Abstract
Introduction
Congenital cardiac valve disease is common, affecting ~1% of the population, with substantial morbidity and mortality, but suboptimal treatment options. Characterization of the specific matrix and valve cell phenotypic abnormalities in these valves could lend insight into disease pathogenesis and potentially pave the way for novel therapies.
Methods
Thirty-five human aortic and pulmonic valves were categorized based on gross and microscopic assessment into control valves (n=21); dysplastic valves, all except one also displaying hemodynamic changes (HEMO/DYSP, n=6); and hemodynamically altered valves (HEMO, n=8). Immunohistochemistry was performed on valve sections and flow cytometry on valvular interstitial cells.
Results
While both hemodynamically altered aortic and pulmonic valves demonstrated increased collagen turnover and cell activation, prolyl 4-hydroxylase and hyaluronan increased in hemodynamically altered aortic valves but decreased in hemodynamically altered pulmonic valves relative to control valves (P<.001). HEMO/DYSP aortic valves demonstrated decreased collagen and elastic fiber synthesis and turnover compared to both hemodynamically altered aortic valves and control aortic valves (each P<.006). Valvular interstitial cells from both hemodynamically altered and HEMO/DYSP pulmonic valves showed altered cell phenotype compared to control valves (each P<.032), especially increased non-muscle myosin. Furthermore, valvular interstitial cells from hemodynamically altered pulmonic valves and HEMO/DYSP aortic and pulmonic valves each demonstrated greater size and complexity compared to control valves (each P<.05).
Conclusions
Dysplastic semilunar valves displayed alterations in collagen and elastic fiber turnover that were distinct from valves similarly exposed to altered hemodynamics as well as to control valves. These results demonstrate that dysplastic valves are not simply valves with gross changes or loss of leaflet layers, but contain complex matrix and cell phenotype changes that, with future study, could potentially be targets for novel nonsurgical treatments.
Keywords: Extracellular matrix, Semilunar valve, Valvular interstitial cell, Dysplastic, Hemodynamics, Congenital valve
1. Introduction
Congenital cardiac valve disease imposes a heavy burden on our society, with a prevalence of approximately 1% [1] and high associated morbidity and mortality. In 2000 alone 213,000 life years were lost before age 65 because of congenital heart disease, nearly equal to the sum of leukemia, prostate cancer, and Alzheimer's disease [2]. The majority of congenital heart disease cases involve the valves and/or septa [3]. Treatment options for patients with congenitally diseased valves are suboptimal at best. While mechanical valves and bioprosthetic valves perform relatively well in adults, they are wrought with complications in children. Bioprosthetic valves quickly calcify and mechanical valves require anti-coagulation, which is not compatible with a child's active lifestyle. Even if these issues could be solved, neither of these valve replacements grows with the child; therefore, these children must undergo multiple open-heart surgeries to replace valve replacements that the children have outgrown. In light of the heavy burden of congenital valve disease, there is a compelling need to better understand the pathology of this disease, as a foundation for the development of improved treatment options.
Prior research on congenital valve disease has largely focused on gross structural and epidemiological aspects. Pathological studies have examined the incidence of congenital valve disease, its association with other anatomic cardiac anomalies, and its gross anatomical features. While these valves are known to be histologically abnormal, the specific matrix changes that occur within these congenitally diseased valves remain largely unknown. The goal of this study was to characterize the matrix composition, matrix turnover, and valve cell phenotype in congenitally diseased semilunar cardiac valves.
2. Material and methods
2.1. Sample set
The research use of this tissue was approved by the institutional review boards at each institution. Patients were enrolled in the study after informed consent was obtained. Inclusion criteria included any patient undergoing valve replacement or cardiac transplantation; exclusion criteria were valve homografts and repeat transplantation. Of the 36 patients at Texas Children's Hospital who were approached for involvement in the study, 35 gave consent and 11 did not have tissue excised (three had valve repairs; no tissue was obtained from the other eight), leaving a total of 24 patients. From these patients, 32 surgically excised semilunar valves were collected [17 aortic valves (AVs; seven were bicuspid) and 15 pulmonic valves (PVs; one bicuspid)]. Three autopsy semilunar valves were obtained from Texas Children's Hospital (two PVs) and Ben Taub General Hospital, Houston, TX (one AV).
Valves were grouped into three categories based on gross structural, histologic architecture, and clinical history. Valves with thin, delicate cusps, three clearly delineated, properly oriented histologic layers, and absence of significant stenosis or regurgitation were designated controls (CTRL, n=21, 12 AVs, nine PVs, ages 2 months to 22 years). Hemodynamically altered valves with an accompanying clinical history of hemodynamic changes (HEMO, n=8, three AVs, five PVs, ages 9–21 years) appeared thickened and fibrotic with three identifiable but variably thickened layers. Valves that were variably thickened and deformed and lacked delineated layers with increased myxoid matrix were defined as dysplastic. All except the youngest of these samples also displayed hemodynamic changes and were designated HEMO/DYSP (n=6, three AVs, three PVs, ages 2–35 years). Samples were subdivided into valves from patients aged ≤2 years (n=13) and aged ≥9 years (n=21), with one outlying HEMO/DYSP AV from a 5-year-old patient. Additionally, a sub-group analysis was performed on valvular interstitial cells (VICs) from pulmonary autografts in the aortic position for >3 years as part of the Ross procedure [4] (n=2; for all other analysis Ross valves were categorized as HEMO PVs). Table 1 summarizes the main clinical and demographic features of the patients in the sample set.
Table 1.
Sample set
| Patient no. |
Age | Sex | Valve | Valve function |
Classification | Pertinent clinical valve history |
|---|---|---|---|---|---|---|
| 1 | 2 years | M | PV | R/S | HEMO/DYSP | TOF |
| 2 | 12 years | M | PV | R | HEMO | Ross |
| 3 | 14 years | F | AV | CTRL | ||
| 3 | 14 years | F | PV | CTRL | ||
| 4 | 9 years | F | PV | R | HEMO | Ross |
| 5 | 18 years | M | AV | R/S | HEMO/DYSP | Balloon valvuloplasty |
| 6 | 5 months | F | AV | CTRL | ||
| 6 | 5 months | F | PV | CTRL | ||
| 7 | 12 years | F | PV | R | HEMO/DYSP | Valvotomy |
| 8 | 3 months | F | AV | CTRL | ||
| 9 | 11 years | F | AV | CTRL | ||
| 10 | 35 years | F | PV | R | HEMO/DYSP | Remote commissurotomy |
| 11 | 14 years | M | AV | CTRL | ||
| 11 | 14 years | M | PV | CTRL | ||
| 12 | 21 years | M | PV | R/S | HEMO | Valvotomy, TOF |
| 13 | 5 years | M | AV | R/S | HEMO/DYSP | Valvotomy |
| 14 | 14 years | M | AV | CTRL | ||
| 14 | 14 years | M | PV | R | HEMO | |
| 15 | 11 years | F | AV | S | HEMO | |
| 15 | 11 years | F | PV | CTRL | ||
| 16 | 2 months | F | AV | CTRL | ||
| 17 | 7 months | F | AV | CTRL | ||
| 17 | 7 months | F | PV | CTRL | ||
| 18 | 19 years | F | PV | R | HEMO | TOF |
| 19 | 2 years | F | AV | CTRL | ||
| 20 | 12 years | M | AV | R/S | HEMO/DYSP | Balloon valvuloplasty |
| 21 | 9 months | M | AV | CTRL |
M=Male, F=female; AV=aortic valve, PV=pulmonic valve; S=stenotic, R=regurgitant, R/S=regurgitant and stenotic; TOF=tetralogy of Fallot.
2.2. Histology and immunohistochemistry
Samples were fixed in 10% formalin, paraffin embedded, and sectioned according to standard procedures. Each sample was stained with hematoxylin and eosin, Movat pentachrome, and immunohistochemically (IHC) with diaminobenzidine visualization for extracellular matrix components and valve cell phenotype (Table 2), as previously described [5] and [6]. Average IHC staining intensity across the leaflet thickness of the mid-leaflet region of each section was quantified using ImageJ software (NIH, Bethesda, MD, USA), which quantifies pixel intensity on a scale of 0–255. Additionally, IHC intensity was separately quantified within superficial plaques and the underlying leaflet. Plaques were identified based on demarcation between the normal valve and superimposed fibrotic tissue on hematoxylin and eosin-stained sections.
Table 2.
Collection of antibodies used in immunohistochemistry and flow cytometry
| Protein | IHC, FC Dilution | Function |
|---|---|---|
| Collagen turnover proteins | ||
| Collagen III (Col III)a | 1:100, – | Reticular collagen |
| Matrix metalloproteinase-13 (MMP13)b |
1:200, – | Collagen degradation |
| Prolyl 4-hydroxylase (P4H)b | 1:200, 1:100 | Marker of collagen synthesis |
| Heat shock protein-47 (HSP47)a |
1:1000, 1:100 | Marker of collagen synthesis |
| Elastic fiber-related proteins | ||
| Lysyl oxidase (LOX)c | 1:250, – | Involved in collagen and elastin crosslinking |
| Fibrillina | 1:400, – | Component of elastic fibers |
| Matrix metalloproteinase-9 (MMP9)d |
1:100, – | Elastic fiber degradation |
| Proteoglycans (PG), glycosaminoglycans (GAG), and glycoproteins | ||
| Hyaluronan (HA)e | 1:250, – | GAG providing compressibility |
| Hyaluronan receptor for endocytosish |
–, 1:100 | Protein involved in HA turnover |
| CD44a | –, 1:100 | Cellular receptor for HA |
| Fibronectinb | –, 1:100 | Extracellular glycoprotein |
| Decorinf | 1:500, – | PG involved in collagen fibrillogenesis |
| Biglycanf | 1:500, – | PG involved in collagen fibrillogenesis |
| Versicang | 1:500, – | PG involved elastic fibrillogenesis and compressibility |
| Valve cell activation/cell phenotype | ||
| VimentinI | –, 1:200 | Intermediate filament of mesenchymal cells |
| Non-muscle myosinj | 1:250, 1:100 | Marker of an “activated” myofibroblast-like phenotype |
| Smooth muscle alpha-actinI | 1:1000, 1:100 | Marker of an “activated” myofibroblast-like phenotype |
FC=Flow cytometry, IHC=immunohistochemistry; “–” indicates marker was not analyzed using either IHC or FC.
Abcam (Cambridge, MA, USA).
Chemicon (Temecula, CA, USA).
Imgenex (San Diego, CA, USA).
Assay Designs (Ann Arbor, MI, USA).
Detected using hyaluronan binding protein (Associates of Cape Cod, Falmouth, MA, USA).
Gift of Dr. Larry Fisher (NIH, Bethesda, MD, USA).
Associates of Cape Cod.
Gift of Dr. Paul Weigel (University of Oklahoma, Oklahoma City, OK, USA).
DakoCytomation (Denmark).
Covance (Berkeley, CA, USA) used for IHC; GeneTex (Irvine, CA, USA) used in FC.
2.3. Flow cytometry
Valvular interstitial cells were isolated from samples procured within 24 h of surgery and cultured as previously described [7]. VICs from 26 surgical samples were analyzed using flow cytometry (13 AVs, 13 PVs). For each cell line, an unstained sample and a negative control (mouse or rabbit IgG) were run alongside specific antibody samples (Table 2). Cells were fixed and stained as described previously [8], but modified with respect to fixation time (>1 h), permeabilization time (10 min), antibody incubation-step times (1 h), and final resuspension volume (250 μl). Samples were run on a FACScan flow cytometer and data analyzed with Cellquest Pro software (both BD, Franklin Lakes, NJ, USA). Each sample was run three times and the median fluorescence recorded. With the use of the isotype control sample, the cell population was identified and gated based on size (forward scatter, FSC) and complexity (side scatter, SSC). This population was then further gated based on fluorescence such that less than 1% fluorescence fell within the gate.
2.4. Statistical analysis
Multifactorial analysis of variance (ANOVA) was performed using SigmaStat (SPSS, Chicago, IL, USA). Non-normally distributed data was rank transformed before the ANOVA. For comparisons between IHC intensity within plaques and the underlying leaflet, paired t tests were performed. In all cases, the level of significance was set at .05. Pearson tests (for normally distributed data) and Spearman rank order tests (for non-normally distributed data) were used to calculate correlations between staining intensities. Significance was set at P≤.0042 for correlations in IHC (12 proteins compared) and P≤.0071 for flow cytometry (seven proteins compared).
3. Results
3.1. Age-related changes in matrix composition of semilunar valves
Analysis of CTRL AVs revealed decreased hyaluronan and proteoglycans versican and biglycan, but not decorin, with age (Fig. 1, P=.002). Markers of collagen turnover (MMP13, P4H, HSP47), Col 3, and smooth muscle alpha-actin (SMaA) also decreased in CTRL AVs with age (Fig. 2, P<.001). CTRL PVs revealed similar age-related changes, with decreased markers of collagen turnover (MMP13, P4H, HSP47) and markers of VIC activation [non-muscle myosin (NMM) and SMaA] (P=.02, data not shown). Expression of proteoglycans and hyaluronan in CTRL PVs of different ages showed similar tendencies as AVs, with decreased versican, biglycan, and hyaluronan, and no change in decorin, but the age-related changes in versican and hyaluronan were smaller than in AVs. No significant differences in mean intensities were detected between CTRL AVs and PVs.
Figure 1.
Expression of proteoglycans and HA in different aged CTRL AVs as determined by histochemical staining for each marker. The data for each group was calculated by averaging the mean intensities for each section. Overall P=.002. Error bars indicate the standard error of the mean.
Figure 2.
Expression of markers of collagen turnover and activated VICs in different aged CTRL AVs. The mean IHC intensity for each group is plotted; error bars indicate the standard error of the mean. Overall P<.001, *P<.05 between age groups for a given marker.
3.2. Matrix changes in pathological semilunar valves
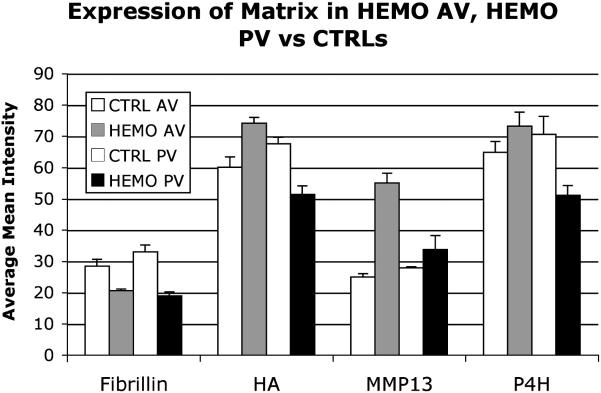
HEMO AVs and PVs aged ≥9 years not only demonstrated altered matrix compared to CTRLs (Fig. 3, HEMO AV vs. CTRL, HEMO PV vs. CTRL each P<.001), but changes were distinct between AV and PV (P<.001). While both HEMO AVs and PVs demonstrated increased MMP13 and NMM, and decreased fibrillin, P4H and hyaluronan increased in HEMO AVs and decreased in HEMO PVs relative to CTRLs. HEMO/DYSP AVs aged ≥9 years demonstrated distinct compositions compared to both HEMO AVs and CTRL AVs (each P<.006; subset of markers shown in Fig. 4), including decreased collagen and elastic fiber synthesis and turnover. The Ross valves within this HEMO PV group displayed changes consistent with HEMO PVs and distinct from HEMO AVs.
Figure 3.
Expression of matrix components in HEMO AVs and PVs from patients aged ≥9 years compared to CTRL AVs and PVs from patients aged ≥9 years. The mean histochemical staining intensity for each marker for each group is plotted; error bars indicate the standard error of the mean. Overall P<.001 for HEMO vs. CTRL for each of AV and PV; for both HEMO and CTRL, AV vs. PVP<.001. HEMO vs. CTRL P<.05 for P4H and HA by Holm–Sidak post hoc testing.
Figure 4.
Expression of matrix components in HEMO/DYSP AVs and HEMO AVs from patients aged ≥9 years compared to CTRL AVs from patients aged ≥9 years. The mean IHC intensity for each marker for each group is plotted; error bars indicate the standard error of the mean. Overall P<.001, HEMO, HEMO/DYSP, and CTRL each significantly different from one another (each P<.006).
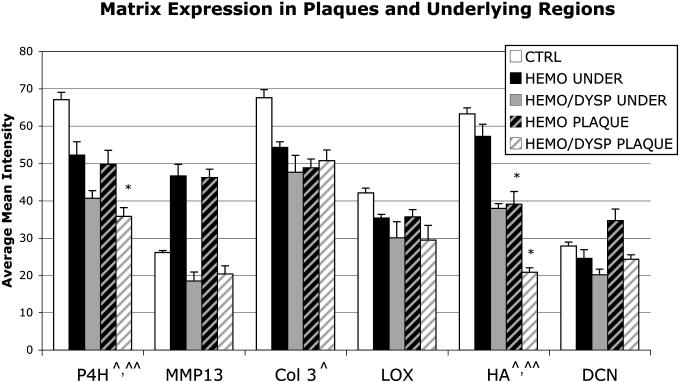
Plaques and underlying regions demonstrated altered matrix compositions relative to CTRL valves (Fig. 5, each P<.001), with alterations distinct for HEMO and HEMO/DYSP (Fig. 5, each P<.013). Both HEMO and HEMO/DYSP plaques and underlying regions displayed decreased P4H, LOX, Col 3, and hyaluronan in plaques and underlying regions, but MMP13 was increased in HEMO plaques and underlying regions, while MMP13 was decreased in HEMO/DYSP plaques. P4H was also greater in HEMO plaques relative to HEMO/DYSP and the decrease in hyaluronan was greater in HEMO/DYSP plaques and underlying regions compared to HEMO. Relative to their respective underlying leaflet regions, HEMO and HEMO/DYSP plaques showed decreased hyaluronan (Fig. 6, P<.001) and MMP9 (P<.05), but increased decorin (P<.002). HEMO plaques also demonstrated decreased HSP47 (P=.013) and decreased NMM (P<.01) relative to underlying leaflet regions.
Figure 5.
Expression of matrix in HEMO and HEMO/DYSP plaques and the regions underlying plaques (UNDER) relative to CTRL valves. The mean histochemical staining intensity for each marker for each group is plotted; error bars indicate the standard error of the mean. Overall plaque vs. CTRL P<.001, underlying regions vs. CTRL P<.001. Pathology (HEMO, HEMO/DYSP, CTRL) was significant for both plaques (P<.001) and underlying regions (P=.001), and HEMO was significantly different than HEMO/DYSP by post hoc testing for both plaques (P<.013) and underlying regions (P<.012). *P<.05 HEMO or HEMO/DYSP vs. CTRL for a given matrix component. ^P<.05 CTRL vs. plaque and ^^P<.05 CTRL vs. underlying plaques for a given matrix component.
Figure 6.
Plaque in a HEMO/DYSP bicuspid AV from an 18-year-old patient demonstrating decreased HA expression (as visualized with histochemical staining) in the plaque relative to the adjacent, underlying leaflet. Scale bar indicates 1 mm. In histochemistry with diaminobenzidine visualization, brown indicates positive staining (i.e., a location in which that protein is expressed). “*” highlights areas of particularly intense staining in the underlying leaflet; “^” highlights an area of relatively light staining within the overlying plaque. In Movat pentachrome staining, elastic fibers appear black, collagen appears yellow, proteoglycans and glycosaminoglycans appear blue/green, and myocardium appears violet. A negative stained control section has been included for comparison and is representative for the other sections and markers (in the negative stained control the same IHC protocol is performed but no primary antibody is applied).
3.3. Remodeling of leaflet layers in pathological semilunar valves
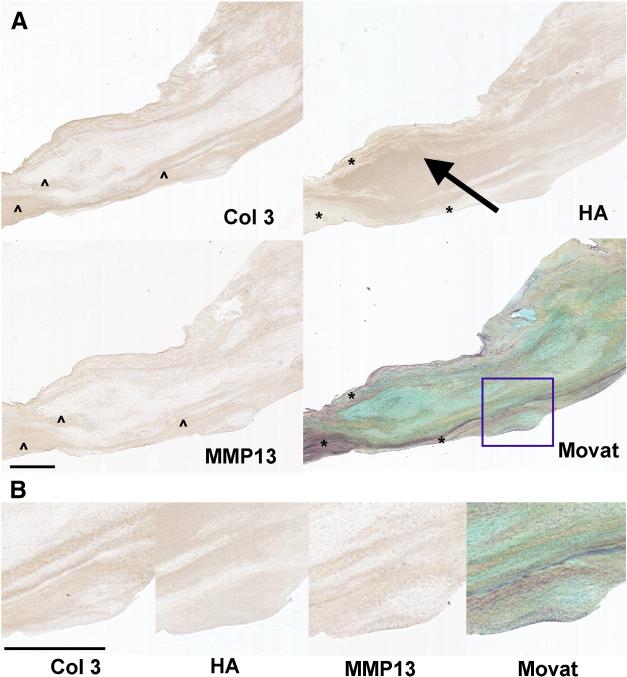
While CTRL valves demonstrated a clearly delineated trilaminar leaflet structure with the Movat stain, accompanied by layer-predominant expression of different matrix components (Fig. 7), the architectural disruption in HEMO/DYSP valves was accompanied by altered localization of matrix components (Fig. 8). For instance, in CTRL valves hyaluronan was largely expressed in the spongiosa layer, with some expression in the elastic fiber-rich ventricularis, but in HEMO/DYSP hyaluronan was abnormally distributed throughout the valve, expanding the central region in some samples and not co-localizing with elastic fibers (Fig. 8 and Fig. 9). In Fig. 8, for example, there was evident co-localization of Col 3 and MMP13, suggestive of collagen remodeling, in precise regions of the leaflet that appeared marbled. Profound abnormalities in marker expression within the central region of the valve are demonstrated in the HEMO/DYSP PV shown in Fig. 9, with high levels of NMM, P4H, LOX, and fibrillin. Acquired hemodynamic remodeling in HEMO/DYSP valves was evidenced by pockets of collagen within the leaflet and accentuated collagen and elastic fibers towards the surface, focally forming plaques (Fig. 8, Fig. 10 and Fig. 11). In several valves with plaques, pockets of strong SMaA expression were noted both within and at edge of the plaque, which co-localized with high levels of MMP13 and biglycan (Fig. 11).
Figure 7.
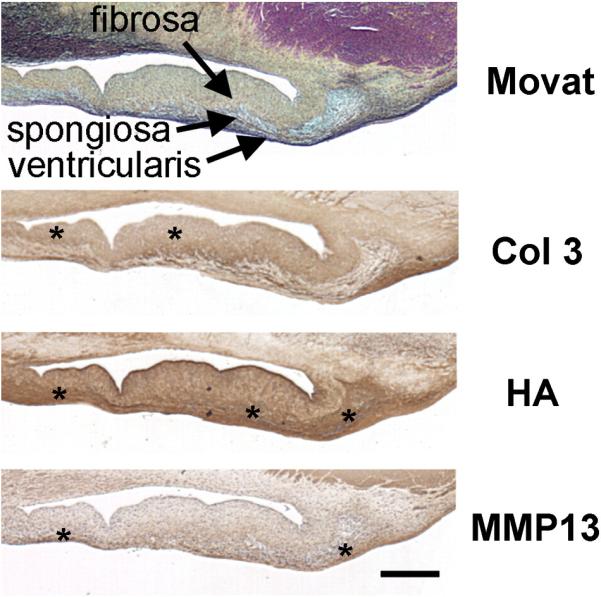
CTRL AV from a 2-year-old patient demonstrating clearly delineated leaflet layers and the layer-specific expression of matrix components characteristic of both AVs and PVs in the CTRL groups. The top panel demonstrates a Movat pentachrome-stained sample (see legend of Fig. 6 for explanation of colors in a Movat stain); the remaining panels demonstrate histochemical staining for different matrix components (Col 3, HA, MMP13). Scale bar indicates 500 μm and applies to all panels of the figure. “*” highlights regions of particularly dark staining in this section, including the edge of the fibrosa and the annular ventricularis for Col 3 and the spongiosa for HA. MMP13 staining in this section appears relatively diffuse with greater staining in the ventricularis.
Figure 8.
(A) HEMO/DYSP bicuspid AV from a 12-year-old patient demonstrating a lack of clearly delineated leaflet layers evident in the Movat-stained section (see legend of Fig. 6 for explanation of colors in a Movat stain), and marbling of Col 3, HA, and MMP13 as demonstrated via histochemical staining for those matrix components. Arrow points to expansion of HA composition in the interior of the leaflet. “*” highlights regions of elastic fibers (evident in the Movat) in which hyaluronan staining is relatively light. “^” highlights areas of Col 3 staining that co-localize with MMP13 staining suggestive of collagen remodeling. Scale bar indicates 1 mm and applies to all panels of the figure. (B) Higher magnification images of the stained sections in Part (A), allowing better visualization of the marbling of matrix components. Portion magnified is indicated by the purple box in the Movat in Part (A) of the figure. Scale bar indicates 1 mm and applies to all panels of the figure.
Figure 9.
(A) HEMO/DYSP PV from a 12-year-old patient demonstrating (via histochemical staining) strong expression of NMM, P4H, LOX, and fibrillin in the spongiosa (arrow). Note also the expansion of HA staining across the thickness of the leaflet. Scale bar indicates 500 μm and applies to all panels of the figure. (B). Images demonstrating the region within the purple box in Part (A) of this figure at higher magnification. Scale bar indicates 500 μm and applies to all panels of the figure.
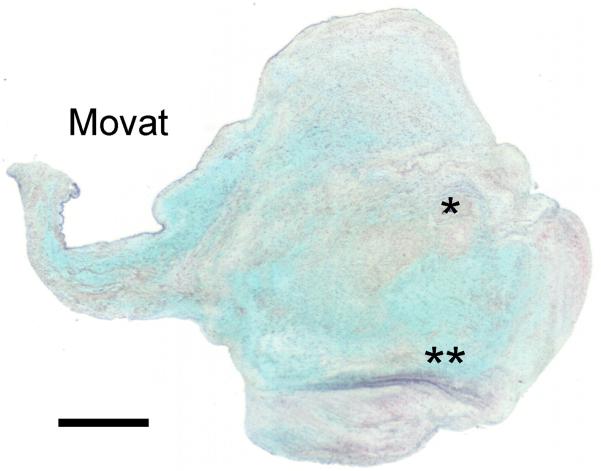
Figure 10.
Movat pentachrome-stained section of a HEMO/DYSP bicuspid AV from a 5-year-old patient demonstrating a nodular region with pockets of collagen (*) and elastic fiber remodeling (**) (see legend of Fig. 6 for explanation of colors in a Movat stain). Scale bar indicates 1 mm.
Figure 11.
IHC-stained sections of a HEMO/DYSP bicuspid AV from an 18-year-old patient illustrating pockets of strong SMaA staining co-localizing with MMP13 and biglycan expression (*). Brackets indicate location of plaques. See legend of Fig. 6 for explanation of colors in a Movat stain. Scale bar indicates 1 mm and applies to all panels of the figure.
3.4. Correlations between matrix components in pathological semilunar valves
While HSP47 moderately correlated with P4H in CTRL AVs and PVs (r=0.64, P<.002) as expected considering their common roles in collagen synthesis, in diseased valves HSP47 showed strong correlations with versican in regions underlying plaques (r=0.91, P<.001). Furthermore, HEMO and HEMO/DYSP valves demonstrated distinct correlations related to collagen synthesis and turnover. While HSP47 correlated with decorin and versican in HEMO valves (r=0.82, P=.002 and r=0.80, P=.003, respectively), MMP13 correlated with decorin and versican in HEMO/DYSP valves (r=0.91, P<.001 andr=0.82, P<.002, respectively) and HSP47 correlated with MMP9 and fibrillin (r=0.76, P=.003 and r=0.76,P=.002, respectively).
3.5. Differences between AV and PV VICs
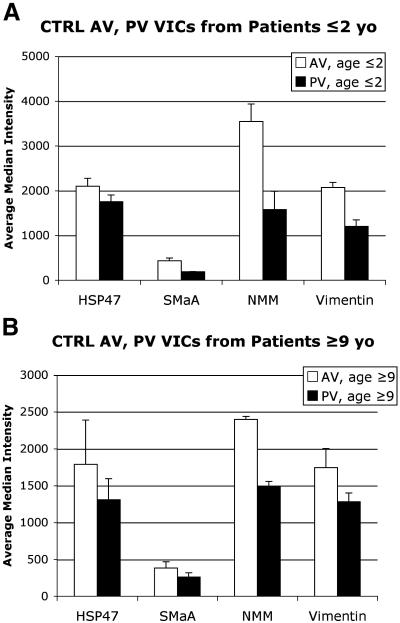
Flow cytometry analysis of VICs from CTRL AVs and PVs revealed greater cell activation and collagen synthesis in AV VICs than in PV VICs (P=.02, Fig. 12).
Figure 12.
Expression of cell phenotype markers in VICs from CTRL AV and PV from patients aged ≤2 years (A) and aged ≥9 years (B) as measured by flow cytometry. The average median intensity for each marker for each group is plotted; error bars indicate the standard error of the mean. P=.02 for overall AV vs. PV comparison (including both age groups).
3.6. Changes in VICs from pathological semilunar valves
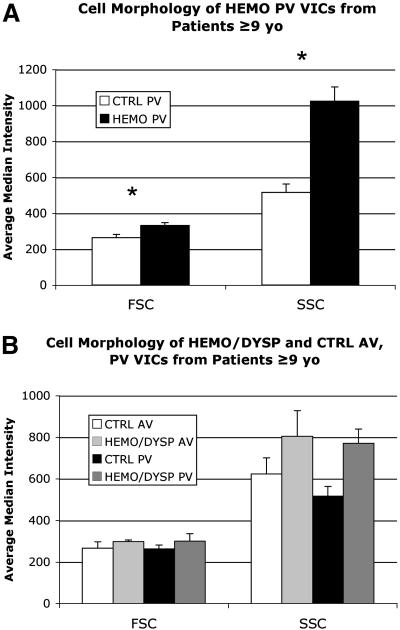
Compared to VICs from CTRL PVs aged ≥9 years, VICs from both HEMO PVs and HEMO/DYSP PVs aged ≥9 years showed significantly different expression in cell phenotype markers (P=.022 and P=.031, respectively), especially increased NMM. Furthermore, VICs from HEMO PVs demonstrated slightly greater size (FSC, P<.001) and dramatically greater complexity (SSC, P<.001) than CTRL PV (Fig. 13A). VICs from HEMO/DYSP AV and PV aged ≥9 years similarly displayed greater size (FSC, P=.041) and complexity (SSC, P<.001) compared to VICs from CTRL AV and PV (Fig. 13B). Analysis of the subset of VICs from Ross valves (PV placed in the AV for at least 3 years) aged ≥9 years compared to VICs from CTRL AVs and PVs aged ≥9 years showed greater NMM and vimentin (P=.016, Fig. 14A) and significantly greater size (P=.013) and complexity (P<.001, Fig. 14B).
Figure 13.
(A) Cell morphology of VICs from HEMO PV of patients aged ≥9 years compared to VICs from CTRL PV of patients aged ≥9 years as measured by flow cytometry. *P<.001 HEMO vs. CTRL (for each variable). FSC=Forward scatter, which indicates cell size. SSC=Side scatter, which indicates cell complexity. (B) Cell morphology of VICs from HEMO/DYSP AV and PV of patients aged ≥9 years compared to VICs from CTRL AV and PV of patients aged ≥9 years as measured by flow cytometry. Comparison of HEMO/DYSP vs. CTRL gave P=.041 for FSC and P<.001 for SSC. For both parts of the graph, the average median intensity for each marker for each group is plotted; error bars indicate the standard error of the mean.
Figure 14.
(A) Expression of cell phenotype markers in VICs from Ross valves (PV placed in the aortic position for >3 years), CTRL AV, and CTRL PV from patients aged ≥9 years as measured by flow cytometry. Overall P=.016. (B) Cell morphology of VICs from Ross valves, CTRL AV, and CTRL PV from patients aged ≥9 years as measured by flow cytometry. For FSC: Ross, AV, PV P=.013, for SSC: Ross, AV, PV P<.001. *P<.05 Ross vs. PV for a given marker by Dunn's post hoc testing. For both parts of the graph the average median intensity for each marker for each group is plotted; error bars indicate the standard error of the mean.
4. Discussion
4.1. Previous studies of matrix composition in congenitally diseased valves
General structure and histology of congenitally diseased valves have occasionally been reported; however, analyses of specific matrix components are rare. In 1979, Baig [9] reported more dermatan sulfate in the 21- to 40-year-old BAV and less hyaluronan and more chondroitin sulfate in the 41- to 60-year-old BAV compared to age-matched controls. A more recent study of adult bicuspid AVs demonstrated increased MMP2 and MMP9 [10]. However, given the age of these patients in these studies, it is difficult to discern how much of these changes was a result of the years of altered hemodynamics [9]. It is also likely that these valves were not significantly dysplastic, unlike bicuspid AVs that present early in life. In terms of basic histologic analyses, Hinton et al. [11] reported disorganized collagen and elastin, proteoglycans in all layers, decreased elastin, and increased collagen in bicuspid AVs from six young patients (ranging from 1 to 184 months). Given the age of these patients, these valves may have been less affected by hemodynamic changes and potentially more dysplastic than those studied by Baig [9]. In 1973, Bharati and Lev [12] analyzed valves in congenital polyvalvular disease (CPVD, a specific congenital valve disease in which all four valves are affected) using basic histology. CPVD valves demonstrated disrupted architecture with expansion of the spongiosa layer that intermingled with a diminished fibrosa, and a lack of elastic fibers, as compared to age-matched normal valves and normal valves exposed to altered hemodynamics[12]. Others have described similar histologic alterations in isolated valvar dysplasia and in CPVD [13],[14] and [15]. These findings sharply contrast with those described in hemodynamically altered valves that have increased elastic fibers and variably increased fibrous tissue with distinct, albeit altered, trilaminar architecture [14]. Rarely have alterations in specific proteins been studied in congenitally diseased valves and none reported for dysplastic valves. Furthermore, none of the above studies assessed alterations in VIC cell phenotype.
4.2. Altered matrix composition and VIC cell phenotype in pathological valves
In the present study, we found that HEMO and HEMO/DYSP valves demonstrated significant and distinct alterations in matrix composition compared to CTRL valves. In HEMO valves, increased VIC activation and collagen turnover (MMP13) were seen in both AVs and PVs, which is consistent with ex vivo studies in which porcine AVs were exposed to increased mechanical stress [16] and [17]. Fibrillin was also decreased in both HEMO AVs and PVs, which may correlate with alterations in elastic fibers, as reported in hemodynamically altered valves [12]. However, hyaluronan and P4H decreased in HEMO PVs and increased in HEMO AVs, suggesting that the AV and PV may respond to altered hemodynamics differently. Ross valves were consistent with HEMO PVs and distinct from HEMO AVs, suggesting that Ross valves maintain PV mechanobiological responses to altered hemodynamics despite placement in the aortic position.
HEMO/DYSP AVs demonstrated distinct compositions relative to HEMO AVs, including decreased collagen and elastic fiber synthesis and turnover, as compared to increased collagen synthesis and turnover in HEMO AVs. Leaflet layer remodeling in HEMO/DYSP included altered localization of matrix components, particularly in the region normally comprising the spongiosa layer, as well as pockets of collagen and elastic fiber remodeling, consistent with previous reports of dysplastic valves [12],[14] and [15]. These findings are also consistent with collagen and elastic fiber disarray previously reported in bicuspid (possibly dysplastic) AVs from young patients [11] and could suggest impaired ability of the valve to remodel in response to stress with potential functional consequences. Distinct patterns of correlations between matrix components related to collagen turnover, elastic fiber turnover, and proteoglycans in HEMO valves relative to HEMO/DYSP valves suggest distinct matrix interactions within these different valves which may contribute to the distinct pathological processes, although much remains to be learned in that regard.
The plaques and regions underlying plaques in HEMO and HEMO/DYSP valves demonstrated distinct matrix compositions relative to CTRL valves, with decreased markers of collagen synthesis and hyaluronan. Particularly notable were the differences between plaques on HEMO as compared to HEMO/DYSP valves, including increased MMP13 in HEMO but decreased MMP13 in HEMO/DYSP plaques relative to CTRLs, and a more substantial decrease in P4H and hyaluronan in HEMO/DYSP than in HEMO plaques relative to CTRLs. Pockets of strong SMaA expression and increased expression of matrix components were also noted within and bordering plaques, suggesting the involvement of VICs with a myofibroblast phenotype in plaque formation. Further work understanding the processes occurring in the leaflet regions underlying plaques and how they may relate to plaque formation is warranted. Overall, the documented abnormalities in elastic and collagen fiber turnover in these congenitally diseased valves suggest potential novel therapeutic targets for the treatment of these valves that should be investigated in future studies, particularly since these matrix defects could alter the leaflet material behavior and thus worsen valve function. If proper matrix composition and turnover could be encouraged pharmacologically, valve function may improve, which could prevent or delay the need for valve replacement. Clearly, however, considerably more work remains in this area.
Not only did the congenitally diseased valves in this study display altered matrix composition, but the VICs contained in these valves displayed alterations in cell phenotype relative to CTRL valves. VICs from HEMO valves particularly demonstrated increased NMM, a marker of VIC activation, consistent with the IHC results. Both VICs from HEMO/DYSP valves and HEMO valves displayed altered cell morphology, including increased size and complexity, relative to CTRL VICs. Analysis of a subset of VICs from Ross valves also demonstrated increased VIC activation, consistent with the higher mechanical stress the PV must experience in the AV position and consistent with previous analyses of tissues from Ross valves [18]. As evident in VICs from HEMO and HEMO/DYSP valves, VICs from Ross valves also demonstrated increased cell size and complexity compared to CTRLs, suggesting these changes in cell morphology may be common to multiple forms of congenital valve disease. The differences in VICs from pathological valves relative to CTRL valves suggest that inherent abnormalities in the VICs could contribute to the documented matrix abnormalities and potentially plaque formation. As such, considerable caution should be exercised before utilizing autologous VICs in a tissue-engineered heart valve for a patient with a congenitally diseased valve.
4.3. Age-related changes and differences between AV and PV among CTRL samples
Analysis of CTRL AVs and PVs demonstrated decreased markers of collagen synthesis and turnover, VIC activation, proteoglycans (particularly in the AV), and hyaluronan with age. Decreased VIC activation and MMP13 are consistent with a previous study reporting decreases in these markers from fetal to ~6-year-old and then to ~50-year-old semilunar valves [19]. Previous work examining age-related matrix changes in porcine valves and their relationship to changes in material properties [20] among other studies suggests that the changes in composition demonstrated in the present study could have important consequences for valve function.
Greater VIC activation and collagen synthesis in VICs from CTRL AVs compared to those from PVs are consistent with previous reports of cell activation and collagen synthesis in VICs from ovine AVs and PVs[21] and vimentin in VICs from human adult semilunar valves [22]. These apparent inherent differences in cell phenotype between the valves could relate to differences in composition between the valves [23].
4.4. Limitations and future studies
While this study provides important, fundamental knowledge by characterizing the specific matrix composition and cell phenotypes within these diseased valves for the first time, a number of study limitations should be noted. First, the limited number of samples for each group made it difficult to obtain statistical significance for some group comparisons. Future collection of additional samples, including more age-matched autopsy samples, will allow narrower age groupings and more statistical power for group comparisons, especially for the Ross valve subgroup analysis. The surgically excised CTRL valves were exposed to abnormal hemodynamic milieu; while utilizing this group for comparisons does not answer the question of how these valves compare to normal valves, it is advantageous in isolating the variable of pathology. The complex conditions of patients with congenitally diseased valves further complicate these analyses. While this study demonstrated significant changes, it is unclear whether these changes are adaptive or maladaptive. Additionally, this study was largely descriptive. Future in vitro studies elucidating cause and effect, identifying signaling pathways involved in the observed matrix changes, and linking remodeling to functional hemodynamic changes will greatly enhance our understanding of the disease and could shed light on potential novel treatment strategies. Nevertheless, the results contained in this study lay the groundwork for future studies.
5. Conclusions
In sum, the results contained in this study provide a detailed characterization of the matrix changes of congenitally diseased semilunar valves. As such, the study adds to our understanding that dysplastic valves are not simply valves with gross changes or a loss of layered leaflet structure microscopically, but these valves contain complex changes in matrix turnover, composition, and valve cell phenotype.
Acknowledgments
The authors appreciate the assistance of the members of the Grande-Allen laboratory, especially Mark Mendenhall, as well as Texas Children's Hospital staff member Karol Arrington. The authors also appreciate the counsel of Dr. Scott Baggett regarding statistics.
References
- [1].LeBlanc JG, Russell JL. Pediatric cardiac surgery in the 1990's. Surg Clin North Am. 1998;78:729–747. doi: 10.1016/s0039-6109(05)70347-9. [DOI] [PubMed] [Google Scholar]
- [2].National Center for Health Statistics: National Heart, Lung, Blood Institute; 2005. [Google Scholar]
- [3].Hoffman J. Congenital heart disease and inheritance. Pediatr Clin North Am. 1990;37:25–43. doi: 10.1016/s0031-3955(16)36830-4. [DOI] [PubMed] [Google Scholar]
- [4].Ross DN. Replacement of aortic and mitral valves with a pulmonary autograft. Lancet. 1967;2:956–958. doi: 10.1016/s0140-6736(67)90794-5. [DOI] [PubMed] [Google Scholar]
- [5].Gupta V, Barzilla JE, Mendez JS, Stephens EH, Lee EL, Collard CD, et al. Abundance and location of proteoglycans and hyaluronan within normal and myxomatous mitral valves. Cardiovasc Pathol. 2009;18:191–197. doi: 10.1016/j.carpath.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Stephens EH, Grande-Allen KJ. Age-related changes in collagen synthesis and turnover in porcine heart valves. J Heart Valve Dis. 2007;16:672–682. [PubMed] [Google Scholar]
- [7].Stephens EH, Carroll JL, Grande-Allen KJ. The use of collagenase III for the isolation of porcine aortic valvular interstitial cells: rationale and optimization. J Heart Valve Dis. 2007;16:175–183. [PubMed] [Google Scholar]
- [8].Blevins TL, Carroll JL, Raza AM, Grande-Allen KJ. Phenotypic characterization of isolated valvular interstitial cell subpopulations. J Heart Valve Dis. 2006;15:815–822. [PubMed] [Google Scholar]
- [9].Baig M. Acid mucopolysaccharides of congenitally defective, rheumatic, and normal human aortic valves. Am J Pathol. 1979;96:771–780. [PMC free article] [PubMed] [Google Scholar]
- [10].Koullias GJ, Korkolis DP, Ravichandran P, Psyrri A, Hatzaras I, Elefteriades JA. Tissue microarray detection of matrix metalloproteinases, in diseased tricuspid and bicuspid aortic valves with or without pathology of the ascending aorta. Eur J Cardiothorac Surg. 2004;26:1098–1103. doi: 10.1016/j.ejcts.2004.07.050. [DOI] [PubMed] [Google Scholar]
- [11].Hinton RB, Jr, Lincoln J, Deutsch GH, Osinska H, Manning PB, Benson DW, et al. Extracellular matrix remodeling and organization in developing and diseased aortic valves. Circ Res. 2006;98:1431–1438. doi: 10.1161/01.RES.0000224114.65109.4e. [DOI] [PubMed] [Google Scholar]
- [12].Bharati S, Lev M. Congenital polyvalvular disease. Circulation. 1973;47:575–586. doi: 10.1161/01.cir.47.3.575. [DOI] [PubMed] [Google Scholar]
- [13].Bartram U, Bartelings M, Kramer H, Gittenberger-de Groot A. Congenital polyvalvular disease: a review. Pediatr Cardiol. 2001;22:93–101. doi: 10.1007/s002460010169. [DOI] [PubMed] [Google Scholar]
- [14].Hyams V, Manion W. Incomplete differentiation of the cardiac valves. Am Heart J. 1968;76:173–182. doi: 10.1016/0002-8703(68)90192-0. [DOI] [PubMed] [Google Scholar]
- [15].Koretzky ED, Moller JH, Korns ME, Schwartz CJ, Edwards JE. Congenital pulmonary stenosis resulting from dysplasia of valve. Circulation. 1969;40:43–53. doi: 10.1161/01.cir.40.1.43. [DOI] [PubMed] [Google Scholar]
- [16].Balachandran K, Konduri S, Sucosky P, Jo H, Yoganathan AP. An ex vivo study of the biological properties of porcine aortic valves in response to circumferential cyclic stretch. Ann Biomed Eng. 2006;34:1655–1665. doi: 10.1007/s10439-006-9167-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Balachandran K, Sucosky P, Jo H, Yoganathan AP. Elevated cyclic stretch alters matrix remodeling in aortic valve cusps: implications for degenerative aortic valve disease. Am J Physiol Heart Circ Physiol. 2009;296:H756–H764. doi: 10.1152/ajpheart.00900.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Rabkin-Aikawa E, Aikawa M, Farber M, Kratz JR, Garcia-Cardena G, Kouchoukos NT, et al. Clinical pulmonary autograft valves: pathologic evidence of adaptive remodeling in the aortic site. J Thorac Cardiovasc Surg. 2004;128:552–561. doi: 10.1016/j.jtcvs.2004.04.016. [DOI] [PubMed] [Google Scholar]
- [19].Aikawa E, Whittaker P, Farber M, Mendelson K, Padera RF, Aikawa M, et al. Human semilunar cardiac valve remodeling by activated cells from fetus to adult: implications for postnatal adaptation, pathology, and tissue engineering. Circulation. 2006;113:1344–1352. doi: 10.1161/CIRCULATIONAHA.105.591768. [DOI] [PubMed] [Google Scholar]
- [20].Stephens EH, de Jonge N, McNeill MP, Durst CA, Grande-Allen KJ. Age-related changes in material behavior of porcine mitral and aortic valves and correlation to matrix composition. Tissue Eng Part A. 2010;16:867–878. doi: 10.1089/ten.tea.2009.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Merryman WD, Liao J, Parekh A, Candiello JE, Lin H, Sacks MS. Differences in tissue-remodeling potential of aortic and pulmonary heart valve interstitial cells. Tissue Eng. 2007;13:2281–2289. doi: 10.1089/ten.2006.0324. [DOI] [PubMed] [Google Scholar]
- [22].Della Rocca F, Sartore S, Guidolin D, Bertiplaglia B, Gerosa G, Casarotto D, et al. Cell composition of the human pulmonary valve: a comparative study with the aortic valve—the VESALIO Project. Vitalitate Exornatum Succedaneum Aorticum labore Ingegnoso Obtinebitur. Ann Thorac Surg. 2000;70:1594–1600. doi: 10.1016/s0003-4975(00)01979-2. [DOI] [PubMed] [Google Scholar]
- [23].Aldous IG, Veres SP, Jahangir A, Lee JM. Differences in collagen cross-linking between the four valves of the bovine heart: a possible role in adaptation to mechanical fatigue. Am J Physiol Heart Circ Physiol. 2009;296:H1898–H1906. doi: 10.1152/ajpheart.01173.2008. [DOI] [PubMed] [Google Scholar]