Abstract
Metabolically engineered strains of the hyperthermophile Pyrococcus furiosus(Topt 95-100°C), designed to produce 3-hydroxypropionate (3HP) from maltose and CO2 using enzymes from the Metallosphaera sedula (Topt73°C) carbon fixation cycle, were examined with respect to the impact of heterologous gene expression on metabolic activity, fitness at optimal and sub-optimal temperatures, gas-liquid mass transfer in gas-intensive bioreactors, and potential bottlenecks arising from product formation. Transcriptomic comparisons of wild-type P. furiosus, a genetically-tractable, naturally-competent mutant (COM1), and COM1-based strains engineered for 3HP production revealed numerous differences after being shifted from 95°C to 72°C, where product formation catalyzed by the heterologously-produced M. sedula enzymes occurred. At 72°C, significantly higher levels of metabolic activity and a stress response were evident in 3HP-forming strains compared to the non-producing parent strain (COM1). Gas-liquid mass transfer limitations were apparent, given that 3HP titers and volumetric productivity in stirred bioreactors could be increased over 10-fold by increased agitation and higher CO2 sparging rates, from 18 mg/L to 276 mg/L and from 0.7 mg/L/hr to 11 mg/L/hr, respectively. 3HP formation triggered transcription of genes for protein stabilization and turnover, RNA degradation, and reactive oxygen species detoxification. The results here support the prospects of using thermally diverse sources of pathways and enzymes in metabolically engineered strains designed for product formation at sub-optimal growth temperatures.
Keywords: 3-Hydroxypropionate, CO2 fixation, Metallosphaera sedula, Pyrococcus furiosus
1. Introduction
Metabolically engineered microorganisms are being developed as alternative production platforms for fuels and chemicals derived from both biological feedstocks, including sugars and cellulosic biomass (Gronenberg et al., 2013), and inorganic carbon (CO2, bicarbonate) (Conrado et al., 2013; Hawkins et al., 2011). With respect to the latter, microorganisms that are capable of fixing CO2 into higher value products could have a particular advantage by reducing carbon emissions through production of industrial chemicals (Hawkins et al., 2013). Target chemicals range from fuel molecules (alcohols and fatty acids) (Lan and Liao, 2013; Peralta-Yahya and Keasling, 2010) to strategic building blocks for chemical upgrading (e.g. 3-hydroxypropionate, acrylate, 1,3-propanediol) (Werpy et al., 2004).
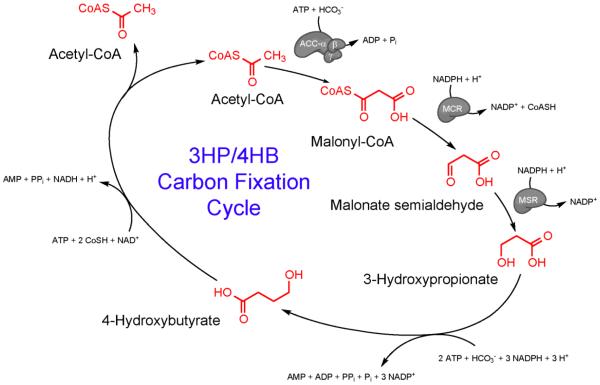
The marine hyperthermophilic archaeon, Pyrococcus furiosus, has been considered as a metabolic engineering host with unique thermally-regulated features (Hawkins et al., 2013). P. furiosus grows optimally at 100°C by fermentation of sugars and peptides (Fiala and Stetter, 1986), but retains metabolic activity at temperatures at least as low as 72°C, thereby creating a potentially novel bioprocessing strategy for producing fuels and chemicals with heterologous enzymes introduced into P. furious with maximum activity around 70°C (Basen et al., 2012). This strategy would exploit the 30°C difference between the host growth temperature and pathway activity to decouple growth from product formation, potentially minimizing metabolic burden of heterologous systems during biomass accumulation and host maintenance energy requirements during product formation. Genetic tools have been developed that allow efficient and rapid chromosomal modifications in a naturally competent mutant of this hyperthermophile, P. furiosus strain COM1 (Lipscomb et al., 2011). The 3-hydroxypropionate/4-hydroxybutyrate (3HP/4HB) carbon fixation cycle from Metallosphaera sedula (Berg et al., 2007), a thermoacidophilic archaeon that grows optimally at 72°C, has been engineered into P. furiosus COM1 for the production of chemicals from CO2 and maltose (Keller et al., 2013). P. furiosus strains engineered to contain the first three steps of the 3HP/4HB cycle (acetyl-CoA/propionyl-CoA carboxylase, malonyl-CoA/succinyl-CoA reductase, and malonate semialdehyde reductase) (see Table 1 and Figure 1) have demonstrated the capacity of the heterologous M. sedula enzymes to incorporate CO2 from either bicarbonate or exogenous gaseous CO2 to form 3HP from cellular pools of acetyl-CoA (Keller et al., 2013).
Table 1.
Primary and accessory enzymes of the M. sedula 3HP/4HB cycle.
| Enzyme Abbrev |
Enzyme | ORF | Reference |
|---|---|---|---|
| ACC/PCC | Acetyl-CoA/Propionyl-CoA carboxylase | Msed_0147 | NCE (Hügler et al., 2003) |
| Msed_0148 | |||
| Msed_1375 | |||
| MCR/SCR | Malonyl-CoA/Succinyl-CoA reductase (NADPH) |
Msed_0709 | R (Kockelkorn and Fuchs, 2009) |
| MSR | Malonate semialdehyde reductase (NADPH) | Msed_1993 | R (Kockelkorn and Fuchs, 2009) |
| CA | Carbonic anhydrase | Msed_0390 | R (Lian, 2014) |
| BPL | Biotin protein ligase | Msed_2010 | R (Lian, 2014) |
NCE – Native cell extract; R – Recombinant enzyme; NP – Native purified enzyme
Figure 1.
3-Hydroxypropionate (3HP)/4-Hydroxybutyrate (4HB) Carbon Fixation Cycle from Metallosphaera sedula, highlighting the portion of the cycle for 3HP production. The first step requires carboxylation of acetyl-CoA via acetyl-CoA carboxylase with bicarbonate as substrate, followed by two reductions to form 3HP. Enzyme abbreviations: ACC – Acetyl-CoA carboxylase; MCR – Malonyl-CoA reductase; MSR – Malonate semialdehyde reductase (Table 1).
There are many bioprocessing issues that need to be examined for P. furiosus as a prospective metabolic engineering host for CO2-based product formation. These include the basal effect of inserting foreign genes into the genome of P. furiosus strain (COM1), metabolic and physiological features of engineered strains of P. furiosus at optimal and sub-optimal growth temperatures, impact of non-native metabolites and pathway intermediates, and substrate delivery challenges due to gas-liquid mass transfer limitations. To begin to address these issues, P. furiosus strains engineered to produce 3HP at 72°C from CO2 and maltose via the first three steps of the 3HP/4HB cycle were examined by comparative transcriptome and microbiological analysis of samples obtained from bioreactor growth at optimal and suboptimal temperatures to gain insights into potential bottlenecks for CO2 utilization as well as to assess this hyperthermophile as a novel metabolic engineering platform.
2. Materials and Methods
2.1. Growth of P. furiosus strains
All strains used in this study are listed in Table 2. P. furiosus (DMSZ3638) was routinely grown anaerobically under N2 at 95°C in a shaking oil bath (90 rpm) in seawater medium containing 1 × base salts, 1 × trace minerals, 10 ×M Na2WO4·2H2O, 0.25 mg/L resazurin, 0.5 g/L cysteine hydrochloride, 0.5 g/L sodium sulfide, and 1 mM potassium phosphate buffer (pH 6.8). For growth in serum bottles, sodium bicarbonate was also added at 1 g/L. However, when grown in bioreactors using gas feeds containing CO2, bicarbonate was omitted from the medium. Various complex media formulations were used that extended the seawater medium base. Routine medium for growth in serum bottles contained 5 g/L yeast extract and 5 g/L maltose (YM5) unless otherwise noted; 250 ×g/L biotin was added to the medium when growing the engineered strains in the bioreactor. Stock solutions were as follows: 5 × base salts, containing, per liter, 140 g NaCl, 17.5 g MgSO4·7H2O, 13.5 g MgCl2·6H2O, 1.65 g KCl, 1.25 g NH4Cl, and 0.7 g CaCl2·2H2O; 1000 × trace minerals, containing, per liter, 1 mL concentrated HCl, 0.5 g Na4EDTA, 2 g FeCl3, 0.05 g H3BO3, 0.05 g ZnCl2, 0.03 g CuCl2·2H2O, 0.05 g MnCl2·4H2O, 0.05 g (NH4)2MoO4, 0.05 g AlK(SO4)2, 0.05 g CoCl2·6H2O, and 0.05 g NiCl2·6H2O (Adams et al., 2001).
Table 2.
P. furiosus strains used in this study
|
P. furiosus
strain |
Parent strain | Expression construct for M. sedula gene |
Genetic marker |
Reference |
|---|---|---|---|---|
| COM1 | - | None | NONE | (Lipscomb et al., 2011) |
| MW56 | COM1 | Pslp-accABC-mcr-msr | Pgdh-pyrF | (Keller et al., 2013) |
| MW76 | COM1 | Pslp-accABC-mcr-msr-bpl-ca | Pgdh-pyrF | This work |
(see Table 1 for enzyme abbreviations)
Pgdh – P. furiosus glutamate dehydrogenase gene promoter
Pslp – P. furiosus S-layer protein gene promoter
Ppep – P. furiosus PEP synthase gene promoter
2.2. Growth in serum bottles
Medium for serum bottle cultures was prepared by adding all of the components and reducing reagents listed above, except for the potassium phosphate buffer. The pH was adjusted to 6.8, after which the potassium phosphate was added and the medium sterile filtered (0.22 ×m), and then aliquoted into serum bottles using a serological pipette. The bottles were sealed with a butyl rubber stopper and aluminum crimp, and the headspace made anaerobic with a vacuum manifold system using three cycles of vacuum and flushing with N2 gas.
2.3. Construction of P. furiosus strain MW76
All P. furiosus transformations were done using uracil prototrophic selection on defined medium, as previously described (Lipscomb et al., 2011), using linearized plasmids or linear splice overlap extension (SOE) PCR products. Strains were further purified by two consecutive transfers on solid medium, and the final strains were verified by PCR and sequencing of the regions containing the chromosomal insertions. The P. furiosus genome regions (3 and 5) used for chromosomal integration of the constructs contain little to no transcriptional activity as determined from analysis of tiling array data (Yoon et al., 2011). Strains used and constructed for this study are listed in Table 2. Strain MW76 was created from strain MW56 by inserting the Ppep-BPL-CA construct into pGL010 between the SphI and AscI sites (Keller et al., 2013). pGL010 contains the Pslp-ACCααγ-MCR-MSR operon and the Pgdh-pyrF marker cassette. The addition of Ppep-BPL-CA produced the plasmid pGL021. P. furiosus COM1 was transformed using linearized plasmid pGL021 to construct strain MW76.
2.4. Bioreactor setup and operation
Medium for bioreactor growth was prepared in situ at time of use in autoclaved 3L Applikon glass bioreactors (ADI 1010/1025; Delft, The Netherlands). A gas mixture of 80% N2, 20% CO2 was sparged into the medium using a 2-×m sparging stone at 15 ml/min. All gas flows were controlled by Matheson rotameters (FM-1050 series, E910; psig – 10; Basking Ridge, NJ, USA) and flow rates were corrected for the composition mixture. The medium pH was allowed to slowly equilibrate at room temperature (about 1 hour), after which it was adjusted to 6.7 and buffered with potassium phosphate. The pH was controlled using 0.5 M NaOH. The bioreactors were inoculated with 5% (vol/vol) cells passaged one time after reviving from glycerol stocks. Growth was measured by cell counts and followed at 95°C until the density reached 1 × 108 ml−1, at which point the temperature was reduced to 72°C. At the temperature switch, the agitation was increased (to 400 or 1,000 rpm) and the gas feed was increased to 50 or 100 ml/min.
2.5. Bioreactor sampling, cell counts, RNA harvesting
During experimental runs, samples were taken for cell counting (1 ml), metabolite analysis (10 ml), cell dry weight measurements (50 ml), and RNA harvesting (200-300 ml). Samples were taken every 90 minutes during the growth phase at 95°C and every 8 hours during the production phase at 72°C, except for RNA harvesting which was done only twice – 1-2 hours after the temperature switch (‘Early’ time point) and 30-40 hours after the temperature switch (‘Late’ time point). Cells were harvested by rapid cooling with dry ice and ethanol to ~10°C and then centrifuged at 6,000 × g for 10 min at 4°C. Cell pellets were lysed with TRIzol reagent (Life Technologies) and total RNA was extracted and purified using RNeasy kits (Qiagen) and stored at −80°C.
2.6. Derivatization and detection of organic acids
Detection of acetate and 3HP in samples of cell-free supernatant was performed by derivatization with dibromoacetophenone (DBAP) to form the phenacyl ester as described previously (Hawkins et al., 2014). For each sample, 500 ×l of supernatant was extracted with ether and derivatized using 100 mM 2,4-dibromoacetophenone. The samples were run on an Atlantis dC18 column (Waters) (3 ×m, 4.6 × 150 mm) at 30°C with a flow rate of 1.5 ml/min using a gradient elution profile. The initial mobile phase composition was 65% Buffer A (0.1% formic acid) and 35% Buffer B (acetonitrile). Samples were eluted with a fifteen minute linear gradient to a final composition of 30% Buffer A and 70% Buffer B. Products were detected by following the absorpance at 254 nm using a single channel of the Waters 2998 photodiode array detector. Peaks were quantified relative to standards prepared in the base YM5 medium.
2.7. P. furiosus oligonucleotide microarray transcriptional response analysis
A spotted, whole-genome hybrid oligonucleotide microarray, with probes for 2,032 P. furiosus COM1 genes and 19 additional probes for heterologous genes from M. sedula 3HP/4HB cycle enzymes, was used as described previously for other microarrays, with 5 replicates of each probe spotted on the slide (Hawkins et al., 2014). Total RNA from two separate biological experiments was pooled and reverse-transcribed (Superscript III, Invitrogen), re-purified, labeled with either Cy3 or Cy5 dye (GE Healthcare), and hybridized to the microarray slides (Corning). Slides were scanned on a GenePix 4000B Microarray Scanner (Molecular Devices, Sunnyvale, CA), and raw intensities were quantitated using GenePix Pro version 6.0. Data normalization and statistical analysis were performed using JMP Genomics 5 (SAS). In general, significant differential transcription was defined to be relative changes in expression of ≥ 2-fold (where a log2 value of ±1 means a 2-fold change) having −log10p-values of ≥ 4.9 (Bonferroni correction equivalent to a p-value of 1.2 × 10−7 for these experiments).
2.8. Microarray data accession number
Microarray data are available through the NCBI Gene Expression Omnibus (GEO) under accession number GSEXXXXX.
3. Results and Discussion
3.1. Impact of heterologous M. sedula genes on recombinant P. furiosus COM1 at 95°C
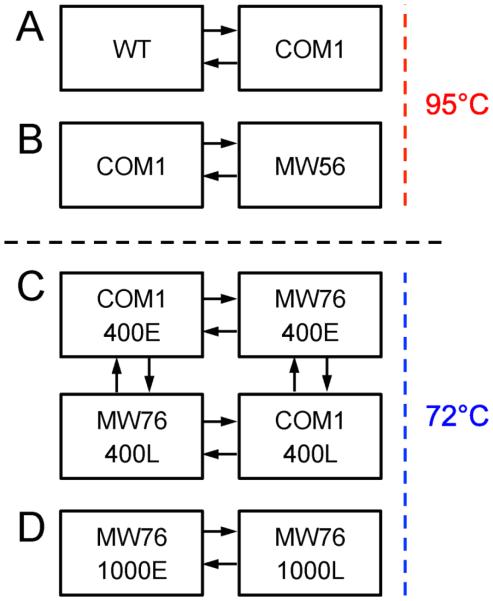
The naturally competent strain of P. furiosus used here for metabolic engineering (COM1) has numerous chromosomal rearrangements, deletions, and single base changes compared to the NCBI sequence of the wild-type strain (DSMZ 3638) (Bridger et al., 2012). However, these changes did not give rise to any phenotypic growth changes, as COM1, like wild-type P. furiosus, appears to grow well at 95°C on complex and minimal media containing cellobiose, malto-oligosaccharides, and/or peptides (Bridger et al., 2012; Fiala and Stetter, 1986). To further assess differences of potential importance in metabolic engineering efforts, a hybrid P. furiosus DNA microarray was used to compare the COM1 transcriptome to the wild-type P. furiosus strain (WT) during growth in 1L bottles at 95°C on complex media containing yeast extract (0.05%), cellobiose (0.35%), and tryptone (0.5%) (see Figure 2 for experimental loop designs). Results showed that the collection of genetic deletions, inactivations, and rearrangements in the naturally competent parent (COM1) led to many differences in transcriptional patterns between the two strains. Overall, 258 genes, or about 10% of the genome, were differentially transcribed 2-fold or more and met the Bonferroni cut-off (–log10(p-value) of 4.9 for this experiment) (see GEO submission for complete data set). Many of these genes are involved in amino acid and protein biosynthesis, central metabolism, and energy metabolism. While it is difficult to correlate transcriptional response to specific chromosomal differences, COM1 metabolism apparently compensates for gene deletions and inactive gene products by shifting transcriptional patterns. The most obvious phenotype change is the high degree of competence that allows for genetic engineering. However, the transcriptomes showed that there is a distinct ’transcriptional phenotype’ in the COM1 strain. This is important to consider when interpreting COM1 response to various stimuli, such as temperature shift, energy sources, and metabolite accumulation, because the response may vary in key ways from what has been observed in wild-type P. furiosus.
Figure 2.
Experimental design overview for the transcriptional response experiments used in this study. See Materials and Methods section and Table 2 for detailed information about strains and growth conditions. A) Dye-flip between P. furiosus Wild-type (WT) and the naturally competent genetic host strain (COM1) grown at 95°C (serum bottles). B) Dye-flip between the genetic parent strain (COM1) and P. furiosus strain MW56 grown at 95°C (serum bottles). C) Four-slide loop comparing the genetic parent strain (COM1) with P. furiosus strain MW76 grown in gas-intensive bioreactor at 72°C with low agitation (400 rpm) at 2.5 h (E - Early) and 40 h (L – Late) after the temperature switch from 95°C. D) Dye-flip between P. furiosus strain MW76 grown in gas-intensive bioreactor at 72°C with high agitation (1000 rpm) at 1 h (E - Early) and 31 h (L – Late) after the temperature switch from 95°C.
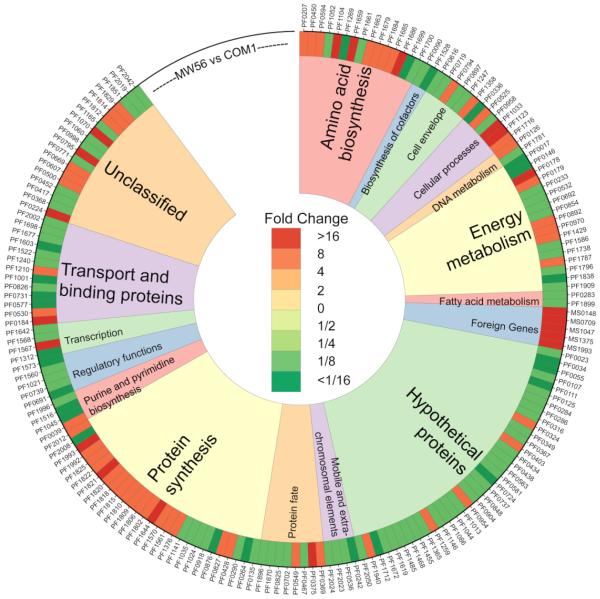
Next, the impact of transcribing and translating five heterologous M. sedula genes for the first three 3HP/4HB cycle steps (see Figure 1) on engineered strains of P. furiosus COM1 was examined. P. furiosus MW56, the COM1 strain engineered to contain genes encoding acetyl-CoA carboxylase (Msed_0147, Msed_0148, Msed_1375), malonyl-CoA reductase (Msed_0709), and malonate semialdehyde reductase (Msed_1993), was compared to the parent strain COM1; both strains were grown in 1L bottles at 95°C on complex media containing yeast extract (0.05%), cellobiose (0.35%), and tryptone (0.5%). Note that at this temperature, the M. sedula enzymes were not functional, since this temperature is more than 20°C above the optimal growth temperature of the thermoacidophile (Keller et al., 2013). Differential transcription of approximately 25% of the genome (205 genes up/293 genes down two-fold or more) was noted (see Figure 3). The five M. sedula genes were transcribed at very high levels, with 4 out of 5 ranking in the 99th percentile in terms of transcript abundance, consistent with the fact that the constitutive, high-expression, S-layer gene promoter (Pslp) was used. The impact of the high transcription levels of the five M. sedula genes at 95°C was apparent from the MW56 transcriptome. Two of the most highly up-regulated genes in MW56 compared to COM1, a ribonucleolytic PilT/VapC homolog (PF1716 – 8-fold up) and an exosome RNA-binding protein (PF1567 – 10.3-fold up), suggest actions to degrade heterologous mRNA. The burden of foreign gene expression was also evident with respect to translation. Transcription levels of ribosomal proteins were much higher for MW56 than for COM1, with 18 out of 62 genes annotated as ribosomal proteins showing 2-fold or greater transcript abundance in MW56 compared to COM1. Conversely, only 5 out of 62 genes annotated as ribosomal proteins had transcript levels 2-fold or greater for COM1 compared to MW56.
Figure 3. Heatmap showing Differential Transcription between P. furiosus COM1 and P. furiosus MW56 strains.
Select genes with log2-fold changes of ≥ ±2.1, sorted by TIGR functional categories. P. furiosus ORF numbers are listed on outer edge. Red indicates higher transcriptional levels for a given gene in MW56 compared to COM1, while green indicates lower transcription levels. See Figure 2 for microarray experimental loop summary.
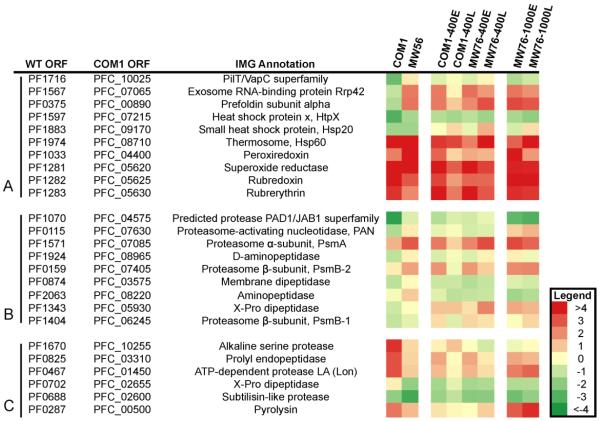
The high expression levels of M. sedula genes encoding for enzymes that function optimally at 70-75°C in a host organism growing at 95°C likely resulted in a cytosolic accumulation of unfolded or misfolded polypeptides. This scenario was consistent with the observed up-regulation of chaperone-related genes, including the prefoldin α-subunit (PF0375) and heat shock protein X (PF1597) (Figure 4A). However, no significant up-regulation was seen for other chaperone encoding genes previously observed during P. furiosus heat shock (Shockley et al., 2003), although the thermosome (Hsp60, PF1974) was constitutively transcribed at very high levels in both strains (95th percentile of transcript abundance). Several protease genes were also up-regulated in MW56, presumably triggered in response to the accumulation of unfolded protein (Figure 4B). These include a putative protease from the PAD1/JAB1 superfamily (PF1070), the proteasome (PF1571, PF0159), proteasome-activating nucleosidase (PAN) (PF0115), D-aminopeptidase (PF1924), a membrane dipeptidase (PF0874), and an aminopeptidase (PF2063). The up-regulation of proteasome subunits and PAN are likely involved in protein turnover of misfolded M. sedula proteins (Maupin-Furlow et al., 2006). Interestingly, a different set of proteases were transcribed at higher levels in COM1 compared to MW56; these proteases are listed in Figure 4C. Only one protease was constitutively present at high levels for both strains, a carboxypeptidase (PF0456), although several others were transcribed at average levels for both conditions, such as the aminopeptidase (PF2063) and the endopeptidase IV (PF1583). The specific proteases responding in MW56 most likely reflect the high rate of heterologous protein production in that strain compared to COM1, and these are geared toward protein turnover instead of routine peptide catabolism.
Figure 4. Heat plot showing normalized transcription levels for select genes in P. furiosus strains.
High transcription levels are shown in red, low transcription in green. The corresponding numbers depicted here are least-squares mean values of transcription relative to the overall average transcription level of zero (yellow). The genes are divided into three groups: protease genes up-regulated on COM1 (top), proteases up-regulated on MW56 (middle), and select stress responsive genes (bottom). Stress response genes include RNA degradation enzymes, protein chaperones, and oxygen/peroxide scavenging molecules.
The possibility of an oxidative stress response from P. furiosus due to high heterologous gene expression was indicated by the high transcript levels for peroxiredoxin (PF1033 – 15-fold higher) in MW56 compared to COM1 (Figure 4A). However, other important oxidative stress response genes, such as superoxide reductase, rubredoxin, and rubrerythrin, were transcribed at constitutively high levels in both strains at 95°C. This was also observed when oxidative stress was induced by the addition of hydrogen-peroxide (Strand et al., 2010) or by gamma-irradiation to P. furiosus (Williams et al., 2007), where these genes were non-responsive to the stress and constitutively produced. Overall, the extensive transcriptional changes observed in MW56 as compared to COM1 indicates that P. furiosus makes numerous adjustments that specifically relate to transcription and translation of the five heterologous genes from M. sedula at 95°C, even though these proteins are not enzymatically functional at 20°C above their optimum (Keller et al., 2013).
3.2. Impact of heterologous M. sedula genes on recombinant P. furiosus COM1 at 72°C
The next issue to consider was how a temperature shift to 72°C to allow the M. sedula enzymes to fold properly and become functional, with the concomitant stimulation of 3HP production in recombinant P. furiosus, impacted the host strain. P. furiosus strain MW76 was used for this experiment. Although based on MW56, MW76 also contained genes for M. sedula carbonic anyhydrase (CA) (Msed_0390) and protein biotin protein ligase (BPL) (Msed_2010); CA was added to generate bicarbonate from CO2, since bicarbonate is the substrate of the M. sedula carboxylase, while BPL was added to catalyze biotinylation of the α-subunit of the same enzyme (see Table 2) (Lian, 2014). Both strains were grown in 3L Applikon bioreactors. RNA for transcriptional analysis was harvested from COM1 and MW76 cultures at two different time points following the temperature shift to 72°C from 95°C. At the same time as the temperature decrease, the agitation was increased from 250 to 400 rpm and gas feed (N2/CO2) was increased from 15 to 50 ml/min (Figure 5, Table 3). For the first time point, sampled 2.5 h after the temperature switch to 72°C, no 3HP production was detectable, while at 40 h 3HP accumulation in the medium was readily detected. These bioreactor runs will be designated as COM1-400 and MW76-400 to distinguish them from subsequent runs; the two time points will be designated as ‘Early’ (E) or ‘Late’ (L), for example COM1-400E or MW76-400L.
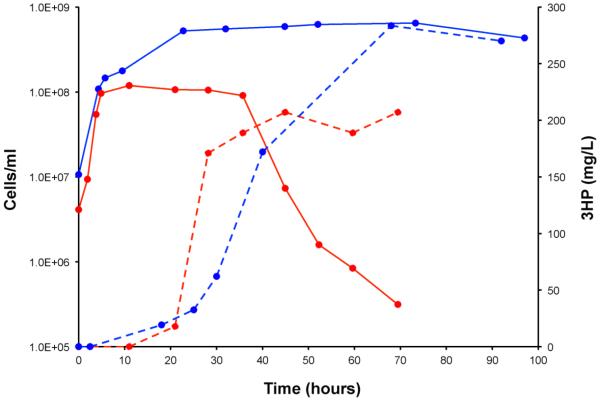
Figure 5. Cell Density and 3HP production levels for selected bioreactor runs.
Solid lines show cell densities (left axis) and dashed lines show 3HP production levels (right axis); MW76-400 is shown in blue and MW76-1000 in red. Note the rapid decrease in cell viability for MW76-1000 (red) compared to MW76-400 (blue), corresponding to higher 3HP production rates.
Table 3.
Bioreactor conditions for runs with P. furiosus strain MW76
| Experiment | Medium | Agitation |
Gas Feed
(ml/min) |
3HP
Productivity (mg/L/hr) |
3HP Titer
(mg/L) |
|---|---|---|---|---|---|
| COM1-400 | 5 g/L YE; 5 g/L maltose | 250 → 400 rpm | 15 (N2/CO2) → 50 (N2/CO2) | 0 | 0 |
| MW76-400 | 5.4 | 276 | |||
| MW76-1000 | 5 g/L YE; 5 g/L maltose | 250 → 1000 rpm | 15 (N2/CO2) → 100 (N2/CO2) | 11 | 210 |
| MW76-1000M | 5 g/L YE; 10 g/L maltose | 250 → 1000 rpm | 15 (N2/CO2) → 100 (N2/CO2) | 8.8 | 193 |
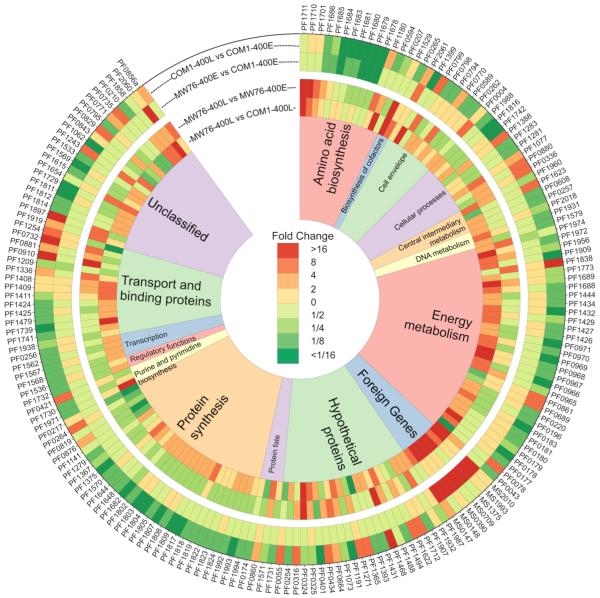
The constitutive transcription of the heterologous genes, the recruitment of translational machinery for production of foreign protein, and the formation of 3HP appeared to stimulate metabolism, even at temperatures substantially below the optimum growth temperature of P. furiosus. This is reflected in the heat plot shown in Figure 6, which includes all genes that showed a transcriptional change of 4-fold or more (log2-fold change ≥ ±2.2) in any of the following contrasts: COM1-400L vs. COM1-400E, MW76-400E vs. COM1-400E, MW76-400L vs. MW76-400E, and MW76-400L vs. COM1-400L (see also Table 3). The COM1 and MW76 transcriptomes were comparable after 2.5 h at 72°C, with only 36 ORFs differentially transcribed 2-fold or greater (see Figure 6). However, as the 72°C incubation time extended to 40 h, the number of ORFs differentially transcribed 2-fold or more between MW76 and COM1 increased to 497, suggesting a significantly different physiological state for the two strains. Among the 283 differentially transcribed ORFs that were higher in MW76 at 40 h, 58 were involved in energy metabolism, 29 were related to protein synthesis, 19 were related to amino acid biosynthesis, and seven corresponded to the M. sedula genes that had been metabolically engineered into the P. furiosus MW76 strain. Conversely, the transcription of many of these genes related to energy metabolism and protein synthesis were down-regulated in COM1 after 40 h at 72°C, while they were transcribed at the same or higher levels in MW76 after 40 h compared to 2.5 h at 72°C. Thus, the addition of constitutively-transcribed, heterologous genes appears to continue driving transcription of genes involved in core metabolic processes even at 72°C in the engineered strain, while the parent strain, COM1, slowed down transcription of the same core metabolic genes as it transitioned to a stationary or dormant phase.
Figure 6. Heatmap showing Differential Transcription for P. furiosus COM1 and MW76 strains.
Select genes with log2-fold changes of ≥ ±2.2, sorted by TIGR functional categories. P. furiosus ORF numbers are listed on outer edge. Each track represents a separate transcriptional comparison, labeled in the top of the figure. See Figure 2 for microarray experiment loop summary.
The cold shock effect observed here is consistent with previous transcriptional studies of the same phenomenon in wild-type P. furiosus (Weinberg et al., 2005). In that study, distinctly different patterns of transcription were observed at 72°C: an early shock (1-2 h), a late shock (>5 h), and a cold-adapted response occurring after many generations at 72°C. Many of the same genes were observed here to exhibit higher levels of transcript abundance during the early time point (2.5 h after the temperature switch). The set of genes involved in the early cold shock includes ORFs related to translation processes, amino acid and energy metabolism, and solute transport. The transcriptional levels of these genes drops off during the later sampling time point (40 h after the temperature switch) for COM1 as the cells transitioned into the late/adapted response, while in MW76 many continue to be transcribed at the same or higher levels even after 40 h.
Genes involved in amino acid synthesis, energy metabolism, DNA metabolism, transcription, and protein synthesis were all down-regulated in COM1 after 40 h at 72°C, indicative of the transition to stationary phase. Amino acid biosynthesis genes belonging to the pyruvate, aspartate, and glutamate families were down-regulated 4- to 32-fold, including lysine biosynthesis protein (PF1681), acetyl-lysine deacetylase (PF1686), aspartate aminotransferase (PF1702), and acetylglutamate/acetylaminoadipate kinase (PF1684). Genes for the V-type ATP synthase, responsible for generating ATP from the sodium motive force were all down-regulated between 2- to 10-fold (PF0177–PF0183). Genes encoding for 30S ribosomal proteins (PF1809–PF1810, PF1644) and 50S subunits (PF1802, PF1807, PF1818, PF1820) were all strongly down-regulated, as were translation initiation factors (PF1295, PF1349). This down-regulation of amino acid biosynthesis, energy conversation, and translation genes was not seen in MW76, indicating that these cellular processes remained active, as a consequence of the constitutively expressed heterologous genes.
Several of the genes that were activated in engineered strains at 95°C were also triggered at 72°C, including genes for protein chaperones, proteases, and RNA-degrading enzymes (Figure 4A). The thermosome (PF1974) was up-regulated 7-fold in MW76 at 40 h compared to 2.5 h, but was also constitutively transcribed at high levels in COM1. However, both the prefoldin α-subunit (PF0375) and the small heat shock protein (PF1883) were up-regulated 2- and 3.5-fold, respectively, in MW76 at 40 h compared to 2.5 h, but not in COM1 at the later time point. Likewise, the proteasome α-subunit (PF1571) was up-regulated 2-fold in MW76 at 40 h compared to 2.5 h, but this was not the case in COM1. There were also two RNA-degradation related genes that were up-regulated in MW76: an exosome RNA-binding protein Rrp42 and an exosome related cleavage and polyadenylation specificity factor (CPSF, PF1405). Thus, even at 72°C, it is likely that RNA transcripts produced by the constitutive, high-lever promoter for foreign gene expression were being targeted for degradation.
3.3. 3HP productivity increases with more efficient CO2 gas mass transfer
The high temperature, gas-intensive bioreactor system was used to assess gas-liquid mass transfer limitations of CO2-dependent 3HP production in engineered P. furiosus strain MW76. The bioreactor runs discussed below are summarized in Table 3. In all cases, the bioreactor was inoculated at 95°C, agitated at 250 rpm, and sparged with 15 ml/min 80% N2– 20% CO2 gas feed mixture during the initial biomass generation phase. When cell densities reached approximately 1 × 108 cells/ml, the reactor was shifted to 72°C to allow M. sedula enzymes to fold and become functional. Maltose concentrations, agitation rates, and gas sparging rates were varied to determine the effect of these parameters on cell growth, product formation and the transcriptome.
The initial comparison was done between COM1 and MW76 (COM1-400 and MW76-400 in Table 3). As the temperature was dropped to 72°C, both the agitation and gas flow rate were increased to 400 rpm and 50 ml/min N2/CO2, respectively. The 3HP productivity rate of MW76 at 400 rpm was 5.4 mg/L/hr with a final 3HP titer of 276 mg/L. This titer was approximately 15-fold more than reported previously, likely the result of the better availability of CO2 in the medium (Keller et al., 2013).
In subsequent runs, several improvements were made to further increase gas-liquid mass transfer of CO2. Both agitation and gas flow were increased to 1,000 rpm and 100 ml/min N2/CO2, and a small pore microbubbler stone (pore size – 2 ×m) was used to increase the interfacial surface area of the gas bubbles. With these changes the 3HP productivity rate doubled (11 mg/L/hr), while the 3HP titer remained approximately the same (210 mg/L) (see MW76-1000 in Table 3). This corresponds with a previous report of 3HP production in recombinant E. coli strains using MCR from Chloroflexus aurantiacus, from the very closely related carbon fixation pathway known as the 3HP bicycle (Rathnasingh et al., 2012). In that case, a titer of 144 mg/L was achieved when the native E. coli acetyl-CoA carboxylase and biotinylase were also overexpressed, along with the C. aurantiacus MCR.
It is interesting that at the higher productivity rates P. furiosus rapidly loses viability once the 3HP level reaches a maximum at around 35 hours (see Figure 5). This precipitous drop in cell density was much sharper than what is normally seen for these engineered P. furiosus strains; the cells in MW76-400 maintained their viability even out to 100 hours (Figure 5). Even when the maltose loading levels were increased to 10 g/L (MW76-1000M, Table 3), the same precipitous die-off was seen at that time, suggesting that nutrient exhaustion is not the primary cause of cell death. We also compared MW76 growth at 400 and 1000 rpm sampled shortly before the die-off (data not shown) to determine whether shear sensitivity was an issue. However, the transcriptomes were nearly identical and provided no evidence to suggest that shear stress alone was contributing to the loss in viability.
The rapid loss of cell viability at the higher 3HP productivities could result from intracellular accumulation of 3HP or other pathway intermediates (e.g. malonate semialdehyde) leading to acute toxicity. It is unlikely that cell death is triggered by M. sedula enzymes interfering with native P. furiosus metabolism or producing toxic byproducts because the enzymes are constitutively produced at high levels in all cases and no evidence of this death is observed at lower agitations (i.e. lower CO2 gas mass transfer). COM1 cells are not adversely affected by even 20 g/L 3HP in the medium if added during exponential growth (data not shown) so if 3HP is the cause of toxicity then it would most likely be due to intracellular build-up resulting from inefficient export of the hydroxyacid. P. furiosus typically ferments sugars to acetate, but it is not clear how acetate is exported or excreted. Excretion of undissociated acids can happen passively; there is evidence of passive or facilitated diffusion for lactate export in Streptococcus mutans (Dashper and Reynolds, 1996). Alternatively, diffusion of dissociated acids across membranes requires active transport mediated by ATP-dependent pumps. Most likely P. furiosus uses some kind of facilitated diffusion of undissociated acids, but it is not known whether native P. furiosus transporters can recognize and excrete 3HP from the cytosol.
In other microorganisms, the response to acid stress at the transcriptional level typically involves genes encoding for protein stabilization, DNA repair, or oxidative stress responsive enzymes (Choi et al., 2000; Warnecke and Gill, 2005). However, in P. furiosus there was no indication of a specific acid stress response. There was no differential regulation of genes encoding for DNA repair enzymes (e.g. RadA or DNA helicase PF0642) and heat shock proteins Hsp20 and Hsp60 were up-regulated at similar levels in the late time point compared to the lower agitation runs. Oxidative stress enzymes were all constitutively transcribed at high levels. Therefore, it remains unclear what mechanism is responsible for the sudden loss of viability at high CO2 gas mass transfer rates, but it is likely caused by build-up of pathway intermediates and will require additional study to further improve strain performance.
4. Future Directions for P. furiosus Metabolic Engineering
Metabolic engineering of heterologous pathways into P. furiosus has opened the door for developing a hyperthermophilic production host that could leverage unique metabolic pathways and temperature-dependent strategies for producing valuable chemicals. Here, we showed that recombinant P. furiosus metabolism is significantly activated at temperatures 30°C below its optimum growth temperature, driven largely by constitutive transcription and translation of heterologous genes, and the subsequent biosynthetic function of the corresponding enzymes. Improving the titers of 3HP will require insights into the causative issues related to loss of cell viability at 72°C. At this point, shear sensitivity to high agitation rates does not appear to be a problem. Also, since P. furiosus is able to withstand exogenous extracellular levels of 3HP of 100 mM or more without significant impact on growth (unpublished data), the 3HP present in the culture supernatant was not an issue. More likely, toxicity related to intracellular accumulation of 3HP, heterologous pathway intermediates (e.g., malonate semialdehyde), or byproducts of P. furiosus native metabolism may play a role. Certainly, evidence of stress response was apparent from transcriptional analysis. Fine-tuning of promoter strength to manipulate heterologous enzyme levels could be beneficial for balancing product formation with export from the cell; this will require examination of transcription mechanisms as they relate to P. furiosus metabolic engineering. It also needs to be established that product formation at sub-optimal temperatures indeed provides a bioenergetic benefit that motivates use of a thermally-based gene regulation strategy. While there are challenging issues that must be addressed, there are many potential advantages to using thermally diverse sources of pathways and enzymes in metabolically engineered thermophiles where temperature can be exploited to favor product formation over native host metabolites.
Acknowledgments
This work was supported grants to RMK and MWWA by the US Department of Energy Research ARPA-E Electrofuels Program (DE-AR0000081) and the US National Science Foundation (CBET-1264052, CBET-1264053). ABH acknowledges support from a US Department of Education GAANN Fellowship and AJL and BMZ acknowledge support from NIH Biotechnology Traineeships (2T32GM008776).
References
- Adams MW, Holden JF, Menon AL, Schut GJ, Grunden AM, Hou C, Hutchins AM, Jenney FE, Kim C, Ma K, Pan G, Roy R, Sapra R, Story SV, Verhagen MF. Key role for sulfur in peptide metabolism and in regulation of three hydrogenases in the hyperthermophilic archaeon Pyrococcus furiosus. J. Bacteriol. 2001;183:716–724. doi: 10.1128/JB.183.2.716-724.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basen M, Sun J, Adams MWW. Engineering a hyperthermophilic archaeon for temperature-dependent product formation. mBio. 2012;3:1–8. doi: 10.1128/mBio.00053-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg IA, Kockelkorn D, Buckel W, Fuchs G. A 3-hydroxypropionate/4-hydroxybutyrate autotrophic carbon dioxide assimilation pathway in Archaea. Science. 2007;318:1782–1786. doi: 10.1126/science.1149976. [DOI] [PubMed] [Google Scholar]
- Bridger SL, Lancaster WA, Poole FL, Schut GJ, Adams MWW. Genome sequencing of a genetically tractable Pyrococcus furiosus strain reveals a highly dynamic genome. J. Bacteriol. 2012;194:4097–4106. doi: 10.1128/JB.00439-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Baumler DJ, Kaspar CW. Contribution of dps to acid stress tolerance and oxidative stress tolerance in Escherichia coli O157: H7. Appl. Environ. Microbiol. 2000;66:3911–3916. doi: 10.1128/aem.66.9.3911-3916.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrado RJ, Haynes CA, Haendler BE, Toone EJ. Electrofuels: a new paradigm for renewable fuels. In: Lee JW, editor. Advanced Biofuels and Bioproducts. Springer; New York: 2013. pp. 1037–1064. [Google Scholar]
- Dashper SG, Reynolds EC. Lactic acid excretion by Streptococcus mutans. Microbiol. 1996;142:33–39. doi: 10.1099/13500872-142-1-33. [DOI] [PubMed] [Google Scholar]
- Fiala G, Stetter K. Pyrococcus furiosus sp. nov. represents a novel genus of marine heterotrophic archaebacteria growing optimally at 100°C. Arch. Microbiol. 1986;145:56–61. [Google Scholar]
- Gronenberg LS, Marcheschi RJ, Liao JC. Next generation biofuel engineering in prokaryotes. Curr. Opin. Chem. Biol. 2013;17:462–471. doi: 10.1016/j.cbpa.2013.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins AB, Adams MWW, Kelly RM. Conversion of 4-hydroxybutyrate to acetyl coenzyme A and its anapleurosis in the Metallosphaera sedula 3-hydroxypropionate/4-hydroxybutyrate carbon fixation pathway. Appl. Environ. Microbiol. 2014;80:2536–2545. doi: 10.1128/AEM.04146-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins AS, McTernan PM, Lian H, Kelly RM, Adams MWW. Biological conversion of carbon dioxide and hydrogen into liquid fuels and industrial chemicals. Curr. Opin. Biotechnol. 2013;24:376–384. doi: 10.1016/j.copbio.2013.02.017. [DOI] [PubMed] [Google Scholar]
- Hawkins A, Han Y, Lian H, Loder A, Menon A, Iwuchukwu I, Keller M, Leuko T, Adams MWW, Kelly RM. Extremely thermophilic routes to microbial electrofuels. ACS Catal. 2011;1:1043–1050. [Google Scholar]
- Keller MW, Schut GJ, Lipscomb GL, Menon AL, Iwuchukwu IJ, Leuko TT, Thorgersen MP, Nixon WJ, Hawkins AS, Kelly RM, Adams MWW. Exploiting microbial hyperthermophilicity to produce an industrial chemical, using hydrogen and carbon dioxide. Proc. Natl. Acad. Sci. U.S.A. 2013 doi: 10.1073/pnas.1222607110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan EI, Liao JC. Microbial synthesis of n-butanol, isobutanol, and other higher alcohols from diverse resources. Bioresource Technology. 2013;135:339–349. doi: 10.1016/j.biortech.2012.09.104. [DOI] [PubMed] [Google Scholar]
- Lian H. Transcriptomic and physiological analysis of a recombinant Pyrococcus furiosus strain metabolically engineered to produce 3-hydroxypropionate from CO2 and maltose; PhD dissertation. North Carolina State University; 2014. [Google Scholar]
- Lipscomb GL, Stirrett K, Schut GJ, Yang F, Jenney FEJ, Scott RA, Adams MWW, Westpheling J. Natural competence in the hyperthermophilic archaeon Pyrococcus furiosus facilitates genetic manipulation: construction of markerless deletions of genes encoding the two cytoplasmic hydrogenases. Appl. Environ. Microbiol. 2011;77:2232–2238. doi: 10.1128/AEM.02624-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maupin-Furlow JA, Humbard MA, Kirkland PA, Li W, Reuter CJ, Wright AJ, Zhou G. Proteasomes from structure to function: perspectives from Archaea. Curr. Top. Dev. Biol. 2006;75:125–169. doi: 10.1016/S0070-2153(06)75005-0. [DOI] [PubMed] [Google Scholar]
- Peralta-Yahya PP, Keasling JD. Advanced biofuel production in microbes. Biotechnol. J. 2010;5:147–162. doi: 10.1002/biot.200900220. [DOI] [PubMed] [Google Scholar]
- Rathnasingh C, Raj SM, Lee Y, Catherine C, Ashok S, Park S. Production of 3-hydroxypropionic acid via malonyl-CoA pathway using recombinant Escherichia coli strains. J. Biotechnol. 2012;157:633–640. doi: 10.1016/j.jbiotec.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Shockley KR, Ward DE, Chhabra SR, Conners SB, Montero CI, Kelly RM. Heat shock response by the hyperthermophilic archaeon Pyrococcus furiosus. Appl. Environ. Microbiol. 2003;69:2365–2371. doi: 10.1128/AEM.69.4.2365-2371.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand KR, Sun C, Li T, Jenney FE, Schut GJ, Adams MWW. Oxidative stress protection and the repair response to hydrogen peroxide in the hyperthermophilic archaeon Pyrococcus furiosus and in related species. Arch. Microbiol. 2010;192:447–459. doi: 10.1007/s00203-010-0570-z. [DOI] [PubMed] [Google Scholar]
- Warnecke T, Gill RT. Organic acid toxicity, tolerance, and production in Escherichia colibiorefining applications. Microb Cell Fact. 2005;4:25. doi: 10.1186/1475-2859-4-25. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=16122392&retmode=ref&cmd=prlinks. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg MV, Schut GJ, Brehm S, Datta S, Adams MWW. Cold shock of a hyperthermophilic archaeon: Pyrococcus furiosus exhibits multiple responses to a suboptimal growth temperature with a key role for membrane-bound glycoproteins. J. Bacteriol. 2005;187:336–348. doi: 10.1128/JB.187.1.336-348.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werpy T, Peterson G, Aden A, Bozell J, Holladay J, White J, Manheim A. Top Value Added Chemicals from Biomass: Volume I--Results of Screening for Potential Candidates from Sugars and Synthesis Gas. U.S. Department of Energy - Energy Efficiency and Renewable Energy. 2004;1:67. [Google Scholar]
- Williams E, Lowe TM, Savas J, DiRuggiero J. Microarray analysis of the hyperthermophilic archaeon Pyrococcus furiosus exposed to gamma irradiation. Extremophiles. 2007;11:19–29. doi: 10.1007/s00792-006-0002-9. [DOI] [PubMed] [Google Scholar]