Abstract
This study tested the hypothesis that enhanced neural arousal in response to performance errors would predict poor affect and coping behaviors in everyday life. Participants were preselected as either low-depressed (LD) or high-depressed (HD) based on a screening questionnaire, and they then completed a laboratory Stroop task while EEG was recorded, followed by a 2-week period of daily reports of affect and coping behaviors. The EEG measure of arousal response to errors was the degree of error-related alpha suppression (ERAS) in the intertrial interval, that is the reduction in alpha power following errors compared with correct responses. ERAS was relatively heightened at frontal sites for the HD versus the LD group, and frontal ERAS predicted lower positive affect, higher negative affect, and less adaptive coping behaviors in the daily reports. Together, the results imply that heightened arousal following mistakes is associated with suboptimal emotion and coping with stressors.
Keywords: depression, EEG, performance errors, coping
Deficits in self-regulation likely contribute to conditions such as depression and anxiety. These deficits may cut across the related domains of emotion regulation, including the ability to regulate negative affect, and cognitive control, which is the ability to adapt attention, performance, and behavior in order to meet task goals. In the present study, we address the possibility that depression-related characteristics of high negative affect, low positive affect, and maladaptive coping behaviors are also associated with exaggerated neural responses to performance mistakes.
The ability to respond effectively to errors, failures, and setbacks is a relevant skill in everyday life and one that appears to be disrupted in depression. For example, people with depression, compared with controls, tend to show greater performance decrements following errors or negative performance feedback (Beats, Sahakian, & Levy, 1996; Elliott, Sahakian, McKay, & Herrod, 1996; Holmes & Pizzagalli, 2007; Murphy, Robbins, & Sahakian, 2003), suggesting a difficulty in recovering from mistakes. Such maladaptive cognitive control may feed into a downward spiral as errors or failures compound.
Deficits in emotional self-regulation also characterize depression and related conditions. Depressed people are more likely to engage in catastrophic thinking and less likely to engage in positive reappraisal of negative events (Garnefski & Kraaij, 2006; Martin & Dahlen, 2005), and depressed or high-risk individuals reported lower positive affect and higher negative affect and self-blame following stressful events, compared with less-depressed or low-risk participants (Compton et al., 2011b; Schneider et al., 2006; see also Myin-Germeys et al., 2003). Another study found that depressed people, compared with controls, showed elevated negative affect for a longer period following negative events (Peeters, Nicolson, Berkhof, Delespaul, & deVries, 2003). An important goal for future research, then, is to determine the relationship between deficient cognitive and emotional self-regulation in depression.
Recent research has aimed to better understand the cognitive and neural mechanisms involved in cognitive control and how they may be disrupted in individuals with depression or other mood disorders. For example, some research has focused on the error-related negativity (ERN), a scalp-recorded event-related potential that appears within 100 ms following an erroneous action and is thought to be generated by the anterior cingulate cortex (Gehring, Goss, Coles, Meyer, & Donchin, 1993; for reviews, see Gehring, Liu, Orr, & Carp, 2012; Simons, 2010). Because of its error-specificity and its putative generation by an anatomical structure known to be crucial in cognitive control (e.g., Gehring et al., 2012), the ERN may index an important component of the cognitive–neural mechanisms of behavioral control, namely the detection of an undesirable outcome.
Several research teams have investigated the possibility that the ERN, and the underlying cognitive process that it reflects, may be disrupted in depressed samples. Yet, results are conflicting. Some reports suggest that the ERN is elevated in depression (e.g., Chiu & Deldin, 2007; Holmes & Pizzagalli, 2010; Tucker, Luu, Frishkoff, Quiring, & Poulsen, 2003), although others suggest that it is dampened (e.g., Ladouceur et al., 2012; Ruchsow et al., 2004; Ruchsow et al., 2006). Still other studies report that depression is associated with no difference in the ERN despite deficits in posterror performance (Compton et al., 2008), that remitted but not acutely depressed participants show an elevated ERN (Georgiadi, Liotti, Nixon, & Liddle, 2011), or that comorbid depression may dampen the elevated ERN that is often associated with anxiety (Weinberg, Klein, & Hajcak, 2012).
Although continuing research on the ERN in depressed samples may, in time, untangle these conflicting results, novel approaches may help to yield additional clues about maladaptive responses to performance mistakes in depression. In the present study, we examined the association between depression (and related affective characteristics) and a novel error-related neural marker, namely error-related alpha suppression (ERAS). ERAS, first demonstrated by Carp and Compton (2009), describes an effect in which performance errors are followed by increased cerebral arousal relative to correct responses.
Across a series of studies, a number of features of ERAS have been consistently described (Carp & Compton, 2009; Compton, Arnstein, Freedman, Dainer-Best, & Liss, 2011a; Compton, Hofheimer, & Kazinka, 2013; Compton, Huber, Levinson, & Zheutlin, 2012). Correct responses are typically followed by a phasic increase in alpha power during the intertrial interval (intertribal interval, ITI), reflecting a period of “mental relaxation” or disengagement during the ITI. The change in alpha power following correct responses displays a quadratic pattern during the ITI, with alpha power increasing and then decreasing again in time for the next stimulus onset. In contrast, following erroneous responses, this quadratic change in alpha power during the ITI is suppressed. Because alpha power is inversely related to arousal or engagement, ERAS implies increased arousal following errors relative to correct trials. In addition to replicating the overall phenomenon of ERAS, several studies have replicated a reliable scalp distribution in which ERAS is maximal over parietal regions (Carp & Compton, 2009; Compton et al., 2011a, 2012, 2013). Even following correct responses, the pattern of alpha power in the ITI is modulated by the degree of conflict inherent in the preceding trial (Compton et al., 2011a, 2012), indicating that postresponse alpha measures may be relevant to understanding ongoing cognitive control processes.
Although the functional meaning of ERAS is still under study, some preliminary evidence suggests that, unlike the ERN, ERAS may reflect a maladaptive reaction to errors. In a study with a large sample size, we found that ERAS predicted the degree of posterror slowing, whereas the ERN predicted posterror accuracy (Carp & Compton, 2009). Specifically, participants who showed greater ERAS tended to show slower performance following mistakes (compared with correct trials), without any benefit to accuracy. In contrast, individuals who showed a greater ERN tended to show better posterror accuracy, as would be expected if the ERN reflects a component of adaptive behavior control. In addition, individual differences in the ERN and ERAS predict cortisol reactivity in opposite directions (Compton et al., 2013). Individuals with a greater ERN tended to show less cortisol reactivity during a cognitive task, whereas those with greater ERAS tended to show greater cortisol reactivity. These findings imply that ERAS may reflect an arousal response to errors that is associated with maladaptive performance outcomes and heightened stress reactivity.
In the present study, therefore, we addressed the hypothesis that individuals with high self-reported levels of depression would show increased levels of alpha suppression following errors. In addition to standard questionnaire measures of depression and anxiety, we also included daily reports of negative and positive affect, reactivity to stress, and coping behaviors (for similar approaches, see Compton et al., 2011b; Compton et al., 2008). Daily reports may have more ecological validity because they are less subject to retrospective biases that occur with broad personality, affect, and coping measures (e.g., Todd, Tennen, Carney, Armeli, & Affleck, 2004). By including these measures, we aimed to address the extent to which ERAS co-occurs with suboptimal affect and coping measured on a daily basis.
Method
Participants
Sixty-two undergraduates completed the study. Participants were selected on the basis of responses on an online screening questionnaire that included the Center for Epidemiological Studies Depression scale (CESD; Radloff, 1977). The screening questionnaire was advertised to all students at the college via message boards. Respondents were invited to participate in the full study if screening questionnaire responses indicated absence of neurological history, normal vision, and absence of current regular use of substances (prescription or illicit) that would affect the central nervous system (such as antidepressants, anxiolytics, etc.), and if CESD scores were either <10 (low-depression group, LD) or > 14 (high-depression group, HD). The LD group included 37 participants (19 male, 18 female) with a mean CESD score of 5.2 (range 0–9.5); the HD group included 25 participants (12 male, 13 female) with a mean CESD score of 23.7 (range 15–38).
Laboratory Task
Participants completed a six-choice Stroop task while EEG was recorded. The Stroop task was selected as one that has often been used in studies of cognitive control and performance monitoring. The task required participants to identify the color of a target word whose meaning was color-incongruent (color-word conflicting with the font color, e.g., “red” in blue font), emotional (e.g., “fail”), or neutral (e.g., “chair”). Participants indicated the color using the first three fingers on each hand, with response mappings in “rainbow order” (red, orange, yellow, green, blue, purple) from left to right across the six keys.
The main task was composed of 10 blocks of trials, in which each block included 30 trials each of the three word types, randomly intermixed (90 trials per block, 900 trials total). Prior to the main task, participants completed 24 practice trials with accuracy feedback. Feedback was not given during the main trial blocks, but break screens between blocks reminded participants of the correct response mapping. On each trial, the target was presented for 150 ms against a black background, followed by a blank screen that was displayed until the participant responded. A1,280-ms intertrial interval (ITI) followed the response; a blank screen was displayed during the ITI.
Electrophysiological Recording and Data Processing
Electrodes were applied using an elastic cap (Quik-Caps) fitted with sintered Ag/AgCl electrodes. Data were recorded continuously from four midline scalp sites (Fz, FCz, Cz, Pz) and three pairs of lateral sites (F3/4, C3/4, and P3/4). For the purposes of examining ERAS, data from the FCz site were not considered so that data from a 3 × 3 grid of electrodes (frontal/central/parietal × left/midline/right) could be analyzed by factorial ANOVA. Signals were amplified by a NuAmps amplifier controlled by Neuroscan software, with a sampling rate of 1,000 Hz and a bandpass of 0.1–40 Hz (−3 dB). Data were referenced online to the left mastoid and digitally rereferenced off-line to the average of left and right mastoids. Eye movements were monitored by electrodes placed above and below the left eye and at the outer canthus of each eye. Recordings from these four sites were used to compute bipolar horizontal and vertical EOG channels off-line.
Artifacts were addressed off-line in three steps. First, upon visual inspection, portions of the EEG record with large nonblink artifacts were manually excluded. Second, the effect of blinks was reduced using the Neuroscan software's regression-based algorithm for ocular artifact reduction. Finally, remaining artifacts in the EEG were identified using a ±150 μv threshold, and corresponding epochs were excluded.
To address alpha power changes following errors versus correct trials, power spectra were computed for five 256-ms epochs beginning at the time of the response and extending throughout the intertrial interval. Division of the ITI into epochs of this length allows for a characterization of how alpha power changes over the course of the ITI (e.g., Carp & Compton, 2009). Power spectra were obtained for each window using the fast Fourier transform and a cosine windowing method. This procedure yielded time-frequency representations of the ITI with a resolution of 256 ms in the time domain and 4 Hz in the frequency domain. Spectra for each window were then averaged separately for the six conditions yielded by crossing trial accuracy (error, correct) and trial type (incongruent, emotion, neutral). Statistical analyses were conducted on log-transformed mean power values in the 10–14 Hz frequency band.
Self-Report Measures
One-time measures
At the end of the lab session, participants completed two self-report scales intended to index aspects of depression and anxiety. The Mood and Anxiety Symptom Questionnaire (MASQ; Watson et al., 1995a,1995b) is a 62-item questionnaire that includes subscales for anhedonic depression (MASQ-AD) and anxious arousal (MASQ-AA). The Penn State Worry Questionnaire (PSWQ; Meyer, Miller, Metzger, & Borkovec, 1990) includes 16 items tapping worry-prone tendencies.
Daily reports
Participants were prompted via e-mail reminder to submit online daily reports after 8 p.m. every evening for 14 days following the lab session. The purpose of the daily reports was to track affect, stress reactivity, and coping behaviors on a daily basis.
The online questionnaire included the Positive and Negative Affect Schedule (PANAS; Watson, Clark, & Tellegen, 1988), in which the participant rated on a 5-point scale the degree to which each of 20 mood-related adjectives described his or her mood that day. Ten adjective ratings were summed to form the negative affect scale (PANAS-NA), and 10 were summed to form the positive affect scale (PANAS-PA).
The questionnaire also included items tapping daily stress experiences. Fifteen items were adapted from the Daily Stress Inventory (DSI; Brantley & Jones, 1989). The inventory asks participant to indicate which of a list of stressors and hassles occurred on that day (e.g., heard some bad news, was late, misplaced something) and, for each event that occurred, how stressful it was perceived to be on a 7-point scale (1 = occurred but was not stressful; 7 = caused me to panic). The DSI-events score is the count of stressors that occurred, whereas the DSI-ratio score reflects reactivity to events by quantifying response per event (sum of stress scores divided by count of stressors). As a simpler index of perceived stress, the participants also rated a single stress item, “Overall, how would you rate the level of stress imposed on you by outside events today?” on a 7-point scale (1 = very few stressors; 7 = many stresses imposed on you today).
Finally, the daily questionnaire included self-reports of 12 coping behaviors. In this section, participants were asked to indicate how much they engaged in each of the list of behaviors on that day, using a 4-point rating scale (1 = not at all; 4 = very much). Items were randomly intermixed, and included three emotion-focused coping items (I criticized myself, I focused on my inadequacies, I blamed myself for situations), three task-focused coping items (I analyzed a problem before reacting, I outlined my priorities, I got control of a situation), three approach-focused coping items (I affiliated with others, I approached things I wanted, I sought out other people), and three avoidance-focused coping items (I avoided a situation, I kept my distance from a situation, I distracted myself to prevent thinking about a situation). Item scores were summed across the three items in each subscale to yield four subscale scores.
Results
Self-Report Measures
Table 1 presents scores on self-report measures for the LD and HD groups. Not surprisingly, participants who were preselected as HD based on CESD scores also had significantly higher worry (PSWQ), anxious arousal (MASQ-AA) and anhedonic depression (MASQ-AD) scores than the LD group at the time of the laboratory session. CESD scores at the time of screening were significant predictors of PSWQ (r = .63, p < .001), MASQ-AD (r = .63, p < .001) and MASQ-AA (r = .39, p = .002) at the time of the lab session.
Table 1.
Mean (SD) Scores on Self-Report Measures
| Depression group |
|||
|---|---|---|---|
| Low | High | t- and p-values | |
| One-time measures |
|||
| PSWQ | 43.6 (11.7) | 59.8 (10.7) | t(60) = 5.52, p < .001 |
| MASQ-AA | 22.6 (5.6) | 27.3 (8.9) | t(60) = 2.59, p < .02 |
| MASQ-AD | 44.5 (10.6) | 61.0 (13.9) | t(60) = 5.33, p < .001 |
| Average daily measures |
|||
| PANAS-PA | 26.0 (6.5) | 21.5 (6.2) | t(58) = –2.65, p < .02 |
| PANAS-NA | 14.8 (3.8) | 17.7 (5.3) | t(58) = 2.50, p < .02 |
| Emotion-focused coping | 4.8 (1.7) | 6.1 (1.7) | t(58) = 2.83, p < .01 |
| Task-focused coping | 6.3 (1.6) | 5.8 (1.4) | ns |
| Approach-focused coping | 7.9 (1.5) | 6.9 (1.5) | t(58) = –2.53, p < .02 |
| Avoidance-focused coping | 5.0 (1.8) | 5.7 (1.9) | ns |
| DSI-events | 4.1 (1.8) | 4.7 (1.6) | ns |
| DSI-ratio | 2.3 (0.6) | 2.7 (0.8) | t(58) = 2.48, p < .02 |
| Single-item stress | 2.5 (0.8) | 3.1 (1.0) | t(58) = 2.52, p < .02 |
Table 1 also presents group differences in daily report variables (averaged across days). The mean number of daily reports was 11.9 days, and this number did not differ between LD and HD groups (p > .90). Two participants (one from each group) failed to submit any daily reports, and are excluded from analyses that involve daily report data. As seen in Table 1, the HD group reported significantly higher daily NA and emotion-focused coping than the LD group, as well as lower PA and approach-focused coping than the LD group. Groups did not differ significantly on task- or avoidance-focused coping. Furthermore, although the groups did not differ in the number of stressful events reported (DSI-events), the HD group had a higher DSI-ratio, reflecting increased reactivity to daily stressors, and they also reported higher perceived stress overall on the single-item measure compared with the LD group.
Together, these comparisons confirm expectations that higher levels of depression co-occur with higher levels of anxiety, negative affect, perceived stress, and self-blame in reaction to events, and lower levels of positive affect and approach-oriented coping.
Behavioral Performance
Accuracy and reaction time (RT) data from the laboratory task revealed expected Stroop interference effects but no group differences in performance. Both accuracy and RT were separately submitted to an ANOVA with trial type (incongruent, emotion, neutral) as a repeated-measures factor and group (LD, HD) as a between-subjects factor. For accuracy (proportion correct), the main effect of trial type, F(2, 120) = 13.77, p < .001, was due to lowest accuracy on incongruent (M = 0.893, SEM = .011), followed by neutral (M = 0.903, SEM = .011) and then emotion trials (M = 0.910, SEM = .011; all pairwise comparisons significant, Bonferroni post hoc, ps < .05). For RT, again the main effect of trial type was significant, F(2, 120) = 93.94, p < .001, due to longer RTs (Bonferroni post hoc, ps < .001) for incongruent trials (M = 682 ms, SEM = 19) compared with both emotion (M = 623 ms, SEM = 18) and neutral trials (M = 627 ms, SEM = 19), which did not differ. Neither the main effect of group nor the trial-type × group interaction was significant for either accuracy or RT (Fs < 1).
Alpha Power Analyses
Log alpha power values were submitted to an ANOVA with repeated-measures factors trial accuracy (correct, error), trial type (incongruent, emotion, neutral), epoch (beginning 0, 256, 512, 768, 1,024 ms after button-press response), anterior-posterior site (frontal, central, parietal), and laterality (left, midline, right hemisphere), as well as the between-subjects factor depression group (LD, HD). Greenhouse–Geisser corrections were applied to correct for violations of sphericity. Eight participants (four LD, four HD) were excluded due to technical difficulties that resulted in missing data for one of the electrode sites.
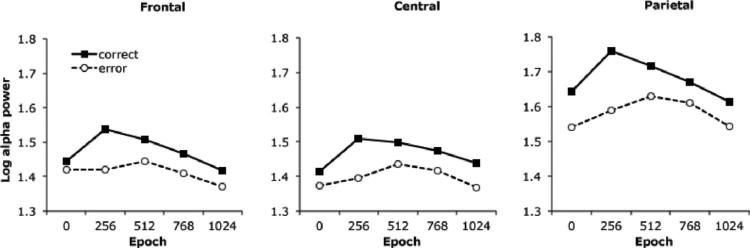
Numerous effects replicated patterns of alpha power that have been previously reported in other datasets (e.g., Carp & Compton, 2009; Compton et al., 2011a) and are briefly summarized here. Confirming the overall phenomenon of ERAS, the main effect of accuracy, F(1, 52) = 35.9, p < .001, reflects lower alpha power following errors (M = 1.47 μV2, SEM = .04) than correct responses (M = 1.54 μV2, SEM = .04). The main effect of epoch, F(4, 208) = 12.1, p < .001, was due to an overall increase and decrease of alpha power across the ITI (quadratic trend across epochs, F(1, 52) = 47.3, p < .001). Furthermore, the main effect of site, F(2, 104) = 43.3, p < .001, reflects higher alpha power (Bonferroni-corrected post hoc, ps < .001) at parietal (M = 1.63 μV2, SEM = .05) than central (M = 1.43 μV2, SEM = .04) and frontal sites (M = 1.44 μV2, SEM = .04), which did not differ significantly. Qualifying all of these main effects were three interaction effects, Accuracy × site, F(2, 104) = 12.3, p < .001; Accuracy × epoch, F(4, 208) = 7.3, p < .001; Accuracy × epoch × site, F(8, 416) = 4.6, p = .001. Means for the three-way interaction are presented in Figure 1. Briefly, ERAS was more pronounced at parietal than frontal and central sites, accounting for the accuracy × site interaction; across all sites, ERAS was most pronounced in the epoch beginning 256 ms after the button-press, accounting for the accuracy × epoch interaction; and ERAS appeared earlier in the ITI at parietal than frontal or central sites, accounting for the 3-way interaction.
Figure 1.
Alpha power across the intertrial interval for correct and error trials. Data are separated into 256-ms epochs beginning at the time of the button-press (Time 0).
The main effect of trial type, F(2, 104) = 4.2, p < .02, also replicated an earlier finding (Compton et al., 2011a); alpha power was lower following incongruent (M = 1.49 μV2, SEM = .04) than neutral trials (M = 1.51 μV2, SEM = .04; Bonferroni post hoc, p < .02); alpha power on emotion trials (M = 1.50 μV2, SEM = .04) was intermediate and did not significantly differ from other two types (ps > .12). Trial type did not interact with any other factors in the ANOVA.
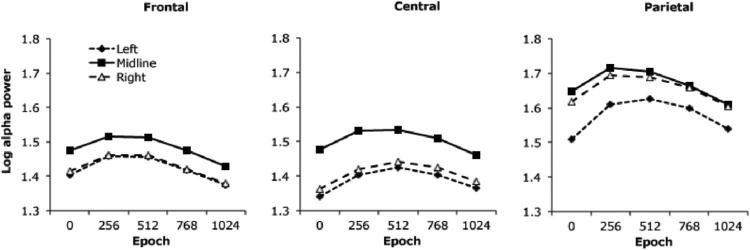
Effects of laterality on alpha power also replicated earlier findings that contrasted left and right hemisphere sites (Compton et al., 2011a), with the addition in this dataset of midline sites. The main effect of laterality, F(2, 104) = 72.5, p < .001, reflected lowest alpha power at left-hemisphere sites (M = 1.46 μV2, SEM = .04), followed by right-hemisphere sites (M = 1.50 μV2, SEM = .04) and then midline sites (M = 1.55 μV2, SEM = .04; all pairwise comparisons significant, Bonferroni-corrected post hoc, ps < .001). This overall laterality effect was qualified by several interactions involving anterior–posterior site and epoch, laterality × site, F(4, 208) = 12.0, p < .001; laterality × epoch, F(8, 416) = 8.6, p < .001; laterality × epoch × site, F(16, 832) = 4.4, p = .001. Means for the three-way interaction are presented in Figure 2. Frontal and central sites were characterized by higher midline than lateral alpha but no left–right asymmetry, whereas an asymmetry emerged at parietal sites, with lower left than right hemisphere alpha power. The asymmetry at the parietal sites was more evident early in the ITI, contributing to the interactions involving epoch.
Figure 2.
Alpha power across the intertrial interval for left-hemisphere, midline, and right-hemisphere sites (collapsed across error and correct trials). Data are separated into 256-ms epochs beginning at the time of the button-press (Time 0).
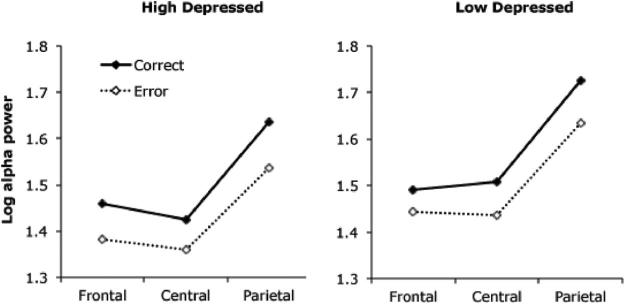
Finally, and most relevant to the present aims, alpha power was influenced by the interaction of accuracy, site, and depression group, F(2, 104) = 3.5, p < .05, partial eta-squared = 0.063. Means for the interaction are presented in Figure 3. Although the accuracy × site interaction was significant for both groups, it was more pronounced in the LD group (LD: accuracy × site, F(2, 64) = 14.1, p < .001; HD: F(2, 40) = 4.1, p < .05), leading to the 3-way effect. For the LD group, ERAS was twice as great at parietal versus frontal sites (frontal ERAS, M = 0.047; parietal ERAS, M = 0.092), whereas for the HD group, frontal ERAS (M = 0.077) was closer to that of parietal ERAS (M = 0.098). The group difference appeared to be driven primarily by the pattern at the frontal sites (increased ERAS for HD vs. LD group), although the accuracy × group interaction did not reach significance for any of the sites considered individually (frontal sites, F(1, 52) = 1.90, p = .17; central and parietal sites, Fs < 1). In sum, in the LD group, the effect of errors on alpha power was more pronounced in parietal versus frontal sites, whereas in the HD group, the effect was more distributed across the sites.
Figure 3.
Alpha power for correct and error trials, separated by depression group and anterior–posterior electrode location.
Because these analyses indicated group differences in the ERAS variable, we sought to confirm whether the group differences were more closely related to individual differences in anxiety or depression, both of which differed between the low- and high-depressed groups at the time of the lab session (see Table 1). To do so, we repeated the ANOVA on alpha power but included a covariate that was either the PSWQ, MASQ-AA, or MASQ-AD score, and examined whether the accuracy × site effect interacted with the covariate. These analyses found a three-way accuracy × site × MASQ-AD interaction, F(2, 104) = 4.3, p < .05, partial eta-squared = 0.076, but no accuracy × site × MASQ-AA interaction (F < 1) nor accuracy × site × PSWQ interaction, F(2, 104) = 1.2, p = .30. These analyses indicate that the pattern of ERAS across the scalp, described in the prior paragraph, appear to be specifically related to anhedonic depression rather than associated anxiety variables.
Correlations Between ERAS and Self-Report Data
The final set of analyses addressed the extent to which ERAS predicted affect and coping measures. According to predictions, participants with greater ERAS, that is greater arousal responses to performance errors, should report poorer affect and coping on the self-report measures. Because of the multidimensionality of the self-report variables (13 measures) and the alpha power data (two accuracy levels, three trial types, five epochs, and nine electrode sites), we first reduced both types of data to a manageable number of variables. This strategy reduces the problem of excessive Type I error probability that would result from computing all possible zero-order correlations.
The self-report variables were entered into a factor analysis using principal component extraction and varimax rotation. Table 2 displays the initial and rotated factor loadings for the first three components, which together account for 73% of the variance. Factor 1 (rotated) appears to index daily negative emotional reactivity, with heavy loadings from the daily DSI-ratio (reactivity) score, overall stress score, NA, emotion-focused coping, and avoidance-focused coping. Factor 2 appears to represent trait negative affect, with heavy loadings from the one-time measures: CESD, PSWQ, MASQ-AD, and MASQ-AA. Factor 3 appears to reflect daily positive emotion and adaptive coping, with heavy loadings from daily PA, task-focused coping, and approach-focused coping.
Table 2.
Factor Loadings for Self-Report Data
| Initial loading |
Varimax rotation |
|||||
|---|---|---|---|---|---|---|
| Variable | Factor 1 | Factor 2 | Factor 3 | Factor 1 | Factor 2 | Factor 3 |
| CESD | .74 | –.13 | .43 | .23 | .82 | –.19 |
| PSWQ | .71 | –.15 | .27 | .30 | .67 | –.24 |
| MASQ-AA | .58 | .12 | .43 | .21 | .70 | .09 |
| MASQ-AD | .66 | –.34 | .39 | .12 | .74 | –.37 |
| DSI-event | .41 | .69 | .31 | .37 | .47 | .62 |
| DSI-ratio | .75 | –.11 | –.45 | .76 | .15 | –.42 |
| Overall stress | .81 | .18 | –.39 | .88 | .22 | –.15 |
| PA | –.49 | .66 | .10 | –.16 | –.27 | .76 |
| NA | .84 | .35 | –.05 | .77 | .49 | .10 |
| Task coping | –.24 | .86 | .22 | .03 | –.02 | .91 |
| Emotion coping | .78 | .33 | –.17 | .78 | .37 | .06 |
| Avoidance coping | .58 | .50 | –.38 | .83 | .06 | .21 |
| Approach coping | –.42 | .77 | –.02 | –.01 | –.32 | .81 |
| Eigenvalue | 5.32 | 2.92 | 1.28 | 3.59 | 2.99 | 2.95 |
| % variance | 40.95 | 22.45 | 9.87 | 27.62 | 22.99 | 22.66 |
Note: Bolded and italicized values indicate rotated factor loadings of .70 or greater.
Log alpha power data were subjected to several steps of data reduction to focus on the variables of interest. First, we focused only on data from the 256-, 512-, and 768-ms epochs, because these are the epochs in which the ERAS effect is most evident, whereas the 0-ms epoch includes time-points before ERAS has begun and the 1,024-ms epoch represents a time period when the effect is trailing off. Within these epochs, we subtracted log alpha power for error trials from log alpha power for correct trials to yield values that represent the magnitude of ERAS for each type × epoch × site condition. We averaged these values across the three trial types (incongruent, emotion, neutral), because there was no indication of depression-related effects that involved trial type in the behavioral data or factorial analysis of alpha power data. We also averaged across laterality (left, center, right hemisphere electrode site) within each region, because the prior analyses indicated no depression-related laterality effects. However, we continued to separate data by anterior–posterior location (frontal, central, parietal), because the ANOVA on the alpha power data indicated a depression-related difference in ERAS scalp distribution in the anterior–posterior dimension. These steps yielded a set of nine variables: ERAS for frontal, central, and parietal sites at each of the 256-, 512-, and 768-ms epochs.
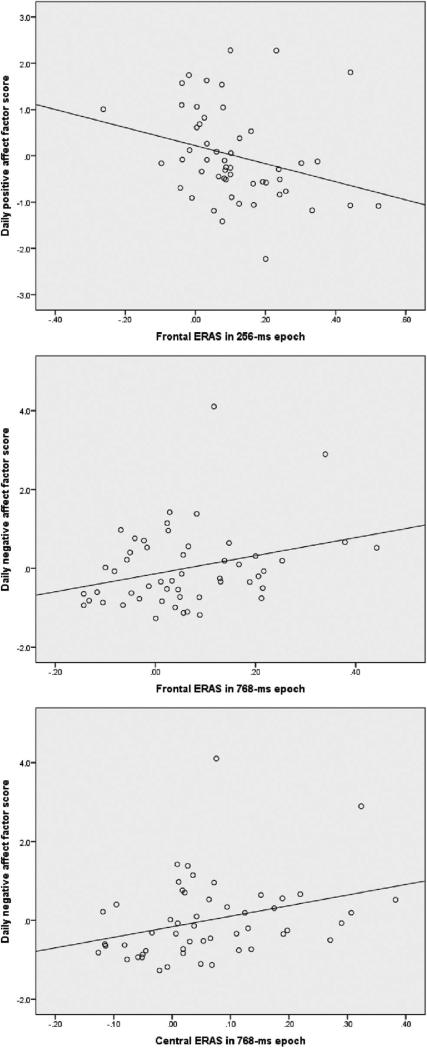
To address the main research question, the factor scores from the self-report measures were correlated with these ERAS measures. Table 3 presents the matrix of correlations. Notably, frontal ERAS in the 256-ms epoch was negatively correlated with the self-report factor representing positive affect. That is, participants with greater ERAS early in the ITI tended to have lower scores on the composite positive affect measure. Second, frontal and central ERAS in the 768-ms epoch were correlated with the daily negative affect factor. In other words, participants with greater ERAS later in the epoch tended to report greater daily negative emotional reactivity. The self-report factor representing trait negative affect was not significantly correlated with any of the three ERAS variables.
Table 3.
Correlations Between Error-Related Alpha Suppression (ERAS) and Self-Report Factors
| Self-report factors |
|||
|---|---|---|---|
| ERAS (Region, Epoch) | Factor 1: Daily negative affect | Factor 2: Trait negative affect | Factor 3: Daily positive affect |
| Frontal, 256-ms | –0.13 | 0.08 | –0.28* |
| Central, 256-ms | –0.10 | 0.04 | –0.18 |
| Parietal, 256-ms | 0.00 | 0.02 | –0.10 |
| Frontal, 512-ms | 0.10 | 0.03 | –0.03 |
| Central, 512-ms | 0.05 | –0.03 | 0.04 |
| Parietal, 512-ms | –0.03 | 0.11 | –0.10 |
| Frontal, 768-ms | 0.30* | –0.04 | –0.10 |
| Central, 768-ms | 0.32* | –0.07 | 0.07 |
| Parietal, 768-ms | 0.09 | 0.03 | 0.05 |
p < .05.
Figure 4 presents scatterplots illustrating the three significant relationships between the ERAS and self-report data. Note that in the scatterplots pertaining to the negative affect variable (Figure 4 middle and bottom panels), there appears to be a single outlying point with a factor score of more than 4.0 on the negative affect factor. Analyses excluding this participant found that the relationships remained significant (correlation between negative affect and frontal ERAS at 768-ms, r = .33, p < .05; parietal ERAS at 768-ms, r = .38, p < .01).
Figure 4.
Scatterplots depicting the significant relationships among self-report factors and error-related alpha suppression (ERAS).
ERN
Although the main focus of the study was on the relationship between ERAS and affective and coping variables, we also examined the ERN for comparison because it has been more extensively studied in relationship to depression and related mood variables. The ERN was defined as the most negative peak between 0 and 100 ms following a button-press in time-locked event-related potential waveforms. Peak amplitude data were submitted to a mixed ANOVA with accuracy (correct, error), site (Fz, FCz, Cz, Pz), trial type (incongruent, emotion, neutral), and group (LD, HD) as factors. Analyses focused on the four midline sites because those sites are the ones known to exhibit the maximal ERN.
The ANOVA revealed expected effects of trial accuracy on the ERN, but no significant effects involving depression group. The main effect of accuracy, F(1, 58) = 104.9, p < .001, was due to more negative amplitudes on error trials (M = −8.40 μV, SEM = 0.77) compared with correct trials (M = −1.51 μV, SEM = 0.35), the expected ERN effect. Means for the main effect of site, F(3, 174) = 14.6, p < .001, and the accuracy × site interaction, F(3, 174) = 16.2, p < .001, are presented in Table 4. The means reflect greatest error–correct differentiation at the FCz site, as is commonly found (Gehring et al., 2012). Finally, an unanticipated trial type × site interaction, F(6, 348) = 4.51, p < .005, was due to greater negativity at anterior (but not posterior) sites for incongruent and emotion compared with neutral trials (see means in Table 5).
Table 4.
Mean (SEM) ERN Peak Amplitudes as a Function of Trial Accuracy and Site
| Trial accuracy |
||||
|---|---|---|---|---|
| Correct | Error | Difference | Average | |
| Fz | –1.02 (0.37) | –8.00 (0.81) | 6.98 | –4.51 |
| FCz | –0.83 (0.41) | –8.67 (0.79) | 7.84 | –4.75 |
| Cz | –0.62 (0.41) | –7.91 (0.80) | 7.29 | –4.27 |
| Pz | –3.57 (0.49) | –9.00 (0.82) | 5.43 | –6.29 |
Table 5.
Mean (SEM) ERN Peak Amplitudes as a Function of Trial Type and Site
| Trial type |
|||
|---|---|---|---|
| Incongruent | Emotion | Neutral | |
| Fz | –4.66 (0.58) | –4.76 (0.56) | –4.11 (0.60) |
| FCz | –4.94 (0.56) | –5.13 (0.61) | –4.19 (0.54) |
| Cz | –4.50 (0.57) | –4.61 (0.59) | –3.69 (0.52) |
| Pz | –6.58 (0.67) | –5.86 (0.56) | –6.41 (0.60) |
Correlations between ERN amplitude (correct-trial peak minus error-trial peak at the FCz site) and the three self-report factors yielded no significant effects. Individual differences in the ERN were also uncorrelated with the ERAS variables, consistent with prior evidence that these two error-related phenomena are relatively independent (Carp & Compton, 2009).
Discussion
The present study is the first to link ERAS to individual differences in self-reported affect and coping. Participants who were preselected for high levels of depression differed from those low in depression in the distribution of ERAS across the scalp, with relatively greater frontal versus parietal ERAS in the HD group. In addition, individual differences in ERAS predicted individual differences in daily reports of affect and coping. The general pattern was an association between frontal ERAS and less optimal daily report outcomes, as ERAS predicted lower scores on a factor reflecting positive affect and adaptive coping strategies as well as predicting higher scores on a factor reflecting daily negative affect and stress reactivity. Generally, the data support an association between arousal responses to performance errors and suboptimal mood and coping.
These findings, together with the null effects involving the ERN, suggest that further study of ERAS may help to develop understanding of the relationship between cognitive control deficits and affective deficits in depression and related conditions. Specifically, when addressing possible error-related neural effects in depression, it may be fruitful to consider the more sustained arousal response that follows an error, that is the ERAS effect, rather than only considering the brief signal marking the initial detection of an error, that is the ERN. For example, it is possible that depressed and nondepressed participants are both efficient at detecting the presence of an error (e.g., Dunn, Dalgleish, Lawrence, & Ogilvie, 2007) but that they differ in the engagement of subsequent processes such as arousal or engagement of corrective action.
In addition, by linking ERAS with suboptimal outcomes—that is, decreased positive affect, increased negative affect, and less beneficial coping—the present data fit with prior findings suggesting that ERAS may reflect a maladaptive arousal response. In prior studies we found that individuals with greater ERAS tended to have both slower posterror response times (Carp & Compton, 2009) and increased cortisol reactivity (Compton et al., 2013). Together, the findings implicate ERAS as an error-related neural process that operates outside of, and perhaps even counter to, an adaptive control system. Future research would benefit from more thorough investigations of the functional meaning of ERAS and how it differs from processes tapped by the more commonly studied ERN.
Although the results generally support an association between ERAS and suboptimal affect and coping, several limitations of the study should be acknowledged. First, the results from the factorial ANOVA of alpha power indicate not a greater ERAS overall in HD compared with LD groups, but rather a different distribution of the ERAS across the scalp between the two groups. Although the direction of the means supports the conclusion that the interaction was driven by relatively greater ERAS across frontal sites in the HD group, compared with the LD group, the statistical results from the relevant post hoc tests on the decomposed interaction preclude a strong conclusion in that regard, as groups did not differ significantly at any individual site considered separately. However, the patterns in the data from both the factorial and correlational analyses suggest that a shift toward more frontal ERAS may be associated with suboptimal mood and coping variables. We did not specifically predict a frontal shift in ERAS associated with depression, and precise neural sources are ambiguous in scalp-recorded EEG data. Nevertheless, because frontal regions have been implicated in both affect regulation and cognitive control (e.g., Pizzagalli, 2011), it will be intriguing for future research to further address the functional meaning of a frontal shift in ERAS.
An additional limitation is that the significant correlations between ERAS and self-report variables emerged only for certain windows within the ITI, such that ERAS earlier in the ITI was more strongly associated with reduced positive affect and ERAS later in the ITI was more strongly associated with increased negative affect. Because there is no a priori reason to predict that different aspects of emotion would be associated with ERAS at different time-points in the ITI, it is probably most prudent to withhold speculation about the epoch-related aspect of the findings pending replication or other further evidence.
Relatedly, in the correlational analyses, ERAS effects were significantly associated with the daily self-report variables (both negative and positive) but were not associated with individual differences in the one-time measures, which function more as trait measures (CESD, MASQ-AD, MASQ-AA, and PSWQ). This pattern emerged even though most of the one-time measures (except the CESD) were collected at the same session as the cognitive task during which ERAS was measured, and the daily report variables were collected over a period of 2 weeks subsequent to that session. This aspect of the data suggests that the daily report variables may have more sensitivity, perhaps due to their greater ecological validity and reduced retrospective biases, compared with the onetime measures (Bolger, Davis, & Rafaeli, 2003).
Although the LD and HD groups were preselected to differ in depression, as indexed by the CESD and confirmed by the MASQAD, they also differed significantly in measures of anxiety (MASQ-AA and PSWQ). This pattern is not surprising, given the high degree of comorbidity between depression and anxiety disorders (McGlinchey & Zimmerman, 2007; Watson, 2005). Although some research groups have been successful in isolating effects of depression versus anxiety on neural functioning (e.g., Heller, Schmidtke, Nitschke, Koven, & Miller, 2002; Weinberg et al., 2012), the present research design did not permit such isolation because of the high level of shared variance between depression and anxiety measures. Indeed, the factor analysis was not able to separate anxiety and depression measures, although it did separate negative and positive affect factors in the daily report data. Therefore, any conclusion about the HD versus LD groups must be seen not as a conclusion about “pure” depression, but about depression as it typically occurs, that is confounded with anxiety.
A final aspect of the results that merits discussion is the relative absence of effects related to the Stroop word type (incongruent, emotion, or neutral). The task produced strong Stroop interference effects, namely slower and less accurate responding on incongruent trials compared with the other two trial types, as well as greater arousal (less alpha power) on incongruent versus neutral trials. However, neither behavioral nor neural responses to the emotion words were especially linked to self-report variables, counter to what might be expected if depressed and anxious individuals allocate more attention to emotional information or to errors made in the context of failure-related emotional cues (e.g., Mineka, Rafaeli, & Yovel, 2003; see also Compton et al., 2011b; Compton et al., 2008). Although the reason for this null effect is unclear, one possibility is that in the present study, the task intermixed emotion, neutral, and incongruent words within the same block rather than grouping the word types in separate blocks. It may be that emotion-word conditions elicit specific depression-related effects only when emotion trials dominate a block, thus, yielding a more tonic emotional or emotion-driven attentional state. Regardless of the explanation in relation to prior findings, the present findings do not support the notion that responses to errors in a momentary emotion context are particularly predictive of depression or other affective characteristics.
Despite these limitations, the present results contribute to growing understanding of the relationship between cognitive and affective characteristics of depression and anxiety. In a sample that included a wide range of self-reported scores on standard depression measures, momentary neural reactions to errors in the lab session predicted both positive and negative affect and coping over the subsequent 2 weeks. In a general sense, these results support the close relationship between cognitive and affective self-regulation, as those with greater arousal responses to errors reported worse affect and less adaptive coping with stressors during the daily report period. Future research should aim to better characterize the neural and cognitive characteristics of alpha suppression following errors so that its relationship with depression can be more fully understood.
Acknowledgments
This research was supported by NIH Grant 1R15MH085 182 to Rebecca J. Compton.
References
- Beats BC, Sahakian BJ, Levy R. Cognitive performance in tests sensitive to frontal lobe dysfunction in the elderly depressed. Psychological Medicine. 1996;26:591–603. doi: 10.1017/s0033291700035662. doi:10.1017/S0033291700035662. [DOI] [PubMed] [Google Scholar]
- Bolger N, Davis A, Rafaeli E. Diary methods: Capturing life as it is lived. Annual Review of Psychology. 2003;54:579–616. doi: 10.1146/annurev.psych.54.101601.145030. doi:10.1146/ annurev.psych.54.101601.145030. [DOI] [PubMed] [Google Scholar]
- Brantley PJ, Jones GN. Daily stress inventory: Professional manual. Psychological Assessment Resources, Inc.; Odessa, FL: 1989. [Google Scholar]
- Carp J, Compton RJ. Alpha power is influenced by performance errors. Psychophysiology. 2009;46:336–343. doi: 10.1111/j.1469-8986.2008.00773.x. doi:10.1111/j.1469-8986.2008.00773.x. [DOI] [PubMed] [Google Scholar]
- Chiu PH, Deldin PJ. Neural evidence for enhanced error detection in major depressive disorder. The American Journal of Psychiatry. 2007;164:608–616. doi: 10.1176/ajp.2007.164.4.608. doi:10.1176/appi.ajp.164.4.608. [DOI] [PubMed] [Google Scholar]
- Compton RJ, Arnstein D, Freedman G, Dainer-Best J, Liss A. Cognitive control in the inter-trial interval: Evidence from EEG alpha power. Psychophysiology. 2011a;48:583–590. doi: 10.1111/j.1469-8986.2010.01124.x. doi:10.1111/j.1469-8986.2010.01124.x. [DOI] [PubMed] [Google Scholar]
- Compton RJ, Arnstein D, Freedman G, Dainer-Best J, Liss A, Robinson MD. Neural and behavioral measures of error-related cognitive control predict daily coping with stress. Emotion. 2011b;11:379–390. doi: 10.1037/a0021776. doi:10.1037/a0021776. [DOI] [PubMed] [Google Scholar]
- Compton RJ, Hofheimer J, Kazinka R. Stress regulation and cognitive control: Evidence relating cortisol reactivity and neural responses to errors. Cognitive, Affective, & Behavioral Neuroscience. 2013;13:152–163. doi: 10.3758/s13415-012-0126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton RJ, Huber E, Levinson AR, Zheutlin A. Is “conflict adaptation” driven by conflict? Behavioral and EEG evidence for the underappreciated role of congruent trials. Psychophysiology. 2012;49:583–589. doi: 10.1111/j.1469-8986.2012.01354.x. doi:10.1111/j.1469-8986.2012.01354.x. [DOI] [PubMed] [Google Scholar]
- Compton RJ, Lin M, Vargas G, Carp J, Fineman S, Quandt LC. Error detection and post-error behavior in depressed undergraduates. Emotion. 2008;8:58–67. doi: 10.1037/1528-3542.8.1.58. doi:10.1037/1528-3542.8.1.58. [DOI] [PubMed] [Google Scholar]
- Compton RJ, Robinson MD, Ode S, Quandt LC, Fineman SL, Carp J. Error-monitoring ability predicts daily stress regulation. Psychological Science. 2008;19:702–708. doi: 10.1111/j.1467-9280.2008.02145.x. doi:10.1111/j.1467-9280.2008.02145.x. [DOI] [PubMed] [Google Scholar]
- Dunn BD, Dalgleish T, Lawrence AD, Ogilvie AD. The accuracy of self-monitoring and its relationship to self-focused attention in dysphoria and clinical depression. Journal of Abnormal Psychology. 2007;116:1–15. doi: 10.1037/0021-843X.116.1.1. doi:10.1037/0021-843X.116.1.1. [DOI] [PubMed] [Google Scholar]
- Elliott R, Sahakian BJ, McKay AP, Herrod JJ. Neuro-psychological impairments in unipolar depression: The influence of perceived failure on subsequent performance. Psychological Medicine. 1996;26:975–989. doi: 10.1017/s0033291700035303. doi:10.1017/S0033291700035303. [DOI] [PubMed] [Google Scholar]
- Garnefski N, Kraaij V. Relationships between cognitive emotion regulation strategies and depressive symptoms: A comparative study of five specific samples. Personality and Individual Differences. 2006;40:1659–1669. doi:10.1016/j.paid.2005.12.009. [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychological Science. 1993;4:385–390. doi:10.1111/j.1467-9280.1993.tb00586.x. [Google Scholar]
- Gehring WJ, Liu Y, Orr JM, Carp J. The error-related negativity (ERN/Ne). In: Luck SJ, Kappenman ES, editors. The Oxford handbook of event-related potentials. Oxford; New York, NY: 2012. pp. 231–291. [Google Scholar]
- Georgiadi E, Liotti M, Nixon NL, Liddle PF. Electro-physiological evidence for abnormal error monitoring in recurrent major depressive disorder. Psychophysiology. 2011;48:1192–1202. doi: 10.1111/j.1469-8986.2011.01198.x. doi:10.1111/j .1469-8986.2011.01198.x. [DOI] [PubMed] [Google Scholar]
- Heller W, Schmidtke JI, Nitschke JB, Koven NS, Miller GA. States, traits, and symptoms: Investigating the neural correlates of emotion, personality, and psychopathology. In: Cervone D, Mischel W, editors. Advances in personality science. Guilford Press; New York, NY: 2002. pp. 106–126. [Google Scholar]
- Holmes AJ, Pizzagalli DA. Task feedback effects on conflict monitoring and executive control: Relationship to subclinical measures of depression. Emotion. 2007;7:68–76. doi: 10.1037/1528-3542.7.1.68. doi:10.1037/1528-3542.7.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes AJ, Pizzagalli DA. Effects of task-relevant incentives on the electrophysiological correlates of error processing in major depressive disorder. Cognitive, Affective, & Behavioral Neuroscience. 2010;10:119–128. doi: 10.3758/CABN.10.1.119. doi:10.3758/CABN.10.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladouceur CD, Slifka JS, Dahl RE, Birmaher B, Axelson DA, Ryan ND. Altered error-related brain activity in youth with major depression. Developmental Cognitive Neuroscience. 2012;2:351–362. doi: 10.1016/j.dcn.2012.01.005. doi:10.1016/j.dcn.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RC, Dahlen ER. Cognitive emotion regulation in the prediction of depression, anxiety, stress, and anger. Personality and Individual Differences. 2005;39:1249–1260. doi:10.1016/j.paid.2005.06.004. [Google Scholar]
- McGlinchey JB, Zimmerman M. Examining a dimensional representation of depression and anxiety disorders’ comorbidity in psychiatric outpatients with item response modeling. Journal of Abnormal Psychology. 2007;116:464–474. doi: 10.1037/0021-843X.116.3.464. doi:10.1037/0021-843X.116.3.464. [DOI] [PubMed] [Google Scholar]
- Meyer TJ, Miller ML, Metzger RL, Borkovec TD. Development and validation of the Penn State Worry Questionnaire. Behaviour Research and Therapy. 1990;28:487–495. doi: 10.1016/0005-7967(90)90135-6. doi:10.1016/0005-7967(90)90135-6. [DOI] [PubMed] [Google Scholar]
- Mineka S, Rafaeli E, Yovel I. Cognitive biases in emotional disorders: Information processing and social-cognitive perspectives. In: Davidson RJ, Scherer KR, Goldsmith HH, editors. Handbook of affective sciences. Oxford University Press; Oxford, UK: 2003. pp. 976–1009. [Google Scholar]
- Murphy FC, Robbins MTW, Sahakian BJ. Neuropsychological impairment in patients with major depressed disorder: The effects of feedback on task performance. Psychological Medicine. 2003;33:455–467. doi: 10.1017/s0033291702007018. doi:10.1017/S0033291702007018. [DOI] [PubMed] [Google Scholar]
- Myin-Germeys I, Peeters F, Havermans R, Nicolson NA, deVries MW, Delespaul P, van Os J. Emotional reactivity to daily life stress in psychosis and affective disorder: An experience sampling study. Acta Psychiatrica Scandinavica. 2003;107:124–131. doi: 10.1034/j.1600-0447.2003.02025.x. doi:10.1034/j.1600-0447.2003.02025.x. [DOI] [PubMed] [Google Scholar]
- Peeters F, Nicolson NA, Berkhof J, Delespaul P, deVries M. Effects of daily events on mood states in major depressive disorder. Journal of Abnormal Psychology. 2003;112:203–211. doi: 10.1037/0021-843x.112.2.203. doi:10.1037/ 0021-843X.112.2.203. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA. Frontocingulate biomarkers of depression: Toward biomarkers of treatment response. Neuropsychopharmacology. 2011;36:183–206. doi: 10.1038/npp.2010.166. doi:10.1038/npp.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. doi:10.1177/014662167700100306. [Google Scholar]
- Ruchsow M, Herrnberger B, Beschoner P, Grön G, Spitzer M, Kiefer M. Error processing in major depressed disorder: Evidence from event-related potentials. Journal of Psychiatric Research. 2006;40:37–46. doi: 10.1016/j.jpsychires.2005.02.002. doi:10.1016/j.jpsychires.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Ruchsow M, Herrnberger B, Wiesend C, Grön G, Spitzer M, Kiefer M. The effect of erroneous responses on response monitoring in patients with major depressed disorder: A study with event-related potentials. Psychophysiology. 2004;41:833–840. doi: 10.1111/j.1469-8986.2004.00237.x. doi:10.1111/j.1469-8986.2004.00237.x. [DOI] [PubMed] [Google Scholar]
- Schneiders J, Nicolson NA, Berkhof J, Feron FJ, van Os J, deVries MW. Mood reactivity to daily negative events in early adolescence: Relationship to risk for psychopathology. Developmental Psychology. 2006;42:543–554. doi: 10.1037/0012-1649.42.3.543. doi:10.1037/0012-1649.42.3.543. [DOI] [PubMed] [Google Scholar]
- Simons RF. The way of our errors: Theme and variations. Psychophysiology. 2010;47:1–14. doi: 10.1111/j.1469-8986.2009.00929.x. doi:10.1111/j.1469-8986.2009.00929.x. [DOI] [PubMed] [Google Scholar]
- Todd M, Tennen H, Carney MA, Armeli S, Affleck G. Do we know how we cope? Relating daily coping reports to global and time-limited retrospective assessments. Journal of Personality and Social Psychology. 2004;86:310–319. doi: 10.1037/0022-3514.86.2.310. doi:10.1037/0022-3514.86.2.310. [DOI] [PubMed] [Google Scholar]
- Tucker DM, Luu P, Frishkoff G, Quiring J, Poulsen C. Frontolimbic response to negative feedback in clinical depression. Journal of Abnormal Psychology. 2003;112:667–678. doi: 10.1037/0021-843X.112.4.667. doi:10.1037/0021-843X.112.4.667. [DOI] [PubMed] [Google Scholar]
- Watson D. Rethinking the mood and anxiety disorders: A quantitative hierarchical model for DSM-V. Journal of Abnormal Psychology. 2005;114:522–536. doi: 10.1037/0021-843X.114.4.522. doi:10.1037/0021-843X.114.4.522. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. doi:10.1037/0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Weber K, Assenheimer JS, Strauss ME, McCormick RA. Testing a tripartite model: II. Exploring the symptom structure of anxiety and depression in student, adult, and patient samples. Journal of Abnormal Psychology. 1995a;104:15–25. doi: 10.1037//0021-843x.104.1.15. doi: 10.1037/0021-843X.104.1.15. [DOI] [PubMed] [Google Scholar]
- Watson D, Weber K, Assenheimer JS, Clark LA, Strauss ME, McCormick RA. Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. Journal of Abnormal Psychology. 1995b;104:3–14. doi: 10.1037//0021-843x.104.1.3. doi:10.1037/0021-843X.104.1.3. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Klein DN, Hajcak G. Increased error-related brain activity distinguishes generalized anxiety disorder with and without comorbid major depressive disorder. Journal of Abnormal Psychology. 2012 doi: 10.1037/a0028270. Advanced online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]