Abstract
Purpose
Renal cell carcinoma with sarcomatoid dedifferentiation (sRCC) is an aggressive malignancy associated with a poor prognosis. While existing literature focuses on patients presenting with metastatic disease, characteristics and outcomes for patients with localized disease are not well described. We aimed to evaluate post-nephrectomy characteristics, outcomes, and predictors of survival in patients with sRCC who presented with clinically localized disease.
Patients and Methods
An IRB-approved review from 1986–2011 identified 77 patients who presented with clinically localized disease, underwent nephrectomy and had sRCC in their primary kidney tumor. Clinical and pathologic variables were captured for each patient. Overall survival (OS) and recurrence-free survival (RFS) were calculated for all patients and those who had no evidence of disease (NED) following nephrectomy, respectively. Comparisons were made with categorical groupings in proportional hazards regression models for univariable and multivariable analyses.
Results
OS for the entire cohort (N=77) at 2 years was 50%. A total of 56 (77%) patients of the 73 who were NED following nephrectomy experienced a recurrence, with a median time to recurrence of 26.2 months. On multivariable analysis, tumor stage, pathologically positive lymph nodes, and year of nephrectomy were significant predictors of both OS and RFS. Limitations include the retrospective nature of this study and relatively small sample size.
Conclusions
Long-term survival for patients with sRCC, even in clinically localized disease is poor. Aggressive surveillance of those who are NED following nephrectomy is essential and further prospective studies evaluating the benefit of adjuvant systemic therapies in this cohort are warranted.
Keywords: renal cell carcinoma, sarcomatoid, nephrectomy
1. INTRODUCTION
Renal cell carcinoma with sarcomatoid dedifferentiation (sRCC) is an aggressive variant of renal cell carcinoma historically associated with a poor prognosis and a median survival of 4–9 months [1–3]. sRCC occurs in 4–32% of all RCC, and is associated with high-grade tumors with an underlying clear-cell epithelial component, although it could occur with any RCC histologic subtype [4–8]. Factors that contribute to aggressive behavior of sRCC are not well understood. Previous studies have noted that approximately 70–80% of patients diagnosed with sRCC initially present with metastatic disease and, as expected, have a worse overall survival than those presenting with localized disease [3, 8]. Given the small numbers of patients who initially present with localized disease, prognostic factors and outcomes for this cohort are largely unknown and to our knowledge, there are no existing studies that specifically focus on this subset of patients.
Our aim was to study the clinical presentation, surgical outcomes, pathologic details, recurrence patterns and treatment, and survival predictors and outcomes in patients with clinically non-metastatic sRCC at presentation who were treated with surgery with curative intent.
2. PATIENTS AND METHODS
2.1 Patients
This was a single-institution retrospective study conducted after IRB approval was obtained. Our database contained information on 273 patients from 1986 to 2011 who were identified as having sRCC. Patients who were lost to follow-up or are currently participating in an unreported clinical trial were excluded. Complete clinical and pathologic data were available for 230 patients who underwent partial or radical nephrectomy and had sRCC in their primary nephrectomy specimen. Of 230 patients, 77 presented with clinically localized disease and comprised the current study cohort.
2.2 Clinical and pathologic characteristics
Patient characteristics and intraoperative details were recorded for all patients at the time of presentation and surgery. Clinical information included age, gender, Eastern Cooperative Oncology Group performance status (ECOG PS), race, associated symptoms and year of nephrectomy. All patients underwent a metastatic evaluation including at least a chest X-ray or CT Chest, and CT Abdomen/pelvis prior to proceeding with surgery. A regional retroperitoneal lymph node dissection was performed at the discretion of the operating surgeon. None of the patients received adjuvant systemic therapy.
Pathologic variables included tumor size, tumor stage, lymph node status, margin status, necrosis, lymphovascular invasion (LVI), histology and percent sarcomatoid component. All available pathology slides were reviewed by dedicated genitourinary pathologists, who performed microscopic visual estimation of the percentage sarcomatoid component. Patients with pathology documenting a “focal” sarcomatoid component were included with the 0–24% group while those reported to have “predominant” or “majority” of the specimen comprised of sRCC were included in the 75–99% group. Patients with 100% sarcomatoid component are considered to be unclassified RCC and therefore were not included in the study. Data on estimated blood loss (EBL), blood transfusions, surgery time and additional organs resected were also recorded for all patients.
2.3 Statistical methods
Overall survival (OS) was measured in months from nephrectomy until death or last follow-up. Patients who were alive at their last contact were censored on that date. Recurrence-free survival (RFS) was calculated for patients who had no evidence of disease (NED) following nephrectomy (RFS evaluable). RFS was measured in months from the date of nephrectomy to recurrence, death, or last follow-up if alive and free of recurrence. Patients who were alive and free of recurrence at last follow-up were censored on that date. Comparisons were made with categorical groupings in proportional hazards regression models for univariable and multivariable analyses. All characteristics of interest were compared for univariable analyses. Due to sample size, a selection of characteristics was determined based on clinical knowledge for multivariable analyses. Results are presented for the “full” model containing tumor stage, lymph node positivity, margin status, LVI, and year of nephrectomy. Then a step-wise backwards selection procedure was also run until only significant characteristics remained in the model. All analyses were performed in SAS 9.3(SAS Institute Inc, Cary, NC). Survival estimates were plotted according to Kaplan-Meier methods using Stata 12.1(StatCorp, College Station, TX).
3. RESULTS
3.1 Clinical and pathological characteristics
This study included 77 patients presenting with clinically localized disease (clinical N0M0). Table 1 shows the detailed patient demographic and pathologic characteristics. Median age at time of surgery was 63 years (range 38–85). Forty-four patients (57%) were male and 52 (68%) were white. Thirty-four (44%) patients had ECOG performance status of 0 and 42 (55%) had ECOG performance status of 1. Seventy (91%) patients presented with at least one relevant symptom: the most common symptoms at presentation were pain (57%), hematuria (45%), and weight loss (23%). Median tumor size was 10 cm (range 1.8–27), 58 (77%) patients had pathologic stage T3 or T4, and 56 (73%) patients had clear cell histology as the parent epithelial component. Nineteen (25%) patients had pathologically positive lymph nodes. Fifteen patients (19%) had LVI, 39 (51%) patients had <25% sarcomatoid component reported in the nephrectomy specimen. Fifteen patients (19%) had a microscopic positive margin: 6 had microscopic soft tissue margin, 4 had microscopic renal vein/IVC margin, 1 had both, and 4 were equivocal/unclear, related to vein margin status, but were still counted as positive for statistical purposes.
Table 1.
Patient Demographics and Pathological Characteristics
| N (%) | ||
|---|---|---|
| All patients | 77 (100) | |
|
| ||
| Clinical Characteristics | ||
|
| ||
| Age – median, years (range) | 63 (38–85) | |
| Age categories (years) | 22–45 | 5 (6) |
| 46–55 | 18 (23) | |
| 56–69 | 35 (45) | |
| 70+ | 19 (25) | |
|
| ||
| Gender | Female | 33 (43) |
| Male | 44 (57) | |
|
| ||
| ECOG Performance Status | 0 | 34 (44) |
| 1 | 42 (55) | |
| >=2 | 1 (1) | |
|
| ||
| Race | White | 52 (68) |
| Hispanic | 16 (21) | |
| Black | 7 (9) | |
| Other | 2 (3) | |
|
| ||
| Symptoms | Any relevant | 70 (91) |
| Pain | 44 (57) | |
| Hematuria | 35 (45) | |
| Weight Loss | 18 (23) | |
| Fever | 6 (8) | |
| Night Sweats | 6 (8) | |
| Other Symptoms | 28 (36) | |
|
| ||
| Pathological Characteristics | ||
|
| ||
| Tumor Size – median, cm (range) | N=68 | 10 (1.8–27) |
|
| ||
| Pathologic Tumor Stage* | T1/T2 | 18 (23) |
| T3 | 42 (55) | |
| T4 | 16 (22) | |
| N (%) | ||
|
| ||
| Pathologic Nodal Stage | N0/Nx | 58 (75) |
| N1 | 19 (25) | |
|
| ||
| Primary Epithelial Histology | ||
| Clear Cell | 56 (73) | |
| Other | 21 (27) | |
|
| ||
| Percent Sarcomatoid Component | 1–24 | 39 (51) |
| 25–49 | 9 (12) | |
| 50–74 | 8 (10) | |
| 75–99 | 12 (16) | |
|
| ||
| Positive Surgical Margins | 15 (19) | |
|
| ||
| Necrosis* | 26 (34) | |
|
| ||
| LVI* | 15 (19) | |
Numbers do not always sum to 77 due to missing information 1 each for tumor stage, necrosis, and LVI and 9 for percent sarcomatoid and tumor size
3.2 Intraoperative characteristics
Table 2 displays intraoperative characteristics of the study patients. Two patients underwent partial nephrectomy and 75 underwent radical nephrectomy. Twenty (27%) of the patients who underwent radical nephrectomy also had an IVC thrombectomy. Median EBL was 800mL (range 0–30,000) and median surgery time was 219 minutes (range 61–910). Forty-two (60%) patients received a blood transfusion. Forty-seven (61%) patients underwent a lymph node dissection and 17 (22%) patients had additional organs resected at time of nephrectomy secondary to direct invasion, most commonly bowel (10 patients).
Table 2.
Intraoperative Details
| Patient Characteristics | N (%) |
|---|---|
| All | 77 (100) |
|
| |
| Partial Nephrectomy | 2 (3) |
| Radical Nephrectomy | 75 (97) |
| Concomitant IVC Thrombectomy | 20 (26) |
| Estimated Blood Loss – median, mL (range) (N=70) | 800 (0–30,000) |
| Transfusion received (N=70) | 42 (60) |
| Transfusion – median, number of units (range) (N=46) | 3 (0, 49) |
| Operative Time – median, minutes (range) (N=64) | 219 (61, 910) |
| LN Dissection | 47 (61) |
| Other Organ Resection | 17 (22) |
| Bowel | 10 (13) |
| IVC | 3 (4) |
| Gallbladder | 1(1) |
| Spleen | 3 (4) |
| Diaphragm | 3 (4) |
| Pancreas | 3 (4) |
3.3 Survival
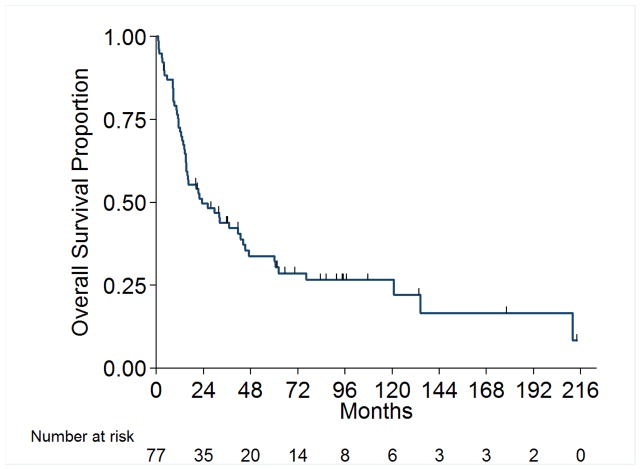
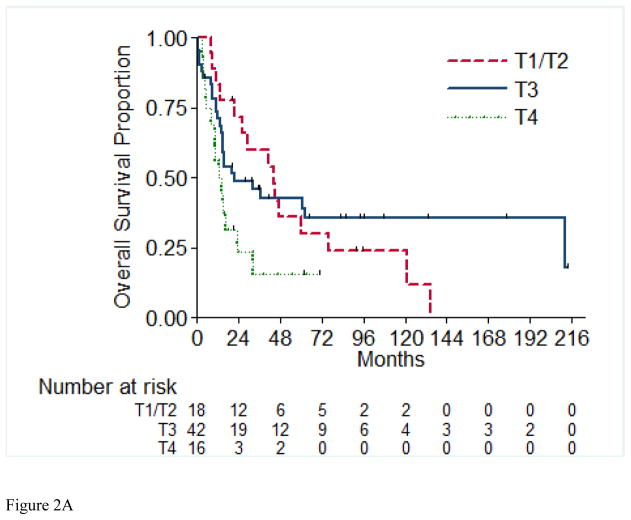
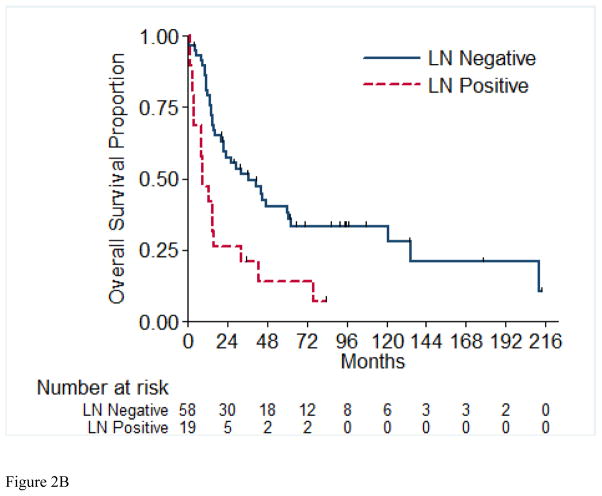
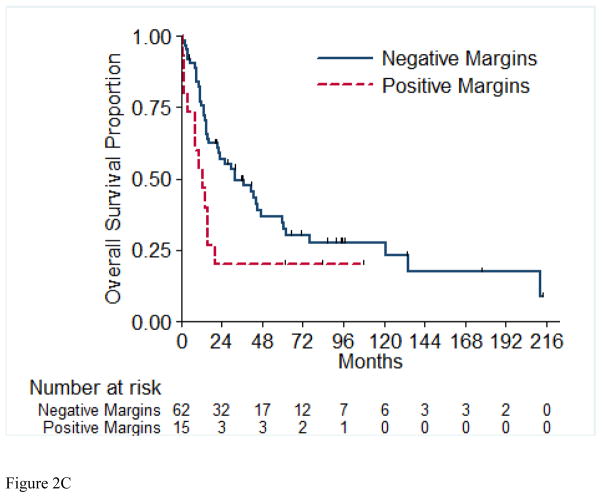
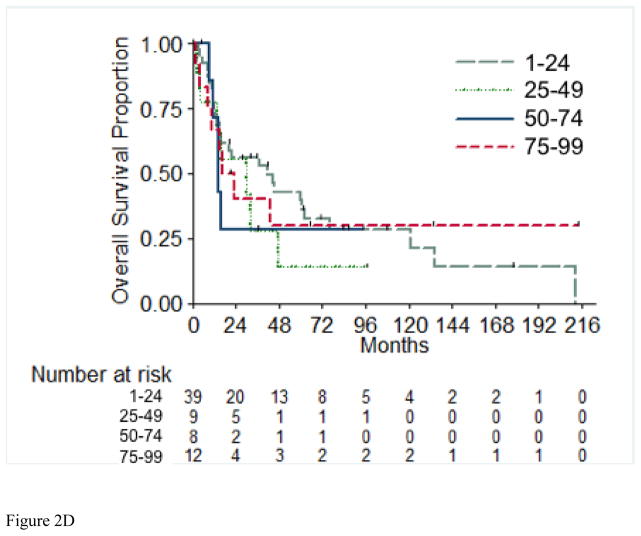
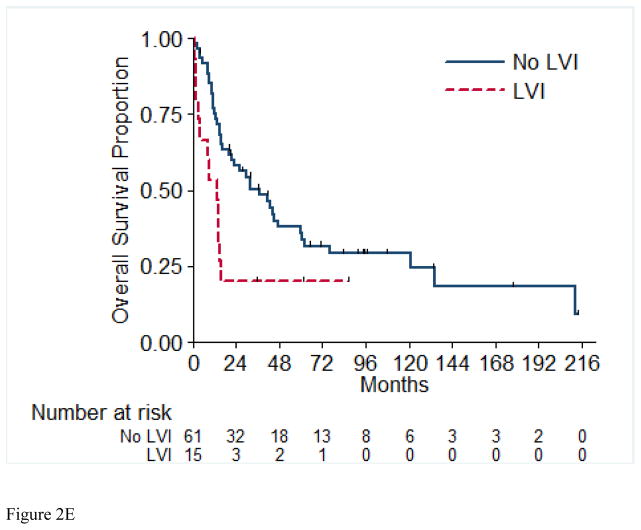
Overall survival (OS) for the entire cohort at 2 years was 50% (SE=6%) (Figure 1). Median follow-up was 20.4 months (range 1.0 – 213.5 months). A total of 55 (71%) patients died, of which 4 had no evidence of disease at time of death. Twenty-two patients (29%) were alive at time of analysis, of which 14 (18%) were alive with no evidence of disease (NED). Table 5 presents the univariable and multivariable analyses for OS. Tumor stage pT4 vs. pT1/2 (p= 0.03), presence of LVI (p= 0.03), and pathologically positive LN (p=0.002), were associated with worse overall survival, while positive margins and percentage sarcomatoid component were not significantly associated with overall survival. Figures 2A through 2E present the Kaplan-Meier OS curves stratified for these variables. Age, ECOG PS, clear-cell histology and the presence of necrosis were also evaluated and were not significant on univariable analysis. When controlling for all included variables, tumor stage, pathologically positive LN, and year of nephrectomy remained significant predictors of overall survival on multivariable analysis (Table 3).
Figure 1.
Overall Survival for All Patients
Table 5.
Analyses of Recurrence-Free Survival (RFS) for Patients with Localized Disease
| Multivariate Analyses | |||||||
|---|---|---|---|---|---|---|---|
| Univariate Analyses | Full Model | Reduced Model | |||||
| Patient Characteristic | Hazard Ratio (95% CI) | P-value | Hazard Ratio (95% CI) | P-value | Hazard Ratio (95% CI) | P-value | |
| Tumor Stage | T3 vs T1/T2 | 1.0 (0.5, 2.0) | 0.96 | 1.7 (1.1, 2.5) | 0.01 | 1.7 (1.2, 2.5) | 0.005 |
| T4 vs T1/T2 | 3.1 (1.5, 6.8) | 0.003 | 2.3 (1.3, 3.9) | 0.002 | 2.5 (1.5, 4.0) | <0.001 | |
| LN Positive | Yes vs No | 2.5 (1.4, 4.6) | 0.003 | 2.0 (1.4, 2.8) | <0.001 | 2.1 (1.5, 2.8) | <0.001 |
| Year of Nephrectomy* | 1996–2005 vs 1986–1995 | 1.1 (0.5, 2.5) | 0.74 | 0.8 (0.5, 1.3) | 0.30 | 0.8 (0.5, 1.3) | 0.30 |
| 2006–2011 vs 1986–1995 | 1.3 (0.5, 3.1) | 0.61 | 1.2 (0.8, 1.9) | 0.31 | 1.2 (0.8, 1.9) | 0.29 | |
| Positive Margin | Yes vs No | 1.8 (1.0, 3.5) | 0.07 | 1.1 (0.7, 1.6) | 0.66 | NI | |
| LVI | Yes vs No | 2.3 (1.2, 4.5) | 0.01 | 1.1 (0.8, 1.6) | 0.48 | NI | |
|
| |||||||
| Age | >55 vs <=55 | 0.8 (0.4, 1.3) | 0.34 | ||||
| ECOG Status | 1 vs 0 | 1.0 (0.6, 1.6) | 0.89 | ||||
| >=2 vs 0 | 1.1 (0.1, 8.1) | 0.94 | |||||
| Clear Cell Histology | Yes vs No | 0.7 (0.4, 1.3) | 0.24 | ||||
| Percent Sarcomatoid | 25–49 vs 1–24 | 1.7 (0.7, 3.8) | 0.23 | ||||
| 50–74 vs 1–24 | 1.9 (0.8, 4.3) | 0.14 | |||||
| 75–99 vs 1–24 | 1.1 (0.5, 2.2) | 0.88 | |||||
| Necrosis | Necrosis Yes vs No | 1.2 (0.7, 2.2) | 0.45 | ||||
RFS is only calculated for patients who both had localized disease and were NED after nephrectomy. With only 56 RFS events among 73 applicable patients, only 5 characteristics could be included in a multivariable model and maintain model stability. Tumor stage, LN positivity, year of nephrectomy, positive margins, and LVI were selected based on known or suspected clinical importance combined with new interest based on univariable analyses. The remaining variables were explored in univariable analyses only.
NI=Not included. After step-wise backward selection, variables that did not remain in the full model due to lack of statistical significance were not included in the final reduced model.
Even though each individual comparison for year of nephrectomy is not significant in the multivariate model, it remains in the model because the overall p-value for any difference among the 3 time groups is significant (p=0.04).
Figure 2.
Overall Survival Stratified by pathologic Tumor Stage (A), pathologic Lymph Node Status (B), Margin Status (C), Percentage Sarcomatoid (D), and Lymphovascular Invasion (E). LN=Lymph Nodes; LVI=Lymphovascular Invasion.
Table 3.
Analyses of Overall Survival (OS) for Patients with Clinically Localized Disease
| Multivariate Analyses | |||||||
|---|---|---|---|---|---|---|---|
| Univariate Analyses | Full Model | Reduced Model | |||||
| Patient Characteristic | Hazard Ratio (95% CI) | P-value | Hazard Ratio (95% CI) | P-value | Hazard Ratio (95% CI) | P-value | |
| Tumor Stage | T3 vs T1/T2 | 0.8 (0.4, 1.6) | 0.55 | 1.6 (1.1, 2.3) | 0.02 | 1.6 (1.1, 2.4) | 0.01 |
| T4 vs T1/T2 | 2.4 (1.1, 5.1) | 0.03 | 1.9 (1.1, 3.2) | 0.02 | 2.0 (1.2, 3.3) | 0.005 | |
| LN Positive | Yes vs No | 2.6 (1.4, 4.8) | 0.002 | 1.9 (1.4, 2.6) | <0.001 | 2.0 (1.5, 2.8) | <0.001 |
| Year of Nephrectomy | 1996–2005 vs 1986–1995 | 0.9 (0.4, 1.9) | 0.73 | 0.8 (0.5, 1.3) | 0.44 | 0.8 (0.5, 1.3) | 0.45 |
| 2006–2011 vs 1986–1995 | 1.0 (0.4, 2.4) | >0.99 | 1.3 (0.9, 2.0) | 0.15 | 1.3 (0.9, 2.0) | 0.16 | |
| Positive Margin | Yes vs No | 1.6 (0.8, 3.2) | 0.20 | 1.0 (0.7, 1.5) | 0.81 | NI | |
| LVI | Yes vs No | 2.1 (1.1, 4.2) | 0.03 | 1.3 (0.9, 1.9) | 0.14 | NI | |
|
| |||||||
| Age | >55 vs <=55 | 1.0 (0.5, 1.7) | 0.87 | NI | |||
| ECOG Status | 1 vs 0 | 0.9 (0.5, 1.6) | 0.69 | NI | |||
| >=2 vs 0 | 1.1 (0.1, 8.1) | 0.94 | NI | ||||
| Clear Cell Histology | Yes vs No | 0.7 (0.4, 1.3) | 0.30 | NI | |||
| Percent Sarcomatoid | 25–49 vs 1–24 | 1.3 (0.5, 3.2) | 0.54 | NI | |||
| 50–74 vs 1–24 | 1.3 (0.5, 3.5) | 0.56 | NI | ||||
| 75–99 vs 1–24 | 1.0 (0.4, 2.1) | 0.93 | NI | ||||
| Necrosis | Yes vs No | 0.8 (0.4, 1.5) | 0.54 | NI | |||
With only 55 deaths among 77 applicable patients, only 5 characteristics could be included in a multivariable model and maintain model stability. Tumor stage, LN positivity, year of nephrectomy, positive margins, and LVI were selected based on known or suspected clinical importance combined with new interest based on univariable analyses. The remaining variables were explored in univariable analyses only.
NI=Not included. After step-wise backward selection, variables that did not remain in the full model due to lack of statistical significance were not included in the final reduced model.
Even though each individual comparison for year of nephrectomy is not significant in the multivariate model, it remains in the model because the overall p-value for any difference among the 3 time groups is significant (p=0.03).
3.4 Recurrences and management
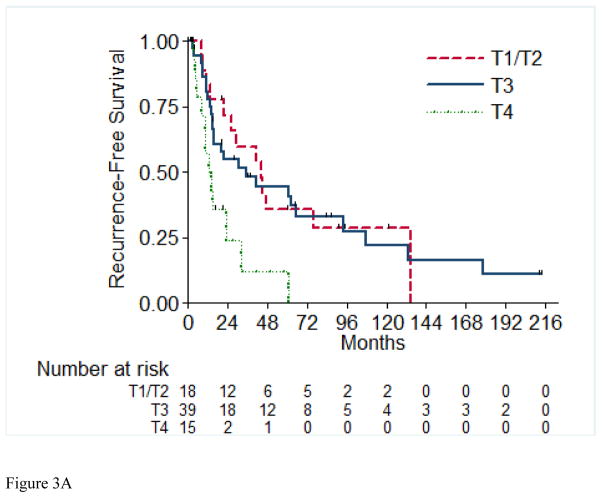
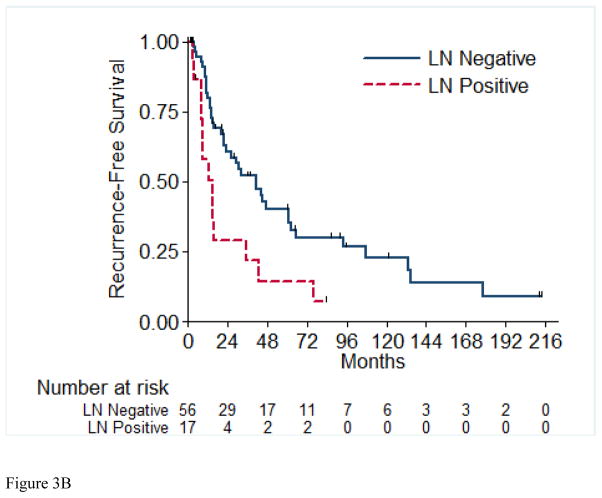
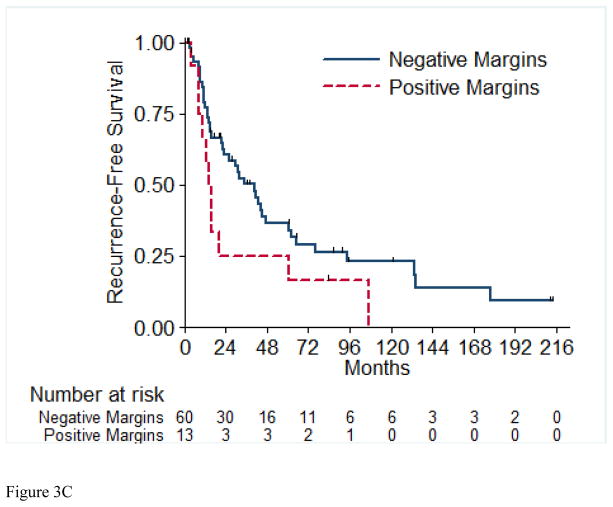
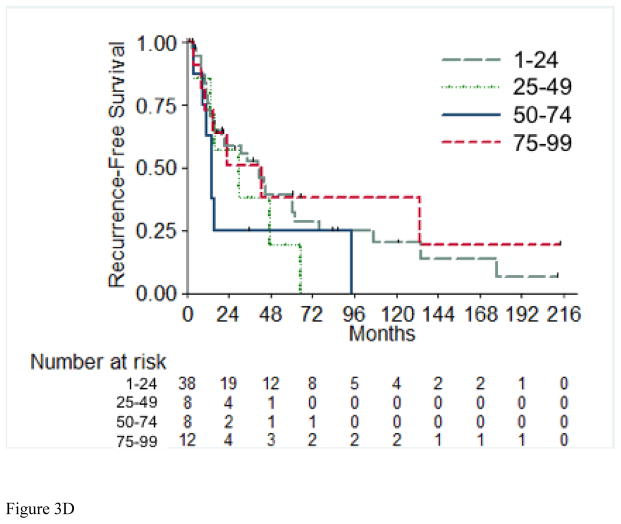
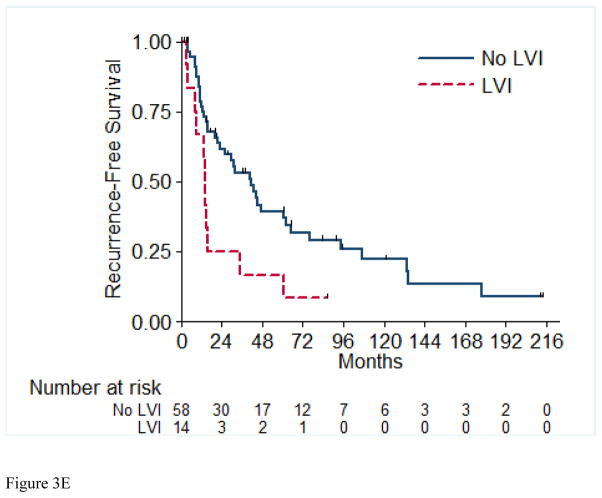
A total of 56 (72%) of the 73 RFS evaluable patients experienced a recurrence, with a median time to recurrence of 26.2 months (95% CI: 15.4, 42.9). Table 4 details the recurrences that occurred in the 56 patients in this cohort. Most patients (72%) presented with a single site of disease at time of initial recurrence. The most common sites of initial recurrence were lung (45%), local (25%), followed by bone (13%) and liver (13%). Most patients (71%) received systemic therapy at time of initial recurrence, and 29% received radiation therapy, while 32% underwent metastasectomy. Of the 56 patients who had recurrence, 10 were still alive at time of analysis. Three of these 10 patients were alive with no evidence of disease (one patient had metastasectomy, one had chemotherapy and metastasectomy, and one had radiation therapy and metastasectomy); the other seven patients are alive with disease. The 2-year RFS estimate was 51% (SE=6%). Table 5 presents the univariable and multivariable hazard ratios for selected clinical characteristics in relation to RFS. On univariable analysis, tumor stage pT4 vs. pT1/2 (p= 0.003), presence of LVI (p= 0.01), and pathologically positive LN (p=0.003) were associated with worse RFS, while positive margins and percentage sarcomatoid component were not significantly associated with RFS. Figures 3A through 3E present the Kaplan-Meier RFS curves stratified for these variables. Age, ECOG PS, clear cell histology and presence of necrosis were also evaluated and were not significant on univariable analysis. Multivariable analysis showed independent predictors of shorter RFS to be higher pathologic tumor stage, pathologically positive LN, and year of nephrectomy.
Table 4.
Recurrences after nephrectomy and their management
| Characteristics | N (%) |
|---|---|
| Recurrence at Last Follow-up | |
| Yes | 56 (73) |
| No | 21 (27) |
|
| |
| Number of Sites Involved at First Recurrence | |
| 1 | 40 (72) |
| ≥ 2 | 12 (21) |
| Missing | 4 (7) |
|
| |
| Organ Sites Involved at First Recurrence | |
| Lung | 25 (45) |
| Local (renal fossa/retroperitoneal nodes) | 14 (25) |
| Bone | 7 (13) |
| Liver | 7 (13) |
| Non-retroperitoneal nodes | 5 (9) |
| Brain | 4 (7) |
| Adrenal | 2 (4) |
| Pancreas | 1 (2) |
|
| |
| Management at time of first recurrence | |
|
| |
| Systemic Therapy | |
| Yes | 40 (71) |
| No | 13 (23) |
| Missing | 3 (6) |
|
| |
| Radiation | |
| Yes | 16 (29) |
| No | 34 (61) |
| Missing | 6 (10) |
|
| |
| Metastasectomy | |
| Yes | 18 (32) |
| No | 36 (64) |
| Missing | 2 (4) |
Figure 3.
Recurrence-Free Survival Stratified by pathologic Tumor Stage (A), pathologic Lymph Node Status (B), Margin Status (C), Percentage Sarcomatoid (D), and Lymphovascular Invasion (E). LN=Lymph Nodes; LVI=Lymphovascular Invasion.
4. DISCUSSION
To our knowledge, we report the first series exclusively evaluating outcomes and predictors of survival and recurrence in patients who presented with clinically localized non-metastatic renal cell carcinoma with sarcomatoid dedifferentiation and underwent surgery with curative intent. To date, published reports have typically included patients with both localized and metastatic disease in the analysis cohort. While some studies report overall survival outcomes separately for patients with initially nonmetastatic disease, the sample sizes are typically small and predictors of outcomes could not be adequately assessed [3, 8].
We have previously reported on 108 patients with RCC with sarcomatoid dedifferentiation treated at our institution between 1987 and 1998, of which only 25 patients had non-metastatic disease at initial presentation [3]. Twenty of these patients (80%) experienced disease recurrence at a median of 5.8 months after surgery, and ultimately died of metastatic disease. Despite the overall dismal outcomes for the localized cohort, these patients still had a significantly longer median overall survival (17 months vs. 7 months) than patients who presented with metastatic disease [3].
Other large studies (>100 patients in each) have also reported on sRCC (including metastatic and non-metastatic patients). Cheville et al [7] studied 120 patients with sRCC and noted that CSS at 2 years was 33.3%. In the subgroup of 66 patients with M0 disease, the 2-year CSS was 49.7%. This cohort has been recently updated by Zhang et al [9], with the 2-year CSS of the 105 M0 patients being slightly worse, at around 42% (as approximated from the Kaplan-Meier curve). De Peralta-Venturina et al [5] investigated 101 patients with sRCC, where 75 patients were considered stage I–III (7 stage I, 6 stage II, 62 stage III). Although not reported per se in the text, the CSS at 2 years from the Kaplan-Meier curve appeared to be around 80% for stage I–II and around 40% for stage III. Shuch et al [8] also described outcomes of 104 patients with sRCC. In a small subgroup of patients with nonmetastatic disease with sRCC, the 2-year survival rate for this cohort of 32 patients was 29.9%. Univariable analysis identified percentage sarcomatoid component, tumor size and ECOG PS as significant predictors of overall survival; however, multivariable analysis did not identify any independent predictors, likely due to the small number of patients in the study. In addition, the authors performed a SEER-17 database analysis of 1005 patients with sRCC. The authors reported 2-year overall survival rates of 44.6% for patients identified as having nonmetastatic, regional disease, keeping in mind all limitations in coding for sRCC in SEER, where sRCC is still considered a separate RCC subtype, a notion that has been changed in recent years. Another limitation of studying sRCC using SEER is the lack of central pathology review, as there are several entities that histologically mimic sRCC.
Our data revealed that patients presenting with sRCC and clinically localized disease have similar outcomes as previously reported in smaller cohorts, with 1, 2, and 5-year OS rates of 72%, 50%, and 34%. We found that higher tumor stage and pathologically positive LN were associated with an increased risk of death and recurrence on multivariable analysis, suggesting the importance of accurate pathologic diagnosis and staging in determining prognosis.
Consistent with other reports, in our focused group of patients with clinically non-metastatic disease, we did not find other variables such as age, ECOG PS, clear cell histology or percent sarcomatoid component to be associated with OS or RFS. It is possible that these factors could be important prognostic variables in patients who present with metastatic disease and have sRCC. Alternatively, the lack of difference could be related to the relatively small sample size.
Despite the improved OS rates and longer median time to recurrence that we report in the current study (compared to historical combined cohorts), outcomes for patients with sRCC remain poor, even if they present with clinically localized disease. Approximately half of our patients developed tumor recurrence and died by 2 years. Of those who had recurrence and were alive at last follow-up, only 3 were alive with NED, and 7 alive with disease. Since a majority of patients who had recurrence received some form of systemic therapy and/or multimodal therapy, no meaningful comparison could be performed to identify a benefit of specific additional therapy.
The use of systemic therapy in patients with sRCC has been investigated, although not specifically in patients who presented with clinically localized disease. The combination of gemcitabine and doxorubicin has been shown to have clinical activity in some patients with advanced sRCC, with an objective response rate of 15.8% as reported in a phase II ECOG-8802 study in 2009 [10]. More recently, other groups have evaluated the benefit of combination therapy with tyrosine kinase inhibitors and other targeted agents [11, 12]. We recently completed a phase II clinical trial at our institution using the combination of bevacizumab, capecitabine and gemcitabine in patients with metastatic or unresectable sRCC. Currently, there are no studies evaluating the use of systemic therapy in the adjuvant setting following nephrectomy for patients with sRCC.
Although the retrospective nature of this study and the small cohort size are limitations of our findings, we report important trends that may have a significant clinical impact. First, clinically localized disease at presentation is associated with better outcome than metastatic disease; however, most patients eventually develop tumor recurrence and die of their disease. Second, surgeries performed for this type of tumor seem to be associated with prolonged operative times, blood transfusions, positive surgical margins, and resection of additional organs, all of which are likely due to the locally aggressive nature and higher stage of this disease at presentation. Third, approximately 25% of patients are found to have pathologically positive lymph nodes at the time of surgery, in the background of clinically negative nodes at preoperative imaging. The high rate of pN1 in our cohort is indeed not surprising, when considering that 77% were pT3-4, 34% had necrosis, 50% were >10cm, all were sarcomatoid, and all were high grade. Having 2 of these 5 risk factors present, puts the risk of pN1 at 20%, with higher pN1 rates seen when more risk factors are present [13]. We have previously shown that detection of sarcomatoid elements on preoperative biopsy is quite limited (<10% detection rate) [14], and therefore we are currently conducting molecular studies to better identify these patients who harbor sRCC. Such information can be used to guide the surgical management, including a thorough lymphadenectomy and wide resection. Fourth, in our cohort of patients, the percentage sarcomatoid component did not seem to impact survival, suggesting that any sarcomatoid component should be noted in the pathology report, and taken seriously postoperatively, as these patients have a high rate of recurrence. Fifth, we suggest that surveillance in the post-operative setting in sRCC should be more aggressive than the current recommendations for non-sRCC, especially for those patients with unfavorable characteristics of higher tumor stage or lymph node involvement, with the hopes of identifying recurrences as soon as possible and treating them as aggressively as possible, using medical and surgical means as appropriate. Finally, further prospective studies are warranted to determine if this particular group of patients with sRCC might benefit from adjuvant or neoadjuvant systemic therapy.
5. CONCLUSIONS
sRCC is an aggressive disease with high rate of recurrence and mortality, even in patients who present with non-metastatic disease. Recognition of this entity on pathological evaluation, careful follow-up, and aggressive surgical and medical therapy are important factors toward improving patient outcomes. We are currently conducting detailed molecular analyses aiming at refining the diagnosis of sRCC preoperatively, in addition to gaining insights into the biology behind its aggressive behavior, and identifying novel targets that can be exploited therapeutically.
Acknowledgments
Supported by MD Anderson’s National Cancer Institute Cancer Center Support Grant P30CA016672 and used the Biostatistics Resource Group.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shuch B, Bratslavsky G, Linehan WM, Srinivasan R. Sarcomatoid renal cell carcinoma: a comprehensive review of the biology and current treatment strategies. The oncologist. 2012;17:46–54. doi: 10.1634/theoncologist.2011-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shuch B, Said J, La Rochelle JC, Zhou Y, Li G, Klatte T, et al. Cytoreductive nephrectomy for kidney cancer with sarcomatoid histology--is up-front resection indicated and, if not, is it avoidable? The Journal of urology. 2009;182:2164–71. doi: 10.1016/j.juro.2009.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mian BM, Bhadkamkar N, Slaton JW, Pisters PW, Daliani D, Swanson DA, et al. Prognostic factors and survival of patients with sarcomatoid renal cell carcinoma. The Journal of urology. 2002;167:65–70. [PubMed] [Google Scholar]
- 4.Kanamaru H, Sasaki M, Miwa Y, Akino H, Okada K. Prognostic value of sarcomatoid histology and volume-weighted mean nuclear volume in renal cell carcinoma. BJU international. 1999;83:222–6. doi: 10.1046/j.1464-410x.1999.00912.x. [DOI] [PubMed] [Google Scholar]
- 5.de Peralta-Venturina M, Moch H, Amin M, Tamboli P, Hailemariam S, Mihatsch M, et al. Sarcomatoid differentiation in renal cell carcinoma: a study of 101 cases. The American journal of surgical pathology. 2001;25:275–84. doi: 10.1097/00000478-200103000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Kuroiwa K, Konomoto T, Kumazawa J, Naito S, Tsuneyoshi M. Cell proliferative activity and expression of cell-cell adhesion factors (E-cadherin, alpha-, beta-, and gamma-catenin, and p120) in sarcomatoid renal cell carcinoma. Journal of surgical oncology. 2001;77:123–31. doi: 10.1002/jso.1082. [DOI] [PubMed] [Google Scholar]
- 7.Cheville JC, Lohse CM, Zincke H, Weaver AL, Leibovich BC, Frank I, et al. Sarcomatoid renal cell carcinoma: an examination of underlying histologic subtype and an analysis of associations with patient outcome. The American journal of surgical pathology. 2004;28:435–41. doi: 10.1097/00000478-200404000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Shuch B, Bratslavsky G, Shih J, Vourganti S, Finley D, Castor B, et al. Impact of pathological tumour characteristics in patients with sarcomatoid renal cell carcinoma. BJU international. 2012;109:1600–6. doi: 10.1111/j.1464-410X.2011.10785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang BY, Thompson RH, Lohse CM, Leibovich BC, Boorjian SA, Cheville JC, et al. Novel prognostic model for patients with sarcomatoid renal cell carcinoma. BJU international. 2014 doi: 10.1111/bju.12781. [DOI] [PubMed] [Google Scholar]
- 10.Haas NBLX, Manola J, Pins M, Liu G, McDermott D, Nanus D, Heath E, Wilding G, Dutcher J. A phase II trial of doxorubicin and gemcitabine in renal cell carcinoma with sarcomatoid features: ECOG 8802. Med Oncol. 2012;29:761–7. doi: 10.1007/s12032-011-9829-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michaelson DMD, Atkins M, et al. Phase II trial of sunitinib and gemcitabine in patients with sarcomatoid and/or poor-risk metastatic renal cell carcinoma. 2010 American Society of Clinical Oncology Genitourinary Cancer Symposium; Orlando, Florida. 2010. [Google Scholar]
- 12.Golshayan ARGS, Heng DY, et al. Metastatic sarcomatoid renal cell carcinoma treated with vascular endothelial growth factor-targeted therapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:235–41. doi: 10.1200/JCO.2008.18.0000. [DOI] [PubMed] [Google Scholar]
- 13.Crispen PL, Breau RH, Allmer C, Lohse CM, Cheville JC, Leibovich BC, et al. Lymph node dissection at the time of radical nephrectomy for high-risk clear cell renal cell carcinoma: indications and recommendations for surgical templates. European urology. 2011;59:18–23. doi: 10.1016/j.eururo.2010.08.042. [DOI] [PubMed] [Google Scholar]
- 14.Abel EJ, Carrasco A, Culp SH, Matin SF, Tamboli P, Tannir NM, et al. Limitations of preoperative biopsy in patients with metastatic renal cell carcinoma: comparison to surgical pathology in 405 cases. BJU international. 2012;110:1742–6. doi: 10.1111/j.1464-410X.2012.11124.x. [DOI] [PubMed] [Google Scholar]