Summary
Drosophila is a genetically tractable system ideal for investigating the mechanisms of aging and developing interventions for promoting healthy aging. Here we describe methods commonly used in Drosophila aging research. These include basic approaches for preparation of diets and measurements of lifespan, food intake and reproductive output. We also describe some commonly used assays to measure changes in physiological and behavioral functions of Drosophila in aging, such as stress resistance and locomotor activity.
Keywords: Aging, Drosophila melanogaster, Lifespan, Reproduction, Locomotor activity, Mitochondrial function, Oxidative stress, Starvation
1. Introduction
Drosophila melanogaster is a widely used model organism that has distinct advantages in aging research, including short lifespan (mean lifespan, 2–3 months), low maintenance requirements, rich genetic resource and ease to perform genetic manipulation (1). More importantly, the Drosophila genome is fully sequenced with more than 50% of fly genes having homologs in humans (2,3). Moreover, more than 75% of known human disease genes, covering a broad range of disorders, have fly homologs (4). These features make Drosophila an ideal model organism for studying the mechanisms of aging and for developing effective aging interventions, which are relevant to aging research in humans.
Lifespan measurement is the basic method used to determine the effects of genetic and nongenetic factors involved in aging (5). A number of issues need to be carefully considered to avoid artifacts or confounds that may cause misinterpretation of the results when conducting lifespan studies. First, genetic background and control lines must be taken into consideration in order to minimize inbreeding depression and heterosis effects on lifespan (6,7). A number of genetic approaches have been developed to generate mutations and manipulate gene expression for aging research in Drosophila, which include insertion mutagenesis by P-element, gene expression alterations by the Gal4-UAS system, inducible gene expression by the gene-switch-Gal4 (GSG)-UAS system, and gene knockdown by RNA interference (RNAi) (8,9). These approaches are instrumental in investigating molecular mechanisms of aging, such as identifying single genes that are involved in modulating lifespan. For examples, methuselah (mth) is the first gene that has been found to increase Drosophila lifespan when mutated by a P-element insertion (10). In genetic studies, however, genetic backgrounds may mask or exaggerate any differences in lifespan between mutant and control animals. To reduce the undesirable effects of genetic backgrounds, experimental lines should be backcrossed for more than five generations to an appropriate wild type control line. Commonly used control lines include Canton S, Oregon R, Dahomey, yw and w1118. When possible, one should use the GSG-UAS system, which allows for conditionally altering gene expression in a temporal and/or tissue specific manner by the compound RU486 added to the food (8). By using this approach, control and mutant flies have the same genetic background and differ only in the food that they are fed. More importantly, the GSG-UAS system can be used to induce changes in gene expression in adult flies, which allows for bypassing any interference or possible lethality effect caused by a mutation during developmental stages.
Second, aging is modulated by both genetic and environmental factors. Diet is a major environmental factor that has a huge impact on lifespan in Drosophila and many other species (11). Two commonly used diets in Drosophila lifespan studies are based on cornmeal and sugar-yeast extract (SY) diets. Diet composition is a critical determinant of lifespan (11). This notion has been confirmed by numerous dietary restriction studies. Dietary restriction (DR) by diluting all or specific nutrients in the diet has been shown to extend lifespan in many species, ranging from yeast, worms, flies, rodents and primates (12). The importance of diet composition has been further demonstrated in recent nutritional geometric studies, which employ diets with various amounts of sugar and yeast extract to show that the carbohydrate-to-protein (C:P) ratio is more critical than single nutrients in determining lifespan in flies (13,14). For example, Lee et al. reported that Drosophila has an optimal mean lifespan on the SY diet with the C:P ratio at 16:1 (13). Yeast extract is the only protein source in SY diets.
Third, food intake should be carried out along with lifespan measurements considering the impact of nutrient intake on lifespan. The purpose of measuring food intake is to determine whether any genetic manipulation or aging intervention affects lifespan by directly influencing aging processes or indirectly through affecting food intake. Two major methods have been widely used to measure food intake in Drosophila. One is the indirect method, which estimates food uptake by measuring the uptake of a dye or radioactive tracer added in the food (15–17). The second method is to directly measure the amount of liquid food consumed by flies using a capillary feeder (CAFE) (18).
Only after carefully considering the confounding factors in lifespan measurements can one start to investigate the mechanisms of aging and develop effective aging interventions. A number of physiological, biochemical and behavioral assays are routinely conducted in these studies. One commonly conducted physiological assay is to measure flies’ resistance to various stressors, such as oxidative stress, starvation, heat or cold shock and desiccation. Lifespan and stress response are often closely associated, and long-lived populations tend to be more stress resistant (19,20). The free radical theory of aging proposes that cumulative damage to biological macromolecules by reactive oxygen species (ROS) leads to irreversible cell damage and an overall functional decline with age (21). Oxidative stress resistance is typically measured by feeding flies paraquat (N, N′-dimethyl-4, 4′-bipyridinium dichloride), which produces various ROS upon ingestion and consequently induces oxidative damage (22). Starvation resistance measurement is the evaluation of the ability of flies to deal with an energy shortage, an event that often occurs in real life environments. Due to the central role of energy for organisms, improving starvation resistance could be one of the mechanisms that play a role in lifespan extension. Starvation assay is typically performed by measuring the survival of adult flies on water only (23). However, longevity is not necessarily tightly correlated with resistance to oxidative stress and starvation resistance. The free radical hypothesis of aging has been challenged by numerous studies showing that animals with higher levels of oxidative damage do not necessarily have shorter lifespan than controls (24). Moreover, long-lived animals including flies show no difference and even sometimes display a decrease in resistance to oxidative stress and starvation (25–28).
The second lifespan-related physiological assay is to measure lifetime reproductive output. The “cost of reproduction” concept in aging argues for a negative correlation between reproductive output and survival due to a ‘trade-off’ in life history traits (29–32). In Drosophila, long-lived flies tend to decrease early reproduction (33), while selection for late life reproduction often identify lines with increased life span (34,35). In addition, sterile flies tend to live longer than their fertile controls (36,37), and long-lived mutants have reduced fecundity or fertility (31). Methods to assess reproductive output include measuring lifetime egg production in once-mated females or progeny number from the mating of females and males.
The third and perhaps most important lifespan-related assay is to assess healthspan. Although the precise definition of healthspan is still controversial, one healthspan parameter is locomotor activity, which can be used to assess changes in an animal’s mobility, circadian rhythm, sleep patterns and even cognitive function in aging (38). The connection between locomotor activity and aging has been well established. For example, aging is associated with a gradual decline in locomotor activities in almost all species tested so far (38–40). Two methods for assessment of locomotor behaviors are commonly employed in Drosophila aging studies. One is the rapid iterative negative geotaxis (RING) assay (Fig. 2), which tests the climbing ability of adult flies (41,42). ‘Negative geotaxis’ refers to an innate escape response elicited by mechanical stimulation, in which flies ascend the wall of a container after being tapped to the bottom of the container. The climbing speed has been demonstrated to decline with age in Drosophila. The other method used to measure locomotor activity takes advantage of the Drosophila Activity Monitoring (DAM) System (43). Typically flies are kept individually in sealed activity tubes placed in the DAM system, and the fly activity is measured by the frequency of an “activity event”, which is recorded each time a fly breaks an infrared light beam across the middle of the activity tube. The activity event data can be used to analyze a wide range of behaviors, such as circadian rhythm, sleep pattern, hypoactivity and hyperactivity (44–46). Similar to humans, Drosophila experiences a decline in sleep time with aging (47). With appropriate modifications, the DAM system is also suitable for monitoring stimulation responses to stimuli, such as noise, vibration, rotation, heat and chemicals (45,46,43). Besides the two relatively simple systems described above, sophisticated video tracking systems have been developed to analyze various fly behaviors, such as movement pattern and courting, which can be potentially used to measure lifetime behavioral changes and locomotor activity related healthspan parameters (48,49).
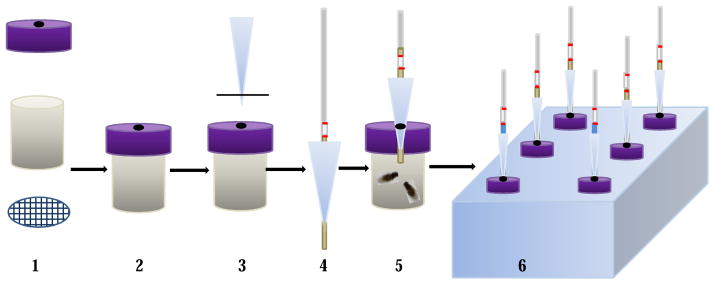
Figure 2.
The setup for the capillary feeder (CAFE) assay. 1. Parts for a fly chamber; 2. The fly chamber; 3. Cut pipette tip; 4. An assembled feeding capillary; 5. An assembled fly feeding chamber with flies; 6. A foam rack holding feeding chambers.
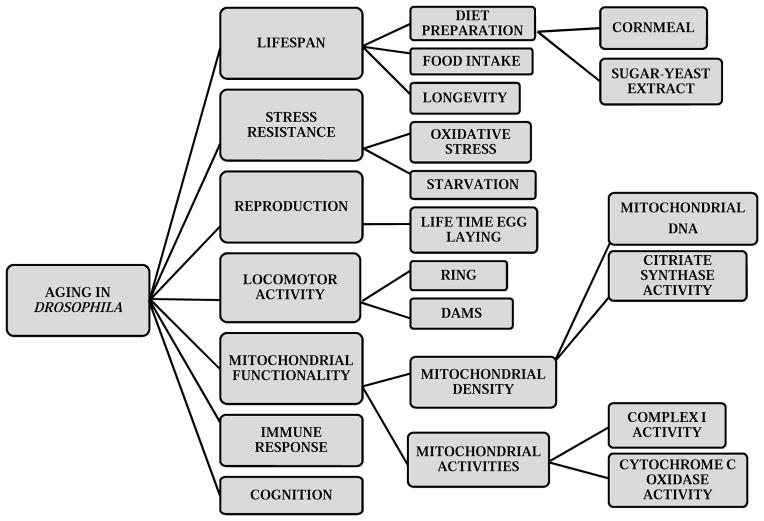
In this review, we will describe some of the basic approaches for aging studies in Drosophila (Fig. 1). We will cover methods to prepare foods and flies for lifespan measurement, and protocols to assess stress resistance, reproductive output and locomotor activity. Comprehensive mitochondrial assays can be found in a recent review (50). The protocols described here are fundamental in investigating the mechanisms by which any genetic factor, dietary intervention and other non-genetic factors influence healthspan and lifespan.
Figure 1.
Flow chart of basic assays to assess lifespan, age-related changes in physiological and behavioral function in Drosophila.
2. Materials
2.1. Diet preparation
Yellow cornmeal
Agar
Active dry yeast (Baker’s yeast)
Dextrose (D-Glucose)
Sugar (Sucrose)
Yeast extract (enzymatic yeast hydrolysate)
Methyl 4-hydroxybenzoate (Tegosept; To make a 10% stock solution, add 86.4 g Tegosept to 800 mL 70% ethanol)
Acid mix (Add distilled water to 836 mL propionic acid to bring the final volume to 1 liter. Add distilled water to 83 mL phosphoric acid to bring the final volume to 1 liter. Mix the two diluted acid solutions to make a 2-liter acid mix.)
2.2. Strains and culture conditions
Fly strains are maintained on the cornmeal diet. For lifespan assays, flies are maintained at 25°C, 60–65% humidity and a 12 h light/12 hr dark cycle in a climate-controlled incubator.
2.3. Food Intake
FD&C Blue #1 dye
Spectrophotometer
Custom-made fly chamber (Fig. 2)
Calibrated 10-μl capillary
2.4. Stress assays
Filter paper discs
Paraquat (Methyl viologen dichloride or 1,1′-dimethyl-4,4′-bipyridinium dichloride)
Sucrose
2.5. Locomotor Activity
RING apparatus
Digital camera
Drosophila Activity Monitor System
3. Methods
3.1. Cornmeal Food
Among many available recipes for cornmeal food, two most commonly used recipes are based on those developed by the Lewis laboratory and the Lakovaara laboratory, respectively (51,52). Detailed recipes can be found in the Bloomington Drosophila Stock Center website (http://flystocks.bio.indiana.edu) (Bloomington, IN, USA). The following procedure is based on the Lewis recipe, which is more commonly used in aging studies. The Lewis recipe contains 17 L distilled water, 93 g agar, 1,716 g cornmeal, 310 g Baker’s Yeast, 517 g sugar 1,033 g Dextrose and 200 mL acid mix, which results in approximately 18 L cornmeal food.
Dissolve 93 g agar in 13 L boiling water. (see Note 1)
Add 1,716 g cornmeal and 310 g active dry yeast slowly to the boiling agar while mixing continuously to avoid clumps. Simmer for an hour while mixing continuously.
Dissolve 517 g sucrose and 1,033 g dextrose in 4 L hot water and add to agar-cornmeal-yeast slurry. Reduce heat and mix until homogenous.
Cool the food to 60–65°C before adding 200 mL acid mix and mix well.
Pour or dispense the food into vials or bottles. (see Note 1)
Cover vials or bottles in the tray with cheese cloth and dry overnight before plugging.
3.2. Sugar-Yeast Extract (SY) Diet
The SY diets with desired amounts of sugar and yeast extract are made by mixing appropriate volumes of the two base solutions, 20% (w/v) sugar and 20% (w/v) yeast extract, both containing 1.5% (w/v) agar (53).
Add 200 g sugar and 15 g agar to 800 mL distilled water, and add 200 g yeast extract and 15 g agar to 800 mL distilled water in autoclavable containers. (see Note 1)
Place food containers in a boiling water bath for an hour (see Note 1). Also boil an extra 500 mL water to be used in step 4.
Remove sugar solution and yeast extract solution from the water bath and cool them down to 60–65 °C before adding 20 mL tegosept to each solution. For aging intervention studies, pharmacological and nutraceutical agents can be added to the sugar solution at this step.
Add ~200 mL water to each solution to bring the final volume to one liter.
Mix proper amounts of sugar solution, yeast extract solution and water to achieve desired sugar and yeast extract contents before pouring into vials or bottles to make SY diets. For example, add equal amounts of sugar solution and yeast extract solution together to make a SY diet with 10% sugar, 10% yeast extract and 1.5% agar, which we refer to as the SY1:1 diet (see Note 1).
3.3. Lifespan Measurement
Fly cultures are performed at 25°C, 60–65% humidity and a 12 h light/12 h dark cycle in a climate-controlled incubator. Proper control strains should be selected in each experiment (see Note 2). The following procedure is based on the protocol using once-mated flies (54,53).
To prepare flies for lifespan experiments, put approximately 50 females and an approximately equal number of males in each 6 oz bottle with cornmeal food (see Note 2). Allow the parental flies to mate and lay eggs for 4–5 days before clearing them out.
Check emerged flies after 10–14 days. Clear any flies that are eclosed on the first day and then collect newly emerged adult flies daily for the next 3 days.
Transfer emerged flies of mixed sexes daily to 6 oz bottles with cornmeal food or a SY diet, such as SY1:1, and let the flies mate for 24 h. Each bottle should have no more than 200 flies. Record the birth date.
After mating, males and females are separated under light CO2 anesthesia. Place 20 males or females in each vial with cornmeal food or the SY diet used in step 3. Set up at least five vials as replicates for each lifespan assay (see Note 2).
After another 24 h, flies are transferred to fresh vials with cornmeal food or desired SY diets.
Transfer flies into fresh vials every 2–3 days (or 3 times a week) and record the number of dead flies at each transfer.
Analyze data with suitable software to calculate mean lifespan and standard error. Plot survival curves by the Kaplan-Meier method and determine statistical differences between groups for mean lifespan by the Mantel–Cox log rank test.
Age-dependent mortality rates can be calculated according to the Gompertz model using the equation, m(t)=α*exp(b*t), where m(t) is the mortality rate at time t, α is the age-independent mortality rate or the initial mortality rate and b is the age-dependent mortality rate.
Maximum lifespan analysis is conducted on the longest-lived 10% of flies in each treatment. p<0.05 is considered statistically significant.
3.4. Food intake
3.4.1. Food tracer method
The method using FD&C blue #1 dye as the food tracer is described here and food intake is quantified as uptake of the blue dye (17).
Prepare the blue diet by mixing FD&C blue #1 dye to cooled cornmeal or SY food to a final concentration of 0.5% and pouring 5 mL food to each vial.
Transfer 5 flies of 7–14 days old to each vial containing the blue diet. Age-matched control flies are transferred to non-dyed food. Allow flies to feed for 30 min. Set up 5–6 vials as replicates for each treatment.
While waiting, dissolve 0.5 g dyed food in 15 mL distilled water. Prepare serial 2-fold dilutions of the stock solution by 8–128 folds. After centrifuging at 12,000× g for 2 min, measure the absorbance of dilutions at 625 nm to generate a standard curve of the absorbance.
Transfer both experimental and control flies in each vial into a 1.5 mL eppendorf tube and then snap freeze in liquid nitrogen (see Note 3).
Separate fly heads from the bodies by briefly vortexing the tube (see Note 3).
Collect and homogenize fly bodies in 200 μl distilled water with a plastic pestle and then add 800 μl distilled water. Centrifuge at 12,000× g for 2 min.
Transfer 0.9 mL supernatant to a new tube and bring to a final volume of 1.5 ml with distilled water, and centrifuge again for 2 min.
Immediately measure the sample absorbance at 625 nm using a spectrophotometer (see Note 3). The absorbance from control flies is used to correct for background absorbance of flies.
Calculate food intake by using the net absorbance and the standard curve generated in step 3.
3.4.2. CAFE method
The CAFE method described here is based on the protocol published by Ja et al. with some modifications (18,55) (see Note 3).
To make a fly chamber (Fig. 4.), cut a 15-mL plastic tube (1.5-cm diameter) to 2-cm height from the end with cap, seal the open end with a nylon mesh, and poke a hole of ~2 mm diameter in the cap to hold a 200 μl pipette tip.
Cut off 1–2 mm from the tip of a 200 μl pipette tip with a sharp blade. Make sure the capillary can go through but not drop through it.
Add 1 mL water in each hole of a foam rack for 15-ml tubes, which typically has 5×5 holes and is used to house fly chambers.
Transfer one or two flies under light CO2 to each chamber and use a 200-μl pipette tip to temporarily block the hole in the cap to prevent flies from escaping. Allow flies to recover from CO2 anesthesia for at least 15 min.
Fill each calibrated 10-μl capillary with 2–3 mm mineral oil first for minimizing food evaporation, and then with 20–30 mm liquid SY food without agar to ensure that the food is dripped out when placed in the fly chamber. Wipe out any food outside the capillary with tissue paper. Mark the food level on the capillary with a marker pen as the starting volume.
Put the filled capillary through a cut 200-μl pipette tip with 2–3 mm of the capillary over the pipette tip, and replace the empty tip on the chamber cap.
Load the CAFE chambers with flies and filled capillaries onto the foam rack with water. Each rack should hold 8–12 chambers. Put the rack in an incubator at 25°C and 70% humidity. Set up 8–16 chambers as replicates for each treatment.
Two capillaries with food are set up in separate chambers without flies and placed in the foam rack to correct for evaporation.
After 24 h feeding, mark the food level on both feeding capillary and evaporation controls. Food intake is recorded as the length of the two marks on the feeding capillary subtracted from the average length of the two marks on evaporation controls.
Convert the length to the volume of food based on calibration of the capillary. Each 5-mm length typically equals to 1 μl of liquid food when using the 10-μl capillary.
Repeat steps 5 to 10 with new capillaries to measure food intake for three consecutive days.
Food intake is calculated by averaging daily food consumption per fly over three days.
3.5. Stress assays
3.5.1. Oxidative Stress Resistance
Oxidative stress resistance is based on flies’ resistance to paraquat feeding (56,10).
Flies are prepared and aged for 7–14 days as described in 3.3.
Prepare paraquat vials by adding 600 μl 20 mM paraquat in 5% sucrose solution into vials, each with 2–3 pieces of 22-mm filter discs (see Note 4).
Transfer 20 flies into each paraquat vial. 6–10 vials are set up as replicates for each treatment.
Record the number of dead flies once every 12 h.
Transfer flies to fresh vials with the paraquat solution every 48 h.
3.5.2. Starvation assay
Follow steps in 3.5.1. substituting the paraquat solution with 600 μl autoclaved water on filter discs.
3.6. Reproductive output
This protocol is used to measure the lifetime egg production of once-mated females (57) (see Note 5).
Fly preparation follows steps 1–4 in 3.3.
Place five once-mated females in each vial on cornmeal or a desired SY diet. Set up 6–8 vials as replicates for each treatment.
Transfer flies daily to fresh food until all flies are dead.
Count the number of dead flies and eggs laid in old vials after each transfer (see Note 5).
To calculate age-specific reproductive output, divide the total number of eggs produced daily by the number of surviving flies.
To calculate the lifetime reproductive output, divide the total amount of eggs produced by the total number of females used for the experiment.
3.7. Locomotor activity
3.7.1. Rapid Iterative Negative Geotaxis (RING) assay
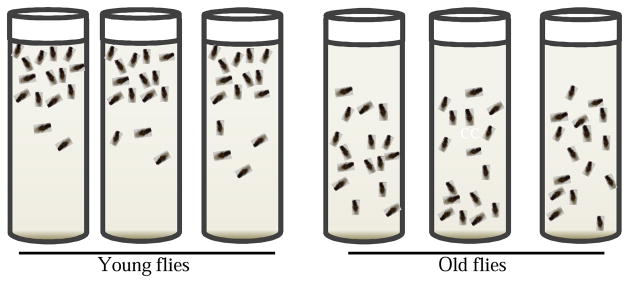
The procedure is based on the RING device and protocol described by Nicols et al.(42) (Fig. 3).
Figure 3.
The rapid iterative negative geotaxis (RING) assay for measuring locomotor activities of young and old flies. Young flies generally climb faster than old flies after tapped to the bottom of the vials.
Prepare flies following steps 1–5 in 3.3.
Transfer 20 flies without anesthesia to each new polystyrene vial and assemble vials into the RING apparatus.
Allow flies to acclimate to the environment for 15–20 minutes.
Place a digital camera ~1 m away in front of the apparatus and focus the camera onto the apparatus, and set a timer to 3 seconds.
Sharply tap the apparatus down on the bench three times to knock all flies down to the bottom of the vial.
Start a 3-sec countdown timer immediately after the third tap.
Take a picture after three sec.
Repeat steps 5–7 after a 1 min rest for 5–6 times.
Upload images onto a computer and calculate the height that each fly climbs in each vial.
Calculate the mean height that flies climb in each group and perform statistical analysis.
3.7.2. Drosophila Activity Monitoring (DAM) System
The procedures are based on the protocol previously described (43).
Before the experiments, set the incubator to desired light cycle and temperature. A typical experiment is run at 25°C and a 12 hr light/12 hr dark cycle for 3–5 days (see Note 6).
Pour desired liquid SY food in a beaker to a depth of approximately 2 to 2.5cm.
Position behavior tubes vertically in the beaker.
Once food solidifies, remove tubes by rotating the ends or by moving them side to side against the food until they are free.
Wipe out any residual food outside the walls of tubes with tissue paper (see Note 6).
Cover the food end of each tube with a cap that has a hole in the middle.
Anesthetize flies under light CO2 and transfer one fly to each behavior tube (see Note 6).
Cap the open end of the tube with a tiny piece of cotton ball.
Place tubes into the activity monitor so that the center of each tube is in the holder.
Wrap a rubber band around every three to four tubes to hold them in place, and put the activity monitor in the environmentally controlled incubator.
Connect the activity monitor to a computer with the DAM system software.
Set the activity reading to any desired interval with the DAM system software.
Start run when all conditions are set. Do not open the incubator while locomotor activity is being measured (see Note 6).
Once the recording is stopped, export the data from the DAM system to a computer for analyzing locomotor activity, circadian rhythm and sleep patterns (45,46,43).
Acknowledgments
This work was supported by the Intramural Research Program at the National Institute on Aging, NIH, to SZ.
Footnotes
Adjust the total volume of the food to be prepared based on whether the cooking is done with an electric stove, steamer or electric kettle. Adjust the amount of agar to achieve desirable and consistent texture of the cornmeal food (52). Additional cornmeal recipes can be found on the Bloomington Stock Center website (www.flystock.bio.indiana.edu). The sugar and yeast extract portions of the SY diets are cooked separately to minimize the brown reaction between sugar and protein, which damages protein and potentially affects the survival of flies. Tegosept and other pharmacological and nutraceutical reagents should be added after the food is cooled down to 60–65 °C to minimize heat-induced loss of activities. The SY diets used in aging studies are recommended to have 1.5% agar, which provides appropriate texture for flies, based on studies by the Partridge lab (53). For dietary restriction studies, 8% cornmeal can be added to the SY diets, which may minimize the confounding effect of water consumption on lifespan (58). Dispense 5–10 ml of food per vial and 50 ml per 6 oz bottle. Cooked food can last up to a few weeks if stored at 4°C.
Age of parental flies should be less than one week and the density of eggs laid in each bottle should be kept between 100 and 200 to minimize the developmental influence on adult lifespan. When sorting out flies for lifespan measurements, do not anesthetize flies for more than 15 min under CO2 as this may result in brain damage or reduced lifespan. A minimum of 5 vials of flies (~ 100 flies) per group should be used for each lifespan experiment to minimize the impact of sample size and obtain enough statistical power (59).
The feeding period in the food tracer method is limited to approximately 30 min to ensure that the ingested dye is mostly, if not all, retained in the fly. The food tracer method can be used for measuring food intake on the solid food. However, flies have to be sacrificed in each measurement. This prevents longitudinal monitoring of food intake, such as days or lifetime of flies, which is valuable for aging studies. The CAFE assay is suitable for such long-term studies, but only applies to liquid diet. Minimize the presence of air bubbles to ensure the flow of the liquid in the capillary. It should be noted that it is still controversial whether feeding the liquid food in the CAFE setup reflects the natural feeding environment of flies (17).
For oxidative stress, adjust paraquat concentrations based on the sensitivity of control lines to oxidative stress in each experiment so that approximately 50% of control flies still survive in day 2 on paraquat treatment. Most commonly used concentrations are 15–30 mM inf 5% sucrose solution.
Reproductive output can also be measured with mixed males and females by counting either egg production or the number of eclosed flies. In reproductive output experiments with once-mated females, flies can be transferred to fresh vials every 2–3 days instead of every day. However, to do so, one should be careful not to count any emerged larvae. Count the egg shells that the larvae leave behind on the food for egg counting.
For measuring locomotor activity with the DAM system, make sure the activity tubes remain moist but without any water droplet before transferring flies (43). Flies should not be touching the food when they are transferred to the activity tube to avoid getting stuck. The center of each tube should be aligned with one another and in the center of the holder in the activity monitor. To achieve appropriate alignment, gently push one side of the test tubes against the wall or a flat surface to align their centers after wrapping the tubes with the rubber bands. Perform the recording for at least 3 to 4 consecutive days to reduce variations in locomotor activity.
References
- 1.Helfand SL, Rogina B. Genetics of aging in the fruit fly, Drosophila melanogaster. Annu Rev Genet. 2003;37:329–348. doi: 10.1146/annurev.genet.37.040103.095211. [DOI] [PubMed] [Google Scholar]
- 2.Adams MD, Celniker SE, Holt RA, et al. The genome sequence of Drosophila melanogaster. Science. 2000;287:2185–2195. doi: 10.1126/science.287.5461.2185. [DOI] [PubMed] [Google Scholar]
- 3.Myers EW, Sutton GG, Delcher AL, et al. A whole-genome assembly of Drosophila. Science. 2000;287:2196–2204. doi: 10.1126/science.287.5461.2196. [DOI] [PubMed] [Google Scholar]
- 4.Reiter LT, Potocki L, Chien S, et al. A systematic analysis of human disease-associated gene sequences in Drosophila melanogaster. Genome Research. 2001;11:1114–1125. doi: 10.1101/gr.169101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finch CE, Ruvkun G. The genetics of aging. Annu Rev Genomics Hum Genet. 2001;2:435–462. doi: 10.1146/annurev.genom.2.1.435. [DOI] [PubMed] [Google Scholar]
- 6.Swindell WR, Bouzat JL. Inbreeding depression and male survivorship in Drosophila: implications for senescence theory. Genetics. 2006;172:317–327. doi: 10.1534/genetics.105.045740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fry JD, Heinsohn SL, Mackay TF. Heterosis for viability, fecundity, and male fertility in Drosophila melanogaster: comparison of mutational and standing variation. Genetics. 1998;148:1171–1188. doi: 10.1093/genetics/148.3.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGuire SE, Mao Z, Davis RL. Spatiotemporal gene expression targeting with the TARGET and gene-switch systems in Drosophila. Sci STKE. 2004;2004:pl6. doi: 10.1126/stke.2202004pl6. [DOI] [PubMed] [Google Scholar]
- 9.Tower J. Transgenic methods for increasing Drosophila life span. Mech Ageing Dev. 2000;118:1–14. doi: 10.1016/s0047-6374(00)00152-4. [DOI] [PubMed] [Google Scholar]
- 10.Lin YJ, Seroude L, Benzer S. Extended life-span and stress resistance in the Drosophila mutant methuselah. Science. 1998;282:943–946. doi: 10.1126/science.282.5390.943. [DOI] [PubMed] [Google Scholar]
- 11.Piper MD, Partridge L, Raubenheimer D, et al. Dietary restriction and aging: a unifying perspective. Cell Metab. 2011;14:154–160. doi: 10.1016/j.cmet.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fontana L, Partridge L, Longo VD. Extending healthy life span--from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee KP, Simpson SJ, Clissold FJ, et al. Lifespan and reproduction in Drosophila: New insights from nutritional geometry. Proc Natl Acad Sci U S A. 2008;105:2498–2503. doi: 10.1073/pnas.0710787105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skorupa DA, Dervisefendic A, Zwiener J, et al. Dietary composition specifies consumption, obesity, and lifespan in Drosophila melanogaster. Aging Cell. 2008;7:478–490. doi: 10.1111/j.1474-9726.2008.00400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carvalho GB, Kapahi P, Benzer S. Compensatory ingestion upon dietary restriction in Drosophila melanogaster. Nat Methods. 2005;2:813–815. doi: 10.1038/nmeth798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edgecomb RS, Harth CE, Schneiderman AM. Regulation of Feeding-Behavior in Adult Drosophila-Melanogaster Varies with Feeding Regime and Nutritional State. Journal of Experimental Biology. 1994;197:215–235. doi: 10.1242/jeb.197.1.215. [DOI] [PubMed] [Google Scholar]
- 17.Wong R, Piper MD, Wertheim B, et al. Quantification of food intake in Drosophila. PLoS One. 2009;4:e6063. doi: 10.1371/journal.pone.0006063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ja WW, Carvalho GB, Mak EM, et al. Prandiology of Drosophila and the CAFE assay. Proc Natl Acad Sci U S A. 2007;104:8253–8256. doi: 10.1073/pnas.0702726104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haigis MC, Yankner BA. The aging stress response. Mol Cell. 2010;40:333–344. doi: 10.1016/j.molcel.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirkwood TBL, Austad SN. Why do we age? Nature. 2000;408:233–238. doi: 10.1038/35041682. [DOI] [PubMed] [Google Scholar]
- 21.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 22.Arking R, Buck S, Berrios A, et al. Elevated paraquat resistance can be used as a bioassay for longevity in a genetically based long-lived strain of Drosophila. Dev Genet. 1991;12:362–370. doi: 10.1002/dvg.1020120505. [DOI] [PubMed] [Google Scholar]
- 23.Huey RB, Suess J, Hamilton H, et al. Starvation resistance in Drosophila melanogaster: testing for a possible ‘cannibalism’ bias. Functional Ecology. 2004;18:952–954. [Google Scholar]
- 24.Salmon AB, Richardson A, Perez VI. Update on the oxidative stress theory of aging: does oxidative stress play a role in aging or healthy aging? Free Radic Biol Med. 2010;48:642–655. doi: 10.1016/j.freeradbiomed.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Force AG, Staples T, Soliman S, et al. Comparative biochemical and stress analysis of genetically selected Drosophila strains with different longevities. Developmental Genetics. 1995;17:340–351. doi: 10.1002/dvg.1020170407. [DOI] [PubMed] [Google Scholar]
- 26.Hoffmann AA, Harshman LG. Desiccation and starvation resistance in Drosophila: patterns of variation at the species, population and intrapopulation levels. Heredity (Edinb) 1999;83( Pt 6):637–643. doi: 10.1046/j.1365-2540.1999.00649.x. [DOI] [PubMed] [Google Scholar]
- 27.Kapahi P, Zid BM, Harper T, et al. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway (May 25, pg 885, 2004) Curr Biol. 2004;14:1789–1789. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 29.Flatt T, Promislow DE. Physiology. Still pondering an age-old question. Science. 2007;318:1255–1256. doi: 10.1126/science.1147491. [DOI] [PubMed] [Google Scholar]
- 30.De Loof A. Longevity and aging in insects: Is reproduction costly; cheap; beneficial or irrelevant? A critical evaluation of the “trade-off” concept. J Insect Physiol. 2011;57:1–11. doi: 10.1016/j.jinsphys.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 31.Flatt T. Survival costs of reproduction in Drosophila. Exp Gerontol. 2011;46:369–375. doi: 10.1016/j.exger.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 32.Tatar M. Reproductive aging in invertebrate genetic models. Ann N Y Acad Sci. 2010;1204:149–155. doi: 10.1111/j.1749-6632.2010.05522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zwaan B, Bijlsma R, Hoekstra RE. Direct Selection on Life-Span in Drosophila-Melanogaster. Evolution. 1995;49:649–659. doi: 10.1111/j.1558-5646.1995.tb02301.x. [DOI] [PubMed] [Google Scholar]
- 34.Hutchinson EW, Shaw AJ, Rose MR. Quantitative Genetics of Postponed Aging in Drosophila-Melanogaster.2. Analysis of Selected Lines. Genetics. 1991;127:729–737. doi: 10.1093/genetics/127.4.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Partridge L, Prowse N, Pignatelli P. Another set of responses and correlated responses to selection on age at reproduction in Drosophila melanogaster. Proceedings of the Royal Society of London Series B-Biological Sciences. 1999;266:255–261. doi: 10.1098/rspb.1999.0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barnes AI, Wigby S, Boone JM, et al. Feeding, fecundity and lifespan in female Drosophila melanogaster. Proc Biol Sci. 2008;275:1675–1683. doi: 10.1098/rspb.2008.0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sgro CM, Partridge L. A delayed wave of death from reproduction in Drosophila. Science. 1999;286:2521–2524. doi: 10.1126/science.286.5449.2521. [DOI] [PubMed] [Google Scholar]
- 38.Iliadi KG, Boulianne GL. Age-related behavioral changes in Drosophila. Ann N Y Acad Sci. 2010;1197:9–18. doi: 10.1111/j.1749-6632.2009.05372.x. [DOI] [PubMed] [Google Scholar]
- 39.Shively CA, Willard SL, Register TC, et al. Aging and physical mobility in group-housed Old World monkeys. Age (Dordr) 2011 doi: 10.1007/s11357-011-9350-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Francois M, Morice AH, Blouin J, et al. Age-related decline in sensory processing for locomotion and interception. Neuroscience. 2011;172:366–378. doi: 10.1016/j.neuroscience.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 41.Gargano JW, Martin I, Bhandari P, et al. Rapid iterative negative geotaxis (RING): a new method for assessing age-related locomotor decline in Drosophila. Exp Gerontol. 2005;40:386–395. doi: 10.1016/j.exger.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 42.Nichols CD, Becnel J, Pandey UB. Methods to assay Drosophila behavior. J Vis Exp. 2012 doi: 10.3791/3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pfeiffenberger C, Lear BC, Keegan KP, et al. Locomotor activity level monitoring using the Drosophila Activity Monitoring (DAM) System. Cold Spring Harb Protoc. 2010;2010 doi: 10.1101/pdb.prot5518. pdb prot5518. [DOI] [PubMed] [Google Scholar]
- 44.Allada R, Chung BY. Circadian organization of behavior and physiology in Drosophila. Annu Rev Physiol. 2010;72:605–624. doi: 10.1146/annurev-physiol-021909-135815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pfeiffenberger C, Lear BC, Keegan KP, et al. Processing sleep data created with the Drosophila Activity Monitoring (DAM) System. Cold Spring Harb Protoc. 2010;2010 doi: 10.1101/pdb.prot5520. pdb prot5520. [DOI] [PubMed] [Google Scholar]
- 46.Pfeiffenberger C, Lear BC, Keegan KP, et al. Processing circadian data collected from the Drosophila Activity Monitoring (DAM) System. Cold Spring Harb Protoc. 2010;2010 doi: 10.1101/pdb.prot5519. pdb prot5519. [DOI] [PubMed] [Google Scholar]
- 47.Koh K, Evans JM, Hendricks JC, et al. A Drosophila model for age-associated changes in sleep:wake cycles. Proc Natl Acad Sci U S A. 2006;103:13843–13847. doi: 10.1073/pnas.0605903103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Branson K, Robie AA, Bender J, et al. High-throughput ethomics in large groups of Drosophila. Nat Methods. 2009;6:451–457. doi: 10.1038/nmeth.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grover D, Yang J, Ford D, et al. Simultaneous tracking of movement and gene expression in multiple Drosophila melanogaster flies using GFP and DsRED fluorescent reporter transgenes. BMC Res Notes. 2009;2:58. doi: 10.1186/1756-0500-2-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frazier AE, Thorburn DR. Biochemical analyses of the electron transport chain complexes by spectrophotometry. Methods Mol Biol. 2012;837:49–62. doi: 10.1007/978-1-61779-504-6_4. [DOI] [PubMed] [Google Scholar]
- 51.Lakovaara S. Malt as a culture medium for Drosophila species. Drosophila Information Service. 1969;44:128. [Google Scholar]
- 52.Lewis EB. A new standard food medium. Drosophila Information Service. 1960;34:117–118. [Google Scholar]
- 53.Bass TM, Grandison RC, Wong R, et al. Optimization of dietary restriction protocols in Drosophila. J Gerontol A Biol Sci Med Sci. 2007;62:1071–1081. doi: 10.1093/gerona/62.10.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun X, Seeberger J, Alberico T, et al. Acai palm fruit (Euterpe oleracea Mart.) pulp improves survival of flies on a high fat diet. Exp Gerontol. 2010;45:243–251. doi: 10.1016/j.exger.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun X, Komatsu T, Lim J, et al. Nutrient-dependent requirement for SOD1 in lifespan extension by protein restriction in Drosophila melanogaster. Aging Cell. 2012 doi: 10.1111/j.1474-9726.2012.00842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arking R, Buck S, Berrios A, et al. Elevated Paraquat Resistance Can Be Used as a Bioassay for Longevity in a Genetically Based Long-Lived Strain of Drosophila. Developmental Genetics. 1991;12:362–370. doi: 10.1002/dvg.1020120505. [DOI] [PubMed] [Google Scholar]
- 57.Boyd O, Weng P, Sun X, et al. Nectarine promotes longevity in Drosophila melanogaster. Free Radic Biol Med. 2011;50:1669–1678. doi: 10.1016/j.freeradbiomed.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ja WW, Carvalho GB, Zid BM, et al. Water- and nutrient-dependent effects of dietary restriction on Drosophila lifespan. Proc Natl Acad Sci U S A. 2009;106:18633–18637. doi: 10.1073/pnas.0908016106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pletcher SD, Khazaeli AA, Curtsinger JW. Why do life spans differ? Partitioning mean longevity differences in terms of age-specific mortality parameters. J Gerontol A Biol Sci Med Sci. 2000;55:B381–389. doi: 10.1093/gerona/55.8.b381. [DOI] [PubMed] [Google Scholar]