Abstract
Visceral Leishmaniasis (VL) is a fatal disease of the internal organs caused by the eukaryotic parasite Leishmania. Control of VL would best be achieved through vaccination. However, this has proven to be difficult partly because the correlates of protective immunity are not fully understood. In contrast, protective immunity against non-fatal cutaneous Leishmaniasis (CL) is well defined and mediated by rapidly recruited, IFN-γ-producing, Ly6C+CD4+ T cells at the dermal challenge site. Protection against CL is best achieved by prior infection or live vaccination with Leishmania major, termed leishmanization. A long-standing question is whether prior CL or leishmanization can protect against VL. Employing an intra-dermal challenge model in mice, we report that cutaneous infection with Leishmania major provides heterologous protection against visceral infection with Leishmania infantum. Protection was associated with a robust CD4+ T cell response at the dermal challenge site and in the viscera. In-vivo labeling of circulating cells revealed that increased frequencies of IFN-γ+CD4+ T cells at sites of infection is due to recruitment or retention of cells in the tissue, rather than increased numbers of cells trapped in the vasculature. Shortly after challenge IFN-γ producing cells were highly enriched for Ly6C+T-bet+ cells in the viscera. Surprisingly, this heterologous immunity was superior to homologous immunity mediated by prior infection with Leishmania infantum. Our observations demonstrate a common mechanism of protection against different clinical forms of leishmaniasis. The efficacy of leishmanization against VL may warrant the introduction of the practice in VL endemic areas or during outbreaks of disease.
INTRODUCTION
The leishmaniases consist of a broad range of cutaneous, mucocutaneous, and visceral diseases caused by different strains of the eukarytotic parasite Leishmania. Leishmania, which is transmitted to the human host by the bite of a sand fly vector, is an obligate intracellular pathogen that establishes chronic infection within phagocytes. Visceral forms of leishmaniasis are fatal if left untreated and while drugs are available they are expensive, highly toxic, and drug resistance is common (1, 2). Visceral leishmaniasis caused by L. donovani is endemic in northern India and East Africa, whereas L. infantum, also known as L. chagasi, is the causative agent of VL in South America and the Mediterranean basin, which has experienced several recent outbreaks (3-6). In some areas, L. infantum infection of dogs has reached epidemic proportions and dogs are believed to be a major reservoir of human disease (7).
No vaccine is currently available for any form of leishmaniasis in people. However, a deliberate, single needle inoculation of infectious L. major into the skin without the disease exacerbating factors co-egested during natural sand fly transmission, termed leishmanization, provides complete and long lasting homologous protection against sand fly transmitted cutaneous disease and has been used extensively as a live vaccine in humans (8-12). Despite its efficacy and the convenience of a single administration, leishmanization has largely been abandoned because of rare adverse reactions at the site of inoculation (13, 14); and the chronic nature of the infection raises concerns should leishmanized individuals become immune-compromised, although there are no reports of reactivation or dissemination of L. major in leishmanized individuals. A more justifiable use of leishmanization would be to vaccinate against strains that cause lethal visceral leishmaniasis (VL), for which the benefits of leishmanization may outweigh any risks. Cross-protection conferred by leishmanization against VL would suggest a common mechanism of resistance against Leishmania species that cause different clinical diseases, and suggest that different Leishmania species share a sufficient number of protective antigens to warrant their use in pan-Leishmania vaccines (15, 16). However, evidence that L. major infection cross-protects against VL in people is rare or difficult to interpret (17-23).
Experimentally, two prior studies have investigated this question and found that leishmanization either provided no protection (24) or enhanced visceral infection (25) following L. infantum challenge. However, these studies employed BALB/c mice that are susceptible to L. major infection due to a defect in the generation of Th1 immunity, a condition not typically observed in people infected with L. major (26). In contrast, leishmanized C57BL/6 mice more closely replicate the immune status of leishmanized humans (11). Therefore, we employed an intra-dermal challenge model of visceral infection caused by L. infantum in C57BL/6 mice leishmanized with L. major (27). We present evidence that leishmanization provides robust protection and similar correlates of protection against both cutaneous and visceral infection. Leishmanization may be a viable strategy for control of visceral disease.
MATERIALS AND METHODS
Parasites
L. major Friedlin strain was isolated from a patient who acquired his infection in the Jordan Valley (MHOM/IL/80/Friedlin). L. infantum (MHOM/ES/92/LLM-320; isoenzyme typed MON-1) was isolated from a patient with VL in Spain and was provided by Diane MacMahon-Pratt. L. infantum-RFP was generated as previously described (28). Parasites were cultured in vitro at 26°C in complete medium 199 (CM199) supplemented with 20% heat-inactivated FCS (Gemini Bio-products), 100 U/ml penicillin, 100μg/ml streptomycin, 2mM L-glutamine, 40mM Hepes, 0.1 mM adenine (in 50mM Hepes), 5mg/ml hemin (in 50% triethanolamine), and 1mg/ml 6-biotin. For L. infantum the CM199 was further supplemented with 2μg/ml 6-Biopterin (Sigma, St Louis). L. infantum and L. major infective-stage metacyclic promastigotes were isolated from stationary cultures (4-6 day old) by centrifugation through a Ficoll-step gradient as described (29). For leishmanization, L. major metacyclic promastigotes were isolated by negative selection of non-infective forms using peanut agglutinin (Vector Laboratories) (30).
Mice
Female C57BL/6 mice were obtained from Taconic. All mice were maintained in the National Institute of Allergy and Infectious Diseases animal care facility under specific pathogen-free conditions.
Leishmanization and challenge
Leishmanized mice were generated by injecting 104 L. major metacyclic promastigotes subcutaneously in the hind footpad in a volume of 40μl and used at 12 to 20 weeks post-primary infection when footpad lesions had completely resolved. Mice with a primary L. infantum infection where generated in the same manner. Naïve mice, leishmanized mice, or L. infantum infected mice were challenged with 2×106 L. infantum metacyclic promastigotes intra-dermally (i.d.) in the ear in a volume of 10μl. In some experiments mice were injected intravenously (i.v) in the tail vein with 2×106 L. infantum metacyclics promastigotes in a volume of 200μl.
Processing of different sites of infection and parasite quantification
Mice were perfused via intra-cardiac injection of 20 ml of cold PBS. Liver perfusion was performed by injection of 6 ml of cold PBS into the liver portal vein. The spleen, and ear draining LN (dLN) were removed, cut with tweezers, homogenized with a syringe plunger, and the cell suspension was filtered through a 70μm strainer. Liver were incubated for 45min with 2ml DMEM containing 250μg/ml of Liberase TL purified enzyme blend (Roche Diagnostic Corp.) and 10μg/ml of DNase. Liver cells were further purified using a Percol gradient (31). In experiments employing direct intracellular staining (dICS), organs were processed in media pre-warmed to 37°C containing 20μg/ml of Brefeldine A (BFA) and incubated at 37°C and 5% CO2 post-processing for a total time in BFA of 4 hours. Ear tissue was prepared as previously described (11). Briefly, ears were removed and placed in 70% ethanol for 2-5 minutes and then allowed to dry. Separated dorsal and ventral sheets of ears were then incubated at 37°C for 90 minutes in 1ml DMEM containing 160μg/ml of Liberase. Following Liberase treatment tissue was homogenized for 3½ minutes in a Medicon using a Medimachine (Becton Dickinson). The tissue homogenate was then flushed from the medicon with 10 ml RPMI media containing 0.05% DNase and filtered using a 50 um-pore-size cell strainer. In experiments employing dICS, BFA at 20μg/ml was added to media pre-warmed to 37°C and the ear homogenate returned to 37°C and 5% CO2 post-processing for a total time in BFA of 4 hours. In some experiments mice were not perfused and red blood cells were removed from the spleen and liver using ACK lysing buffer for 5min at room temperature. Cells from each tissue were re-suspended in CM199 with 6-biopterin.
Parasite loads were determined by two fold serial dilutions in 96-well flat bottom microtiter plates, by overlaying 100μl of the diluted tissue suspension onto 50 μl NNN medium containing 20% defibrinated rabbit blood. The dilutions were made in duplicate. The plates were scored microscopically for growth and the number of parasites in each tissue was determined from the highest dilution at which parasites could be grown out after 7-10 days incubation at 26°C. In some experiments 25μg/ml of hygromycin B (Sigma) was added to the media to discriminate between parasites used for live vaccination versus parasites used for challenge.
Re-stimulation of tissue-derived cells for cytokine analysis by flow cytometry
Tissue derived cells were re-stimulated as described previously (11). Briefly, single-cell suspensions were incubated at 37°C in 5% CO2 for 12-14 hours in flat-bottom 48-well plates with 0.5-1×106 T cell-depleted (Miltenyi Biotech) naïve spleen cells (APCs), with or without 50 μg/ml freeze-thaw Leishmania antigen (L.m.-Ag and or L.i.-Ag) in a total volume of 1ml. During the final 4-6 hours of culture, 1μg/ml of Brefeldin A (Sigma) was added. Cells were then washed and labeled with Live/Dead fixable AQUA at a 1:500 dilution of the manufacturer suggested stock solution (Invitrogen) to exclude dead cells and anti-Fc III/II (CD16/32) receptor Ab (2.4G2), followed by PE-Cy7 anti-mouse CD4 (RM4-5), and in some experiments APC-efluor 780 CD8a (53-6.7) for 20 minutes. In some experiments cells were stained with PerCp.Cy5.5 anti-CD4 (RM4-5), FITC anti-CD44 (IM7), APC-eFluor 780 anti-Ly6C (HK1.4) and PE-Cy7 anti-CD62L (MEL-14) for 20 minutes. Cells were then fixed with BD Cytofix/Cytoperm (BD Biosciences) according to the manufacturer instructions and stained with FITC anti-IFN-γ (XMG1.2), Per-CP eFluor710 anti-TNF-α (MP6-XT22), and in some experiments APC anti-TcR-β (145-2C11) and/or PE anti-human Granzyme B (GRB04, Invitrogen) and/or V500 anti-CD3 (145-2C11). In some experiments, samples were treated with the Foxp3 Fixation/permeabilization Buffer (eBioscience) per manufacturer's instructions, and subsequently stained for 1 hour at 4°C with PE anti-IFN-γ (XMG1.2) and eFluor 660 anti-Tbet (eBio4B10) antibodies. Isotype controls employed were rat IgG1 (R3-34) and rat IgG2b (A95-1 or eBR2a). All Abs were from eBiosciences or BD Biosciences. Data were collected using FacsDIVA software on a FacsCANTO flow cytometer (BD Biosciences), and analyzed using FlowJo software (TreeStar). Forward-scatter and side-scatter width was employed to exclude cell doublets from analysis.
Perfusion and in-vivo staining of circulating cells
In-vivo labeling of circulating cells was done as described previously (32). Briefly, 0.6μg of anti-TCR-β BV421 (clone H57-597 Biolegend) was injected i.v in 200μl PBS via the tail vein. Three minutes after the injection, mice were sacrificed by isofluorane asphyxiation and perfused via intra-cardiac injection of 20 ml of cold PBS. Liver perfusion was performed by injection of 6 ml of cold PBS into the liver portal vein. Organs were then harvested as described above. Following re-stimulation and intracellular staining the frequency of TcR-β+ cells labeled by i.v. administration of TcR-β antibody was not reduced on T cells from the liver (30.0+/−13 TcR-β+ before re-stimulation versus 29.6+/−12 TcR-β+ after re-stimulation and intracellular staining, n=14) and only slightly reduced on cells from the spleen (39.2+/−13 TcR-β+ before re-stimulation versus 34.0+/−12 TcR-β+ after re-stimulation and intracellular staining, n=12).
Statistics
Unless otherwise noted, data were compared using the Mann-Whitney test. Comparisons between multiple groups were done using ANOVA with Holm-Sidek's post test for comparisons between multiple groups. All p-values are two-sided. Statistical calculations were done in Graphpad PRISM 5.0c (www.graphpad.com). For figures depicting pooled data, error bars represent the standard error of the mean (SEM). For figures in which data points represent individual replicates error bars represent the standard deviation of the mean (SD). **** p<0.0001; *** 0.0001< p<0.001; ** 0.001<p<0.01; * 0.01<p<0.05.
Ethics Statement
All animal experiments were performed under the LPD-68E Animal Study Protocol approved by the NIAID Animal Care and Use Committee using guidelines established by the Animal Welfare Act and the PHS Policy on Humane Care and Use of Laboratory Animals.
RESULTS
The course of L. infantum infection in C57BL/6 mice following intra-dermal inoculation of the ear
In order to study cross protection against visceral infection, we challenged naïve or leishmanized C57BL/6 mice with 2×106 L. infantum parasites via the intra-dermal route in the ear. Although higher than the number of parasites deposited during natural sand fly transmission (33), we employed 2×106 parasites because this was the lowest dose at which L. infantum parasites disseminated from the skin to the spleen and liver in all naïve mice tested (Figure 1A). While no murine model replicates the development of progressive, fatal visceral leishmaniasis in people, the i.d. challenge model in C57BL/6 mice does allow for an immunological assessment of protective immunity in the skin, the physiological site of challenge (27). In addition, the i.d. challenge model results in the delivery of low doses of parasites into the visceral organs, something that is also likely to occur during natural infection, and far different from the massive doses that are rapidly delivered to the viscera during conventional i.v. challenge.
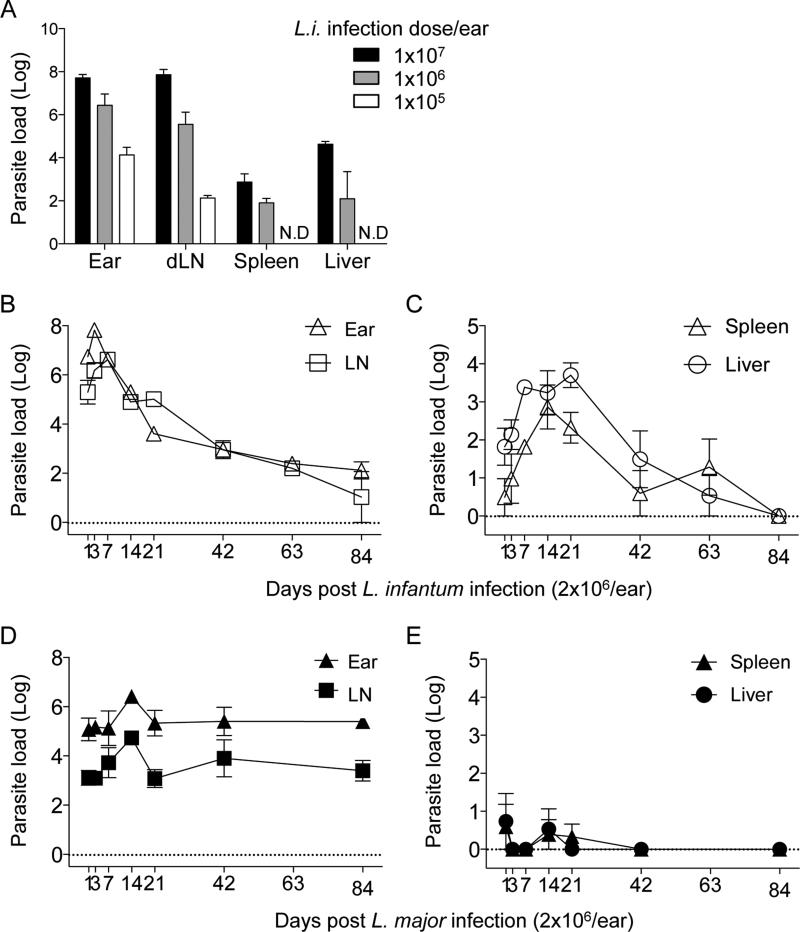
Figure 1. The course of primary infection with L. infantum or L. major in C57Bl/6 mice following intra-dermal inoculation of the ear.
Naïve mice were infected with the indicated doses of L. infantum (A) or with 2×106 L. infantum (B and C) or L. major (D and E) metacyclic promastigotes in the left ear pinnae. Parasite loads in individual ears and draining lymph nodes (A, B and D) or spleens and liver (A, C and E) was determined at 1 week (A) or the indicated time points (B-E) post-infection. n=4 (A) or n=3 (B-E) mice per time point. Representative results from 2 independent experiments. Mean and SEM are shown.
We initially determined using limiting dilution analysis (LDA) the course of L. infantum infection in naïve C57BL/6 mice following i.d. inoculation, since this has not been reported previously (Fig. 1B and C). Following i.d. inoculation, parasites were found in the ear, dLN, liver and spleen. In the ear and dLN parasites initially increased in number followed by a decline between 7 and 42 days, at which point parasite numbers stabilized for the remainder of the experiment. In the liver and spleen, parasites underwent significant expansion between 1 and 14-21 days p.i., peaking at 1737 in the spleen and 5011 in the liver. Parasite numbers then underwent a gradual reduction between 21 and 63 days, and by day 84, parasites were no longer detected in the liver or spleen. This course of infection was in contrast to infection with L. major, which did not expand in the viscera but established stable, chronic infections in the skin and dLN following high dose inoculation of the ear (Fig. 1 D and E).
Leishmanization with L. major provides protection against visceral infection
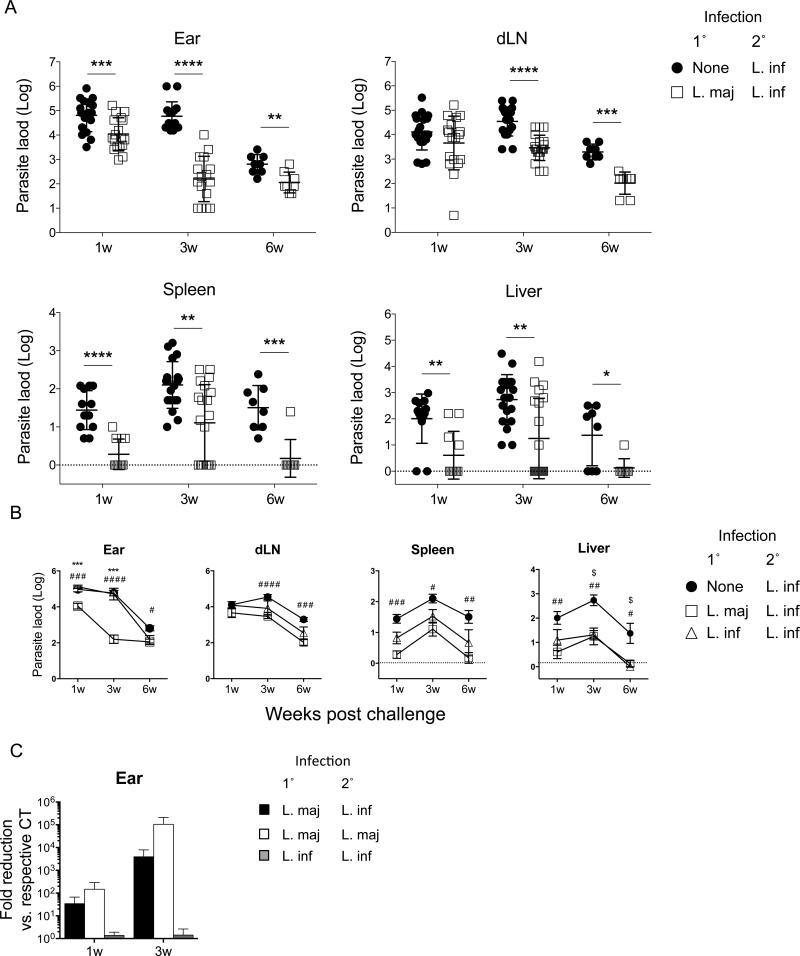
Twelve to sixteen weeks post leishmanization with L. major, mice were challenged i.d. in one ear with L. infantum. Analysis of parasite loads in the ear, dLN, liver, and spleen of leishmanized animals revealed significant control of parasite numbers at all sites of challenge and at all time points tested versus control animals, with the exception of the dLN at 1 week post-challenge (Fig. 2A). At 3 weeks post challenge, 40% (8/20) of the spleens and 55% (11/20) of the livers from leishmanized animals had undetectable numbers of parasites and this increased to 88% (7/8) at both sites by 6 weeks post-challenge. To compare the efficacy of prior infection with L. major versus L. infantum to protect against challenge with L. infantum, mice chronically infected with either strain were challenged with drug resistant L. infantum parasites. At 1, 3, or 6 weeks post challenge parasites LDA was performed under drug pressure to eliminate the Leishmania employed for leishmanization. Leishmanization with L. major provided equivalent or enhanced protection versus leishmanization with L. infantum (Fig. 2B). This was most significant in the ear where the heterologous protection against L. infantum was similar to the level of protection against homologous challenge with L. major (Fig. 2C). At all time points tested, with the exception of the 6 weeks time point in the liver when control mice are beginning to clear parasites from this organ, protection was associated with significantly greater numbers of leishmanized versus control mice exhibiting sterilizing immunity in the spleen and liver as determined by the inability to culture organisms from these tissues (p≤0.036, Fisher's exact test). So far as we are aware, these observations demonstrate the first experimental confirmation that leishmanization with L. major provides protection against visceral infection.
Figure 2. Primary infection with L. major protects against visceral infection with L. infantum.
Naïve mice or mice with a healed L. major (A-C) or L. infantum (B and C only) primary infection were challenged with 2×106 L. infantum (A-C) or L. major (C only) metacyclics in the ear. (A and B) Parasite loads in individual ears, dLNs, spleens or livers at the indicated time points post-challenge. Data are the pool of 5 independent experiments including at least 2 time points per experiment. Mean and SD (A) or SEM (B) are shown. In (A). *0.01 < p < 0.05, **0.001 < p < 0.01, ***0.0001 < p < 0.001, ****p<0.0001. In (B), ***0.0001 < p < 0.001, refer to differences between L. major/L. infantum and L. infantum/L. infantum; #0.01< p < 0.05, ##0.001 < p < 0.01, ###0.0001 < p < 0.001, ####p < 0.0001 refert to differences between naive/L. infantum and L. major/L. infantum; $0.01 < p < 0.05 refers to differences between naive/L. infantum and L. infantum/L. infantum. (C) Mean (+/-SEM)fold parasite load reduction at the site of secondary challenge (ear) in mice with the indicated primary and secondary challenge infections versus the respective controls.
Leishmanization is associated with a rapid and robust CD4+ T cell response in the skin and visceral organs following challenge
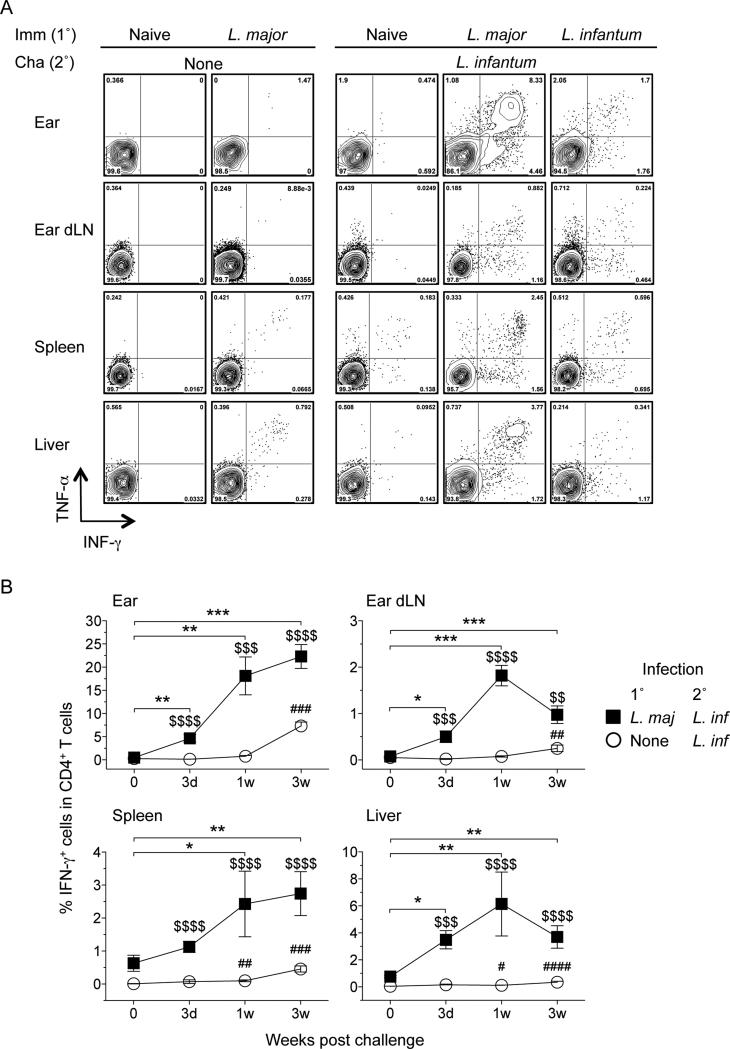
IFN-γ producing CD4+ T cells mediate the protective response conferred by leishmanization against homologous challenge with L. major (34, 35). In addition, the rapidity of the CD4+ response is the clearest correlate of protection against sand fly transmitted disease (11, 12). Therefore, we analyzed IFN-γ production by CD4+ T cells at early time points following heterologous challenge with L. infantum and found increased frequencies of CD4+ T cells with the capacity to make IFN-γ in the skin, dLN, spleen and liver of leishmanized versus control animals (Fig. 3A and 3B). As previously reported (11) a rapid CD4+ T cells response in the skin was observed within 3 days of i.d. challenge (Fig. 3B). We also observed post challenge responses in the viscera of leishmanized mice that were significantly greater than pre-challenge responses starting at day 3 (liver) or one-week (spleen) post challenge with L. infantum. This early response was similar to the response to homologous challenge with L. major (Fig. S1, A and B), suggesting that the early protective responses to L. infantum or L. major are the same despite the different clinical outcomes of infection with these two parasites. In addition, IFN-γ production was elicited equally well employing freeze-thawed antigen from L. infantum or L. major (Ratio of response L.m.:L.i. 1:0.95, n=4, p=0.54, paired t-test), suggesting these two parasites also share immunogenic MHC class II restricted epitopes, as suggested by work with the sterol 24-c-methyltransferase protein (36).
Figure 3. Kinetic of IFN-γ producing CD4+ T cells in the skin and viscera after challenge infection with L. infantum.
At the indicated time post-challenge, cells from mice as shown in Figure 2A were re-stimulated in vitro with APCs plus a mixture of L.m.- and L.i.-antigen and analyzed by flow cytometry. A) Representative intracellular staining contour plots for cytokine-producing CD4+TCRβ+ T at 1 week post-challenge (B) Kinetic of IFN-γ production by CD4+TCRb+ T cells in ears, dLNs, spleens, and livers. *0.01 < p < 0.05, **0.001 < p < 0.01, ***0.0001 < p < 0.001 refer to differences between leishmanized unchallenged mice and L. major/L. infantum; #0.01 < p < 0.05, ##0.001 < p < 0.01, ###0.0001 < p < 0.001, ####p < 0.0001 refer to differences between naive/L. infantum and naive control mice; $$0.001 < p < 0.01, $$$0.0001 < p < 0.001, $$$$p < 0.0001 refer to differences between naive/L. infantum and L. major/L. infantum; n = 4–5 mice per group per time point. Data are the pool of five independent experiments including at least two time points (day 3, 1 wk, and/or 3 wk) per experiment.
Localization of IFN-γ+CD4+ T cells associated with the liver and spleen employing in vivo i.v. staining
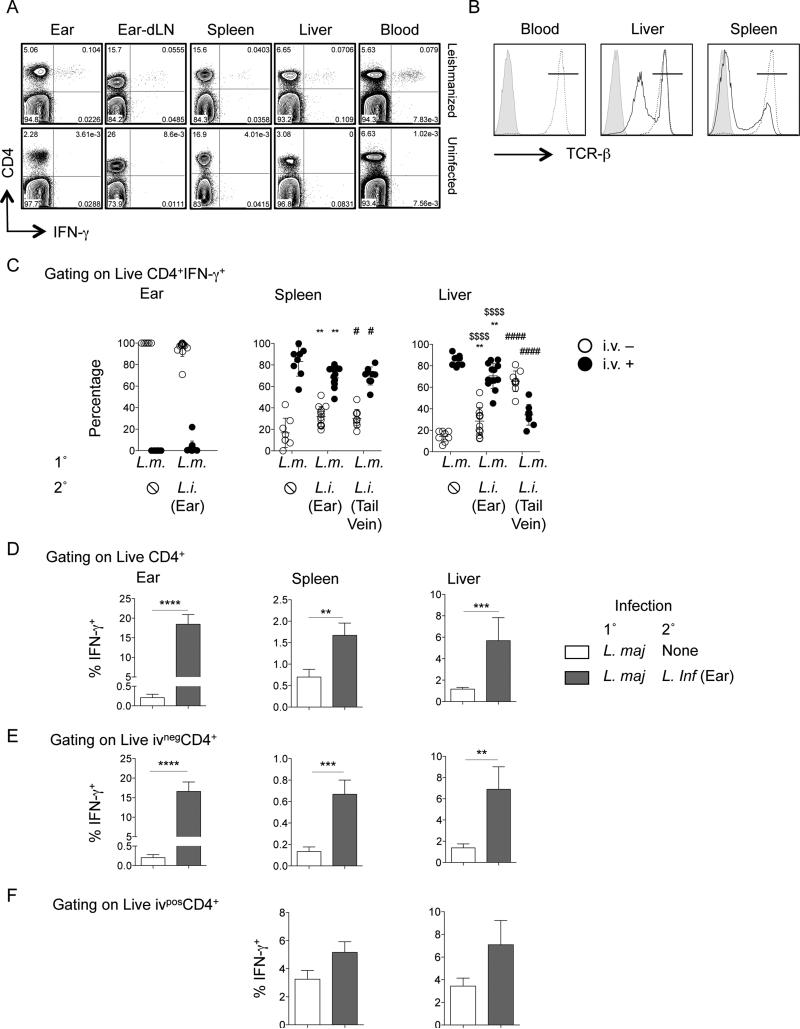
Leishmanized animals had low but detectable frequencies of CD4+ T cells with the capacity to make IFN-γ upon stimulation in the spleen and liver prior to challenge (Fig. 4A). However, similar cells were also found in the circulation making it difficult to conclude whether or not these cells were residing in the tissue prior to challenge or were simply trapped in the organ vasculature. In addition, trapped circulating cells may contribute to the increased frequencies of IFN-γ+CD4+ T cells detected in the spleen and liver following challenge (Figure 3B). Although we routinely perfuse our mice, and perfusion does reduce the frequency of IFN-γ+ cells in the liver and spleen (Fig. S2), recent observations have demonstrated that perfusion can be incomplete (32, 37). Therefore, in order to discern cytokine production from cells present in visceral tissues versus those trapped in the vasculature we in-vivo labeled circulating cells prior to euthanasia and perfusion (32). This technique labels cells on the luminal side of the liver and spleen vasculature as well as cells in the splenic red pulp. This analysis is important in the context of vaccine studies to determine if antigen-specific cells have actually infiltrated infected tissues to mediate effector function versus those that are circulating. Analysis of live CD4+ T cells from the blood following in-vivo labeling revealed greater than 99% were i.v.+ as expected (Fig. 4B). In the liver and spleen we found both i.v.− and i.v.+ cells, despite whole body and liver specific perfusion (Fig. 4B). We then determined the frequency of cytokine producing cells that were in the tissue versus those trapped in the vasculature of the ear, spleen, and liver prior to and at 1 week following i.d. challenge, the peak of the CD4+IFN-γ T cell response. We also employed i.v. challenge with 2×106 L. infantum, which delivers large numbers of parasites directly into the visceral organs, particularly the liver (27), to act as a positive control for antigen driven recruitment and/or retention of IFN-γ+CD4+ cells in the spleen and liver. Both prior to and following dermal challenge, the vast majority of IFN-γ+ cells in the ear were i.v.− (Fig. 4C, left panel), demonstrating these cells were residing in the tissue. In contrast, the majority of IFN-γ+CD4+ cells in the spleen and liver were i.v.+, despite perfusion (i.v.− versus i.v.+ p<0.001), demonstrating that these cells were trapped, circulating cells. This suggests that the majority of Leishmania-specific cytokine production attributed to cells associated with the visceral organs in Figure 3B is due to cells trapped in the vasculature, not cells that are actually mediating effector function in the organ. While i.v. tail vein challenge did not significantly change the data obtained from the spleen, there was a dramatic increase in the frequency of IFN-γ+CD4+ T cells from the liver that were i.v.−, suggesting that antigen load can drive the recruitment and/or retention of IFN-γ producing cells residing in the liver parenchyma (Fig. 4C, right panel). Despite the fact that the majority of IFN-γ+CD4+ T cells associated with the viscera were trapped i.v.+ circulating cells, dermal challenge significantly increased the frequency of IFN-γ+CD4+ cells that were i.v.− versus pre-challenge mice in both the spleen and liver (compare open circles in Fig. 4C). This was most dramatic in the liver following tail vein challenge (Fig. 4C, right panel). In order to correlate responses in the actual tissue with the reductions in parasite numbers mediated by leishmanization observed in Figure 1, we also determined the frequency of cytokine producing cells within the total, i.v.−, or i.v.+ CD4+ T cell populations following challenge (Fig. 4, D-F). In both the spleen and liver there was a significant increase in the frequency of IFN-γ+ cells within the i.v.− population following i.d. challenge (Fig. 4E), demonstrating that leishmanization mediates a rapid response in the visceral organs similar to that observed at the dermal site of challenge. Analysis of i.v.+ cells revealed a trend towards increased frequencies of IFN-γ+ cells following dermal challenge but this did not reach significance. These observations demonstrate that leishmanization with L. major mediates an early CD4+ T cells response in visceral sites of infection following i.d. challenge with L. infantum.
Figure 4. In vivo i.v. staining reveals localization of IFN-γ+CD4+ T cells in the spleen and the liver.
(A) Prechallenge production of IFN-γ by CD4+TCRβ+ T cells from different organs of uninfected or leishmanized mice following ex vivo Ag restimulation as described in Fig. 3. (B) Representative histograms of i.v staining with anti–TCRβ-BV421 on CD4+ T cells in the blood, spleen and liver. (C) Proportion of i.v.− or i.v.+ cells within the IFN-γ+ CD4+ T cell population (following ex vivo Ag restimulation) from the ear, spleen, or liver of leishmanized mice following no challenge (Lm-🛇), i.d. challenge in the ear (Lm-Li (Ear)), or i.v. challenge in the tail vein (Lm-Li (Tail Vein) with 2 × 106 L. infantum metacyclics. **0.001 < p < 0.01, ***0.0001 < p < 0.001, ****p < 0.0001 refer to differences between Lm-🛇 and Lm-Li (Ear); #0.01 < p < 0.05, ####p < 0.0001 refer to differences between Lm-🛇 and Lm-Li (Tail Vein); $$$$p < 0.0001 refers to differences between Lm-Li (Ear) and Lm-Li (Tail Vein). (D–F) Percentage of IFN-γ+ cells in the total CD4+ T cell population (D), the i.v−CD4+ T cell population (E), or the i.v.+CD4+ T cell population (F). One week after challenge data point is shown. Data are the pool of three independent experiments employing four to five mice per experiment (two experiments employed tail vein challenge). Li, L. infantum; Lm, L. major.
intravenous−, IFN-γ-producing cells in the viscera following challenge are enriched for Ly6C expression
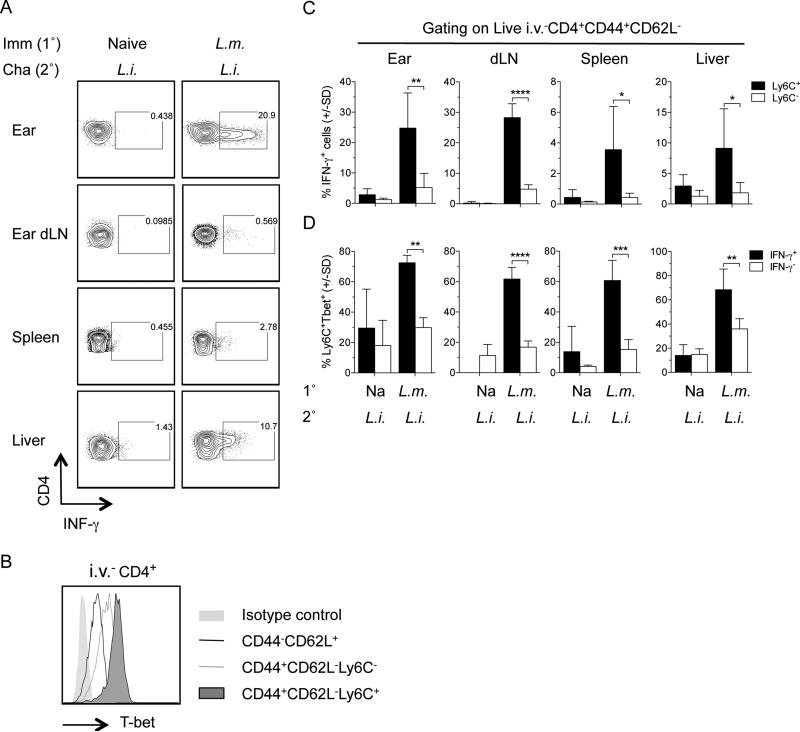
We have previously reported that Leishmanization maintains high frequencies of short-lived CD44+CD62L−T-bet+Ly6C+ CD4+ TEFF cells that are required for protection against re-infection with L. major in the ear dermis. The cells are not memory cells reactivated by secondary challenge, and while they produce cytokine in-vivo in an antigen-dependent manner, they can be rapidly recruited to dermal sites of challenge in an antigen independent manner (35). Therefore, we wished to determine if IFN-γ producing cells in the viscera at 3 days following dermal challenge of Leishmanized mice with L. infantum also expressed the Ly6C+ TEFF phenotype (Figure 5). We employed direct intracellular staining (dICS) to identify IFN-γ producing cells (35) (Fig. 5A). Because dICS does not employ ex-vivo antigen or pharmacological re-stimulation, assessment of Ly6C and T-bet expression using this methodology likely reflects patterns of in-vivo expression. Non-circulating i.v.−CD44+CD62L−CD4+ T cells that expressed Ly6C were T-bet high, as described previously (35) (Fig. 5B), and were highly enriched for IFN-γ producing cells compared to Ly6C− cells (Fig. 5C). In addition, the frequency of T-bet+Ly6C+ expressing cells was significantly higher among IFN-γ-producing i.v.−CD44+CD62L−CD4+ T cells versus IFN-γ non-producing i.v.−CD44+CD62L−CD4+ T cells (Fig. 5D). In contrast, IFN-γ production in naïve mice challenged with L. infantum was very low and no significant difference was observed between Ly6C+ and Ly6C− cells. This data demonstrates that, similar to L. major challenge of the ear, IFN-γ production is highly associated with Ly6C expressing CD4 T cells in the viscera at acute time points following challenge with L. infantum.
Figure 5. i.v.−CD44+CD62L−Ly6C+ CD4+ T cells in the viscera of leishmanized mice are enriched for IFN-γ producing cells following challenge.
Naïve or Leishmanized animals were challenged with L. infantum as described in Figure 2. On day 3 post-challenge CD4+TcRβ+ T cells were analyzed for CD44, CD62L, Ly6C, T-bet, and IFN-γ expression employing dICS. (A) Representative dot plots of IFN-γ production by i.v.−CD4+ T cells. B) Representative histogram of T-bet expression by the indicated cell populations in the spleen. C) Percentage of IFN-γ+ cells within live i.v.−CD4+CD44+CD62L−Ly6C+ or live i.v.−CD4+CD44+CD62L−Ly6C− populations. D) Percentage of Ly6C+T-bet+ cells within the live i.v.−CD4+CD44+CD62L−IFN-γ+ or live i.v.−CD4+CD44+CD62L−IFN-γ− population. In C and D, n=4 mice/group. Data are representative of 2 independent experiments. *0.01 < p < 0.05, **0.001 < p < 0.01, ***0.0001 < p < 0.001, ****p < 0.0001.
Following challenge responding CD8 T cells are predominantly GzB+ but IFN-γ
While Th1 CD4+ T cells are associated with mediating protection against primary and secondary infection with L. major (35, 38), the role of CD8+ T cells in Leishmaniasis remains controversial and is dependent upon the dose, strain, and infection setting (primary or secondary) (1, 38-41). In visceral infection with L. donovani, CD8+ T cells have been shown to be both protective and dispensable (40, 42). We determined IFN-γ and Granzyme B (GzB) production by CD8+ T cells following challenge with L. infantum and, similar to observations in BALB/c mice (24), found a very low frequency of IFN-γ+ cells regardless of the site of analysis (Fig. 6, A and B). In contrast, high frequencies of cells were found to be GzB+. Similar observations were made following challenge with L. major (Fig. S3). The postulated role of granzyme B in Leishmaniasis is evolving. Granzyme AxB−/− double knockout C57BL/6 mice are completely resistant to L. major infection (43) while CD8+GzB+ T cells have been associated with pathology, not parasite killing, during cutaneous L. braziliensis infection (41, 44, 45). Despite the high frequency of GzB+CD8+ cells we were unable to detect GzB by ELISA following antigen stimulation (data not shown). Employing in-vivo labeling of circulating cells we detected an increase in the frequency of GzB+ CD8+ T cells in the viscera following challenge, although this did not reach significance (Fig. S4).
Figure 6. Kinetic of GzB producing CD8+ T cells in the skin and viscera after challenge with L. infantum.
At indicated time post-challenge cells were re-stimulated in vitro with APCs plus a mix of L.m.- and L.i.-Ags and analyzed by flow cytometry. A) Representative intracellular staining contour plots for GzB- and IFN-γ-producing CD8+TCRβ+ T at 3 days post-challenge. (B) Kinetic of GzB production by CD8+TCRβ+ T cells in the ears, dLNs, spleens, and livers. *0.01 < p < 0.05, **0.001 < p < 0.01 refer to differences between leishmanized unchallenged mice and L. major/L. infantum; #0.01 < p < 0.05, ##0.001 < p < 0.01 refer to differences between naive/L. infantum and naive control mice; $$$$p < 0.0001 refer to differences between naive/L. infantum and L. major/L. infantum; n = 4–5 mice per group per time point. Data are the pool of three (ears) or four (other sites) independent experiments.
DISCUSSION
Our results demonstrate for the first time, so far as we are aware, that leishmanization protects against heterologous visceral infection. Protection was associated with rapid activation and recruitment of IFN-γ producing Th1 cells at both the cutaneous site of challenge and visceral sites of infection, demonstrating that protection conferred by leishmanization is not site specific. Similar to what we have previously reported for L. major challenge in the skin, IFN-γ production was highly associated with CD44+CD62L−Tbet+Ly6C+ CD4+ T cells within the i.v.− population in the viscera. At early time points following challenge these cells are recruited, infection-dependent, pre-existing TEFF cells, not memory cells reactivated by challenge (35). Our observations suggest that the correlates of protective immunity against CL and VL may be sufficiently similar to warrant a pan-Leishmania vaccine provided the vaccine can replicate the correlates of immunity observed in leishmanized individuals (11, 12, 35). Our data also provides the best evidence to date that heterologous immunity can be identical, or even superior, to homologous immunity.
The controlled trials demonstrating the efficacy of leishmanization in humans is restricted to protection against CL caused by L. major or L. tropica. However, epidemiological studies suggest prior infection of humans with L. major may provide cross-protection against VL caused by L. donovani. Two longitudinal studies of VL in Sudan demonstrated that despite the presence of both leishmanin-skin test (LST) positive and negative individuals at the beginning of each study, all cases of VL were reported amongst the LST negative group (17, 18). Because LST positivity was highly associated with prior residence in an L. major endemic area, the authors hypothesized that prior infection with L. major provides protection against VL caused by L. donovani. Related observations in Sri Lanka reported that districts with high levels of CL caused by a naturally attenuated strain of L. donovani are almost devoid of VL (19). Experimentally, susceptible BALB/c mice infected with the attenuated L. donovani strain had significantly reduced parasite burden in the liver upon challenge with the L. donovani strain responsible for Sri Lankan VL (46). In a vervet monkey model of disease, Gicheru et. al. (47) reported the inverse result, that subclinical infection with L. donovani provided complete protection against cutaneous leishmaniasis following experimental challenge with L. major.
Experimental cross-protection mediated by infection (24, 25, 48-54) as well as T cell cross-reactivity with antigens from different Leishmania species (15, 16) has been studied extensively in animal models, primarily in an effort to define proteins that could be used in a pan-Leishmania vaccine. Genome sequencing has also revealed that 90% of the genome is conserved between L. major and L. infantum, and 99% of the genes are syntenic (55, 56), suggesting a strong likelihood of shared antigens. Remarkably, only two experimental studies have investigated the cross-protection mediated by primary infection with L. major against subsequent visceral infection (24, 25). These studies employed BALB/c mice chronically infected with L. major and showed no protection against either i.v. or i.d. challenge with L. infantum. In contrast, we were able to demonstrate the protective effect of leishmanization against visceral infection employing C57BL/6 mice with a healed L. major infection, which more closely replicates the immune status of leishmanized humans. Our observations are limited to an assessment of protection against infection, not disease, because mice infected i.d. with visceralizing strains of Leishmania produce minimal pathology in the viscera. In addition, the i.d. model of infection does not establish long-term infection in naïve mice as determined by the detection of parasites in the liver and spleen by LDA at 12 weeks p.i. (Fig 1). Although this may be viewed as a limitation of the model, the use of the more conventional high dose i.v. challenge model would by-pass the potentially important initial step of parasite deposition in the skin, the natural site of infection. In addition, it should be noted that in humans the immunobiology of asymptomatic infection with visceralizing parasites is under-studied, and the scarcity of parasites in the viscera may in fact be a common finding in the field (57).
While the ideal challenge model would employ exposure to the bites of L. infantum-infected sand flies, we have yet to establish a reliable sand fly transmission model of L. infantum in our laboratory. Rather, we employed relatively early time points, i.e. 1 week post-needle challenge, to determine protective immunity in the viscera as early control of parasite numbers in the skin following needle challenge has proven to be an informative correlate of protection against cutaneous disease following challenge with L. major infected sand flies (11, 12).
Our observations suggest that the majority of cytokine production typically associated with adaptive T cell immunity in the spleen and liver is from circulating cells trapped in the vascular or the splenic red pulp at the time of euthanasia. Despite this observation, we were able to employ a combination of in-vivo labeling of circulating cells and pre-challenge leishmanized mice to demonstrate that leishmanization does mediate a rapid Leishmania-specific immune response in visceral tissue in response to dermal challenge with L. infantum. The use of the in-vivo labeling technique would appear to be critical in future studies to determine the relative contribution of circulating cells versus tissue resident cells to total cytokine production. It is important to note that even before challenge, we found low frequencies of i.v.− cells with the capacity to make IFN-γ in the uninfected ear, non-draining LN, blood, liver and spleen of leishmanized mice, similar to what we have reported previously (35). Whether the i.v.−IFN-γ+CD4+ T cells in the viscera are tissue resident memory T cells (TRM) as has been reported for the lung (58) skin (59) and vaginal mucosa (60), or patrolling cells as has also been reported for the skin (61), is unknown. Where as pre-existing Ag-specific T cells are not required for the recruitment of highly protective Leishmania-specific T cells into a site of non-specific tissue damage (35), this does not preclude the possibility that they may enhance protection, regardless of their patrolling or resident nature (59). Extensive studies have identified a role for pre-existing CD8+ TRM cells in protective immunity (62). We found strong evidence for the existence of i.v.−, resident, Leishmania-specific GzB+CD8+ T cells in the viscera of leishmanized mice prior to challenge. The role of these cells in protection will be the topic of future research.
Our data reinforces the rationale for re-introducing the practice of leishmanization under conditions where the benefits, such as protection against fatal visceral disease, are deemed to outweigh the risks associated with administering live L. major. Numerous genetically modified or avirulent Leishmania strains have been studied in an effort to replicate the protective effect of leishmanization while reducing or controlling for the virulence of the parasite (52, 63-68). How effective these modified strains are compared to leishmanization with wild type L. major has not been addressed, with a single exception (68). Past observations (34, 35, 69, 70) suggest that attenuation resulting in sterile clearance rather than persistence will compromise long-term protection, especially against the more stringent conditions of infected sand fly challenge. The ideal situation would be to leishmanize with a parasite that minimizes pathology but also persists over the long term. An additional drawback of leishmanizing against VL is the wisdom of introducing L. major in areas where the parasite is not endemic, such as northeast India. However, L. major strains that establish persistent infection in the mammalian host but cannot produce transmissible infections in the sand fly midgut, such as various lpg knock out parasites, have already been developed that would address these concerns (67, 71-73).
Supplementary Material
Acknowledgements
We thank Kim Beacht, Melanie Faivre-Charmoy, and Flavia L. Ribeiro-Gomes for assistance with experiments. We especially thank Kristin Anderson and David Masopust (University of Minnesota) and Dan Barber (NIAID) for help with the i.v. staining protocol.
References
- 1.Kaye P, Scott P. Leishmaniasis: complexity at the host-pathogen interface. Nature reviews. Microbiology. 2011;9:604–615. doi: 10.1038/nrmicro2608. [DOI] [PubMed] [Google Scholar]
- 2.Sundar S, Chakravarty J. An update on pharmacotherapy for leishmaniasis. Expert opinion on pharmacotherapy. 2015;16:237–252. doi: 10.1517/14656566.2015.973850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giorgobiani E, Chitadze N, Chanturya G, Grdzelidze M, Jochim RC, Machablishvili A, Tushishvili T, Zedginidze Y, Manjgaladze MK, Iashvili N, Makharadze MP, Zakaraya T, Kikaleishvili K, Markhvashvili I, Badashvili G, Daraselia T, Fay MP, Kamhawi S, Sacks D. Epidemiologic aspects of an emerging focus of visceral leishmaniasis in Tbilisi, Georgia. PLoS neglected tropical diseases. 2011;5:e1415. doi: 10.1371/journal.pntd.0001415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Varani S, Cagarelli R, Melchionda F, Attard L, Salvadori C, Finarelli AC, Gentilomi GA, Tigani R, Rangoni R, Todeschini R, Scalone A, Di Muccio T, Gramiccia M, Gradoni L, Viale P, Landini MP. Ongoing outbreak of visceral leishmaniasis in Bologna Province, Italy, November 2012 to May 2013. Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2013;18:20530. [PubMed] [Google Scholar]
- 5.Gramiccia M, Scalone A, Di Muccio T, Orsini S, Fiorentino E, Gradoni L. The burden of visceral leishmaniasis in Italy from 1982 to 2012: a retrospective analysis of the multi-annual epidemic that occurred from 1989 to 2009. Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2013;18:20535. [PubMed] [Google Scholar]
- 6.Arce A, Estirado A, Ordobas M, Sevilla S, Garcia N, Moratilla L, de la Fuente S, Martinez AM, Perez AM, Aranguez E, Iriso A, Sevillano O, Bernal J, Vilas F. Re-emergence of leishmaniasis in Spain: community outbreak in Madrid, Spain, 2009 to 2012. Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2013;18:20546. doi: 10.2807/1560-7917.es2013.18.30.20546. [DOI] [PubMed] [Google Scholar]
- 7.Palatnik-de-Sousa CB, Day MJ. One Health: the global challenge of epidemic and endemic leishmaniasis. Parasites & vectors. 2011;4:197. doi: 10.1186/1756-3305-4-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Melby PC. Experimental leishmaniasis in humans: review. Reviews of infectious diseases. 1991;13:1009–1017. doi: 10.1093/clinids/13.5.1009. [DOI] [PubMed] [Google Scholar]
- 9.Schlein Y, Warburg A, Schnur LF, Le Blancq SM, Gunders AE. Leishmaniasis in Israel: reservoir hosts, sandfly vectors and leishmanial strains in the Negev, Central Arava and along the Dead Sea. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1984;78:480–484. doi: 10.1016/0035-9203(84)90067-1. [DOI] [PubMed] [Google Scholar]
- 10.Nadim A, E. J. a. M. M. The experience of leishmanization in the Islamic Republic of Iran. Eastern Mediterranean Health Journal. 1997 [Google Scholar]
- 11.Peters NC, Kimblin N, Secundino N, Kamhawi S, Lawyer P, Sacks DL. Vector transmission of leishmania abrogates vaccine-induced protective immunity. PLoS pathogens. 2009;5:e1000484. doi: 10.1371/journal.ppat.1000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peters NC, Bertholet S, Lawyer PG, Charmoy M, Romano A, Ribeiro-Gomes FL, Stamper LW, Sacks DL. Evaluation of recombinant Leishmania polyprotein plus glucopyranosyl lipid A stable emulsion vaccines against sand fly-transmitted Leishmania major in C57BL/6 mice. Journal of immunology. 2012;189:4832–4841. doi: 10.4049/jimmunol.1201676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khamesipour A, Abbasi A, Firooz A, Mohammadi AM, Eskandari SE, Jaafari MR. Treatment of cutaneous lesion of 20 years' duration caused by leishmanization. Indian journal of dermatology. 2012;57:123–125. doi: 10.4103/0019-5154.94280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nadim A, Javadian E, Tahvildar-Bidruni G, Ghorbani M. Effectiveness of leishmanization in the control of cutaneous leishmaniasis. Bulletin de la Societe de pathologie exotique et de ses filiales. 1983;76:377–383. [PubMed] [Google Scholar]
- 15.Goto Y, Bhatia A, Raman VS, Liang H, Mohamath R, Picone AF, Vidal SE, Vedvick TS, Howard RF, Reed SG. KSAC, the first defined polyprotein vaccine candidate for visceral leishmaniasis. Clinical and vaccine immunology : CVI. 2011;18:1118–1124. doi: 10.1128/CVI.05024-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duthie MS, Raman VS, Piazza FM, Reed SG. The development and clinical evaluation of second-generation leishmaniasis vaccines. Vaccine. 2012;30:134–141. doi: 10.1016/j.vaccine.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zijlstra EE, el-Hassan AM, Ismael A, Ghalib HW. Endemic kala-azar in eastern Sudan: a longitudinal study on the incidence of clinical and subclinical infection and post-kala-azar dermal leishmaniasis. The American journal of tropical medicine and hygiene. 1994;51:826–836. doi: 10.4269/ajtmh.1994.51.826. [DOI] [PubMed] [Google Scholar]
- 18.Khalil EA, Zijlstra EE, Kager PA, El Hassan AM. Epidemiology and clinical manifestations of Leishmania donovani infection in two villages in an endemic area in eastern Sudan. Tropical medicine & international health : TM & IH. 2002;7:35–44. doi: 10.1046/j.1365-3156.2002.00832.x. [DOI] [PubMed] [Google Scholar]
- 19.Ranasinghe S, Zhang WW, Wickremasinghe R, Abeygunasekera P, Chandrasekharan V, Athauda S, Mendis S, Hulangamuwa S, Matlashewski G, Pratlong F. Leishmania donovani zymodeme MON-37 isolated from an autochthonous visceral leishmaniasis patient in Sri Lanka. Pathogens and global health. 2012;106:421–424. doi: 10.1179/2047773212Y.0000000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manson-Bahr PE, Heisch RB, Garnham PC. Studies in leishmanifasis in East Africa. IV. The Montenegro test in kala-azar in Kenya. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1959;53:380–383. doi: 10.1016/0035-9203(59)90038-0. [DOI] [PubMed] [Google Scholar]
- 21.H.A S. Hematologic and Immunologic Studies on Natural and Induced Leishmaniasis in Paretics. The American journal of tropical medicine and hygiene. 1943;s1-23:53–58. [Google Scholar]
- 22.Adler S, Gunders AE. Immunity to Leishmania Mexicana Following Spontaneous Recovery from Oriental Sore. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1964;58:274–277. doi: 10.1016/0035-9203(64)90041-0. [DOI] [PubMed] [Google Scholar]
- 23.Manson-Bahr PE. Immunity in kala-azar. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1961;55:550–555. doi: 10.1016/0035-9203(61)90078-5. [DOI] [PubMed] [Google Scholar]
- 24.Streit JA, Recker TJ, Filho FG, Beverley SM, Wilson ME. Protective immunity against the protozoan Leishmania chagasi is induced by subclinical cutaneous infection with virulent but not avirulent organisms. Journal of immunology. 2001;166:1921–1929. doi: 10.4049/jimmunol.166.3.1921. [DOI] [PubMed] [Google Scholar]
- 25.Nation CS, Dondji B, Stryker GA. Previous exposure to a low infectious dose of Leishmania major exacerbates infection with Leishmania infantum in the susceptible BALB/c mouse. Parasitology research. 2012;111:1407–1415. doi: 10.1007/s00436-012-2899-5. [DOI] [PubMed] [Google Scholar]
- 26.Sacks D, Noben-Trauth N. The immunology of susceptibility and resistance to Leishmania major in mice. Nature reviews. Immunology. 2002;2:845–858. doi: 10.1038/nri933. [DOI] [PubMed] [Google Scholar]
- 27.Ahmed S, Colmenares M, Soong L, Goldsmith-Pestana K, Munstermann L, Molina R, McMahon-Pratt D. Intradermal infection model for pathogenesis and vaccine studies of murine visceral leishmaniasis. Infection and immunity. 2003;71:401–410. doi: 10.1128/IAI.71.1.401-410.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Romano A, Inbar E, Debrabant A, Charmoy M, Lawyer P, Ribeiro-Gomes F, Barhoumi M, Grigg M, Shaik J, Dobson D, Beverley SM, Sacks DL. Cross-species genetic exchange between visceral and cutaneous strains of Leishmania in the sand fly vector. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:16808–16813. doi: 10.1073/pnas.1415109111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spath GF, Beverley SM. A lipophosphoglycan-independent method for isolation of infective Leishmania metacyclic promastigotes by density gradient centrifugation. Experimental parasitology. 2001;99:97–103. doi: 10.1006/expr.2001.4656. [DOI] [PubMed] [Google Scholar]
- 30.Sacks DL, Hieny S, Sher A. Identification of cell surface carbohydrate and antigenic changes between noninfective and infective developmental stages of Leishmania major promastigotes. Journal of immunology. 1985;135:564–569. [PubMed] [Google Scholar]
- 31.Ramalingam TR, Pesce JT, Sheikh F, Cheever AW, Mentink-Kane MM, Wilson MS, Stevens S, Valenzuela DM, Murphy AJ, Yancopoulos GD, Urban JF, Jr., Donnelly RP, Wynn TA. Unique functions of the type II interleukin 4 receptor identified in mice lacking the interleukin 13 receptor alpha1 chain. Nature immunology. 2008;9:25–33. doi: 10.1038/ni1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson KG, Mayer-Barber K, Sung H, Beura L, James BR, Taylor JJ, Qunaj L, Griffith TS, Vezys V, Barber DL, Masopust D. Intravascular staining for discrimination of vascular and tissue leukocytes. Nature protocols. 2014;9:209–222. doi: 10.1038/nprot.2014.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kimblin N, Peters N, Debrabant A, Secundino N, Egen J, Lawyer P, Fay MP, Kamhawi S, Sacks D. Quantification of the infectious dose of Leishmania major transmitted to the skin by single sand flies. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:10125–10130. doi: 10.1073/pnas.0802331105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zaph C, Uzonna J, Beverley SM, Scott P. Central memory T cells mediate long-term immunity to Leishmania major in the absence of persistent parasites. Nature medicine. 2004;10:1104–1110. doi: 10.1038/nm1108. [DOI] [PubMed] [Google Scholar]
- 35.Peters NC, Pagan AJ, Lawyer PG, Hand TW, Henrique Roma E, Stamper LW, Romano A, Sacks DL. Chronic parasitic infection maintains high frequencies of short-lived Ly6C+CD4+ effector T cells that are required for protection against re-infection. PLoS pathogens. 2014;10:e1004538. doi: 10.1371/journal.ppat.1004538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goto Y, Bhatia A, Raman VS, Vidal SE, Bertholet S, Coler RN, Howard RF, Reed SG. Leishmania infantum sterol 24-c-methyltransferase formulated with MPL-SE induces cross-protection against L. major infection. Vaccine. 2009;27:2884–2890. doi: 10.1016/j.vaccine.2009.02.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sakai S, Kauffman KD, Schenkel JM, McBerry CC, Mayer-Barber KD, Masopust D, Barber DL. Cutting edge: control of Mycobacterium tuberculosis infection by a subset of lung parenchyma-homing CD4 T cells. Journal of immunology. 2014;192:2965–2969. doi: 10.4049/jimmunol.1400019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okwor IB, Jia P, Mou Z, Onyilagha C, Uzonna JE. CD8+ T cells are preferentially activated during primary low dose leishmania major infection but are completely dispensable during secondary anti-Leishmania immunity. PLoS neglected tropical diseases. 2014;8:e3300. doi: 10.1371/journal.pntd.0003300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uzonna JE, Joyce KL, Scott P. Low dose Leishmania major promotes a transient T helper cell type 2 response that is down-regulated by interferon gamma-producing CD8+ T cells. The Journal of experimental medicine. 2004;199:1559–1566. doi: 10.1084/jem.20040172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stager S, Rafati S. CD8(+) T cells in leishmania infections: friends or foes? Frontiers in immunology. 2012;3:5. doi: 10.3389/fimmu.2012.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Novais FO, Carvalho LP, Graff JW, Beiting DP, Ruthel G, Roos DS, Betts MR, Goldschmidt MH, Wilson ME, de Oliveira CI, Scott P. Cytotoxic T cells mediate pathology and metastasis in cutaneous leishmaniasis. PLoS pathogens. 2013;9:e1003504. doi: 10.1371/journal.ppat.1003504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bunn PT, Stanley AC, de Labastida Rivera F, Mulherin A, Sheel M, Alexander CE, Faleiro RJ, Amante FH, Montes De Oca M, Best SE, James KR, Kaye PM, Haque A, Engwerda CR. Tissue requirements for establishing long-term CD4+ T cell-mediated immunity following Leishmania donovani infection. Journal of immunology. 2014;192:3709–3718. doi: 10.4049/jimmunol.1300768. [DOI] [PubMed] [Google Scholar]
- 43.Eisert V, Munster U, Simon MM, Moll H. The course of Leishmania major infection in mice lacking granzyme-mediated mechanisms. Immunobiology. 2002;205:314–320. doi: 10.1078/0171-2985-00134. [DOI] [PubMed] [Google Scholar]
- 44.Faria DR, Souza PE, Duraes FV, Carvalho EM, Gollob KJ, Machado PR, Dutra WO. Recruitment of CD8(+) T cells expressing granzyme A is associated with lesion progression in human cutaneous leishmaniasis. Parasite immunology. 2009;31:432–439. doi: 10.1111/j.1365-3024.2009.01125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santos Cda S, Boaventura V, Ribeiro Cardoso C, Tavares N, Lordelo MJ, Noronha A, Costa J, Borges VM, de Oliveira CI, Van Weyenbergh J, Barral A, Barral-Netto M, Brodskyn CI. CD8(+) granzyme B(+)-mediated tissue injury vs. CD4(+)IFNgamma(+)-mediated parasite killing in human cutaneous leishmaniasis. The Journal of investigative dermatology. 2013;133:1533–1540. doi: 10.1038/jid.2013.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCall LI, Zhang WW, Ranasinghe S, Matlashewski G. Leishmanization revisited: immunization with a naturally attenuated cutaneous Leishmania donovani isolate from Sri Lanka protects against visceral leishmaniasis. Vaccine. 2013;31:1420–1425. doi: 10.1016/j.vaccine.2012.11.065. [DOI] [PubMed] [Google Scholar]
- 47.Gicheru MM, Olobo JO, Anjili CO. Heterologous protection by Leishmania donovani for Leishmania major infections in the vervet monkey model of the disease. Experimental parasitology. 1997;85:109–116. doi: 10.1006/expr.1996.4117. [DOI] [PubMed] [Google Scholar]
- 48.Alexander J, Phillips RS. Leishmania tropica and Leishmania mexicana: cross-immunity in mice. Experimental parasitology. 1978;45:93–100. doi: 10.1016/0014-4894(78)90049-8. [DOI] [PubMed] [Google Scholar]
- 49.Perez H, Arredondo B, Machado R. Leishmania mexicana and Leishmania tropica: cross immunity in C57BL/6 mice. Experimental parasitology. 1979;48:9–14. doi: 10.1016/0014-4894(79)90049-3. [DOI] [PubMed] [Google Scholar]
- 50.Lainson R, Shaw JJ. Leishmaniasis in Brazil: XII. Observations on cross-immunity in monkeys and man infected with Leishmania mexicana mexicana, L. m. amazonensis, L. braziliensis braziliensis, L. b. guyanensis and L. b. panamensis. The Journal of tropical medicine and hygiene. 1977;80:29–35. [PubMed] [Google Scholar]
- 51.Porrozzi R, Teva A, Amaral VF, Santos da Costa MV, Grimaldi G., Jr. Cross-immunity experiments between different species or strains of Leishmania in rhesus macaques (Macaca mulatta). The American journal of tropical medicine and hygiene. 2004;71:297–305. [PubMed] [Google Scholar]
- 52.Dey R, Dagur PK, Selvapandiyan A, McCoy JP, Salotra P, Duncan R, Nakhasi HL. Live attenuated Leishmania donovani p27 gene knockout parasites are nonpathogenic and elicit long-term protective immunity in BALB/c mice. Journal of immunology. 2013;190:2138–2149. doi: 10.4049/jimmunol.1202801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dey R, Natarajan G, Bhattacharya P, Cummings H, Dagur PK, Terrazas C, Selvapandiyan A, McCoy JP, Jr., Duncan R, Satoskar AR, Nakhasi HL. Characterization of cross-protection by genetically modified live-attenuated Leishmania donovani parasites against Leishmania mexicana. Journal of immunology. 2014;193:3513–3527. doi: 10.4049/jimmunol.1303145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mizbani A, Taheri T, Zahedifard F, Taslimi Y, Azizi H, Azadmanesh K, Papadopoulou B, Rafati S. Recombinant Leishmania tarentolae expressing the A2 virulence gene as a novel candidate vaccine against visceral leishmaniasis. Vaccine. 2009;28:53–62. doi: 10.1016/j.vaccine.2009.09.114. [DOI] [PubMed] [Google Scholar]
- 55.Rochette A, Raymond F, Ubeda JM, Smith M, Messier N, Boisvert S, Rigault P, Corbeil J, Ouellette M, Papadopoulou B. Genome-wide gene expression profiling analysis of Leishmania major and Leishmania infantum developmental stages reveals substantial differences between the two species. BMC genomics. 2008;9:255. doi: 10.1186/1471-2164-9-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peacock CS, Seeger K, Harris D, Murphy L, Ruiz JC, Quail MA, Peters N, Adlem E, Tivey A, Aslett M, Kerhornou A, Ivens A, Fraser A, Rajandream MA, Carver T, Norbertczak H, Chillingworth T, Hance Z, Jagels K, Moule S, Ormond D, Rutter S, Squares R, Whitehead S, Rabbinowitsch E, Arrowsmith C, White B, Thurston S, Bringaud F, Baldauf SL, Faulconbridge A, Jeffares D, Depledge DP, Oyola SO, Hilley JD, Brito LO, Tosi LR, Barrell B, Cruz AK, Mottram JC, Smith DF, Berriman M. Comparative genomic analysis of three Leishmania species that cause diverse human disease. Nature genetics. 2007;39:839–847. doi: 10.1038/ng2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Singh OP, Hasker E, Sacks D, Boelaert M, Sundar S. Asymptomatic Leishmania infection: a new challenge for Leishmania control. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2014;58:1424–1429. doi: 10.1093/cid/ciu102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Teijaro JR, Turner D, Pham Q, Wherry EJ, Lefrancois L, Farber DL. Cutting edge: Tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. Journal of immunology. 2011;187:5510–5514. doi: 10.4049/jimmunol.1102243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Glennie ND, Yeramilli VA, Beiting DP, Volk SW, Weaver CT, Scott P. Skin-resident memory CD4+ T cells enhance protection against Leishmania major infection. J. Exp. Med. 2015;212:1405–14. doi: 10.1084/jem.20142101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Iijima N, Iwasaki A. T cell memory. A local macrophage chemokine network sustains protective tissue-resident memory CD4 T cells. Science. 2014;346:93–98. doi: 10.1126/science.1257530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gebhardt T, Whitney PG, Zaid A, Mackay LK, Brooks AG, Heath WR, Carbone FR, Mueller SN. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature. 2011;477:216–219. doi: 10.1038/nature10339. [DOI] [PubMed] [Google Scholar]
- 62.Mueller SN, Gebhardt T, Carbone FR, Heath WR. Memory T cell subsets, migration patterns, and tissue residence. Annual review of immunology. 2013;31:137–161. doi: 10.1146/annurev-immunol-032712-095954. [DOI] [PubMed] [Google Scholar]
- 63.Selvapandiyan A, Dey R, Nylen S, Duncan R, Sacks D, Nakhasi HL. Intracellular replication-deficient Leishmania donovani induces long lasting protective immunity against visceral leishmaniasis. Journal of immunology. 2009;183:1813–1820. doi: 10.4049/jimmunol.0900276. [DOI] [PubMed] [Google Scholar]
- 64.Zhang WW, Matlashewski G. Characterization of the A2-A2rel gene cluster in Leishmania donovani: involvement of A2 in visceralization during infection. Molecular microbiology. 2001;39:935–948. doi: 10.1046/j.1365-2958.2001.02286.x. [DOI] [PubMed] [Google Scholar]
- 65.Mendez S, Tabbara K, Belkaid Y, Bertholet S, Verthelyi D, Klinman D, Seder RA, Sacks DL. Coinjection with CpG-containing immunostimulatory oligodeoxynucleotides reduces the pathogenicity of a live vaccine against cutaneous Leishmaniasis but maintains its potency and durability. Infection and immunity. 2003;71:5121–5129. doi: 10.1128/IAI.71.9.5121-5129.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Silvestre R, Cordeiro-Da-Silva A, Santarem N, Vergnes B, Sereno D, Ouaissi A. SIR2-deficient Leishmania infantum induces a defined IFN-gamma/IL-10 pattern that correlates with protection. Journal of immunology. 2007;179:3161–3170. doi: 10.4049/jimmunol.179.5.3161. [DOI] [PubMed] [Google Scholar]
- 67.Liu D, Okwor I, Mou Z, Beverley SM, Uzonna JE. Deficiency of Leishmania phosphoglycans influences the magnitude but does not affect the quality of secondary (memory) anti-Leishmania immunity. PloS one. 2013;8:e66058. doi: 10.1371/journal.pone.0066058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Davoudi N, Khamesipour A, Mahboudi F, McMaster WR. A dual drug sensitive L. major induces protection without lesion in C57BL/6 mice. PLoS neglected tropical diseases. 2014;8:e2785. doi: 10.1371/journal.pntd.0002785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Uzonna JE, Wei G, Yurkowski D, Bretscher P. Immune elimination of Leishmania major in mice: implications for immune memory, vaccination, and reactivation disease. Journal of immunology. 2001;167:6967–6974. doi: 10.4049/jimmunol.167.12.6967. [DOI] [PubMed] [Google Scholar]
- 70.Belkaid Y, Hoffmann KF, Mendez S, Kamhawi S, Udey MC, Wynn TA, Sacks DL. The role of interleukin (IL)-10 in the persistence of Leishmania major in the skin after healing and the therapeutic potential of anti-IL-10 receptor antibody for sterile cure. The Journal of experimental medicine. 2001;194:1497–1506. doi: 10.1084/jem.194.10.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kamhawi S, Ramalho-Ortigao M, Pham VM, Kumar S, Lawyer PG, Turco SJ, Barillas-Mury C, Sacks DL, Valenzuela JG. A role for insect galectins in parasite survival. Cell. 2004;119:329–341. doi: 10.1016/j.cell.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 72.Secundino N, Kimblin N, Peters NC, Lawyer P, Capul AA, Beverley SM, Turco SJ, Sacks D. Proteophosphoglycan confers resistance of Leishmania major to midgut digestive enzymes induced by blood feeding in vector sand flies. Cellular microbiology. 2010;12:906–918. doi: 10.1111/j.1462-5822.2010.01439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dobson DE, Kamhawi S, Lawyer P, Turco SJ, Beverley SM, Sacks DL. Leishmania major survival in selective Phlebotomus papatasi sand fly vector requires a specific SCG-encoded lipophosphoglycan galactosylation pattern. PLoS pathogens. 2010;6:e1001185. doi: 10.1371/journal.ppat.1001185. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.