Abstract
Objective:
Migraine, particularly with aura, is a risk factor for ischemic stroke. Recent data in migraine mutant mice suggest that cerebral hyperexcitability associated with migraine accelerates recruitment of ischemic penumbra into the core, resulting in faster infarct growth compared with wild type. We hypothesized that individuals with a history of migraine are more likely to exhibit increased recruitment of ischemic tissue into the infarct in acute stroke.
Methods:
In this retrospective case-control study, we identified participants with reliably documented migraine history, measured lesion volumes on diffusion-weighted and perfusion-weighted MRI obtained within 72 hours of symptom onset, calculated the proportion of ischemic tissue on perfusion-weighted imaging (PWI) hyperintense on diffusion-weighted imaging (DWI), and compared the proportion of patients with no-mismatch pattern defined as DWI lesion >83% of PWI lesion.
Results:
Migraineurs (n = 45) were younger, more often female, less likely to have vascular risk factors, and more often had cervical artery dissection, but otherwise did not differ from controls (n = 27). A significantly larger proportion of migraineurs had no-mismatch pattern, indicating that the entire perfusion defect was recruited into the infarct by the time of MRI (22% vs 4% of migraineurs and controls, respectively; p = 0.044). The difference was even more prominent in migraineurs with aura (36% vs 4%, p = 0.019). The association between migraine and no-mismatch pattern persisted after adjustment for time to MRI (p = 0.041).
Conclusions:
This case-control study supports the hypothesis that a history of migraine, particularly with aura, is associated with a no-mismatch pattern during acute ischemic stroke, consistent with data obtained in migraine mutant mice.
Recent experimental data suggest that genetic susceptibility to migraine aura may be a factor rendering the brain tissue more sensitive to ischemia.1 Transgenic mice expressing familial hemiplegic migraine type 1 (FHM1) mutations, frequently used as animal models for migraine, develop accelerated infarct growth because of faster anoxic depolarization and more frequent peri-infarct spreading depressions during acute focal cerebral ischemia.1 Mutant animals require higher blood flow to survive focal ischemia, and develop larger infarcts and worse neurologic outcomes compared with wild type. In addition, larger infarcts were observed in migraineurs with aura in a recent preliminary study.2 We, therefore, sought to determine whether patients with acute ischemic stroke and a history of migraine develop faster infarct growth as a reflection of increased tissue vulnerability to ischemia.
METHODS
Study population.
We retrospectively studied consecutive patients (age >18 years) with imaging-confirmed acute ischemic stroke admitted within 72 hours of symptom onset at a single tertiary care hospital (Massachusetts General Hospital) between 2003 and 2014, with approval from the Institutional Review Board. A priori inclusion criteria were (1) diffusion- and perfusion-weighted MRI within 72 hours of symptom onset and (2) patients whose records included a physician note that explicitly stated a history of migraine (with or without aura, or unspecified) and controls whose records explicitly stated that the participant had no history of migraine. Cases could not be matched with controls for age and sex because of the need to have migraine status documented in the chart.
Image analysis.
Experienced neurologists blinded to case-control assignments manually outlined regions that were hyperintense on diffusion-weighted images (DWI) and hypointense on apparent diffusion coefficient (ADC) maps and hyperintense regions on mean transit time (MTT) maps using MRIcron software (University of Nottingham, UK). We determined the arterial territory involved using standard templates based on infarct distribution on DWI. We also recorded whether there was intracranial arterial occlusion on CT or magnetic resonance angiograms obtained at the same session with MRI. We considered an occlusion proximal when it was located within the first 3 segments of the intracranial arterial system.
Study endpoint.
We hypothesized that migraineurs would show faster recruitment of hypoperfused but viable tissue (i.e., DWI-MTT mismatch) into the infarct (DWI lesion) during acute ischemic stroke.3,4 We reasoned that by the time MRI was done a larger proportion of migraineurs compared to controls would develop a no-mismatch pattern. We defined no-mismatch as DWI lesion volume >83% of perfusion-weighted imaging (PWI) lesion volume.5,6 We excluded participants if DWI and PWI volumes were both less than 10 mL because of the poor reliability of volumetric analyses in this subset.7 None of the patients had MRI before a reperfusion intervention.
Statistical analysis.
We used χ2 or Fisher exact tests for groupwise comparisons among categorical variables and Mann-Whitney U test for comparisons among numerical variables. To assess the association between migraine status and tissue vulnerability to ischemia, we used a logistic regression model in which no-mismatch was the dependent variable and stroke features with a univariate p < 0.150 were the covariates. A p value of <0.05 was considered statistically significant. All analyses were performed using SPSS 16.0.
RESULTS
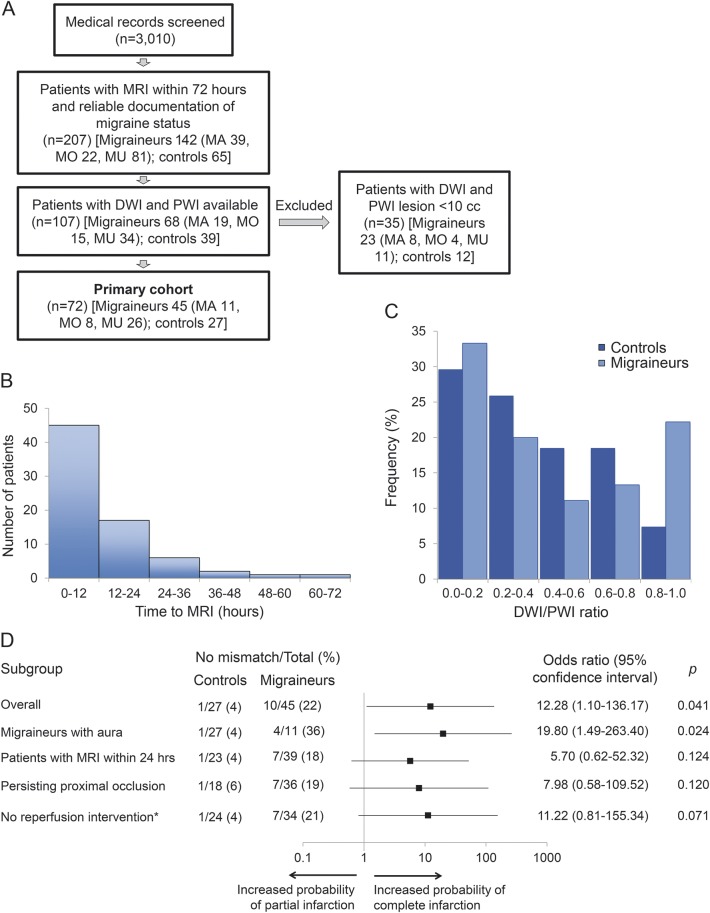
We identified 207 patients with reliable documentation of migraine status and an MRI within 72 hours of symptom onset confirming acute infarction (figure, A). Patients with a history of migraine (n = 142) were younger, predominantly female, more likely to have uncommon etiologies such as cervical artery dissection, and less likely to have multiple vascular risk factors as compared to those who did not have migraine (n = 65). All these associations were in the same direction in migraineurs with aura.
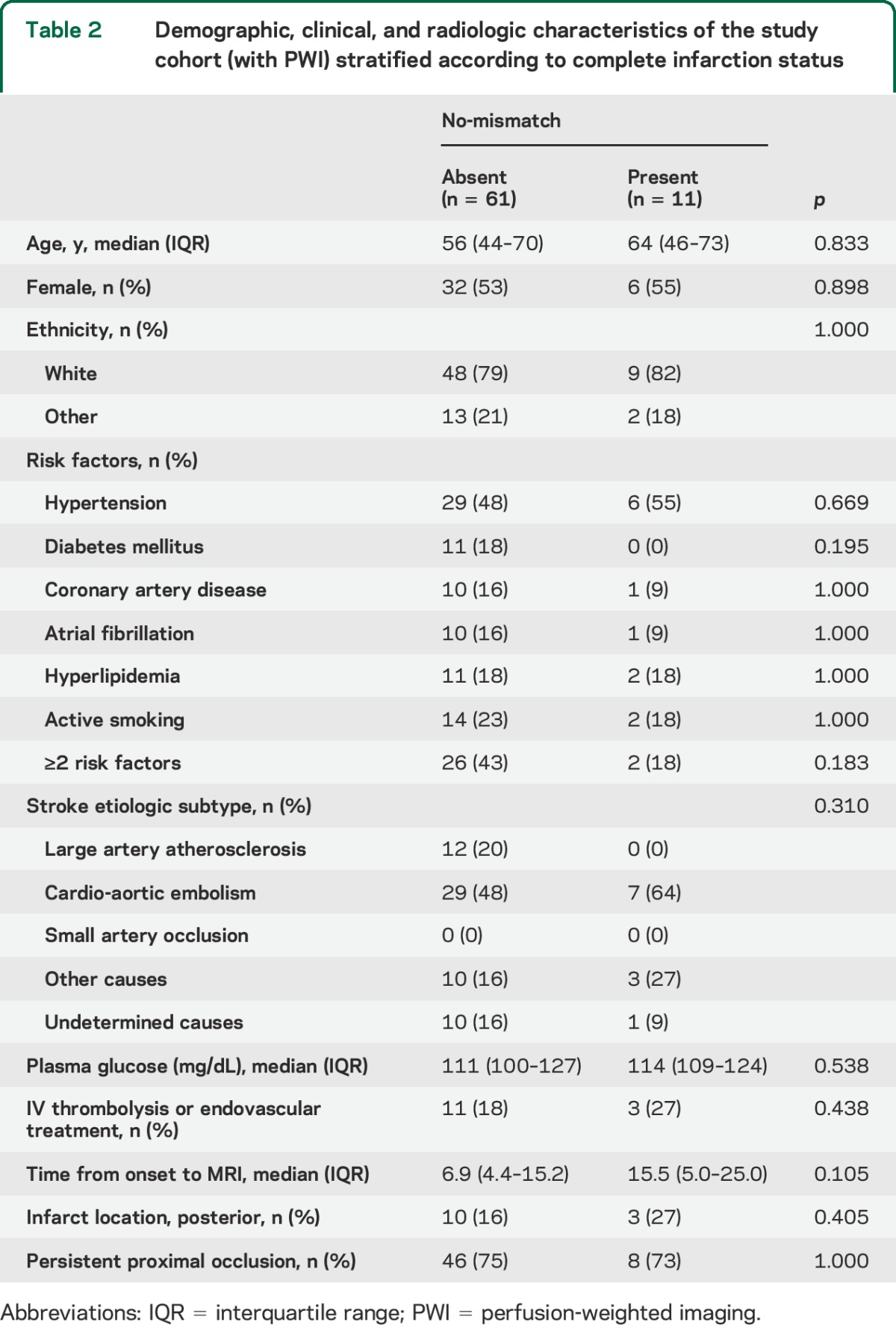
Figure. Study flowchart, time to MRI, and DWI/PWI distribution for the analysis of no-mismatch pattern.
(A) Study flowchart. MA = migraine with aura; MO = migraine without aura; MU = migraine of unknown type. (B) Histogram of time from symptom onset to MRI. (C) Frequency histogram for diffusion-weighted imaging (DWI)/perfusion-weighted imaging (PWI) ratio in controls and migraineurs showed a bimodal distribution in migraineurs. (D) Subgroup analyses for no-mismatch pattern adjusted for time to MRI. Horizontal bars represent odds ratio and 95% confidence interval. *Includes IV thrombolysis and endovascular treatments.
Of the 207 patients, 107 had both DWI and PWI. Thirty-five of these 107 patients had lesions <10 mL and were excluded from further analyses (figure, A). The remaining cohort of 45 migraineurs (11 with aura) and 27 control participants showed similar demographic, clinical, and radiologic findings noted in the excluded participants, except that the exclusion of small lesions eliminated lacunar infarcts from the etiologic subtypes (table 1). Median time from symptom onset to MRI was approximately 7 hours (figure, B).
Table 1.
Demographic, clinical, and radiologic characteristics of the study cohort (with PWI) stratified according to migraine history
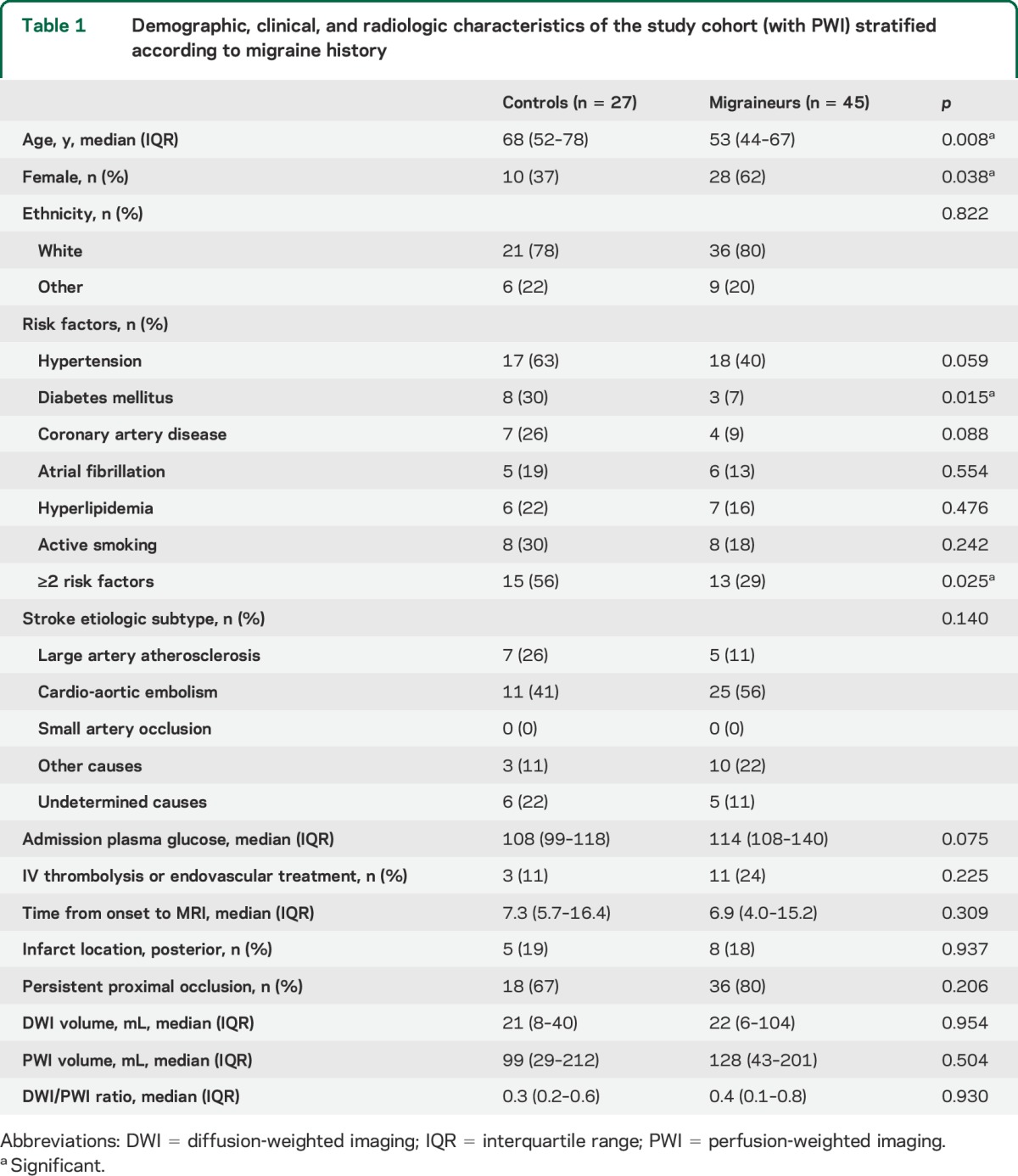
The frequency histogram of DWI/PWI ratios displayed a bimodal distribution in migraineurs (figure, C). In contrast, the proportion of nonmigraineurs continuously decreased as DWI/PWI ratio increased. A receiver operating characteristic curve analysis revealed that the optimal operating point for DWI/PWI ratio to discriminate migraineurs from nonmigraineurs was 0.82. There was a significantly higher proportion of migraineurs with no-mismatch compared with controls (22% vs 4%, respectively; p = 0.044). The relationship was even stronger in migraine patients with aura (36% vs 4%, respectively; p = 0.019; figure, D), but did not reach significance for migraine without aura. Among all covariates (table 2), only time from symptom onset to MRI showed a borderline association with no-mismatch (p = 0.105). The association between DWI/PWI ratio and migraine status was significant when adjusted for time to MRI (p = 0.041; figure, D). There was a trend towards a similar relationship between migraine and tissue outcome in more homogeneous subgroups, such as in patients with MRI obtained within 24 hours of stroke onset, patients with persistent proximal occlusion, or patients who did not receive reperfusion therapies (figure, D).
Table 2.
Demographic, clinical, and radiologic characteristics of the study cohort (with PWI) stratified according to complete infarction status
DISCUSSION
Our data suggest a novel link between migraine and human stroke by showing that a history of migraine, particularly with aura, may accelerate loss of viable tissue at risk for infarction during acute cerebral ischemia. Compared to controls, a significantly higher proportion of migraineurs displayed no-mismatch early after stroke onset (median 7 hours), a pattern suggestive of malignant progression in which penumbra is rapidly recruited into the ischemic core. The risk was particularly high in migraineurs with aura, which is in agreement with experimental findings in transgenic mouse models of enhanced genetic susceptibility to migraine aura.1 Importantly, the relationship observed between migraine and no-mismatch persisted when adjusted for time from symptom onset to imaging, a well-known determinant of the conversion of penumbra into definite infarction.
This study was subject to selection and documentation bias; a history of migraine is more likely to be inquired in young patients with no known risk factors. Migraine status is also more likely to be documented in medical records if it is present than absent. Nevertheless, the present migraine cohort was similar to previously reported larger stroke cohorts with migraine in age and sex, risk factor profile, presumed stroke etiology, and involved vascular territory, suggesting that retrospective design did not introduce an important bias towards selection of a particular risk population.8,9 It was not possible to retrieve data on migraine features (e.g., active vs remote history of migraine, attack frequency or severity, aura with each attack vs only occasional auras, aura without headache) that might influence the rate of progression to infarction. Small sample size and retrospective design also precluded reliable assessment of the effect of different modes of migraine prophylaxis. Future prospective studies will address these hypotheses.
Brain regions abnormal on MTT maps indicate both critically and noncritically hypoperfused tissue. More frequent no-mismatch based on the MTT criterion suggests that even moderately oligemic tissue is at risk of infarction in migraineurs, further supporting the notion of increased tissue sensitivity to ischemia. Although statistically significant, the association between tissue outcome and migraine status was not absolute; 78% of the patients with migraine did not exhibit a no-mismatch, suggesting that increased risk of no-mismatch originated from a subset of highly susceptible patients, perhaps those with a genetic predisposition to develop anoxic and spreading depolarizations. Importantly, although our a priori time to MRI cutoff was <72 hours of symptom onset, the vast majority of patients in our final cohort had times to MRI <36 hours. Indeed, secondary analyses using 48 hours time to MRI cutoff yielded the same conclusion (odds ratio 18.2 for no-mismatch in migraineurs with aura compared with controls; p = 0.025). Therefore, long inclusion times to MRI did not confound our conclusions in this hypothesis-generating study.
This study raises several important hypotheses10 to be tested in subsequent studies. First, our findings support a shorter therapeutic window for reperfusion in migraineurs due to rapid loss of viable and salvageable tissue at risk. Second, higher sensitivity to ischemia may require more stringent monitoring and management of stroke risk factors and possibly antithrombotic prophylaxis in migraineurs. Third, migraine prophylaxis may reduce tissue vulnerability to ischemic injury by suppressing the susceptibility to spreading depolarizations.11
Supplementary Material
GLOSSARY
- ADC
apparent diffusion coefficient
- DWI
diffusion-weighted imaging
- FHM1
familial hemiplegic migraine type 1
- MTT
mean transit time
- PWI
perfusion-weighted imaging
Footnotes
Editorial, page 1920
AUTHOR CONTRIBUTIONS
Jerome Mawet: drafting/revising the manuscript, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, acquisition of data, and statistical analysis. Katharina Eikermann-Haerter: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, acquisition of data, statistical analysis, and study supervision. Kwang-Yeol Park: analysis or interpretation of data, accepts responsibility for conduct of research and final approval, acquisition of data, and statistical analysis. Johanna Helenius: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, acquisition of data, and statistical analysis. Ali Daneshmand: analysis or interpretation of data, accepts responsibility for conduct of research and final approval, acquisition of data, and statistical analysis. Lea Pearlman: analysis or interpretation of data, accepts responsibility for conduct of research and final approval, and acquisition of data. Ross Avery: analysis or interpretation of data and accepts responsibility for conduct of research and final approval. Andrea Negro: analysis or interpretation of data, accepts responsibility for conduct of research and final approval, and acquisition of data. Murat Velioglu: analysis or interpretation of data and accepts responsibility for conduct of research and final approval. Ethem M. Arsava: drafting/revising the manuscript, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, acquisition of data, and statistical analysis. Hakan Ay: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, acquisition of data, and study supervision. Cenk Ayata: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and final approval, and study supervision.
STUDY FUNDING
Supported by the American Heart Association (10SDG2610275), the Claflin Distinguished Award from the Massachusetts General Hospital, NIH (NS061505, NS055104), Fondation Leducq, The Heitman Foundation, The Ellison Foundation, Institut Servier, Philippe Foundation, and Thérèse and René Planiol Foundation for The Study of The Brain.
DISCLOSURE
J. Mawet was funded by travel grants from Institut Servier, Philippe Foundation, Thérèse and René Planiol Foundation for The Study of The Brain, and Association CADASIL; received travel, accommodation, and meeting expenses from Linde and Bayer; and received travel, accommodation, and meeting expenses for lectures or educational activities not funded by industry. K. Eikermann-Haerter, K. Park, J. Helenius, A. Daneshmand, L. Pearlman, R. Avery, A. Negro, M. Velioglu, E. Arsava, H. Ay, and C. Ayata report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Eikermann-Haerter K, Lee JH, Yuzawa I, et al. Migraine mutations increase stroke vulnerability by facilitating ischemic depolarizations. Circulation 2012;125:335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nahas SJ, Dave HN. Association of history of migraine with aura and larger infarct volume in acute stroke. Cephalalgia 2013;33(8 Suppl):P88. [Google Scholar]
- 3.Ay H, Koroshetz WJ, Vangel M, et al. Conversion of ischemic brain tissue into infarction increases with age. Stroke 2005;36:2632–2636. [DOI] [PubMed] [Google Scholar]
- 4.Gokcay F, Arsava EM, Baykaner T, et al. Age-dependent susceptibility to infarct growth in women. Stroke 2011;42:947–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albers GW, Thijs VN, Wechsler L, et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution (DEFUSE) study. Ann Neurol 2006;60:508–517. [DOI] [PubMed] [Google Scholar]
- 6.Davis SM, Donnan GA, Parsons MW, et al. Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo-controlled randomised trial. Lancet Neurol 2008;7:299–309. [DOI] [PubMed] [Google Scholar]
- 7.Ay H, Arsava EM, Vangel M, et al. Interexaminer difference in infarct volume measurements on MRI: a source of variance in stroke research. Stroke 2008;39:1171–1176. [DOI] [PubMed] [Google Scholar]
- 8.Schurks M, Rist PM, Bigal ME, Buring JE, Lipton RB, Kurth T. Migraine and cardiovascular disease: systematic review and meta-analysis. BMJ 2009;339:b3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rist PM, Diener HC, Kurth T, Schurks M. Migraine, migraine aura, and cervical artery dissection: a systematic review and meta-analysis. Cephalalgia 2011;31:886–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mawet J, Kurth T, Ayata C. Migraine and stroke: in search of shared mechanisms. Cephalalgia 2015;35:165–181. [DOI] [PubMed] [Google Scholar]
- 11.Eikermann-Haerter K, Lee JH, Yalcin N, et al. Migraine prophylaxis, ischemic depolarizations, and stroke outcomes in mice. Stroke 2015;46:229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.