Abstract
Background
Prolonged pathogen shedding and increased duration of illness associated with infections in immunosuppressed individuals put close human immunodeficiency virus (HIV)–negative contacts of HIV-infected persons at increased risk of exposure to infectious pathogens.
Methods
We calculated incidence and longitudinal prevalence (number of days per year) of influenzalike illness (ILI), diarrhea, and nonspecific febrile illness during 2008 from a population-based surveillance program in the urban slum of Kibera (Kenya) that included 1830 HIV-negative household contacts of HIV-infected individuals and 13 677 individuals living in exclusively HIV-negative households.
Results
For individuals ≥5 years old, incidence was significantly increased for ILI (risk ratio [RR], 1.47; P < .05) and diarrhea (RR, 1.41; P < .05) in HIV-negative household contacts of HIV-infected individuals compared with exclusively HIV-negative households. The risk of illness among HIV-negative persons was directly proportional to the number of HIV-infected persons living in the home for ILI (RR, 1.39; P < .05) and diarrhea (RR, 1.36; P < .01). We found no increased rates of illness in children <5 years old who lived with HIV-infected individuals.
Conclusions
Living with HIV-infected individuals is associated with modestly increased rates of respiratory and diarrheal infections in HIV-negative individuals >5 years old. Targeted interventions are needed, including ensuring that HIV-infected persons are receiving appropriate care and treatment.
Keywords: HIV, surveillance, home-based counseling and testing, household transmission, Africa, Kibera, nonspecific febrile illness, respiratory infection, pneumonia, influenzalike illness, ILI, acute lower respiratory infection, ALRI, diarrhea, nonspecific febrile illness, incidence rates, urban slum
During a variety of bacterial, viral, and parasitic infections, human immunodeficiency virus (HIV)–infected patients seem to shed pathogens, in some cases, for longer durations [1–3] and in larger numbers than presumably nonimmunosuppressed HIV-negative patients [4]. The duration of illnesses may also be longer for HIV-infected patients than for HIV-negative patients, as has been shown for influenza [5]. By increasing the infectious period of illnesses and the dose and duration of pathogens shed, the risk of transmission to close contacts may be increased.
We analyzed data from population-based surveillance conducted by the Kenya Medical Research Institute (KEMRI) and the Centers for Disease Control and Prevention (CDC) for infectious disease syndromes and their causes and noninfectious conditions in Kibera, an urban slum in Nairobi, Kenya [6]. In 2008, home-based HIV-testing and counseling were offered to residents of the surveillance area [7], and HIV test results were linked to data from infectious disease surveillance of consenting adults. We examined rates of influenza-like illness (ILI), diarrhea, nonspecific febrile illness, and the noninfectious condition of burn injuries in HIV-negative household members living in households with HIV-infected individuals compared with those living in households without HIV-infected individuals.
METHODS
Surveillance Site
KEMRI/CDC has conducted population-based, infectious disease surveillance (PBIDS) since 2006, in the villages of Gatwikira and Soweto West in Kibera, a slum in Nairobi, Kenya. Population ranges between 25 000 and 29 000 persons within a surveillance area of 0.37 km2 (mean population density, 77 000 persons per square kilometer). The site comprises semipermanent houses and shops with communal pit latrines in a mazelike arrangement with dirt paths and open sewers. HIV seroprevalence among adults living within the surveillance area is 14% [7].
Household and Clinic Surveillance
The ongoing PBIDS program has been described elsewhere [6]. Briefly, all participating households are located within 1 km from the study clinic, known as Tabitha Clinic (operated by Carolina for Kibera). Persons who have resided within the surveillance area for ≥4 calendar months are invited to enroll in the study. Participants who consistently share a common “cooking pot” are defined as members of the same household. Members of each household are assigned a unique identification number and agree to household interviews every 2 weeks, conducted by community interviewers. At each reporting round, participants are asked standard questions about recent occurrences of illnesses, including fever, diarrhea, and respiratory illness, with follow-up questions about severity, length of illness, and type of care sought. Burn injuries occurring within the last 2 weeks, representing a noninfectious comparison outcome, are also documented but without follow-up questions. For adults and older children not available at the time of interview and for children <5 years old, a proxy knowledgeable about the participant's health is interviewed. All surveillance participants have access to free care for acute medical conditions at the study clinic.
Written informed consent was obtained for data collection at the clinics and households; for minors, consent was obtained from a parent or guardian. The protocol and consent forms were reviewed and approved by the Ethical Review Boards of KEMRI (protocol 932) and the Institutional Review Board of the CDC (protocol 4566).
Home-Based HIV Testing and Counseling
From 1 January to 31 December 2008, residents of the surveillance area were offered rapid HIV tests through home-based testing and counseling (HBTC). All individuals aged ≥18 years old and emancipated minors (aged 13–17 years) enrolled in PBIDS were given the option to consent and participate. Dependent minors were offered HIV tests but required parental consent and independent assent. Children (aged ≤13 years) were only offered testing if their biological mothers were HIV positive or were deceased. The acceptance rate among guardians of these children was very high (96%). Overall, 82.8% of all those eligible accepted testing. Informed consent was required to participate in HBTC and to allow data from HBTC to be linked to PBIDS data. Procedures, including counseling and follow-up testing, have been described elsewhere [7].
Inclusion/Exclusion Criteria for Analysis
If a household had ≥1 resident who had HIV tests processed and results were exclusively negative, all individuals in the household were considered to be living in an exclusively HIV-negative household. For a household with ≥1 person testing positive for HIV-infection, all household members except for the HIV-infected individual (the index case) were considered to be household contacts of an HIV-infected individual.
All individuals who tested negative for HIV and children <13 years old who were not tested were included in the analysis. Untested individuals ≥13 years old were excluded from the analysis owing to the risk of exposure to HIV by sexual transmission. In addition, in households with an HIV-infected member, untested children <2 years old were excluded because we did not have data to determine the relationship of each member of the household to one another (eg, biological mother, father, or child) and thus were unable to rule out vertical transmission of HIV. Given that mortality for untreated HIV-infected infants in sub-Saharan Africa is substantial by age 2 years [8], we assumed that untested children surviving past this age were HIV negative and had minimal risk of acquisition until age 13 years; thus, we categorized children 2–13 years old, who were not tested, as not HIV infected.
Case Definitions
Case definitions were fulfilled based on data collected from the fortnightly household visits. A case of ILI was defined as reported fever or “hotness of body” with either cough or sore throat. Diarrhea was defined as ≥3 stools that were looser than normal or ≥1 episode of blood in stools during a 24-hour period; discrete cases of diarrheal illness were preceded by ≥3 symptom-free days. Nonspecific febrile illness was defined as any report during the household visit of fever (“hotness of body”) within the previous 2 weeks without evidence of another infection defined as cough, difficulty breathing, ILI, or diarrhea. A symptom-free period of ≥7 days was used to define discrete cases for both ILI and febrile illness. A burn injury was defined as any report of a burn during the study period with ≥14 days of no previous report of a burn.
Analysis
The study period for incidence and longitudinal prevalence of these conditions was 1 January to 31 December 2008. Incidence rates were calculated as the number of discrete episodes of an illness over the total person-years of observation (PYO) and reported in units of episodes per PYO. Based on previous analyses of data from PBIDS suggesting significant decay in recall for events occurring more than a few days before the interview [9], we counted only symptoms of illnesses reported to be occurring on the day of visit and during the 3 previous days (days 0–3) for children aged <5 years and the 4 previous days (days 0–4) for persons aged ≥5 years. There was no recall limit for burn injuries because the specific date of injury within that reporting round is not asked.
The denominators (reported in PYO for incidence calculations) were adjusted according to the boundaries of the recall limit. For home visit rounds during which the HIV-infected index case patient was reported as migrated out of or not present within the surveillance area, the illness data and respective PYO for all members of the household were excluded from the analysis. We also excluded PYO for reporting rounds during which an interview was not conducted. For identifying symptom-free days to define discrete episodes, we used the entire reporting period without recall bias restrictions.
We modeled incidence and longitudinal prevalence of illnesses at the household level using Poisson regression (PROC GENMOD; SAS version 9.1; SAS Institute) in 2 different cohort methods. First, we compared HIV-negative individuals living with only HIV-negative household members with those living with ≥1 HIV-infected household member. We then compared individuals living in exclusively HIV-negative households with HIV-negative individuals living with 1, 2, or ≥3 HIV-infected household members, modeled as a continuous variable, to determine a dose-dependent relationship. We controlled for intra-household correlation using generalized estimating equations and controlled for household size as a covariate in the analysis. We assumed the “independent” correlation matrix structure in these analyses.
RESULTS
During the study period, 27 821 persons and 6858 households were enrolled in PBIDS. HIV tests were performed in 7427 individuals residing in 4064 households. There were 3466 households with exclusively HIV-negative residents. The mean household size was 6.3 persons (95% confidence interval [CI], 6.2–6.4). These households included 6608 untested individuals ≥13 years old (52% of all persons ≥13 years old in these households), who were excluded from analysis (Table 1). A total of 6704 HIV-negative individuals (≥13 years old) and 6973 untested children <13 years old (total of 13 677) were included in the analysis.
Table 1.
Percentage of Adults Tested for HIV in Households Included in the Analysisa
| HIV-Infected Household Contacts, No. | Households, No. | Adults in Household Not Tested, % |
|---|---|---|
| 0 | 3466 | 52 |
| 1 | 484 | 63 |
| 2 | 104 | 65 |
| 3 | 10 | 66 |
| Total | 4064 | 53 |
Abbreviation: HIV, human immunodeficiency virus.
Adults were defined as persons ≥13 years old.
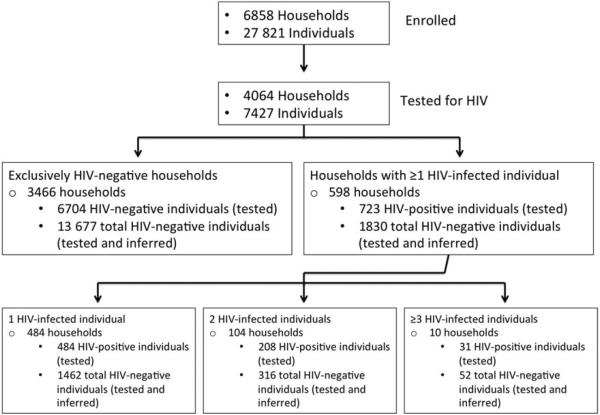
There were 598 households with ≥1 person who tested HIV positive. The mean household size was 7.1 persons (95% CI, 6.8–7.4), significantly larger than households with exclusively HIV-negative residents. These households included 723 individuals testing positive for HIV; 1264 untested adults ≥13 years old (representing 64% of all persons ≥13 years old in these households), and 264 untested children ≤2 years old were excluded from the analysis. A total of 473 HIV-negative individuals and 1357 untested children <13 years old (and >2 years old) were included in the analysis (Figure 1).
Figure 1.
Number of human immunodeficiency virus (HIV)–negative individuals included in each comparison group (exclusively HIV-negative households vs households with ≥1 HIV-infected member).
Influenzalike Illness
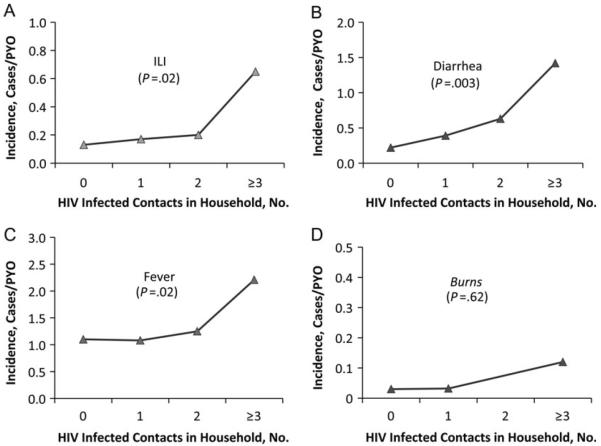
Among persons aged ≥5 years, there were 0.19 cases of ILI (95% CI, .12–.34) and 0.81 days (95% CI, .43–1.51) of ILI per PYO for household contacts of ≥1 HIV-infected individual compared with 0.13 cases (95% CI, .08–.21) and 0.52 days (95% CI, .31–.88) of ILI per PYO for persons living in exclusively HIV-negative households. This represents a statistically significant 1.51-fold higher incidence and a 1.55-fold greater longitudinal prevalence. When results were stratified by the number of HIV-infected individuals, there was a significant 1.44-fold increase and a 1.53-fold increase in longitudinal prevalence for individuals ≥5 years old with each additional HIV-infected household member. No significant differences were seen for children <5 years old (Table 2; Figure 2A).
Table 2.
Rates of ILI in HIV-Negative Household Contacts of HIV-Infected Individuals and Individuals in Exclusively HIV-Negative Households in Kibera, Nairobi
| HIV-Infected Household Contacts, No. |
HIV-Infected Household Contacts, No. |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| ILI Rates by Age Group | 0 | ≥1 | Risk Ratio | P Value | 1 | 2 | ≥3 | Risk Ratio | P Value |
| Age <5 y | |||||||||
| ILI cases, No. | 483 | 56 | … | … | 44 | 11 | 1 | … | … |
| PYO | 614.7 | 89.4 | … | … | 68.0 | 19.5 | 1.9 | … | … |
| Incidence (95% CI), cases/PYO | 1.00 (.77–1.29) | 0.81 (.55–1.17) | 0.80 (.60–1.07) | .14 | 1.09 (.59–2.06) | 0.93 (.44–1.98) | 1.39 (.23–8.54) | 0.85 (.68–1.04) | .12 |
| Longitudinal prevalence (95% CI), d/PYO | 3.9 (2.94–5.31) | 3.1 (2.06–4.54) | 0.77 (.58–1.04) | .09 | 3.99 (2.05–7.77) | 3.65 (1.51–8.85) | 6.06 (.96–38.0) | 0.83 (.66–1.04) | .11 |
| Age ≥5 y | |||||||||
| ILI cases, No. | 268 | 50 | … | … | 37 | 9 | 4 | … | … |
| PYO | 2443.1 | 309.9 | … | … | 250.9 | 50.9 | 8.0 | … | … |
| Incidence (95% CI), cases/PYO | 0.13 (.08–.21) | 0.19 (.12–.34) | 1.51 (1.06–2.16) | .02 | 0.17 (.07–.43) | 0.20 (.05–.79) | 0.65 (.21–1.99) | 1.44 (1.06–1.94) | .02 |
| Longitudinal prevalence (95% CI), d/PYO | 0.52 (.31–.88) | 0.81 (.43–1.51) | 1.55 (1.03–2.34) | .03 | 0.62 (.22–1.73) | 0.97 (.20–4.66) | 2.99 (.88–10.18) | 1.53 (1.07–2.18) | .02 |
Abbreviations: CI, confidence interval; d, days; HIV, human immunodeficiency virus; ILI, influenzalike illness; PYO, person-years of observation.
Figure 2.
Incidence of acute illnesses for persons ≥5 years old, stratified by number of human immunodeficiency virus (HIV)–infected household members. Abbreviations: ILI, influenzalike illness; PYO, person-years of observation.
Diarrheal Illness
Among persons ≥5 years old living with ≥1 HIV-infected individual, there were 0.31 cases (95% CI, .21–.44) and 0.84 days (95% CI, .56–1.24) of diarrheal illness per PYO compared with 0.22 cases (95% CI, .17–.27) and 0.56 days (95% CI, .43–.72) per PYO for individuals living in exclusively HIV-negative households. This was a statistically significant 1.42-fold increase in incidence and a 1.49-fold increase in longitudinal prevalence per PYO. In the analysis stratified by number of HIV-infected individuals, the incidence of diarrhea was significantly increased by 1.38-fold and longitudinal prevalence by 1.44-fold for each additional HIV-infected individual in the household. No significant differences were found for children <5 years old (Table 3; Figure 2B).
Table 3.
Rates of Diarrhea in HIV-Negative Household Contacts of HIV-Infected Individuals and Individuals in Exclusively HIV-Negative Households in Kibera, Nairobi
| HIV-Infected Household Contacts, No. |
HIV-Infected Household Contacts, No. |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Diarrhea Rates by Age Group | 0 | ≥1 | Risk Ratio | P Value | 1 | 2 | ≥3 | Risk Ratio | P Value |
| Age, <5 y | |||||||||
| Diarrhea cases, No. | 1539 | 209 | … | … | 148 | 57 | 4 | … | … |
| PYO | 614.7 | 89.4 | … | … | 68.0 | 19.5 | 1.9 | … | … |
| Incidence (95% CI), cases/PYO | 2.81 (2.37–3.33) | 2.64 (2.01–3.46) | 0.94 (.75–1.17) | .58 | 2.59 (1.54–4.39) | 3.45 (1.99 5.99) | 2.97 (.51 17.29) | 0.99 (.85 1.15) | .88 |
| Longitudinal prevalence (95% CI), d/PYO | 7.59 (6.32–9.11) | 7.19 (5.34–9.67) | 0.95 (.74–1.21) | .66 | 6.96 (3.99–12.12) | 8.85 (4.71–16.61) | 6.44 (1.31–31.55) | 0.99 (.83–1.16) | .87 |
| Age ≥5 y | |||||||||
| Diarrhea cases, No. | 488 | 87 | … | … | 62 | 20 | 5 | … | … |
| PYO | 2443.1 | 309.9 | 250.9 | 50.9 | 8.0 | ||||
| Incidence, (95% CI), cases/PYO | 0.22 (.17–27) | 0.31 (.21–44) | 1.42 (1.07–1.89) | .02 | 0.39 (.24–.63) | 0.63 (.26–1.51) | 1.42 (.46–1.40) | 1.38 (1.12–1.71) | .003 |
| Longitudinal prevalence (95% CI), d/PYO | 0.56 (.43–72) | 0.84 (.56–1.24) | 1.49 (1.10–2.03) | .01 | 1.04 (.62–1.74) | 1.61 (.66–3.95) | 4.56 (1.69–12.33) | 1.44 (1.16–1.78) | .001 |
Abbreviations: CI, confidence interval; d, days; HIV, human immunodeficiency virus; PYO, person-years of observation.
Nonspecific Febrile Illness
For persons ≥5 years old, there were statistically significant increases of 1.15-fold (95% CI, 1.02–1.29) in incidence and 1.17-fold (95% CI, 1.02–1.35) in longitudinal prevalence for each additional HIV-infected individual in the household in the analysis stratified by number of HIV-infected individuals; however, there was no significant increase in incidence or longitudinal prevalence in the non-stratified analysis (Table 4; Figure 2C). For children <5 years old living with ≥1 HIV-infected individual, there were 4.00 cases (95% CI, 3.36–4.77) of nonspecific febrile illness per PYO compared with 4.71 cases (95% CI, 4.18–5.30) for those living in exclusively HIV-negative households, a significant 0.85-fold decrease in incidence. However, this was an isolated finding for children <5 years old, because there were no significant differences in longitudinal prevalence in this nonstratified analysis or in incidence and longitudinal prevalence for the analysis stratified by number of HIV-infected individuals.
Table 4.
Rates of Nonspecific Febrile Illness in HIV-Negative Household Contacts of HIV-Infected Individuals and Individuals in Exclusively HIV-Negative Households in Kibera, Nairobi
| HIV-Infected Household Contacts, No. |
HIV-Infected Household Contacts, No. |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Rates of Febrile Illness by Age Group | 0 | ≥1 | Risk Ratio | P Value | 1 | 2 | ≥3 | Risk Ratio | P Value |
| Age <5 y | |||||||||
| Fever cases, No. | 2474 | 304 | … | … | 217 | 82 | 5 | … | … |
| PYO | 614.7 | 89.4 | … | … | 68.0 | 19.5 | 1.9 | … | … |
| Incidence (95% CI), cases/PYO | 4.71 (4.18–5.30) | 4.00 (3.36–4.77) | 0.85 (.74–98) | .02 | 4.29 (3.13–5.89) | 5.56 (3.74–8.26) | 4.62 (2.06–10.36) | 0.92 (.83–1.02) | .12 |
| Longitudinal prevalence (95% CI), d/PYO | 13.59 (11.79–15.66) | 12.20 (9.98–14.91) | 0.89 (.77–1.05) | .18 | 13.16 (9.08–19.06) | 17.78 (11.67–27.03) | 15.02 (6.36–35.49) | 0.96 (.85–1.08) | .49 |
| Age ≥5 y | |||||||||
| Fever cases, No. | 2266 | 321 | … | … | 248 | 58 | 15 | … | … |
| PYO | 2443.1 | 309.9 | … | … | 250.9 | 50.9 | 8.0 | … | … |
| Incidence (95% CI), cases/PYO | 1.10 (.96–1.27) | 1.26 (1.05–1.52) | 1.14 (.99–1.33) | .07 | 1.08 (.79–1.47) | 1.25 (.84–1.85) | 2.21 (1.04–4.71) | 1.15 (1.02–1.29) | .02 |
| Longitudinal prevalence (95% CI), d/PYO | 3.50 (2.92–4.19) | 4.07 (3.25–5.10) | 1.16 (.99–1.37) | .07 | 3.19 (2.27–4.47) | 3.68 (2.31–5.85) | 7.29 (3.50–15.19) | 1.17 (1.02–1.35) | .03 |
Abbreviations: CI, confidence interval; d, days; HIV, human immunodeficiency virus; PYO, person-years of observation.
Burn Injuries
Burn injuries, a significant noninfectious problem within the surveillance area [10], was included in this analysis to detect analytic bias, because HIV-infected household members would not be expected to contribute to risk in HIV-negative residents. For individuals ≥5 and <5 years old, there were no significant increases in burn incidence or prevalence. The analysis stratified by number of HIV-infected household members also failed to find an association between household HIV status and burn injury incidence in either age category (Table 5; Figure 2D).
Table 5.
Rates of Burn Injuries in HIV-Negative Household Contacts of HIV-Infected Individuals and Individuals in Exclusively HIV-Negative Households in Kibera, Nairobi
| HIV-Infected Household Contacts, No. |
HIV-Infected Household Contacts, No. |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Burn Injury Rates by Age Group | 0 | ≥1 | Risk Ratio | P Value | 1 | 2 | ≥3 | Risk Ratio | P Value |
| Age <5 y | |||||||||
| Burn cases, No. | 101 | 16 | … | … | 12 | 4 | 0 | … | … |
| PYO | 614.7 | 89.4 | 68.0 | 19.5 | 1.9 | … | … | ||
| Incidence (95% CI), cases/PYO | 0.16 (.13–.19) | 0.18 (.11–.28) | 1.09 (.64–1.85) | .57 | 0.18 (.11–.29) | 0.21 (.11–.45) | NA | 1.06 (.34–3.28) | .89 |
| Age ≥5 y | |||||||||
| Burn cases, No. | 77 | 9 | … | … | 8 | 0 | 1 | … | … |
| PYO | 2443.1 | 309.9 | … | … | 250.9 | 50.9 | 8.0 | … | … |
| Incidence (95% CI), cases/PYO | 0.03 (.02–.04) | 0.03 (.01–.05) | 0.92 (.46–1.84) | .85 | 0.032 (.014–.061) | NA | 0.12 (.06–.62) | 0.53 (.07–.26) | .62 |
Abbreviations: CI, confidence interval; d, days; HIV, human immunodeficiency virus; NA, not applicable; PYO, person-years of observation.
DISCUSSION
The findings of these analyses of population-based data in an urban slum in Nairobi suggest that living with HIV-infected persons may be one of many community and environmental components that increase rates of acute respiratory and diarrheal illnesses for HIV-negative individuals ≥5 years old. Evidence of increased rates of infections with each additional HIV-infected individual living in the household suggests that the effect is dose dependent. In contrast to the infectious syndromes analyzed, the incidence of noninfectious condition (burn injury) was not significantly elevated in HIV-negative household contacts of HIV-infected individuals. We have not identified any other studies that have systematically compared the incidence of acute infections in immunocompetent individuals living within the context of an HIV-infected household member(s). However, studies on the health of HIV-negative household members living with HIV-infected individuals receiving cotrimoxazole prophylaxis showed a significant reduction in overall mortality and morbidity after prophylaxis was started, implying that failure to prevent infections in HIV-infected individuals has a measurable impact on the health of close contacts [11]. Our results add further evidence that HIV may be a risk factor for transmission of diarrheal and respiratory illness that could amount to considerable extra disease burden across a large population.
A biological basis for the observed increased transmission of diarrheal and respiratory illness is built on evidence for increased incidence and prolonged shedding of pathogens in HIV-infected hosts. During Cryptosporidium outbreaks, HIV-infected individuals have been shown to shed greater numbers of oocysts and have diarrhea for a longer duration than HIV-negative individuals, and immunocompromised individuals may have chronic transmissible cryptosporidiosis for months [12]. Respiratory pathogens (eg, influenza) in the immunocompromised host can also result in longer periods of shedding and longer durations of illness [13–16]. Kibera is a densely populated and impoverished urban slum with inadequate sanitation, poor air quality, and lack of access to clean water. Given these features that increase the efficiency of transmission of respiratory and orofecal infections, small increases in duration of pathogen shedding due to HIV-infection could translate into higher disease incidence.
Although our analysis showed increased rates of infectious syndromes in individuals ≥5 years old living with HIV-infected persons, the same analysis for children <5 years old did not show a similar association. One possibility is that children <5 years old, who are immunologically naive to many pathogens, experience high incidence of acute diarrheal and respiratory infections; given the wide variety of pathogen exposure opportunities in an urban slum, additional pathogen exposure in the household may not add measurably to increased risk of illness [17, 18]. Given substantial environmental exposure to respiratory and diarrheal pathogens for young children, another consideration is that children <5 years old might tend to be the index case in the household [19–22]. Household-reported rates of ILI in our surveillance, for instance, are >8-fold greater for children 1–2 years old than for adults 18–34 years old (6.6 vs 0.87 cases per PYO) [23]. The increased exposure of children <5 years old to diarrheal and respiratory pathogens from HIV-infected individuals ≥5 years old would therefore not be expected to significantly increase rates of illness as we found in our model of adult-to-adult transmission.
Our analysis of nonspecific febrile illness did not follow the same clear pattern of increased rates of illness in individuals ≥5 years old that we observed for diarrheal and respiratory illnesses. We found an isolated, significant decrease in febrile illness incidence (but not longitudinal prevalence) for children <5 years old living with HIV -infected persons that was not found when results were stratified by number of HIV-infected persons, and a significant increase in incidence and longitudinal prevalence in individuals ≥5 years old only when results were stratified by number of HIV-infected individuals. There is a wide variety of causes of febrile illness in Africa, including vector-borne diseases, such as malaria, rickettsiosis, dengue, and Chikungunya, as well as other environmentally linked diseases, such as leptospirosis or typhoid fever, that would probably not be affected by household exposure to an HIV-infected individual [24, 25]. It is also possible that there was a change in the epidemiology of a specific, direct-contact viral or bacterial pathogen owing to behavior modification after the diagnosis of an HIV infection that could have influenced the incidence of this syndrome. Overall, these results suggest that further investigations into the causes of nonspecific febrile illness in this study population are needed to explain these findings.
Interventions to improve the health of HIV-infected populations in areas of resource-limited settings and high HIV prevalence should be evaluated in future studies for evidence of parallel reduction in a variety of infectious syndromes among HIV-negative household members. Highly active antiretroviral therapy has greatly reduced morbidity and the incidence of infections among HIV-infected individuals in sub-Saharan Africa, and the World Health Organization has recently updated its recommendation for initiation of treatment at much higher CD4 cell counts than in previous guidelines [26–28]. The implementation of these new guidelines, as well as other interventions aggressively promoting highly active antiretroviral therapy to manage or prevent HIV infection could have substantial impact on community-level health. Moreover, water quality and hand washing interventions focused on HIV-infected populations and urban slum populations have individually shown dramatic reductions in diarrheal illness in their respective target groups and should also be evaluated for their additive effects in preventing diarrhea in HIV-infected individuals and their household contacts living in urban slums like Kibera [29–31]. Although the effectiveness of pneumococcal and influenza vaccines in HIV-infected persons is well documented, the effect of community transmission dynamics for these pathogens in a population with high HIV prevalence warrants further investigation [32, 33].
Because a substantial proportion (64%) of adults in households determined to be exclusively HIV negative were untested, it is likely that some HIV-positive households were misclassified. However, such misclassification would have biased toward the null (ie, households incorrectly classified as HIV negative would have been subject to more opportunistic infections, diminishing the calculated risk ratios); thus, it is conceivable that the effects we observed represented underestimates. Moreover, data on the progression of HIV infection that could modify the effect we were measuring, such as longitudinal CD4 cell counts, viral load, or cotrimoxazole and antiretroviral use, were unavailable for analysis.
In 2008–2009, HBTC was rapidly increasing access to HIV care, which could have decreased our rates of household transmission. However, as with the previous limitation, omission of these variables would have biased results toward the null, further underestimating the true morbidity of untreated HIV-infection in our analysis. Follow-up studies assessing the effects of aggressive HIV treatment or antibiotic prophylaxis in this population would elucidate the potentially protective effects of chronic disease management in the epidemiology of acute infections. The only potential misclassification that would not bias toward the null was our inability to be certain that persons testing negative for HIV remained uninfected throughout the observation period, especially individuals living with HIV-infected household members, among whom there might be a higher incidence of HIV infection. To minimize this effect, we restricted the observation period to a single year after the completion of HBTC, not only to limit the cumulative incidence of seroconversion in the group but also, in the event of seroconversion, to exclude the period of profound, chronic immunosuppression occurring later in disease progression, which we assume is the main driver of increased rates of infections in household with HIV-infected individuals.
Households with ≥1 individual who tested HIV positive were significantly larger in number than households with exclusively HIV-negative residents, introducing the possibility that increased rates of infectious illness were due to increased opportunities for exposure to ill persons (HIV infected or not) in the household. However, after controlling for household size in our analysis, we demonstrated that for all significant differences found in our analyses, the presence of an HIV-infected person in the household was an independent risk factor for illness occurring in non–HIV-infected members of household and that this independent risk increases with the number of HIV-infected persons in the household.
Reporting bias could also have been introduced by a household proxy either overreporting or underreporting the illness of a household member based on their knowledge of that member's HIV status. Furthermore, a lack data on household socioeconomic features limited our ability to address potential confounders in our study population, such as economic hardship, stigma, or caregiver fatigue, which are associated with overall poorer health outcomes and could preferentially affect households living with an HIV-infected member [34–36].
Our findings provide an initial step for quantifying increased transmission of acute infectious pathogens among HIV-negative individuals when ≥1 member of the household is HIV infected. This is a “proof of concept” that should inform further studies and interventions aimed at disease prevention in this unique and expanding milieu of poverty, high HIV prevalence, and poor sanitation. Characterizing the community-wide benefits of maintaining the health of HIV-infected individuals and preventing new HIV infections could have major implications for the 35 million HIV-infected individuals, their family members, and potentially other persons within communities with high HIV prevalence.
Acknowledgments
We thank Emmaculate Lebo and Mark Katz for assistance with the influenza data and analysis and Kevin De Cock for manuscript suggestions. We also thank all the community interviewers in Kibera (Kenya) for their hard work in gathering the data for this ongoing study.
Financial support. All funding was provided by the CDC and Kenya Medical Research Institute.
Footnotes
Publisher's Disclaimer: The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Safrin S, Rush JD, Mills J. Influenza in patients with human immunodeficiency virus infection. Chest. 1990;98:33–7. doi: 10.1378/chest.98.1.33. [DOI] [PubMed] [Google Scholar]
- 2.Pozio E, Rezza G, Boschini A, et al. Clinical cryptosporidiosis and human immunodeficiency virus (HIV)-induced immunosuppression: findings from a longitudinal study of HIV-positive and HIV-negative former injection drug users. J Infect Dis. 1997;176:969–75. doi: 10.1086/516498. [DOI] [PubMed] [Google Scholar]
- 3.Chandwani S, Borkowsky W, Krasinski K, Lawrence R, Welliver R. Respiratory syncytial virus infection in human immunodeficiency virus-infected children. J Pediatr. 1990;117(2 pt 1):251–4. doi: 10.1016/s0022-3476(05)80539-6. [DOI] [PubMed] [Google Scholar]
- 4.Goodgame RW, Genta RM, White AC, Chappell CL. Intensity of infection in AIDS-associated cryptosporidiosis. J Infect Dis. 1993;167:704–9. doi: 10.1093/infdis/167.3.704. [DOI] [PubMed] [Google Scholar]
- 5.Evans KD, Kline MW. Prolonged influenza A infection responsive to rimantadine therapy in a human immunodeficiency virus-infected child. Pediatr Infect Dis J. 1995;14:332–4. doi: 10.1097/00006454-199504000-00022. [DOI] [PubMed] [Google Scholar]
- 6.Feikin DR, Olack B, Bigogo GM, et al. The burden of common infectious disease syndromes at the clinic and household level from population-based surveillance in rural and urban Kenya. PLoS One. 2011;6:e16085. doi: 10.1371/journal.pone.0016085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalal W, Feikin DR, Amolloh M, et al. Home-based HIV testing and counseling in rural and urban Kenyan communities. J Acquir Immune Defic Syndr. 2013;62:e47–54. doi: 10.1097/QAI.0b013e318276bea0. [DOI] [PubMed] [Google Scholar]
- 8.Newell ML, Coovadia H, Cortina-Borja M, et al. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet. 2004;364:1236–43. doi: 10.1016/S0140-6736(04)17140-7. [DOI] [PubMed] [Google Scholar]
- 9.Feikin DR, Audi A, Olack B, et al. Evaluation of the optimal recall period for disease symptoms in home-based morbidity surveillance in rural and urban Kenya. Int J Epidemiol. 2010;39:450–8. doi: 10.1093/ije/dyp374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong JM, Nyachieo D, Benzekri N, et al. Sustained high incidence of injuries from burns in a densely populated urban slum in Kenya: an emerging public health priority. Burns. 2014;40:1194–200. doi: 10.1016/j.burns.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mermin J, Lule J, Ekwaru JP, et al. Cotrimoxazole prophylaxis by HIV-infected persons in Uganda reduces morbidity and mortality among HIV-uninfected family members. AIDS. 2005;19:1035–42. doi: 10.1097/01.aids.0000174449.32756.c7. [DOI] [PubMed] [Google Scholar]
- 12.Mengesha B. Cryptosporidiosis among medical patients with the acquired immunodeficiency syndrome in Tikur Anbessa Teaching Hospital, Ethiopia. East Afr Med J. 1994;71:376–8. [PubMed] [Google Scholar]
- 13.Gessner BD, Shindo N, Briand S. Seasonal influenza epidemiology in sub-Saharan Africa: a systematic review. Lancet Infect Dis. 2011;11:223–35. doi: 10.1016/S1473-3099(11)70008-1. [DOI] [PubMed] [Google Scholar]
- 14.Weinstock DM, Gubareva LV, Zuccotti G. Prolonged shedding of multidrug-resistant influenza A virus in an immunocompromised patient. N Engl J Med. 2003;348:867–8. doi: 10.1056/NEJM200302273480923. [DOI] [PubMed] [Google Scholar]
- 15.Klimov AI, Rocha E, Hayden FG, Shult PA, Roumillat LF, Cox NJ. Prolonged shedding of amantadine-resistant influenzae A viruses by immunodeficient patients: detection by polymerase chain reaction-restriction analysis. J Infect Dis. 1995;172:1352–5. doi: 10.1093/infdis/172.5.1352. [DOI] [PubMed] [Google Scholar]
- 16.Gooskens J, Jonges M, Claas EC, Meijer A, Kroes AC. Prolonged influenza virus infection during lymphocytopenia and frequent detection of drug-resistant viruses. J Infect Dis. 2009;199:1435–41. doi: 10.1086/598684. [DOI] [PubMed] [Google Scholar]
- 17.Feikin DR, Jagero G, Aura B, et al. High rate of pneumococcal bacteremia in a prospective cohort of older children and adults in an area of high HIV prevalence in rural western Kenya. BMC Infect Dis. 2010;10:186. doi: 10.1186/1471-2334-10-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Writing Committee of the WHO Consultation on Clinical Aspects of Pandemic (H1N1) 2009 Influenza. Bautista E, Chotpitayasunondh T, Gao Z, et al. Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. N Engl J Med. 2010;362:1708–19. doi: 10.1056/NEJMra1000449. [DOI] [PubMed] [Google Scholar]
- 19.Kim CY, Breiman RF, Cosmas L, et al. Secondary household transmission of 2009 pandemic influenza A (H1N1) virus among an urban and rural population in Kenya, 2009–2010. PLoS One. 2012;7:e38166. doi: 10.1371/journal.pone.0038166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Viboud C, Boëlle PY, Cauchemez S, et al. Risk factors of influenza transmission in households. Br J Gen Pract. 2004;54:684–9. [PMC free article] [PubMed] [Google Scholar]
- 21.Monto AS. Interrupting the transmission of respiratory tract infections: theory and practice. Clin Infect Dis. 1999;28:200–4. doi: 10.1086/515113. [DOI] [PubMed] [Google Scholar]
- 22.Glatman-Freedman A, Portelli I, Jacobs SK, et al. Attack rates assessment of the 2009 pandemic H1N1 influenza A in children and their contacts: a systematic review and meta-analysis. PLoS One. 2012;7:e50228. doi: 10.1371/journal.pone.0050228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katz MA, Lebo E, Emukule G, et al. Epidemiology, seasonality, and burden of influenza and influenza-like illness in urban and rural Kenya, 2007–2010. J Infect Dis. 2012;206(suppl 1):S53–60. doi: 10.1093/infdis/jis530. [DOI] [PubMed] [Google Scholar]
- 24.Maina AN, Knobel DL, Jiang J, et al. Rickettsia felis infection in febrile patients, western Kenya, 2007–2010. Emerg Infect Dis. 2012;18:328–31. doi: 10.3201/eid1802.111372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sergon K, Yahaya AA, Brown J, et al. Seroprevalence of Chikungunya virus infection on Grande Comore Island, union of the Comoros, 2005. Am J Trop Med Hyg. 2007;76:1189–93. [PubMed] [Google Scholar]
- 26.Akileswaran C, Lurie MN, Flanigan TP, Mayer KH. Lessons learned from use of highly active antiretroviral therapy in Africa. Clin Infect Dis. 2005;41:376–85. doi: 10.1086/431482. [DOI] [PubMed] [Google Scholar]
- 27.Manosuthi W, Chaovavanich A, Tansuphaswadikul S, et al. Incidence and risk factors of major opportunistic infections after initiation of antiretroviral therapy among advanced HIV-infected patients in a resource-limited setting. J Infect. 2007;55:464–9. doi: 10.1016/j.jinf.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 28.Hirnschall G, Harries AD, Easterbrook PJ, Doherty MC, Ball A. The next generation of the World Health Organization's global antiretroviral guidance. J Int AIDS Soc. 2013;16:18757. doi: 10.7448/IAS.16.1.18757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peletz RL, Mahin T, Elliot M, et al. Water, sanitation, and hygiene interventions to improve health among people living with HIV/AIDS: a systematic review. AIDS. 2013;27:2593–601. doi: 10.1097/QAD.0b013e3283633a5f. [DOI] [PubMed] [Google Scholar]
- 30.Lule JR, Mermin J, Ekwaru JP, et al. Effect of home-based water chlorination and safe storage on diarrhea among persons with human immunodeficiency virus in Uganda. Am J Trop Med Hyg. 2005;73:926–33. [PubMed] [Google Scholar]
- 31.Pickering AJ, Davis J, Blum AG, et al. Access to waterless hand sanitizer improves student hand hygiene behavior in primary schools in Nairobi, Kenya. Am J Trop Med Hyg. 2013;89:411–8. doi: 10.4269/ajtmh.13-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pedersen RH, Lohse N, Østergaard L, Søgaard OS. The effectiveness of pneumococcal polysaccharide vaccination in HIV-infected adults: a systematic review. HIV Med. 2011;12:323–33. doi: 10.1111/j.1468-1293.2010.00892.x. [DOI] [PubMed] [Google Scholar]
- 33.Beck CR, McKenzie BC, Hashim AB, et al. Influenza vaccination for immunocompromised patients: systematic review and meta-analysis by etiology. J Infect Dis. 2012;206:1250–9. doi: 10.1093/infdis/jis487. [DOI] [PubMed] [Google Scholar]
- 34.Earnshaw VA, Smith LR, Chaudoir SR, Amico KR, Copenhaver MM. HIV stigma mechanisms and well-being among PLWH: a test of the HIV stigma framework. AIDS Behav. 2013;17:1785–95. doi: 10.1007/s10461-013-0437-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kipp W, Matukala Nkosi T, Laing L, Jhangri GS. Care burden and self-reported health status of informal women caregivers of HIV/AIDS patients in Kinshasa, Democratic Republic of Congo. AIDS Care. 2006;18:694–7. doi: 10.1080/13548500500294401. [DOI] [PubMed] [Google Scholar]
- 36.Rajaraman D, Russell S, Heymann J. HIV/AIDS, income loss and economic survival in Botswana. AIDS Care. 2006;18:656–62. doi: 10.1080/09540120500287010. [DOI] [PubMed] [Google Scholar]