Abstract
PURPOSE
Plaque brachytherapy is a common form of treatment for uveal melanoma, and the Collaborative Ocular Melanoma Study (COMS) employed I-125. Recently, Ru-106 has been reintroduced for plaque brachytherapy in the United States. We reviewed our experience treating uveal melanoma with Ru-106 plaque brachytherapy using COMS planning techniques, hypothesizing we would observe similar outcomes to those in the COMS.
METHODS AND MATERIALS
Medical records of patients undergoing Ru-106 plaque brachytherapy were reviewed retrospectively. Patient, tumor, and treatment characteristics were recorded. Outcomes including visual acuity, local tumor recurrence, salvage treatment, metastasis and survival were recorded. Cox regression analyses were used to determine factors associated with local tumor recurrence and enucleation.
RESULTS
Twenty-eight patients were studied. Median age was 60 and 50% were men. Median tumor base diameter and height were 9.4 and 2.6 mm. Ophthalmic complications were rare. Local tumor recurrence and enucleation occurred in 13 and 4 patients, respectively. Local tumor recurrence was associated with low visual acuity in the tumor-bearing eye, posterior tumors, small plaque size, and difference in plaque-tumor diameter <6 mm. Enucleation was associated with low visual acuity and posteriorly located tumor. Estimated 5-year rate of death and metastasis was 18.5% and 11.4%.
CONCLUSIONS
Among patients treated with Ru-106 plaque brachytherapy using COMS planning techniques, we found a greater than expected rate of local tumor recurrence. Planning Ru-106 plaque brachytherapy should be done carefully at centers that have previously used COMS protocols and I-125.
Keywords: Ru-106, I-125, eye plaque, plaque brachytherapy, uveal melanoma, cancer, choroid, radiation
Introduction
Plaque brachytherapy is widely accepted as an effective treatment for uveal melanoma. Prospective trials have demonstrated that plaque brachytherapy is able to control growth of the primary tumor while conserving the eye and preserving useful vision (1, 2). The Collaborative Ocular Melanoma Study (COMS), a prospective randomized trial, found no difference in the survival of patients treated with plaque brachytherapy or enucleation of the eye (1). For this reason, plaque brachytherapy is an acceptable option for treatment of localized uveal melanoma. When treatment is recommended, most patients in the United States choose some form of radiation therapy as primary therapy of their uveal melanoma.
Although Co-60 was the original isotope used worldwide for more than 30 years, most prospective studies of plaque brachytherapy for uveal melanoma have used I-125 (or much less often Pd-103), despite the availability of many other radioisotopes for treatment (1–3).
Ru-106 has and continues to be used extensively in Europe. Theoretical advantages of Ru-106 plaques include a thin profile that facilitates placement, as well as the limited depth of penetration by the emitted beta-particles. The latter factor has lead most to consider Ru-106 appropriate for melanomas less than 5 mm high. Ru-106 has been unavailable in the United States for the last 5 years, but has recently been re-released for use.
During the brief period of time that Ru-106 was available for ophthalmic brachytherapy in the United States, we used this isotope for treatment of select patients with uveal melanomas less than 5 mm high. We used brachytherapy planning protocols similar to what is used for I-125. Given the re-release of this Ru-106 in the United States, we sought to review our experience and treatment outcomes. We hypothesized that the outcome of treatment would be similar to reports from other centers, and similar to the outcomes from the COMS trials. As a result of our findings we undertook an in-depth analysis of factors associated with outcome.
Methods
Patients
This retrospective clinical research study was carried out with permission from the institutional review board (WA0380-12) of Memorial Sloan Kettering Cancer Center. Patients with uveal melanoma ≤5 mm high treated with Ru-106 brachytherapy at our institution were identified through review of the brachytherapy treatment-planning databases.
Patient and tumor characteristics analyzed included age at diagnosis, visual acuity, intraocular pressure, tumor size (largest base diameter and height), shape (dome, collar button, placoid), uveal location (ciliary body and/or choroid), anterior border (ciliary body, ora serrata to equator, posterior to equator), posterior border (ora serrata to equator or posterior to equator), distance to avascular fovea and optic nerve, and whether retinal detachment was present. Tumors were staged according to the COMS and American Joint Committee on Cancer (AJCC) criteria.
Treatment parameters were recorded, including BEBIG (Eckert & Ziegler Bebig s.a., Brussels, Belgium) Ru-106 ophthalmic applicator model (CCA, CIA, CIB, CCX), prescribed radiation dose (75–85 Gy to the apex of the tumor without a minimum or maximum dose to the sclera, based on our experience with I-125) and delivered radiation dose (to tumor apex and sclera surface), duration of brachytherapy, and use of adjuvant transpupillary thermotherapy (TTT, at the time of plaque removal, and/or 3–6 months after removal).
The outcome of treatment was recorded and included time from the end of brachytherapy to visual acuity <20/200, local tumor recurrence, salvage local therapy (TTT, photodynamic therapy, proton radiotherapy, and/or enucleation), melanoma metastasis, and death from melanoma or other cause. Local tumor recurrence events were independently reviewed and agreed upon by a radiation oncologist and an ophthalmic oncologist and were based on the COMS criteria (15% increase in height or 250 micrometer increase in tumor boundary on two consecutive occasions). Tumor region of the recurrence (horizontal/tumor margin, vertical/diffuse) was noted. Reason for enucleation and pathologic findings were noted. Visual acuity and intraocular pressure at last follow-up were recorded.
Statistical analysis
Summary statistics are presented. Kaplan-Meier methods were used to estimate rates of local recurrence, enucleation, metastasis, and death. Univariable Cox regression analyses of factors associated with local recurrence and enucleation are presented. Multivariable analyses were not undertaken because of the small sample size. All analyses were performed using WinSTAT for Excel® (version 2009.1; Microsoft Corporation, Redmond, WA) and figures were created with GraphPad Prism® software, version 6.02 (GraphPad Software, Inc., La Jolla, CA).
Results
Twenty-eight patients underwent Ru-106 plaque brachytherapy between 2000 and 2008 as their first treatment for uveal melanoma. There were 14 men and 14 women. Median age was 60 years (range, 28–82). Median tumor largest base diameter and height were 9.4 and 2.6 mm, respectively (range, 5.8–12.0 and 1.5–3.9, respectively). Median prescription and delivered radiation dose at tumor apex was 75.0 and 75.5 Gy, respectively (range, 75–85 and 66.8–89.3, respectively). Median radiation dose to the sclera surface was 238.7 Gy (range, 181.7–372.5). Median follow-up was 71 months (range, 10–95). Twelve patients harbored tumors meeting criteria for COMS small size, while 16 met criteria for medium size. T-staging by AJCC was T1a in 20, T1b in 4, and T2a in 4; no patients had clinical evidence of nodal or metastatic disease at diagnosis. Demographic, ophthalmic, tumor, and treatment characteristics are presented in Table 1.
Table 1.
Demographic, ophthalmic, tumor and treatment characteristics with proportions of patients experiencing local tumor recurrence and enucleation among the cohort studied
| Local tumor recurrence |
Enucleation | |||||||
|---|---|---|---|---|---|---|---|---|
| N | Yes | No | Yes | No | ||||
| Demographic characteristics | ||||||||
| Gender | ||||||||
| Male | 14 | 50% | 9 | 5 | 64.3% | 3 | 11 | 21% |
| Female | 14 | 50% | 4 | 10 | 28.6% | 1 | 13 | 7% |
| Age (years) | ||||||||
| <50 | 5 | 18% | 3 | 2 | 60.0% | 1 | 4 | 20% |
| 50–69 | 14 | 50% | 7 | 7 | 50.0% | 3 | 11 | 21% |
| ≥70 | 9 | 32% | 3 | 6 | 33.3% | 0 | 9 | 0% |
| Ophthalmic characteristics | ||||||||
| Visual acuity in eye with tumor | ||||||||
| ≥20/20 | 1 | 4% | 0 | 1 | 0.0% | 0 | 1 | 0% |
| 20/25–20/40 | 19 | 68% | 8 | 11 | 42.1% | 1 | 18 | 5% |
| 20/50–20/160 | 5 | 18% | 2 | 3 | 40.0% | 1 | 4 | 20% |
| ≤20/200 | 3 | 11% | 3 | 0 | 100.0% | 2 | 1 | 67% |
| Visual acuity in fellow eye | ||||||||
| ≥20/20 | 2 | 7% | 1 | 1 | 50.0% | 0 | 2 | 0% |
| 20/25–20/40 | 25 | 89% | 11 | 14 | 44.0% | 3 | 22 | 12% |
| 20/50–20/160 | 1 | 4% | 1 | 0 | 100.0% | 1 | 0 | 100% |
| ≤20/200 | 0 | 0% | 0 | 0 | 0 | 0 | ||
| Tumor characteristics | ||||||||
| Tumor apical height (mm) | ||||||||
| <2.5 | 11 | 39% | 6 | 5 | 54.5% | 1 | 10 | 9% |
| 2.5–5.0 | 17 | 61% | 7 | 10 | 41.2% | 3 | 14 | 18% |
| Longest basal dimension (mm) | ||||||||
| <4.5 | 0 | 0% | 0 | 0 | 0.0% | 0 | 0 | 0% |
| 4.5–8.0 | 5 | 18% | 3 | 2 | 60.0% | 1 | 4 | 20% |
| 8.1–11.0 | 14 | 50% | 7 | 12 | 36.8% | 2 | 17 | 11% |
| 11.1–14.0 | 4 | 14% | 3 | 1 | 75.0% | 1 | 3 | 25% |
| Distance to proximal edge of optic disc (mm) | ||||||||
| <2 | 1 | 4% | 1 | 0 | 100.0% | 0 | 1 | 0% |
| 2.1–4.0 | 7 | 25% | 5 | 2 | 71.4% | 3 | 4 | 43% |
| 4.1–6.0 | 7 | 25% | 5 | 2 | 71.4% | 0 | 7 | 0% |
| 6.1–8.0 | 4 | 14% | 1 | 3 | 25.0% | 1 | 3 | 25% |
| >8 | 9 | 32% | 1 | 8 | 11.1% | 0 | 9 | 0% |
| Distance to center of foveal avascular zone (mm) | ||||||||
| 0 | 4 | 14% | 4 | 0 | 100.0% | 2 | 2 | 50% |
| 0.1–2.0 | 4 | 14% | 2 | 2 | 50.0% | 1 | 3 | 25% |
| 2.1–5.0 | 3 | 11% | 3 | 0 | 100.0% | 1 | 2 | 33% |
| 5.1–8.0 | 6 | 21% | 3 | 3 | 50.0% | 0 | 6 | 0% |
| >8.0 | 1 | 39% | 1 | 10 | 9.1% | 0 | 11 | 0% |
| 1 | ||||||||
| Location of anterior tumor border | ||||||||
| Ciliary body | 4 | 14% | 0 | 4 | 0.0% | 0 | 4 | 0% |
| Equator to ora serrata | 1 | 36% | 3 | 7 | 30.0% | 0 | 10 | 0% |
| 0 | ||||||||
| Posterior to equator | 1 | 50% | 10 | 4 | 71.4% | 4 | 10 | 29% |
| 4 | ||||||||
| Location of posterior tumor border | ||||||||
| Equator to ora serrata | 1 | 4% | 0 | 1 | 0.0% | 0 | 1 | 0% |
| Posterior to equator | 2 | 96% | 13 | 14 | 48.1% | 4 | 23 | 15% |
| 7 | ||||||||
| Retina detached over tumor | ||||||||
| No | 2 | 82% | 9 | 14 | 39.1% | 2 | 21 | 9% |
| 3 | ||||||||
| Yes | 5 | 18% | 4 | 1 | 80.0% | 2 | 3 | 40% |
| Tumor shape | ||||||||
| Dome | 2 | 89% | 11 | 14 | 44.0% | 4 | 21 | 16% |
| 5 | ||||||||
| Collar button | 1 | 4% | 1 | 0 | 100.0% | 0 | 1 | 0% |
| Placoid | 2 | 7% | 1 | 1 | 50.0% | 0 | 2 | 0% |
| Treatment characteristics | ||||||||
| Delivered dose at tumor apex (Gy) | ||||||||
| 65.0–80.0 | 1 | 68% | 7 | 12 | 36.8% | 2 | 17 | 11% |
| 9 | ||||||||
| 80.1–85.0 | 7 | 25% | 5 | 2 | 71.4% | 1 | 6 | 14% |
| 85.1–90.0 | 2 | 7% | 1 | 1 | 50.0% | 1 | 1 | 50% |
| Delivered dose to sclera (Gy) | ||||||||
| <293.0 | 2 | 79% | 11 | 11 | 50.0% | 2 | 20 | 9% |
| 2 | ||||||||
| 293.0–342.9 | 2 | 7% | 1 | 1 | 50.0% | 1 | 1 | 50% |
| 343.0–409.9 | 4 | 14% | 1 | 3 | 25.0% | 1 | 3 | 25% |
| ≥410 | 0 | 0% | 0 | 0 | 0.0% | 0 | 0 | 0% |
| Plaque model (physical diameter, active diameter in mm) | ||||||||
| CCX (11.6, 9.5) | 1 | 4% | 1 | 0 | 100.0% | 0 | 1 | 0% |
| CCA (15.3, 13.0) | 2 | 79% | 12 | 10 | 54.5% | 4 | 18 | 18% |
| 2 | ||||||||
| CIA (15.3, 13.0) | 3 | 11% | 0 | 3 | 0.0% | 0 | 3 | 0% |
| CIB (20.2, 17.8) | 2 | 7% | 0 | 2 | 0.0% | 0 | 2 | 0% |
| Plaque serial number | ||||||||
| CCX-104 | 1 | 4% | 1 | 0 | 100.0% | 0 | 1 | 0% |
| CCA-571 | 9 | 32% | 5 | 4 | 55.6% | 1 | 8 | 11% |
| CCA-992 | 1 | 39% | 5 | 6 | 45.5% | 2 | 9 | 18% |
| 1 | ||||||||
| CCA-892 | 2 | 7% | 2 | 0 | 100.0% | 1 | 1 | 50% |
| CIA-173 | 2 | 7% | 0 | 2 | 0.0% | 0 | 2 | 0% |
| CIA-156 | 1 | 4% | 0 | 1 | 0.0% | 0 | 1 | 0% |
| CIB-307 | 2 | 7% | 0 | 2 | 0.0% | 0 | 2 | 0% |
| Difference in plaque diameter and tumor largest base diameter | ||||||||
| <6 mm | 1 | 57% | 10 | 6 | 62.5% | 3 | 13 | 19% |
| 6 | ||||||||
| ≥6 mm | 1 | 43% | 3 | 9 | 25.0% | 1 | 11 | 8% |
| 2 | ||||||||
| Adjuvant transpupillary thermotherapy | ||||||||
| Yes | 8 | 29% | 2 | 6 | 25.0% | 1 | 7 | 13% |
| No | 2 | 71% | 11 | 9 | 55.0% | 3 | 17 | 15% |
| 0 | ||||||||
Ophthalmic complications after treatment were rare. No patient developed neovascular glaucoma. Among 24 patients retaining the treated eye, 8 (33.3%) developed significant vision loss (acuity <20/200). In all but one of these patients, vision loss occurred after local tumor recurrence and additional local therapy. Therefore, preservation of vision (acuity ≥20/200) was 93.3% among patients without recurrence not undergoing additional local therapy. No patient required enucleation for ophthalmic complications.
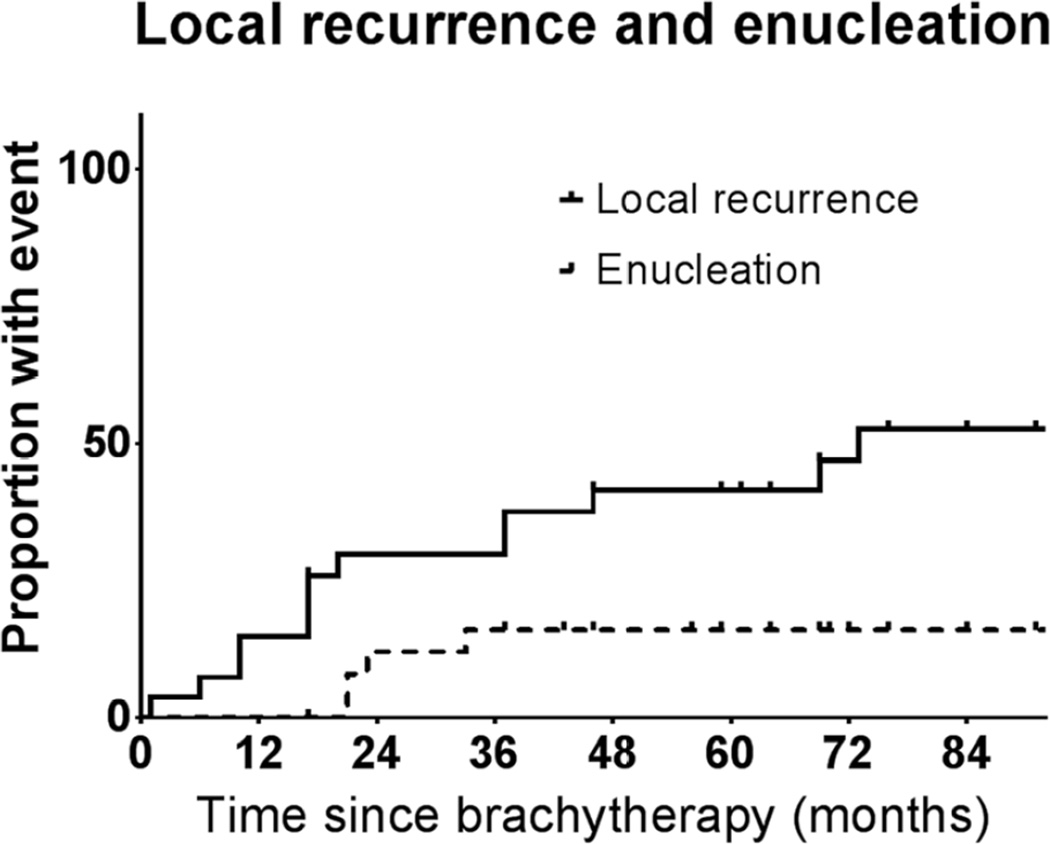
Local tumor recurrence was more common than expected. Figure 1 depicts the probability of local recurrence and enucleation after brachytherapy. Thirteen (46.4%) patients were found to have local tumor recurrence after brachytherapy. In 8 (61.5%) patients recurrence was horizontal/marginal and in 5 (38.5%) patients the recurrence was vertical/diffuse. All patients underwent salvage therapy, with modalities presented in Table 2. Among 13 patients with tumor growth, 4 underwent enucleation, 10 underwent TTT, 2 underwent photodynamic therapy, and 1 underwent reirradiation with protons; some patients underwent more than one salvage therapy. In all patients that underwent enucleation, viable melanoma was found on histopathologic analysis.
Fig. 1.
Local recurrence and enucleation after Ru-106 brachytherapy for uveal melanoma.
Table 2.
Salvage therapy after local tumor recurrence with Ru-106 brachytherapy
| Salvage local therapy* | N | % |
|---|---|---|
| Enucleation | 4 | 14.3 |
| Transpupillary thermotherapy | 10 | 35.7 |
| Photodynamic therapy | 2 | 7.1 |
| Proton therapy | 1 | 3.6 |
Some patients underwent more than one salvage local therapy.
Because of the high frequency of local treatment failure, further analysis was carried out to determine if any factors were associated with this event. Table 1 presents the characteristics, tumors, and treatment of patients that experienced local tumor recurrence and enucleation and those who did not. Five years after treatment, the estimated risks of local recurrence and enucleation were 41.5% (95% confidence interval [CI], 20.2–61.7%) and 16% (95% CI, 0.7–31.3%), respectively.
When the variables presented in Table 1 were tested in a univariable Cox regression model, six were found to be significantly associated with local tumor recurrence: low visual acuity in eye with tumor (HR, 2.4; 95% CI, 1.7–3.1; p = 0.02), tumor close to the proximal edge of the optic disc (HR, 2.4; 95% CI, 1.5–2.4; p = 0.005), tumor close to the center of the foveal avascular zone (HR, 2.1; 95% CI, 1.7–2.5; p = 0.0001), posterior tumor border close to posterior pole (HR, 4.8; 95% CI, 3.6–6.0; p = 0.009), smaller plaque size (HR, 3.6; 95% CI, 2.4–4.8; p = 0.04), and difference in plaque diameter and tumor largest base diameter <6 mm (HR, 6.2; 95% CI, 4.7–7.8; p = 0.02). When the variables in Table 3 were tested in a univariable Cox regression model, three were found to be significantly associated with enucleation: low visual acuity in eye with tumor (HR, 4.1; 95% CI, 2.9–5.2; p = 0.02), low visual acuity in fellow eye (HR, 11.2; 95% CI, 8.8–13.5; p = 0.04), and tumor close to center of foveal avascular zone (HR, 3.2; 95% CI, 2.2–4.2; p = 0.02). Notably, gender, age, tumor apical height and longest basal dimension, location of posterior border, retinal detachment over tumor, tumor shape, radiation dose at tumor apex and sclera, plaque serial number, and adjuvant TTT were not associated with local tumor recurrence or enucleation.
Table 3.
Previously reported series of ≥400 patients treated with Ru-106 brachytherapy for uveal melanoma
| Institution | Eyes treated (n=) |
Treatment period (years) |
Radiation dose prescribed |
Radiation dose delivered |
Planning margins | Median follow-up (months) |
Local tumor recurrence (n=) |
Crude recurrence rate |
Enucleated (n=) |
Crude enucleation rate |
|---|---|---|---|---|---|---|---|---|---|---|
| St Erik's Eye Hospital, Stockholm | 579 | 1979–2003 | 100 Gy to 1 mm beyond tumor apex | Median 100 Gy to tumor apex | Not reported | 82 among living 55 among deceased | 79 | 14% | 106 | 18% |
| Royal Liverpool University Hospital | 458 | 1993–2001 | 80–100 Gy to tumor apex; ≥300 Gy to sclera | Median 100–115 Gy to tumor apex; median 400–500 Gy to sclera | At least 2 mm around tumor; 15.3 mm plaque if tumor base diameter <10 mm; 20.2 mm plaque if tumor base diameter ≥10 mm | 46.8 | 9 | 2% | 8 | 2% |
| Leiden University Medical Centre | 425 | 1993–2004 | 400–800 Gy to sclera | 100–150 Gy to tumor apex | Not reported | 50 for survivors | 16 | 4% | 17 | 4% |
| Present study | 28 | 2000–2008 | 75–85 Gy to tumor apex | Median 75.5 Gy to tumor apex | At least 2 mm around tumor | 71 | 13 | 46% | 4 | 14% |
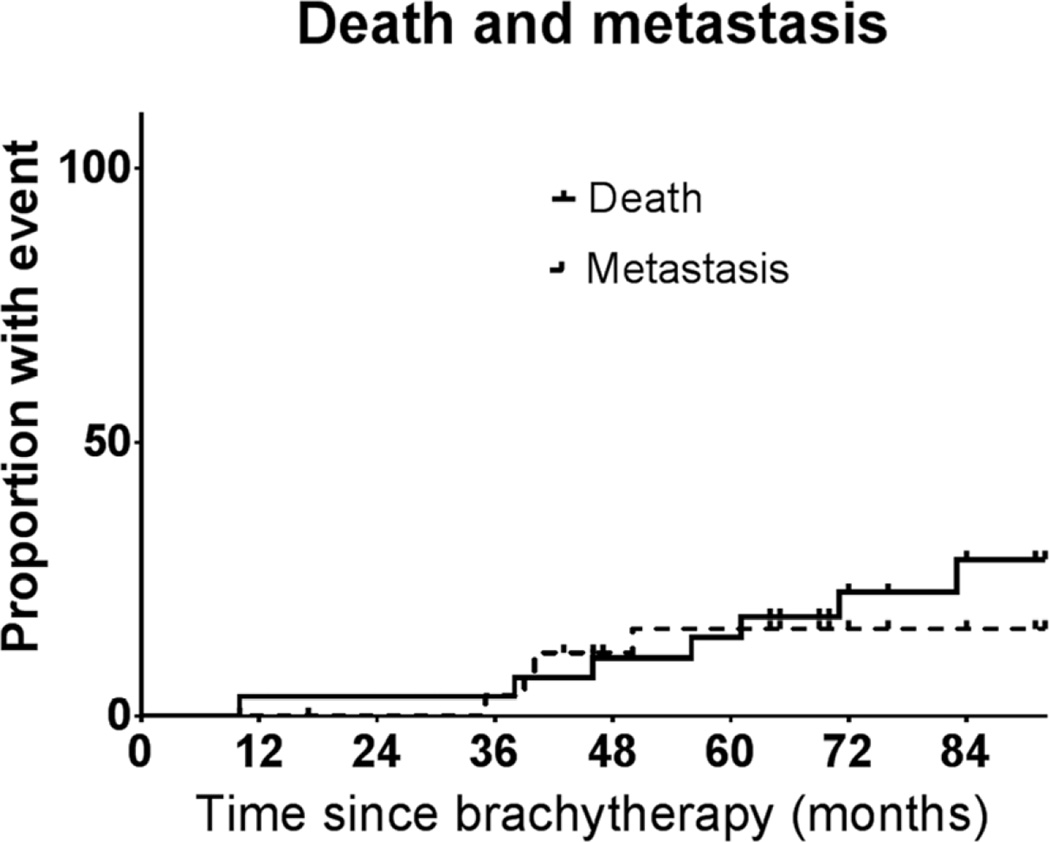
The 5-year estimated rates of death and metastasis were 18.5% (95% CI, 1.6–49.3%) and 11.4% (95% CI, 0.1–49.7%).
Discussion
This study was designed to assess the outcome of Ru-106 plaque brachytherapy in patients with uveal melanoma. We found that treatment was well tolerated and ophthalmic complications were infrequent. However, we also found a high frequency of local tumor recurrence necessitating additional local therapy. We found that local tumor recurrence was significantly more common in patients with worse visual acuity in the tumor-bearing eye, in patients with posteriorly located tumors (near to the optic nerve and avascular foveal zone), and in patients treated with smaller plaques, and when the difference between the plaque diameter and tumor diameter was <6 mm. We found that enucleation was significantly more common in patients with worse visual acuity in the tumor-bearing and fellow eye, and in patients with tumors near the avascular foveal zone. While some of these findings have been previously reported (4), for the first time we report that tumor-plaque margin <6 mm is associated with local tumor recurrence after Ru-106 plaque brachytherapy.
Previous studies of Ru-106 have generally shown very low rates of local tumor recurrence. Table 3 presents the three largest independent studies each reporting on over 400 patients treated with Ru-106 plaque brachytherapy. With a similar duration of follow-up as the present study, local treatment failure in these studies was 2–14%. These studies reported that tumor size (5, 6) and posterior location (7) may be associated with treatment failure. Our study did not recapitulate the former finding (likely because of a narrow range of tumor sizes), but did corroborate the latter finding. In the Liverpool series, the brachytherapy planning technique is reported; however, in the other series, details regarding the planning techniques are not indicated. Importantly, it appears that in previous studies the tumor apex received doses of radiation ≥33% higher than those in the present study. Within the present study we did not find an association between radiation dose and local tumor recurrence, however, given the considerable differences in local tumor recurrence rates between the present study and prior studies using a higher dose of radiation, a relationship between local tumor recurrence and radiation dose may exist.
The effect of radiation dose may be of particular importance when considering the differences in dose distribution between Ru-106 and I-125 plaques that we have based our brachytherapy planning techniques on. Investigators in Cleveland conducted a dosimetric comparison between I-125 and Ru-106 eye plaques. In this study, when using plaques of the same size (18 mm) and radiation doses prescribed to the same depth (3 or 5 mm), greater lateral constriction of isodoses at the edge of the plaque were observed with Ru-106. At some isodose levels this difference was up to 20%, suggesting that for similarly sized plaques, with radiation prescribed to the same distance from the plaque, the dose at the edge of the plaque could be considerably lower with Ru-106 compared with I-125 (8). The reason for this difference is in part because of the way the Ru-106 plaque is manufactured. Ru-106 plaques have no isotope along the edge of the plaque. This detail is important and should be kept in mind during brachytherapy planning.
While differences in radiation dose distribution from Ru-106 and I-125 plaques have been previously appreciated, never before have clinical outcomes suggested this factor is of importance. The present study is therefore novel, because of the demonstrated association between higher rates of local tumor recurrence and narrow tumor-plaque edge margin. The clinical significance implication of a low radiation dose at the edge of the Ru-106 plaque is further substantiated by the observation that local treatment failure occurred most often at the periphery in most cases (61.5%). This observation supports the dosimetric observations described above, specifically that the dose at the edge of Ru-106 is lower than a similarly designed I-125 plaque. This suggests that brachytherapy planning protocols used for I-125 are not sufficient for Ru-106 plaque brachytherapy.
The present series suggests a higher rate of local tumor recurrence than observed with I-125 in the COMS, or other prospective studies. The 5-year rate of local tumor recurrence in the present study was 41.5% (95% CI, 20.2–61.7%), while in the COMS 5-year local tumor recurrence was 10.3% (95% CI, 8.0–13.2%) (4). Although comparison of outcomes between studies is problematic, the 95% CIs of the results of these datasets do not overlap, suggesting the differences in these results may be significant. Moreover, other groups have reported higher rates of failure with Ru-106, compared to I-125. In 1999, investigators in London reported on 140 patients with uveal melanoma treated with proton therapy or plaque brachytherapy using I-125 or Ru-106. These investigators observed a significantly higher rate of local tumor recurrence with Ru-106 (10.7%) compared with I-125 (4.2%) (9). Similarly, investigators in Philadelphia reported on 354 patients with uveal melanoma ≥8 mm thick treated with plaque brachytherapy. They observed a significantly higher risk of enucleation after brachytherapy with Ru-106, compared with I-125 (10). While the present study did not compare the outcome of patients treated with Ru-106 to those treated with I-125 because of the presence of selection bias, prior studies comparing outcomes of Ru-106 and I-125 brachytherapy have reported inferior outcomes with Ru-106. We speculate that centers (such as ours) may have observed higher rates of local tumor recurrence because the difference in dosimetry between Ru-106 and I-125 plaques was not accounted for during brachytherapy planning.
Our study has several potential limitations. First, the retrospective nature of the design limits the quality of the data gathered. However, detailed clinical records have been maintained at our center for many years, and the major endpoints studied here are unlikely to have been affected because of the study design. Second, the cohort is small. As Ru-106 was only available in the United States for a few years, it is likely that the present study is one of the larger cohorts of patients treated in the United States, where most physicians plan ophthalmic brachytherapy based on techniques developed in the COMS using I-125. It is possible that our outcomes could be related to the early and limited use of Ru-106 at our center. The present contribution is important to the field because it emphasizes the need to adapt to the nuances of brachytherapy using Ru-106 in ophthalmic brachytherapy, and not to rely on treatment-planning methods used with other isotopes.
Fig. 2.
Death and metastasis after Ru-106 brachytherapy for uveal melanoma.
In conclusion, our study was designed to ascertain the outcome of Ru-106 in patients with uveal melanoma. We found that ophthalmic complications were infrequent, but local tumor recurrence was more common than expected. We found that posteriorly located tumors are more likely to recur after brachytherapy, as previously reported. For the first time, we report that the difference in size between tumor diameter and plaque diameter is associated with local tumor recurrence. As a modifiable factor in Ru-106 plaque brachytherapy, we encourage others to pay close attention to this factor when using Ru-106 plaques. The results suggest that margins used with I-125 cannot be applied to Ru-106 plaques, and as a result we will now adopt a strategy of 3 mm margins around the tumor when selecting the appropriate Ru-106 plaque, for a dose of 85 Gy at the tumor apex. An alternative approach would be to prescribe higher tumor apex doses to account for lateral dose constriction with this isotope, as has been done in European centers. Further study will be necessary to determine the optimal planning technique to maximize local tumor control and minimize ophthalmic complications.
Acknowledgements
The authors acknowledge Lawrence A. Herman for editorial assistance.
Glossary
- COMS
Collaborative Ocular Melanoma Study
- AJCC
American Joint Committee on Cancer
- TTT
transpupillary
- CI
confidence interval
- HR
hazard ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no financial disclosures.
References
- 1.Collaborative Ocular Melanoma Study G. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma: V. Twelve-year mortality rates and prognostic factors: COMS report No. 28. Arch Ophthalmol. 2006;124:1684–1693. doi: 10.1001/archopht.124.12.1684. [DOI] [PubMed] [Google Scholar]
- 2.Char DH, Quivey JM, Castro JR, et al. Helium ions versus iodine 125 brachytherapy in the management of uveal melanoma. A prospective, randomized, dynamically balanced trial. Ophthalmology. 1993;100:1547–1554. doi: 10.1016/s0161-6420(93)31446-6. [DOI] [PubMed] [Google Scholar]
- 3.Finger PT, Chin KJ, Duvall G, et al. Palladium-103 ophthalmic plaque radiation therapy for choroidal melanoma: 400 treated patients. Ophthalmology. 2009;116:790–796. doi: 10.1016/j.ophtha.2008.12.027. 796 e791. [DOI] [PubMed] [Google Scholar]
- 4.Jampol LM, Moy CS, Murray TG, et al. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma: IV. Local treatment failure and enucleation in the first 5 years after brachytherapy. COMS report no. 19. Ophthalmology. 2002;109:2197–2206. doi: 10.1016/s0161-6420(02)01277-0. [DOI] [PubMed] [Google Scholar]
- 5.Bergman L, Nilsson B, Lundell G, et al. Ruthenium brachytherapy for uveal melanoma, 1979–2003: survival and functional outcomes in the Swedish population. Ophthalmology. 2005;112:834–840. doi: 10.1016/j.ophtha.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 6.Damato B, Patel I, Campbell IR, et al. Local tumor control after 106Ru brachytherapy of choroidal melanoma. Int J Radiat Oncol Biol Phys. 2005;63:385–391. doi: 10.1016/j.ijrobp.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 7.Verschueren KM, Creutzberg CL, Schalij-Delfos NE, et al. Long-term outcomes of eye-conserving treatment with Ruthenium(106) brachytherapy for choroidal melanoma. Radiother Oncol. 2010;95:332–338. doi: 10.1016/j.radonc.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 8.Wilkinson DA, Kolar M, Fleming PA, et al. Dosimetric comparison of 106Ru and 125I plaques for treatment of shallow (<or=5 mm) choroidal melanoma lesions. Br J Radiol. 2008;81:784–789. doi: 10.1259/bjr/76813976. [DOI] [PubMed] [Google Scholar]
- 9.Wilson MW, Hungerford JL. Comparison of episcleral plaque and proton beam radiation therapy for the treatment of choroidal melanoma. Ophthalmology. 1999;106:1579–1587. doi: 10.1016/S0161-6420(99)90456-6. [DOI] [PubMed] [Google Scholar]
- 10.Shields CL, Naseripour M, Cater J, et al. Plaque radiotherapy for large posterior uveal melanomas (>or =8-mm thick) in 354 consecutive patients. Ophthalmology. 2002;109:1838–1849. doi: 10.1016/s0161-6420(02)01181-8. [DOI] [PubMed] [Google Scholar]