Excessive fluid resuscitation can lead to a build-up of fluid in the interstitium, which, if left unchecked, may progress to secondary increased abdominal pressures, abdominal compartment syndrome (ACS) and, ultimately, several systemic impairments and organ dysfunction. Bowel ischemia has been theorized to occur in ACS but has not been reported in the literature. Accordingly, this article describes multiple instances of secondary ACS in a four-year-old girl during treatment for severe burn and inhalation injury.
Keywords: Abdominal compartment syndrome, Burns, Ischemic bowel, Pediatrics
Abstract
Abdominal compartment syndrome (ACS) is a known complication of the large-volume resuscitation that burn patients receive. Bowel ischemia has been theorized to occur in ACS but has yet to be described in the literature. The authors report an occurrence of late bowel obstruction related to ACS-associated bowel ischemia in a burn patient.
A four-year-old previously well girl sustained 70% total body surface area burns with inhalation injury. The areas injured were the anterior neck, circumferential torso from neck to waist, left arm, left thigh and two-thirds of her right thigh. Fluid resuscitation was initially administered using the modified Parkland formula. Her transfer to the regional burn unit from a local hospital was complicated by early septic shock from a line infection, which increased her resuscitation fluid requirements. Infection ultimately led to multiple instances of ACS. Intervention with percutaneous drainage led to immediate improvement; however, the episodes of ACS resulted in a late small bowel obstruction secondary to stricture, requiring a laparotomy and bowel resection.
Abstract
Le syndrome du compartiment abdominal (SCA) est une complication connue du volume liquidien important que reçoivent les patients brûlés pendant la réanimation. Théoriquement, l’ischémie intestinale est liée au SCA, mais elle n’a jamais été décrite dans des publications scientifiques. Les auteurs présentent un cas d’obstruction intestinale tardive liée à une ischémie intestinale associée à un SCA chez une patiente brûlée.
Une fillette de quatre ans auparavant en santé a subi des brûlures sur 70 % de la surface totale de son corps, de même que des brûlures par inhalation. Elle était brûlée sur la partie antérieure du cou, la circonférence du torse entre le cou et la taille, le bras gauche, la cuisse gauche et les deux tiers de la cuisse droite. La réanimation liquidienne a d’abord été administrée selon la formule de Parkland modifiée. Le transfert de la patiente d’un hôpital local à l’unité régionale des brûlés a été compliqué par un choc septique précoce causé par une infection liée à un cathéter, ce qui a accru la réanimation liquidienne. L’infection a provoqué de multiples SCA. Un drain percutané a favorisé une amélioration clinique immédiate de la patiente, mais les épisodes de SCA ont entraîné une obstruction tardive de l’intestin grêle attribuable à un rétrécissement, laquelle a exigé une laparotomie et une résection intestinale.
Abdominal compartment syndrome (ACS) secondary to fluid resuscitation has been well documented in the literature; however, the development of bowel ischemia and late sequelae after treated ACS has only been described in theory (1). Bowel ischemia can lead to a significant increase in morbidity, and can be fatal if left untreated (2). The reported mortality of secondary ACS from burn injury ranges from 40% to 100% (3,4). In the clinical picture of a severely burned child, bowel ischemia is unexpected and may be missed. The present report reviews the pathophysiology of ACS in burn injury and discusses the potential for bowel ischemia and the consequences thereof.
Fluid resuscitation is required to prevent hypovolemic shock secondary to the inflammatory response to burn injury (5). At our institution, this is typically based on the Parkland formula: 4 mL/percentage of total body surface area/kg over 24 h. Due to diffusion and estimated compartment volumes, it is predicted that approximately two-thirds of resuscitation crystalloid enters the interstitial space (6). In the peritoneal cavity, this can lead to an increase in intra-abdominal pressure (IAP). IAPs can be measured by bladder pressures and have been the traditional marker for severity of ACS. A normal physiological IAP ranges from 5 mmHg to 7 mmHg (7 mmH2O to 10 mmH2O) (7).
ACS is defined as a sustained IAP >20 mmHg, leading to organ dysfunction (8). IAPs are measured by bladder pressures and have been the traditional marker for impending ACS or severity of ACS. However, measuring abdominal perfusion pressure (APP = mean arterial pressure [MAP] − IAP) has been hypothesized to be a better indicator of circulation to the abdominal viscera, and an APP <60 mmHg has been associated with higher mortality after the onset of ACS (4).
High abdominal pressures lead to several systemic impairments: cephalad movement of the diaphragm leads to cardiac and lung compression, reduced venous return and, subsequently, contributes to hypoxemia, hypercapnia, atelectasis and ventilation-perfusion mismatch. ACS will also compress renal vessels, activating sympathetic drive and the renin-angiotensin system; these effects contribute to a decrease in urine output. Primarily, renal vasoconstriction leads to a significant decrease in urine output, and is typically the first indicator of the onset of ACS – oliguria is noted at IAPs >15 mmHg and anuria at IAPs of 30 mmHg. Reports document a decrease in mesenteric blood flow at 10 mmHg IAP; intestinal mucosa perfusion decreases at 20 mmHg IAP, and celiac and superior mesenteric artery flow is compromised at IAPs >40 mmHg. To further exacerbate the effects on gastric circulation, the increased pressure may compress mesenteric veins, impairing drainage and exacerbating ACS, ultimately leading to further gut hypoperfusion, ischemic bowel, decreased intramural pH and worsening lactic acidosis (1,4,9,10). In the context of tissue injury consistent with severe burn trauma, inflammatory responses can also exacerbate an ischemic bowel. The inflammatory cytokines released will increase capillary permeability, leading to more edema and higher IAP (11). This is a vicious cycle in which edema results in injury, which in turn worsens edema.
CASE PRESENTATION
A previously healthy four-year-old girl was treated for 70% total body surface area flame burns. She sustained full-thickness burns involving her circumferential torso, from the neck to the waist, and circumferential burns to both arms and thighs. She also exhibited signs of an inhalation injury. The child was initially resuscitated at a local hospital before being transferred to the regional burn unit >24 h later. At the local hospital, fluid resuscitation with the modified Parkland formula (4 mL/percentage of total body surface area /kg) was started, using a calculated total of 5600 mL of Ringer’s lactate over a 24 h period. The initial rate was titrated to 200 mL/h to 230 mL/h to maintain urine output between 1 mL/kg/h and 2 mL/kg/h. Following transfer to the regional burn centre, the child developed a Gram-positive bacteremia, later determined to be Staphylococcus pneumoniae related to a central line placed at the referring centre. This eventually led to recurring sepsis and was a significant contributing factor to the onset of ACS. On presentation to the burn unit, she was hypotensive (80/40 mmHg) and tachycardic (heart rate >190 beats/min), consistent with signs of septic shock, requiring vasopressor support. Despite large-volume resuscitation, urine output decreased; this led to the eventual use of albumin at the burn unit, and an infusion of 25% albumin at 50 mL/h was administered.
Escharatomies were performed on her left leg, arm, hand, chest and abdomen. Early burn excision occurred the morning after admission to the burn unit. In a second surgery on the day after, the burn sites on the thorax and left leg were further debrided and covered with allograft. One week after admission, the allograft was removed and she received a widely meshed split-thickness autograft to her back, autograft to her left arm and Integra (bovine type I collagen, Integra Life Sciences, USA) for her anterior trunk. The estimated blood loss during the surgery was 150 mL; no blood products were given and urine output was >1 mL/kg/h. The surgery was tolerated well, and the patient was brought back to the pediatric intensive care unit (PICU) in stable condition.
For the next two days, she exhibited stable systolic pressures between 110 mmHg and 140 mmHg, and a urine output varying from 2 mL/kg/h to 8 mL/kg/h. On the 10th day after her admission, the patient was noted to have a distended abdomen; bladder pressures measured between 22 cmH2O to 24 cmH2O (16 mmHg to 18 mmHg), and urine output decreased from 1.0 mL/kg/h to 1.5 mL/kg/h to <0.5 mL/kg/h. Throughout this time, blood pressures remained constant, with an average MAP of 80 mmHg. An abdominal x-ray revealed grossly distended air-filled loops of bowel suggestive of narcotic-related ileus, and as such she was initially treated with neostigmine, which resolved the ileus and reduced the distention. Throughout this episode, urine output remained consistently >1 mL/kg/h. Bladder pressures were not immediately measured because normal organ function resumed after the administration of neostigmine. Routine bladder pressures measured after the administration of neostigmine showed IAPs to be consistently <20 mmHg.
Three days later, she was taken to the operating room for a scheduled major dressing change and debridement of Pseudomonas-infected Integra on her chest and abdomen. Immediately before the operation, her IAP had increased to 22 mmHg, and she presented with a severely distended abdomen and no bowel sounds, indicating a recurrence of her ACS. During surgery, the infected Integra was replaced with Acticoat (Smith & Nephew, United Kingdom) and burn gauze.
With her abdominal wound infection, it was believed that the risk of introducing bacteria into the abdomen at this stage was prohibitively high to be using a peritoneal drain, and other techniques were initiated to reduce her IAP – paralysis, diuresis and use of colloid. Despite this treatment, her symptoms remained; therefore, a percutaneous drain was inserted the next day, and 810 mL of clear amber fluid was removed. Following the fluid removal, there was immediate improvement in peak inspiratory pressures from >40 mmHg to <30 mmHg, and a drop in bladder pressures from 20 mmHg to 6 mmHg (Figure 1). The drained fluid was replaced 1:1 using 5% albumin intravenously. The next day, an abdominal computed tomography showed multiple edematous organs consistent with hypotensive ischemia and no signs of obstruction.
Figure 1).
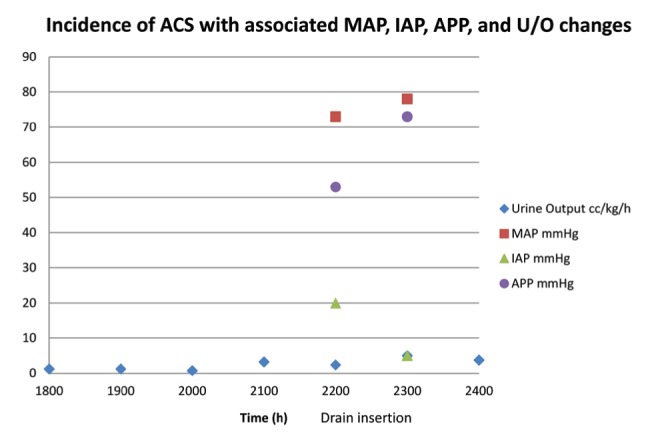
The incidence of abdominal compartment syndrome (ACS) was tracked on this day by a steadily declining urine output (U/O). Because of clinical signs of distension, loss of bowel sounds and a measured bladder pressure of 20 mmHg (27 cmH2O), a percutaneous drain was inserted just after 21:00, and 800 mL of clear amber fluid was collected from the drain. Subsequent parameters showed significant improvement in abdominal perfusion, suggesting that the ACS has been resolved and organ function (U/O) has returned to normal. APP Abdominal perfusion pressure; IAP Intra-abdominal pressure; MAP Mean arterial pressure
The drain was removed four days later because the ACS had resolved. One week after the drain was removed, the patient was found to again have a distended abdomen, no bowel sounds, a decrease in urine output from 3 mL/kg/h to <1 mL/kg/h, and a bladder pressure of 21 mmHg. This third occurrence of ACS was likely associated with a blood culture positive for Streptococcus, and was treated with peritoneal drainage. An additional 800 mL of clear amber fluid was drained from the peritoneum, which resulted in immediate improvement in urine output back to 3 mL/kg/h, and bladder pressure of 5 mmHg. The drain was left to continuously drain for five days, during which her IAP remained steadily between 9 mmHg and 13 mmHg. She went on to further grafting and eventually was discharged from the PICU to the regular ward in stable condition for rehabilitation. She did not experience any episodes of intra-abdominal infection. Several weeks after her third episode of ACS resolved, the patient’s course on the wards was complicated by several days of irregular, nephrotic range proteinuria, which spontaneously resolved before a kidney biopsy was performed. She had normal kidney function for the remainder of her stay in the hospital.
Forty-five days after the third incidence of ACS, the patient, now on the ward, complained of nausea and vomiting. Before this, she had had no difficulty with tolerating her tube feeds, and she was tolerating a full diet. A small-bowel follow-through showed slightly delayed transit time, but no obstructions. Over the next day, the patient developed abdominal distention, sepsis with Escherichia coli in the urine and blood, and required readmission to PICU. A new computed tomography scan of the abdomen was performed, which revealed small bowel obstruction, and arrangements were made for an urgent laparotomy. At the time of laparotomy, multiple adhesions were mobilized, and a stricture was found in the mid-jejunum; signs of proximal dilation (Figure 2), and thickening and hypertrophy of the small bowel suggested that this was a relatively chronic process (Figure 3). A 2.5 cm segment was excised, and an end-to-end anastamosis was completed. The pathology report of the excised section showed signs of nonspecific chronic inflammation of the mucosa, a focal crypt abscess, fibrinoid exudation and foreign body-type giant cell infiltration, and attributed the intestinal injury to ischemia and iatrogenic causes.
Figure 2).
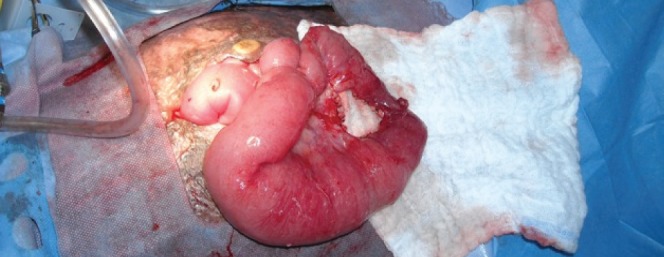
Signs of proximal dilation
Figure 3).
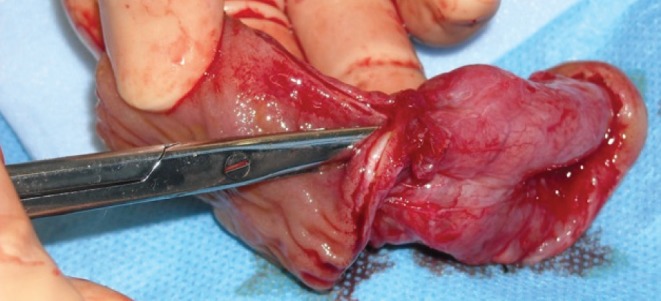
Thickening and hypertrophy of the small bowel
The procedure was tolerated well by the patient, and the remainder of her hospital stay was not marred by any other major issues. Five months after admission, the patient recovered from her burn injuries and surgeries with no further complications. She was discharged after her initial admission with no long-term physiological complications and remains healthy, with no current renal or gastrointestinal complications.
DISCUSSION
ACS develops because excessive fluid resuscitation leads to a build-up of fluid in the interstitium, resulting in high IAP, increasing afterload and disrupting splanchnic perfusion. In the present case, multiple hits of ischemia likely led to the chronic inflammation of the small intestine, which resulted in an obstruction.
There were no risk factors in the patient’s history that would have predisposed her to a small bowel obstruction, but her initial admission was complicated by septic shock from an infected central line. This led to challenges with resuscitation because her septic shock required more intravenous fluid than calculated using the Parkland formula.
During her course in the hospital, three incidences of ACS with significant organ dysfunction were noted; these correlated with a precipitous drop in urine output, abdomen distention and absent bowel sounds. Although blood pressures remained level with a rising IAP, it is possible that the abdominal organs sustained some generalized ischemic injury in this time. The measured bladder pressures during the episodes of ACS did not always exceed 20 mmHg, but impaired abdominal perfusion may have still occurred because of a consistently lower MAP in this patient. Thus, whereas the IAPs were not always sufficiently high to diagnose ACS, APPs were sufficiently low (<60 mmHg) to cause the dysfunction associated with ACS. As a result of these variations in pressures, it is possible for there to be ACS-related organ dysfunction below the defined IAP. In a healthy pediatric patient, blood pressures are naturally lower. In this patient, such incidences would have led to reduced bowel perfusion, increased inflammation and ischemic changes.
The introduction of a percutaneous drain in one instance of ACS led to an immediate improvement in urine output of >2 mL/kg/h, a decrease in IAP of 15 mmHg and an increase in MAP of 5 mmHg. This led to a proportionally larger increase in APP from 53 mmHg to 73 mmHg, raising the pressure to above the ACS threshold of 60 mmHg.
The use of percutaneous drainage to ameliorate the effect of increased IAP has been previously described in the burn literature (12). In a pilot study, Latenser et al (13) concluded it was a ‘safe and effective’ way to reduce intra-abdominal pressure and prevent ACS. Additionally, both a recent systematic review (14), and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome (15) recommended that percutaneous catheter drainage precede decompressive laparotomy, especially in children. The treatment for this child followed these current recommendations and treatment algorithm from the World Society of the Abdominal Compartment Syndrome (15), including attempts to restrict the amount of intravenous fluid, use of gastric decompression, neostigmine and, eventually, percutaneous drainage. Because the child responded to the drainage there was no need to continue onto the open laparotomy. At the time of the bowel surgery, it was not possible to confirm with 100% certainty the cause for the stricture. It is very unusual for an isolated stricture to occur with a catheter insertion. In a study involving >2500 diagnostic peritoneal lavage catheters similarly inserted, Nagy et al (16) reported only one bowel injury and no strictures. As well, the presence of the inflammation and the absence of documented vascular changes is consistent with the histopathology expected with hypoperfusion.
CONCLUSION
ACS and small bowel obstructions have both been extensively documented separately in literature, but the present report provides the first causative link between the two. The increased abdominal pressure from aggressive fluid resuscitation, in a septic patient, may have led to ischemic injury of the peritoneal viscera. The ischemia could not be immediately recognized because the patient was asymptomatic; nevertheless, the chronic inflammation led to an unexpected small bowel obstruction requiring surgical intervention. Therefore, clinicians must be cognizant of potential bowel ischemia in a patient with ACS, and recognize that the final morbid sequela of ischemic injury can arise many months after the first incidence of ACS.
Footnotes
DISCLOSURES: The authors have no financial disclosures or conflicts of interest to declare.
REFERENCES
- 1.Greenhalgh DG, Warden GD. The importance of intra-abdominal pressure measurements in burned children. Trauma Acute Surg. 1994;36:685–90. doi: 10.1097/00005373-199405000-00015. [DOI] [PubMed] [Google Scholar]
- 2.Wilson MD, Dzielwulski P. Severe gastrointestinal hemorrhage and ischemic necrosis of the small bowel in a child with 70% full-thickness burns: A case report. Burns. 2001;27:763–6. doi: 10.1016/s0305-4179(01)00044-4. [DOI] [PubMed] [Google Scholar]
- 3.Hershberger RC, Hunt JL, Arnoldo BD, Purdue GF. Abdominal compartment syndrome in the severely burned patient. J Burn Care Res. 2007;28:708–14. doi: 10.1097/BCR.0b013E318148C988. [DOI] [PubMed] [Google Scholar]
- 4.Schein M, Ivatury R. Intra-abdominal hypertension and the abdominal compartment syndrome. Br J Surg. 1998;85:1027–8. doi: 10.1046/j.1365-2168.1998.00831.x. [DOI] [PubMed] [Google Scholar]
- 5.Rose BD. Clinical Physiology of Acid-Base and Electrolyte Disorders. New York: Mc-Graw Hill; 2001. [Google Scholar]
- 6.Marini JJ. Critical Care Medicine. Philadelphia: Lippincott Williams & Wilkins; 2012. [Google Scholar]
- 7.Sanchez NC, Tenofsky PL, Dort JM, Shen LY, Helmer SD, Smith RS. What is normal intra-abdominal pressure? Am Surg. 2001;67:243–8. [PubMed] [Google Scholar]
- 8.Malbrain ML, Cheatham ML, Kirkpatrick A, et al. Results from the International Conference of Experts on Intra-abdominal Hypertension and Abdominal Compartment Syndrome. Intens Care Med. 2006;32:1722–32. doi: 10.1007/s00134-006-0349-5. [DOI] [PubMed] [Google Scholar]
- 9.Saaiq M. Abdominal compartment syndrome. J Postgraduate Med Inst. 2006;20:297–301. [Google Scholar]
- 10.Newcombe J, Mathur M, Eike JC. Abdominal compartment syndrome in children. Crit Care Nurse. 2012;32:51–61. doi: 10.4037/ccn2012761. [DOI] [PubMed] [Google Scholar]
- 11.Vegar-Brozovic V, Stoic-Brezak J. Pathophysiology of abdominal compartment syndrome. Transplant Proc. 2006;38:833–5. doi: 10.1016/j.transproceed.2006.01.077. [DOI] [PubMed] [Google Scholar]
- 12.Parra MW, Al-Khayat H, Smith HG, Cheatham ML. Paracentesis for resuscitation-induced abdominal compartment syndrome: An alternative to decompressive laparotomy in the burn patient. J Trauma. 2006;60:1119–21. doi: 10.1097/01.ta.0000217274.48792.4d. [DOI] [PubMed] [Google Scholar]
- 13.Latenser BA, Kowal-Vern A, Kimball D, Chakrin A, Dujovny N. A pilot study comparing percutaneous decompression with decompressive laparotomy for acute abdominal compartment syndrome in thermal injury. J Burn Care Rehabil. 2002;23:190–5. doi: 10.1097/00004630-200205000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Strang SG, Van Lieshout EM, Breederveld RS, Van Waes OJ. A systematic review on intra-abdominal pressure in severely burned patients. Burns. 2014;40:9–16. doi: 10.1016/j.burns.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Kirkpatrick AW, Roberts DJ, De Waele J, et al. Pediatric Guidelines Sub-Committee for the World Society of the Abdominal Compartment Syndrome Intra-abdominal hypertension and the abdominal compartment syndrome: Updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intens Care Med. 2013;39:1190–206. doi: 10.1007/s00134-013-2906-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagy KK, Roberts RR, Joseph KT, et al. Experience with over 2500 diagnostic peritoneal lavages. Injury. 2000;31:479–82. doi: 10.1016/s0020-1383(00)00010-3. [DOI] [PubMed] [Google Scholar]


