Abstract
BACKGROUND
Miscarriage is the spontaneous loss of a pregnancy before 12 weeks (early miscarriage) or from 12 to 24 weeks (late miscarriage) of gestation. Miscarriage occurs in one in five pregnancies and can have considerable physiological and psychological implications for the patient. It is also associated with significant health care costs. There is evidence that potentially preventable infections may account for up to 15% of early miscarriages and up to 66% of late miscarriages. However, the provision of associated screening and management algorithms is inconsistent for newly pregnant women. Here, we review recent population-based studies on infections that have been shown to be associated with miscarriage.
METHODS
Our aim was to examine where the current scientific focus lies with regards to the role of infection in miscarriage. Papers dating from June 2009 with key words ‘miscarriage’ and ‘infection’ or ‘infections’ were identified in PubMed (292 and 327 papers, respectively, on 2 June 2014). Relevant human studies (meta-analyses, case–control studies, cohort studies or case series) were included. Single case reports were excluded. The studies were scored based on the Newcastle – Ottawa Quality Assessment Scale.
RESULTS
The association of systemic infections with malaria, brucellosis, cytomegalovirus and human immunodeficiency virus, dengue fever, influenza virus and of vaginal infection with bacterial vaginosis, with increased risk of miscarriage has been demonstrated. Q fever, adeno-associated virus, Bocavirus, Hepatitis C and Mycoplasma genitalium infections do not appear to affect pregnancy outcome. The effects of Chlamydia trachomatis, Toxoplasma gondii, human papillomavirus, herpes simplex virus, parvovirus B19, Hepatitis B and polyomavirus BK infections remain controversial, as some studies indicate increased miscarriage risk and others show no increased risk. The latest data on rubella and syphilis indicate increased antenatal screening worldwide and a decrease in the frequency of their reported associations with pregnancy failure. Though various pathogens have been associated with miscarriage, the mechanism(s) of infection-induced miscarriage are not yet fully elucidated.
CONCLUSIONS
Further research is required to clarify whether certain infections do increase miscarriage risk and whether screening of newly pregnant women for treatable infections would improve reproductive outcomes.
Keywords: miscarriage, infection, female tract, pregnancy
Introduction
Miscarriage is one of the most common yet under-studied adverse pregnancy outcomes. In the majority of cases the effects of a miscarriage on women's health are not serious and may be unreported. However in the most serious cases symptoms can include pain, bleeding and a risk of haemorrhage. Feelings of loss and grief are also common and the psychology and mental health of those affected can suffer (Engelhard et al., 2001).
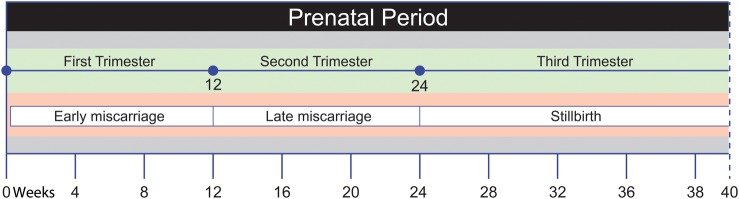
For the purposes of this review ‘miscarriage’ is defined as the spontaneous loss of a pregnancy during the first 24 weeks of gestation (Fig. 1). For most women, a miscarriage is an individual event and will be followed by a successful pregnancy (‘spontaneous miscarriage’, termed ‘miscarriage’ from this point onwards). A small number (0.5–1%) of women wishing to have children may experience three or more successive miscarriages, a condition known as ‘recurrent miscarriage’ (Bulletti et al., 1996). ‘Early miscarriage’ is defined as pregnancy loss during the first trimester of pregnancy (less than 12 weeks of gestation) and occurs in up to one in five pregnancies. ‘Late miscarriage’ occurs during the second trimester (12–24 weeks of gestation) and is less common, occurring in 1–2% of pregnancies (Hay, 2004). Fetal death from the 25th week of gestation onwards is defined as stillbirth, an outcome taken into consideration in some of the studies included here, however it is not the main focus of this review.
Figure 1.
Adverse pregnancy outcomes across the three trimesters of pregnancy.
Although miscarriage is considered the most common adverse pregnancy outcome, worldwide figures are not available. In 2012–2013 there were 729 674 live births recorded in England and Wales (Office for National Statistics, 2012). Loss of one in five pregnancies suggests that this figure is accompanied by ∼200 000 miscarriages. Statistics from England and Wales for 2012/13 report that 39 800 miscarriages resulted in a hospital stay (Office for National Statistics, 2012). In an Australian prospective cohort including 14 247 women aged 18–23 years, the rate of miscarriage varied from 11.3 to 86.5 per 100 live births amongst different groups; overall, miscarriage occurred in 25% of the women in the study when the women were 31–36 years old (Hure et al., 2012).
Aetiology of miscarriage
The causes of miscarriage are often unknown. However, in ∼50% of early miscarriages the fetus exhibits chromosomal aberrations such as a structural alteration or abnormal chromosomal numbers (Eiben et al., 1990; Suzumori and Sugiura-Ogasawara, 2010). Several other factors have been associated with increased risk of miscarriage. The age of both parents has a significant role as the risk of an adverse pregnancy outcome is increased if the parents are 35 years old or older and it is 50% higher if the mother is 42 years of age (Fretts et al., 1995; Nybo Andersen et al., 2000; de la Rochebrochard and Thonneau, 2002; Slama et al., 2005; Maconochie et al., 2007). In addition, factors such as ethnic origin, psychological state of the mother, very low or very high pre-pregnancy BMI, feelings of stress, use of non-steroidal anti-inflammatory drugs, smoking and alcohol consumption have also been associated with significantly higher rates of miscarriage (Coste et al., 1991; Nielsen et al., 2001; Sopori, 2002; Lashen et al., 2004; Maconochie et al., 2007). Moreover, it has been reported that women whose first pregnancy resulted in miscarriage are at a higher risk of the second pregnancy resulting in miscarriage compared with women who had a live birth (Kashanian et al., 2006). Finally, a number of infections have been linked to miscarriage (Benedetto et al., 2004) and to other adverse outcomes, such as stillbirth (Goldenberg and Thompson, 2003) and preterm delivery (Garland et al., 2002). Specifically, 15% of early miscarriages and 66% of late miscarriages have been attributed to infections (Srinivas et al., 2006; Baud et al., 2008). In a recent study, 78% of 101 tissue samples from miscarriage were infected with bacteria (chorioamnionitis), whereas all the control samples from medically induced abortions were uninfected (Allanson et al., 2010).
Methods
The aim of this review is to summarize present knowledge regarding the role of infection in miscarriage. In order to combine the most recent findings regarding infection and a potential association with miscarriage, we focused on studies published in the past 5 years. Our aim was to investigate current evidence regarding high-risk pathogens and scientific research trends. In PubMed, using the key words ‘miscarriage’ combined with ‘infection’ and ‘infections’, with ‘human’, ‘English language’ and ‘2009-present’ filters, articles published in the past 5 years were identified. The search returned a total of 292 and 327 papers for ‘miscarriage infection’ and ‘miscarriage infections’ respectively (up to 02/06/2014). From these, single case reports and studies in animals were excluded. A total of 44 studies investigated the association of different pathogens with miscarriage and the findings are presented in this review. The studies were also scored by two individuals independently based on the Newcastle - Ottawa Quality Assessment Scale for case control studies. The score of random studies was further evaluated by two more individuals.
Results
Infections associated with miscarriage
An overview of all the studies analysed is presented in Supplementary Table SI, including pathogen(s) investigated, outcome of the study and an estimation on the strength of each study, as described in Methods. Some of the most common caveats addressed in this review were variation in sample size and detection techniques, whether multivariate analysis was implemented or not and variation in study design.
Bacterial infections
Bacterial vaginosis
In healthy women, the normal genital tract flora consists for the most part of Lactobacillus species bacteria (Lamont et al., 2011). Other potentially virulent organisms, such as Gardnerella vaginalis, group B streptococci, Staphylococcus aureus, Ureaplasma urealyticum (U. urealyticum) or Mycoplasma hominis (M. hominis) occasionally displace lactobacilli as the predominant organisms in the vagina, a condition known as bacterial vaginosis (BV) (Eschenbach, 1993; Casari et al., 2010). BV is present in 24–25% of women of reproductive age (Ralph et al., 1999; Wilson et al., 2002) and causes a rise in the vaginal pH from the normal value of 3.8–4.2 up to 7.0. It is usually asymptomatic but may result in a vaginal discharge, which can be grey in colour with a characteristic ‘fishy’ odour. BV is diagnosed using microscopic examination of vaginal swab samples for ‘clue cells’ and/or Nugent criteria and is commonly treated with antibiotics, such as metronidazole (Donders et al., 2014). Change of sexual partner, a recent pregnancy, use of an intrauterine contraceptive device and antibiotic treatment have been identified as plausible causes of BV (Hay, 2004; Smart, 2004). BV has been associated with premature delivery (Hay et al., 1994) and with miscarriage (Donders et al., 2009; Rocchetti et al., 2011; Tavo, 2013).
In a retrospective study from Albania, U. urealyticum and M. hominis were present in 54.3 and 30.4% of the patients (150 hospitalized women, presenting with infertility, who had had a miscarriage or medically induced abortion, Tavo, 2013). The prevalence of both pathogens was significantly higher among women with a history of miscarriage (U. urealyticum: P = 0.04 and M. hominis: P = 0.02) and women who reported more than one miscarriage (P = 0.02 for both pathogens). This study however has some weaknesses, as it is not clear whether the comparisons made were with non-infected women with a miscarriage history or non-infected women with no miscarriage history and the method by which prevalence of microbes was tested is not specified.
Data on the prevalence of group B streptococci and pregnancy outcome in 405 Brazilian women with gestational age between 35 and 37 weeks was published in 2011 (Rocchetti et al., 2011). Overall, 25.4% of women were positive for Streptococcus agalactiae and infection was associated, among other factors, with a history of miscarriage (odds ratio (OR) 1.875; 95% confidence interval (CI) 1.038–3.387).
Association of BV and particularly M. hominis and U. urealyticum was reported from a study from Turkey (Bayraktar et al., 2010). In total 50 pregnant women with BV symptoms were tested for M. hominis and U. urealyticum and observed until end of pregnancy. The pregnancy outcomes of 50 asymptomatic pregnant women were used as controls. Miscarriage was reported in 12 symptomatic women, in 8 of which M. hominis and/or U. urealyticum infection was confirmed. However, the definition of miscarriage used in this study was ‘less’ than 36 weeks. Furthermore, comparative analysis between the two groups was not carried out.
Ureaplasma urealyticum was also detected in 25% of 101 gestational tissue samples (chorion, amnion, umbilical cord) from miscarriage cases that were otherwise normal. Second most common pathogens were M. hominis and group B streptococci at 11.1%, whereas all controls were not infected (Allanson et al., 2010).
In a further study using a cohort of 759 Belgian pregnant women following microbiological evaluation of vaginal flora, 8.4% of participants in the cohort presented with BV and were not treated (Donders et al., 2009). BV was positively correlated with miscarriage, as 2% of positive women miscarried before 25 weeks gestation; with an OR of 6.6 (OR 6.6; 95% CI 2.1–20.9). An absence of lactobacilli was also associated with miscarriage (less than 25 weeks; OR 4.9; 95% CI 1.4–16.9, Donders et al., 2009).
These studies indicate an association of BV with miscarriage. As BV is treatable, screening programmes for pregnant women can be used to prevent adverse pregnancy outcome. Current guidelines from the USA advise against screening asymptomatic pregnant women (U.S. Preventive Services, 2008). The same principle is applied in Canada (Yudin and Money, 2008) and the UK as of November 2014 (UK National Screening Committee, 2014). A recent Cochrane review, including 7847 women in 21 trials, found decreased risk of late miscarriage when antibiotic treatment was administered (relative risk (RR) 0.20; 95% CI 0.05–0.76; two trials, 1270 women, fixed-effect, I² = 0%). As the authors highlight, further studies are required to establish the effect of screening programmes to prevent adverse pregnancy outcomes (Brocklehurst et al., 2013).
Brucellosis
Bacteria of the genus Brucella can infect a variety of wild and domesticated mammals. Cattle and deer are susceptible to Brucella abortus (B. abortus) whereas Brucella melitensis affects goats and sheep, causing fever and abortion; a disease known as brucellosis (Atluri et al., 2011; Moreno, 2014). Humans can contract infection via consumption of unpasteurised dairy products (Corbel, 1997). Infection is detected via bacterial isolation from blood samples or serology (CDC—Centre for Disease and Prevention, 2012a).
Kurdoglu and colleagues in Turkey (Kurdoglu et al., 2010), conducted a case–control study examining the miscarriage rate of 342 pregnant women with brucellosis compared with 33 936 uninfected women of similar socioeconomic status treated in the same hospital. The researchers concluded that 24.14% of infected pregnant women miscarried versus 7.59% of the controls. This result however could be influenced by statistical power, as the cases are ∼100 times smaller than the control group.
The seroprevalence of brucellosis among 445 miscarriage cases and 445 control pregnant Jordanian women with no history of miscarriage consecutively recruited, matched for age, socioeconomic status and area of residence, was not significantly different (Abo-shehada and Abu-Halaweh, 2011). In the paper the researchers state that a sample of 441 was adequate as the prevalence of brucellosis is 8% in high-risk patients in contact with livestock (Abo-Shehada et al., 1996), though their reference for statistical power could not be reviewed. The overall prevalence was similar in both groups; 1% in controls and 1.8% in cases.
The evidence suggests brucellosis is still a risk factor for miscarriage in areas where the infection is endemic in farm animals. This is in accordance with older studies that have reported high miscarriage rates among women with brucellosis (Lulu et al., 1988; Khan et al., 2001).
Chlamydia trachomatis
Chlamydia trachomatis, an obligate intracellular bacterium, is the most common sexually transmitted bacterial disease worldwide (Howie et al., 2011). The prevalence of the disease is high, estimated at 101 million new cases in 2005 worldwide (World Health Organisation, 2011). Though in women it is often asymptomatic, untreated C. trachomatis infection can result in mucopurulent cervicitis (Brunham et al., 1984), acute urethral syndrome (Stamm et al., 1980) and pelvic inflammatory disease (PID) (Paavonen and Lehtinen, 1996). Chlamydia trachomatis infection is a known risk factor for ectopic pregnancy and preterm birth (Martin et al., 1982; Hillis et al., 1997; Egger et al., 1998; Kovács et al., 1998; Bakken et al., 2007; Shaw et al., 2011). Diagnosis is carried out by PCR on vaginal swab samples and treatment includes the administration of antibiotics, such as tetracyclines, azithromycin or erythromycin (Brocklehurst and Rooney, 2000; MedlinePlus, 2014).
The most recent case–control study investigating a potential association of C. trachomatis and miscarriage was published in 2011 (Baud et al., 2011). Using an enzyme-linked immunosorbent assay (ELISA) to detect C. trachomatis antibodies in sera, as well as a standard vaginal swab for C. trachomatis detection by PCR, on 145 cases and 261 controls, a positive association with miscarriage was observed. Immunoglobulin (Ig)G antibodies against C. trachomatis were present in higher levels in the miscarriage group (15.2%) than in the controls (7.3%; P = 0.018). The same pattern was observed for IgA antibodies only after adjustment for age, origin, education and number of sexual partners. Furthermore, C. trachomatis was detected using PCR in the placentae from cases more often than those from controls (4.0 and 0.7% respectively, P = 0.026). Subsequently, an observational study from Finland on 4920 women with genital tract infections has suggested that late complications can occur in C. trachomatis infected pregnant women (Kortekangas-Savolainen et al., 2012). However there was no control group in this study and neither were the terms ‘early’ or ‘late’ pregnancy defined, therefore limiting extrapolation of the findings.
In a study from Serbia, 21.3% of 54 miscarriage cases were shown to have persistent C. trachomatis infection as determined by levels of sera IgA against C. trachomatis major outer membrane protein (Arsovic et al., 2014). The authors suggest an association between persistent C. trachomatis infection and miscarriage, however these cases were compared only against patients with tubal infertility and not uninfected pregnant women.
Chlamydia trachomatis has been studied extensively and a lot of data are available for this infection from over three decades of research. Contradicting studies have been published, resulting in conflicting evidence regarding the role of C. trachomatis in miscarriage (Feist et al., 1999; Wilkowska-Trojniel et al., 2009). Taking into account the most recent findings and the increase in screening programmes worldwide, such as the screening offered to all pregnant women in the USA (CDC, 2014), public awareness of the possible risk of C. trachomatis infection to a future pregnancy might be advisable.
Mycoplasma genitalium
Mycoplasma genitalium is a sexually transmitted bacterium, known to cause urethritis, cervicitis and PID, but infection can also be asymptomatic (Taylor-Robinson and Jensen, 2011). It has been suggested that M. genitalium can enhance human immunodeficiency virus (HIV) infection and transmission (Napierala Mavedzenge and Weiss, 2009); diagnosis is via PCR on urine samples (CDC, 2012b) and treatments include azithromycin and doxycycline (Horner et al., 2014). The only published study of this infection, is a case–control study from the USA on 392 women with miscarriage before 22 weeks of gestation and 802 healthy pregnant controls, and used data from participants originally enrolled in another study. Overall, M. genitalium had a prevalence of 5.9% but no association with miscarriage was found (Short et al., 2010).
Q fever
Q fever is a zoonotic infection, caused by the bacterium Coxiella burnetii (Maurin and Raoult, 1999). Infection is most commonly observed in humans who come into close proximity to livestock. Coxiella burnetii is usually transmitted via inhalation of infectious aerosols from animal fluids (Maurin and Raoult, 1999; van der Hoek et al., 2010). Infection is asymptomatic in half of all cases in adults but can present as an unspecific illness combined with pneumonia or hepatitis. Q fever is confirmed via PCR on blood samples (CDC, 2013). Recommended treatment in symptomatic adults and children is doxycycline administration. In pregnant women, Q fever infection has been associated with adverse pregnancy outcomes, as in a recent report from the United States Centres for Disease Control and Prevention (CDC) (Anderson et al., 2013). However, as the authors note, studies investigating serological evidence of infection and miscarriage have produced contradictory results. Screening of pregnant women is not currently recommended in the European Union (Munster et al., 2012).
Two Danish studies, one in 2012 and the second in 2013, concluded that C. burnetii is not linked to miscarriage (Nielsen et al., 2012, 2013). Both used randomized sera samples from the Danish National Birth Cohort. The first study was powered to detect whether infection could be associated with miscarriage. The presence of infection was investigated in a case group of 218 women with miscarriage (loss of pregnancy prior to 22 weeks of gestation) compared with 482 healthy pregnancies. The second study focused on pregnancy outcomes of 397 women exposed to cattle and sheep (high risk of exposure to C. burnetii infection) versus 459 women that had no contact with animals. Coxiella burnetii prevalence was 5% in cases and 6% in controls of the first study, whereas in the second study 19.5% of all women were positive, however 87% of these women had contact with livestock. Nielsen and colleagues (Nielsen et al., 2012) reported one positive miscarriage case (0.46%) and 3 (0.67%) seropositive among controls whereas in the second study two miscarriages were positive (Nielsen et al., 2013). These results suggest that, despite presence of C. burnetii infection especially in pregnant women in proximity with cattle and sheep, this bacterium does not seem to be widely associated with adverse pregnancy outcome, although individual cases have been reported.
Syphilis
Syphilis is a bacterial infection that can be transmitted sexually or via contact with the blood of an infected person. It is caused by Treponema pallidum, diagnosed using PCR, and is treated with antibiotics (Cohen et al., 2013). Stage one symptoms include a highly contagious sore that develops during stage two to a rash accompanied by sore throat. The third and final stage is tertiary syphilis, which is not contagious but is very harmful.
Casal and colleagues (Casal et al., 2012) assessed risk factors associated with syphilis and pregnancy outcomes in a Brazilian population. The cases consisted of women positive for syphilis, 169 with live births and 68 who had an adverse pregnancy outcome. This included miscarriage, stillbirth and neonatal death grouped together. The control group of women negative for syphilis included 219 women who had live births and 83 with adverse pregnancy outcome. Syphilis was significantly associated with history of miscarriage (OR 3.31; CI 2.20–4.99; P < 0.0001) after testing using a multiple regression model. Most of the pregnancies resulting in live births were not completely asymptomatic when infection was present, resulting in outcomes such as prematurity, low birthweight and respiratory problems, among others. They also observed that maternal syphilis was associated with illegal drugs, alcohol, no counselling on syphilis, sexual activity initiation at 16 years of age or younger, two or more sexual partners during the preceding 1.5 years, life in a household with a low income and poorer sanitation; all factors that may also have a detrimental impact on reproductive outcome.
A study from China reported that, following a screening programme aiming to prevent mother-to-child syphilis transmission, the adverse pregnancy outcomes including miscarriage were reduced from 27.3% in 2003 to 8.2% in 2011 (Hong et al., 2014).
The effect of syphilis on pregnancy has been a subject of interest for almost 100 years; general consensus is that syphilis can have a devastating effect on fetuses resulting in miscarriage, stillbirth and congenital transmission (Temmerman et al., 1992; Oswal and Lyons, 2008). Syphilis screening programmes are in effect in the USA and EU (CDC, 2014; Janier et al., 2014).
Viral infections
Herpes virus infections
The Herpes family of DNA viruses includes a number of pathogenic viruses of humans (Human Herpes Viruses/HHV) that can remain latent in the host and can reactivate (Whitley and Roizman, 2001). Two members of this family, HSV-1 (HHV1) and HSV-2 (HHV-2) establish latency in neuronal cells and on reactivation can cause herpes genitalis or labialis (Margolis et al., 2007). Cytomegalovirus (CMV) (HHV-5) is also a very common virus, acquired by most people during childhood (Chisholm and Lopez, 2011). CMV infects mostly myeloid cells and is never eradicated from the body (Koch et al., 2006). Herpes viruses can be diagnosed using PCR in sera samples (Singh et al., 2005).
HSV-1 and HSV-2
HSV1 and/or HSV2 DNA were detected in 43.5% of 95 frozen trophoblastic tissue samples from Greek women with spontaneous pregnancy loss compared with 16.7% of women undergoing elective abortion (n = 35, P = 0.03, Fisher's exact test) (Kapranos and Kotronias, 2009). Using in situ hybridization HSV DNA was detected in the trophoblast of 18 out of 25 HSV positive cases. The authors concluded that HSV seems to have a role in early miscarriage, although they did not distinguish between the two types of HSV.
These data are supported by a more recent study from Korea (Kim, et al., 2012b). The authors of this study tested sera of 500 pregnant women for HSV-2 and 85 (17%) were seropositive. Most of the women in both groups also tested positive for rubella, varicella zoster (HHV-3) and hepatitis B (HEPB), however the authors adjusted for this. Of HSV-2 seropositive women, 38.8% had a history of miscarriage compared with 29.6% of the control group (P < 0.05).
A possible association of HSV1 and HSV2 with miscarriage cannot be ascertained from these reports and further studies are required.
Human CMV/HHV-5
Hadar and colleagues studied a group of seropositive 59 women with peri-conceptional CMV infection, which occurred between 4 weeks prior to the last reported menstrual period and up to 3 weeks after the expected date of the period. Out of these women, four had miscarriages before undergoing amniocentesis to confirm intrauterine infection. The remaining patients either elected to terminate the pregnancy or gave birth to live infants. No conclusion could be drawn with regards to miscarriage association as no controls were included in this study (Hadar et al., 2010).
Data from a Malaysian study (Saraswathy et al., 2011) showed that anti-CMV IgG antibody was detected in 84% of healthy pregnant women as well as women with adverse pregnancy outcome, including 17 cases of miscarriage.
Despite the lack of recent studies supporting an association of CMV with miscarriage, in vitro studies have shown that CMV infection can result in placental dysfunctions (see below). However, further studies are required to elucidate the true role of CMV in adverse pregnancy outcomes.
Human papillomavirus
Human papillomaviruses (HPV) comprise a group of over 150 different types of small DNA viruses some of which cause common sexually transmitted infections (Cutts et al., 2007). Sexually transmitted HPV infection has a prevalence rate of 11.7% in the general female population of reproductive age (Bruni et al., 2010). According to CDC, sexually transmitted HPV prevalence nationwide in the USA among women 14–59 years old was 42.5% in 2003–2006, an estimation based on positive servicovaginal swab tests (Hariri et al., 2011). Persistent infection with high-risk types of HPV (the most prevalent being HPV 16/18 worldwide) have been associated with cervical cancer, and others (HPV 6/11) with genital warts (Cutts et al., 2007; Crosbie et al., 2013). The vast majority of infections are asymptomatic and clear naturally without causing long-term disease and a vaccine is now available for types 6, 11, 16 and 18 (Cutts et al., 2007). HPV infection cannot be diagnosed by blood tests, however PCR on cervical cell samples is used to determine specific viral genotypes following a positive Papanicolaou (PAP)-test (Molijn et al., 2005).
The results of recent studies into the effects of HPV infection upon miscarriage are contradictory (Perino et al., 2011; Skoczyński et al., 2011; Yang et al., 2013). A study in China on the effect of HPV on the pregnancy outcome of IVF treated patients found no difference in miscarriage rates between women with abnormal cervical cytology who had a positive high risk HPV test (n = 56) and those who tested negative for high-risk HPV (n = 56, Yang et al., 2013). A second study from Poland tested for 33 HPV genotypes and also for specific HPV 16/18 DNA presence in placentae from miscarriages (n = 51) and from term deliveries from women who showed no signs of systemic infection (but were not tested, n = 78). They found HPV DNA in 17.7% of miscarriage cases and in 24.4% control placentae. A total of 11.8% of miscarriages and 12.8% of normal placentae were positive for HPV 16/18, but none of these differences reached statistical significance (Skoczyński et al., 2011). Both of these studies suggest that HPV infection in women has no effect on pregnancy outcome, although no more than 150 women were examined in either study.
Conversely, results from a 2011 study significantly associated male partner HPV infection with miscarriage rate in 199 couples attending IVF clinics in Italy (66.7% in HPV infected couples versus 15% of controls with no HPV infection, P < 0.01, Perino et al., 2011). The researchers also identified that all pregnancies in couples where both partners were infected resulted in miscarriage (n = 9).
These studies present contradictory data, however the first two examined infection in female partners whereas the second one investigated male partners. Interestingly, an older study (Hermonat et al., 1997) reported HPV DNA presence in 15/25 early miscarriage samples compared with 3/15 first trimester elective abortion samples. Further well-designed, adequately powered studies are required to fully elucidate the role of HPV as a potential risk factor for miscarriage, whilst considering the role of an infected male partner as there are indications of a potential role in early miscarriage (Garolla et al., 2011).
Parvovirus infection
Parvoviruses belong to the Parvoviridae family and are very small single stranded DNA viruses that infect invertebrates and vertebrates (Cotmore et al., 2014). Of interest to studies of miscarriage are Adeno-associated virus (AAV), Parvovirus B19 (B19V) and Bocavirus (BC).
AAV
Antibodies against several serotypes of AAV show infection in various tissues, but it is asymptomatic (Gao et al., 2004). AAV needs the help of a helper virus, adenovirus, to replicate. Despite this, 80% of the human population is seropositive for AAV, as diagnosed by PCR (Gonçalves, 2005).
No association of AAV infection with serotypes 2, 3 and 5 with recurrent miscarriage (defined as two or more) was found in couples with subfertility (Schlehofer et al., 2012). A total of 146 semen samples as well as 134 endocervical samples from couples attending a fertility clinic were tested for the presence of AAV DNA and 14.9% of female and 19.9% of male samples were positive. No associations with other infectious pathogens, semen quality or subsequent fertility issues were indicated.
In another study (Pereira, 2010), the presence of AAV was examined in 81 patients, divided into three groups: 13 medically induced abortions, 29 miscarriages and 39 ‘undetermined’ (including 66 decidua and 52 ovarian biopsies from the same patients). AAV DNA was detected in 23/81 (28.4%) of cases for at least one of the decidual or ovular fragments. Furthermore, 22/68 (32.3%) of spontaneous and 7.7% (1/13) of elective abortions (classified according to patient information) tested positive. The authors grouped cases with confirmed type of abortion and observed 28.6% (12/42) and 2.4% (1/42) AAV positive ‘for spontaneous and medically induced abortion, respectively (P < 0.05)’. The classification of samples used as well as definition of the various groups compared in this study are unclear from the paper description, thus interpretation is challenging. The authors suggest a ‘casual association’ of AAV to miscarriage.
Despite the detection of AAV DNA in some miscarriage cases, there is inconclusive evidence for a role for this virus in miscarriage.
Parvovirus B19 (B19V)
Parvovirus B19 (B19V) is a small virus capable of causing different diseases in humans, such as ‘Fifth disease’ during childhood (Young and Brown, 2004). It is estimated that ∼50% of young men and women have antibodies against B19V, determined via serology tests (Broliden et al., 2006). The remaining 50% of women are at risk of developing infection during pregnancy, which can lead to non-immune hydrops fetalis, a well-established cause of fetal death (Silingardi et al., 2009).
A recent study from Northern Ireland examined 3921 women of reproductive age and 33.5% of them were at risk of infection as they had no antibodies against B19V (Watt et al., 2013). Though fetal loss was reported in infected women with confirmed presence of the virus in miscarried fetuses, no increased association with miscarriage was observed. However, the authors reported ‘inadequate follow-up’ of pregnancies potentially associated with B19V infection.
In an earlier study of 72 pregnant women with B19V, it was noted that the risk of vertical transmission is higher if infection occurs by gestational week 20. Six out of eight cases of fetal loss observed were ‘attributed to B19V infection’ without further elucidation. No conclusions regarding the association were reached by the researchers (Bonvicini et al., 2011).
A higher percentage of IgM antibodies indicating recent infection was observed in women with adverse pregnancy outcomes (22.72%, n = 88) compared with 4.5% observed in 88 control healthy pregnant women (Brkic et al., 2011). Interestingly, anti B19V IgG antibodies were higher in controls than cases (70.5 and 53.4% respectively, P = 0.046). An important limitation of this study is that the adverse pregnancy outcome included miscarriage, non-immune hydrops fetalis and intrauterine fetal death, thus the association of miscarriage alone with B19V is not clear.
In a study from Nigeria, B19V prevalence among pregnant women was estimated at 40.7%, as 111 out of 273 patients in the study had detectable levels of either IgG or IgM antibodies, however these were not associated with a history of miscarriage (Emiasegen et al., 2011).
From the above, it is evident that a case–control study on women with miscarriage versus healthy pregnant controls, statistically powered to elucidate the role of B19V in miscarriage is required, as there are indications of high prevalence in pregnancy and fetal infection.
Bocavirus (BC)
The human BC is a newly discovered member of the parvovirus family detected in 93% of sera of children older than 3 years old (Karalar et al., 2010). In a study on 535 fetal biopsies (120 miscarriages, 169 intrauterine fetal deaths and 246 induced abortions), even though only 10% of women were seronegative, none of the fetuses tested positive and the authors concluded that BC could not have a possible role in miscarriage (Riipinen et al., 2010).
HIV
HIV is a retrovirus, and is most commonly transmitted via unprotected sexual intercourse or sharing of equipment for intravenous drug use. There are two types of HIV, HIV-1 and HIV-2, with the first being the most common (Gnann et al., 1987). The virus infects several cell types of the host immune system, such as CD4+ T lymphocytes (Miedema et al., 1988; Embretson et al., 1993), macrophages (Orenstein et al., 1997) and dendritic cells (Gringhuis et al., 2010). Worldwide, the World Health Organization (WHO) estimates that 34 million people are living with HIV, diagnosed by HIV viral load blood tests (PCR) (World Health Organisation, 2013a). Anti-retroviral treatment delays the onset of severe symptoms and protects the patient from opportunistic infections, which are the main cause of death among HIV-positive patients (Dybul et al., 2002).
A 2013 study from Nigeria examined 2381 pregnancies in 1702 women positive for HIV compared with 2381 pregnant non-infected women from the same hospitals. Following preterm delivery, miscarriage was significantly associated with HIV positivity (OR: 1.37; CI: 1.1–2.3). This association was retained after adjustment for several confounding variables such as age, parity, history of miscarriage and others. The infected women in this study were all receiving anti-retroviral treatment, however different regimes were used during the years in which the study was conducted. Limitations of this study include the number of controls not being clearly stated and lack of testing for other sexually transmitted diseases (Ezechi et al., 2013).
In a study in Zambia 1229 HIV-positive pregnant women were followed up (Kim et al., 2012a, b). The ratio of miscarriages to live births was 3.1/100 and CD4 counts less than 350 cells/mm2 were significantly associated with miscarriage. The women were recruited during both first and second trimesters and none of the women who miscarried had received anti-retroviral treatment. The study did not compare the cases with uninfected pregnant women.
In a study of 382 Ugandan/Zimbabwean HIV-infected pregnant women undergoing multiple anti-retroviral therapies (Gibb et al., 2012), miscarriage and medically induced abortions occurring prior to 22 weeks of gestation were assessed as one factor, not separate outcomes. Of note, fetal death after 22 weeks was classified as stillbirth. Therefore, no conclusions regarding miscarriage specifically can be drawn from this study. A study from India on 69 HIV-infected and 345 non-infected women demonstrated higher miscarriage/stillbirth risk amongst the infected group, however again there was no distinction between medically induced abortions and miscarriages (Darak et al., 2011). In a retrospective analysis from Germany, 42% of HIV-positive women attending an outpatient clinic for preconception counselling became pregnant and only one miscarried (Gingelmaier et al., 2011).
HIV status was associated with miscarriage in a study of 1,218 pregnant women from Uganda (De Beaudrap et al., 2013). However, they defined stillbirth as the delivery of a non-living fetus ≥28 weeks gestation; and miscarriage as the delivery of a non-viable fetus either at <28 weeks gestation or weighing <500 g. Furthermore, as stillbirth and miscarriage were grouped together as one outcome, no definite conclusions regarding miscarriage can be drawn from this study.
To summarize, evidence suggests that HIV infection negatively affects pregnancy; however, anti-retroviral treatment can reduce the risk of adverse outcomes (Zolopa et al., 2009; Friedman et al., 2011). Furthermore, HIV has been associated with BV which could have a detrimental role on pregnancy outcome (Ledru et al., 1997). The presence of multiple diseases could further compromise a pregnancy. As most of the studies suggest, consultation and monitoring of HIV-positive women who wish to become pregnant is desirable. Women during their first antenatal visit are offered HIV tests in the UK, USA and EU (European Centre for Disease Prevention and Control, 2010; UK National Screening Committee, 2013; CDC, 2014).
Polyomavirus BK
Polyomavirus BK infects up to 90% of the general population via an unknown transmission route and is usually asymptomatic with the exception of immunocompromised individuals (Hirsch and Steiger, 2003). Antibodies against the virus can be detected using sera samples and PCR, urine cytology and viral immunostaining (Masutani, 2014).
Recent studies have investigated a potential role of BK virus infection on adverse pregnancy outcomes. A study on patients with unexplained villitis (infection of the placental villi associated with adverse pregnancy outcomes) detected no BK in placenta from miscarriages (Cajaiba et al., 2011). The authors state that ‘For cases with diffuse villitis, the gestational age ranged from 31 to 41 weeks (average 37.2 weeks)’. It seems therefore more suitable to address these cases as stillbirths, not miscarriages. In another study from Italy, samples from five miscarried fetuses with chorioamnionitis and miscarriages due to chromosomal abnormalities (controls), BK was detected in fetal organs (Boldorini et al., 2010). Though this provides possible proof of vertical transmission of the virus, as it was detected in four out of five chromosomally abnormal controls and three out of five cases, the authors concluded that BK infection does not have a role in miscarriage. In accordance with the first study, the fetuses were between the 15th and the 28th week of gestation, so some of them were stillbirths according to our review's classification. Moreover, the fetuses were not matched for gestational age. In both studies, the numbers were small and no early miscarriages were tested. The question whether BK virus could be associated with miscarriage requires therefore further investigation.
Dengue fever
Dengue fever is a disease caused by four viruses of the single stranded RNA flaviviridae genus (DEN1-4), transmitted via mosquito bites usually in tropical and sub-tropical climates worldwide. WHO estimates 40–50 million new cases every year. Dengue is a flu-like illness with no vaccination and treatment currently available. Diagnosis is difficult as symptoms resemble other diseases, however usual approaches include DNA and antibody detection in serum samples using PCR and ELISA, respectively (CDC, 2012).
The role of dengue fever in miscarriage was examined in a prospective study from Malaysia on 115 women with miscarriage up to 22 weeks of gestation and 296 healthy pregnant controls. This study found significant association of recent dengue fever infection with miscarriage after adjusting for confounders such as maternal age, gestational age, parity and ethnicity (5.3% in cases versus 1.7% in controls, adjusted OR 4.2, 95% CI 1.2–14, P = 0.023, Tan et al., 2012).
In a case series report from Sri Lanka, two out of fifteen pregnant women experienced fetal death at 24 and 35 weeks of gestation, however the study provides no evidence of vertical transmission to the fetuses (Kariyawasam and Senanayake, 2010). In another case series report from French Guiana the authors reported two late miscarriages in 53 pregnant women with dengue fever. However, the infection could not be connected to the adverse pregnancy outcome (Basurko et al., 2009).
A systematic review on 30 studies concluded that it is unclear whether dengue fever is associated with adverse pregnancy outcomes (Pouliot et al., 2010). Based on recent evidence however, we can conclude that dengue fever seems to be a risk factor for miscarriage; therefore it is advisable to raise awareness regarding protective measures in high-risk areas and for people travelling to those areas.
HEPB and HEPC
The HEPB virus is a member of the Hepadnavirus family of small DNA viruses and the HEPC virus is a member of the flaviviridae genus of single stranded RNA viruses. Both viruses cause liver inflammation and disease and are both found in body fluids. HEPB is often resolved within a couple of months, however HEPC can develop into a chronic disease. Both diseases are diagnosed using blood serological tests (Gretch, 1997; Krajden et al., 2005).
In a case–control study from China, 75 couples that received assisted reproduction treatment were followed up, divided into a group with one partner diagnosed with chronic HEPB infection and a control group with both parents seronegative for HEPB (Ye et al., 2014). The early miscarriage rate (gestational week range not specified) was 44% in the case group compared with 9.1% in the control group (P = 0.043, Fisher's exact test). Highest miscarriage rates (60%) were observed when mothers were seropositive and fathers seronegative (P = 0.03). Using PCR, HEPB DNA was detected in 6/62 ‘abandoned embryos’ from the case group, whereas all embryos of the control group were negative. These results suggest a possible role of chronic HEPB infection in miscarriage.
Conversely, a cross-sectional study from Yemen examined the association of miscarriage with HEPB and HEPC infection in pregnant women, and found that 10.8% of women were positive for HEPB (95% CI: 8.0–14.0%) and 8.5% for HEPC (95% CI: 6.0–11.5%). No association of infection with miscarriage was apparent after multivariate analysis (Murad et al., 2013).
These studies raise questions regarding the role of persistent HEPB infection during pregnancy. At the moment, screening programmes of pregnant women for HEPB and HEPC during their first antenatal visit in the USA and UK aim to prevent adverse pregnancy outcome (UK National Screening Committee, 2013; CDC, 2014).
Rubella
Rubella is a mild childhood disease that, if acquired during the first 16 weeks of gestation, can result in miscarriage and serious fetal defects (Banatvala and Brown, 2004). A vaccine has been available for several years resulting in significant reduction in new cases according to the latest WHO progress report (Reef et al., 2011). Regardless of this progress, it is important to be aware that there remain a number of unvaccinated pregnant women in Europe and worldwide that do not have access to vaccination and who are still at risk of adverse pregnancy outcome due to rubella (Metcalf et al., 2011; Muscat et al., 2014).
Influenza virus
A study of the 1918 Influenza pandemic concluded that it resulted in a decrease of live births due not only to high mortality but also to an increase of early miscarriages in pregnant women who were infected by the virus (Bloom-Feshbach et al., 2011). In a case series report regarding the H1N1 Influenza A pandemic, six women were admitted to intensive care and had adverse pregnancy outcomes, however only one seriously ill patient had a spontaneous abortion as four cases occurred during the third trimester (Oluyomi-Obi et al., 2010).
Protozoan infections
Malaria
Malaria is caused by infection with protozoa of the genus Plasmodium (P. falciparum, P. vivax, P. malariae, P. ovale), is transmitted via mosquito bites and is endemic in more than 100 countries in Africa, Asia and South America (World Health Organisation, 2013b). In 2012 there were an estimated 207 million cases of malaria resulting in an approximately 627 000 deaths (90% of all malaria deaths occur in sub-Saharan Africa) (World Health Organisation, 2013b). Symptoms include fever, sweats, headache and diarrhoea and can be treated using different drugs depending on the symptoms and the specific pathogen causing the disease such as atovaquone plus proguanil or doxycycline (Kar and Kar, 2010). Malaria parasites are identified by microscopit examination of patients' blood samples. In 2007, 54.7 million pregnancies occurred in areas with endemic P. falciparum malaria and a further 70.5 million in areas with exceptionally low malaria transmission or with P. vivax only (Dellicour et al., 2010). Plasmodium can bind chondroitin sulphate A expressed on trophoblast and this is what causes local parasitaemia in the placenta (Agbor-Enoh et al., 2003). Maternal disease is most severe in primigravida women, and it reduces with each pregnancy as immunity builds up to those parasites that target the placenta (Fried et al., 1998).
Women with asymptomatic and symptomatic malaria (single episode before 14 weeks of gestation) are at a higher risk of miscarriage (adjusted OR 2.70, 95% CI 2.04–3.59 and 3.99, 95% CI 3.10–5.13, respectively). This study included 3527 women with miscarriage and 14 087 women that gave birth to live babies in Thailand. The risk ratios were not different for both P. falciparum and P. vivax (McGready et al., 2012).
De Beaudrap et al. (2013) also studied malaria during pregnancy in Uganda. In 1218 pregnant women no association of malaria with adverse pregnancy outcome was shown but an association with HIV status was demonstrated, as described above.
An association of malaria with adverse pregnancy outcomes, and more specifically miscarriage, is evident from the above studies. Prevention measures and screening of pregnant women at risk of malaria infection are advised.
Toxoplasmosis
Prevalence of Toxoplasmosis differs across the world, from 20–40% in the UK and USA (Food Standards Agency, 2012) to ∼70% in tropical countries (Klaren and Kijlstra, 2002). In a recent study from London, 17.32% of 2610 samples tested were seropositive (Flatt and Shetty, 2013). Even though most patients are asymptomatic, immunocompromised individuals are susceptible to developing severe disease and women who become infected whilst pregnant can pass the infection vertically (Jones et al., 2001). The presence of T. gondii is confirmed by antibody detection.
Alvarado-Esquivel et al. (2014) showed that 6.7% of 326 women with a history of miscarriage had been exposed to T. gondii. This study however did not include a control group with no history of miscarriage. Miscarriage or stillbirth occurred in 28 out of 190 pregnant cases with toxoplasmosis presenting in England and Wales between 2008 and 2012, however these are data from the surveillance programme currently in place and not part of a study (Halsby et al., 2014). In a study on serum samples from 100 women who had miscarried, 86% of which were during the first trimester of pregnancy, 55% were seropositive for IgG against T. gondii (Vado-Solis et al., 2012), however, no comparison to uninfected pregnant women was made. A meta-analysis of several Mexican studies also indicates that infection rates are higher in women with miscarriage (Galvan-Ramirez et al., 2012). Despite this, as the authors highlight, only three out of the 132 studies included in their systematic review, were focused on women who had a miscarriage.
The likelihood of association of T. gondii infection resulting in miscarriage is highlighted by the present review of recent studies. Taking into account the significant worldwide prevalence of this protozoan infection, screening of pregnant women is recommended if it is established that this infection presents a significant risk for adverse pregnancy outcome.
How do infections lead to miscarriage?
Pregnancy is a complex process involving multiple cell types and regulated by several sophisticated mechanisms, which are still not fully clarified despite years of research. To examine the negative impact of infections to pregnancy, we first need to understand how a normal, successful pregnancy is established.
Maternal-fetal interface: morphology, implantation process and the role of the immune system
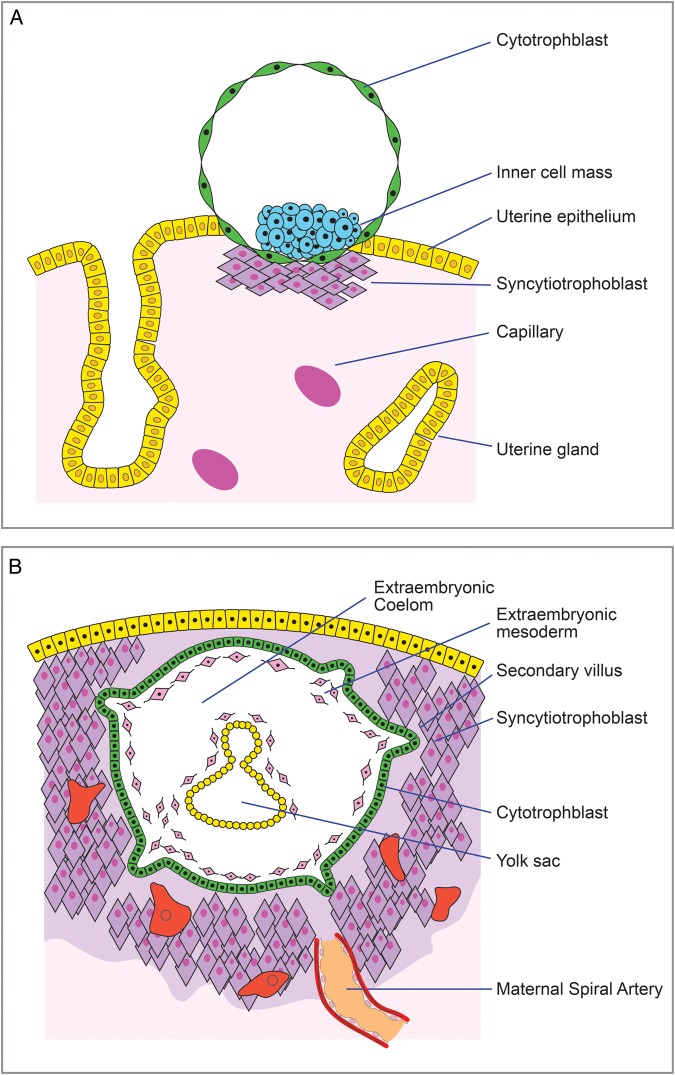
The human endometrium is composed of several different cell types, including luminal and glandular epithelial cells, stroma with stromal fibroblastic cells, immune cells and blood vessels. During every menstrual cycle, in response to ovarian estrogen and progesterone via a process called ‘decidualisation’, the endometrial stromal compartment undergoes morphological and structural transformation to become receptive to implantation. Prior to implantation, the trophoblast differentiates into the growing blastocyst as it travels from the Fallopian tube to the uterus. The ‘implantation window’, during which the uterus is receptive to the embryo, is usually between 6 and 12 days after ovulation (Rashid et al., 2011). The blastocyst attaches to the receptive endometrium utilizing adhesion proteins, called integrins, during the implantation window (Fig. 2A, Merviel et al., 2001). Placenta formation begins as the trophoblast comes into contact with the epithelium and differentiates further into syncytiotrophoblast that invades the epithelial layer. Various other molecules, both from the maternal and fetal side, are involved in this process (reviewed in Dimitriadis et al., 2005; Tranguch et al., 2005; Achache and Revel, 2006; Chen et al., 2009). Syncytiotrophoblasts, supported by the decidualised stroma (Godbole et al., 2011), penetrate the endometrium and surround the embryo, whilst it embeds itself in the decidual stroma. A second trophoblast layer, the cytotrophoblast, is an inner layer without contact with the maternal cells. During the trophoblast invasion, cavities called lacunae develop, which, as they get filled with maternal blood, bring the maternal circulation into contact with the placental villi, thus marking the onset of placental circulation that includes exchange of nutrients and waste between the embryo and mother. At days 10–12 of gestation, the embryo is completely embedded in the endometrium, the epithelium has grown over it and the implantation process is complete (Fig. 2B). The three placental zones are now distinguishable: the early chorionic plate near the embryo, the intervillous space with the villous trees and the primitive basal plate in contact with the maternal endometrium (Pijnenborg et al., 1980, 1981). Simultaneously, endovascular trophoblast cells stemming from the basal plate invade the walls of the spiral arteries, replacing the maternal muscular and endothelial cells with trophoblast cells, transforming the arteries into large diameter and low resistance blood vessels (Lyall, 2005).
Figure 2.
Implantation of blastocyst in the maternal endometrium. (A) During the implantation window (Day 6–12 post conception), the blastocyst adheres to the endometrium, and the placenta formation commences as the syncytiotrophoblast develops and invades the endometrium. (B) On Days 10–12 the implantation is completed as the embryo is encapsulated within the maternal tissue and the endometrial spiral arteries have been transformed into low resistance blood vessels, thus marking the onset of the placental blood flow.
The role of the immune system in a successful pregnancy is crucial (Fig. 3A). Whilst the immune tolerance of the semi-allogeneic fetus is maintained, several components of the immune system fulfil their designated roles in preparation for implantation as well as during gestation (Entrican, 2002; Chaouat et al., 2004). Natural killer (NK) cells, macrophages and dendritic cells have all been detected in the feto–maternal interface (Guleria and Pollard, 2000; Moffett-King, 2002; Gardner and Moffett, 2003). Cytokines such as interleukin (IL-10), colony stimulating factor (CSF-1) and transforming growth factor-β among others have been linked with the implantation process and are expressed in uterine cells (Altman et al., 1990; Guleria and Pollard, 2000; Thaxton and Sharma, 2010). Implantation induces an inflammatory response because of invasion and damage of maternal tissue, with many cells undergoing apoptosis (Jerzak and Bischof, 2002; Joswig et al., 2003). Conversely, inflammatory cytokines such as interferon-γ and tumour necrosis factor alpha (TNF-α) are not usually expressed in the placenta and have been associated with abortion in mouse models (Entrican, 2002).
Figure 3.
Healthy and infected feto–maternal interface. (A) During a healthy pregnancy, the interaction between maternal decidua, vasculature and immune cells (macrophages, uterine natural killer cells and dendritic cells) with fetal trophoblast and syncytial cells is the cornerstone of establishment and progression of pregnancy. Molecules such as interleukin (IL-10), colony stimulating factor (CSF-1) and transforming growth factor-β are essential for trophoblast invasion during the implantation process and are expressed by uterine cells. (B) Infections can disrupt the balance of feto–maternal interactions. Plasmodium falciparum can infect trophoblast cells entering via the maternal bloodstream. Cytomegalovirus and Listeria monocytogenes are examples of viral and bacterial infections known to interfere with trophoblast cells.
Abnormal implantation, placentation or blood vessel transformation are thought to result in miscarriage (Michel et al., 1990; Ball et al., 2006). An active infection could interfere with the pregnancy by affecting any of the above-mentioned processes as well as disrupt the immune balance, whether it resulted in placental and fetal infection or not.
Examples of where we understand the mechanism of infection-induced miscarriage
For most of the pathogens where an association has been demonstrated, the exact mechanism that leads from infection to miscarriage is unknown. Bacteria, protozoa and viruses utilize different mechanisms to infect their host and each one seems to induce a unique cascade of events in the feto–maternal interface, most of which remains to be determined. Our knowledge is derived mostly from animal studies and data on human pregnancies are scarce.
Multiple mechanisms can be utilized by pathogens to cross the placental barrier. Plasmodium, as mentioned previously, enters the host via the maternal circulation and can infect and multiply in the trophoblast (Fig. 3B), even though its natural target cells are red blood cells (Agbor-Enoh et al., 2003; Moreno-Pérez et al., 2013). However, this mechanism of crossing the placental barrier is specific to malaria. Listeria monocytogenes uses two bacterial surface proteins called internalin A and B to invade the placenta, after passing from the intestinal barrier to the maternal circulation (Fig. 3B, Vázquez-Boland et al., 2001; Lecuit et al., 2004; Disson et al., 2008). The presence of pathogenic organisms in the placenta induces a maternal immune response to infection that could result in miscarriage.
The susceptibility of placenta and fetus to several viruses has been investigated, as trophoblast cells have been identified as targets and viruses such as AAV and CMV have been detected in fetal tissue. CMV has been shown in vitro to replicate in trophoblast cells (Fig. 3B), in addition to epithelial, stromal cells and macrophages that are known target cells of the virus (Minton et al., 1994; Fisher et al., 2000; Sinzger et al., 2008). In trophoblasts, CMV can induce an inflammatory response that increases apoptosis (Chou et al., 2006). CMV has also been shown to activate TNF-α, again leading to cell death (Chan et al., 2002). TNF-α is normally expressed in low levels by the placenta (Entrican, 2002); in the mouse CBA × DBA/2 model TNF-α was shown to increase fetal resorption via activation of NK cells, macrophages, and Th1-type cytokines (Clark et al., 1998). Furthermore, decreased levels of implantation-associated matrix metalloproteinases 2 and 9 (MMP2 and MMP9) in the early pregnancy villi of women with CMV indicate compromised invasive capability, that could result in miscarriage (Tao et al., 2011). These results suggest that CMV infection could lead to placental dysfunction as well as suggest possible routes of fetal infection resulting in miscarriage.
Bacterial infections initiate different responses from the immune system compared with viruses but gram-negative and gram-positive bacteria are both capable of activating the innate immune system (Takeuchi et al., 1999; Yoshimura et al., 1999). Most of our knowledge regarding bacterial infections and pregnancy comes from studies in mouse models. Nitric oxide and prostaglandins produced in the presence of bacterial lipopolysaccharides (LPS) were shown to be associated with embryonic resorption, as inhibition of this pathway reversed the effect in mice (Aisemberg et al., 2010). Poor uterine receptivity and implantation failure due to exposure to bacterial LPS was also reported in another study in mice (Deb et al., 2005).
Bacteria, viruses and protozoa utilize various mechanisms to infect fetal and maternal tissues (Fig. 3B), a few of which have been elucidated yet several remain unknown. These pathways are possibly implicated in miscarriage caused by infection. Further research is however required, as understanding the exact mechanisms behind infection-induced miscarriages could lead to effective treatment and thus prevention.
Conclusions
A plethora of bacterial, viral and protozoan infectious agents have been investigated to determine whether they are associated with an increased risk of miscarriage. The evidence presented in this review shows that infections such as BV, malaria, CMV, dengue fever, brucellosis and HIV may adversely affect pregnancy outcome. In contrast, there is no current evidence to suggest that C. burnetii, adeno-associated virus, Bocavirus, Hepatitis C and M. genitalium are associated with miscarriage. More importantly though, the lack of consensus regarding the effects of C. trachomatis, T. gondii, HPV, HSV1, HSV2, Polyomavirus BK, Hepatitis B and B19V infection reveals a gap in knowledge that future research should address, as these pathogens could potentially be harmful to early pregnancy development (Table I). This issue is of particular importance for public health practitioners as it could alter current policies of prevention of infection, diagnosis and treatment in pregnant women.
Table I.
Summary of pathogens and their association with miscarriage.
| Bacteria | Viruses | Protozoa | |
|---|---|---|---|
| Associated with miscarriage |
|
|
|
| Little or no evidence for association with miscarriage |
|
|
|
| Conflicting evidence for association with miscarriage |
|
|
|
HIV, human immunodeficiency virus.
Even in diseases such as malaria and rubella, where a causative role is established, the underlying molecular cause of miscarriage is still unknown. The mechanism that has been proposed to explain how CMV infection could undermine a pregnancy could apply to other intracellular pathogens. Chlamydia trachomatis and U. urealyticum have been detected in placental cells, therefore they could cause a similar response (Joste et al., 1994; Baud, et al., 2011). It is well established that pregnancy is a balance between tolerance and rejection, as the maternal immune system is re-programmed to tolerate the allogeneic (paternal) fetal antigens (Thellin and Heinen, 2003). An active infection could destabilize this balance resulting in rejection, especially if it leads to a serious illness of the mother. Evidently, further research is required to understand the causes of pregnancy failure.
In severe maternal infection, such as with influenza, HIV, dengue fever and malaria, the maternal response may result in miscarriage instead of a direct placental infection effect. However, pathogens such as Plasmodium parasites and Dengue fever's Flavivirus are known to be detected in fetal tissue and placenta, as are a plethora of other pathogens (Table II). This is of particular importance, as proof of vertical transmission that could interfere with an ongoing pregnancy is more likely to result in miscarriage than a maternal infection. Examination of fetal tissues from infected mothers is essential to clarify whether vertical transmission is possible for pathogens as this has not yet been elucidated and it is evident from recent studies that an association is likely: for example, brucellosis, Mycoplasma genitalium and Coxiella burnetii infections. A very significant issue is fetal specimen contamination in cases with presence in the vagina of common viruses, such as HPV and HSV, as this does not equate to causation of miscarriage.
Table II.
Summary of the sites of detection of pathogens in the studies in the review.
One interesting outcome of our review was that studies regarding infections and pregnancy outcome were conducted worldwide. Despite this, it seems that most of the studies were from countries in the developing world where the prevalence of specific diseases is higher.
A commonly observed limitation of the studies presented in this review was that few studies tested for the presence of other pathogens except for the one of interest. Several pathogens are often associated with one another, such as HIV with BV and malaria (Ledru et al., 1997; Taha et al., 1998; De Beaudrap et al., 2013).
Furthermore, the definition of terms, such as miscarriage and stillbirth, may differ from the ones generally accepted in some studies, as mentioned previously. For example, loss of pregnancy up to 36 weeks was considered miscarriage (Bayraktar et al., 2010), or in other cases less than 22 gestational weeks (Nielsen et al., 2012). Universal terminology guidelines are required to establish effective scientific communication.
The impact of immunization, if a vaccine is available such as in the case of HPV, could be detrimental in cases of infection-induced miscarriage. However, vaccine development for some of the pathogens of interest in this review is a complicated process and has been unsuccessful so far (Hafner et al., 2008; Mouquet and Nussenzweig, 2013).
To our knowledge, there are no EU guidelines regarding screening for infectious diseases in pregnancy. Current screening guidelines in the UK include offering tests for HepB, HIV, rubella (testing for susceptibility) and syphilis to pregnant women (Public Health England, 2015). Systematic screening for infections such as BV or CMV is not currently recommended in the UK. Without accounting for cost, screening for pathogens highlighted as high risk for miscarriage in this review should be reconsidered as an option worldwide.
New policies including public education to raise awareness and screening programmes for appropriate pathogens associated with adverse pregnancy outcomes could result in a decrease in the number of miscarriages.
Supplementary data
Supplementary data are available at http://humupd.oxfordjournals.org/.
Authors' roles
S.G. drafted the manuscript, substantially contributed to conception and design, analysis and interpretation of data. N.W. contributed to manuscript preparation and critically revised important intellectual content. K.C. critically revised important intellectual content. G.E. critically revised important intellectual content. S.E.M.H. substantially contributed to conception and design, and critically revised important intellectual content. A.W.H. substantially contributed to conception and design, and critically revised important intellectual content.
Funding
S.G. is funded by the MRC Centre for Reproductive Health and Tommy's Charity. N.W. is funded by the Biotechnology and Biological Sciences Research Council (BBSRC)/Zoetis Industrial Partnership award. G.E. is funded by the Scottish Government Rural and Environment Science and Analytical Services Division (RESAS). Funding to pay the Open Access publication charges for this article was provided by RCUK UK Open Access Fund.
Conflict of interest
The authors have no conflict of interests in relation to this work.
Supplementary Material
Acknowledgements
The authors would like to acknowledge Sharon Cameron's critical review of the manuscript and suggestions, Ronnie Grant for the electronic drawing of the figures in the present review, Dr Colin Duncan for his expert opinion on statistics and Miss Eleni Fitsiou for proofreading the manuscript.
References
- Abo-Shehada MN, Abu-Halaweh M. Seroprevalence of Brucella species among women with miscarriage in Jordan. East Mediterr Health J 2011;17:871–874. [DOI] [PubMed] [Google Scholar]
- Abo-Shehada MN, Odeh JS, Abu-Essud M, Abuharfeil N. Seroprevalence of Brucellosis among high risk people in Northern Jordan. Int J Epidemiol 1996;25:450–454. [DOI] [PubMed] [Google Scholar]
- Achache H, Revel A. Endometrial receptivity markers, the journey to successful embryo implantation. Hum Reprod Update 2006;12:731–746. [DOI] [PubMed] [Google Scholar]
- Agbor-Enoh ST, Achur RN, Valiyaveettil M, Leke R, Taylor DW, Gowda DC. Chondroitin sulfate proteoglycan expression and binding of Plasmodium falciparum-infected erythrocytes in the human placenta during pregnancy. Infect Immun 2003;71:2455–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aisemberg J, Vercelli C, Wolfson M, Salazar AI, Osycka-Salut C, Billi S, Ribeiro ML, Farina M, Franchi AM. Inflammatory agents involved in septic miscarriage. Neuroimmunomodulation 2010;17:150–152. [DOI] [PubMed] [Google Scholar]
- Allanson B, Jennings B, Jacques A, Charles AK, Keil AD, Dickinson JE. Infection and fetal loss in the mid-second trimester of pregnancy. Aust N Z J Obstet Gynaecol 2010;50:221–225. [DOI] [PubMed] [Google Scholar]
- Altman DJ, Schneider SL, Thompson DA, Cheng HL, Tomasi TB. A transforming growth factor beta 2 (TGF-beta 2)-like immunosuppressive factor in amniotic fluid and localization of TGF-beta 2 mRNA in the pregnant uterus. J Exp Med 1990;172:1391–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado-Esquivel C, Pacheco-Vega SJ, Hernández-Tinoco J, Centeno-Tinoco MM, Beristain-García I, Sánchez-Anguiano LF, Liesenfeld O, Rábago-Sánchez E, Berumen-Segovia LO. Miscarriage history and Toxoplasma gondii infection: a cross-sectional study in women in Durango City, Mexico. Eur J Microbiol Immunol (Bp) 2014;4:117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson A, Bijlmer H, Fournier P-E, Graves S, Hartzell J, Kersh GJ, Limonard G, Marrie TJ, Massung RF, McQuiston JH et al. Diagnosis and management of Q fever—United States, 2013: recommendations from CDC and the Q Fever Working Group. MMWR Recomm Rep 2013;62:1–30. [PubMed] [Google Scholar]
- Arsovic A, Nikolov A, Sazdanovic P, Popovic S, Baskic D. Prevalence and diagnostic significance of specific IgA and anti-heat shock protein 60 Chlamydia trachomatis antibodies in subfertile women. Eur J Clin Microbiol Infect Dis 2014;33:761–766. [DOI] [PubMed] [Google Scholar]
- Atluri VL, Xavier MN, de Jong MF, den Hartigh AB, Tsolis RM. Interactions of the human pathogenic Brucella species with their hosts. Annu Rev Microbiol 2011;65:523–541. [DOI] [PubMed] [Google Scholar]
- Bakken IJ, Skjeldestad FE, Nordbø SA. Chlamydia trachomatis infections increase the risk for ectopic pregnancy: a population-based, nested case-control study. Sex Transm Dis 2007;34:166–169. [DOI] [PubMed] [Google Scholar]
- Ball E, Bulmer JN, Ayis S, Lyall F, Robson SC. Late sporadic miscarriage is associated with abnormalities in spiral artery transformation and trophoblast invasion. J Pathol 2006;208:535–542. [DOI] [PubMed] [Google Scholar]
- Banatvala JE, Brown DWG. Rubella. Lancet 2004;363:1127–1137. [DOI] [PubMed] [Google Scholar]
- Basurko C, Carles G, Youssef M, Guindi WEL. Maternal and foetal consequences of dengue fever during pregnancy. Eur J Obstet Gynecol Reprod Biol 2009;147:29–32. [DOI] [PubMed] [Google Scholar]
- Baud D, Regan L, Greub G. Emerging role of Chlamydia and Chlamydia-like organisms in adverse pregnancy outcomes. Curr Opin Infect Dis 2008;21:70–76. [DOI] [PubMed] [Google Scholar]
- Baud D, Goy G, Jaton K, Osterheld M-C, Blumer S, Borel N, Vial Y, Hohlfeld P, Pospischil A, Greub G. Role of Chlamydia trachomatis in miscarriage. Emerg Infect Dis 2011;17:1630–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayraktar MR, Ozerol IH, Gucluer N, Celik O. Prevalence and antibiotic susceptibility of Mycoplasma hominis and Ureaplasma urealyticum in pregnant women. Int J Infect Dis 2010;14:e90–e95. [DOI] [PubMed] [Google Scholar]
- Benedetto C, Tibaldi C, Marozio L, Marini S, Masuelli G, Pelissetto S, Sozzani P, Latino MA. Cervicovaginal infections during pregnancy: epidemiological and microbiological aspects. J Matern Fetal Neonatal Med 2004;16 Suppl 2:9–12. [DOI] [PubMed] [Google Scholar]
- Bloom-Feshbach K, Simonsen L, Viboud C, Mølbak K, Miller MA, Gottfredsson M, Andreasen V. Natality decline and miscarriages associated with the 1918 influenza pandemic: the Scandinavian and United States experiences. J Infect Dis 2011;204:1157–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldorini R, Allegrini S, Miglio U, Nestasio I, Paganotti A, Veggiani C, Monga G, Pietropaolo V. BK virus sequences in specimens from aborted fetuses. J Med Virol 2010;82:2127–2132. [DOI] [PubMed] [Google Scholar]
- Bonvicini F, Puccetti C, Salfi NCM, Guerra B, Gallinella G, Rizzo N, Zerbini M. Gestational and fetal outcomes in B19 maternal infection: a problem of diagnosis. J Clin Microbiol 2011;49:3514–3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brkic S, Bogavac MA, Simin N, Hrnjakovic-Cvetkovic I, Milosevic V, Maric D. Unusual high rate of asymptomatic maternal parvovirus B19 infection associated with severe fetal outcome. J Matern Fetal Neonatal Med 2011;24:647–649. [DOI] [PubMed] [Google Scholar]
- Brocklehurst P, Rooney G. Interventions for treating genital chlamydia trachomatis infection in pregnancy. Cochrane Database Syst Rev 2000:1–123. [DOI] [PMC free article] [PubMed]
- Brocklehurst P, Gordon A, Heatley E, Milan S. Antibiotics for treating bacterial vaginosis in pregnancy (Review). Cochrane Database Syst Rev 2013. [DOI] [PMC free article] [PubMed]
- Broliden K, Tolfvenstam T, Norbeck O. Clinical aspects of parvovirus B19 infection. J Intern Med 2006;260:285–304. [DOI] [PubMed] [Google Scholar]
- Brunham RC, Paavonen J, Stevens CE, Kiviat N, Kuo CC, Critchlow CW, Holmes KK. Mucopurulent cervicitis—the ignored counterpart in women of urethritis in men. N Engl J Med 1984;311:1–6. [DOI] [PubMed] [Google Scholar]
- Bruni L, Diaz M, Castellsagué X, Ferrer E, Bosch FX, de Sanjosé S. Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J Infect Dis 2010;202:1789–1799. [DOI] [PubMed] [Google Scholar]
- Bulletti C, Flamigni C, Giacomucci E. Reproductive failure due to spontaneous abortion and recurrent miscarriage. Hum Reprod Update 1996;2:118–136. [DOI] [PubMed] [Google Scholar]
- Cajaiba MM, Parks WT, Fuhrer K, Randhawa PS. Evaluation of human polyomavirus BK as a potential cause of villitis of unknown etiology and spontaneous abortion. J Med Virol 2011;83:1031–1033. [DOI] [PubMed] [Google Scholar]
- Casal C, Araújo EDC, Corvelo TCDO. Risk factors and pregnancy outcomes in women with syphilis diagnosed using a molecular approach. Sex Transm Infect 2012;89:257–261. [DOI] [PubMed] [Google Scholar]
- Casari E, Ferrario A, Morenghi E, Montanelli A. Gardnerella, Trichomonas vaginalis, Candida, Chlamydia trachomatis, Mycoplasma hominis and Ureaplasma urealyticum in the genital discharge of symptomatic fertile and asymptomatic infertile women. New Microbiol 2010;33:69–76. [PubMed] [Google Scholar]
- Centres for Disease Control and Prevention—CDC. Dengue fever 2012.
- Centres for Disease Control and Prevention—CDC. Q fever 2013.
- Centres for Disease Control and Prevention—CDC. Brucellosis 2012a.
- Centres for Disease Control and Prevention—CDC. Mycoplasma genitalium 2012b.
- Centres for Disease Control and Prevention—CDC. Sexually Transmitted Diseases Treatment Guidelines, 2010. MMWR Recomm Rep 2010;59:1–110. [PubMed] [Google Scholar]
- Chan G, Hemmings DG, Yurochko AD, Guilbert LJ. Human cytomegalovirus-caused damage to placental trophoblasts mediated by immediate-early gene-induced tumor necrosis factor-α. Am J Pathol 2002;161:1371–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaouat G, Ledée-Bataille N, Dubanchet S, Zourbas S, Sandra O, Martal J. Th1/Th2 paradigm in pregnancy: paradigm lost? Cytokines in pregnancy/early abortion: reexamining the Th1/Th2 paradigm. Int Arch Allergy Immunol 2004;134:93–119. [DOI] [PubMed] [Google Scholar]
- Chen Q, Zhang Y, Lu J, Wang Q, Wang S, Cao Y, Wang H, Duan E. Embryo–uterine cross-talk during implantation: the role of Wnt signaling. Mol Hum Reprod 2009;15:215–221. [DOI] [PubMed] [Google Scholar]
- Chisholm C, Lopez L. Cutaneous infections caused by Herpesviridae: a review. Arch Pathol Lab Med 2011;135:1357–1362. [DOI] [PubMed] [Google Scholar]
- Chou D, Ma Y, Zhang J, McGrath C, Parry S. Cytomegalovirus infection of trophoblast cells elicits an inflammatory response: a possible mechanism of placental dysfunction. Am J Obstet Gynecol 2006;194:535–541. [DOI] [PubMed] [Google Scholar]
- Clark DA, Chaouat G, Arck PC, Mittruecker HW, Levy GA. Cytokine-dependent abortion in CBA×DBA/2 mice is mediated by the procoagulant fgl2 prothrombinase [correction of prothombinase]. J Immunol 1998;160:545–549. [PubMed] [Google Scholar]
- Cohen SE, Klausner JD, Engelman J, Philip S. Syphilis in the modern era: an update for physicians. Infect Dis Clin North Am 2013;27:705–722. [DOI] [PubMed] [Google Scholar]
- Corbel MJ. Brucellosis: an overview. Emerg Infect Dis 1997;3:213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste J, Job-Spira N, Fernandez H. Risk factors for spontaneous abortion: a case-control study in France. Hum Reprod 1991;6:1332–1337. [DOI] [PubMed] [Google Scholar]
- Cotmore SF, Agbandje-McKenna M, Chiorini JA, Mukha DV, Pintel DJ, Qiu J, Soderlund-Venermo M, Tattersall P, Tijssen P, Gatherer D et al. The family Parvoviridae. Arch Virol 2014;159:1239–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosbie EJ, Einstein MH, Franceschi S, Kitchener HC. Human papillomavirus and cervical cancer. Lancet 2013;382:889–899. [DOI] [PubMed] [Google Scholar]
- Cutts FT, Franceschi S, Goldie S, Castellsague X, De Sanjose S, Garnett G, Edmunds WJ, Claeys P, Goldenthal KL, Harperi DM et al. Human papillomavirus and HPV vaccines: a review. Bull World Health Organ 2007;85:719–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darak S, Janssen F, Hutter I. Fertility among HIV-infected Indian women: the biological effect and its implications. J Biosoc Sci 2011;43:19–29. [DOI] [PubMed] [Google Scholar]
- De Beaudrap P, Turyakira E, White LJ, Nabasumba C, Tumwebaze B, Muehlenbachs A, Guérin PJ, Boum Y, McGready R, Piola P. Impact of malaria during pregnancy on pregnancy outcomes in a Ugandan prospective cohort with intensive malaria screening and prompt treatment. Malar J 2013;12:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De la Rochebrochard E, Thonneau P. Paternal age and maternal age are risk factors for miscarriage; results of a multicentre European study. Hum Reprod 2002;17:1649–1656. [DOI] [PubMed] [Google Scholar]
- Deb K, Chaturvedi MM, Jaiswal YK. Gram-negative bacterial LPS induced poor uterine receptivity and implantation failure in mouse: alterations in IL-1beta expression in the preimplantation embryo and uterine horns. Infect Dis Obstet Gynecol 2005;13:125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellicour S, Tatem AJ, Guerra CA, Snow RW, Ter Kuile FO. Quantifying the number of pregnancies at risk of malaria in 2007: a demographic study. PLoS Med 2010;7:e1000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitriadis E, White CA, Jones RL, Salamonsen LA. Cytokines, chemokines and growth factors in endometrium related to implantation. Hum Reprod Update 2005;11:613–630. [DOI] [PubMed] [Google Scholar]
- Disson O, Grayo S, Huillet E, Nikitas G, Langa-Vives F, Dussurget O, Ragon M, Le Monnier A, Babinet C, Cossart P et al. Conjugated action of two species-specific invasion proteins for fetoplacental listeriosis. Nature 2008;455:1114–1118. [DOI] [PubMed] [Google Scholar]
- Donders GG, Van Calsteren K, Bellen G, Reybrouck R, Van Den Bosch T, Riphagen I, Van Lierde S. Predictive value for preterm birth of abnormal vaginal flora, bacterial vaginosis and aerobic vaginitis during the first trimester of pregnancy. BJOG 2009;116:1315–1324. [DOI] [PubMed] [Google Scholar]
- Donders GG, Zodzika J, Rezeberga D. Treatment of bacterial vaginosis: what we have and what we miss. Expert Opin Pharmacother 2014;15:645–657. [DOI] [PubMed] [Google Scholar]
- Dybul M, Fauci A, Bartlett J. Guidelines for using antiretroviral agents among HIV-infected adults and adolescent. Ann Intern Med 2002;137:381–433. [DOI] [PubMed] [Google Scholar]
- Egger M, Low N, Smith GD, Lindblom B, Herrmann B. Screening for chlamydial infections and the risk of ectopic pregnancy in a county in Sweden: ecological analysis. BMJ 1998;316:1776–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiben B, Bartels I, Bähr-Porsch S, Borgmann S, Gatz G, Gellert G, Goebel R, Hammans W, Hentemann M, Osmers R. Cytogenetic analysis of 750 spontaneous abortions with the direct-preparation method of chorionic villi and its implications for studying genetic causes of pregnancy wastage. Am J Hum Genet 1990;47:656–663. [PMC free article] [PubMed] [Google Scholar]
- Embretson J, Zupancic M, Ribas J, Burke A, RACZ P, TENNER-RACZ K, HAASE AT. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature 1993;362:359–362. [DOI] [PubMed] [Google Scholar]
- Emiasegen SE, Nimzing L, Adoga MP, Ohagenyi AY, Lekan R. Parvovirus B19 antibodies and correlates of infection in pregnant women attending an antenatal clinic in central Nigeria. Mem Inst Oswaldo Cruz 2011;106:227–231. [DOI] [PubMed] [Google Scholar]
- Engelhard IM, van den Hout MA, Arntz A. Posttraumatic stress disorder after pregnancy loss. Gen Hosp Psychiatry 2001;23:62–66. [DOI] [PubMed] [Google Scholar]
- Entrican G. Immune regulation during pregnancy and host-pathogen interactions in infectious abortion. J Comp Pathol 2002;126:79–94. [DOI] [PubMed] [Google Scholar]
- Eschenbach D. Bacterial vaginosis and anaerobes in obstetric-gynecologic infection. Clin Infect Dis 1993;16 Suppl 4:S282–S287. [DOI] [PubMed] [Google Scholar]
- European Centre for Disease Prevention and Control. HIV testing—increasing uptake and effectiveness in the European Union 2010.
- Ezechi OC, Gab-Okafor CV, Oladele DA, Kalejaye OO, Oke BA, Ujah IO. Pregnancy, obstetric and neonatal outcomes in HIV positive Nigerian women. Int J Gynecol Obstet 2013;119:S345. [PubMed] [Google Scholar]
- Feist A, Sydler T, Gebbers JJ, Pospischil A, Guscetti F. No association of Chlamydia with abortion. J R Soc Med 1999;92:237–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher S, Genbacev O, Maidji E, Pereira L. Human cytomegalovirus infection of placental cytotrophoblasts in vitro and in utero: implications for transmission and pathogenesis. J Virol 2000;74:6808–6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatt A, Shetty N. Seroprevalence and risk factors for toxoplasmosis among antenatal women in London: a re-examination of risk in an ethnically diverse population. Eur J Public Health 2013;23:648–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food Standards Agency. Risk profile in relation to toxoplasma in the food chain 2012.
- Fretts RC, Schmittdiel J, McLean FH, Usher RH, Goldman MB. Increased maternal age and the risk of fetal death. N Engl J Med 1995;333:953–957. [DOI] [PubMed] [Google Scholar]
- Fried M, Nosten F, Brockman A, Brabin BJ, Duffy PE. Maternal antibodies block malaria. Nature 1998;395:851–852. [DOI] [PubMed] [Google Scholar]
- Friedman RK, Bastos FI, Leite IC, Veloso VG, Moreira RI, Cardoso SW, Vasconcelos de Andrade ÂC, Sampaio MC, Currier J, Grinsztejn B. Pregnancy rates and predictors in women with HIV/AIDS in Rio de Janeiro, Southeastern Brazil. Rev Saude Publica 2011;45:373–381. [DOI] [PubMed] [Google Scholar]
- Galvan-Ramirez MDLL, Troyo R, Roman S, Calvillo-Sanchez C, Bernal-Redondo R. A systematic review and meta-analysis of Toxoplasma gondii infection among the Mexican population. Parasit Vectors 2012;5:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G, Vandenberghe LH, Alvira MR, Lu Y, Calcedo R, Zhou X, Wilson JM. Clades of Adeno-associated viruses are widely disseminated in human tissues. J Virol 2004;78:6381–6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner L, Moffett A. Dendritic cells in the human decidua. Biol Reprod 2003;69:1438–1446. [DOI] [PubMed] [Google Scholar]
- Garland SM, Ní Chuileannáin F, Satzke C, Robins-Browne R. Mechanisms, organisms and markers of infection in pregnancy. J Reprod Immunol 2002;57:169–183. [DOI] [PubMed] [Google Scholar]
- Garolla A, Pizzol D, Foresta C. The role of human papillomavirus on sperm function. Curr Opin Obstet Gynecol 2011;23:232–237. [DOI] [PubMed] [Google Scholar]
- Gibb DM, Kizito H, Russell EC, Chidziva E, Zalwango E, Nalumenya R, Spyer M, Tumukunde D, Nathoo K, Munderi P et al. Pregnancy and infant outcomes among HIV-infected women taking long-term art with and without tenofovir in the DART trial. PLoS Med 2012;9:e1001217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingelmaier A, Wiedenmann K, Sovric M, Mueller M, Kupka MS, Sonnenberg-Schwan U, Mylonas I, Friese K, Weizsaecker K. Consultations of HIV-infected women who wish to become pregnant. Arch Gynecol Obstet 2011;283:893–898. [DOI] [PubMed] [Google Scholar]
- Gnann JW, McCormick JB, Mitchell S, Nelson JA, Oldstone MB. Synthetic peptide immunoassay distinguishes HIV type 1 and HIV type 2 infections. Science 1987;237:1346–1349. [DOI] [PubMed] [Google Scholar]
- Godbole G, Suman P, Gupta SK, Modi D. Decidualized endometrial stromal cell derived factors promote trophoblast invasion. Fertil Steril 2011;95:1278–1283. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Thompson C. The infectious origins of stillbirth. Am J Obstet Gynecol 2003;189:861–873. [DOI] [PubMed] [Google Scholar]
- Gonçalves MAFV. Adeno-associated virus: from defective virus to effective vector. Virol J 2005;2:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gretch DR. Diagnostic tests for hepatitis C. Hepatology 1997;26:43S–46S. [DOI] [PubMed] [Google Scholar]
- Gringhuis SI, van der Vlist M, van den Berg LM, den Dunnen J, Litjens M, Geijtenbeek TBH. HIV-1 exploits innate signaling by TLR8 and DC-SIGN for productive infection of dendritic cells. Nat Immunol 2010;11:419–426. [DOI] [PubMed] [Google Scholar]
- Guleria I, Pollard JW. The trophoblast is a component of the innate immune system during pregnancy. Nat Med 2000;6:589–593. [DOI] [PubMed] [Google Scholar]
- Hadar E, Yogev Y, Melamed N, Chen R, Amir J, Pardo J. Periconceptional cytomegalovirus infection: pregnancy outcome and rate of vertical transmission. Prenat Diagn 2010;30:1213–1216. [DOI] [PubMed] [Google Scholar]
- Hafner L, Beagley K, Timms P. Chlamydia trachomatis infection: host immune responses and potential vaccines. Mucosal Immunol 2008;1:116–130. [DOI] [PubMed] [Google Scholar]
- Halsby K, Guy E, Said B, Francis J, O'Connor C, Kirkbride H, Morgan D. Enhanced surveillance for toxoplasmosis in England and Wales, 2008–2012. Epidemiol Infect 2014;142:1653–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri S, Unger ER, Sternberg M, Dunne EF, Swan D, Patel S, Markowitz LE. Prevalence of genital human papillomavirus among females in the United States, the National Health And Nutrition Examination Survey, 2003–2006. J Infect Dis 2011;204:566–573. [DOI] [PubMed] [Google Scholar]
- Hay P. Bacterial vaginosis and miscarriage. Curr Opin Infect Dis 2004;17:41–44. [DOI] [PubMed] [Google Scholar]
- Hay PE, Lamont RF, Taylor-Robinson D, Morgan DJ, Ison C, Pearson J. Abnormal bacterial colonisation of the genital tract and subsequent preterm delivery and late miscarriage. BMJ 1994;308:295–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermonat PL, Han L, Wendel PJ, Quirk JG, Stern S, Lowery CL, Rechtin TM. Human papillomavirus is more prevalent in first trimester spontaneously aborted products of conception compared to elective specimens. Virus Genes 1997;14:13–17. [DOI] [PubMed] [Google Scholar]
- Hillis SD, Owens LM, Marchbanks PA, Amsterdam LF, Mac Kenzie WR. Recurrent chlamydial infections increase the risks of hospitalization for ectopic pregnancy and pelvic inflammatory disease. Am J Obstet Gynecol 1997;176:103–107. [DOI] [PubMed] [Google Scholar]
- Hirsch HH, Steiger J. Polyomavirus BK. Lancet Infect Dis 2003;3:611–623. [DOI] [PubMed] [Google Scholar]
- Hong F-C, Yang Y-Z, Liu X-L, Feng T-J, Liu J-B, Zhang C-L, Lan L-N, Yao M-Z, Zhou H. Reduction in mother-to-child transmission of syphilis for 10 years in Shenzhen, China. Sex Transm Dis 2014;41:188–193. [DOI] [PubMed] [Google Scholar]
- Horner P, Blee K, Adams E. Time to manage Mycoplasma genitalium as an STI: but not with azithromycin 1 g! Curr Opin Infect Dis 2014;27:68–74. [DOI] [PubMed] [Google Scholar]
- Howie SEM, Horner PJ, Horne AW. Chlamydia trachomatis infection during pregnancy: known unknowns. Discov Med 2011;12:57–64. [PubMed] [Google Scholar]
- Hure AJ, Powers JR, Mishra GD, Herbert DL, Byles JE, Loxton D. Miscarriage, preterm delivery, and stillbirth: large variations in rates within a cohort of Australian women. PLoS One 2012;7:e37109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janier M, Hegyi V, Dupin N, Unemo M, Tiplica G, Potočnik M, French P, Patel R. IUSTI: 2014 European Guideline on the Management of Syphilis Int Union Against Sex Transm Infect 2014;1–29. [DOI] [PubMed]
- Jerzak M, Bischof P. Apoptosis in the first trimester human placenta: the role in maintaining immune privilege at the maternal-foetal interface and in the trophoblast remodelling. Eur J Obstet Gynecol Reprod Biol 2002;100:138–142. [DOI] [PubMed] [Google Scholar]
- Jones JL, Lopez A, Wilson M, Schulkin J, Gibbs R. Congenital toxoplasmosis: a review. Obstet Gynecol Surv 2001;56:296–305. [DOI] [PubMed] [Google Scholar]
- Joste NE, Kundsin RB, Genest DR. Histology and Ureaplasma urealyticum culture in 63 cases of first trimester abortion. Am J Clin Pathol 1994;102:729–732. [DOI] [PubMed] [Google Scholar]
- Joswig A, Gabriel H-D, Kibschull M, Winterhager E. Apoptosis in uterine epithelium and decidua in response to implantation: evidence for two different pathways. Reprod Biol Endocrinol 2003;1:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapranos NC, Kotronias DC. Detection of herpes simplex virus in first trimester pregnancy loss using molecular techniques. In Vivo 2009;23:839–842. [PubMed] [Google Scholar]
- Kar S, Kar S. Control of malaria. Nat Rev Drug Discov 2010;9:511–512. [DOI] [PubMed] [Google Scholar]
- Karalar L, Lindner J, Schimanski S, Kertai M, Segerer H, Modrow S. Prevalence and clinical aspects of human bocavirus infection in children. Clin Microbiol Infect 2010;16:633–639. [DOI] [PubMed] [Google Scholar]
- Kariyawasam S, Senanayake H. Dengue infections during pregnancy: case series from a tertiary care hospital in Sri Lanka. J Infect Dev Ctries 2010;4:767–775. [DOI] [PubMed] [Google Scholar]
- Kashanian M, Akbarian AR, Baradaran H, Shabandoust SH. Pregnancy outcome following a previous spontaneous abortion (miscarriage). Gynecol Obstet Invest 2006;61:167–170. [DOI] [PubMed] [Google Scholar]
- Khan MY, Mah MW, Memish ZA. Brucellosis in pregnant women. Clin Infect Dis 2001;32:1172–1177. [DOI] [PubMed] [Google Scholar]
- Kim H-Y, Kasonde P, Mwiya M, Thea DM, Kankasa C, Sinkala M, Aldrovandi G, Kuhn L. Pregnancy loss and role of infant HIV status on perinatal mortality among HIV-infected women. BMC Pediatr 2012a;12:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim ID, Chang HS, Hwang KJ. Herpes simplex virus 2 infection rate and necessity of screening during pregnancy: a clinical and seroepidemiologic study. Yonsei Med J 2012b;53:401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaren VNA, Kijlstra A. Toxoplasmosis, an overview with emphasis on ocular involvement. Ocul Immunol Inflamm 2002;10:1–26. [DOI] [PubMed] [Google Scholar]
- Koch S, Solana R, Dela Rosa O, Pawelec G. Human cytomegalovirus infection and T cell immunosenescence: a mini review. Mech Ageing Dev 2006;127:538–543. [DOI] [PubMed] [Google Scholar]
- Kortekangas-Savolainen O, Mäkinen J, Koivusalo K, Mattila K. Hospital-diagnosed late sequelae after female Chlamydia trachomatis infections in 1990–2006 in Turku, Finland. Gynecol Obstet Invest 2012;73:299–303. [DOI] [PubMed] [Google Scholar]
- Kovács L, Nagy E, Berbik I, Mészáros G, Deák J, Nyári T. The frequency and the role of Chlamydia trachomatis infection in premature labor. Int J Gynaecol Obstet 1998;62:47–54. [DOI] [PubMed] [Google Scholar]
- Krajden M, McNabb G, Petric M. The laboratory diagnosis of hepatitis B virus. Can J Infect Dis Med Microbiol 2005;16:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurdoglu M, Adali E, Kurdoglu Z, Karahocagil MK, Kolusari A, Yildizhan R, Kucukaydin Z, Sahin HG, Kamaci M, Akdeniz H. Brucellosis in pregnancy: a 6-year clinical analysis. Arch Gynecol Obstet 2010;281:201–206. [DOI] [PubMed] [Google Scholar]
- Lamont RF, Sobel JD, Akins RA, Hassan SS, Chaiworapongsa T, Kusanovic JP, Romero R. The vaginal microbiome: new information about genital tract flora using molecular based techniques. BJOG 2011;118:533–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashen H, Fear K, Sturdee DW. Obesity is associated with increased risk of first trimester and recurrent miscarriage: matched case-control study. Hum Reprod 2004;19:1644–1646. [DOI] [PubMed] [Google Scholar]
- Lecuit M, Nelson DM, Smith SD, Khun H, Huerre M, Vacher-Lavenu M-C, Gordon JI, Cossart P. Targeting and crossing of the human maternofetal barrier by Listeria monocytogenes: role of internalin interaction with trophoblast E-cadherin. Proc Natl Acad Sci USA 2004;101:6152–6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledru S, Méda N, Ledru E, Bazie AJ, Chiron JP. HIV-1 infection associated with abnormal vaginal flora morphology and bacterial vaginosis. Lancet 1997;350:1251–1252. [DOI] [PubMed] [Google Scholar]
- Lulu AR, Araj GF, Khateeb MI, Mustafa MY, Yusuf AR, Fenech FF. Human brucellosis in Kuwait: a prospective study of 400 cases. Q J Med 1988;66:39–54. [PubMed] [Google Scholar]
- Lyall F. Priming and remodelling of human placental bed spiral arteries during pregnancy—a review. Placenta 2005;26:S31–S36. [DOI] [PubMed] [Google Scholar]
- Maconochie N, Doyle P, Prior S, Simmons R. Risk factors for first trimester miscarriage-results from a UK-population-based case-control study. BJOG 2007;114:170–186. [DOI] [PubMed] [Google Scholar]
- Margolis TP, Imai Y, Yang L, Vallas V, Krause PR. Herpes simplex virus type 2 (HSV-2) establishes latent infection in a different population of ganglionic neurons than HSV-1: role of latency-associated transcripts. J Virol 2007;81:1872–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DH, Koutsky L, Eschenbach DA, Daling JR, Alexander ER, Benedetti JK, Holmes KK. Prematurity and perinatal mortality in pregnancies complicated by maternal Chlamydia trachomatis infections. JAMA 1982;247:1585–1588. [PubMed] [Google Scholar]
- Masutani K. Current problems in screening, diagnosis and treatment of polyomavirus BK nephropathy. Nephrology (Carlton) 2014;19(Suppl 3):11–16. [DOI] [PubMed] [Google Scholar]
- Maurin M, Raoult D. Q fever. Clin Microbiol Rev 1999;12:518–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGready R, Lee SJ, Wiladphaingern J, Ashley EA, Rijken MJ, Boel M, Simpson JA, Paw MK, Pimanpanarak M, Mu O et al. Adverse effects of falciparum and vivax malaria and the safety of antimalarial treatment in early pregnancy: a population-based study. Lancet Infect Dis 2012;12:388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MedlinePlus. MedlinePlus—Chlamydia trachomatis 2014. [Google Scholar]
- Merviel P, Challier JC, Carbillon L, Foidart JM, Uzan S. The role of integrins in human embryo implantation. Fetal Diagn Ther 2001;16:364–371. [DOI] [PubMed] [Google Scholar]
- Metcalf CJE, Munayco CV, Chowell G, Grenfell BT, Bjørnstad ON. Rubella metapopulation dynamics and importance of spatial coupling to the risk of congenital rubella syndrome in Peru. J R Soc Interface 2011;8:369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel MZ, Khong TY, Clark DA, Beard RW. A morphological and immunological study of human placental bed biopsies in miscarriage. Br J Obstet Gynaecol 1990;97:984–988. [DOI] [PubMed] [Google Scholar]
- Miedema F, Petit A, Terpstra FG, Schattenkerk JK, de Wolf F, Al BJ, Roos M, Lange J, Danner SA, Goudsmit J et al. Immunological abnormalities in human immunodeficiency virus (HIV)-infected asymptomatic homosexual men. HIV affects the immune system before CD4+ T helper. J Clin Invest 1988;82:1908–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minton EJ, Tysoe C, Sinclair JH, Sissons JG. Human cytomegalovirus infection of the monocyte/macrophage lineage in bone marrow. J Virol 1994;68:4017–4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett-King A. Natural killer cells and pregnancy. Nat Rev Immunol 2002;2:656–663. [DOI] [PubMed] [Google Scholar]
- Molijn A, Kleter B, Quint W, van Doorn L-J. Molecular diagnosis of human papillomavirus (HPV) infections. J Clin Virol 2005;32 Suppl 1:S43–S51. [DOI] [PubMed] [Google Scholar]
- Moreno E. Retrospective and prospective perspectives on zoonotic brucellosis. Front Microbiol 2014;5:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Pérez DA, Ruíz JA, Patarroyo MA. Reticulocytes: plasmodium vivax target cells. Biol Cell 2013;105:251–260. [DOI] [PubMed] [Google Scholar]
- Mouquet H, Nussenzweig MC. HIV: roadmaps to a vaccine. Nature 2013;496:441–442. [DOI] [PubMed] [Google Scholar]
- Munster J, Steggerda L, Leenders A, Aarnoudse J, Hak E. Screening for Coxiella burnetii infection during pregnancy: pros and cons according to the Wilson and Jungner criteria. Euro Surveill 2012;17:1–5. [PubMed] [Google Scholar]
- Murad EA, Babiker SM, Gasim GI, Rayis DA, Adam I. Epidemiology of hepatitis B and hepatitis C virus infections in pregnant women in Sana'a, Yemen. BMC Pregnancy Childbirth 2013;13:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscat M, Shefer A, Ben Mamou M, Spataru R, Jankovic D, Deshevoy S, Butler R, Pfeifer D. The state of measles and rubella in the WHO European Region, 2013. Clin Microbiol Infect 2014;20:12–18. [DOI] [PubMed] [Google Scholar]
- Napierala Mavedzenge S, Weiss HA. Association of Mycoplasma genitalium and HIV infection: a systematic review and meta-analysis. AIDS 2009;23:611–620. [DOI] [PubMed] [Google Scholar]
- Nielsen GL, Sørensen HT, Larsen H, Pedersen L. Risk of adverse birth outcome and miscarriage in pregnant users of non-steroidal anti-inflammatory drugs: population based observational study and case-control study. BMJ 2001;322:266–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen SY, Hjøllund NH, Andersen AMN, Henriksen TB, Kantsø B, Krogfelt KA, Mølbak K. Presence of antibodies against coxiella burnetii and risk of spontaneous abortion: a nested case-control study. PLoS One 2012;7:e31909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen SY, Andersen A-MN, Mølbak K, Hjøllund NH, Kantsø B, Krogfelt KA, Henriksen TB. No excess risk of adverse pregnancy outcomes among women with serological markers of previous infection with Coxiella burnetii: evidence from the Danish National Birth Cohort. BMC Infect Dis 2013;13:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nybo Andersen AM, Wohlfahrt J, Christens P, Olsen J, Melbye M. Maternal age and fetal loss: population based register linkage study. BMJ 2000;320:1708–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Office for National Statistics. Live births England and Wales 2012 2012. Available at: http://www.ons.gov.uk/ons/rel/vsob1/birth-summary-tables-england-and-wales/2012/stb-births-in-england-and-wales-2012.html (15 February 2015, date last accessed).
- Oluyomi-Obi T, Avery L, Schneider C, Kumar A, Lapinsky S, Menticoglou S, Zarychanski R. Perinatal and maternal outcomes in critically ill obstetrics patients with pandemic H1N1 Influenza A. J Obstet Gynaecol Can 2010;32:443–447. 448–452. [DOI] [PubMed] [Google Scholar]
- Orenstein J, Fox C, Wahl S. Macrophages as a source of HIV during opportunistic infections. Science 1997;276:1857–1861. [DOI] [PubMed] [Google Scholar]
- Oswal S, Lyons G. Syphilis in pregnancy. Contin Educ Anaesthesia Crit Care Pain 2008;8:224–227. [Google Scholar]
- Paavonen J, Lehtinen M. Chlamydial pelvic inflammatory disease. Hum Reprod Update 1996;2:519–529. [DOI] [PubMed] [Google Scholar]
- Pereira C. Molecular detection of adeno-associated virus in cases of spontaneous and intentional human abortion. J Med Virol 2010;1693:1689–1693. [DOI] [PubMed] [Google Scholar]
- Perino A, Giovannelli L, Schillaci R, Ruvolo G, Fiorentino FP, Alimondi P, Cefal E, Ammatuna P. Human papillomavirus infection in couples undergoing in vitro fertilization procedures: impact on reproductive outcomes. Fertil Steril 2011;95:1845–1848. [DOI] [PubMed] [Google Scholar]
- Pijnenborg R, Dixon G, Robertson WB, Brosens I. Trophoblastic invasion of human decidua from 8 to 18 weeks of pregnancy. Placenta 1980;1:3–19. [DOI] [PubMed] [Google Scholar]
- Pijnenborg R, Bland JM, Robertson WB, Dixon G, Brosens I. The pattern of interstitial trophoblastic invasion of the myometrium in early human pregnancy. Placenta 1981;2:303–316. [DOI] [PubMed] [Google Scholar]
- Pouliot SH, Xiong X, Harville E, Paz-Soldan V, Tomashek KM, Breart G, Buekens P. Maternal dengue and pregnancy outcomes: a systematic review. Obstet Gynecol Surv 2010;65:107–118. [DOI] [PubMed] [Google Scholar]
- Public Health England. Pregnancy Screening Guidelines UK 2015. Available at: https://www.gov.uk/infectious-diseases-in-pregnancy-screening-programme-overview (30 May 2015, date last accessed).
- Ralph SG, Rutherford AJ, Wilson JD. Influence of bacterial vaginosis on conception and miscarriage in the first trimester: cohort study. BMJ 1999;319:220–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid NA, Lalitkumar S, Lalitkumar PG, Gemzell-Danielsson K. Endometrial receptivity and human embryo implantation. Am J Reprod Immunol 2011;66:23–30. [DOI] [PubMed] [Google Scholar]
- Reef SE, Strebel P, Dabbagh A, Gacic-Dobo M, Cochi S. Progress toward control of rubella and prevention of congenital rubella syndrome—worldwide, 2009. J Infect Dis 2011;204:S24–S27. [DOI] [PubMed] [Google Scholar]
- Riipinen A, Väisänen E, Lahtinen A, Karikoski R, Nuutila M, Surcel HM, Taskinen H, Hedman K, Söderlund-Venermo M. Absence of human bocavirus from deceased fetuses and their mothers. J Clin Virol 2010;47:186–188. [DOI] [PubMed] [Google Scholar]
- Rocchetti TT, Marconi C, Rall VLM, Borges VTM, Corrente JE, Da Silva MG. Group B streptococci colonization in pregnant women: risk factors and evaluation of the vaginal flora. Arch Gynecol Obstet 2011;283:717–721. [DOI] [PubMed] [Google Scholar]
- Saraswathy TS, Az-Ulhusna A, Asshikin RN, Suriani S, Zainah S. Seroprevalence of cytomegalovirus infection in pregnant women and associated role in obstetric complications: a preliminary study. Southeast Asian J Trop Med Public Health 2011;42:320–322. [PubMed] [Google Scholar]
- Schlehofer JR, Boeke C, Reuland M, Eggert-Kruse W. Presence of DNA of adeno-associated virus in subfertile couples, but no association with fertility factors. Hum Reprod 2012;27:770–778. [DOI] [PubMed] [Google Scholar]
- Shaw JL V, Wills GS, Lee KF, Horner PJ, McClure MO, Abrahams VM, Wheelhouse N, Jabbour HN, Critchley HOD, Entrican G et al. Chlamydia trachomatis infection increases fallopian tube PROKR2 via TLR2 and NFκB activation resulting in a microenvironment predisposed to ectopic pregnancy. Am J Pathol 2011;178:253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short VL, Jensen JS, Nelson DB, Murray PJ, Ness RB, Haggerty CL. Mycoplasma genitalium among young, urban pregnant women. Infect Dis Obstet Gynecol 2010;2010:984760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silingardi E, Santunione AL, Rivasi F, Gasser B, Zago S, Garagnani L. Unexpected intrauterine fetal death in parvovirus B19 fetal infection. Am J Forensic Med Pathol 2009;30:394–397. [DOI] [PubMed] [Google Scholar]
- Singh A, Preiksaitis J, Ferenczy A, Romanowski B. The laboratory diagnosis of herpes simplex virus infections. Can J Infect Dis Med Microbiol 2005;16:92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinzger C, Digel M, Jahn G. Cytomegalovirus cell tropism. Curr Top Microbiol Immunol 2008;325:63–83. [DOI] [PubMed] [Google Scholar]
- Skoczyński M, Goździcka-Józefiak A, Kwaśniewska A. Prevalence of human papillomavirus in spontaneously aborted products of conception. Acta Obstet Gynecol Scand 2011;90:1402–1405. [DOI] [PubMed] [Google Scholar]
- Slama R, Bouyer J, Windham G. Influence of paternal age on the risk of spontaneous abortion. Am J Epidemiol 2005;161:816–823. [DOI] [PubMed] [Google Scholar]
- Smart S. Social and sexual risk factors for bacterial vaginosis. Sex Transm Infect 2004;80:58–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sopori M. Effects of cigarette smoke on the immune system. Nat Rev Immunol 2002;2:372–377. [DOI] [PubMed] [Google Scholar]
- Srinivas SK, MA Y, Sammel MD, Chou D, McGrath C, Parry S, Elovitz MA. Placental inflammation and viral infection are implicated in second trimester pregnancy loss. Am J Obstet Gynecol 2006;195:797–802. [DOI] [PubMed] [Google Scholar]
- Stamm WE, Wagner KF, Amsel R, Alexander ER, Turck M, Counts GW, Holmes KK. Causes of the acute urethral syndrome in women. N Engl J Med 1980;304:409–415. [DOI] [PubMed] [Google Scholar]
- Suzumori N, Sugiura-Ogasawara M. Genetic factors as a cause of miscarriage. Curr Med Chem 2010;17:3431–3437. [DOI] [PubMed] [Google Scholar]
- Taha TE, Hoover DR, Dallabetta GA, Kumwenda NI, Mtimavalye LA, Yang LP, Liomba GN, Broadhead RL, Chiphangwi JD, Miotti PG. Bacterial vaginosis and disturbances of vaginal flora: association with increased acquisition of HIV. AIDS 1998;12:1699–1706. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 1999;11:443–451. [DOI] [PubMed] [Google Scholar]
- Tan PC, Soe MZ, Lay K, Wang SM, de Sekaran S, Omar SZ. Dengue infection and miscarriage: a prospective case control study. PLoS Negl Trop Dis 2012;6:e1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao L, Suhua C, Juanjuan C, Zongzhi Y, Juan X, Dandan Z. In vitro study on human cytomegalovirus affecting early pregnancy villous EVT's invasion function. Virol J 2011;8:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavo V. Prevalence of Mycoplasma hominis and Ureaplazma urealyticum among women of reproductive age in Albania. Med Arch 2013;67:25. [DOI] [PubMed] [Google Scholar]
- Taylor-Robinson D, Jensen JS. Mycoplasma genitalium: from chrysalis to multicolored butterfly. Clin Microbiol Rev 2011;24:498–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temmerman M, Lopita MI, Sanghvi HCG, Sinei SKF, Plummer FA, Piot P. The role of maternal syphilis, gonorrhoea and HIV-1 infections in spontaneous abortion. Int J STD AIDS 1992;3:418–422. [DOI] [PubMed] [Google Scholar]
- Thaxton JE, Sharma S. Interleukin-10: a multi-faceted agent of pregnancy. Am J Reprod Immunol 2010;63:482–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thellin O, Heinen E. Pregnancy and the immune system: between tolerance and rejection. Toxicology 2003;185:179–184. [DOI] [PubMed] [Google Scholar]
- Tranguch S, Daikoku T, Guo Y, Wang H, Dey SK. Molecular complexity in establishing uterine receptivity and implantation. Cell Mol Life Sci 2005;62:1964–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Preventive Services. Bacterial Vaginosis in Pregnancy to Prevent Preterm Delivery: Screening 2008.
- UK National Screening Committee. Infectious Diseases in Pregnancy Screening across the UK 2013. Available at: http://www.screening.nhs.uk/infectiousdiseases-compare (30 May 2015, date last accessed).
- UK National Screening Committee. The UK NSC recommendation on Bacterial vaginosis screening in pregnancy (currently under review). 2014. Available at: http://www.screening.nhs.uk/bacterialvaginosis (20 April 2015, date last accessed).
- Vado-Solis IA, Suarez-Solis VM, Jimenez-Delgadillo B, Zavala-Velazquez JE, Segura JC. Toxoplasma gondii presence in women with spontaneous abortion in Yucatan, Mexico. J Parasitol 2012;99:383–385. [DOI] [PubMed] [Google Scholar]
- van der Hoek W, Dijkstra F, Schimmer B, Schneeberger PM, Vellema P, Wijkmans C, ter Schegget R, Hackert V, van Duynhoven Y. Q fever in the Netherlands: an update on the epidemiology and control measures. Euro Surveill 2010;15:57–60. [PubMed] [Google Scholar]
- Vázquez-Boland JA, Kuhn M, Berche P, Chakraborty T, Domínguez-Bernal G, Goebel W, González-Zorn B, Wehland J, Kreft J. Listeria pathogenesis and molecular virulence determinants. Clin Microbiol Rev 2001;14:584–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt AP, Brown M, Pathiraja M, Anbazhagan A, Coyle PV. The lack of routine surveillance of Parvovirus B19 infection in pregnancy prevents an accurate understanding of this regular cause of fetal loss and the risks posed by occupational exposure. J Med Microbiol 2013;62:86–92. [DOI] [PubMed] [Google Scholar]
- Whitley RJ, Roizman B. Herpes simplex virus infections. Lancet 2001;357:1513–1518. [DOI] [PubMed] [Google Scholar]
- Wilkowska-Trojniel M, Zdrodowska-Stefanow B, Ostaszewska-Puchalska I, Redźko S, Przepieść J, Zdrodowski M. The influence of Chlamydia trachomatis infection on spontaneous abortions. Adv Med Sci 2009;54:86–90. [DOI] [PubMed] [Google Scholar]
- Wilson JD, Ralph SG, Rutherford AJ. Rates of bacterial vaginosis in women undergoing in vitro fertilisation for different types of infertility BJOG 2002;109:714–717. [DOI] [PubMed] [Google Scholar]
- World Health Organisation (WHO). Prevalence and incidence of selected sexually transmitted infections. 2011.
- World Health Organisation (WHO). HIV 2013a. Available at: http://www.who.int/mediacentre/factsheets/fs360/en/index.html (15 February 2015, date last accessed).
- World Health Organisation (WHO). World Malaria Report 2013. 2013b.
- Yang R, Wang Y, Qiao J, Liu P, Geng L, Guo Y. Does human papillomavirus infection do harm to in-vitro fertilization outcomes and subsequent pregnancy outcomes? Chin Med J (Engl) 2013;126:683–687. [PubMed] [Google Scholar]
- Ye F, Liu Y, Jin Y, Shi J, Yang X, Liu X, Zhang X, Lin S, Kong Y, Zhang L. The effect of hepatitis B virus infected embryos on pregnancy outcome. Eur J Obstet Gynecol Reprod Biol 2014;172:10–14. [DOI] [PubMed] [Google Scholar]
- Yoshimura A, Lien E, Ingalls RR, Tuomanen E, Dziarski R, Golenbock D. Cutting edge: recognition of Gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J Immunol 1999;163:1–5. [PubMed] [Google Scholar]
- Young NS, Brown KE. Parvovirus B19. N Engl J Med 2004;350:586–597. [DOI] [PubMed] [Google Scholar]
- Yudin MH, Money DM. Screening and management of bacterial vaginosis in pregnancy. J Obstet Gynaecol Can 2008;30:702–716. [DOI] [PubMed] [Google Scholar]
- Zolopa A, Andersen J, Powderly W, Sanchez A, Sanne I, Suckow C, Hogg E, Komarow L. Early antiretroviral therapy reduces AIDS progression/death in individuals with acute opportunistic infections: a multicenter randomized strategy trial. PLoS One 2009;4:e5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.