Abstract
We have investigated several in silico and in vitro methods in order to improve our ability to predict potential drug interactions of antibiotics. Our focus was to identify those antibiotics that activate PXR and induce CYP3A4 in human hepatocytes and intestinal cells. Human PXR activation was screened using reporter assays in HepG2 cells, kinetic measurements of PXR activation were made in DPX-2 cells, and induction of CYP3A4 expression and activity was verified by quantitative PCR, immunoblotting and testosterone 6β-hydroxylation in primary human hepatocytes and LS180 cells. We found that in HepG2 cells CYP3A4 transcription was activated strongly (>10-fold) by rifampin and troleandomycin; moderately (> 7-fold) by dicloxacillin, tetracycline, clindamycin, griseofulvin and (> 4-fold) by erythromycin; weakly (>2.4-fold) by nafcillin, cefaclor and sulfisoxazole; and (>2-fold) by cefadroxil and penicillin V. Similar though not identical results were obtained in DPX-2 cells. CYP3A4 mRNA and protein expression were induced by these antibiotics to differing extents in both liver and intestinal cells. CYP3A4 activity was significantly increased by rifampin (9.7-fold), nafcillin and dicloxacillin (5.9-fold), and weakly induced (2-fold) by tetracycline, sufisoxazole, troleandomycin and clindamycin. Multiple pharmacophore models and docking indicated a good fit for dicloxacillin and nafcillin in PXR. These results suggest that in vitro and in silico methods can help to prioritize and identify antibiotics that are most likely to reduce exposures of medications (such as oral contraceptive agents) which interact with enzymes and transporters regulated by PXR. In summary, nafcillin, dicloxacillin, cephradine, tetracycline, sulfixoxazole, erythromycin, clindamycin, and griseofulvin exhibit a clear propensity to induce CYP3A4 and warrant further clinical investigation.
Introduction
The cytochromes P450 (CYPs) are responsible for the metabolism of over 90% of existing pharmaceuticals including over 50% of therapeutically important drugs (Williams et al., 2004; de Groot, 2006). Clinically relevant adverse drug reactions occur due to consumption of multiple xenobiotics that interact with CYP3A4 as substrates, inhibitors or inducers (Yao and Levy, 2002; Marechal et al., 2006; Harmsen et al., 2007). CYP3A4 inducers can cause autoinduction of clearance, or enhance clearance and decrease therapeutic efficacy of coadministered medications. The nuclear hormone receptor PXR/SXR, NR1l2 (pregnane X receptor/steroid and xenobiotic receptor), mediates transcriptional induction of CYP3A4 by many xenobiotics and endobiotics (Bertilsson et al., 1998; Blumberg et al., 1998; Kliewer et al., 1998). Ligand activators of PXR include a growing list of structurally and pharmacologically diverse endobiotics (e.g., pregnanes, glucocorticoids, some bile acids), vitamins E and K2, and xenobiotics including drugs (e.g., rifampin and protease inhibitors) and environmental contaminants (e.g., organochlorine pesticides and polychlorinated biphenyls) (Ekins et al., 2007). Upon ligand binding, hPXR binds its cognate response elements in the 5’-flanking region of the CYP3A4 gene to activate its transcription.
Activation of PXR is recognized as the molecular mechanism responsible for many drug interactions (Sinz et al., 2006). Accordingly, this led to the development of high throughput cell based assays to test whether xenobiotics would induce CYP3A4 promoter-Iuciferase reporter plasmids in a PXR-dependent fashion (Moore and Kliewer, 2000). However, this powerful assay only screens for whether a test compound can ligand-activate PXR and induce CYP3A4 transcription in the context of the transfected host cell. It does not reveal whether activation of CYP3A4 transcription ultimately results in induction of CYP3A4 mRNA and protein in hepatocytes, nor whether CYP3A4 activity is also increased, since some inducers are also CYP3A4 inhibitors (Piscitelli and Gallicano, 2001). It should also be noted that there are species differences in CYP3A induction that could be due to differences in PXR (LeCluyse, 2001). The gold standard screening assay for induction of hepatic CYP3A4 protein and activity remains primary cultures of human hepatocytes (Luo et al., 2002). Since induction of CYP3A4 in the intestine contributes to some drug interactions, and since induction of PXR targets can be ligand, promoter and tissue specific (Koch et al., 2002) it is important to test for CYP3A4 induction potential in intestine with human intestinal cell lines, such LS180s.
A variety of computational models ranging from pharmacophores (Ekins and Erickson, 2002; Schuster and Langer, 2005), quantitative structure activity relationships (QSAR) and ligand docking into a PXR crystal structure (Gao et al., 2007; Lemaire et al., 2007) have all been used to predict PXR ligand binding. These computational methods generally focused on diverse structures for agonists, rarely using close structural analogs (Ekins et al., 2007). Accurate predictions can be difficult due to the size and flexibility of the human PXR ligand binding domain, however the combination of models for searching molecule databases represents a rapid way to prescreen molecules prior to in vitro testing as demonstrated previously using pharmacophores for other proteins (Ekins et al., 2005a; Chang et al., 2006) as originally suggested with the first human PXR pharmacophore (Ekins and Erickson, 2002).
The prototypical human PXR ligand, rifampin, is a potent activator of human PXR (Moore et al., 2000), and causes numerous drug-interactions (Finch et al., 2002). Surprisingly, the majority of antibiotics have not been tested as potential PXR ligands despite the fact that: 1) antibiotics have a long history of use (Bud, 2007), 2) they represent some of the most widely prescribed medications for treating infections (Cizman, 2003; Eng et al., 2003), 3) are frequently taken with other medications (Pai et al., 2006), and 4) there is anecdotal data that some antibiotics can increase metabolism and decrease efficacy of coadministered medications, (particularly oral contraceptives (OCs) (Weaver and Glasier, 1999). For example, nafcillin significantly lowered the levels of the CYP3A4 substrate nifedipine (Lang et al., 2003). Flucloxacillin in combination with cyclosporine caused rejection in three of the seven kidney transplant patients (Cynke et al., 1999). There are also reports that women taking OCs who begin antibiotic regimens are more likely to experience contraception failure and breakthrough bleeding (Weaver and Glasier, 1999). However, there is clearly confusion in advising patients which antibiotics are likely to cause these drug interactions and there is a need for some evidence-based guidelines (DeRossi and Hersh, 2002). Therefore, we designed a study whose aim was to improve our ability to predict potential antibiotic interactions by identifying with in silico and in vitro methods those antibiotics that activate PXR and induce CYP3A4 in human hepatocytes and intestinal cells.
Material and Methods
Reagents
Rifampin, penicillins (PNCV, Penicillin V; NFC, nafcillin; DXC, dicloxacillin; AXC, amoxicillin; APC, ampicillin), cephems (CFDX, cefadroxil; CPRD, cephradine; CPLX, cephalexin; CFCL, cefaclor; CFXM, cefuroxime), tetracyclines (TCL, tetracycline; DXCL, toxycycline; MCL, minocycline; DCCL, demeclocycline), sulfonamides (SXZ, sufisoxazole; SMXZ, sulfamethoxazole), macrolides (ERM, erythromycin; TAO, troleandomycin) and others (GSF, griseofulvin; CMC, clindamycin) (Supplemental Table 1) were all purchased from Sigma Chemical Co. (St. Louis, MO) and were the highest purity available. Rifampin was used in a concentration of 10 µM. All other antibiotics were in a concentration of 50 µM unless otherwise specified (see DPX2 cells).
Plasmids
pSG5-hPXR was generously provided by Dr. Steven Kliewer (University of Texas Southwestern Medical Center). The reporter plasmid (CYP3A4 +53 to 362(7836/7208)-LUC), hereafter called CYP3A4PXRE2-LUC, was prepared by Dr. Rommel Tirona (Vanderbilt University) as previously described (Tirona et al., 2003).
Reporter Gene Assay using HepG2 cells
HepG2 cells were maintained in minimum Eagle’s medium supplemented with 10% fetal bovine serum (Hyclone Laboratories, Logan, UT), 1 % penicillin/streptomycin and 1 % L-glutamine. Cells were plated in 24-well plates at 3 × 105 cells per well. Twenty-four hours later, they were transfected overnight by calcium phosphate precipitation with 300 ng of PXRE2-CYP3A4-LUC reporter plasmid in the presence or absence of either pSG5-hPXR (100 ng) expression plasmid or empty vector plasmid. The next day, cells were washed once with medium and incubated with fresh medium containing serum with or without antibiotics. Twenty-four hours later, cells were harvested, lysed, and centrifuged at 1500 g for 5 min, and luciferase activities were determined on an aliquot of supernatant according to the manufacturer’s instructions (Luciferase Assay System, Promega, Madison, WI) using an automated luminometer (model OPTOCOMP 1 (MGM Instruments, Hamden, CT). LUC activities were normalized to protein concentration. All experiments were performed at least twice in triplicate.
Reporter Gene Assay using DPX-2 cells
The DPX-2 cells were a generous gift of Puracyp (Carlsbad, CA). We employed DPX-2 cells in a reporter gene assay for PXR as we have recently described (Ekins et al., 2007). All test articles were used in the following final concentrations: 1, 5, 10, 20, and 50µM except for rifampin which was used throughout at the single concentration of 10 µM. Rifampin was the positive comparator, and DMSO was used as the negative control (Ekins et al., 2007).
EC50 and Emax values were also estimated as described previously (Ekins et al., 2007). Relative induction scores (RIS) were computed as described by Ripp et al (Ripp et al., 2006), using prior published values for Cmax and fraction unbound to calculate Cmaxunb.
Preparation of Primary Cultures of Human Hepatocytes and LS180 cells
Livers were provided by the Liver Tissue Procurement and Distribution System (NIH Contract #N01-DK-9-2310) and by the Cooperative Human Tissue Network. Livers were procured from donor organs that were not suitable for whole organ transplantation or from remaining tissue after reduced allograft transplantation. Donor livers were flushed, in situ, and maintained with Belzar’s UW solution. Hepatocytes were isolated within 24 h of cross-clamp. Reasons for not using tissues for transplantation included traumatic damage, errors in organ harvest, brief anoxic periods, or macro- or microsteatosis. Human hepatocytes were isolated essentially as described elsewhere (Strom et al., 1996). Human hepatocyte preparations 1180, 1183, 1188 and 1201 were used for the antibiotic studies, and human hepatocyte preparations 812, 818 and 819 for the screen of other PXR activators.
Cells were plated on collagen-coated six-well plates maintained in Modified Williams E for 48 h, and then treated with drugs for 48 h. Media was then aspirated, total lysate harvested or Trizol solution (Invitrogen) added for RNA isolation. First-strand cDNA was synthesized from 2 µg of total RNA according to the manufacturer’s instructions (SuperScript Preamplification System for FirstStrand cDNA Synthesis, Invitrogen).
LS180 cells were maintained in RPMI-1640 supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin and 1% L-glutamine. Cells were plated on six-well plates for 24 h and then treated with drugs for 48 h. Media was then aspirated, and total lysate harvested for protein analysis.
Quantitative PCR for hCYP3A4 mRNA
Specific PCR primers of hCYP3A4 and glyceraldehyde-3-phosphate dehydrogenase (hGAPDH) were designed with PRIMER3 (http://www.genome.wi.mit.edu/cgi-bin/primer/primer3.cgi) The sequence homology and specificity were checked by using BLASTn (http://www.ncbi.nlm.nih.gov). The sequence of these primers were as follows: hCYP3A4.F, 5’-CACAGATCCCCCTGAAATTAAGCTTA −3’; hCYP3A4.R, 5’AAAATTCAGGCTCCACTTACGGTG −3’; hGAPDH.F, 5’ACCACAGTCCATGCCATCAC −3’; hGAPDH.R, 5’TCCACCACCCTGTTGCTGT A −3’.
Real-time PCR quantitation was carried using a QuantiTect SYBR Green PCR kit (QIAGEN, Chatsworth, CA) according to the manufacturer’s instructions. Amplification was done with the ABI PRISM 7900HT Sequence Detection System (PE Applied Biosystems, Foster City, CA). The hCYP3A4 and hGAPDH primers were used in realtime PCR amplifications in which the initial activation step was conducted at 95°C for 15 min, and was followed by 40 cycles in which each cycle consisted of denaturation at 92°C for 30 s, annealing at 60°C for 30 s and synthesis at 72°C for 60 s. Specificity of amplification was confirmed in each case by performing melt curve analysis. The relative amounts of hCYP3A4 mRNAs in each human liver sample were normalized to the hGAPDH values to control for quality of the mRNA. Quantitative PCR values were determined for CYP3A4 mRNA levels using the comparative CT (DDCT) method.
Immunoblot Analysis
Total lysates were recovered from primary cultures of human hepatocytes and LS180 cells. Protein was estimated by using the Bio-Rad protein assay with bovine serum albumin as the standard. 5 µg and 25 µg of total lysate from human hepatocytes and LS180, respectively, were separated on 10% polyacrylamide gels, and immunoblotted with monoclonal anti-CYP3A4 K03 (Schuetz et al., 1996) and anti-mouse secondary antibody coupled with peroxidase. The blot was developed with the ECL detection system (Amersham Biosciences, Piscataway, NJ).
CYP3A4 Enzymatic Activity
The activity of CYP3A4 was measured by formation of 6β-hydroxytestosterone in intact cultured hepatocytes, as described (Kostrubsky et al., 1999). After a 48 h exposure to chemicals, the culture medium was replaced with fresh Williams E medium containing 200 µM testosterone. The cells were incubated for 30 min at 37°C, and media taken for measurement of 6β-hydroxytestosterone and cells scraped and pelleted for immunoblot analysis. 6β-hydroxytestosterone in the medium was measured by HPLC with the following modifications: culture medium (100 µI) was diluted with methanol (1:1, v/v) and injected into a LiChrospher 100 RP-18 column (4.6 × 250 mm, 5 µm) with a mobile phase of methanol/water (60:40) at a flow rate of 1.2 ml/min. The eluent was detected by its absorbance at 242 nm and quantified by comparing the absorbance to a standard curve of 6β-hydroxytestosterone prepared in Williams E medium.
In silico modeling: Pharmacophores
The computational molecular modeling studies were carried out using Catalyst™ in Discovery Studio 2.0 (Accelrys, San Diego, CA) running on a Centrino Duo processor (Intel, Santa Clara, CA) in a Dell Latitude D630 Laptop. Pharmacophore models attempt to describe the arrangement of key features that are important for biological activity and their generation has been widely described (Ekins et al., 2007). Previously generated pharmacophores for PXR agonists (Ekins and Erickson, 2002; Bachmann et al., 2004; Ekins et al., 2007) were employed to generate predictions for antibiotics tested in this study. These pharmacophores represent 1. the original PXR pharmacophore using 12 diverse ligands (Ekins and Erickson, 2002) with EC50 data from competition binding assays or CV1 cells, which has previously been used to predict affinity of imidazoles (Bachmann et al., 2004; Ekins et al., 2007), 2. A pharmacophore using 30 steroids with EC50 data from HepG2 cells using a reporter assay that may define a unique site within PXR (Ekins et al., 2007), and 3. a pharmacophore using 31 diverse ligands (Ekins et al., 2007) with EC50 data from HepG2 cells using a reporter assay (Sinz et al., 2006). The structures of antibiotics were sketched in ChemDraw for Excel (CambridgeSoft, Cambridge MA) and exported as sdf files. In Catalyst, the 3-D molecular structures were produced using up to 255 conformers with the best conformer generation method, allowing a maximum energy difference of 20 kcal/mol. Using the Ligand Pharmacophore Mapping protocol the ‘Best Mapping’ was performed with the ‘rigid fitting method’ and maximum omitted features = 0. The quality of the molecule mapping to the pharmacophore is determined by the fit value, with a higher fit value representative of a better fit and dependent on the proximity of the features to pharmacophore centroids and the weights assigned to each feature.
In silico modeling: Docking
A selection of the antibiotics were docked into the co-crystallized structure of PXR with SR12813 (PDB ID: 1NRL)(Watkins et al., 2003) using FlexX (BioSolveIT, GmbH, Sankt Augustin)(Kramer et al., 1999; Zhang et al., 2007). The FlexX program considers ligand flexibility by an incremental ligand placement technique while the receptor is considered as rigid. For each ligand, 30 different docked poses were generated and the best pose was selected based on the FlexX score. The active site was defined as the amino acid residues within 6Å of the co-crystallized ligand.
Statistical Analysis
Differences between two groups (control vs drug treatment) were analyzed using a two-sided two-sample t-test.
Results
Activation of PXR in a Reporter Gene Assay using HepG2 cells
We first screened for antibiotic activation of PXR using a CYP3A4-LUC reporter assay in the human hepatoblastoma HepG2 cells. PXR was activated strongly (>10-fold) by rifampin and troleandomycin; moderately (> 7-fold) by dicloxacillin, tetracycline, clindamycin and griseofulvin; moderately (> 4-fold) by erythromycin and weakly ( >2.4-fold) by nafcillin, cefaclor, sulfisoxazole (Table 1).
Table 1.
Maximum Fold increase in PXR activation observed experimentally in HepG2 and DPX-2 cells
| Antibiotics |
HepG2 cells |
DPX-2 cells† |
|---|---|---|
| Rifamycins | ||
| Rifampin | 10.5 ± 0.50** | 9.4 ± 2.6 |
| Penicillins | ||
| Penicillin V | 2.03 ± 0.24** | 1.09 ± 0.14 |
| Nafcillin | 2.91 ± 0.25** | 1.58 ± 0.41 |
| Dicloxacillin | 7.89 ± 0.53** | 4.51 ± 1.4 |
| Amoxicillin | 1.30 + 0.05** | 3.76 ± 1.12 |
| Ampicillin | 1.64 + 0.18** | 1.01 ± 0.45 |
| Cephems | ||
| Cefadroxil | 2.07 ± 0.02** | 1.14 ± 0.07 |
| Cephalexin | 1.27 + 0.06* | 1.93±0.24 |
| Cephradine | 1.83 ± 0.15** | 7.42 ± 0.48 |
| Cefaclor | 2.44 ± 0.28** | 1.45 ± 0.3 |
| Cefuroxime | 1.06 ± 0.14 | 0.29 ± 0.04 |
| Tetracyclines | ||
| Tetracycline | 7.35 ± 0.58** | 1.21 ± 0.15 |
| Doxycycline | 1.40 + 0.07** | 2.67 ± 0.35 |
| Minocycline | 1.64 + 0.03** | 1.15 ± 0.17 |
| Demeclocycline | 1.40 + 0.09** | 3.08 ± 0.47 |
| Sulfonamides | ||
| Sulfisoxazole | 2.15 ± 0.08** | 2.61 ± 0.60 |
| Sulfamethoxazole | 0.99 ± 0.07 | 2.27 ± 0.10 |
| Macrolides | ||
| Erythromycin | 4.32 + 0.65** | 3.56 ± 0.72 |
| Troleandomycin (TAO) | 13.26 + 0.98** | 10.06 ± 1.36 |
| Lincosamides | ||
| Clindamycin | 7.05 ± 0.27** | 9.71 ± 0.44 |
| Others | ||
| Griseofulvin | 9.27 ± 1.11** | 2.27 ± 0.08 |
p< 0.05 compared to DMSO controls
p< 0.01 compared to DMSO controls
Statistical comparisons were not made with DMSO controls for DPX-2 cells. Instead, EC50 and Emax values were estimated from dose-response data (see Table 2).
Activation of PXR in a Reporter Gene Assay using DPX-2 cells
In order to determine more precise PXR binding affinities for these drugs, we next turned to the DPX-2 cells because we have previously validated their utility in combining computational and experimental data to model the interactions a series of PXR agonists and antagonists with PXR (Ekins et al., 2007). The estimated Emax and EC50 values along with experimentally observed maximum fold induction are listed in Table 2. Rifampin (10µM) elicited an increase in PXR activity averaging 9.4 fold; dicloxacillin, 4.5 fold; amoxicillin, 3.8 fold; cephradine, 7.4 fold; doxycycline, 2.7 fold; democlocycline, 3.1 fold; sulfisoxazole, 2.6 fold; sulfamethoxazole, 2.3 fold; troleandomycin, 10 fold; griseofulvin, 2.3 fold; and clindamycin, 9.7 fold (Table 2). Table 2 also depicts RIS values for those antibiotics exhibiting >2 fold induction in DPX-2 cells.
Table 2.
Cmax, Cmaxunb, and estimated Emax, EC50, and RIS values for antibiotics exhibiting >2 fold PXR activation in DPX-2 cells
| Antibiotic | Cmax* | Cmaxunb* | Emax† | EC50*† | RIS‡ | Clinical Significance¶ |
|---|---|---|---|---|---|---|
| Dicloxacillin | 31.9 | 1.3 | 13.8 | 104.9 | 0.17 | Yes |
| Cephradine | 26–69 | 22.1–58.7 | 9.8 | 20.7 | 5.1–7.3 | Yes |
| Doxycycline | 6.8 | 0.8 | 2.5 | 6.1 | 0.3 | Yes |
| Griseofulvin | 1.1–5.7 | 0.18–0.91 | 2.1 | 0.96 | 0.3–1.0 | Yes |
| Clindamycin | 40.5 | 2.6 | 15.6 | 30.8 | 1.21 | Yes |
| Sulfisoxazole | 411–935 | 61.7–140 | 2.3 | 2.4 | 2.2 | Yes |
| Sulfamethoxazole | 146.5 | 68.9 | 2.1 | 0.9 | 2.1 | Yes |
| Erythromycin | 1.2 | 0.2 | 8.7 | 72.9 | 0.02 | No |
| Triacetyloleandomycin | 2.44 | ? | 11.3 | 7.9 | ? |
Concentrations in µM
Estimated from concentration-response data
Calculated as:
Assuming RIS < 0.05, not likely; 0.05–0.1, possible; and > 0.1, likely.
unknown
Induction of CYP3A4 mRNA, protein and activity in Primary Human Hepatocytes and LS180 Human Intestinal Cells
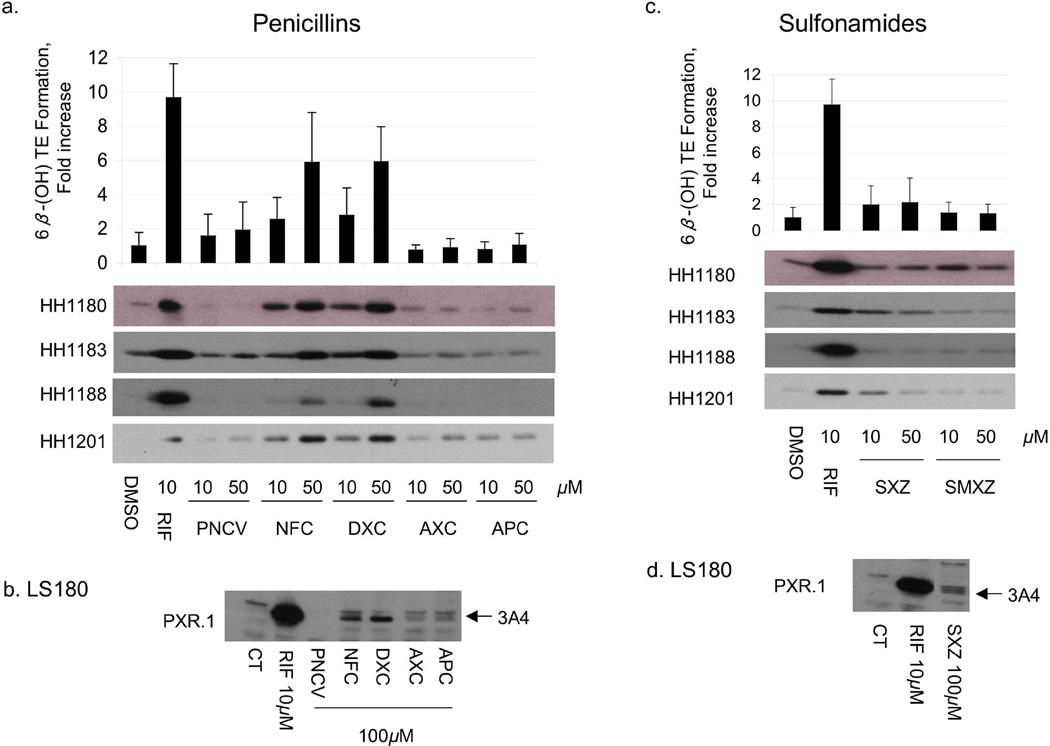
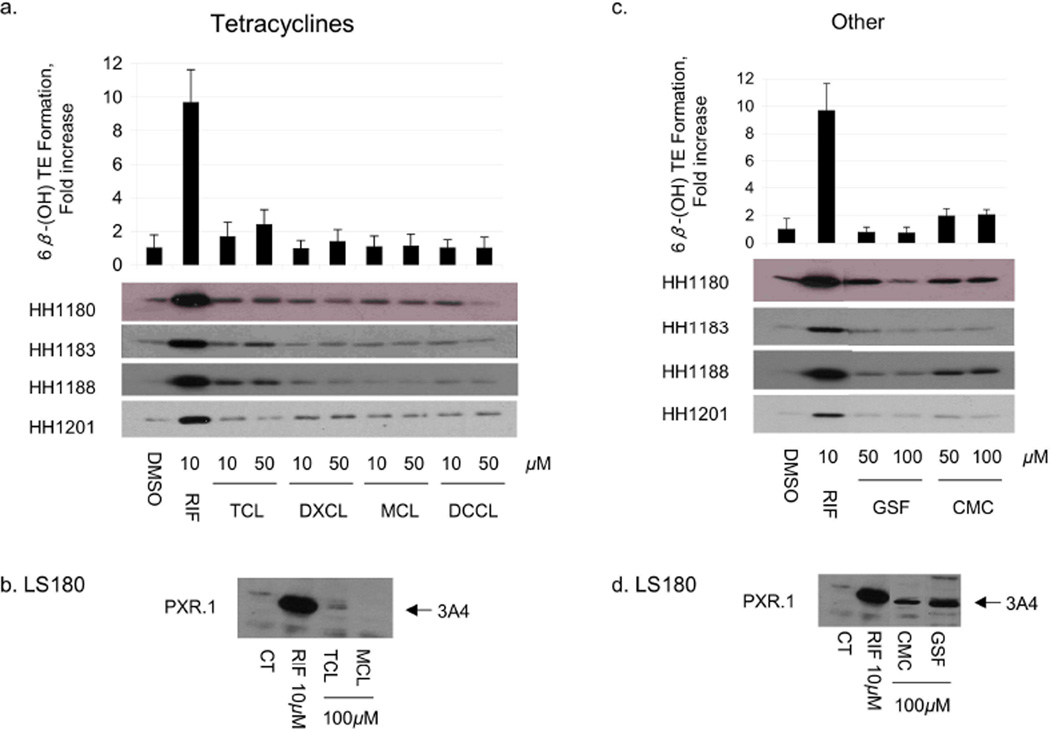
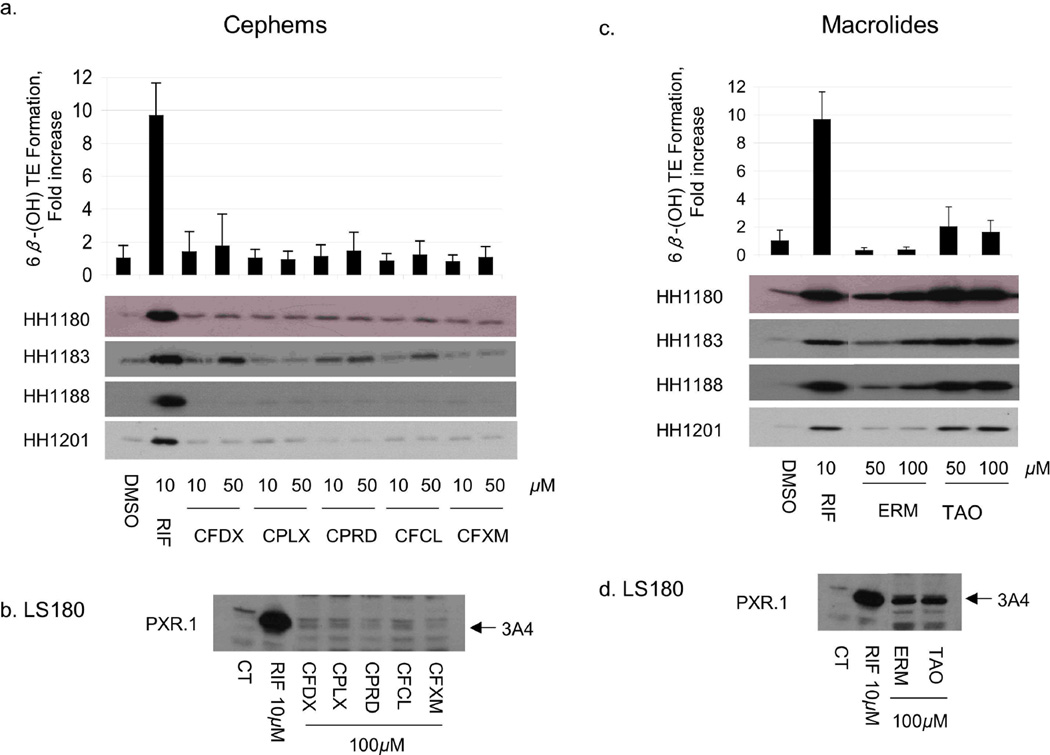
To verify that antibiotics that transactivated PXR also resulted in increased CYP3A4, primary human hepatocytes and LS180 (human intestinal) cells were treated with the same antibiotics. CYP3A4 mRNA and protein were measured in both cell types, whereas testosterone 6β-hydroxylase activity was measured only in hepatocytes (Figs 1–3). Among the penicillins (Fig 1a and Table 3), nafcillin and dicloxacillin resulted in statistically significant increases in all CYP3A4 measures, approaching 6-fold induction of testosterone 6β-hydroxylase activity for both. Penicillin V resulted in an almost two fold increase in CYP3A4 activity. CYP3A4 protein was also induced in LS180 cells treated with these same penicillin antibiotics (Fig 1b). Among the sulfonamides, sulfisoxazole weakly increased CYP3A4 protein in primary human hepatocytes and LS180 cells (Fig 1c,d), with a corresponding 2-fold increase in CYP3A4 activity (Table 3). Findings among the cephems were somewhat mixed. In primary human hepatocytes cefadroxil increased CYP3A4 mRNA and protein 1.89 fold, but these effects were not statistically significant. On the other hand, although cephalexin caused a significant increase in CYP3A4 mRNA (1.67 fold), this did not result in measurable increases in CYP3A4 protein nor activity.. Finally, cephradine increased CYP3A4 mRNA 2.34 fold, slightly increased CYP3A4 protein, and significantly increased CYP3A4 activity (Fig 2a,b, Table 3). Cefaclor caused a slight increase in CYP3A4 protein, a finding in accord with its PXR activation in HepG2 cells, though it did not appear to significantly alter any other parameter. TAO and erythromycin, which both activated PXR in HepG2 cells and DPX-2 cells (Table 1), induced CYP3A4 mRNA and protein in primary human hepatocytes and LS180 cells (Fig 2c,d). However, only TAO increased CYP3A4 activity (Table 3). All tetracyclines produced significant increases in hepatocyte CYP3A4mRNA, however only tetracycline increased CYP3A4 protein. Effects on CYP3A4 activity were statistically significant for doxycycline. Both griseofulvin and clindamycin induced CYP3A4 protein in LS180 cells (Fig 3d). However, only clindamycin caused any increase (2-fold) in CYP3A4 protein or activity in primary human hepatocytes (Fig 3c and Table 3).
Fig 1.
Effect of penicillins and sulfonamides on CYP3A4 protein expression and activity in human hepatocytes and in LS180 cells. Primary human hepatocytes (HH) from donors 1180, 1183, 1188 and 1201 were treated with vehicle (DMSO), RIF, penicillins, or sulfonamides at the indicated concentrations for 48 h. HH were incubated with testosterone immediately before harvest, and the medium was analyzed for the formation of 6β-hydroxytestosterone. The fold change (mean ± S.D. (top panels a,c) in 6β-hydroxytestosterone formation rate (nmol/min/mg protein) for drug vs vehicle treated cells (in triplicate) of a representative experiment are shown. The same cells were lysed and 5 µg analyzed by immunoblot for CYP3A4 (bottom panels a, c). LS180 cells stably expressing PXR.1 were treated for 48 h with the indicated concentrations of drug or 0.1% DMSO as the vehicle control (CT). Total cell lysate (25 µg) was analyzed by immunoblot analysis for CYP3A4 protein (panels b,d). Abbreviations for drugs are indicated in Supplemental Table 1.
Fig 3.
Effect of tetracyclines, clindamycin, and griseofulvin on CYP3A4 protein expression and activity in human hepatocytes and in LS180 cells. Primary human hepatocytes (HH) from donors 1180, 1183, 1188 and 1201 were treated with vehicle (DMSO), RIF, penicillins, or sulfonamides at the indicated concentrations for 48 h. HH were incubated with testosterone ilmediately before harvest, and the medium was analyzed for the formation of 6β-hydroxytestosterone. The fold change (mean ± S.D. (top panels a,c) in 6β-hydroxytestosterone formation rate (nmol/min/mg protein) for drug vs vehicle treated cells (in triplicate) of a representative experiment are shown. The same cells were lysed and 5 µg analyzed by immunoblot for CYP3A4 (bottom panels a, c). LS180 cells stably expressing PXR.1 were treated for 48 h with the indicated concentrations of drug or 0.1% DMSO as the vehicle control (CT). Total cell lysate (25 µg) was analyzed by immunoblot analysis for CYP3A4 protein (panels b,d). Abbreviations for drugs are indicated in Supplemental Table 1.
Table 3.
Effects of antibiotics on CYP3A4 mRNA and protein expression and CYP3A4 activity in primary human hepatocytes.
| Antibiotic* | QT-PCR CYP3A4/GAPDH mRNA** |
Protein Expression |
6β-(OH) testosterone formation rate† |
|
|---|---|---|---|---|
| Rifamycins | ||||
| Rifampin | 10.33 ± 5.2‡ | +++++ | 9.67 ± 1.99‡ | |
| Penicillins | ||||
| Penicillin V | 1.84 ± 0.12 | − | 1.92 ± 1.64‡ | |
| Nafcillin | 6.60 ± 2.06¶ | +++ | 5.88 ± 2.91* | |
| Dicloxacillin | 7.70 ± 4.00 | ++++ | 5.91 ± 2.05‡ | |
| Amoxicillin | 1.01 ± 0.31 | − | 0.87 ± 0.56 | |
| Ampicillin | 1.14 ± 0.21 | − | 1.03 ± 0.70 | |
| Cephems | ||||
| Cefadroxil | 1.89 ± 0.89 | ++ | 1.75 ± 1.93 | |
| Cephalexin | 1.67 ± 0.15¶ | − | 0.91 ± 0.54 | |
| Cephradine | 2.34 ± 1.13 | + | 1.43 ± 1.16¶ | |
| Cefaclor | 1.4 ± 0.40 | + | 1.19 ± 0.87 | |
| Cefuroxime | 1.16 ± 0.1* | − | 1.04 ± 0.68 | |
| Tetracyclines | ||||
| Tetracycline | 3.64 ± 1.58‡ | ++ | 2.38 ± 0.91 | |
| Doxycycline | 2.80 ± 0.66¶ | − | 1.38 ± 0.73‡ | |
| Minocycline | 1.88 ± 0.16¶ | − | 1.09 ± 0.74 | |
| Demeclocycline | 1.70 ± 0.19¶ | − | 0.99 ± 0.66 | |
| Sulfonamides | ||||
| Sulfisoxazole | 4.05 ± 1.55‡ | ++ | 2.15 ± 1.90¶ | |
| Sulfamethoxazole | 2.51 ± 0.40¶ | − | 1.20 ± 0.73 | |
| Macrolides | ||||
| Erythromycin | 3.25 ± 0.44¶ | ++++ | 0.33 ± 0.20¶ | |
| Triacetyloleandomycin | 8.15 ± 2.74¶ | +++++ | 2.03 ± 1.40‡ | |
| Lincosamides | ||||
| Clindamycin | 4.85 ± 2.33‡ | +++ | 1.96 ± 0.52 | |
| Others | ||||
| Griseofulvin | 3.20 ± 0.86 | ++ | 0.77 ± 0.38 | |
Rifampin was used in a concentration of 10 µM; all others were used at a concentration of 50 µM.
Fold increase over vehicle control.
nmol/min/mg protein.
p<0.05
p<0.01
Fig 2.
Effect of cephems and macrolides on CYP3A4 protein expression and activity in human hepatocytes and in LS180 cells. Primary human hepatocytes (HH) from donors 1180, 1183, 1188 and 1201 were treated with vehicle (DMSO), RIF, penicillins, or sulfonamides at the indicated concentrations for 48 h. HH were incubated with testosterone immediately before harvest, and the medium was analyzed for the formation of 6β-hydroxytestosterone. The fold change (mean ± S.D. (top panels a,c) in 6β-hydroxytestosterone formation rate (nmol/min/mg protein) for drug vs vehicle treated cells (in triplicate) of a representative experiment are shown. The same cells were lysed and 5 µg analyzed by immunoblot for CYP3A4 (bottom panels a, c). LS180 cells stably expressing PXR.1 were treated for 48 h with the indicated concentrations of drug or 0.1% DMSO as the vehicle control (CT). Total cell lysate (25 µg) was analyzed by immunoblot analysis for CYP3A4 protein (panels b,d). Abbreviations for drugs are indicated in Supplemental Table 1.
There was broad though imperfect agreement between drugs that activated PXR in either HepG2 or DPX-2 cells and those also inducing CYP3A4 mRNA, protein, or activity. CYP3A4 mRNA, protein or activity did not always mirror that of PXR activation. Only for rifampin, nafcillin and dicloxacillin did the magnitude of PXR activation closely match the increases in CYP3A4 mRNA, protein, and activity.
In silico predictions for antibiotics binding to PXR as agonists
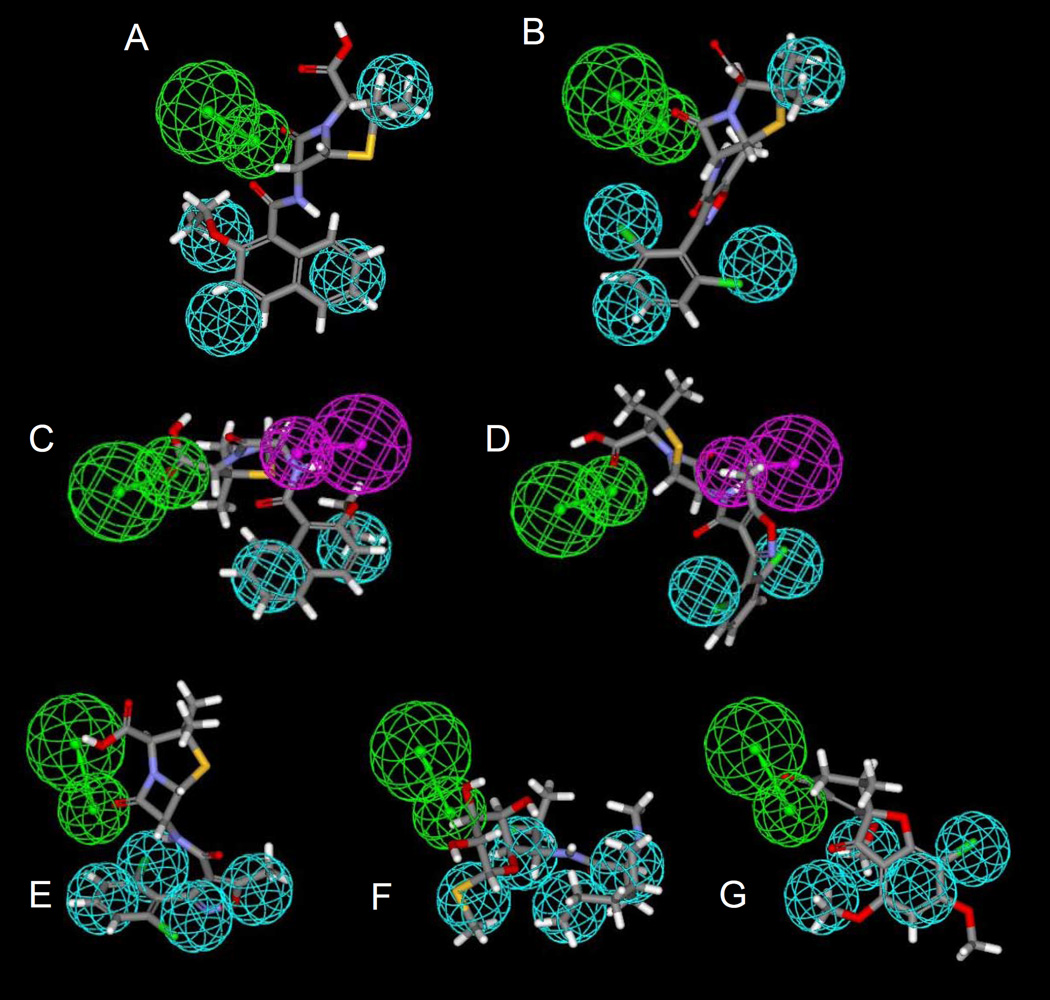
Previously generated pharmacophores for human PXR agonists were used to predict whether antibiotics were likely to bind PXR. The original PXR pharmacophore consisted of 4 hydrophobes and a hydrogen bond acceptor feature and was found to map 5 of the antibiotics (Table 4), one of which, RIF, was originally in the pharmacophore training set. Nafcillin (Fig 4A), dicloxacillin (Fig 4B), erythromycin and troleandomycin mapped to the widely dispersed features well. The diverse (n=31) pharmacophore consisted of 2 hydrophobes, a hydrogen bond acceptor and a hydrogen bond donor feature and mapped to 16 of the antibiotics, one of which, RIF, was also in the pharmacophore training set. Tetracycline, sulfisoxazole, sulfmethazole, troleandomycin and griseofulvin did not map to the features. Interestingly nafcillin fit well (Fig 4C) and dicloxacillin had the lowest fit value (Fig 4D). The steroidal (n=30) pharmacophore consisted of 4 hydrophobes and a hydrogen bond acceptor feature and surprisingly was found to map 4 of the antibiotics, dicloxacillin (Fig 4E), troleandomycin, clindamycin (Fig4F) and griseofulvin (Fig 4G). Twelve of the antibiotics were also docked (erythromycin failed to dock) into one of the PXR crystal structures 1NRL, and were then scored using FlexX (Khandelwal et al., 2007). The lower the FlexX score, the better the complimentarity between ligand and receptor. All the penicillins and cephems docked and scored well apart from cefuroxime, tetracycline, doxycycline, and clindamycin which had poorer scores.
Table 4. Using Computational docking and pharmacophore methods to predict antibiotics that are likely to be PXR agonists.
The techniques involved in pharmacophore mapping and docking are described in the Materials and Methods. Empty values represent no fit to the pharmacophore using the parameters as described.
| Fit Value (units) | ||||
|---|---|---|---|---|
| Antibiotic | Original (n = 12) Pharmacophore (Ekins and Erickson, 2002; Bachmann et al., 2004) |
Diverse (n = 31) Pharmacophore (Ekins et al., 2007) |
Steroidal (n = 30) Pharmacophore (Ekins et al., 2007) |
Docking FlexX Score (Khandelwal et al., 2007) |
| Rifampin | 1.368 * | 2.696 * | - | ND |
| Penicillin V | - | 2.01 | - | ND |
| Nafcillin | 1.105 | 4.438 | - | −17.996 |
| Dicloxacillin | 2.991 | 1.71 | 8.342 | −17.441 |
| Amoxicillin | - | 1.899 | - | −18.464 |
| Ampicillin | - | 2.633 | - | −17.44 |
| Cefadroxil | - | 2.139 | - | −19.998 |
| Cephalexin | - | 2.897 | - | −18.107 |
| Cephradine | - | 2.084 | - | −15.906 |
| Cefaclor | - | 2.87 | - | −18.925 |
| Cefuroxime | - | 4.659 | - | −10.542 |
| Tetracycline | - | - | - | −12.247 |
| Doxycycline | - | 4.697 | - | −10.707 |
| Minocycline | - | 2.12 | - | ND |
| Demeclocycline | - | 2.602 | - | ND |
| Sulfisoxazole | - | - | - | ND |
| Sulfmethoxazole | - | - | - | ND |
| Erythromycin | 2.216 | 4.503 | - | failed |
| Troleandomycin | 1.288 | * | 2.412 | ND |
| Clindamycin | - | 5.289 | 3.536 | −11.83 |
| Griseofulvin | - | - | 9.598 | ND |
ND = not determined
molecule in pharmacophore training set
molecule failed to map to all pharmacophore features
Fig 4.
Molecules mapped to PXR pharmacophores. A. nafcillin B. dicloxacillin mapped to the original PXR pharmacophore; C. nafcillin D. dicloxacillin mapped to the diverse (n=31) PXR pharmacophore; E. dicloxacillin, F. clindamycin, G. griseofulvin mapped to the steroidal (n=30) PXR pharmacophore. Spheres represent: Hydrophobic features (cyan), hydrogen bond acceptor and vector (green), hydrogen bond donor and vector (purple).
Discussion
We employed HepG2 cells to conduct a primary screen, and DPX-2 cells to make precise kinetic measurements, of PXR activation and CYP3A4 transactivation by 21 antibiotics. We then verified whether these same 21 antibiotics induced CYP3A4 mRNA, protein and testosterone 6β-hydroxylase activity in primary human hepatocytes and the human intestinal LS180 cell line. However, caution is required when extrapolating PXR transactivation outcomes to predict increases in CYP3A4 activity or increases in activities associated with other known PXR target genes. Although it is likely that some other PXR target genes will be induced by the same antibiotic therapies, it is also the case that PXR mediated gene activation is ligand, promoter, and cell-type specific.
There was broad though imperfect correlation between transactivation assays and in vitro CYP3A4 message, protein or activity associated with cell pretreatment observed with this series of antibiotics, however similar disparate results have also been reported for fourteen more therapeutically diverse enzyme-inducing agents (Luo et al., 2002). Admittedly, the inconsistent results within and across models hampers the reliability with which in vivo outcomes can be predicted. Rifampin, our active comparator, is, of course, an exception to this problem, since rifampin markedly increased each parameter in every system. Since there are many clinical drug interaction studies with erythromycin further clinical studies would not be necessary. However, inconsistencies notwithstanding, several other antibiotics yielded sufficiently large changes in all but one or two parameters that their effects on the disposition kinetics of low clearance drugs with narrow therapeutic margins may warrant reappraisal in the context of clinical trials. Our findings suggest that nafcillin, dicloxacillin, cephradine, tetracycline, sulfisoxazole, clindamycin, and griseofulvin warrant further scrutiny in vivo in the clinic. Results for doxycycline, minocycline, demeclocycline and sulfamethoxazole are simply too ambiguous to suggest calling for clinical re-evaluation at this time. Penicillin V, amoxicillin, and ampicillin appear relatively devoid of effects on CYP3A4. Though TAO was even more active than erythromycin, its use is extremely limited in the U.S.
Antibiotics are some of the most widely prescribed drugs (Cizman, 2003; Eng et al., 2003). Their interactions with low-dose estrogen-containing OCs as a potential cause of unplanned pregnancy has been a concern among health professionals for three decades. There is considerable confusion in the marketplace as to which antibiotics may decrease the effectiveness of oral contraceptives. Back et al., (Back et al., 1988) reported epidemiological data showing that 63 women had become pregnant in a 17 year period in Great Britain while taking OCs, and 70% of them became pregnant while taking either penicillins or tetracyclines plus OCs. Subsequently similar reports were described from New Zealand (Sparrow, 1989) and Holland (Kovacs et al., 1989). However, in the intervening 20 years there has been no verification that the relationship was causal. Moreover, earlier studies suggested that ampicillin had no effect on oral contraceptive effectiveness (Friedman et al., 1980; Joshi et al., 1980). Other than the rifamycins, no antibiotics have been shown to alter the pharmacokinetics of ethinylestradiol in humans (Shenfield and Griffin, 1991). Soon after implicating antibiotics as a potential cause of OC failure, Back et al., (Back et al., 1991) allowed that the OC failure rate among antibiotic users may not be substantively greater than the natural failure rate of OCs.
Our results show that dicloxacillin is a highly efficacious hPXR activator as well as an inducer of CYP3A4 mRNA and protein, and that it can significantly increase the rate of 6β-hydroxytestosterone formation. Nafcillin is comparable for all parameters except PXR transactivation in DPX-2 cells. The findings for these two penicillins are consistent with reports of in vivo of drug interactions between these same antibiotics and CYP3A substrates. For example, Lang et al., (Lang et al., 2003) reported that 5-day treatment with nafcillin markedly increased the total plasma clearance of the CYP3A4 substrate nifedipine by 245% in healthy controls. In a controlled trial using subjects who were infection-free, Krstenansky et al., reported that dicloxacillin decreased warfarin-induced prothrombin times (Krstenansky et al., 1987). Additional evidence that nafcillin might also increase warfarin metabolism has also been published (Cropp and Bussey, 1997).
However, there are also additional reports of antibiotic drug-interactions, in general with OCs. Rifampin, the most potent antibiotic activator of hPXR, was found as early as 1971 to decrease OC effectiveness. Among women taking rifampin and OCs, 75% experienced intermenstrual bleeding, and 6% became pregnant (Reimers and Jezek, 1971). Rifampin’s effects on the disposition kinetics of ethinylestradiol resulting in a substantial decrease in AUC have been thoroughly studied (Barditch-Crovo et al., 1999). Likewise, van Dijke and Weber (van Dijke and Weber, 1984) reported an array of case studies involving women taking concomitant griseofulvin and OCs who reported menstrual cycle disturbances and pregnancies. Based on the reported findings in humans, it is certain that rifampin can decrease the effectiveness of OCs, and it is probable that dicloxacillin and nafcillin may also behave similarly as a result of PXR activation and/or increased CYP3A4 activity. Nevertheless, a myriad of review papers and position statements caution about the potential loss of effectiveness of OCs associated with antibiotic use in general.
Our in vitro results predict that penicillin V and the aminopenicillins, ampicillin and amoxicillin are unlikely to affect the metabolism of CYP3A4 substrates in humans. However, our in vitro results affirm that drug interactions are likely to occur with some other penicillins including nafcillin and dicloxacillin, an isoxazolyl penicillin. Using LS180 cells, primary human hepatocytes, BHK21 cells and pig kidney epithelial cells. Huwyler et al., (Huwyler et al., 2006) also recently reported that clinically relevant doses of flucloxacillin (not assessed in the current study) activates PXR and induces CYP3A4 and P-gp.
Although the macrolide antibiotics erythromycin and troleandomycin were efficacious PXR activators, having both increased mRNA expression of CYP3A4, they both failed to induce CYP3A4 activity, consistent with their ability to also act as CYP3A4 inhibitors (McConn et al., 2004; Atkinson et al., 2005). Interestingly TAO had previously been shown to have an EC50 of 8.9 µM while erythromycin was inactive in a PXR transactivation assay (Sinz et al., 2006).
Similarly, though clindamycin elicited substantial increases in PXR activation, CYP3A4mRNA, and protein, its failure to produce a significant increase in the rate of 6β-hydroxytestosterone formation could reflect its modest ability to inhibit CYP3A4 (Wynalda et al., 2003).
Computational approaches could represent a method to filter molecules prior to in vitro testing. Our computational analyses consisted of using multiple pharmacophores and docking into a single PXR structure. Rifampicin was present in two of the pharmacophore models and therefore it does not merit discussion as a true prediction. However, the originally published pharmacophore based on 12 agonists (Ekins and Erickson, 2002; Bachmann et al., 2004) proved remarkably selective in only mapping nafcillin, dicloxacillin, erythromycin and troleandomycin. The steroidal pharmacophore (with identical features) was equally selective again scoring dicloxacillin highly along with griseofulvin and clindamycin. This was surprising as the model was derived from a set of steroidal analogs suggested to map a specific region of the PXR binding site (Ekins et al., 2007). The more structurally diverse (n= 31) pharmacophore was less discriminatory but scored nafcillin higher than other penicillins. It is interesting to note that dicloxacillin is the only antibiotic that is mapped by all three pharmacophores. A selection of the antibiotic molecules are shown to map very well to the pharmacophore features (Fig 4). The use of multiple pharmacophores used in this way may be useful to gather a consensus prediction that could counteract the large flexible binding site, improving the overall confidence in predictions. Docking and scoring with FlexX scored the penicillins and cephems similarly and failed to dock the large erythromycin. We have also recently suggested that docking methods may need combination with other QSAR or computational methods, in order to improve predictions due to the flexibility of the protein and large binding site that could accommodate multiple pharmacophores (Khandelwal et al., 2007). This study represents a step in that direction.
In our transactivation studies in DPX-2 cells, a wide enough array of antibiotic concentrations was used to permit EC50 and Emax estimates which could then be used to compute a RIS (Ripp et al., 2006). By using this strategy we identified seven out of twenty one (33%) antibiotics with RIS values that would predict clinically significant enzyme induction (i.e. decrease in target drug AUC) using the following RIS criteria (ours): likely, if an RIS score was 0.1–0.5.; possible, for an RIS from 0.05–0.1; and not likely for an RIS < 0.05. In contrast, Sinz et al. found evidence that only about 5% of a diverse array of 170 drugs that activate PXR are likely to cause clinically significant enzyme induction (Sinz et al., 2006). It may be that some classes of drugs, such as antibiotics, show an enrichment in the number of molecules likely to show significant induction via PXR due to their possession and arrangement of key molecular features needed for interaction in the ligand binding pocket, which may be similar to those required for the therapeutic target/s. It must also be considered that antibiotics are more prone to cause drug interactions because both the doses administered and the concentrations achieved in gut and systemically are high relative to most orally administered drugs. Hence, the potential for this class of drugs to activate PXR and transactive target genes is a function not only of structure, but also of antibiotic exposure.
PXR transcriptionally activates a growing list of drug detoxification genes as can be shown visually as a network of direct interactions (Ekins et al., 2005b) radiating from a central node using Ingenuity Pathways Analysis (Supplemental Fig 1). These genes include numerous transporters and drug metabolism genes (Rosenfeld et al., 2003) leading to altered clearance of drugs that are substrates for these gene products. PXR activation by antibiotics might therefore be capable of precipitating DDIs by mechanisms other than an increase in CYP3A4 activity. We should also consider that some antibiotics such as the macrolides also inhibit P-gp (Eberl et al., 2007) and this could counteract the induction effect.
Our evaluation of twenty one antibiotics using PXR transactivation in HepG2 and DPX-2 cells, CYP3A4 mRNA and protein in human hepatocytes, CYP3A4 protein in LS180 cells, and CYP3A4 activity (testosterone 6β-hydroxylase activity) in primary human hepatocytes, identified a large number of antibiotics that were active in all or most of these in vitro assays for CYP3A4 induction. Rifampin, the active comparator molecule, was highly active in every assay. Dicloxacillin was also highly active in every assay while nafcillin was highly active in every assay apart from transactivation in DPX-2 cells. These in vitro findings correspond well with the in silico pharmacophore predictions for the two penicillins and in vivo studies of CYP3A4 induction by these antibiotics in humans. TAO was also highly active in every assay. Erythromycin, clindamycin, and griseofulvin were also active in all assays apart from testosterone 6β-hydroxylase activity in primary human hepatoctyes, which may be an artifact of their simultaneous inhibition of CYP3A4. Though not as active as the foregoing, sulfisoxazole was active in every assay and tetracycline was active in every assay except for PXR transactivation in DPX-2 cells while cephradine was active in every assay except for PXR transactivation in HepG2 cells. Penicillin V, amoxicillin, and ampicillin were overall devoid of activity in these systems, and results with the remaining cephems, tetracyclines, and sulfamethoxazole were inconsistent and modest across these assays.
We conclude by suggesting multiple in vitro and in silico approaches are necessary to reliably predict the likelihood of clinically significant CYP3A4 induction via PXR. Among the extensive array of antibiotics that we tested nafcillin, dicloxacillin, cephradine, tetracycline, sulfixoxazole, erythromycin, clindamycin, and griseofulvin exhibit a clear propensity to induce human CYP3A4 in vitro, and their potential to alter the disposition kinetics of drugs with narrow limits of efficacy such as ethinylestradiol in OCs warrants further investigation in the context of clinical trials.
Supplementary Material
Supplemental Fig 1. Downstream Direct interaction network for human PXR showing connected genes using Ingenuity Pathways Analysis 5.0 (Ingenuity Systems). Human receptors (rectangles), transporters (trapezoid) and enzymes (diamonds) are shown connected to PXR (NR1I2) and highlighted in yellow.
Acknowledgements
We are grateful to Mr. Dave Baran for technical assistance with DPX-2 cells. S.E. gratefully acknowledges Dr. Akash Khandelwal for providing docking scores and Dr. Cheng Chang for initial preliminary work on antibiotic predictions with PXR computational models. Ingenuity Systems Inc. graciously provided SE access to Ingenuity Pathways Analysis™ and Accelrys kindly provided Discovery Studio Catalyst 2.0.
Supported by: This work is supported in part by the NIH Grant GM60346, the NIH P30 CA21765 Cancer Center Support grant and by the American Lebanese Syrian Associated Charities (ALSAC).
List of abbreviations
- APC
ampicillin
- AXC
amoxicillin
- CFCL
cefaclor
- CFDL
cefadroxil
- CFXM
cefuroxime
- CPLX
cephalexin
- CPRD
cephradine
- CMC
clindamycin
- DCCL
demeclocycline
- DXC
dicloxacillin
- DXCL
doxycycline
- ERM
erythromycin
- GSF
griseofulvin
- MCL
minocycline
- NFC
nafcillin
- OCs
oral contraceptives
- PNCV
penicillin V
- RIF
rifampin
- SMXZ
sulfamethoxazole
- SXZ
sulfisoxazole
- TCL
tetracycline
- TAO
triacetyloleandomycin
Contributor Information
Kazuto Yasuda, Department of Pharmaceutical Sciences, St. Jude Children’s Research Hospital, Memphis, Tennessee 38105.
Aarati Ranade, Department of Pharmaceutical Sciences, School of Pharmacy, University of Pittsburgh, Pittsburgh, Pennsylvania 15261.
Raman Venkataramanan, Department of Pharmaceutical Sciences, School of Pharmacy, University of Pittsburgh, Pittsburgh, Pennsylvania 15261.
Stephen Strom, Department of Pharmaceutical Sciences, School of Pharmacy, University of Pittsburgh, Pittsburgh, Pennsylvania 15261.
Jonathan Chupka, Department of Pharmacology, The University of Toledo College of Pharmacy, Toledo, Ohio 43606.
Sean Ekins, Collaborations in Chemistry, Jenkintown, PA 19046, Department of Pharmaceutical Sciences, University of Maryland, 20 Penn Street, Baltimore, MD 21201, and Department of Pharmacology, University of Medicine and Dentistry of New Jersey, Robert Wood Johnson Medical School, Piscataway, NJ 08854.
Erin Schuetz, Department of Pharmaceutical Sciences, St. Jude Children’s Research Hospital, Memphis, Tennessee 38105.
Kenneth Bachmann, Department of Pharmacology, The University of Toledo College of Pharmacy, Toledo, Ohio 43606.
References
- Atkinson A, Kenny JR, Grime K. Automated assessment of time-dependent inhibition of human cytochrome P450 enzymes using liquid chromatography-tandem mass spectrometry analysis. Drug Metab Dispos. 2005;33:1637–1647. doi: 10.1124/dmd.105.005579. [DOI] [PubMed] [Google Scholar]
- Bachmann K, Patel H, Batayneh Z, Slama J, White D, Posey J, Ekins S, Gold D, Sambucetti L. PXR and the regulation of apoA1 and HDL-cholesterol in rodents. Pharmacol Res. 2004;50:237–246. doi: 10.1016/j.phrs.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Back DJ, Grimmer SF, Orme ML, Proudlove C, Mann RD, Breckenridge AM. Evaluation of Committee on Safety of Medicines yellow card reports on oral contraceptive-drug interactions with anticonvulsants and antibiotics. Br J Clin Pharmacol. 1988;25:527–532. doi: 10.1111/j.1365-2125.1988.tb03341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back DJ, Tjia J, Martin C, Millar E, Mant T, Morrison P, Orme M. The lack of interaction between temafloxacin and combined oral contraceptive steroids. Contraception. 1991;43:317–323. doi: 10.1016/0010-7824(91)90070-v. [DOI] [PubMed] [Google Scholar]
- Barditch-Crovo P, Trapnell CB, Ette E, Zacur HA, Coresh J, Rocco LE, Hendrix CW, Flexner C. The effects of rifampin and rifabutin on the pharmacokinetics and pharmacodynamics of a combination oral contraceptive. Clin Pharmacol Ther. 1999;65:428–438. doi: 10.1016/S0009-9236(99)70138-4. [DOI] [PubMed] [Google Scholar]
- Bertilsson G, Heidrich J, Svensson K, Asman M, Jendeberg L, Sydow-Backman M, Ohlsson R, Postlind H, Blomquist P, Berkenstam A. Identification of a human nuclear receptor defines a new signaling pathway for CYP3A induction. Proc Natl Acad Sci U S A. 1998;95:12208–12213. doi: 10.1073/pnas.95.21.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg B, Sabbagh W, Jr, Juguilon H, Bolado J, Jr, van Meter CM, Ong ES, Evans RM. SXR, a novel steroid and xenobiotic-sensing nuclear receptor. Genes Dev. 1998;12:3195–3205. doi: 10.1101/gad.12.20.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bud R. Antibiotics: the epitome of a wonder drug. Bmj. 2007;334(Suppl 1):s6. doi: 10.1136/bmj.39021.640255.94. [DOI] [PubMed] [Google Scholar]
- Chang C, Bahadduri PM, Polli JE, Swaan PW, Ekins S. Rapid Identification of P-glycoprotein Substrates and Inhibitors. Drug Metab Dispos. 2006;34:1976–1984. doi: 10.1124/dmd.106.012351. [DOI] [PubMed] [Google Scholar]
- Cizman M. The use and resistance to antibiotics in the community. Int J Antimicrob Agents. 2003;21:297–307. doi: 10.1016/s0924-8579(02)00394-1. [DOI] [PubMed] [Google Scholar]
- Cropp JS, Bussey HI. A review of enzyme induction of warfarin metabolism with recommendations for patient management. Pharmacotherapy. 1997;17:917–928. [PubMed] [Google Scholar]
- Cynke E, Binet I, Haefeli WE, Thiel G. Flucloxacillin and cyclosporine:an unrecognised but relevant interaction in renal transplant recipients. Kidney International. 1999;55:1156. (abstract) [Google Scholar]
- de Groot MJ. Designing better drugs: predicting cytochrome P450 metabolism. Drug Discov Today. 2006;11:601–606. doi: 10.1016/j.drudis.2006.05.001. [DOI] [PubMed] [Google Scholar]
- DeRossi SS, Hersh EV. Antibiotics and oral contraceptives. Dent Clin North Am. 2002;46:653–664. doi: 10.1016/s0011-8532(02)00017-4. [DOI] [PubMed] [Google Scholar]
- Eberl S, Renner B, Neubert A, Reisig M, Bachmakov I, Konig J, Dorje F, Murdter TE, Ackermann A, Dormann H, Gassmann KG, Hahn EG, Zierhut S, Brune K, Fromm MF. Role of p-glycoprotein inhibition for drug interactions: evidence from in vitro and pharmacoepidemiological studies. Clin Pharmacokinet. 2007;46:1039–1049. doi: 10.2165/00003088-200746120-00004. [DOI] [PubMed] [Google Scholar]
- Ekins S, Chang C, Mani S, Krasowski MD, Reschly EJ, Iyer M, Kholodovych V, Ai N, Welsh WJ, Sinz M, Swaan PW, Patel R, Bachmann K. Human pregnane X receptor antagonists and agonists define molecular requirements for different binding sites. Mol Pharmacol. 2007;72:592–603. doi: 10.1124/mol.107.038398. [DOI] [PubMed] [Google Scholar]
- Ekins S, Erickson JA. A pharmacophore for human pregnane-X-receptor ligands. Drug Metab Dispos. 2002;30:96–99. doi: 10.1124/dmd.30.1.96. [DOI] [PubMed] [Google Scholar]
- Ekins S, Johnston JS, Bahadduri P, D’Souzza VM, Ray A, Chang C, Swaan PW. In Vitro And Pharmacophore Based Discovery Of Novel hPEPT1 Inhibitors. Pharm Res. 2005a;22:512–517. doi: 10.1007/s11095-005-2505-y. [DOI] [PubMed] [Google Scholar]
- Ekins S, Kirillov E, Rakhmatulin EA, Nikolskaya T. A Novel Method for Visualizing Nuclear Hormone Receptor Networks Relevant to Drug Metabolism. Drug Metab Dispos. 2005b;33:474–481. doi: 10.1124/dmd.104.002717. [DOI] [PubMed] [Google Scholar]
- Eng JV, Marcus R, Hadler JL, Imhoff B, Vugia DJ, Cieslak PR, Zell E, Deneen V, McCombs KG, Zansky SM, Hawkins MA, Besser RE. Consumer attitudes and use of antibiotics. Emerging Inf Dis. 2003:9. doi: 10.3201/eid0909.020591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch CK, Chrisman CR, Baciewicz AM, Self TH. Rifampin and rifabutin drug interactions: an update. Arch Intern Med. 2002;162:985–992. doi: 10.1001/archinte.162.9.985. [DOI] [PubMed] [Google Scholar]
- Friedman CI, Huneke AL, Kim MH, Powell J. The effect of ampicillin on oral contraceptive effectiveness. Obstet Gynecol. 1980;55:33–37. [PubMed] [Google Scholar]
- Gao YD, Olson SH, Balkovec JM, Zhu Y, Royo I, Yabut J, Evers R, Tan EY, Tang W, Hartley DP, Mosley RT. Attenuating pregnane X receptor (PXR) activation: a molecular modelling approach. Xenobiotica. 2007;37:124–138. doi: 10.1080/00498250601050412. [DOI] [PubMed] [Google Scholar]
- Harmsen S, Meijerman I, Beijnen JH, Schellens JH. The role of nuclear receptors in pharmacokinetic drug-drug interactions in oncology. Cancer Treat Rev. 2007;33:369–380. doi: 10.1016/j.ctrv.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Huwyler J, Wright MB, Gutmann H, Drewe J. Induction of cytochrome P450 3A4 and P-glycoprotein by the isoxazolyl-penicillin antibiotic flucloxacillin. Curr Drug Metab. 2006;7:119–126. doi: 10.2174/138920006775541534. [DOI] [PubMed] [Google Scholar]
- Joshi JV, Joshi UM, Sankholi GM, Krishna U, Mandlekar A, Chowdhury V, Hazari K, Gupta K, Sheth UK, Saxena BN. A study of interaction of low-dose combination oral contraceptive with Ampicillin and Metronidazole. Contraception. 1980;22:643–652. doi: 10.1016/0010-7824(80)90089-x. [DOI] [PubMed] [Google Scholar]
- Khandelwal A, Krasowski MD, Reschly EJ, Sinz M, Swaan PW, Ekins S. A Comparative Analysis of Quantitative Structure Activity Relationship Methods and Docking For Human Pregnane X Receptor Activation. submitted. 2007 [Google Scholar]
- Kliewer SA, Moore JT, Wade L, Staudinger JL, Watson MA, Jones SA, McKee DD, Oliver BB, Willson TM, Zetterstrom RH, Perlmann T, Lehmann JM. An orphan nuclear receptor activated by pregnanes defines a novel steroid signalling pathway. Cell. 1998;92:73–82. doi: 10.1016/s0092-8674(00)80900-9. [DOI] [PubMed] [Google Scholar]
- Koch I, Weil R, Wolbold R, Brockmoller J, Hustert E, Burk O, Nuessler A, Neuhaus P, Eichelbaum M, Zanger U, Wojnowski L. Interindividual variability and tissue-specificity in the expression of cytochrome P450 3A mRNA. Drug Metab Dispos. 2002;30:1108–1114. doi: 10.1124/dmd.30.10.1108. [DOI] [PubMed] [Google Scholar]
- Kostrubsky VE, Ramachandran V, Venkataramanan R, Dorko K, Esplen JE, Zhang S, Sinclair JF, Wrighton SA, Strom SC. The use of human hepatocyte cultures to study the induction of cytochrome P-450. Drug Metab Dispos. 1999;27:887–894. [PubMed] [Google Scholar]
- Kovacs GT, Riddoch G, Duncombe P, Welberry L, Chick P, Weisberg E, Leavesley GM, Baker HW. Inadvertent pregnancies in oral contraceptive users. Med J Aust. 1989;150:549–551. doi: 10.5694/j.1326-5377.1989.tb136691.x. [DOI] [PubMed] [Google Scholar]
- Kramer B, Rarey M, Lengauer T. Evaluation of the FLEXX incremental construction algorithm for protein-ligand docking. Proteins. 1999;37:228–241. doi: 10.1002/(sici)1097-0134(19991101)37:2<228::aid-prot8>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Krstenansky PM, Jones WN, Garewal HS. Effect of dicloxacillin sodium on the hypoprothrombinemic response to warfarin sodium. Clin Pharm. 1987;6:804–806. [PubMed] [Google Scholar]
- Lang CC, Jamal SK, Mohamed Z, Mustafa MR, Mustafa AM, Lee TC. Evidence of an interaction between nifedipine and nafcillin in humans. Br J Clin Pharmacol. 2003;55:588–590. doi: 10.1046/j.1365-2125.2003.01789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeCluyse EL. Pregnane X receptor: molecular basis for species differences in CYP3A induction by xenobiotics. Chem Biol Interact. 2001;134:283–289. doi: 10.1016/s0009-2797(01)00163-6. [DOI] [PubMed] [Google Scholar]
- Lemaire G, Benod C, Nahoum V, Pillon A, Boussioux AM, Guichou JF, Subra G, Pascussi JM, Bourguet W, Chavanieux A, Balaguer P. Discovery of a highly active ligand of human Pregnane X Receptor: a case study from pharmacophore modeling and virtual screening to “in vivo” biological activity. Mol Pharmacol. 2007;72:572–581. doi: 10.1124/mol.106.033415. [DOI] [PubMed] [Google Scholar]
- Luo G, Cunningham M, kim S, Burn T, Lin J, Sinz M, Hamilton GA, Rizzo C, Jolley S, Gilbert D, Downey A, Mudra D, Graham R, Carroll K, Xie J, Madan A, Parkinson A, Christ D, Selling B, LeCluyse EL, Gan L-S. CYP3A4 induction by drugs: correlation between a pregnane X receptor reporter gene assay and CYP3A4 expression in human hepatocytes. Drug Metab Dispos. 2002;30:795–804. doi: 10.1124/dmd.30.7.795. [DOI] [PubMed] [Google Scholar]
- Marechal JD, Yu J, Brown S, Kapelioukh I, Rankin EM, Wolf CR, Roberts GC, Paine MJ, Sutcliffe MJ. In silico and in vitro screening for inhibition of cytochrome P450 CYP3A4 by co-medications commonly used by patients with cancer. Drug Metab Dispos. 2006;34:534–538. doi: 10.1124/dmd.105.007625. [DOI] [PubMed] [Google Scholar]
- McConn DJ, 2nd, Lin YS, Allen K, Kunze KL, Thummel KE. Differences in the inhibition of cytochromes P450 3A4 and 3A5 by metabolite-inhibitor complex-forming drugs. Drug Metab Dispos. 2004;32:1083–1091. doi: 10.1124/dmd.32.10.. [DOI] [PubMed] [Google Scholar]
- Moore JT, Kliewer SA. Use of the nuclear receptor PXR to predict drug interactions. Toxicology. 2000;153:1–10. doi: 10.1016/s0300-483x(00)00300-0. [DOI] [PubMed] [Google Scholar]
- Moore LB, Parks DJ, Jones SA, Bledsoe RK, Consler TG, Stimmel JB, Goodwin B, Liddle C, Blanchard SG, Willson TM, Collins JL, Kliewer SA. Orphan nuclear receptors constitutive androstane receptor and pregnane X receptor share xenobiotic and steroid ligands. J Biol Chem. 2000;275:15122–15127. doi: 10.1074/jbc.M001215200. [DOI] [PubMed] [Google Scholar]
- Pai MP, Momary KM, Rodvold KA. Antibiotic drug interactions. Med Clin North Am. 2006;90:1223–1255. doi: 10.1016/j.mcna.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Piscitelli SC, Gallicano KD. Interactions among drugs for HIV and opportunistic infections. N Engl J Med. 2001;344:984–996. doi: 10.1056/NEJM200103293441307. [DOI] [PubMed] [Google Scholar]
- Reimers D, Jezek A. [The simultaneous use of rifampicin and other antitubercular agents with oral contraceptives] Prax Pneumol. 1971;25:255–262. [PubMed] [Google Scholar]
- Ripp SL, Mills JB, Fahmi OA, Trevena KA, Liras JL, Maurer TS, de Morais SM. Use of immortalized human hepatocytes to predict the magnitude of clinical drug-drug interactions caused by CYP3A4 induction. Drug Metab Dispos. 2006;34:1742–1748. doi: 10.1124/dmd.106.010132. [DOI] [PubMed] [Google Scholar]
- Rosenfeld JM, Vargas R, Jr, Xie W, Evans RM. Genetic profiling defines the xenobiotic gene network controlled by the nuclear receptor pregnane X receptor. Mol Endocrinol. 2003;17:1268–1282. doi: 10.1210/me.2002-0421. [DOI] [PubMed] [Google Scholar]
- Schuetz EG, Schinkel AH, Relling MV, Schuetz JD. P-glycoprotein: A major determinant of rifampicin-inducible expression of cytochrome P4503A in mice and humans. Proc Natl Acad Sci USA. 1996;93:4001–4005. doi: 10.1073/pnas.93.9.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster D, Langer T. The identification of ligand features essential for PXR activation by pharmacophore modeling. J Chem Inf Model. 2005;45:431–439. doi: 10.1021/ci049722q. [DOI] [PubMed] [Google Scholar]
- Shenfield GM, Griffin JM. Clinical pharmacokinetics of contraceptive steroids. An update. Clin Pharmacokinet. 1991;20:15–37. doi: 10.2165/00003088-199120010-00002. [DOI] [PubMed] [Google Scholar]
- Sinz M, Kim S, Zhu Z, Chen T, Anthony M, Dickinson K, Rodrigues AD. Evaluation of 170 xenobiotics as transactivators of human pregnane X receptor (hPXR) and correlation to known CYP3A4 drug interactions. Curr Drug Metab. 2006;7:375–388. doi: 10.2174/138920006776873535. [DOI] [PubMed] [Google Scholar]
- Sparrow MJ. Pregnancies in reliable pill takers. N Z Med J. 1989;102:575–577. [PubMed] [Google Scholar]
- Strom S, Pisarov LA, Dorko K, Thompson MT, Schuetz JD, Schuetz EG. Use of human hepatocytes to study P450 gene induction. Academic Press; 1996. [DOI] [PubMed] [Google Scholar]
- Tirona RG, Lee W, Leake BF, Lan LB, Cline CB, Lamba V, Parviz F, Duncan SA, Inoue Y, Gonzalez FJ, Schuetz EG, Kim RB. The orphan nuclear receptor HNF4alpha determines PXR- and CAR-mediated xenobiotic induction of CYP3A4. Nat Med. 2003;9:220–224. doi: 10.1038/nm815. [DOI] [PubMed] [Google Scholar]
- van Dijke CP, Weber JC. Interaction between oral contraceptives and griseofulvin. Br Med J (Clin Res Ed) 1984;288:1125–1126. doi: 10.1136/bmj.288.6424.1125-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins RE, Davis-Searles PR, Lambert MH, Redinbo MR. Coactivator binding promotes the specific interaction between ligand and the pregnane X receptor. J Mol Biol. 2003;331:815–828. doi: 10.1016/s0022-2836(03)00795-2. [DOI] [PubMed] [Google Scholar]
- Weaver K, Glasier A. Interaction between broad-spectrum antibiotics and the combined oral contraceptive pill. A literature review. Contraception. 1999;59:71–78. doi: 10.1016/s0010-7824(99)00009-8. [DOI] [PubMed] [Google Scholar]
- Williams JA, Hyland R, Jones BC, Smith DA, Hurst S, Goosen TC, Peterkin V, Koup JR, Ball SE. Drug-drug interactions for UDP-glucuronosyltransferase substrates: a pharmacokinetic explanation for typically observed low exposure (AUCi/AUC) ratios. Drug Metab Dispos. 2004;32:1201–1208. doi: 10.1124/dmd.104.000794. [DOI] [PubMed] [Google Scholar]
- Wynalda MA, Hutzler JM, Koets MD, Podoll T, Wienkers LC. In vitro metabolism of clindamycin in human liver and intestinal microsomes. Drug Metab Dispos. 2003;31:878–887. doi: 10.1124/dmd.31.7.878. [DOI] [PubMed] [Google Scholar]
- Yao C, Levy RH. Inhibition-based metabolic drug-drug interactions: predictions from in vitro data. J Pharm Sci. 2002;91:1923–1935. doi: 10.1002/jps.10179. [DOI] [PubMed] [Google Scholar]
- Zhang T, Zhou JH, Shi LW, Zhu RX, Chen MB. 3D–QSAR studies with the aid of molecular docking for a series of non-steroidal FXR agonists. Bioorg Med Chem Lett. 2007;17:2156–2160. doi: 10.1016/j.bmcl.2007.01.079. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Fig 1. Downstream Direct interaction network for human PXR showing connected genes using Ingenuity Pathways Analysis 5.0 (Ingenuity Systems). Human receptors (rectangles), transporters (trapezoid) and enzymes (diamonds) are shown connected to PXR (NR1I2) and highlighted in yellow.