Abstract
Objectives:
Afzal is a common smokeless tobacco product (STP) available illegally in Oman. This study aimed to assess pH and moisture levels and determine cancer-enhancing factors in a randomly selected sample of Afzal.
Methods:
This study was carried out at the Sultan Qaboos University in Muscat, Oman, between April and December 2013. A package of Afzal was purchased from a single provider and divided into samples. The pH and moisture content of the samples were measured according to the protocols of the Centers for Disease Control and Prevention. Gas chromatography-mass spectrometry was used to analyse nicotine levels and ion-exchange chromatography (IC) was used to determine concentrations of nitrate, nitrite, chloride, fluoride, bromide, sulphate and phosphate anions.
Results:
The samples had an alkaline pH of 10.46 with high levels of total (48,770.00 µg per g of STP [µg/g]) and unionised (48,590.00 µg/g) nicotine. The concentration of nitrate (8,792.20 µg/g) was alarmingly high. The chloride concentration (33,170.80 µg/g) showed a surge on IC chromatography. The moisture content percentage was 52.00%.
Conclusion:
The moisture content percentage and chloride concentration of Afzal was consistent with those of other STPs. In contrast, nitrite, sulphate and phosphate concentrations were below reported levels of other STPs. All anion concentrations were below the maximum daily limit set by international health organisations. However, the high concentrations of nitrite, nitrate and nicotine and the elevated alkaline pH observed in the analysed Afzal samples suggest that STP users will face health risks as a result of their use.
Keywords: Smokeless Tobaccos, Gas Chromatography-Mass Spectrometry, Ion-Exchange Chromatography, Carcinogens, Nicotine, Anions, Oman
Advances in Knowledge
- This study shows that Afzal, an illegal smokeless tobacco product (STP) commonly available in Oman, contains high levels of nitrites, nitrates and nicotine and an elevated alkaline pH.
Application to Patient Care
- This study may encourage health officials and relevant government departments to continue increasing awareness among young Afzal users about the potential health risks associated with this STP.
- Health practitioners should be aware of Afzal use among patients and the medical complications that could arise as a result.
According to the International Agency for Research on Cancer, smokeless tobacco products (STPs) are carcinogenic.1 Understanding the chemical composition of these products is therefore the first step in assessing their potential toxicity.2 The majority of STPs contain compounds that are potentially detrimental to health, including tobacco-specific nitrosamines (TSNAs); nitrosodimethylamine; polycyclic aromatic hydrocarbons (e.g. benzo(a)pyrene); and heavy metals.3 Additionally, they generally have elevated alkaline pH and moisture levels and contain nicotinic secondary metabolites and anions such as nitrite (NO2−) and chloride (Cl−), which may enhance cancer formation.4 The most harmful carcinogens in STPs are TSNAs, which normally form during the preservation, fermentation, maturation and storage stages of the tobacco process.3,5 The presence of certain agents in tobacco—including nicotine, elevated moisture levels, a high pH level and NO2− and nitrate (NO3−) concentrations—usually facilitates the accumulation of high concentrations of TSNAs.6 As such, STPs must be analysed and their compounds quantified in order to estimate their potential health risk.
The nicotine content of a specific STP is the primary determinant of both its appeal and cause of addiction among users.7 The absorption of nicotine is strongly associated with the pH and moisture content of the product.8 The naturally occurring form of nicotine is the unprotonated form, which is easily absorbed by the oral mucus membranes into the blood stream and delivered directly to the brain.2 Within STPs, an alkaline pH value helps make more of the total nicotine absorbable.9 Furthermore, the moisture content of an STP enhances microbial activity, resulting in an elevated formation of TSNAs.10,11 Microflora in the buccal cavity cause the reduction of NO3− to NO2−, which is more toxic to humans.12 In turn, NO2− forms the nitrosating agent of the alkaloids in the tobacco (mainly nicotine and its secondary metabolites), which leads to the formation of carcinogenic nitrosamines.4
Afzal is a commonly used STP available illegally in Oman. In order to educate consumers, public health officials and regulatory policy-makers regarding the levels of toxicants in Afzal, analyses of the contents of this STP are needed. Previous research has determined the composition of heavy metals in Afzal.13 The aim of this study was therefore to measure pH and moisture levels and assess cancer-causing agents in a random sample of Afzal.
Methods
This study was carried out at the Sultan Qaboos University (SQU) in Muscat, Oman, between April and December 2013. A single package of 4.00 kg of Afzal was purchased from a single source in order to maintain uniformity. The pH levels and moisture content of the sample were tested on the day of purchase according to the methods described below. The product was subsequently labelled with the date of purchase as well as the original pH level and moisture content so that any changes that occurred after storage could be noted. The sample was then kept refrigerated at 4 °C in plastic bags until analysis.
In order to assess pH levels, the package of Afzal was divided into three samples of 2.00 g each. Each sample was then mixed with 20 mL of deionised water. The pH levels were measured using a pH meter (Benchtop pH Meter, Mettler-Toledo International Inc., Columbus, Ohio, USA) with two-point calibration to an accuracy of two decimal places using standard pH buffers (4.00 and 7.00). The pH was measured with continuous stirring at the first five, 15 and 30 minutes to ensure no variation in the results. The mean pH value was obtained in accordance with standard protocols from the Centers for Disease Control and Prevention (CDC).14 Three samples of 5.00 g each of Afzal were dried to determine their moisture content. This was measured by obtaining the difference in weight of the samples before and after they had been dried in an oven for three hours at 99 ± 1 °C. The mean moisture value was reported as a percentage of the original weight and compared to CDC protocols.14
Ion-exchange chromatography (IC) analysis was performed for NO2−, NO3−, Cl−, fluoride (F−), bromide (Br−), phosphate (PO42−) and sulphate (SO42−) ions. Dried Afzal samples were ground into a homogenous powder and three samples of 1.00 g each were transferred into three 50 mL centrifuge tubes. Subsequently, 20 mL of Milli-Q® water (Merck KGaA, Darmstadt, Germany) was added to each tube. Samples were sonicated for 30 minutes followed by centrifugation at 3,000 revolutions per minute (rpm) for 30 minutes. Finally, the samples were filtered through a 0.45 μm cellulose acetate polytetrafluoroethylene membrane nylon syringe filter (Whatman®, Sigma-Aldrich Corp., St. Louis, Missouri, USA) to remove solids before the solution was injected into the 881 Compact IC Pro Anion System with an 858 Professional Sample Processor (Metrohm AG, Ionenstrasse, Switzerland). In order to ensure the accuracy of the readings, each sample was injected into the IC instrument twice. Each sample was diluted five-fold before IC analysis. The IC method used was validated and applied by the Environmental Engineering Laboratory of the College of Engineering at SQU.
The conditions of the analysis were as follows: column size of 250.00 × 4.00 mm; flow rate of 0.7 mL/minute; suppressed conductivity detector; ambient temperature of 21–23 °C; pressure of 13.7 megapascals; and a sample size of 20 µL. The eluent was prepared by dissolving 678,400.00 µg (3.2 mM/L) of 98% pure sodium carbonate (VWR International Ltd., Lutterworth, Leicestershire, UK) and 168,000.00 µg (1.0 mM/L) of sodium bicarbonate with 99% purity (VWR International Ltd.) in 2 L of deionised ultrapure water. The suppressor regenerating solution used was 50 mM of analytical-grade sulphuric acid (Honeywell Specialty Chemicals Seelze GmbH, Seelze, Germany). In order to form a five-point calibration curve, 1,000 parts per million of each anion was concentrated using six anion standard concentrations (Fluka® Analytical, Sigma-Aldrich Corp.). The NO2− standard was laboratory-prepared from 98% pure sodium NO2− (Merck KGaA). Deionised water at a purity of 18 megohms (Ultrapure Private Limited, Singapore) was used throughout the analysis.
The mean total nicotine value of Afzal samples was obtained from gas chromatography-mass spectrometry (GC-MS) analysis. The nicotine analysis procedure was performed as previously described.15,16 A total of 5 mL of a 2 M solution of sodium hydroxide and 50 mL of extraction solution (methyl tert-butyl ether) with quinoline as an internal standard was added to a 1.00 g sample of Afzal. Sample vials were shaken on an orbital shaker at 160 rpm for two hours. The resulting extract was transferred to a 2 mL autosampler vial. The analysis was performed by injecting 1 mL from each vial into a Clarus® 600 Gas Chromatograph (PerkinElmer Inc., Waltham, Massachusetts, USA) fitted with an Rtx®-5MS capillary column (Restek Corp., Bellefonte, Pennsylvania, USA) of 30.00 m × 0.25 mm × 0.25 μm film thickness at a maximum temperature of 350 °C and coupled to a Clarus® 600C Mass Spectrometer (PerkinElmer Inc.). Ultra-high-purity helium (>99.9%) was used as a carrier gas at a constant flow of 1.0 mL/minute. The injection, transfer line and ion source temperatures were 290, 280 and 280 °C, respectively. The ionising energy was 70 electron volts. The injected volume for each sample was 1 μL with a split ratio of 80:1. The oven temperature was initially 80 °C for five minutes and then accelerated at a rate of 10 °C per minute to 280 °C for 30 minutes. Unknown compounds were identified by comparing the spectra obtained with National Institute of Standards and Technology (NIST) Software, Version 11 (United States Department of Commerce, Gaithersburg, Maryland, USA) and NIST Mass Spectral Library, Version 14 (United States Department of Commerce). All chemicals used for GC-MS analysis were of analytical grade (Sigma-Aldrich Corp.).
For method validation purposes, blank samples of deionised water were run 10 times to calculate the limits of detection (LOD) and limits of quantitation (LOQ) of the tested analytes. Blank reagent samples of the tested anions were also used to subtract the results of all tested anion standards and samples. The LOD was calculated using the following equation:
Where SDblank is the standard deviation for the signal recorded on the blank for the corresponding element studied and Concnsample is the concentration in μg/L of the respective sample aspired. The Inet value was calculated as below:
Where Isample and Iblank are the signal intensities recorded for the sample and blank, respectively. The LOQ was approximately 10 times the LOD for all studied anions. Each analysed standard and sample was injected three times. This reflected high precision as it was automatically calculated using the relative standard deviation for each analyte. Six-point calibration standards curves were plotted at 50, 100, 150, 200, 250 and 300 parts per billion for each analyte.
The percentage of unprotonated nicotine was calculated using the pH and the pKa value of the pyrolic nitrogen of nicotine (pKa = 8.02), which was substituted into the Henderson-Hasselbalch equation.14 The total amount of unprotonated nicotine was then calculated by multiplying the percentage of unprotonated nicotine by the total nicotine as follows:14
Where WISTD is the weight of the internal standard (6,250.00 µg), Wsample is the weight of the Afzal sample (1.00 g), Anicotine is the area of nicotine from the chromatogram and AISTD is the area of the internal standard. Unprotonated nicotine was calculated according to the following Henderson-Hasselbalch equation:17
Where B is the amount of unprotonated nicotine, BH+ is the amount of ionised nicotine, pKa is 8.02 and pH is 10.46. The percentage of unprotonated nicotine was calculated according to the following formula:
This was then used to calculate the total free nicotine as per the below formula:
Excel spreadsheet software, Version 2010 (Microsoft Corp., Redmond, Washington, USA) was used for the statistical analysis. For each Afzal sample, the mean pH value, moisture content and nicotine concentrations were calculated. This study did not require ethical approval as it involved a chemical analysis of Afzal.
Results
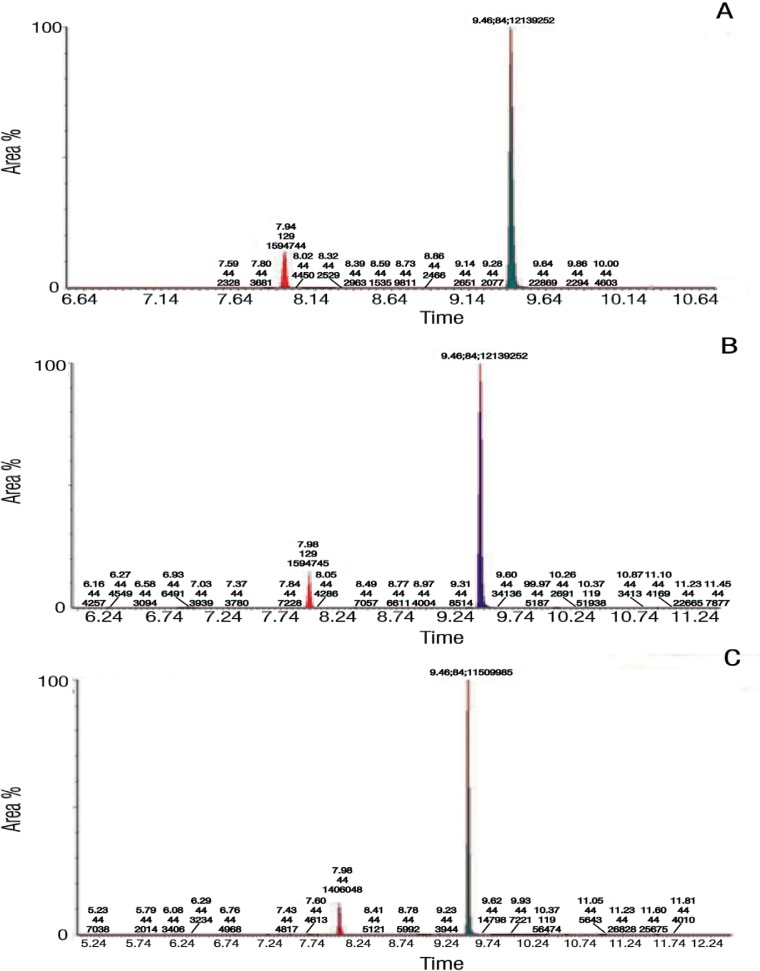
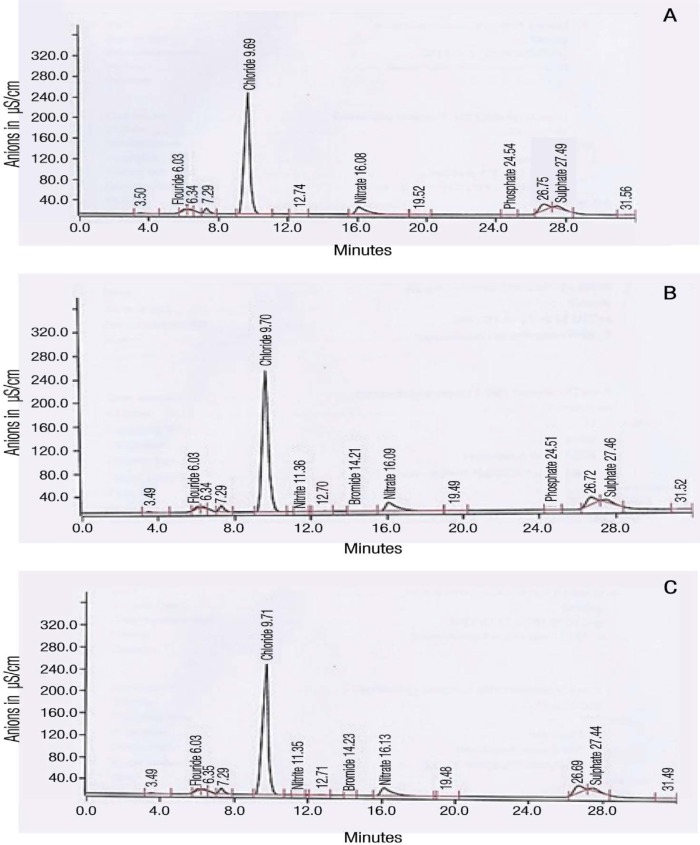
The minimum detection limit values of the samples revealed satisfactory sensitivity of the IC method. The calibration of the linearity (r value) of the tested anion standards ranged between 0.996–0.999. The chromatograms from both the GC-MS and IC analyses validated the methods, as evidenced by the consistency and repeatability of the results [Figures 1 and 2].
Figure 1A–C:
Gas chromatography-mass spectrometry graphs of the nicotine in the (A) first, (B) second and (C) third Afzal samples. The long peak represents nicotine while the short one represents quinoline (the internal standard). The numbers on the peaks represent the retention time. The spectra mass value is represented in the area under the peak.
Figure 2A–C:
Ion-exchange chromatograms of the anions in the (A) first, (B) second and (C) third Afzal samples. Each sample was injected twice into the instrument. There was a high chloride peak in all samples. Numbers on top of each anion peak represent the retention time.
µS/cm = microSiemens/cm.
The Afzal samples had a mean pH of 10.46 and moisture content of 2.60 g. The calculated moisture content percentage was 52.00%. With regards to nicotine, the mean total nicotine and unprotonated nicotine levels were 48,770.00 µg per g of STP (µg/g) and 48,590.00 µg/g, respectively. The percentage of unprotonated nicotine was 99.64%. The chromatograms of the Afzal nicotine analysis showed two peaks—a long peak representing nicotine and a shorter one representing the internal standard [Figure 1]. The ion-exchange chromatograms also showed a long peak for Cl− [Figure 2]. Table 1 shows the anion concentrations within the Afzal samples. Cl− concentrations were highest at 33,170.00 µg/g while NO2− was the lowest at 6.22 µg/g. The nitrate concentration (8,792.20 µg/g) was alarmingly high. The order of the ion concentrations from highest to lowest was as follows: Cl−, NO3−, SO42−, F−, Br−, PO42− and NO2−.
Table 1:
Anion concentrations in Afzal samples determined by ion-exchange chromatography
| Afzal sample* | Anion levels in µg/g | ||||||
|---|---|---|---|---|---|---|---|
| Fluoride | Chloride | Nitrite | Bromide | Nitrate | Phosphate | Sulphate | |
| 1A | 16.73 | 1,639.21 | 0.29 | 4.49 | 432.49 | 1.98 | 73.23 |
| 1B | 16.67 | 1,639.71 | ND | ND | 432.91 | 1.92 | 74.59 |
| 2A | 17.11 | 1,677.85 | 0.36 | 4.30 | 447.34 | 1.77 | 75.06 |
| 2B | 17.05 | 1,678.62 | 0.32 | 4.30 | 446.46 | ND | 76.59 |
| 3A | 17.05 | 1,656.96 | 0.30 | 1.91 | 438.56 | ND | 76.05 |
| 3B | 17.06 | 1,658.87 | 0.30 | 1.90 | 439.90 | ND | 75.12 |
| Mean concentration ± SD | 16.95 ± 0.19 | 1,658.54 ± 17.36 | 0.31 ± 0.03 | 3.38 ± 1.35 | 439.61 ± 6.38 | 1.89 ± 0.11 | 75.11 ± 1.17 |
| Concentration | 339.00 | 33,170.80 | 6.22 | 67.60 | 8,792.20 | 37.80 | 1,502.20 |
| Instrument detection limit in ppm | 0.02 | 0.02 | 0.03 | 0.02 | 0.02 | 0.03 | 0.03 |
ND = not detected; SD = standard deviation; ppm = parts per million.
Three Afzal samples were tested, of which each was injected twice into the ion-exchange chromatography instrument (A and B).
Discussion
In the current study, samples of Afzal were found to have an alkaline pH; this is known to encourage the formation of TSNAs, which are potent carcinogens.6 An alkaline pH environment also plays an important role in causing nicotine to become more absorbable, thus increasing its addictiveness.8 The elevated alkaline pH of Afzal noted in the current study may therefore increase health risks for users, including the possibility of heightened addiction rates and carcinogenicity. Substances such as slaked lime are often added to the tobacco blend during the curing process, thereby increasing the alkaline pH.7 The Afzal samples in this study had a higher pH value (pH 10.46) than the value described in the quality standards used by GOTHIATEK® (Swedish Match, Stockholm, Sweden) for snus (pH 8.50), a moist powder tobacco which is highly regulated and monitored by the Swedish National Food Act.10 It has been shown that the pH value of snus is affected by its storage temperature.10 Similarly, a pH range of 9.2–10.0 has been reported for other STPs including khaini and gul from India, toombak from Sudan and snuff products from South Africa.15 However, traditional Alaskan Iq’mik appears to have the highest alkalinity (pH 11.00).18 Bearing in mind the pKa of nicotine, any increase in pH levels would favour transformation of the nicotine to its unprotonated form.
Moisture content is another major factor enhancing the permeability of both nicotine and TSNAs into the mucosal membrane of the mouth, since both are water-soluble.7,19 Higher nicotine levels are found in moist snuff in comparison to the dry form.1 Additionally, it has been found that American moist snuff contains higher levels of NO2− and Cl− than the dry type.2 Thus, the observed moisture content of Afzal in the current study (52.00%), which was similar to that of the American moist snuff (51.00%), can be expected to contain similarly elevated nicotine, NO2− and Cl− levels. The moisture content of the Afzal samples in the current study was also higher than that reported for the Indian tobacco Bidi (10.26%), but within the range of snus (45.00–60.00%).2,10,20
Nicotine content in STPs is considered low at ≤7,500.00 µg/g, moderate between 10,300.00– 11,400.00 µg/g and high at >11,400.00 µg/g.21 This is worth noting since, besides addiction, nicotine may cause other physiological effects such as an increase in pulse rate, blood pressure and plasma free fatty acids and the mobilisation of blood sugar and catecholamines in the blood.22,23 A high nicotine content has been found in toombak (40,600.00 µg/g) and Iq’mik (42,700.00 µg/g).18,24 However, the nicotine content of Afzal in the current study exceeded these values (48,770.00 µg/g); as evidenced by Henningfield et al., nearly all of its nicotine content would have been in the unprotonated form because of its high pH level.21 As is the case with snus, STPs with higher unprotonated nicotine content hold the highest market share.25
All of the anions analysed in this study were below the recommended daily intake limits determined by international health organisations (F− = 33.30 µg/kg/day; Cl− = 9,000.00 µg/kg/day; NO2− = 60.00 µg/kg/day; Br− = 400.00 µg/kg/day; NO3− = 370.00 µg/kg/day; PO42− = 13,330.00 µg/kg/day; and SO42− = 8,330.00 µg/kg/day).26–32 Although anions are vital to the human body, they can be toxic at excessive levels. Sodium chloride is often added to tobacco blends to improve the flavour of the product and act as a preservative.2,10 However, high quantities of this compound may contribute to several disease mechanisms, including chronic inflammation, tumour promotion and cocarcinogenesis (mutations in the gastric epithelium).33 Although the Cl− content of Afzal in the current study showed a high peak compared to other anions, it was below the recommended daily uptake.27 In the USA, STPs were found to contain higher Cl− levels in the moist form (95,000.00 µg/g) than the dry form (23,100.00 µg/g); this finding was similar to the levels in moist snus sold in the USA at the same time.2 The reported Cl− content in unused snus (35,300.00 µg/g) was close to that of Afzal as observed in the current study (33,170.80 µg/g).19 Quantities of Swedish snus are well monitored as they are typically sold in 1.00 g tea bag-sized sachets;19 in contrast, Afzal users usually self-determine doses (typically a pinch-sized amount per single dose ranging from 1.00–2.00 g) which are then repeated as desired.13 As a result, Cl- consumption will increase due to repeated Afzal use during the day.16 Few studies have investigated the other anions tested in this study (including F−, Br−, SO42− and PO42−). Stepanov et al. analysed SO42− (4,560.00–12,300.00 µg/g) and PO42− (309.00–1,300.00 µg/g) levels in American STPs; their findings were higher than anion levels indicated in the current study (1,502.20 µg/g and 37.80 µg/g, respectively).4
NO3− is an endogenous component of tobacco which originates from the presence of inorganic fertilisers in the soil.34 In saliva, it is converted to NO2− (the more toxic form) by the oral microflora and NO3− reductase, leading to various toxic products such as methaemoglobin, which reduces oxygen transport and can lead to cyanosis.35,36 In addition, the formed NO2− promotes the conversion into nitrosating agents which in turn facilitate the formation of endogenous TSNAs from tobacco alkaloids and dietary amines.4 Besides the oral reduction of NO3− to NO2−, there is a natural endogenous formation of NO3− processes in other areas of the gastrointestinal tract.12 The NO3− content reported in unused snus (1,220.00 µg/g) was much lower than that of the Afzal samples in the current study (8,792.20 µg/g).19 Borgerding et al. reported that the NO2− value of American STPs was >20.00 μg/g, although Hoffmann et al. have reported that values range much higher in moist snuff.2,37 Stepanov et al. reported that newer STPs generally contain <10 μg/g of NO2−.4 However, the NO2− content of Afzal found in the current study (6.22 µg/g) was below all of these levels, including the GOTHIATEK® limits for snus (7.00 µg/g).38 Nevertheless, Afzal use is potentially harmful due its elevated NO3− content which can be reduced in the body to NO2− and subsequently to carcinogens (in the form of TSNAs). Critically, elevated NO3− levels have been found to result in an increased risk of gastric cancer.39 Many tobacco manufacturing companies are now attempting to produce STPs with low NO3− and NO2− levels in order to reduce their toxicity and limit TSNA formation during tobacco processing.4
Conclusion
Afzal was analysed chemically for toxicants that enhance cancer formation. There was a surge in both nicotine and Cl− content, with alarmingly elevated NO3− levels. The levels of certain factors, including pH value, nicotine content and NO3−, were above those reported for other international STPs, while the levels of Cl− and moisture content were consistent and the levels of NO2−, SO42− and PO42− were below those found in other STPs. All tested anions were below the maximum recommended daily intake advised by international organisations. However, the frequent use of Afzal poses a health risk to users which may potentially manifest as cancer.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
References
- 1.World Health Organization International Agency for Research on Cancer IARC monographs on the evaluation of carcinogenic risks to humans: Volume 89 - Smokeless tobacco and some tobacco-specific N-nitrosamines. From: www.monographs.iarc.fr/ENG/Monographs/vol89/mono89.pdf Accessed: Aug 2015. [PMC free article] [PubMed]
- 2.Borgerding MF, Bodnar JA, Curtin GM, Swauger JE. The chemical composition of smokeless tobacco: A survey of products sold in the United States in 2006 and 2007. Regul Toxicol Pharmacol. 2012;64:367–87. doi: 10.1016/j.yrtph.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 3.National Cancer Institute Smoking and tobacco control monographs: Monograph 2 - Smokeless tobacco or health: An international perspective. From: www.cancercontrol.cancer.gov/brp/tcrb/monographs/2/index.html Accessed: Aug 2015.
- 4.Stepanov I, Jensen J, Hatsukami D, Hecht SS. New and traditional smokeless tobacco: Comparison of toxicant and carcinogen levels. Nicotine Tob Res. 2008;10:1773–82. doi: 10.1080/14622200802443544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rickert WS, Joza PJ, Trivedi AH, Momin RA, Wagstaff WG, Lauterbach JH. Chemical and toxicological characterization of commercial smokeless tobacco products available on the Canadian market. Regul Toxicol Pharmacol. 2009;53:121–33. doi: 10.1016/j.yrtph.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Zakiullah MS, Saeed M, Muhammad N, Khan SA, Gul F, Khuda F, et al. Assessment of potential toxicity of a smokeless tobacco product (naswar) available on the Pakistani market. Tob Control. 2012;21:396–401. doi: 10.1136/tc.2010.042630. [DOI] [PubMed] [Google Scholar]
- 7.Prabhakar V, Jayakrishnan G, Nair SV, Ranganathan B. Determination of trace metals, moisture, pH and assessment of potential toxicity of selected smokeless tobacco products. Indian J Pharm Sci. 2013;75:262–9. doi: 10.4103/0250-474X.117398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lauterbach JH, Bao M, Joza PJ, Rickert WS. Free-base nicotine in tobacco products: Part II - Determination of free-base nicotine in the aqueous extracts of smokeless tobacco products and the relevance of these findings to product design parameters. Regul Toxicol Pharm. 2011;59:8–18. doi: 10.1016/j.yrtph.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Tomar SL, Henningfield JE. Review of the evidence that pH is a determinant of nicotine dosage from oral use of smokeless tobacco. Tob Control. 1997;6:219–25. doi: 10.1136/tc.6.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rutqvist LE, Curvall M, Hassler T, Ringberger T, Wahlberg I. Swedish snus and the GothiaTek® standard. Harm Reduct J. 2011;8:11. doi: 10.1186/1477-7517-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ammigan N, Nagabhushan M, Nair UJ, Amonkar AJ, Bhide SV. Effect of nutritional status on mutagenicity of urine excreted by rats treated with standard/experimental carcinogens. Indian J Exp Biol. 1990;28:711–13. [PubMed] [Google Scholar]
- 12.Hsu J, Arcot J, Lee NA. Nitrate and nitrite quantification from cured meat and vegetables and their estimated dietary intake in Australians. Food Chem. 2009;115:334–9. doi: 10.1016/j.foodchem.2008.11.081. [DOI] [Google Scholar]
- 13.Al-Mukhaini N, Ba-Omar T, Eltayeb E, Al-Shehi A. Determination of heavy metals in the common smokeless tobacco Afzal in Oman. Sultan Qaboos Univ Med J. 2014;14:e349–55. [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention Notice regarding requirement for annual submission of the quantity of nicotine contained in the smokeless tobacco products manufactured, imported, or packaged in the United States. Fed Regist. 1999;64:14086–96. [PubMed] [Google Scholar]
- 15.Stanfill SB, Connolly GN, Zhang L, Jia LT, Henningfield JE, Richter P, et al. Global surveillance of oral tobacco products: Total nicotine, unionised nicotine and tobacco-specific N-nitrosamines. Tob Control. 2011;20:e2. doi: 10.1136/tc.2010.037465. [DOI] [PubMed] [Google Scholar]
- 16.Lawler TS, Stanfill SB, Zhang L, Ashley DL, Watson CH. Chemical characterization of domestic oral tobacco products: Total nicotine, pH, unprotonated nicotine and tobacco-specific N-nitrosamines. Food Chem Toxicol. 2013;57:380–6. doi: 10.1016/j.fct.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention Laboratory protocol to measure the quantity of nicotine contained in smokeless tobacco products manufactured, imported, or packaged in the United States. Federal Register. 2009;62:24116–19. [PubMed] [Google Scholar]
- 18.Hearn BA, Renner CC, Ding YS, Vaughan-Watson C, Stanfill SB, Zhang L, et al. Chemical analysis of Alaskan Iq’mik smokeless tobacco. Nicotine Tob Res. 2013;15:1283–8. doi: 10.1093/ntr/nts270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Digard H, Gale N, Errington G, Peters N, McAdam K. Multi-analyte approach for determining the extraction of tobacco constituents from pouched snus by consumers during use. Chem Cent J. 2013;7:55. doi: 10.1186/1752-153X-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhisey RA. Chemistry and toxicology of smokeless tobacco. Indian J Cancer. 2012;49:364–72. doi: 10.4103/0019-509X.107735. [DOI] [PubMed] [Google Scholar]
- 21.Henningfield JE, Radzius A, Cone EJ. Estimation of available nicotine content of six smokeless tobacco products. Tob Control. 1995;4:57–61. doi: 10.1136/tc.4.1.57. [DOI] [Google Scholar]
- 22.Dani JA, Heinemann S. Molecular and cellular aspects of nicotine abuse. Neuron. 1996;16:905–8. doi: 10.1016/S0896-6273(00)80112-9. [DOI] [PubMed] [Google Scholar]
- 23.Benowitz NL. Nicotine and smokeless tobacco. CA Cancer J Clin. 1988;38:244–7. doi: 10.3322/canjclin.38.4.244. [DOI] [PubMed] [Google Scholar]
- 24.Idris AM, Ibrahim SO, Vasstrand EN, Johannessen AC, Lillehaug JR, Magnusson B, et al. The Swedish snus and the Sudanese toombak: Are they different? Oral Oncol. 1998;34:558–66. doi: 10.1016/S1368-8375(98)00047-5. [DOI] [PubMed] [Google Scholar]
- 25.Wallström M. Harm and harm reduction in smokeless tobacco users. An in vitro and clinical study. From: gupea.ub.gu.se/handle/2077/23719 Accessed: Aug 2015.
- 26.World Health Organization Environmental health criteria 36: Fluorine and fluorides. From: www.inchem.org/documents/ehc/ehc/ehc36.htm Accessed: Aug 2015.
- 27.World Health Organization Chloride in drinking-water: Background document for development of WHO guidelines for drinking-water quality. From: www.who.int/water_sanitation_health/dwq/chloride.pdf Accessed: Aug 2015.
- 28.European Commission Reports of the Scientific Committee for Food (thirty-eighth series): Opinions of the Scientific Committee for Food on - Nitrates and nitrite. From: ec.europa.eu/food/safety/docs/labelling_nutrition-special_groups_food-children-scf_reports_38_en.pdf Accessed: Aug 2015.
- 29.Reinik M, Tamme T, Roasto M, Juhkam K, Jurtsenko S, Tenńo T, et al. Nitrites, nitrates and N-nitrosoamines in Estonian cured meat products: Intake by Estonian children and adolescents. Food Addit Contam. 2005;22:1098–105. doi: 10.1080/02652030500241827. [DOI] [PubMed] [Google Scholar]
- 30.Food and Agriculture Organization of the United Nations and World Health Organization Bromide ion. From: www.inchem.org/documents/jmpr/jmpmono/v88pr03.htm Accessed: Aug 2015.
- 31.National Research Council . Recommended Dietary Allowances. 10th ed. Washington, USA: National Academy Press; 1989. p. 186. [Google Scholar]
- 32.World Health Organization Sulfate in drinking water: Background document for development of WHO guidelines for drinking-water quality. From: www.who.int/water_sanitation_health/dwq/chemicals/sulfate.pdf Accessed: Aug 2015.
- 33.Takahashi M, Nishikawa A, Furukawa F, Enami T, Hasegawa T, Hayashi Y. Dose-dependent promoting effects of sodium chloride (NaCI) on rat glandular stomach carcinogenesis initiated with N-methyl-N′-nitro-N-nitrosoguanidine. Carcinogenesis. 1994;15:1429–32. doi: 10.1093/carcin/15.7.1429. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization Nitrate and nitrite in drinking water: Background document for development of WHO guidelines for drinking-water wuality. From: www.who.int/water_sanitation_health/dwq/chemicals/nitratenitrite2ndadd.pdf Accessed: Aug 2015.
- 35.Gangolli SD, van den Brandt P, Feron VJ, Janzowsky C, Koeman JH, Speijers GJ, et al. Nitrate, nitrite and N-nitroso compounds. Eur J Pharmacol. 1994;292:1–38. doi: 10.1016/0926-6917(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 36.Jaffé ER. Methaemoglobinaemia in the differential diagnosis of cyanosis. Hosp Pract (Off Ed) 1985;20:92–6. doi: 10.1080/21548331.1985.11703207. [DOI] [PubMed] [Google Scholar]
- 37.Hoffmann D, Djordjevic MV, Fan J, Zang E, Glynn T, Connolly GN. Five leading U.S. commercial brands of moist snuff in 1994: Assessment of carcinogenic N-nitrosamines. J Natl Cancer Inst. 1995;87:1862–9. doi: 10.1093/jnci/87.24.1862. [DOI] [PubMed] [Google Scholar]
- 38.Swedish Match GOTHIATEK® standard: GOTHIATEK® limits for undesired components. From: www.swedishmatch.com/en/Snus-and-health/GOTHIATEK/GOTHIATEK-standard/ Accessed: Aug 2015.
- 39.Joossens JV, Hill MJ, Elliott P, Stamler R, Lesaffre E, Dyer A, et al. Dietary salt, nitrate and stomach cancer mortality in 24 countries: Euopean Cancer Prevention (ECP) and the INTERSALT Cooperative Research Group. Int J Epidemiol. 1996;25:494–504. doi: 10.1093/ije/25.3.494. [DOI] [PubMed] [Google Scholar]