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, et al. Body-mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363:2211–2219. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of u.S. Adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- De Pergola G, Silvestris F. Obesity as a major risk factor for cancer. J Obes. 2013;2013:291546. doi: 10.1155/2013/291546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreccio C, Gonzalez C, Milosavjlevic V, Marshall G, Sancha AM, Smith AH. Lung cancer and arsenic concentrations in drinking water in chile. Epidemiology. 2000;11:673–679. doi: 10.1097/00001648-200011000-00010. [DOI] [PubMed] [Google Scholar]
- Ferreccio C, Sancha AM. Arsenic exposure and its impact on health in chile. J Health Popul Nutr. 2006;24:164–175. [PubMed] [Google Scholar]
- Ferreccio C, Yuan Y, Calle J, Benitez H, Parra RL, Acevedo J, et al. Arsenic, tobacco smoke, and occupation: Associations of multiple agents with lung and bladder cancer. Epidemiology. 2013;24:898–905. doi: 10.1097/EDE.0b013e31829e3e03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha Mazumder DN, Steinmaus C, Bhattacharya P, von Ehrenstein OS, Ghosh N, Gotway M, et al. Bronchiectasis in persons with skin lesions resulting from arsenic in drinking water. Epidemiology. 2005;16:760–765. doi: 10.1097/01.ede.0000181637.10978.e6. [DOI] [PubMed] [Google Scholar]
- Haldane JB. The estimation and significance of the logarithm of a ratio of frequencies. Ann Hum Genet. 1956;20:309–311. doi: 10.1111/j.1469-1809.1955.tb01285.x. [DOI] [PubMed] [Google Scholar]
- Henley SJ, Flanders WD, Manatunga A, Thun MJ. Leanness and lung cancer risk: Fact or artifact? Epidemiology. 2002;13:268–276. doi: 10.1097/00001648-200205000-00006. [DOI] [PubMed] [Google Scholar]
- IARC. A review of human carcinogens: Arsenic, metals, fibres, and dusts. 100c. Lyon: 2012. [Google Scholar]
- Karim MR, Rahman M, Islam K, Mamun AA, Hossain S, Hossain E, et al. Increases in oxidized low-density lipoprotein and other inflammatory and adhesion molecules with a concomitant decrease in high-density lipoprotein in the individuals exposed to arsenic in bangladesh. Toxicol Sci. 2013;135:17–25. doi: 10.1093/toxsci/kft130. [DOI] [PubMed] [Google Scholar]
- Kovalchik S. Validity of adult lifetime self-reported body weight. Public Health Nutr. 2009;12:1072–1077. doi: 10.1017/S1368980008003728. [DOI] [PubMed] [Google Scholar]
- Liu S, Sun Q, Wang F, Zhang L, Song Y, Xi S, et al. Arsenic induced over-expression of inflammatory cytokines based on human urothelial cell model in vitro and urinary secretion of individuals chronically exposed to arsenic. Chem Res Toxicol. 2014 doi: 10.1021/tx5002783. In press. [DOI] [PubMed] [Google Scholar]
- Loomba R, Yang HI, Su J, Brenner D, Barrett-Connor E, Iloeje U, et al. Synergism between obesity and alcohol in increasing the risk of hepatocellular carcinoma: A prospective cohort study. Am J Epidemiol. 2013;177:333–342. doi: 10.1093/aje/kws252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manabe I. Chronic inflammation links cardiovascular, metabolic and renal diseases. Circ J. 2011;75:2739–2748. doi: 10.1253/circj.cj-11-1184. [DOI] [PubMed] [Google Scholar]
- Marseglia L, Manti S, D’Angelo G, Nicotera A, Parisi E, Di Rosa G, et al. Oxidative stress in obesity: A critical component in human diseases. Int J Mol Sci. 2015;16:378–400. doi: 10.3390/ijms16010378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall G, Ferreccio C, Yuan Y, Bates MN, Steinmaus C, Selvin S, et al. Fifty-year study of lung and bladder cancer mortality in chile related to arsenic in drinking water. J Natl Cancer Inst. 2007;99:920–928. doi: 10.1093/jnci/djm004. [DOI] [PubMed] [Google Scholar]
- Melak D, Ferreccio C, Kalman D, Parra R, Acevedo J, Perez L, et al. Arsenic methylation and lung and bladder cancer in a case-control study in northern chile. Toxicol Appl Pharmacol. 2014;274:225–231. doi: 10.1016/j.taap.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. [accessed June 2, 2014];Critical aspects of epa’s iris assessment of inorganic arsenic: Interim report. 2014 Available: www.nap.edu/catalog.php?record_id=18594.
- NRC. Arsenic in drinking water 2001 update. Washington, DC: National Research Council; 2001. Subcommittee to Update the 1999 Arsenic in Drinking Water Report. [Google Scholar]
- Perry GS, Byers TE, Mokdad AH, Serdula MK, Williamson DF. The validity of self-reports of past body weights by u.S. Adults. Epidemiology. 1995;6:61–66. doi: 10.1097/00001648-199501000-00012. [DOI] [PubMed] [Google Scholar]
- Qi Y, Zhang M, Li H, Frank JA, Dai L, Liu H, et al. Autophagy inhibition by sustained overproduction of il6 contributes to arsenic carcinogenesis. Cancer Res. 2014;74:3740–3752. doi: 10.1158/0008-5472.CAN-13-3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey KA, Bosco A, McKenna KL, Carter KW, Elliot JG, Berry LJ, et al. In utero exposure to arsenic alters lung development and genes related to immune and mucociliary function in mice. Environ Health Perspect. 2013;121:244–250. doi: 10.1289/ehp.1205590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- Rothman KJ. The estimation of synergy or antagonism. Am J Epidemiol. 1976;103:506–511. doi: 10.1093/oxfordjournals.aje.a112252. [DOI] [PubMed] [Google Scholar]
- Smith A, Lopipero P, Bates M, Steinmaus C. Arsenic epidemiology and drinking water standards. Science. 2002;296:2145–2146. doi: 10.1126/science.1072896. [DOI] [PubMed] [Google Scholar]
- Smith AH, Marshall G, Yuan Y, Liaw J, Ferreccio C, Steinmaus C. Evidence from chile that arsenic in drinking water may increase mortality from pulmonary tuberculosis. Am J Epidemiol. 2011;173:414–420. doi: 10.1093/aje/kwq383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AH, Marshall G, Liaw J, Yuan Y, Ferreccio C, Steinmaus C. Mortality in young adults following in utero and childhood exposure to arsenic in drinking water. Environ Health Perspect. 2012;120:1527–1531. doi: 10.1289/ehp.1104867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmaus CM, Ferreccio C, Acevedo Romo J, Yuan Y, Cortes S, Marshall G, et al. Drinking water arsenic in northern chile: High cancer risks 40 years after exposure cessation. Cancer Epidemiol Biomarkers Prev. 2013;22:623–630. doi: 10.1158/1055-9965.EPI-12-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokar EJ, Benbrahim-Tallaa L, Ward JM, Lunn R, Sams RL, 2nd, Waalkes MP. Cancer in experimental animals exposed to arsenic and arsenic compounds. Crit Rev Toxicol. 2010;40:912–927. doi: 10.3109/10408444.2010.506641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kruijsdijk RC, van der Wall E, Visseren FL. Obesity and cancer: The role of dysfunctional adipose tissue. Cancer Epidemiol Biomarkers Prev. 2009;18:2569–2578. doi: 10.1158/1055-9965.EPI-09-0372. [DOI] [PubMed] [Google Scholar]
- Waalkes MP, Ward JM, Diwan BA. Induction of tumors of the liver, lung, ovary and adrenal in adult mice after brief maternal gestational exposure to inorganic arsenic: Promotional effects of postnatal phorbol ester exposure on hepatic and pulmonary, but not dermal cancers. Carcinogenesis. 2004;25:133–141. doi: 10.1093/carcin/bgg181. [DOI] [PubMed] [Google Scholar]
- Wang W, Xie Z, Lin Y, Zhang D. Association of inorganic arsenic exposure with type 2 diabetes mellitus: A meta-analysis. J Epidemiol Community Health. 2014;68:176–184. doi: 10.1136/jech-2013-203114. [DOI] [PubMed] [Google Scholar]
- Xu Y, Zhao Y, Xu W, Luo F, Wang B, Li Y, et al. Involvement of hif-2alpha-mediated inflammation in arsenite-induced transformation of human bronchial epithelial cells. Toxicol Appl Pharmacol. 2013;272:542–550. doi: 10.1016/j.taap.2013.06.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.