Abstract
Inflammation is increasingly recognized as being a critical contributor to both normal development and injury outcome in the immature brain. The focus of this Review is to highlight important differences in innate and adaptive immunity in immature versus adult brain, which support the notion that the consequences of inflammation will be entirely different depending on context and stage of CNS development. Perinatal brain injury can result from neonatal encephalopathy and perinatal arterial ischaemic stroke, usually at term, but also in preterm infants. Inflammation occurs before, during and after brain injury at term, and modulates vulnerability to and development of brain injury. Preterm birth, on the other hand, is often a result of exposure to inflammation at a very early developmental phase, which affects the brain not only during fetal life, but also over a protracted period of postnatal life in a neonatal intensive care setting, influencing critical phases of myelination and cortical plasticity. Neuroinflammation during the perinatal period can increase the risk of neurological and neuropsychiatric disease throughout childhood and adulthood, and is, therefore, of concern to the broader group of physicians who care for these individuals.
Introduction
The CNS is an immune-privileged site, which is appropriate for an organ with limited regenerative capacity.1 However, its immune privilege is severely undermined once inflammation is established, and it is now clear that both peripheral and central immune signals can induce inflammatory responses within the CNS during the perinatal period. During this time, the immature brain passes through several essential stages of CNS development, and activation of the immune system during fetal and neonatal life affects critical phases of brain development, with long-lasting consequences for neurological and mental health.2,3
Brain injury in the perinatal period occurs in at least four clinical settings: neonatal encephalopathy in term infants, neonatal stroke, encephalopathy of prematurity (Box 1), and systemic infections (Figure 1). Neonatal encephalopathy often results from intrapartum asphyxia leading to hypoxia–ischaemia, and affects the brain globally. Perinatal arterial ischaemic stroke induces a focal brain lesion with a core and a penumbra similar to those in adult stroke.4 Encephalopathy of prematurity is caused by exposure of the very immature brain to inflammatory triggers during fetal and postnatal life. In addition, CNS inflammation can occur as a result of systemic infections arising at any time during pregnancy or neonatal life; such inflammation can affect brain development directly or act in concert with the above-described insults.
Box 1. Clinical settings of perinatal brain injury.
Neonatal encephalopathy at term (>36 weeks’ gestational age), also known as hypoxic–ischaemic encephalopathy
3/1,000 live births
Presentation: encephalopathy and changes in muscle tone
Acute injury results in damage to basal ganglia; subacute injury can be restricted to watershed regions
The insult causes primary energy depletion, and brain injury develops during the secondary and tertiary phases112,121
Neurons are the primary target in selectively vulnerable regions
Infections and inflammation can precede and predispose to neonatal encephalopathy (sensitization),56 and brain injury itself initiates an immune response
Outcomes depend on severity of injury
Therapeutic hypothermia is now standard of care for babies if neonatal encephalopathy is recognized by 6 h of life
Perinatal arterial ischaemic stroke
1/2,300 live births
Presentation: focal motor seizures in first day of life
Focal injury detected on MRI in arterial (70%) or venous (30%) distribution
Perinatal stroke is caused by thrombus or embolus formation that can be associated with infection; inflammation contributes to progression of brain injury in the penumbral area4,122
Affects babies in utero (20 weeks’ gestation) and up to first 28 days of life
50% of affected infants have disability that includes epilepsy, behavioural deficits, learning disorders and cerebral palsy
Currently no available therapies, except for anticoagulation therapy in sinovenous thrombosis
Encephalopathy of prematurity
Preterm birth occurs in one in eight deliveries; about 5–10% have moderate to severe brain injury
Damage is primarily to oligodendroglial precursors, but grey matter dysmaturation also occurs; the very immature brain is exposed to inflammation during fetal life
Inflammation results in preterm birth, and the neonate might further be exposed to hypotension, hypoxia, inflammation, surgery, hypocarbia or hypercarbia during the intensive care period123,124
Injury often goes undetected in the nursery
Diffuse cortical white matter injury and cerebellar hypoplasia often observed
Affects babies from 23–36 weeks’ gestational age
Results in cerebral palsy, cognitive disabilities, visual dysfunction, hearing impairments and epilepsy
No specific therapies
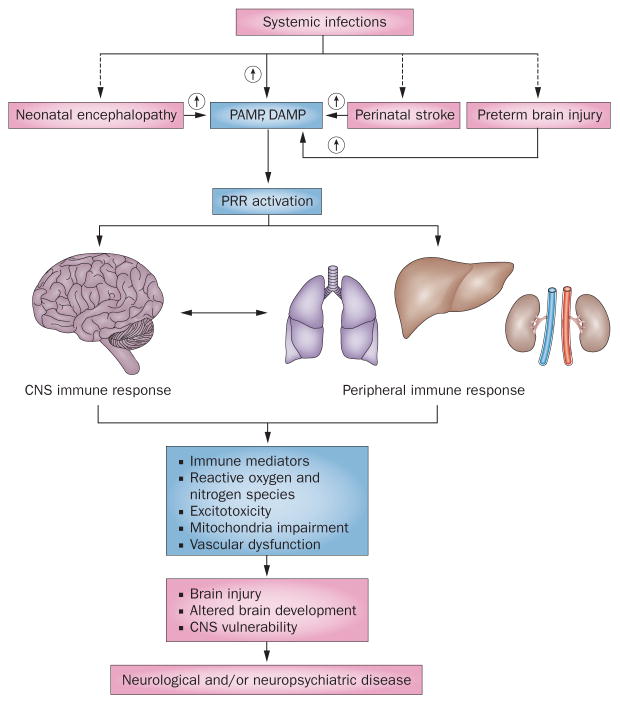
Figure 1.
Inflammation in the developing brain. Neonatal encephalopathy, perinatal stroke, preterm brain injury and systemic infections trigger release of PAMP and DAMP, which activate PRRs. Under some conditions, systemic infection can also be an antecedent of the other insults (dashed arrows). PRRs trigger inflammation in the periphery and in the brain. Inflammation can act in concert with hypoxia–ischaemia to induce activation of immune mediators, reactive oxygen and nitrogen species, excitotoxicity, mitochondrial impairment and vascular disruption. These effectors can cause brain injury directly, interfere with brain development and modulate CNS vulnerability, all of which may contribute to neurological and neuropsychiatric disease. Abbreviations: DAMP, damage-associated molecular pattern; PAMP, pathogen-associated molecular pattern; PRR, pattern recognition receptor.
In this Review, we will summarize how different perinatal insults activate the immune system and trigger peripheral and central responses that involve immune mediators (cytokines and chemokines), reactive oxygen species (ROS), reactive nitrosative species, excitotoxicity, mitochondrial impairment, and vascular integrity. We propose that the combined actions of these effectors can cause brain injury directly, modulate vulnerability or interfere with CNS development, thereby contributing to neurological or psychiatric disease (Figure 1).
PRRs in the innate immune response
Pattern recognition receptors (PRRs) of the innate immune system provide the organism with an intrinsic mechanism to distinguish self from non-self antigens and defend against invading microbes and viruses.5,6 These receptors recognize different classes of pathogens, as well as endogenous molecules released from, or expressed by, damaged tissues. Thus, PRRs could be involved in both infection-induced injury and inflammatory responses that result from hypoxia–ischaemia or neonatal stroke. To date, the Toll-like receptor (TLR) family has been the most studied PRR type in the developing brain.
In the neonatal mouse, TLR1-9 mRNAs are constitutively expressed in the forebrain,7 and several TLR genes are expressed in the choroid plexus.8 TLR2 expression is relatively low before birth.7,9 TLR3 and TLR8 have been suggested to regulate embryonic brain development, given that artificial upregulation of these molecules inhibits neurite outgrowth10 and reduces cell proliferation in vitro.9
Glial TLR3 protein expression is increased in infants with periventricular white matter injury, suggesting that abnormal TLR3 expression influences developmental processes in the immature brain.11 Moreover, in rodents, stimulation of TLRs during pregnancy or in the neonatal period results in robust inflammatory responses in the fetal and newborn brain, including marked microglial proliferation12 and increased cytokine expression.13,14 Prolonged periods of TLR stimulation result in notable blood–brain barrier (BBB) changes,15 and infiltration of peripheral immune cells can further contribute to inflammation in the brain.
The choroid plexus—the site of the blood–cerebrospinal fluid (CSF) barrier in the brain—has been considered as a possible access route for peripheral immune signals and cells into the CNS. In neonatal mice, immune stimulation via administration of specific TLR ligands altered the expression of mRNAs encoding choroid plexus TLRs and the tight junction protein occludin.8 In addition, the TLR2 ligand Pam3CSK4 induced the transcription of the tumour necrosis factor (TNF) gene, and also dramatically increased leukocyte diapedesis into the CSF.
Convincing data from several different species and experimental conditions show that activation of TLR3 or TLR4 in mid to late pregnancy, or during the early neonatal period, results in brain injury similar to that seen in human infants, including loss of myelin, astrogliosis and microgliosis, and disruption of thalamocortical function (Table 1). Other TLRs in the immature brain have been less well studied, but existing data suggest that TLR2 activation has detrimental effects on grey and white matter (Table 1).
Table 1.
Effects of TLR2, TLR3 and TLR4 activation on the developing brain
| Subjects | Target | Treatment | Effects |
|---|---|---|---|
| Mice, P3–11 | TLR2 | Pam3CSK4 5 mg/kg, i.p, daily injections | Elevated IL-1β, IL-6, CXCL1 and CCL2 in brain; transient decrease in grey and white matter volume Brain deficits resolved by P50128 |
| Cultured cells (E9 chick, E14 mouse), P4 mice | TLR3 | Poly(I:C) 20–100 μg/ml, (in vitro); Poly(I:C) 3 mg/kg intrathecally to mice | Growth cone collapse and irreversible inhibition of neurite extension in culture experiments; righting reflex and negative geotaxis impaired at P9 No neuropathological analysis of the forebrain129 |
| Pregnant mice, E9 or E17 | TLR3 | Poly(I:C) 5 mg/kg, i.v. | Poly(I:C) at E9 impaired sensorimotor gating and reduced dopamine D1 receptors in adulthood Poly(I:C) at E17 impaired working memory, potentiated locomotor reaction, and reduced hippocampal N-methyl-D-aspartate receptor type 1 expression130 |
| Pregnant spiny mice, E20 | TLR3 | Poly(I:C) 0.5 mg/kg, s.c. | Impairments in non-spatial memory and learning tasks and motor activity in off-spring at P100 Decreased reelin expression, increased GFAP expression and increased numbers of activated microglia, specifically in the hippocampus131 |
| Pregnant SD rats, E10.5 | TLR4 | LPS 1 mg/kg, i.p. | TNF increased in mesencephalon in P21 offspring Substantia nigra volume and number of tyrosine hydroxylase-positive cells reduced132 |
| Fetal sheep, 70% of gestation | TLR4 | LPS 1 μg/kg, i.v. several doses over 5 days | Infrequent neural injury, but injury more common in cerebral white matter Corticospinal tract cross-sectional area reduced by 30% Very high LPS dose for fetal sheep133 |
| Fetal sheep, 70% of gestation | TLR4 | LPS 100 ng/kg, i.v., single bolus | Focal inflammation and cystic lesions in periventricular white matter in two of five animals, but with no neuron-specific injury Loss of astrocytes and oligodendrocytes in white matter75 |
| Pregnant Wistar rats, E19 and E20 | TLR4 | LPS 0.3 mg/kg, i.p. | Increased IL-1β in brain at P1 Increased cell death, astrogliosis and hypomyelination at P7 Delays in neonatal behaviour; myelination and most motor deficits normalized by adulthood76 |
| Pregnant Sprague–Dawley rats, E15 | TLR4 | LPS 0.1 mg/kg, i.p. | Reduction in complexity and spine numbers of cortical and hippocampal pyramidal neurons up to P60134 |
| Mice, P3–P11 | TLR4 | LPS 0.3 mg/kg, i.p., daily | Grey matter volume, myelin basic protein, CNPase staining area and number of Olig2+ cells decreased in white matter at P12135 |
| Fetal sheep, 70% of gestation | TLR4 | LPS 200 ng/kg, i.v., single bolus | Reduced white matter and cortical volumes shown by MRI and histology Maturation deficits in fetal EEG activity89 |
| Pregnant rabbits, E28 | TLR4 | LPS 20 mg/kg, i.u. | PET and immunohistochemical findings indicate newborn rabbits from LPS-treated mothers to have reduced cortical 5-HT and disruption of 5-HT-regulated thalamocortical development136 |
| Pregnant rats, E15 | TLR4 | LPS 0.25 mg/kg, i.p. | Reduced social preference and exploration behaviours in offspring; affected genes that regulate migration of GABAergic interneurons137 |
| Pregnant mice, E17 | TLR4 | LPS 50 μg for each dam, i.u. | At P90–P100 disturbances in the circadian rhythm in the offspring, with longer time spent in non-rapid eye movement sleep states during the dark cycle compared with controls (measured by EEG and electromyography)138 |
Abbreviations: 5-HT, 5-hydroxytryptamine; CNPase, 2′,3′-cyclic nucleotide 3′ phosphodiesterase; E, embryonic day; GABA, γ-aminobutyric acid; GFAP, glial fibrillary acidic protein; i.p., intraperitoneal; i.u., intrauterine; i.v., intravenous; LPS, lipopolysaccharide; Olig2, oligodendrocyte lineage transcription factor 2; P, postnatal day; Poly(I:C), polyinosinic:polycytidylic acid; TLR, Toll-like receptor; TNF, tumour necrosis factor. Chemokines are abbreviated according to the international classification.
Hypoxia–ischaemia and stroke at term
Experimental studies
Hypoxia–ischaemia triggers inflammatory processes that can continue for several weeks after the initial insult. The initial phase of inflammation (Figures 2 and 3) targets the region of injury, combats invading microbes and limits infectious processes to benefit the host, albeit at the cost of ‘bystander’ brain injury.16 Several studies have shown that interventions that attenuate the early inflammatory phase confer neuroprotection (Table 2). After this early proinflammatory phase, the immune system shifts to favour an anti-inflammatory response, which is followed by a repair phase (Figure 2).
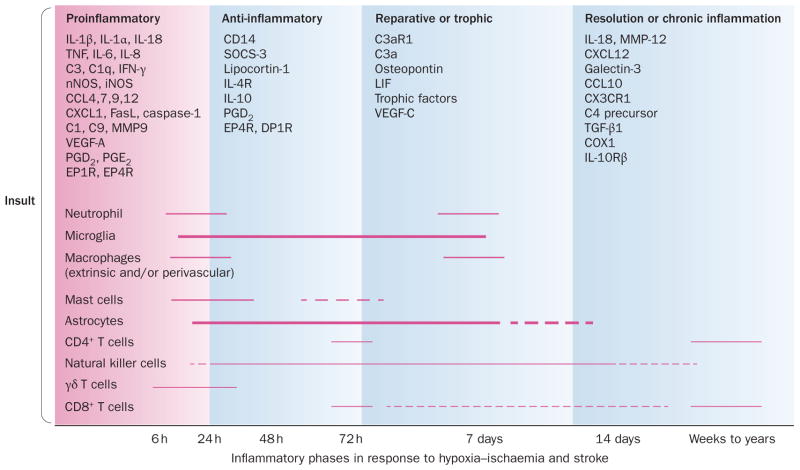
Figure 2.
Stages of inflammation in the immature brain after hypoxia–ischaemia and stroke. The hypoxic or ischaemic insult triggers a proinflammatory response followed by anti-inflammatory and reparative phases. These events result either in resolution of inflammation or in chronic inflammation. The critical phases of inflammation are regulated by multiple cytokines, chemokines, prostaglandins and other immune mediators, leading to activation and participation of inflammatory cells that are part of both innate and adaptive immune responses. The figure is a summary based on multiple experimental and clinical studies.4,17,19,21,28,31–33,57–59,122,125 Abbreviations: C, complement; CD, cluster of differentiation; COX, cyclooxygenase; DPR, prostaglandin D receptor; EPR, prostaglandin E receptor; FasL, Fas ligand; iNOS, inducible NOS; LIF, leukemia inhibitory factor; MMP, matrix metalloproteinase; nNOS, neuronal NOS; NOS, nitric oxide synthase; PG, prostaglandin; SOCS, suppressor of cytokine signalling; TGF, transforming growth factor; TNF, tumour necrosis factor; VEGF, vascular endothelial growth factor. Chemokines are abbreviated according to the new classification.
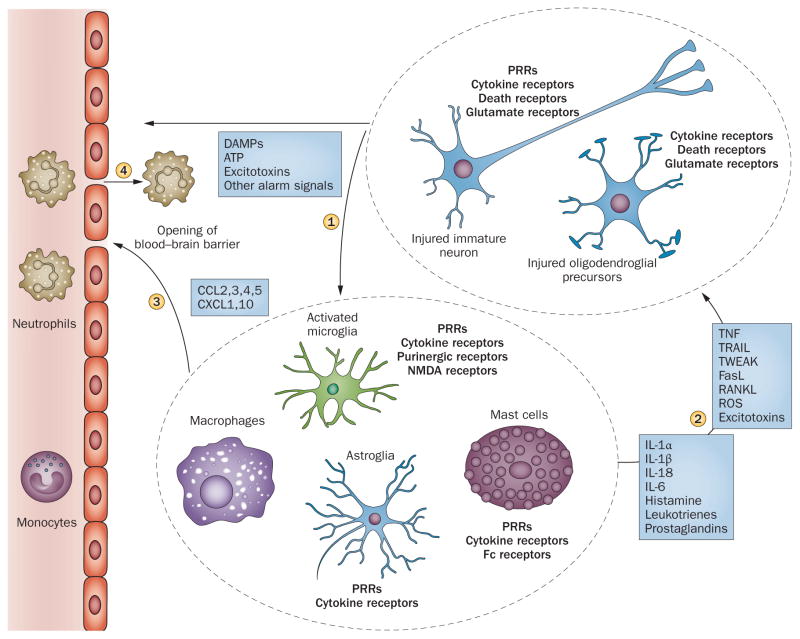
Figure 3.
Early innate response to hypoxia–ischaemia. Immune effector cells (microglia, macrophages, astroglia, mast cells) sense alarm signals from injured parenchymal cells via PRRs and cytokine receptors (1). The triggered innate immune response has proinflammatory and toxic influences on the neurons, oligodendroglial precursors (2) and vascular bed (3); increased blood–brain barrier permeability contributes to the recruitment of immune cells from the periphery (4). Abbreviations: DAMP, damage-associated molecular pattern; NMDA, N-methyl-D-aspartate; PRR, pattern recognition receptor; RANKL, receptor activator of nuclear factor-κB ligand; ROS, reactive oxygen species; TNF, tumour necrosis factor; TRAIL, TNF-related apoptosis-inducing ligand; TWEAK, TNF-like weak inducer of apoptosis. Chemokines are abbreviated according to the new classification.
Table 2.
Immunomodulatory therapeutic possibilities for perinatal brain injury
| Experimental insult and animal model | Intervention | Outcome |
|---|---|---|
| Corticosteroids (DEX) | ||
| HI, P7 rats | DEX given between P1 and P3 | Exacerbated brain damage, decreased glutamate reuptake; role of microglia not specifically addressed90 |
| HI, P7 rats | DEX given 24 h and 4 h before the insult | Neuroprotection, increased brain VEGF production; role of microglia not specifically addressed139 |
| HI + LPS, P7 rats | DEX given 24 h and 4 h before the insult | Neuroprotection and decreased CXCR4 receptor density140,141 |
| Minocycline | ||
| HI, P7 rats | Minocycline given either immediately before or after the insult | Marked neuroprotection; role of microglia not specifically addressed34 |
| HI, P8 mice | Minocycline given either immediately before or 12 h before the insult | Exacerbated brain damage; role of microglia not specifically addressed142 |
| Ibo, P5 mice | Repeated minocycline injections either before or after Ibo | Protection of GM and WM; reduced microglial density143 |
| LPS (intracerebral), P5 rats | Minocycline given for 3 days, starting 12 h before insults | Protection, reduced microglial activation144 |
| Hyperoxia, P6 rats | Minocycline given during exposure to hyperoxia | Protection, long-lasting reduction in microglial activation145 |
| Melatonin | ||
| Ibo, P5 mice | Melatonin administered immediately after Ibo | Reduced microglial density, beneficial effect on WM146 |
| Experimentally induced stroke, P7 rats | Melatonin given as either a single dose before ischaemia or a double-dose regimen, combining one before ischaemia and one 24 h after reperfusion | Improved myelination, no effect on infarct size, reduced microglial density147 |
| UAL, E18 rats (growth restriction model) | Melatonin given from P0 to P3 | Improved myelination, reduced microglial density148 |
| UCO, E92 sheep | Melatonin infused for 6 h after UCO | Reduced cell death, oxidative stress and microglial density149 |
| UCO, E130 sheep | Melatonin infused to the ewe for 2 h, before and after UCO | Protection, reduced microglial density150 |
| HI (βNTP at 40% baseline for 12.5 min), P0 piglets | Combined melatonin–hypothermia treatment | Neuroprotection, did not affect microglial density151 |
| EPO | ||
| HI, P3 rats | EPO given once a day during the first week after HI (P3 to P10) and then 3 times/week until P25 | Improved WM microstructure, no effect on cortex; role of inflammation not specifically addressed152 |
| HI, P7 rats | EPO was studied as an add-on to hypothermia | No overall protection by EPO or hypothermia, alone or in combination; role of microglia not specifically addressed153 |
| Stroke, P7 rats | EPO given at reperfusion, 24 h, and 7 days after stroke | Lasting decreased brain damage and improved function, neurogenesis enhanced; role of inflammation not specifically addressed154 |
| Ibo, P5 mice | Single dose of EPO 1 h after Ibo | Protection in GM and WM; role of inflammation not specifically addressed155 |
| UCO E165–172, macaques | EPO given on days 1, 2, 3, and 7 after UCO + hypothermia | Improved anisotropy and cognitive functions; role of inflammation not specifically addressed156 |
| Cyclooxygenase inhibitors | ||
| IL-1β P1–P5 and Ibo P5, mice | Nimesulide (COX-2) or indomethacin (COX-1+2) given in combination with IL-1β | Blockade of IL-1β-induced sensitization of brain injury and inflammatory response in the brain91 |
| IL-1β P1–P5 and Ibo P5, mice | Tianeptine given for 5 days before Ibo | Blockade of IL-1β-induced sensitization in GM and WM, no effect in absence of IL-1β; effect on microglia not specifically addressed157 |
| Pifithrin-μ | ||
| HI, P7 rats | Pifithrin-μ given after HI | Protection of GM and WM, reduced microglial density158 |
| Cromolyn | ||
| IL-9 P1-P5 and Ibo P5, mice | Cromoglycate given 1 h before Ibo | Blockade of IL-9-induced sensitization, protection in WM and GM, but no effect in the absence of IL-9; reduced MC density41 |
| HI, P7 rats | Cromoglycate given before and/or following HI | Neuroprotection, inhibition of microglial activation, and MC migration18,42 |
| Innate defence regulatory peptide | ||
| LPS and HI, P8–9 mice | Innate defence regulatory peptide 1018 given 3 h after HI | Reduced injury in WM and GM, microarray analysis demonstrated decrease in proinflammatory and cell-death-related pathways159 |
| NAC | ||
| Maternal LPS, E19, rat NAC | in drinking water from E17 to birth | Prevented oxidative stress and restored long-term potentiation in the hippocampus and spatial recognition performance (effects found only in males)160 |
| Newborn piglets, hypoxia | NAC given as bolus + 24 h infusion, started 5 min after reoxygenation | Attenuated caspase-3 and lipid hydroperoxide in the cortex, short (48 h) recovery period161 |
| HI, P7 rats | NAC, daily until sacrifice, hypothermia for 2 h post HI | Reduced brain infarct volume and improved behavioural outcome, assessed up to 4 weeks after HI162 |
| Intrauterine LPS, E28 rabbit | NAC administered in dendrimers as a single dose within 6 h after birth | D-NAC taken up by astroglia and microglia; reductions in motor deficits, oxidative injury, expression of proinflammatory genes, microglial activation, and loss of WM and GM163 |
| TNF receptor blockade (etanercept) | ||
| HI, P7 rats | Etanercept given immediately after HI | Etanercept detectable in the brain after intraperitoneal administration, reduced the neuroprotective effect of NF-κB inhibition164 |
| IL-1b + Ibo, P1–P5, mice | Etanercept given before or after Ibo | Reduced brain damage by 50%; protective only when given after combined insult165 |
| IL-1ra | ||
| HI, P7 rats | IL-1ra, intracerebroventricular, before or after HI | Improved brain wet weight, but neuropathology not assessed28 |
| LPS, P5 rats | Co-administration of IL-1ra with LPS | Improved myelination, reduction of lateral ventricle enlargement; neuroinflammation not investigated166 |
| HI, P7 rats | Intracerebroventricular injection of IL-1ra 2 h after HI | Reduced cell death and caspase 3 activity in the hippocampus and cortex; reduced NF-κB activity, iNOS and COX-2167 |
| LPS (E20–E22) + HI (P1), rats | IL-1ra treatment every 12 h from P1 to P9 | Normalized motor function, exploratory behaviour, and density of immature neurons and astrocytes29 |
| TAT-NBD | ||
| HI, P7 rats | TAT-NBD given up to 12 h after HI | Neuroprotection, including improved long-term motor and cognitive outcome when given within 6 h after HI; effect independent of cytokines168 |
| LPS + HI, P7 rats | Intranasal delivery of TAT-NBD 10 min after HI | Prevents brain injury after LPS + HI, blocks NF-κB signalling; not neuroprotective in HI alone169 |
| Simvastatin | ||
| HI, P7 rats | Pre-HI treatment | Neuroprotective, improved behaviour; effect on microglia not addressed; only male rats investigated170 |
| HI, P7, rats | Pre-HI treatment | Improved GM and WM injury, reduced microglial activation171 |
| PTB mice | Pravastatin or simvastatin given 24 h before and 2 h after LPS intravaginal administration | Protected cortical neurons in the fetus, protection mediated by Akt/PKB signalling pathways; effect on microglia not addressed172 |
| Histone deacetylase inhibitors (TSA, valproate) | ||
| Hippocampal Ibo, P7 rats | Injected daily from day after surgery until adulthood | Improved some behavioural characteristics, but not anxiety; did not protect against hippocampal lesions; neuroinflammation not specifically addressed173 |
| LPS + HI, P8–9 mice | TSA or valproate given at the same time as LPS | Valproate increased mortality; TSA reduced GM and WM injury and improved learning in the fear conditioning test in females, but did not affect number of microglia after injury174 |
| Unilateral carotid artery ligation, P12 rats | Treatment with valproate, TSA or vehicle for 2 weeks after insult | Both TSA and valproate increased neurogenesis, but valproate also increased mortality and impaired weight gain; neuroinflammation not specifically addressed175 |
Abbreviations: βNTP, β-nucleotide triphosphate; Akt/PKB, protein kinase B; COX, cyclooxygenase; DEX, dexamethasone; d-NAC, dendrimer NAC; E, embryonic day; EPO, erythropoietin; GM, grey matter; HI, hypoxia–ischaemia; Ibo, ibotenate; iNOS, inducible nitric oxide synthase; LPS, lipopolysaccharide; MC, mast cells; NAC, N-acetylcysteine; NF-κB, nuclear factor-κB; P, postnatal day; PTB, preterm birth; TAT-NBD, Tat-NEMO-binding domain; TSA, trichostatin A; UAL, uterine artery ligation; UCO, umbilical cord occlusion; VEGF, vascular endothelial growth factor; WM, white matter. Chemokines and their receptors are named and abbreviated according to the international classification.
Hypoxia–ischaemia induces rapid activation of microglia and mast cells in the rodent brain.17,18 During early reperfusion, neutrophils accumulate in post-capillary venules, and myeloid cells, T cells and natural killer cells infiltrate injured areas of the brain during the delayed recovery phase.19–21 For example, in Lys-EGFP-ki mice,22 a transgenic strain that enables the study of neuroinflammation, myeloid cell infiltration peaked at 24 h after hypoxia–ischaemia, and the infiltrating cells consisted predominantly of monocytes (CD11b+EGFP+Gr1lo/− Ly6Cint/hi); granulocytes accounted for about 10% of infiltrating cells. By contrast, in a neonatal stroke model, neutrophil infiltration was negligible.23 Consistent with these findings, the BBB was found to undergo transient opening at 6–24 h after hypoxia–ischaemia,24 whereas in the neonatal stroke model, the opening of the BBB was restricted.23
Both intrinsic and infiltrating cells produce proinflammatory cytokines and chemokines (Figures 2 and 3).18–20 These cells also produce ROS, release excitatory amino acid agonists, and death receptor agonists including TNF, FasL, RANKL, TRAIL and TWEAK,25 which could further contribute to cell death (Figure 3).
The initial inflammatory response is thought to depend on activation of innate immune receptors. TLRs are induced during recovery from neonatal hypoxia–ischaemia, and knocking out Tlr2 in mice provides neuro protection.7 In contrast to findings from TLR knockout mice, the majority of studies have found that deletion of two TLR adaptor proteins, MyD88 and TRIF, does not confer neuroprotection in neonatal hypoxia–ischaemia.26,27 Indirect evidence from rodents suggests that NOD-like receptors that activate the inflammasome are important in neonatal injury. For example, hypoxia–ischaemia increases IL-1 production,28 and administration of the IL-1 receptor antagonist IL-1ra ameliorates damage induced by hypoxia–ischaemia alone28 or together with lipopolysaccharide (LPS) challenge.29 Moreover, neonatal mice with a homozygous deletion of caspase-130 or IL-1831 are resistant to hypoxia–ischaemia (Table 2).
The specific contributions of the different cell types to brain inflammation and cell death mechanisms in the immature brain continue to be the focus of many investigations. Below, we provide a summary of the findings to date.
Neutrophils
Depletion of neutrophils prior to hypoxia–ischaemia reduces injury, indicating that these cells contribute to injury progression, at least during (or just after) hypoxia–ischaemia. By contrast, neutrophil-targeted treatment after hypoxia–ischaemia is ineffective.19 Neither genetic nor pharmacological inhibition of NADPH—which is required for phagocytosis by neutrophils— confers neuro protection in neonatal mice,32 and modification of chemokine–neutrophil signalling, a pathway that protects against stroke in adult rats, exacerbates injury after neonatal focal stroke.23 Microglia The pathophysiological role of microglia continues to be debated. Both hypoxia–ischaemia and neonatal stroke result in notable microglial activation in the neonatal brain.17,33 The classic view—that these cells exert toxic effects, at least in the initial phase after hypoxia–ischaemia—is supported by several findings. First, IL-18 is produced preferentially by activated microglia, and genetic deletion of IL-18 confers protection.31 Second, minocycline reduces the microglial response and reduces injury.34 Last, caspase-1 is predominantly expressed in microglia after hypoxia–ischaemia, and genetic deletion of caspase-1 attenuates brain injury.30 It should be noted, however, that none of the above-described interventions target microglia selectively. In the neonatal stroke model, microglial activation seems to predominate over extrinsic recruitment of monocytes. 33 Pharmacological depletion of microglia prior to neonatal stroke aggravates rather than improves outcome, and exacerbates the release of inflammatory cytokines, suggesting that at least a subpopulation of microglia have beneficial effects.35
Microglial phagocytosis of debris has been suggested to be critical for tissue recovery during the delayed phase after neonatal stroke;35 this hypothesis is further supported by the finding that CD36 scavenger receptor deletion worsens injury in the neonatal mice.36 The divergent results could relate to differing microglial phenotypes: depending on their phagocytic activity, some microglia might participate in acute early proinflammatory responses and aggravate injury, whereas others might be involved in the late anti-inflammatory responses and protect against injury (Figure 2). Microglia might assume distinct functional phenotypes during recovery from hypoxia–ischaemia, similar to the M1, M2a and M2b phenotypes suggested in other models.37,38
Mast cells
Despite a limited understanding of the contribution of mast cells to normal brain development, their involvement in several aspects of brain injury has now been established.39,40 Data from experimental models of cerebral ischaemia and trauma have implicated mast cells as early contributors to BBB dysfunction, oedema and haemorrhage in the adult brain.39 The immature brain features higher number of mast cells than does the adult brain, suggesting that mast cells might have an even more important role in the response to injury and/or inflammation in the neonatal brain.
Activated mast cells contribute to excitotoxic injury in neonatal mice by exacerbating transforming growth factor β1 (TGF-β1) toxicity, potentially via a histaminergic mechanism.41 In neonatal rats, mast cells have been identified as the first responders to hypoxic–ischaemic brain damage by undergoing early degranulation and releasing TNF, and migration and/or proliferation of mast cells in the pia and infarct area remains enhanced for days to weeks.18,42 Acute treatment with the mast cell stabilizer sodium cromoglycate prevents early mast cell activation and degranulation in this model, commensurate with markedly improved neuroprotection.18
Mast cell activation in response to neonatal stroke has been independently confirmed in the neonatal transient stroke model.43 Mast cells seem to promote inflammation acutely after injury in the neonatal brain, but their contributions to the ongoing evolution of damage and reparative processes have not yet been studied.
Adaptive immune response
Neonatal hypoxia–ischaemia leads to central and peripheral activation of CD11b+ and CD11c+ antigen-presenting cells and the costimulatory molecules CD86 and MHC-II. This co-activation indicates active antigen presentation in the damaged hemisphere and in the spleen.21 Infiltrating antigen-presenting cells and T lymphocytes have been observed in the damaged cerebral hemisphere up to 7 months after the initial insult (Figure 2).21
The site of the T-cell trafficking into the brain in hypoxia–ischaemia has not been established, but recent studies suggests that leukocyte entry via the choroid plexus is an important mechanism for resolution of CNS inflammation,44 and that the infiltration augments recruitment of anti-inflammatory monocyte-derived macrophages.45 Administration of the TLR2 ligand Pam3CSK4 to mice at postnatal day 8 (P8) results in a dramatic increase in leukocyte numbers in the CSF, presumably owing to entry the choroid plexus.8 Furthermore, the choroid plexus has been recently recognized as an important immunological compartment, enriched with CNS-specific CD4+ T cells.
Further support for the notion that T cells have an important role in ischaemic brain injury comes from the finding that adult lymphocyte-deficient mice are protected against ischaemic injury.46 Stroke studies in chimaeric mice have shown that this protection is attributable to T cells rather than B cells.47
Early-life LPS exposure influences the generation of neuroprotective regulatory T cells48 and modifies the inflammatory responses to autoimmune disease in mice.49 Maternal polyinosinic:polycytidylic acid (Poly[I:C]) exposure alters adaptive immunity in offspring by priming their T cells towards a T helper 17 (TH17) phenotype.50 The inflammatory responses following activation of TH1 cells are also toxic to premyelinating oligodendrocytes,51 suggesting that αβ T cells can have a role in the pathogenesis of injury to the immature brain.
In summary, adaptive immune cells are detected in the immature brain after hypoxia–ischaemia in the delayed and tertiary phases, although their roles are not yet elucidated. Recent data, however, indicate that these cells can have toxic effects as well as being important for resolution of inflammation.
Synergy between TLRs and hypoxia–ischaemia
In addition to a direct effect on brain development, TLR activation makes the immature brain more susceptible to insults (Figure 1). For example, the LPS-induced increase in vulnerability to neonatal hypoxia–ischaemia52 is dependent on TLR4, which functions as a sensor of LPS, and MyD88, an adapter protein used by most TLRs to activate nuclear factor-κB (NF-κB; Figure 4).26,53
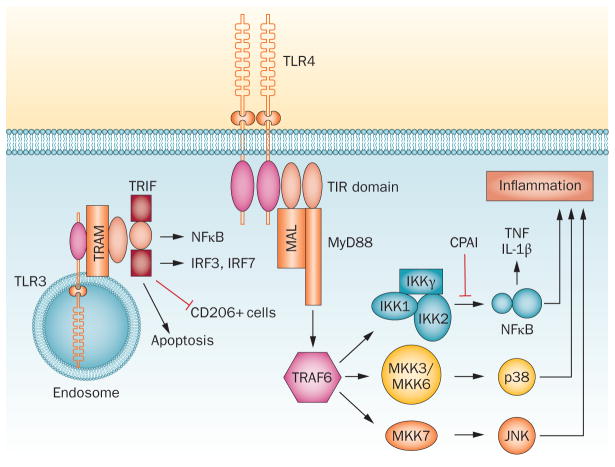
Figure 4.
Mechanisms of TLR4 and TLR3 sensitization. TLR4 increases vulnerability of the immature CNS through activation of the MyD88-dependent pathway, leading to NF-κB-dependent production of IL-1β and TNF and activation of JNK. Endosomal TLR3 induces sensitization through TRIF-dependent activation of NF-κB, IRF and apoptosis, and inhibition of potentially cytoprotective CD206+ cells.27 LPS and hypoxia–ischaemia induce proteolytic activity of tPA, but this can be blocked by CPAI, which reduces NF-κB signalling, microglial activation, and production of proinflammatory cytokines in the brain.126 JNK inhibition also significantly reduces neuroinflammation, blood–brain barrier leakage and oligodendrocyte progenitor apoptosis127 after LPS sensitization. Abbreviations: CPAI, plasminogen activator protein-1; IRF, interferon regulatory factor; JNK, c-Jun N-terminal kinase; LPS, lipopolysaccharide; Myd88, myeloid differentiation factor 88; NF-κB, nuclear factor-κB; TLR, Toll-like receptor; TNF, tumour necrosis factor; tPA, tissue plasminogen activator; TRIF, TIR-domain-containing adapter-inducing IFN-β.
Although MyD88 knockout and wild-type mice show similar numbers of microglia after LPS insult, the cytokine–chemokine response to LPS is blunted in the transgenic mice, suggesting that the activation state of the microglia, rather than their absolute number, is the main factor affecting the inflammatory response to LPS.26
Anticytokine therapy can curtail TLR-dependent vulnerability to injury; for example, blockade of the TNF cluster54 and treatment with IL-1ra28 or anti-NF-κB peptides55 all reduce brain injury (for mechanisms, see Figure 4). Similarly, we have demonstrated that Poly(I:C) increases the vulnerability of the immature brain to hypoxia–ischaemia in a TRIF-dependent manner.27 Interestingly, this effect is associated not with a change in proinflammatory CD86+ cells but, rather, a decrease in reparative CD206+ immune cells (Figure 4).27
Clinical studies
Neonatal encephalopathy and chorioamnionitis
Despite abundant preclinical evidence supporting a role for inflammation in neonatal encephalopathy and neonatal stroke at term, these findings await full validation in clinical studies.56 Neonatal encephalopathy is accompanied by elevation of IL-6, IL-8 and IL-1β in the CSF,57 and raised levels of these cytokines in both CSF and blood58 are associated with adverse neurological outcome. Furthermore, increased levels of several cytokines in the neonatal blood at term correlate strongly with the likelihood of cerebral palsy.59 It is unclear, however, whether inflammation is a result or a cause of the encephalopathy and neurodevelopmental sequelae. Distinct age-related susceptibility to injury of particular cell populations and mechanisms controlling local inflammation and immune cell infiltration might contribute to the development of the causal pathways. Indeed, nonclassical pathways of complement activation are not fully developed in term infants, and are likely to exert important age-dependent effects on the inflammatory response.60
Some human studies have shed light on the role of prior infection in subsequent brain damage, such as that seen in cerebral palsy. In a case–control study from the Kaiser Permanente Medical Care Program, a chart review of children with moderate to severe spastic or dyskinetic cerebral palsy evaluated the association between clinical chorioamnionitis and risk of cerebral palsy. The study found that chorioamnionitis or placental infection conferred a fourfold overall increased risk of cerebral palsy in term infants.61 Among singleton births, the population attributable fraction of chorioamnionitis for cerebral palsy was 11%, and was even higher (27%) for spastic quadriplegia. Multiple logistic regression analyses identified chorioamnionitis, intrauterine growth restriction, maternal black ethnicity, and maternal age >25 years as independent risk factors for cerebral palsy.
In a recent study of term babies with neonatal encephalopathy who had signs of maternal infection (chorioamnionitis) or infant infection (sepsis), a clear dichotomy in outcomes depending on the source of infection was observed.62 Neonates exposed to chorioamnionitis had a reduced risk of brain injury and adverse outcomes, whereas newborns with sepsis had an elevated risk of predominantly watershed injury, as detected with MRI.62 The better outcomes among neonates exposed to chorioamnionitis might be explained by the timing of the prenatal infection, which could have preconditioned the developing brain against subsequent injury induced by hypoxia–ischaemia, as has been reported in animal studies.63 However, the exact mechanisms underlying the protective effect remain unclear. In addition to the studies of placental infection, a recent study revealed that inflammation on the fetal side of the placenta was associated with elevated maternal IL-6 and IL-8 at delivery and fetal IL-1β, IL-6, IL-8, and TNF in the umbilical cord at birth, as well as worse neurological outcome at 6 months.64
Therapeutic hypothermia and inflammation
The advent of therapeutic hypothermia has brought improvements in clinical outcomes for babies with neo natal encephalopathy. Hypothermia affects several physiological parameters, one of which is the inflammatory response. In one small study from Japan, neonates treated with hypothermia had lower blood levels of high mobility group box 1 (HMGB1) than did untreated encephalopathic babies. HMGB1 is a DNA-binding protein that regulates transcription of genes encoding a number of inflammatory cytokines.65
In a study evaluating biomarkers of injury in encephalopathic newborns, some of whom underwent therapeutic hypothermia, elevated glial fibrillary acidic protein, IL-1, IL-6, IL-8, TNF, interferon and vascular endothelial growth factor (VEGF) levels at 6–24 h were associated with abnormal neurological outcomes, although the number of participants was limited in the outcome group owing to low sample size.66 Serial measurements revealed that the levels of these molecules were not affected by hypothermia. Another study compared circulating leukocytes and serum chemokines between infants under therapeutic hypothermia and a normothermic group. In the hypothermia group, total white blood cells and certain subclasses of leukocytes were markedly depleted, and levels of chemokines, CCL2 and IL-8 were correlated negatively with leukocyte count.67 These data indicate that hypothermia is immunosuppressive, which can be hazardous if neonatal sepsis occurs while the infant is under therapeutic hypothermia.
Stroke
With regard to perinatal arterial ischaemic stroke, the evidence is mixed. In a population-based, case–control study from Kaiser Permanente, 13 infants with stroke were identified, along with 86 randomly selected controls. 68 Several polymorphisms were tested, including variation in IL6, LTA, TNF, Leiden variant of coagulation factor V (F5), coagulation factor II (F7) and MTRR, and apolipoprotein E (APOE) alleles ε2 and ε4. Proinflammatory polymorphisms were not associated with stroke, whereas APOE*ε4 was seen more often in infants with stroke than in controls. This study substantiated an earlier hospital-based cohort study of 49 newborns with stroke, in which 31 polymorphisms, including those representing pathways of inflammation, thrombosis, vascular tone and cellular adhesion, were evaluated.69 In this study, no variant allele was found to be significantly more common in the stroke cohort than in the general population.
In a study evaluating risk factors for perinatal stroke in full-term infants, multivariate analysis revealed that risk factors independently associated with stroke included prolonged rupture of membranes (OR 3.8, 95% CI 1.1–12.8), chorioamnionitis (OR 3.4, 95% CI 1.1–10.5), and history of infertility and pre-eclampsia.70 The risk of perinatal stroke increased dramatically when multiple risk factors were present. A more recent study from the Netherlands substantiated these findings in 52 infants: multivariate analysis indicated increased risk associated with maternal fever (OR 10.2, 95% CI 1.3–78.5) and early-onset sepsis and/or meningitis (OR 5.8, 95% CI 1.1–31.9).71 Other risk factors were low Apgar score at 5 min, and hypoglycaemia, again suggesting multifactorial vulnerability.
Translational therapies
Other important aspects of the inflammatory response must be considered before therapies can be developed. Sex could be an important factor, as the response to therapy with 2-iminobiotin, an antioxidative and anti-inflammatory agent that acts as an inducible nitric oxide synthase inhibitor, protects female but not male neonates against hypoxia–ischaemia in a rat model, suggesting a sex-specific mechanism of protection.72 In addition, intrinsic developmental differences in BBB basement membrane and extracellular matrix formation could contribute to the maintenance of barrier integrity after acute neonatal arterial stroke.23 Taken together, these data suggest the need for a modified approach to any translational therapy, in which aspects such as sex and BBB permeability are taken into consideration. Clinical studies are needed to determine the best therapeutic approaches for term newborns with encephalopathy and stroke.
Inflammation and the preterm brain
Experimental models of preterm brain injury
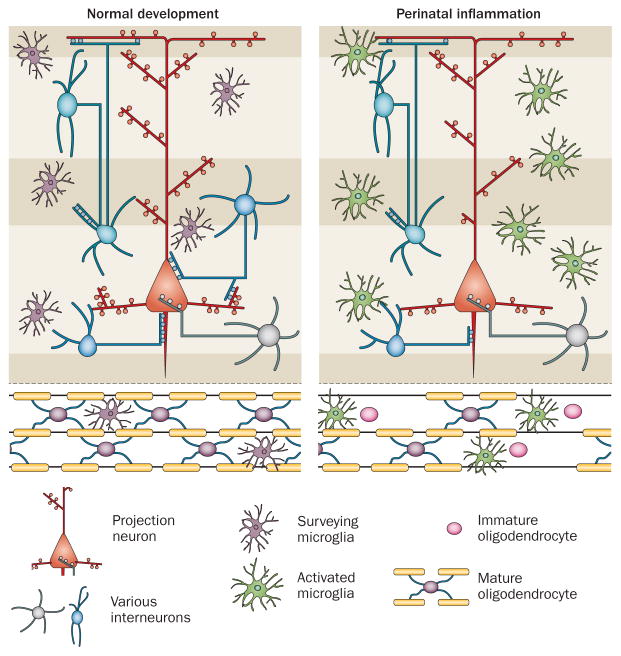
Numerous studies have assessed the effects of microglial activation as a response to experimental infection or inflammation challenges, including infectious agents (Escherichia coli, Ureaplasma parvum, respiratory syncytial virus and cytomegalovirus), sterile inflammatory insults (TLR2, TLR3 or TLR4 agonists, IL-1β and IL-6), experimentally induced hypoxia–ischaemia, chronic hypoxia, or excitotoxic insults. These challenges have been tested across a large array of species (sheep, rabbit, rat, mouse, rat, piglet and guinea pig) to mimic exposures during various stages of pregnancy and neonatal life,3,73 and the insults resulting from these challenges have been consistently shown to lead to white matter damage, often defined as myelin protein deficits. These deficits are accompanied by increased density of microglia and macrophages,17,74–77 collectively pointing to a probable role for microglial activation in the pathophysiology of white matter damage (Figure 5 and Table 1).
Figure 5.
Effects of perinatal inflammation on brain development. Infants born at extremely low gestational age have a markedly increased risk of brain dysfunction, which is attributable to damage and developmental impairment in both white and grey matter. In such situations, microglia become activated. The resulting CNS inflammation impairs oligodendrocyte precursor maturation, which leads to a myelination defect. Furthermore, the cerebral cortex and deep grey matter will be affected by impairments in interneuron survival, axonal integrity, neurite branching, spine density, and synaptogenesis, leading to abnormal connectivity and brain microstructure.
Panels of M1–M2a–M2b markers in microglia have been validated in well-characterized in vitro conditions, 38,78 providing candidate molecules for involvement in progressive and temporal microglial activation that can be tested in future in vivo studies. Until recently, most experimental studies in the perinatal brain only evaluated the number and morphology of microglia at a small number of time points (acute, or acute and ‘long-term’), without assessing markers of differential activation and/or function over a protracted period of time. Timing is a key parameter that determines the predominant microglial phenotype and impact on lesion progression, as recently shown in adult mouse models of stroke,79 brain trauma,80 spinal cord injury and multiple sclerosis.81 Microglial phenotype clearly depends on the type of injury, location (grey versus white matter), and temporal profile of toxic proinflammatory cytokines, and pro-repair and anti-inflammatory cytokines with neuroprotective properties.
Endogenous stem cells
Injuries that elicit neuroinflammation in the brain alter the composition of the pools of neural precursors in both the subventricular zone (SVZ) and the hippocampal subgranular zone (SGZ). In neonatal rats and mice, hypoxia–ischaemia stimulates an increase in the proliferation of SVZ neural precursors that is followed by an increase in the production of neurons and glia, which then also migrate towards regions of injury.82,83 The mechanisms regulating this expansion of the neural precursor pool remain incompletely understood, although self-renewal, proliferation and fate specification of neural precursors are heavily influenced by the cytokines that are produced after injury (Table 3). For example, IL-6, which is markedly upregulated in developmental brain injury, enhances the growth, self-renewal and tripotentiality of neural precursors in the SVZ in vitro.84,85
Table 3.
Effects of microglial cytokines on neural precursors of subventricular and subgranular zones
| Cytokine | Effect on subventricular zone | Effect on subgranular zone |
|---|---|---|
| IL-1α | Not known | Stimulates proliferation176 |
| IL-1β | Maintains stemness177 | Inhibits proliferation178 |
| TNF | TNF receptor 1 inhibits proliferation179 | TNF receptor 1 binding inhibits neural precursor proliferation,180 whereas TNF receptor 2 binding promotes neural precursor proliferation180 |
| IL-6 | Increases neural precursor proliferation and self-renewal84 | Decreases neural precursor proliferation176 and suppresses differentiation of neurons181 |
| Vascular endothelial growth factor C (VEGF-C) | Promotes neural stem cell self-renewal and proliferation182 and enhances oligodendroglial precursor proliferation183 | Not known |
| IFN-γ | Decreases neural precursor proliferation and self-renewal184 | Not known |
| Nitric oxide | Decreases neural precursor proliferation and self-renewal185 | Increases neural precursor proliferation186 |
| Transforming growth factor β (TGF-β) | Increases neural precursor proliferation187 | Increases neural precursor proliferation188 |
Abbreviation: TNF, tumour necrosis factor.
IL-6 induces expression of cyclooxygenase 2 (COX-2) and, thereby, the production of prostaglandins, such as prostaglandin E2. The effects of prostaglandin E2 on neural precursors were evaluated, and this compound was found to increase neural precursor expansion in the SVZ.84 By contrast, indomethacin, which inhibits COX-2, decreased the initial reactive increase in neural precursors in SVZ after hypoxia–ischaemia. Indomethacin reduced the numbers of reactive microglia within and surrounding the SVZ, and reduced IL-6 production after hypoxia–ischaemia.84 These data strongly implicate neuroinflammation— in particular, involvement of IL-6—in the increased expansion of primitive neural precursors in the SVZ after neonatal brain injury.
Whereas SVZ neural precursors proliferate in response to injury, the opposite effect is often observed for the neural precursors in the SGZ: decreased neurogenesis in the hippocampus has been reported in murine LPS and Poly(I:C) models of prenatal inflammation. In a recently published study, LPS was administered to neonatal mice at P5, and its effects on microglio genesis, inflammation and neurogenesis in the developing hippo campus were examined.12 LPS administration acutely, but transiently, increased the proliferation of resident microglia, leading to an increase in numbers of both M1 and M2 type micro glia. Neonatal LPS exposure did not lead to recruitment of peripheral monocyte-derived macrophages to the hippo campus. Microglial cell accumulation was followed by transient inhibition of hippocampal neuronal differentiation, due to specific effects on the type 3 neural precursors, that persisted for 2 weeks after LPS administration.
Oligodendroglial development
Postmortem studies of infants with white matter damage demonstrate that late oligodendrocyte progenitor cells (OPCs) are extremely vulnerable to injury.86 Indeed, the age of highest incidence of white matter damage in the premature infant directly correlates with a predominance of late OPCs in the immature brain.
Hypomyelination can eventually be observed as the brain matures. Mechanisms implicated in hypomyelination include death of OPCs,87 oligodendrocyte maturation blockade without notable cell death,88 and depletion of the pool of proliferating OPCs.87 In animals subjected to infectious, inflammatory, hypoxic, hypoxic–ischaemic or excitotoxic insults during the perinatal period, myelination deficits are seen in association with neuroinflammation,75–77,89–91 suggesting a causal link similar to that seen in human preterm infants.
Neurons are classically regarded as the principal source of glutamate in the brain, but a number of studies have shown that microglia, when activated by proinflammatory stimuli, release substantial amounts of glutamate.92 Several intrinsic properties of OPCs, such as high intracellular iron levels93 and low superoxide dismutases levels,94 render these cells vulnerable to injury mediated by excess glutamate. Multiple lines of in vitro and in vivo evidence support the hypothesis that α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) or kainate-type glutamate receptors are primarily responsible for glutamate-mediated death of OPCs.95 Like immature oligodendrocytes, which are sensitive to high concentrations of glutamate, the late OPCs are extremely vulnerable to kainate-receptor-mediated and AMPA-receptor-mediated excitotoxic death.96
The susceptibility of the brain to white matter lesions after intracerebral injections of AMPA into the pericallosal white matter seems to be age-dependent,94 which is in line with the preferential expression of calcium-permeable AMPA receptors by late OPCs.97 These data have led to the view that the late OPCs are intrinsically vulnerable to injury. In contrast to the classic N-methyl-d-aspartate-receptor-mediated excitotoxic death of neurons that occurs within a few hours, oligodendroglial death after hypoxia–ischaemia in the perinatal brain occurs over 24–48 h.95,96 Glutamate has been demonstrated to activate AMPA and kainate receptors to increase Ca2+ influx in late OPCs, resulting in Bax trans location to the mitochondria, cytochrome c release, activation of caspase-9 and caspase-3, nuclear fragmentation, and cell death.98,99
TNF was one of the first cytokines to be shown to be elevated in premature infants with white matter injury,100 and is consistently found to be strongly upregulated in the neonatal CNS after injury. As with glutamate, TNF seems to exert differential effects depending on the stage of development. TNF is not toxic for OPCs; in fact, it stimulates their proliferation by acting on TNF receptor 2 (TNFR2).101 By contrast, as oligodendroglial cells mature, they become more sensitive to the toxic effects of TNF, which are mediated by TNFR1.102 Like glutamate-mediated OPC cytotoxicity, TNF-mediated OPC death occurs in a dose-dependent manner that requires Bax translocation from the cytosol to the mitochondria.103
In an animal model of systemic inflammation induced by daily intraperitoneal injections of IL-1β during the first 5 days of life, the number of unmyelinated axons was increased and the number of large myelinated fibres was decreased.77 The myelination impairment was associated with reduced fractional anisotropy on MRI and a number of behavioural deficits.
Cytokines of the IL-6 family have potent effects on OPCs. An in vitro study demonstrated that IL-6 could induce cell cycle withdrawal and maturation of OPCs.104 Elevated levels of IL-6, therefore, could contribute to depletion of the pool of proliferating OPCs and premature maturation of the existing precursors into myelinating oligodendrocytes, thereby contributing to dysmyelination. Genetically engineered mice that over-expressed IL-6 developed severe neurological symptoms that include ataxia, tremor, seizures, and severe astrogliosis and microgliosis.105 The white matter was not analysed in these mice.
Neuronal migration and survival
Until recently, migration of cortical neurons in the human brain was thought to be complete by 24–26 weeks of gestation. However, a 2011 study demonstrated that migration of interneurons to the neo-cortex is not complete until term,106 suggesting that events coinciding with preterm delivery could affect this migration. Indeed, these migrating neurons might be influenced by factors released by neighbouring activated microglia, potentially leading to neuronal death or aberrant migration.107
Synaptogenesis
Chronic hypoxia during the neonatal period has been shown to reduce brain size in mice, largely due to decreased cortical volumes.108 A transcriptome analysis of a mouse model of sublethal postnatal hypoxia demonstrated that hypoxia suppresses the expression of genes involved in synaptic maturation, postsynaptic function and neurotransmission.109 Microglia have critical developmental roles in axonal growth and pruning, and in synaptic pruning and function; future studies must determine whether neuroinflammation and microglial activation have deleterious effects on such key events for brain connectivity and function.
Epigenetic changes
Epigenetic changes are crucial for every aspect of normal development and brain function. Epigenetic modifications include enzymatic regulation of transcription through modification of permissive tags (acetylation, methylation, ubiquitination, phosphorylation and sumoylation) on histones or DNA, or microRNA-mediated regulation of translation. In humans, acetylation regulates transcription of up to 5% of the genome, and a notable proportion of human genes can be regulated by at least one microRNA.110 Epigenetic modifications are an essential mechanism by which injury and destructive prenatal environmental factors can lead to long-term disturbances of brain development, including cognitive, motor and behavioural impairments.111
Changes in epigenetic marks and microRNA expression could have key roles in the long-term consequences of perinatal brain insults, such as the failure of OPCs to adequately mature and differentiate. In addition, if these epigenetic changes occur in inflammatory cells such as microglia, they could represent an innate immune cell memory that would alter microglial function for months or years after the perinatal insult.112 As such, epigenetic modifications are emerging as novel and promising targets for neuroprotection (Table 2).
Clinical evidence
Abnormal brain development and brain damage might result from infection in utero or perinatally, or from inflammation triggered by a variety of causes (including ischaemic insults). A large study from Switzerland reported that neonatal sepsis substantially contributed to neurodevelopmental impairment in extremely preterm infants, independently of other risk factors.113
Chorioamnionitis can lead to a fetal inflammatory response and white matter damage.114 Systematic reviews115,116 suggested a link between chorioamnionitis and cystic periventricular leukomalacia and cerebral palsy; although this association was later disputed, more-recent studies have substantiated the initial association. 113 The confusion resulted primarily from the inability to define chorioamnionitis and to document outcome with adequate quantitative MRI techniques coupled with standardized follow-up assessments.
In the absence of infection, intermittent or sustained systemic inflammation might be more detrimental to the brain than is inflammation of shorter duration (such as is seen in infectious diseases).117 This hypothesis emphasizes the importance of ‘systemic’ inflammation affecting the brain—a process that has been documented in a mouse model of perinatal white matter damage,77 in which systemic inflammation blocks oligodendrocyte maturation, resulting in persistent myelination defects. Similarly, in a rat model of neonatal stroke,33 cytokine and chemokine levels are initially raised in plasma, and subsequently in the brain. This phenomenon might explain the secondary damage that accompanies stroke in the newborn.
The concept of sustained damage also embraces the theory of tertiary brain damage, according to which children born prematurely have elevated levels of inflammatory mediators in their plasma long after birth.118 The inflammation might persist through TLR-mediated signalling (as discussed above; Table 1), which is developmentally regulated. Epigenetic mechanisms might also contribute to this protracted event by shifting the balance between proinflammatory and anti-inflammatory gene regulation.112
Immunomodulatory therapies
A variety of drugs targeting neuroinflammation have been tested in animal models of perinatal brain injury (Table 2). The magnitude of neuroprotection observed in these studies has been quite variable, and sometimes the results are inconsistent between animal models and research cohorts (as seen, for example, in the case of minocycline). However, several compounds (for example, corticosteroids, melatonin, erythropoietin, COX inhibitors, cromolyn, histamine receptor blockers, N-acetylcysteine, etanercept, IL-1ra, simvastatin, and certain histone deacetylase inhibitors) that are already in clinical use for other indications have shown promising neuroprotective properties. In addition, innate defence regulatory peptides are currently being tested in clinical trials for other indications.
Two major issues must be taken into account during the investigation of potential immunomodulatory therapies for use in neonates. First, it is not known whether all the candidate drugs can cross the BBB, and additional testing in relevant in vivo models will be necessary to address this key point. Second, neonates at risk of developing brain damage are generally fragile, and their brains are undergoing major developmental changes that will determine the long-term cognitive and motor outcome. Consequently, the safety of compounds to be tested in neonates cannot be directly extrapolated from studies performed in adults or older children.
Most of the drugs tested, with the exception of TNF soluble receptor, IL-1ra and cromolyn, have multiple or even pleiotropic effects that go beyond pure anti-inflammatory effects, including antioxidant and antiapoptotic properties, neuronal activity modulation, mitochondrial protection, and induction of angiogenesis. Therefore, it is difficult to definitively link the neuroprotective properties of these drugs with their anti-inflammatory properties.
Most of the drugs that have been tested in the context of perinatal neuroinflammation have been assessed in a limited number of rodent models, without validation in gyrencephalic animals. The two notable exceptions are melatonin and erythropoietin, which are being tested in clinical trials in preterm infants, and in conjunction with hypothermia in term infants. A recent randomized trial based on a relatively small number of patients has shown that preterm infants exposed to erythropoietin to reduce transfusion needs had a better cognitive outcome than infants exposed to placebo.119 In addition, in an analysis of secondary outcomes of a large randomized clinical trial, exposure of preterm infants to high dose of erythropoietin was associated with reduced brain damage on MRI.120 While awaiting the complete results of these promising trials, present and future research will aim to define more-targeted approaches incorporating the multiple phenotypes of inflammatory cells, leading to modulatory therapies rather than indiscriminate anti-inflammatory strategies.
Conclusions
Perinatal brain injury and developmental abnormalities can be caused by a number of conditions—for example, neonatal encephalopathy, perinatal arterial ischaemic stroke, premature birth, and systemic infections—that can affect the developing brain during fetal life, birth, and the postnatal period. Even though these clinical conditions are very different with respect to aetiology and clinical context, inflammation seems to be an important contributor to the pathogenetic cascade.
Inflammation can both have a priming effect (sensitization or preconditioning) and participate in early or late injury, as well as in repair and recovery after the insult. A number of immunomodulatory interventions that target inflammation have proved effective in experimental models, and might have translational potential.
A great deal of information has been gathered over the past decade about the innate immune response after exposure to TLR agonists and during the early phases of injury, in particular after hypoxia–ischaemia and stroke, but our understanding of inflammation during the perinatal period is still incomplete. More research is urgently needed to understand the role of adaptive immunity, especially during the late stages of disease, and its potential causative role in cognitive impairments acquired by preterm infants. Furthermore, the long-term consequences of inflammation during early fetal and postnatal life, including the possible involvement of epigenetic regulation and T cells, remain to be elucidated. Finally, more work needs to be done to confirm preclinical findings in humans.
Key points.
Perinatal brain injuries result from a spectrum of conditions, including neonatal encephalopathy, arterial ischaemic stroke, prematurity, and systemic infections
Depending on the timing and context, inflammation can prime the brain for injury or exert protective actions
Pattern recognition receptors, such as Toll-like receptors on innate immune cells (microglia, mast cells and macrophages), are important participants in the early phases of injury, and can increase CNS vulnerability (sensitization)
Inflammation during preterm labour and intensive care of premature infants affects the very immature brain during critical phases of brain development, with serious consequences for myelination and cortical development
Understanding the involvement of inflammation in perinatal brain injury can aid identification of new strategies for prevention and treatment that could reduce neurological and neuropsychiatric morbidities in maturing infants
Review criteria.
We searched PubMed for articles published in English from January 1950 to August 2014, with the terms “inflammation”, “mitochondria and brain”, “neonatal brain injury”, “preconditioning”, “sensitization”, “immune and neonatal brain”, “adaptive immunity and immature brain”, “innate immunity and immature brain”, “neonatal hypoxia–ischaemia”, “neonatal encephalopathy”, “neonatal neuroprotection”, “neonatal neuroinflammation”, “neonatal brain oxidative stress”, and “neonatal stroke”. We selected articles reporting clinical, experimental and preclinical findings relevant to understanding of the role of inflammation in brain development and brain injury.
Acknowledgments
All authors have received financial support from Leducq Foundation (DSRR_P34404). H.H. and P.G. have received a grant from Wellcome Trust programme (WT094823MA). NIH has funded D.M.F. (NS033997, 082330), Z.S.V. (RO1NS44025, NS76726) and S.J.V. (R21NS083425). The Swedish Medical Research Council has funded C.M. (VR2012-3500) and H.H. (VR2012-2992). ALF-LUA has funded C.M. (ALFGBG432291) and H.H. (ALFGBG426401). P.G. and C.M. have received the European Union grant FP7 (Neurobid, HEALTHF2-2009-241778). C.M. and H.H. have received funding from the Swedish Brain Foundation has funded (FO2013-095 for C.M., FO2013-0035 for H.H.), the Wilhelm & Martina Lundgren Foundation and the Frimurarna Barnhusdirektionen Foundation. Other funders include the Byggmästare Olle Engkvist Foundation (H.H.), ERA-Net, EU (VR 2014-7551; P.G. and H.H.), DHU PROTECT, INSERM, University Paris 7, (P.G.), the Fondation Graca de Monaco (P.G.), and the Fondation de Spoelberch (P.G.).
Footnotes
Competing interests
The authors declare no competing interests.
Author contributions
All authors researched the data for the article, provided substantial contributions to discussions of the content, and wrote the article.
References
- 1.Galea I, Bechmann I, Perry VH. What is immune privilege (not)? Trends Immunol. 2007;28:12–18. doi: 10.1016/j.it.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Meyer U, Feldon J, Dammann O. Schizophrenia and autism: both shared and disorder-specific pathogenesis via perinatal inflammation? Pediatr Res. 2011;69:26R–33R. doi: 10.1203/PDR.0b013e318212c196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hagberg H, Gressens P, Mallard C. Inflammation during fetal and neonatal life: implications for neurologic and neuropsychiatric disease in children and adults. Ann Neurol. 2012;71:444–457. doi: 10.1002/ana.22620. [DOI] [PubMed] [Google Scholar]
- 4.Fernandez–Lopez D, Natarajan N, Ashwal S, Vexler ZS. Mechanisms of perinatal arterial ischemic stroke. J Cereb Blood Flow Metab. 2014;34:921–932. doi: 10.1038/jcbfm.2014.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 6.Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stridh L, Smith PL, Naylor AS, Wang X, Mallard C. Regulation of Toll-like receptor 1 and -2 in neonatal mice brains after hypoxia–ischemia. J Neuroinflammation. 2011;8:45. doi: 10.1186/1742-2094-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stridh L, Ek CJ, Wang X, Nilsson H, Mallard C. Regulation of Toll-like receptors in the choroid plexus in the immature brain after systemic inflammatory stimuli. Transl Stroke Res. 2013;4:220–227. doi: 10.1007/s12975-012-0248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lathia JD, et al. Toll-like receptor 3 is a negative regulator of embryonic neural progenitor cell proliferation. J Neurosci. 2008;28:13978–13984. doi: 10.1523/JNEUROSCI.2140-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma Y, et al. Toll-like receptor 8 functions as a negative regulator of neurite outgrowth and inducer of neuronal apoptosis. J Cell Biol. 2006;175:209–215. doi: 10.1083/jcb.200606016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vontell R, et al. Toll-like receptor 3 expression in glia and neurons alters in response to white matter injury in preterm infants. Dev Neurosci. 2013;35:130–139. doi: 10.1159/000346158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith PL, Hagberg H, Naylor AS, Mallard C. Neonatal peripheral immune challenge activates microglia and inhibits neurogenesis in the developing murine hippocampus. Dev Neurosci. 2014;36:119–131. doi: 10.1159/000359950. [DOI] [PubMed] [Google Scholar]
- 13.Cai Z, Pan ZL, Pang Y, Evans OB, Rhodes PG. Cytokine induction in fetal rat brains and brain injury in neonatal rats after maternal lipopolysaccharide administration. Pediatr Res. 2000;47:64–72. doi: 10.1203/00006450-200001000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Garay PA, Hsiao EY, Patterson PH, McAllister AK. Maternal immune activation causes age- and region-specific changes in brain cytokines in offspring throughout development. Brain Behav Immun. 2013;31:54–68. doi: 10.1016/j.bbi.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stolp HB, et al. Effect of minocycline on inflammation-induced damage to the blood–brain barrier and white matter during development. Eur J Neurosci. 2007;26:3465–3474. doi: 10.1111/j.1460-9568.2007.05973.x. [DOI] [PubMed] [Google Scholar]
- 16.Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 17.McRae A, Gilland E, Bona E, Hagberg H. Microglia activation after neonatal hypoxic-ischemia. Brain Res Dev Brain Res. 1995;84:245–252. doi: 10.1016/0165-3806(94)00177-2. [DOI] [PubMed] [Google Scholar]
- 18.Jin Y, Silverman AJ, Vannucci SJ. Mast cells are early responders after hypoxia–ischemia in immature rat brain. Stroke. 2009;40:3107–3112. doi: 10.1161/STROKEAHA.109.549691. [DOI] [PubMed] [Google Scholar]
- 19.Palmer C, Roberts RL, Young PI. Timing of neutrophil depletion influences long-term neuroprotection in neonatal rat hypoxic–ischemic brain injury. Pediatr Res. 2004;55:549–556. doi: 10.1203/01.PDR.0000113546.03897.FC. [DOI] [PubMed] [Google Scholar]
- 20.Bona E, et al. Chemokine and inflammatory cell response to hypoxia–ischemia in immature rats. Pediatr Res. 1999;45:500–509. doi: 10.1203/00006450-199904010-00008. [DOI] [PubMed] [Google Scholar]
- 21.Winerdal M, et al. Long lasting local and systemic inflammation after cerebral hypoxic ischemia in newborn mice. PLoS ONE. 2012;7:e36422. doi: 10.1371/journal.pone.0036422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith PL, Ek J, Hagberg H, Mallard C. Peripheral myeloid cells invade the brain following neonatal hypoxic–ischemic insult. Proc. 43rd Annual Meeting of the Society for Neuroscience 813.10; 2013. [Google Scholar]
- 23.Fernandez–Lopez D, et al. Blood–brain barrier permeability is increased after acute adult stroke but not neonatal stroke in the rat. J Neurosci. 2012;32:9588–9600. doi: 10.1523/JNEUROSCI.5977-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ek JC, et al. Brain barrier properties and cerebral blood flow in neonatal mice exposed to cerebral hypoxia–ischemia. J Cereb Blood Flow Metab. doi: 10.1038/jcbfm.2014.255. http://dx.doi.org/10.1038/jcbfm.2014.255. [DOI] [PMC free article] [PubMed]
- 25.Kichev A, et al. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) signaling and cell death in the immature central nervous system after hypoxia–ischemia and inflammation. J Biol Chem. 2014;289:9430–9439. doi: 10.1074/jbc.M113.512350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X, et al. Lipopolysaccharide sensitizes neonatal hypoxic–ischemic brain injury in a MyD88-dependent manner. J Immunol. 2009;183:7471–7477. doi: 10.4049/jimmunol.0900762. [DOI] [PubMed] [Google Scholar]
- 27.Stridh L, et al. Toll-like receptor-3 activation increases the vulnerability of the neonatal brain to hypoxia–ischemia. J Neurosci. 2013;33:12041–12051. doi: 10.1523/JNEUROSCI.0673-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hagberg H, et al. Enhanced expression of interleukin (IL)-1 and IL-6 messenger RNA and bioactive protein after hypoxia–ischemia in neonatal rats. Pediatr Res. 1996;40:603–609. doi: 10.1203/00006450-199610000-00015. [DOI] [PubMed] [Google Scholar]
- 29.Girard S, et al. Postnatal administration of IL-1Ra exerts neuroprotective effects following perinatal inflammation and/or hypoxic–ischemic injuries. Brain Behav Immun. 2012;26:1331–1339. doi: 10.1016/j.bbi.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu XH, et al. Mice deficient in interleukin-1 converting enzyme are resistant to neonatal hypoxic–ischemic brain damage. J Cereb Blood Flow Metab. 1999;19:1099–1108. doi: 10.1097/00004647-199910000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Hedtjarn M, et al. Interleukin-18 involvement in hypoxic–ischemic brain injury. J Neurosci. 2002;22:5910–5919. doi: 10.1523/JNEUROSCI.22-14-05910.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doverhag C, et al. Pharmacological and genetic inhibition of NADPH oxidase does not reduce brain damage in different models of perinatal brain injury in newborn mice. Neurobiol Dis. 2008;31:133–144. doi: 10.1016/j.nbd.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 33.Denker SP, et al. Macrophages are comprised of resident brain microglia not infiltrating peripheral monocytes acutely after neonatal stroke. J Neurochem. 2007;100:893–904. doi: 10.1111/j.1471-4159.2006.04162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arvin KL, et al. Minocycline markedly protects the neonatal brain against hypoxic–ischemic injury. Ann Neurol. 2002;52:54–61. doi: 10.1002/ana.10242. [DOI] [PubMed] [Google Scholar]
- 35.Faustino JV, et al. Microglial cells contribute to endogenous brain defenses after acute neonatal focal stroke. J Neurosci. 2011;31:12992–13001. doi: 10.1523/JNEUROSCI.2102-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woo MS, et al. Genetic deletion of CD36 enhances injury after acute neonatal stroke. Ann Neurol. 2012;72:961–970. doi: 10.1002/ana.23727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Colton C, Wilcock DM. Assessing activation states in microglia. CNS Neurol Disord Drug Targets. 2010;9:174–191. doi: 10.2174/187152710791012053. [DOI] [PubMed] [Google Scholar]
- 38.Chhor V, et al. Characterization of phenotype markers and neuronotoxic potential of polarised primary microglia in vitro. Brain Behav Immun. 2013;32:70–85. doi: 10.1016/j.bbi.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lindsberg PJ, Strbian D, Karjalainen–Lindsberg ML. Mast cells as early responders in the regulation of acute blood–brain barrier changes after cerebral ischemia and hemorrhage. J Cereb Blood Flow Metab. 2010;30:689–702. doi: 10.1038/jcbfm.2009.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nelissen S, et al. The role of mast cells in neuroinflammation. Acta Neuropathol. 2013;125:637–650. doi: 10.1007/s00401-013-1092-y. [DOI] [PubMed] [Google Scholar]
- 41.Mesples B, Fontaine RH, Lelievre V, Launay JM, Gressens P. Neuronal TGF-β1 mediates IL-9/mast cell interaction and exacerbates excitotoxicity in newborn mice. Neurobiol Dis. 2005;18:193–205. doi: 10.1016/j.nbd.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 42.Jin Y, Silverman AJ, Vannucci SJ. Mast cell stabilization limits hypoxic–ischemic brain damage in the immature rat. Dev Neurosci. 2007;29:373–384. doi: 10.1159/000105478. [DOI] [PubMed] [Google Scholar]
- 43.Biran V, et al. Stroke induces histamine accumulation and mast cell degranulation in the neonatal rat brain. Brain Pathol. 2008;18:1–9. doi: 10.1111/j.1750-3639.2007.00092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwartz M, Baruch K. The resolution of neuroinflammation in neurodegeneration: leukocyte recruitment via the choroid plexus. EMBO J. 2014;33:7–22. doi: 10.1002/embj.201386609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shechter R, London A, Schwartz M. Orchestrated leukocyte recruitment to immune-privileged sites: absolute barriers versus educational gates. Nat Rev Immunol. 2013;13:206–218. doi: 10.1038/nri3391. [DOI] [PubMed] [Google Scholar]
- 46.Hurn PD, et al. T- and B-cell-deficient mice with experimental stroke have reduced lesion size and inflammation. J Cereb Blood Flow Metab. 2007;27:1798–1805. doi: 10.1038/sj.jcbfm.9600482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17:796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ellestad KK, et al. Early life exposure to lipopolysaccharide suppresses experimental autoimmune encephalomyelitis by promoting tolerogenic dendritic cells and regulatory T cells. J Immunol. 2009;183:298–309. doi: 10.4049/jimmunol.0803576. [DOI] [PubMed] [Google Scholar]
- 49.Li XL, et al. Neonatal endotoxin exposure suppresses experimental autoimmune encephalomyelitis through regulating the immune cells responsivity in the central nervous system of adult rats. Biochem Biophys Res Commun. 2010;398:302–308. doi: 10.1016/j.bbrc.2010.06.086. [DOI] [PubMed] [Google Scholar]
- 50.Mandal M, Marzouk AC, Donnelly R, Ponzio NM. Maternal immune stimulation during pregnancy affects adaptive immunity in offspring to promote development of TH17 cells. Brain Behav Immun. 2011;25:863–871. doi: 10.1016/j.bbi.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 51.Horiuchi M, Itoh A, Pleasure D, Itoh T. MEK–ERK signaling is involved in interferon-γ-induced death of oligodendroglial progenitor cells. J Biol Chem. 2006;281:20095–20106. doi: 10.1074/jbc.M603179200. [DOI] [PubMed] [Google Scholar]
- 52.Eklind S, et al. Bacterial endotoxin sensitizes the immature brain to hypoxic–ischaemic injury. Eur J Neurosci. 2001;13:1101–1106. doi: 10.1046/j.0953-816x.2001.01474.x. [DOI] [PubMed] [Google Scholar]
- 53.Lehnardt S, et al. The toll-like receptor TLR4 is necessary for lipopolysaccharide-induced oligodendrocyte injury in the CNS. J Neurosci. 2002;22:2478–2486. doi: 10.1523/JNEUROSCI.22-07-02478.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kendall GS, et al. TNF gene cluster deletion abolishes lipopolysaccharide-mediated sensitization of the neonatal brain to hypoxic ischemic insult. Lab Invest. 2011;91:328–341. doi: 10.1038/labinvest.2010.192. [DOI] [PubMed] [Google Scholar]
- 55.Yang D, et al. Intranasal delivery of cell-penetrating anti-NF-κB peptides (Tat-NBD) alleviates infection-sensitized hypoxic–ischemic brain injury. Exp Neurol. 2013;247:447–455. doi: 10.1016/j.expneurol.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grether JK, Nelson KB. Maternal infection and cerebral palsy in infants of normal birth weight. JAMA. 1997;278:207–211. [PubMed] [Google Scholar]
- 57.Sävman K, Blennow M, Gustafson K, Tarkowski E, Hagberg H. Cytokine response in cerebrospinal fluid after birth asphyxia. Pediatr Res. 1998;43:746–751. doi: 10.1203/00006450-199806000-00006. [DOI] [PubMed] [Google Scholar]
- 58.Bartha AI, et al. Neonatal encephalopathy: association of cytokines with MR spectroscopy and outcome. Pediatr Res. 2004;56:960–966. doi: 10.1203/01.PDR.0000144819.45689.BB. [DOI] [PubMed] [Google Scholar]
- 59.Nelson KB, Dambrosia JM, Grether JK, Phillips TM. Neonatal cytokines and coagulation factors in children with cerebral palsy. Ann Neurol. 1998;44:665–675. doi: 10.1002/ana.410440413. [DOI] [PubMed] [Google Scholar]
- 60.Lassiter HA, Watson SW, Seifring ML, Tanner JE. Complement factor 9 deficiency in serum of human neonates. J Infect Dis. 1992;166:53–57. doi: 10.1093/infdis/166.1.53. [DOI] [PubMed] [Google Scholar]
- 61.Wu YW, et al. Chorioamnionitis and cerebral palsy in term and near-term infants. JAMA. 2003;290:2677–2684. doi: 10.1001/jama.290.20.2677. [DOI] [PubMed] [Google Scholar]
- 62.Jenster M, et al. Maternal or neonatal infection: association with neonatal encephalopathy outcomes. Pediatr Res. 2014;76:93–99. doi: 10.1038/pr.2014.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eklind S, Mallard C, Arvidsson P, Hagberg H. Lipopolysaccharide induces both a primary and a secondary phase of sensitization in the developing rat brain. Pediatr Res. 2005;58:112–116. doi: 10.1203/01.PDR.0000163513.03619.8D. [DOI] [PubMed] [Google Scholar]
- 64.Armstrong-Wells J, et al. Inflammatory predictors of neurologic disability after preterm premature rupture of membranes. Am J Obstet Gynecol. doi: 10.1016/j.ajog.2014.09.016. http://dx.doi.org/10.1016/j.ajog.2014.09.016. [DOI] [PMC free article] [PubMed]
- 65.Nakamura T, Yamada S, Yoshioka T. Brain hypothermic therapy dramatically decreases elevated blood concentrations of high mobility group box 1 in neonates with hypoxic–ischemic encephalopathy. Dis Markers. 2013;35:327–330. doi: 10.1155/2013/327604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chalak LF, et al. Biomarkers for severity of neonatal hypoxic–ischemic encephalopathy and outcomes in newborns receiving hypothermia therapy. J Pediatr. 2014;164:468–474.e1. doi: 10.1016/j.jpeds.2013.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jenkins DD, et al. Altered circulating leukocytes and their chemokines in a clinical trial of therapeutic hypothermia for neonatal hypoxic ischemic encephalopathy. Pediatr Crit Care Med. 2013;14:786–795. doi: 10.1097/PCC.0b013e3182975cc9. [DOI] [PubMed] [Google Scholar]
- 68.Gelfand AA, Croen LA, Torres AR, Wu YW. Genetic risk factors for perinatal arterial ischemic stroke. Pediatr Neurol. 2013;48:36–41. doi: 10.1016/j.pediatrneurol.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miller SP, et al. Candidate gene polymorphisms do not differ between newborns with stroke and normal controls. Stroke. 2006;37:2678–2683. doi: 10.1161/01.STR.0000244810.91105.c9. [DOI] [PubMed] [Google Scholar]
- 70.Lee J, et al. Maternal and infant characteristics associated with perinatal arterial stroke in the infant. JAMA. 2005;293:723–729. doi: 10.1001/jama.293.6.723. [DOI] [PubMed] [Google Scholar]
- 71.Harteman JC, et al. Risk factors for perinatal arterial ischaemic stroke in full-term infants: a case–control study. Arch Dis Child Fetal Neonatal Ed. 2012;97:F411–F416. doi: 10.1136/archdischild-2011-300973. [DOI] [PubMed] [Google Scholar]
- 72.Nijboer CH, et al. Gender-specific neuroprotection by 2-iminobiotin after hypoxia–ischemia in the neonatal rat via a nitric oxide independent pathway. J Cereb Blood Flow Metab. 2007;27:282–292. doi: 10.1038/sj.jcbfm.9600342. [DOI] [PubMed] [Google Scholar]
- 73.Boksa P. Effects of prenatal infection on brain development and behavior: a review of findings from animal models. Brain Behav Immun. 2010;24:881–897. doi: 10.1016/j.bbi.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 74.Tahraoui SL, et al. Central role of microglia in neonatal excitotoxic lesions of the murine periventricular white matter. Brain Pathol. 2001;11:56–71. doi: 10.1111/j.1750-3639.2001.tb00381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mallard C, Welin AK, Peebles D, Hagberg H, Kjellmer I. White matter injury following systemic endotoxemia or asphyxia in the fetal sheep. Neurochem Res. 2003;28:215–223. doi: 10.1023/a:1022368915400. [DOI] [PubMed] [Google Scholar]
- 76.Rousset CI, et al. Maternal exposure to LPS induces hypomyelination in the internal capsule and programmed cell death in the deep gray matter in newborn rats. Pediatr Res. 2006;59:428–433. doi: 10.1203/01.pdr.0000199905.08848.55. [DOI] [PubMed] [Google Scholar]
- 77.Favrais G, et al. Systemic inflammation disrupts the developmental program of white matter. Ann Neurol. 2011;70:550–565. doi: 10.1002/ana.22489. [DOI] [PubMed] [Google Scholar]
- 78.Michelucci A, Heurtaux T, Grandbarbe L, Morga E, Heuschling P. Characterization of the microglial phenotype under specific proinflammatory and anti-inflammatory conditions: effects of oligomeric and fibrillar amyloid-β. J Neuroimmunol. 2009;210:3–12. doi: 10.1016/j.jneuroim.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 79.Hu X, et al. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012;43:3063–3070. doi: 10.1161/STROKEAHA.112.659656. [DOI] [PubMed] [Google Scholar]
- 80.Wang G, et al. Microglia/macrophage polarization dynamics in white matter after traumatic brain injury. J Cereb Blood Flow Metab. 2013;33:1864–1874. doi: 10.1038/jcbfm.2013.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Miron VE, et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci. 2013;16:1211–1218. doi: 10.1038/nn.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Plane JM, Liu R, Wang TW, Silverstein FS, Parent JM. Neonatal hypoxic–ischemic injury increases forebrain subventricular zone neurogenesis in the mouse. Neurobiol Dis. 2004;16:585–595. doi: 10.1016/j.nbd.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 83.Yang Z, et al. Sustained neocortical neurogenesis after neonatal hypoxic/ischemic injury. Ann Neurol. 2007;61:199–208. doi: 10.1002/ana.21068. [DOI] [PubMed] [Google Scholar]
- 84.Covey MV, Loporchio D, Buono KD, Levison SW. Opposite effect of inflammation on subventricular zone versus hippocampal precursors in brain injury. Ann Neurol. 2011;70:616–626. doi: 10.1002/ana.22473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Covey MV, Levison SW. Leukemia inhibitory factor participates in the expansion of neural stem/progenitors after perinatal hypoxia/ischemia. Neuroscience. 2007;148:501–509. doi: 10.1016/J.NEUROSCIENCE.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Back SA, et al. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J Neurosci. 2001;21:1302–1312. doi: 10.1523/JNEUROSCI.21-04-01302.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Haynes RL, et al. Nitrosative and oxidative injury to premyelinating oligodendrocytes in periventricular leukomalacia. J Neuropathol Exp Neurol. 2003;62:441–450. doi: 10.1093/jnen/62.5.441. [DOI] [PubMed] [Google Scholar]
- 88.Billiards SS, et al. Myelin abnormalities without oligodendrocyte loss in periventricular leukomalacia. Brain Pathol. 2008;18:153–163. doi: 10.1111/j.1750-3639.2007.00107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dean JM, et al. Delayed cortical impairment following lipopolysaccharide exposure in preterm fetal sheep. Ann Neurol. 2011;70:846–856. doi: 10.1002/ana.22480. [DOI] [PubMed] [Google Scholar]
- 90.Chang KH, Yeh CM, Yeh CY, Huang CC, Hsu KS. Neonatal dexamethasone treatment exacerbates hypoxic–ischemic brain injury. Mol Brain. 2013;6:18. doi: 10.1186/1756-6606-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Favrais G, Schwendimann L, Gressens P, Lelievre V. Cyclooxygenase-2 mediates the sensitizing effects of systemic IL-1-beta on excitotoxic brain lesions in newborn mice. Neurobiol Dis. 2007;25:496–505. doi: 10.1016/j.nbd.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 92.Barger SW, Goodwin ME, Porter MM, Beggs ML. Glutamate release from activated microglia requires the oxidative burst and lipid peroxidation. J Neurochem. 2007;101:1205–1213. doi: 10.1111/j.1471-4159.2007.04487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ozawa H, Nishida A, Mito T, Takashima S. Immunohistochemical study of ferritin-positive cells in the cerebellar cortex with subarachnoidal hemorrhage in neonates. Brain Res. 1994;651:345–348. doi: 10.1016/0006-8993(94)90717-x. [DOI] [PubMed] [Google Scholar]
- 94.Folkerth RD, et al. Developmental lag in superoxide dismutases relative to other antioxidant enzymes in premyelinated human telencephalic white matter. J Neuropathol Exp Neurol. 2004;63:990–999. doi: 10.1093/jnen/63.9.990. [DOI] [PubMed] [Google Scholar]
- 95.Follett PL, Rosenberg PA, Volpe JJ, Jensen FE. NBQX attenuates excitotoxic injury in developing white matter. J Neurosci. 2000;20:9235–9241. doi: 10.1523/JNEUROSCI.20-24-09235.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ness JK, Romanko MJ, Rothstein RP, Wood TL, Levison SW. Perinatal hypoxia–ischemia induces apoptotic and excitotoxic death of periventricular white matter oligodendrocyte progenitors. Dev Neurosci. 2001;23:203–208. doi: 10.1159/000046144. [DOI] [PubMed] [Google Scholar]
- 97.Talos DM, et al. Developmental regulation of α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor subunit expression in forebrain and relationship to regional susceptibility to hypoxic/ischemic injury. II. Human cerebral white matter and cortex. J Comp Neurol. 2006;497:61–77. doi: 10.1002/cne.20978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ness JK, Scaduto RC, Jr, Wood TL. IGF-I prevents glutamate-mediated bax translocation and cytochrome C release in O4+ oligodendrocyte progenitors. Glia. 2004;46:183–194. doi: 10.1002/glia.10360. [DOI] [PubMed] [Google Scholar]
- 99.Simonishvili S, Jain MR, Li H, Levison SW, Wood TL. Identification of Bax-interacting proteins in oligodendrocyte progenitors during glutamate excitotoxicity and perinatal hypoxia–ischemia. ASN Neuro. 2013;5:e00131. doi: 10.1042/AN20130027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Deguchi K, Oguchi K, Takashima S. Characteristic neuropathology of leukomalacia in extremely low birth weight infants. Pediatr Neurol. 1997;16:296–300. doi: 10.1016/s0887-8994(97)00041-6. [DOI] [PubMed] [Google Scholar]
- 101.Arnett HA, et al. TNFα promotes proliferation of oligodendrocyte progenitors and remyelination. Nat Neurosci. 2001;4:1116–1122. doi: 10.1038/nn738. [DOI] [PubMed] [Google Scholar]
- 102.Cammer W, Zhang H. Maturation of oligodendrocytes is more sensitive to TNFα than is survival of precursors and immature oligodendrocytes. J Neuroimmunol. 1999;97:37–42. doi: 10.1016/s0165-5728(99)00045-4. [DOI] [PubMed] [Google Scholar]
- 103.Pang Y, Zheng B, Fan LW, Rhodes PG, Cai Z. IGF-1 protects oligodendrocyte progenitors against TNFα-induced damage by activation of PI3K/Akt and interruption of the mitochondrial apoptotic pathway. Glia. 2007;55:1099–1107. doi: 10.1002/glia.20530. [DOI] [PubMed] [Google Scholar]
- 104.Valerio A, et al. Soluble interleukin-6 (IL-6) receptor/IL-6 fusion protein enhances in vitro differentiation of purified rat oligodendroglial lineage cells. Mol Cell Neurosci. 2002;21:602–615. doi: 10.1006/mcne.2002.1208. [DOI] [PubMed] [Google Scholar]
- 105.Campbell IL, et al. Transgenic models to assess the pathogenic actions of cytokines in the central nervous system. Mol Psychiatry. 1997;2:125–129. doi: 10.1038/sj.mp.4000225. [DOI] [PubMed] [Google Scholar]
- 106.Xu G, et al. Late development of the GABAergic system in the human cerebral cortex and white matter. J Neuropathol Exp Neurol. 2011;70:841–858. doi: 10.1097/NEN.0b013e31822f471c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Leviton A, Gressens P. Neuronal damage accompanies perinatal white-matter damage. Trends Neurosci. 2007;30:473–478. doi: 10.1016/j.tins.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 108.Stewart WB, Ment LR, Schwartz M. Chronic postnatal hypoxia increases the numbers of cortical neurons. Brain Res. 1997;760:17–21. doi: 10.1016/s0006-8993(97)00271-0. [DOI] [PubMed] [Google Scholar]
- 109.Curristin SM, et al. Disrupted synaptic development in the hypoxic newborn brain. Proc Natl Acad Sci USA. 2002;99:15729–15734. doi: 10.1073/pnas.232568799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bhalala OG, Srikanth M, Kessler JA. The emerging roles of microRNAs in CNS injuries. Nat Rev Neurol. 2013;9:328–339. doi: 10.1038/nrneurol.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ozanne SE, Constancia M. Mechanisms of disease: the developmental origins of disease and the role of the epigenotype. Nat Clin Pract Endocrinol Metab. 2007;3:539–546. doi: 10.1038/ncpendmet0531. [DOI] [PubMed] [Google Scholar]
- 112.Fleiss B, Gressens P. Tertiary mechanisms of brain damage: a new hope for treatment of cerebral palsy? Lancet Neurol. 2012;11:556–566. doi: 10.1016/S1474-4422(12)70058-3. [DOI] [PubMed] [Google Scholar]
- 113.Schlapbach LJ, et al. Impact of sepsis on neurodevelopmental outcome in a Swiss National Cohort of extremely premature infants. Pediatrics. 2011;128:e348–e357. doi: 10.1542/peds.2010-3338. [DOI] [PubMed] [Google Scholar]
- 114.Chau V, McFadden DE, Poskitt KJ, Miller SP. Chorioamnionitis in the pathogenesis of brain injury in preterm infants. Clin Perinatol. 2014;41:83–103. doi: 10.1016/j.clp.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 115.Wu YW, Colford JM., Jr Chorioamnionitis as a risk factor for cerebral palsy: a meta-analysis. JAMA. 2000;284:1417–1424. doi: 10.1001/jama.284.11.1417. [DOI] [PubMed] [Google Scholar]
- 116.Shatrov JG, et al. Chorioamnionitis and cerebral palsy: a meta-analysis. Obstet Gynecol. 2010;116:387–392. doi: 10.1097/AOG.0b013e3181e90046. [DOI] [PubMed] [Google Scholar]
- 117.Dammann O, Leviton A. Intermittent or sustained systemic inflammation and the preterm brain. Pediatr Res. 2014;75:376–380. doi: 10.1038/pr.2013.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lin CY, et al. Altered inflammatory responses in preterm children with cerebral palsy. Ann Neurol. 2010;68:204–212. doi: 10.1002/ana.22049. [DOI] [PubMed] [Google Scholar]
- 119.Ohls RK, et al. Cognitive outcomes of preterm infants randomized to darbepoetin, erythropoietin, or placebo. Pediatrics. 2014;133:1023–1030. doi: 10.1542/peds.2013-4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Leuchter RH, et al. Association between early administration of high-dose erythropoietin in preterm infants and brain MRI abnormality at term-equivalent age. JAMA. 2014;312:817–824. doi: 10.1001/jama.2014.9645. [DOI] [PubMed] [Google Scholar]
- 121.Azzopardi D, et al. Prognosis of newborn infants with hypoxic–ischemic brain injury assessed by phosphorus magnetic resonance spectroscopy. Pediatr Res. 1989;25:445–451. doi: 10.1203/00006450-198905000-00004. [DOI] [PubMed] [Google Scholar]
- 122.Vexler ZS, Yenari MA. Does inflammation after stroke affect the developing brain differently than adult brain? Dev Neurosci. 2009;31:378–393. doi: 10.1159/000232556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8:110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dammann O, Leviton A. Maternal intrauterine infection, cytokines, and brain damage in the preterm newborn. Pediatr Res. 1997;42:1–8. doi: 10.1203/00006450-199707000-00001. [DOI] [PubMed] [Google Scholar]
- 125.Taniguchi H, Anacker C, Wang Q, Andreasson K. Protection by vascular prostaglandin E2 signaling in hypoxic–ischemic encephalopathy. Exp Neurol. 2014;255:30–37. doi: 10.1016/j.expneurol.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yang D, et al. Plasminogen activator inhibitor-1 mitigates brain injury in a rat model of infection-sensitized neonatal hypoxia–ischemia. Cereb Cortex. 2013;23:1218–1229. doi: 10.1093/cercor/bhs115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang LW, Tu YF, Huang CC, Ho CJ. JNK signaling is the shared pathway linking neuroinflammation, blood–brain barrier disruption, and oligodendroglial apoptosis in the white matter injury of the immature brain. J Neuroinflammation. 2012;9:175. doi: 10.1186/1742-2094-9-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Du X, et al. Systemic stimulation of TLR2 impairs neonatal mouse brain development. PLoS ONE. 2011;6:e19583. doi: 10.1371/journal.pone.0019583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cameron JS, et al. Toll-like receptor 3 is a potent negative regulator of axonal growth in mammals. J Neurosci. 2007;27:13033–13041. doi: 10.1523/JNEUROSCI.4290-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Meyer U, Nyffeler M, Yee BK, Knuesel I, Feldon J. Adult brain and behavioral pathological markers of prenatal immune challenge during early/middle and late fetal development in mice. Brain Behav Immun. 2008;22:469–486. doi: 10.1016/j.bbi.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 131.Ratnayake U, Quinn TA, Castillo-Melendez M, Dickinson H, Walker DW. Behaviour and hippocampus-specific changes in spiny mouse neonates after treatment of the mother with the viral-mimetic Poly I:C at mid-pregnancy. Brain Behav Immun. 2012;26:1288–1299. doi: 10.1016/j.bbi.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 132.Ling Z, et al. In utero bacterial endotoxin exposure causes loss of tyrosine hydroxylase neurons in the postnatal rat midbrain. Mov Disord. 2002;17:116–124. doi: 10.1002/mds.10078. [DOI] [PubMed] [Google Scholar]
- 133.Duncan JR, et al. White matter injury after repeated endotoxin exposure in the preterm ovine fetus. Pediatr Res. 2002;52:941–949. doi: 10.1203/00006450-200212000-00021. [DOI] [PubMed] [Google Scholar]
- 134.Baharnoori M, Brake WG, Srivastava LK. Prenatal immune challenge induces developmental changes in the morphology of pyramidal neurons of the prefrontal cortex and hippocampus in rats. Schizophr Res. 2009;107:99–109. doi: 10.1016/j.schres.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 135.Wang X, et al. White matter damage after chronic subclinical inflammation in newborn mice. J Child Neurol. 2009;24:1171–1178. doi: 10.1177/0883073809338068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kannan S, et al. Decreased cortical serotonin in neonatal rabbits exposed to endotoxin in utero. J Cereb Blood Flow Metab. 2011;31:738–749. doi: 10.1038/jcbfm.2010.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Oskvig DB, Elkahloun AG, Johnson KR, Phillips TM, Herkenham M. Maternal immune activation by LPS selectively alters specific gene expression profiles of interneuron migration and oxidative stress in the fetus without triggering a fetal immune response. Brain Behav Immun. 2012;26:623–634. doi: 10.1016/j.bbi.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Adler DA, et al. Circadian cycle-dependent EEG biomarkers of pathogenicity in adult mice following prenatal exposure to in utero inflammation. Neuroscience. 2014;275:305–313. doi: 10.1016/j.neuroscience.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Feng Y, Rhodes PG, Bhatt AJ. Dexamethasone pre-treatment protects brain against hypoxic–ischemic injury partially through up-regulation of vascular endothelial growth factor A in neonatal rats. Neuroscience. 2011;179:223–232. doi: 10.1016/j.neuroscience.2011.01.050. [DOI] [PubMed] [Google Scholar]
- 140.Tuor UI, Simone CS, Barks JD, Post M. Dexamethasone prevents cerebral infarction without affecting cerebral blood flow in neonatal rats. Stroke. 1993;24:452–457. doi: 10.1161/01.str.24.3.452. [DOI] [PubMed] [Google Scholar]
- 141.Felszeghy K, Banisadr G, Rostene W, Nyakas C, Haour F. Dexamethasone downregulates chemokine receptor CXCR4 and exerts neuroprotection against hypoxia/ischemia-induced brain injury in neonatal rats. Neuroimmunomodulation. 2004;11:404–413. doi: 10.1159/000080151. [DOI] [PubMed] [Google Scholar]
- 142.Tsuji M, Wilson MA, Lange MS, Johnston MV. Minocycline worsens hypoxic–ischemic brain injury in a neonatal mouse model. Exp Neurol. 2004;189:58–65. doi: 10.1016/j.expneurol.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 143.Dommergues MA, Plaisant F, Verney C, Gressens P. Early microglial activation following neonatal excitotoxic brain damage in mice: a potential target for neuroprotection. Neuroscience. 2003;121:619–628. doi: 10.1016/s0306-4522(03)00558-x. [DOI] [PubMed] [Google Scholar]
- 144.Fan LW, Pang Y, Lin S, Rhodes PG, Cai Z. Minocycline attenuates lipopolysaccharide-induced white matter injury in the neonatal rat brain. Neuroscience. 2005;133:159–168. doi: 10.1016/j.neuroscience.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 145.Schmitz T, et al. Minocycline protects the immature white matter against hyperoxia. Exp Neurol. 2014;254:153–165. doi: 10.1016/j.expneurol.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 146.Husson I, et al. Melatoninergic neuroprotection of the murine periventricular white matter against neonatal excitotoxic challenge. Ann Neurol. 2002;51:82–92. doi: 10.1002/ana.10072. [DOI] [PubMed] [Google Scholar]
- 147.Villapol S, et al. Melatonin promotes myelination by decreasing white matter inflammation after neonatal stroke. Pediatr Res. 2011;69:51–55. doi: 10.1203/PDR.0b013e3181fcb40b. [DOI] [PubMed] [Google Scholar]
- 148.Olivier P, et al. Melatonin promotes oligodendroglial maturation of injured white matter in neonatal rats. PLoS ONE. 2009;4:e7128. doi: 10.1371/journal.pone.0007128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Welin AK, et al. Melatonin reduces inflammation and cell death in white matter in the mid-gestation fetal sheep following umbilical cord occlusion. Pediatr Res. 2007;61:153–158. doi: 10.1203/01.pdr.0000252546.20451.1a. [DOI] [PubMed] [Google Scholar]
- 150.Yawno T, et al. Mechanisms of melatonin-induced protection in the brain of late gestation fetal sheep in response to hypoxia. Dev Neurosci. 2012;34:543–551. doi: 10.1159/000346323. [DOI] [PubMed] [Google Scholar]
- 151.Robertson NJ, et al. Melatonin augments hypothermic neuroprotection in a perinatal asphyxia model. Brain. 2013;136:90–105. doi: 10.1093/brain/aws285. [DOI] [PubMed] [Google Scholar]
- 152.van de Looij Y, et al. Multi-modal assessment of long-term erythropoietin treatment after neonatal hypoxic–ischemic injury in rat brain. PLoS ONE. 2014;9:e95643. doi: 10.1371/journal.pone.0095643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Fang AY, Gonzalez FF, Sheldon RA, Ferriero DM. Effects of combination therapy using hypothermia and erythropoietin in a rat model of neonatal hypoxia–ischemia. Pediatr Res. 2013;73:12–17. doi: 10.1038/pr.2012.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gonzalez FF, et al. Erythropoietin increases neurogenesis and oligodendrogliosis of subventricular zone precursor cells after neonatal stroke. Stroke. 2013;44:753–758. doi: 10.1161/STROKEAHA.111.000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Keller M, et al. Erythropoietin is neuroprotective against NMDA-receptor-mediated excitotoxic brain injury in newborn mice. Neurobiol Dis. 2006;24:357–366. doi: 10.1016/j.nbd.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 156.Traudt CM, et al. Concurrent erythropoietin and hypothermia treatment improve outcomes in a term nonhuman primate model of perinatal asphyxia. Dev Neurosci. 2013;35:491–503. doi: 10.1159/000355460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Plaisant F, et al. Neuroprotective properties of tianeptine: interactions with cytokines. Neuropharmacology. 2003;44:801–809. doi: 10.1016/s0028-3908(03)00066-2. [DOI] [PubMed] [Google Scholar]
- 158.Nijboer CH, et al. Targeting the p53 pathway to protect the neonatal ischemic brain. Ann Neurol. 2011;70:255–264. doi: 10.1002/ana.22413. [DOI] [PubMed] [Google Scholar]
- 159.Bolouri H, et al. Innate defense regulator peptide 1018 protects against perinatal brain injury. Ann Neurol. 2014;75:395–410. doi: 10.1002/ana.24087. [DOI] [PubMed] [Google Scholar]
- 160.Lante F, et al. Neurodevelopmental damage after prenatal infection: role of oxidative stress in the fetal brain. Free Radic Biol Med. 2007;42:1231–1245. doi: 10.1016/j.freeradbiomed.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 161.Liu JQ, Lee TF, Chen C, Bagim DL, Cheung PY. N-acetylcysteine improves hemodynamics and reduces oxidative stress in the brains of newborn piglets with hypoxia–reoxygenation injury. J Neurotrauma. 2010;27:1865–1873. doi: 10.1089/neu.2010.1325. [DOI] [PubMed] [Google Scholar]
- 162.Jatana M, Singh I, Singh AK, Jenkins D. Combination of systemic hypothermia and N-acetylcysteine attenuates hypoxic–ischemic brain injury in neonatal rats. Pediatr Res. 2006;59:684–689. doi: 10.1203/01.pdr.0000215045.91122.44. [DOI] [PubMed] [Google Scholar]
- 163.Kannan S, et al. Dendrimer-based postnatal therapy for neuroinflammation and cerebral palsy in a rabbit model. Sci Transl Med. 2012;18:130ra46. doi: 10.1126/scitranslmed.3003162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Nijboer CH, Heijnen CJ, Groenendaal F, van Bel F, Kavelaars A. Alternate pathways preserve tumor necrosis factor-α production after nuclear factor-κB inhibition in neonatal cerebral hypoxia–ischemia. Stroke. 2009;40:3362–3368. doi: 10.1161/STROKEAHA.109.560250. [DOI] [PubMed] [Google Scholar]
- 165.Aden U, et al. Systemic inflammation sensitizes the neonatal brain to excitotoxicity through a pro-/anti-inflammatory imbalance: key role of TNFα pathway and protection by etanercept. Brain Behav Immun. 2010;24:747–758. doi: 10.1016/j.bbi.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 166.Pang Y, Cai Z, Rhodes PG. Disturbance of oligodendrocyte development, hypomyelination and white matter injury in the neonatal rat brain after intracerebral injection of lipopolysaccharide. Brain Res Dev Brain Res. 2003;140:205–214. doi: 10.1016/s0165-3806(02)00606-5. [DOI] [PubMed] [Google Scholar]
- 167.Hu X, et al. Activation of nuclear factor-κB signaling pathway by interleukin-1 after hypoxia/ischemia in neonatal rat hippocampus and cortex. J Neurochem. 2005;93:26–37. doi: 10.1111/j.1471-4159.2004.02968.x. [DOI] [PubMed] [Google Scholar]
- 168.Nijboer CH, et al. Strong neuroprotection by inhibition of NF-κB after neonatal hypoxia–ischemia involves apoptotic mechanisms but is independent of cytokines. Stroke. 2008;39:2129–2137. doi: 10.1161/STROKEAHA.107.504175. [DOI] [PubMed] [Google Scholar]
- 169.Yang D, et al. Intranasal delivery of cell-penetrating anti-NF-κB peptides (Tat-NBD) alleviates infection-sensitized hypoxic–ischemic brain injury. Exp Neurol. 2013;247:447–455. doi: 10.1016/j.expneurol.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Balduini W, De Angelis V, Mazzoni E, Cimino M. Simvastatin protects against long-lasting behavioral and morphological consequences of neonatal hypoxic/ischemic brain injury. Stroke. 2001;32:2185–2191. doi: 10.1161/hs0901.094287. [DOI] [PubMed] [Google Scholar]
- 171.Li A, et al. Simvastatin attenuates hypomyelination induced by hypoxia–ischemia in neonatal rats. Neurol Res. 2010;32:945–952. doi: 10.1179/016164110X12670144737774. [DOI] [PubMed] [Google Scholar]
- 172.Pedroni SM, et al. Complement inhibition and statins prevent fetal brain cortical abnormalities in a mouse model of preterm birth. Biochim Biophys Acta. 2014;1842:107–115. doi: 10.1016/j.bbadis.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 173.Sandner G, et al. The HDAC inhibitor phenylbutyrate reverses effects of neonatal ventral hippocampal lesion in rats. Front Psychiatry. 2011;1:153. doi: 10.3389/fpsyt.2010.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Fleiss B, Nilsson MK, Blomgren K, Mallard C. Neuroprotection by the histone deacetylase inhibitor trichostatin A in a model of lipopolysaccharide-sensitised neonatal hypoxic–ischaemic brain injury. J Neuroinflammation. 2012;9:70. doi: 10.1186/1742-2094-9-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.George S, et al. Impact of trichostatin A and sodium valproate treatment on post-stroke neurogenesis and behavioral outcomes in immature mice. Front Cell Neurosci. 2013;7:123. doi: 10.3389/fncel.2013.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.McPherson CA, Aoyama M, Harry GJ. Interleukin (IL)-1 and IL-6 regulation of neural progenitor cell proliferation with hippocampal injury: differential regulatory pathways in the subgranular zone (SGZ) of the adolescent and mature mouse brain. Brain Behav Immun. 2011;25:850–862. doi: 10.1016/j.bbi.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kokovay E, et al. VCAM1 is essential to maintain the structure of the SVZ niche and acts as an environmental sensor to regulate SVZ lineage progression. Cell Stem Cell. 2012;11:220–230. doi: 10.1016/j.stem.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 178.Green HF, et al. A role for interleukin-1β in determining the lineage fate of embryonic rat hippocampal neural precursor cells. Mol Cell Neurosci. 2012;49:311–321. doi: 10.1016/j.mcn.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 179.Iosif RE, et al. Suppression of stroke-induced progenitor proliferation in adult subventricular zone by tumor necrosis factor receptor 1. J Cereb Blood Flow Metab. 2008;28:1574–1587. doi: 10.1038/jcbfm.2008.47. [DOI] [PubMed] [Google Scholar]
- 180.Iosif RE, et al. Tumor necrosis factor receptor 1 is a negative regulator of progenitor proliferation in adult hippocampal neurogenesis. J Neurosci. 2006;26:9703–9712. doi: 10.1523/JNEUROSCI.2723-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 182.Calvo CF, et al. Vascular endothelial growth factor receptor 3 directly regulates murine neurogenesis. Genes Dev. 2011;25:831–844. doi: 10.1101/gad.615311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bain JM, Moore L, Ren Z, Simonishvili S, Levison SW. Vascular endothelial growth factors A and C are induced in the SVZ following neonatal hypoxia–ischemia and exert different effects on neonatal glial progenitors. Transl Stroke Res. 2013;4:158–170. doi: 10.1007/s12975-012-0213-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Li L, Walker TL, Zhang Y, Mackay EW, Bartlett PF. Endogenous interferon γ directly regulates neural precursors in the non-inflammatory brain. J Neurosci. 2010;30:9038–9050. doi: 10.1523/JNEUROSCI.5691-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Cheng A, Wang S, Cai J, Rao MS, Mattson MP. Nitric oxide acts in a positive feedback loop with BDNF to regulate neural progenitor cell proliferation and differentiation in the mammalian brain. Dev Biol. 2003;258:319–333. doi: 10.1016/s0012-1606(03)00120-9. [DOI] [PubMed] [Google Scholar]
- 186.Zhu DY, Liu SH, Sun HS, Lu YM. Expression of inducible nitric oxide synthase after focal cerebral ischemia stimulates neurogenesis in the adult rodent dentate gyrus. J Neurosci. 2003;23:223–229. doi: 10.1523/JNEUROSCI.23-01-00223.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ma M, et al. Intranasal delivery of transforming growth factor-beta1 in mice after stroke reduces infarct volume and increases neurogenesis in the subventricular zone. BMC Neurosci. 2008;9:117. doi: 10.1186/1471-2202-9-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.He Y, et al. ALK5-dependent TGF-β signaling is a major determinant of late-stage adult neurogenesis. Nat Neurosci. 2014;17:943–952. doi: 10.1038/nn.3732. [DOI] [PMC free article] [PubMed] [Google Scholar]