Abstract
Loss of huntingtin (HTT), the Huntington's disease (HD) protein, was previously shown to cause axonal transport defects. Within axons, HTT can associate with kinesin-1 and dynein motors either directly or via accessory proteins for bi-directional movement. However, the composition of the vesicle-motor complex that contains HTT during axonal transport is unknown. Here we analyze the in vivo movement of 16 Rab GTPases within Drosophila larval axons and show that HTT differentially influences the movement of a particular sub-set of these Rab-containing vesicles. While reduction of HTT perturbed the bi-directional motility of Rab3 and Rab19-containing vesicles, only the retrograde motility of Rab7-containing vesicles was disrupted with reduction of HTT. Interestingly, reduction of HTT stimulated the anterograde motility of Rab2-containing vesicles. Simultaneous dual-view imaging revealed that HTT and Rab2, 7 or 19 move together during axonal transport. Collectively, our findings indicate that HTT likely influences the motility of different Rab-containing vesicles and Rab-mediated functions. These findings have important implications for our understanding of the complex role HTT plays within neurons normally, which when disrupted may lead to neuronal death and disease.
Introduction
The Huntington's disease (HD) protein, huntingtin (HTT), is a ubiquitously expressed protein that is enriched in the brain (1). HTT is conserved across evolution and loss of HTT function causes embryonic lethality in mice indicating that it is essential for development (2). Although many roles for HTT have been proposed, the main function of HTT is still elusive. Early studies using yeast two-hybrid analysis showed that HTT associates with several proteins termed huntingin associated proteins (HAPs) including HAP1 (3). HTT is transported bi-directionally within axons (4,5). HTT associates with dynactin (a regulator of dynein) and the dynein intermediate chain (DIC) (a subunit of the dynein motor) (6) via HAP1 (7,8). Biochemical associations between HTT and the anterograde motor kinesin-1 via interactions between HAP1 and the light chain subunit of kinesin (KLC) (9) have also been shown. Genetic evidence indicates that HTT has functional interactions with both kinesin-1 and dynein in vivo (10). Loss of HTT causes axonal transport defects (10) and perturbs the transport of brain-derived neurotrophic factor (BDNF) through disruption of the HTT-HAP1-dynactin complex (11). Collectively, these data suggest that HTT may act as a linker to form a functional vesicle complex with motor proteins during axonal transport. However the composition of the vesicle in which HTT is contained in during axonal transport is unknown.
We previously showed that HTT mediates the movement of Rab11-containing vesicles during axonal transport (12). Rabs are members of the Ras family of monomeric G proteins that cycle between an active GTP-bound state and an inactive GDP-bound state to regulate intracellular transport (13,14). When bound to GTP, Rab proteins bind lipid membranes via a prenylated cytoplasmic tail domain (15,16). Rab proteins are known to control membrane trafficking in both the secretory and endocytic pathways; affecting exocytosis, endocytosis, endosome recycling (17,18), vesicle budding (19) and tethering and docking of vesicles (20). Functions for Rabs in neurite outgrowth, elongation and polarization have also been suggested (21,22). Work has shown that some Rabs can bind to motor subunits directly or via adaptor or effector proteins, and interactions between myosin (actin motors) and kinesin and dynein [microtubule (MT) motors] have also been shown (23,24). Rab27A is thought to mediate the transport of melanosomes in melanocytes by interactions with Myosin Va (24) and Rabs 3, 6, 9, 11 and 27 are proposed to associate with the MT motor machinery to facilitate intracellular trafficking of compartments (23). However, the mechanistic details and the functional implications behind these interactions remain elusive.
Previously, we showed that, under physiological conditions, reduction of HTT perturbed the bi-directional movement of Rab11-containing vesicles, while no effect was seen on the motility of Rab5-containing vesicles within larval axons (12). Since there are more than 23 neuronal Rab proteins and several recycling endosomal Rab proteins, we used in vivo imaging coupled with high-resolution quantitative analysis and Drosophila genetics to directly test the hypothesis that HTT transports a particular sub-set of Rab-containing vesicles within axons. Our observations, done under physiological conditions, provide compelling evidence that HTT is required for the normal transport of a specific sub-set of Rabs. Our data suggest a potential mechanism by which altered axonal transport of particular Rab-containing vesicles caused by the loss of HTT function could be an early precursor to HD.
Results
Huntingtin regulates the axonal movement of a particular sub-set of Rab-containing vesicles
Previous work showed that HTT associates with MT motors kinesin-1 and dynein for bi-directional movement (6,8). Indeed, an mRFP-tagged, non-pathogenic form of human HTT (hHTTex1-15Q-mRFP) showed robust bi-directional movement within Drosophila larval axons (Supplementary Material, Fig. S1A) suggesting that HTT undergoes fast axonal transport. However, the composition of the vesicle that HTT is present on and moves during axonal transport is unknown. We previously discovered that reduction of Drosophila HTT perturbed the movement of Rab11-containing vesicles, but not Rab5-containing vesicles suggesting that HTT may regulate the movement of a particular sub-set of vesicles (12). However there are at least 23 other neuronal Rab proteins in Drosophila, 21 of which are conserved in mammals (25).
To test the hypothesis that HTT regulates the movement of a particular sub-set of Rab-containing vesicles, we systematically examined the movement of 16 GFP/YFP-tagged neuronal Rabs (26) in the context of 30% HTT. We used a UAS-RNAi-HTT line that reduces endogenous Drosophila HTT mRNA levels by 70% with only 30% of HTT remaining as observed by q-PCR (10). Work has shown that HTT function is conserved in Drosophila (5). Similar to vertebrate HTT, Drosophila HTT contains five regions of strong homology, including HEAT repeats, which are involved in intracellular trafficking (27). We used a stringent statistical analysis (statistically significant changes at 99% coefficient) to identify Rab proteins that were most significantly affected by 70% HTT reduction. Results from our extensive analysis are summarized in Table 1 and Supplementary Material, Table S1.
Table 1.
Summary of our in vivo movement data with 70% reduction of HTT (30% HTT) in the context of Rab-containing vesicles
| YFP-Rab | Compartment | Transport phenotypes |
Motility after RNAi-HTT (Segmental velocity) | % Vesicle populations after HTT reduction | Pause duration (s) | Pause frequency |
Run length (µm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| With HTT | W/O HTT | Anterograde | Retrograde | Stationary | Anterograde | Retrograde | Reversing | Anterograde | Retrograde | Anterograde | Retrograde | Anterograde | Retrograde | ||
| 1 | ER/Golgi | Normal | Normal | Unchanged | Unchanged | ||||||||||
| 2 | ErR/Golgi | Normal | Normal | ↑0.005 | Unchanged | ns | ns | ns | ns | ns | ↓ 0.001 | ns | ns | ns | ns |
| 3 | Synaptic | Normal | Stalled puncta | ↓ P = 5.9E-15 | ↓ P = 4.1E-20 | ↑ P = 6.7E-13 | ns | ns | ↓ P = 9.4E-12 | ↑ P = 1.65E-6 | ↑ P = 4.65E-6 | ns | ↑ P = 3.68E-5 | ↓ P = 7.29E-7 | ↓ P = 1.18E-8 |
| 7 | Late endosome | Normal | Stalled puncta | Unchanged | ↓0.001 | ↑ P = 0.01 | ns | ns | ns | ns | ns | ns | ns | ↓ P = 0.002 | ns |
| 8 | Recycling/Synaptic | Normal | Diffuse | N/A | N/A | ||||||||||
| 9 | Recycling/Synaptic | Diffuse | Diffuse | N/A | N/A | ||||||||||
| 14 | Recycling | Normal | Normal | Unchanged | Unchanged | ||||||||||
| 18 | Synaptic | Diffuse | Diffuse | N/A | N/A | ||||||||||
| 19 | Recycling | Blocks | Blocks | ↓0.009 | ↓0.004 | ns | ns | ns | ns | ↓ P = 0.004 | ns | ns | ns | ↓ 3.83E-4 | ↓ 0.009 |
| 21 | Recycling | Normal | Normal | Unchanged | Unchanged | ||||||||||
| 23 | Recycling | Diffuse | Diffuse | N/A | N/A | ||||||||||
| 26 | Recycling | Blocks | Blocks | Unchanged | Unchanged | ↑ P = 3.67E-5 | ns | ns | ↓ 0.001 | ns | ns | ns | ns | ns | ns |
| 27 | Synaptic | Normal | Normal | Unchanged | Unchanged | ||||||||||
| 32 | Recycling | Normal | Normal | Unchanged | Unchanged | ||||||||||
| 35 | Untyped | Diffuse | Diffuse | N/A | N/A | ||||||||||
| X1 | Recycling | Normal | Normal | Unchanged | Unchanged | ns | ns | ns | ns | ↓ 9.96E-9 | ns | ns | ns | ns | ns |
The compartment type, axonal transport phenotype and the effects of HTT reduction on the in vivo motility for all Rab-containing vesicles are compared with wild-type (100% HTT). Statistically significant changes at 99% coefficient for in vivo motility dynamic parameters such as segmental velocities (µm/sec2, Wilcoxon–Mann–Whitney rank sum test), vesicle population % (two-tailed Student t-test), pause durations (sec, Wilcoxon–Mann–Whitney rank sum test), pause frequencies (sec−1, Wilcoxon–Mann–Whitney rank sum test) and run length (µm, Wilcoxon–Mann–Whitney rank sum test) are shown with arrows depicting significant decreases and arrows depicting significant increases compared with wild-type (100% HTT). Note that a stringent significance threshold of P < 0.01 (99% confidence) was used to determine significant changes. ns = no significance. N/A = not analyzed as diffused and no puncta were observed. Normal = vesicle motility with HTT reduction (30% HTT) comparable to 100% HTT. Blocks = YFP containing axonal blockage phenotype. Stalled puncta = stalled YFP puncta (not axonal blocks). Diffused = YFP was diffused or cytoplasmic within axons in contrast to being vesicular. Untyped = undefined compartment. The in vivo motility data parameters for all Rab-containing vesicles examined are shown in Supplementary Material, Table S1.
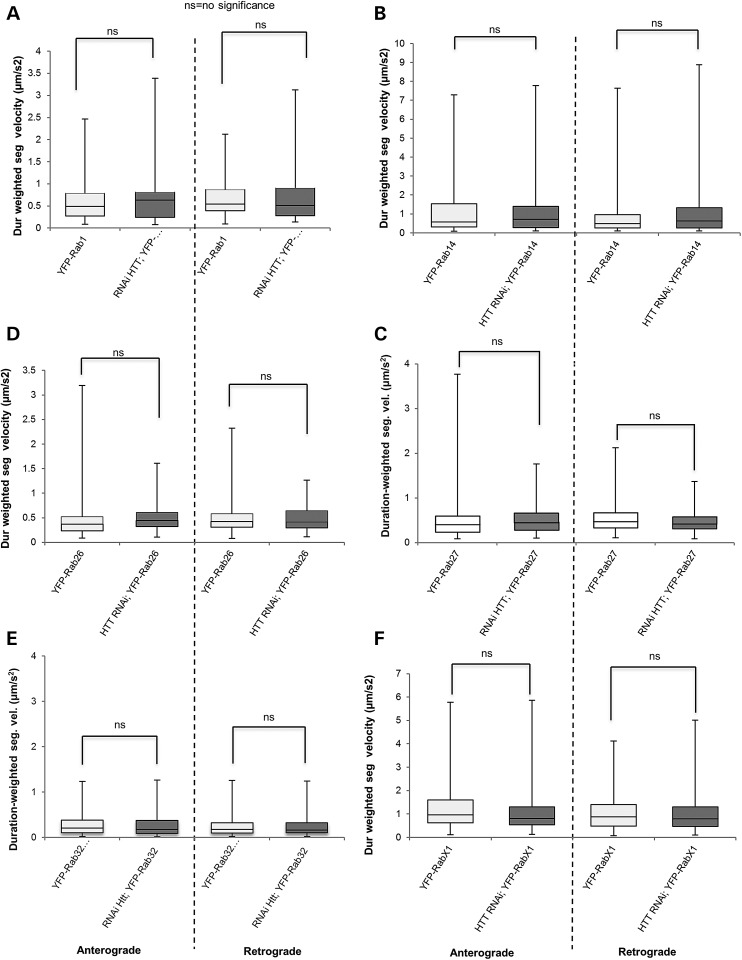
There are 33 known Rab proteins in Drosophila and 27 of these are related to mammalian Rabs (26). The other Drosophila Rabs not conserved in mammals are named Rab X1, X2, X3, X4, X5 and X6 (26). Of the 23 neuronal Drosophila Rab proteins, previous work showed that 6 are specific to neurons, while 17 are ubiquitously expressed, but show strong enrichment in neurons (25). Rabs 1, 2, 14 and 18 are thought to be located on ER-Golgi compartments (28–32). Rab5 is found in early endosomes, Rab7 in late endosomes and Rabs 3, 8, 9, 18 and 27 are contained in synaptic vesicles. Rabs 8, 9, 11, 19, 21, 23, 26, 32 and X1 are all found in recycling endosomes, while Rabs 4 and 35 are thought to be in an as yet unidentified compartment (33). Note that some Rabs are found in more than one compartment. For example, Rabs 8 and 9 are thought to be in recycling endosomes and synaptic vesicles and Rab18 is found in both ER-Golgi and synaptic vesicle compartments. Here we specifically examined 16 neuronal Rab proteins of which 15 are conserved in mammals. 7 Rab proteins were not analyzed as these Rabs, the GAL4 driver and the RNAi-HTT line are all on the X chromosome. A side-by-side comparison of Rab-containing vesicle movement within Drosophila larval axons showed that different Rab-containing vesicles, even those within the same compartment type, move at varying velocities (Supplementary Material, Table S2). Thus, the motility of Rab proteins found in the same compartment appear to be differentially regulated.
Our systematic analysis revealed that HTT influences the movement of several Rab proteins during axonal transport, but the affected Rab proteins were not restricted to a single specific compartment (Supplementary Material, Fig. S5). Additionally, HTT regulated the movement of Rab-containing vesicles in three ways: (1) reduction of HTT impaired both the anterograde and retrograde motility of some Rabs (Rab3 and 19) (P < 0.01 to P < 0.0001), but not others (Rab1, 14, 23, 26, 32, 35, X1) (Figs 1 and 3), (2) reduction of HTT impaired the movement of Rab7, but only in the retrograde direction (Fig. 2C and D, P < 0.001) and (3) reduction of HTT stimulated the anterograde motility of Rab2 (Fig. 2A and B, P < 0.01). Collectively, our analysis, done under physiological conditions, suggests that HTT differentially regulates the movement of a particular sub-set of Rab-containing vesicles during axonal transport (Supplementary Material, Fig. S5), defining a novel role for normal HTT function in Rab motility.
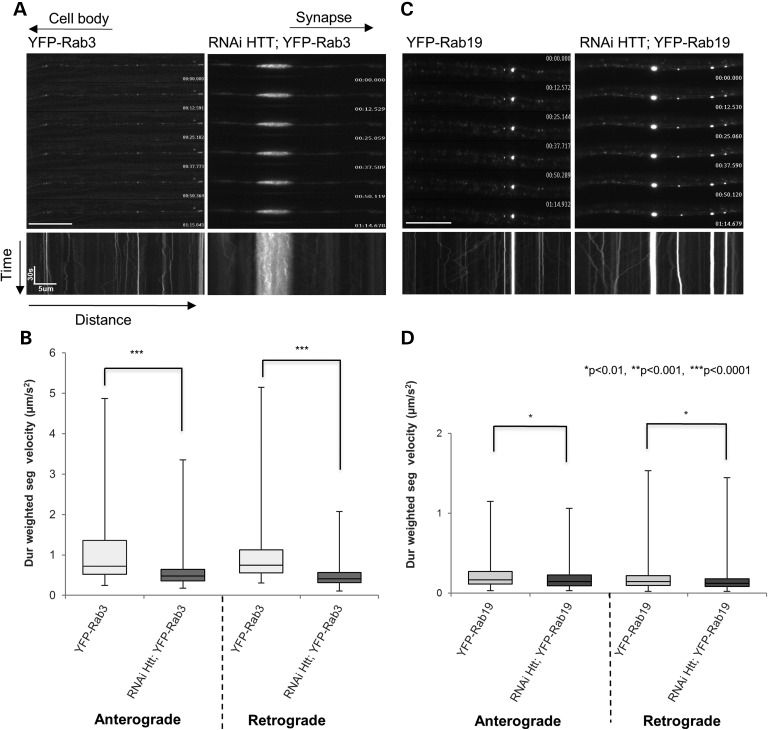
Figure 1.
Reduction of HTT perturbs the bi-directional movement of Rab3 and Rab19-containing vesicles within larval axons. (A) Representative movie montages and the corresponding kymographs of YFP-Rab3 in larval axons with endogenous (100%) HTT (left) or with 30% HTT (right, 70% reduction in RNAi-HTT;YFP-Rab3). The horizontal arrows depict the direction of the cell body and synapse. The arrows depict time (sec) and distance traveled (microns) for the kymograph. Note the large YFP containing axonal blockage in RNAi-HTT;YFP-Rab3. (B) Box plots depict duration-weighted velocities for both anterograde and retrograde moving Rab3-containing vesicles in the context of 100% (light) or 30% (dark) HTT. Box plots outline the distribution of duration-weighted segmental velocities for each genotype. The horizontal bar represents the median. The upper and lower box edges represent 75% percentile (i.e. upper quartile) and 25% percentile (i.e. lower quartile), respectively. Note that 70% reduction of HTT significantly decreases (P < 0.0001) both the anterograde and retrograde velocities of Rab3-containing vesicles (C) Representative movie montages and corresponding kymographs of YFP-Rab19 in larval axons with 100% endogenous HTT (left) or with 30% HTT (right, RNAi-HTT;YFP-Rab19). Note the stalled YFP puncta in RNAi-HTT;YFP-Rab19. (D) Box plots depicts Rab19 duration-weighted velocities (anterograde and retrograde) in the context of 100% and 30% HTT. Note that reduction of HTT significantly decreases (P < 0.01) both the anterograde and retrograde velocities of Rab19-containing vesicles. (*P < 0.01, **P < 0.001, ***P < 0.0001). Scale bars are 10 μm. Forty movies from 10 larvae were analyzed for each genotype. Vesicle velocity distributions were compared using the non-parametric Wilcoxon–Mann–Whitney rank sum test.
Figure 3.
Reduction of HTT has no effect on vesicle velocities of other neuronal Rab-containing vesicles. (A–F) Box plots depict duration-weighted segmental velocities (anterograde and retrograde) of YFP-Rab1 (A), YFP-Rab14 (B), YFP-Rab-26 (C), YFP-Rab-27 (D), YFP-Rab-32 (E), YFP-Rab-X1 (F) in the context 100% HTT or with 30% HTT (RNAi HTT;YFP-Rab1, RNAi HTT;YFP-Rab14, RNAi HTT;YFP-Rab26, RNAi HTT;YFP-Rab27, RNAi HTT;YFP-Rab32, RNAi HTT;YFP-RabX1). Note that no significant changes are observed to either anterograde or retrograde velocities (P > 0.01 = no significance = ns). Forty movies from 10 larvae were analyzed for each genotype. Vesicle velocity distributions were compared using the non-parametric Wilcoxon–Mann–Whitney rank sum test.
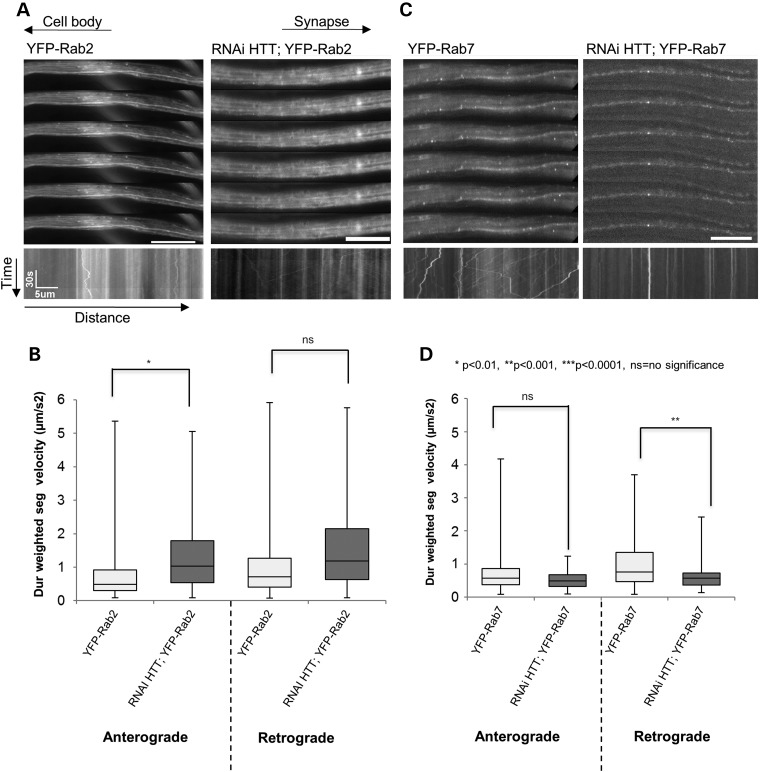
Figure 2.
Reduction of HTT perturbs the retrograde movement of Rab7-containing vesicles and stimulates the anterograde movement of Rab2-containing vesicles. (A) Representative movie montages and corresponding kymographs of YFP-Rab2 in larval axons with 100% endogenous HTT (left) or with 30% HTT (right, 70% reduction in RNAi-HTT;YFP-Rab2). The horizontal arrows depict the direction of the cell body and synapse. The arrows depict time (sec) and distance traveled (microns) for the kymograph. (B) Box plots depict duration-weighted velocities (anterograde and retrograde) for Rab2-containing vesicles in the context of 100% (light) and with 30% (dark) HTT. Note that reduction of HTT significantly stimulates (P < 0.01) the anterograde velocities of Rab2-containing vesicles and no effect is seen for the retrograde velocities. (C) Representative movie montages and corresponding kymographs of YFP-Rab7 in larval axons with endogenous HTT (left) or with 30% HTT (right, RNAi-HTT;YFP-Rab7). (D) Box plots depicts Rab7 duration-weighted velocities (anterograde and retrograde) in the context of 100% (light) and 30% (dark) HTT. Note that reduction of HTT significantly perturbs only (P < 0.001) the retrograde velocities of Rab7-containing vesicles and no effect is seen for the anterograde velocities. (*P < 0.01, **P < 0.001, ***P < 0.001, ns = no significance). Scale bars are 10 μm. Forty movies from 10 larvae were analyzed for each genotype. Vesicle velocity distributions were compared using the non-parametric Wilcoxon–Mann–Whitney rank sum test.
Reduction of HTT perturbs the bi-directional axonal movement of Rab3 and Rab19-containing vesicles
We previously found that reduction of HTT perturbed the bi-directional motility of Rab-11-containing vesicles, but not Rab5-containing vesicles (12). Since Rab11 is in recycling endosomes, we first examined whether the motility of other Rab proteins found in recycling endosomes was also perturbed by reduction of HTT. While Rab 8, 19, 21, 26, 32 and X1 were punctate and showed bi-directional motility, Rab 9 and 23 exhibited a more diffuse localization pattern within larval axons (Supplementary Material, Fig. S2). Strikingly, reduction of HTT only perturbed the bi-directional motility of Rab19, while no changes were detected in the motility of other Rab proteins found within recycling endosomes (Figs 1 to 3).
Within larval axons, Rab19-containing vesicles moved at an average velocity of 0.978 ± 0.053 µm/s (mean ± SEM) anterogradely and 1.079 ± 0.228 µm/s retrogradely. These vesicle velocities are comparable to other vesicle velocities that undergo axonal transport. Reduction of HTT by 70% (only 30% of HTT is present) significantly decreased both the average anterograde (0.832 ± 0.053 µm/s) and retrograde (0.727 ± 0.052 µm/s) velocities of Rab19-containing vesicles (P < 0.01) (compare YFP-Rab19 to RNAi-HTT;YFP-Rab19 in Fig. 1C and D). These decreases in velocities were concomitant with significant decreases in vesicle run lengths (Supplementary Material, Fig. S3E). The average anterograde run length of Rab19-containing vesicles decreased from 4.501 ± 0.481 µm to 2.602 ± 0.213 µm (P < 0.0001) while the retrograde run length decreased from 3.009 ± 0.312 µm to 2.103 ± 0.138 µm (P < 0.001). However, reduction of HTT did not significantly affect the overall populations of moving Rab19-containing vesicles (Supplementary Material, Fig. S3H). Taken together, our in vivo analysis indicates that HTT does not specifically influence the motility of all Rab proteins in the recycling endosome compartment but that the axonal motility of only a particular sub-set of Rab proteins found in recycling endosomes are influenced by HTT.
Interestingly, our systematic analysis revealed that HTT also influenced the bi-directional motility of Rab-containing vesicles found in other compartments. HTT significantly perturbed the motility of Rab3-containing vesicles, but not Rab 9 or 27, which are thought to be contained in synaptic vesicles (33). Rab 3, 9 and 27 showed robust bi-directional movement with velocities comparable for fast axonal transport (Figs. 1 and 3C). HTT reduction significantly decreased the average anterograde velocity of Rab3-containing vesicles from 1.094 ± .053 µm/s to 0.586 ± 0.034 µm/s (P < 0.0001) while the average retrograde velocity decreased from 1.098 ± 0.056 µm/s to 0.498 ± 0.025 µm/s (P < 0.0001) (compare YFP-Rab3 to RNAi-HTT;YFP-Rab3 in Fig. 1A and B). Additionally, large YFP containing axonal blockages were observed in these larval axons (Fig. 1A), in contrast to the stalled puncta seen in RNAi-HTT;YFP-Rab19 larval axons (Fig. 1B). These decreases in velocity were concomitant with decreases in vesicle run length and increases in vesicle pause frequency and pause duration. The average vesicle run length of anterograde Rab3-containing vesicles decreased from 4.786 ± 0.319 µm to 1.785 ± 0.143 µm (P < 0.0001) while the average retrograde run length decreased from 4.636 ± 0.325 µm to 1.502 ± 0.100 µm (P < 0.0001) (Supplementary Material, Fig. S3A). Similarly, the average anterograde pause durations [from 5.715 ± 0.204 s to 8.621 ± 0.559 s (P < 0.0001)], the average anterograde pause frequencies [0.024 ± 0.001 s−1 to 0.03 ± 0.002 s−1 (P < 0.01)], the average retrograde pause durations [5.994 ± 0.246 s to 8.101 ± 0.383 s (P < 0.0001)] and the average retrograde pause frequencies [0.024 ± 0.001 s−1 to 0.032 ± 0.002 s−1 (P < 0.0001)] significantly increased with reduction of HTT (Supplementary Material, Fig. S3B and C). Cargo population analysis showed significant increases in the total percent of stalled Rab3 vesicles with reduction of HTT (30.02 ± 2.88% to 64.64 ± 3.20%, P < 0.0001). Significant decreases were seen in the percent of reversing vesicles (47.83 ± 3.008% to 20.45 ± 1.945%, P < 0.0001) with reduction of HTT (Supplementary Material, Fig. S3D). Thus, HTT not only regulates the movement of a sub-set of Rab proteins in recycling endosomes, but also regulates the movement of Rab proteins found in synaptic vesicles. Taken together, our results suggest that HTT can control the axonal motility of Rab proteins contained in different compartments under physiological conditions.
Reduction of HTT perturbs the retrograde motility of Rab7-containing vesicles
We previously found that reduction of HTT had no effect on the motility of Rab5-containing vesicles, which defines early endosomes (12). However, our in vivo analysis showed that HTT influenced the motility of Rab7-containing vesicles, which defines late endosomes (Fig. 2C and D). Surprisingly, however, only the retrograde motility of Rab7-containing vesicles was significantly affected by HTT reduction, while the anterograde motility was not significantly altered. Reduction of HTT significantly decreased the average retrograde velocities to 0.608 ± 0.060 µm/s from 0.994 ± 0.058 µm/s (P < 0.001) (Fig. 2C and D). This change was concomitant with decreases in vesicle run lengths as reduction of HTT decreased the retrograde run length from 5.543 ± 0.488 µm to 2.245 ± 0.249 µm (P < 0.001) (Supplementary Material, Fig. S4E). While the anterograde run length was also decreased (from 4.05 ± 0.538 µm to 1.788 ± 0.214 µm, P < 0.01), this change did not significantly affect the overall anterograde motility (Supplementary Material, Figs. S4E and 2D). Although cargo population analysis did not reveal any significant changes with HTT reduction, there were definite trends showing an increase in the stationary cargo population, with decreases in both retrograde and reversing cargo populations (Supplementary Material, Fig. S4H). Although it is unclear as to whether these affected Rab7-containing vesicles are lysosomes or autophagosomes, (since Rab7 is present on both lysosomes and autophagosomes, (34), previous work showed that HTT reduction perturbed only the retrograde motility and net run lengths of autophagosomes in cultured DRG neurons (35). Our observations are also consistent with work that showed that Rab7 mutants thought to cause Charcot-Marie Tooth syndrome 2B (CMT2B), a neurodegenerative disease that results in axonal neuropathy, altered trafficking and signaling of retrograde NGF/TrkA trophic signals (36). Thus, collectively, our results suggest that HTT not only regulates the movement of different Rab-containing vesicles, but also specifically influences the retrograde motility of a particular Rab-containing vesicle.
Reduction of HTT stimulates the anterograde movement of Rab2-containing vesicles
We also examined the motility of Rab proteins that are thought to be located within the ER/Golgi compartment since these show strong neuronal enrichment (25). We found that, while Rab18 displayed a very diffused localization, Rabs 1 and 2 showed some bi-directional motility with a diffused component. Rab14 displayed robust bi-directional movement (Supplementary Material, Fig. S2, Fig. 2A). Reduction of HTT had no effect on Rab 1, 14 or 18. However, reduction of HTT affected Rab2 movement and this affect was strikingly opposite to what we saw with other Rabs; i.e. stimulation of anterograde Rab2 movement with reduction of HTT (Fig. 2A and B). The anterograde velocities of Rab2 vesicles significantly increased from 0.734 ± 0.066 µm/s to 0.997 ± 0.081 µm/s (P = 0.005) (Fig. 2B) with reduction of HTT. Although, there is a trend showing an increase in retrograde velocity, these increases were not significant under our stringent criteria of 99% coefficiency. No significant changes were seen in the anterograde run lengths, pause frequencies or cargo populations. However, a trend towards reduced pause durations was seen for anterogradely moving Rab2-containing vesicles in the context of reduced HTT, while the retrograde pause duration were significantly reduced (Supplementary Material, Fig. S4B). Since axons and presynaptic terminals are thought to contain smooth or tubular ER from ultrastructural studies (37,38), perhaps HTT could regulate the anterograde motility of an axonal Rab2-containing tubular ER compartment to presynaptic terminals. Indeed, immunofluorescence of the ER retention signal KDEL in larval segmental nerves revealed tubular ER within axons (Supplementary Material, Fig. S4) reminiscent of Rab2 (Fig. 2A, Supplementary Material, Fig. S4), similar to what has been recently observed (39).
Huntingtin is present with moving Rab-containing vesicles
Since 70% reduction of HTT altered the movement of several Rab-containing vesicles within larval axons in vivo, we tested the hypothesis that HTT and Rab proteins are present on the same moving vesicle during active transport within axons. To directly visualize the composition of moving vesicles during axonal transport under physiological conditions, we used simultaneous dual-view imaging of both HTT (red) and different Rab proteins (green) in Drosophila larval segmental nerves (Fig. 4). We analyzed the co-localization of the entire trajectory of a moving particle during the entire time frame of a movie to differentiate meaningful from spurious co-localization. Only particles containing both the red and green proteins will move in the same trajectory which, when merged, will show co-localization in yellow. Using these criteria, we found that HTT co-localized with all of the Rab proteins that were affected by reduction of HTT function.
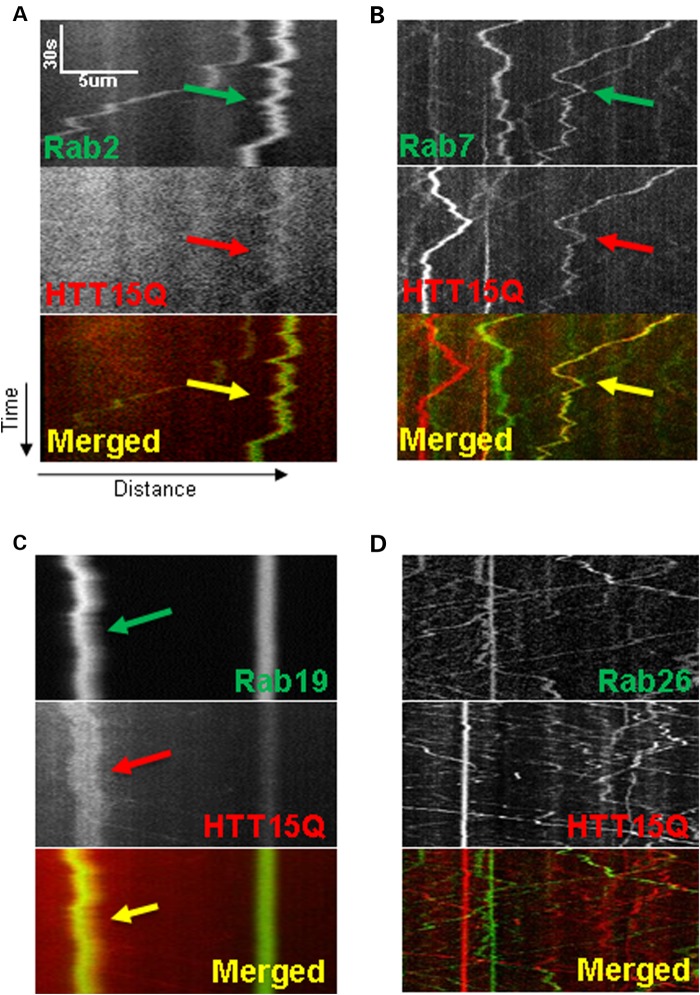
Figure 4.
Simultaneous dual-channel imaging reveals HTT co-localizes with Rab proteins on moving vesicles. (A–D) Representative kymographs of movies captured using simultaneous dual-channel imaging separated by channel into GFP/YFP-Rab proteins (top), HTT15Q-mRFP (middle) and merged (bottom). Arrows indicate co-localized vesicle trajectories from kymographs. Rab2 (A), 7 (B) or 19 (C) (green), shows co-localization with HTT (red) on moving vesicles. Conversely, Rab26 (D) (whose motility was not affected by reduction of HTT), shows no co-localization with HTT15Q. Note that even the stationary vesicles in either green (Rab26) or red (HTT15Q) do not co-localize. Kymographs from 10 movies from 5 larvae were quantified for each genotype.
HTT was present with moving Rab19-containing vesicles (Fig. 4C) (15% of Rab19 tracks co-localized with HTT, 40 tracks analyzed). Both anterograde and retrograde Rab19-containing vesicles contained HTT. HTT was also present on moving Rab7-containing vesicles (Fig. 4B) (24.1% Rab7 co-localization, 54 tracks analyzed). Interestingly, only the retrogradely moving Rab7-containing vesicles contained HTT, consistent with our observation that loss of HTT function only affected the retrograde velocities of Rab7 (Fig. 2B). HTT was present on Rab2-containing anterograde vesicles (Fig. 4A) (20.9% Rab2 co-localization, 43 tracks analyzed), the population of Rab2 vesicles that was affected by reduction of HTT (Fig. 2B). HTT was not present on moving Rab26-containing vesicles (0.8% Rab26 co-localization, 119 tracks analyzed), a Rab-vesicle that was not affected by reduction of HTT (Fig. 4D). Collectively, these observations suggest that Rab and HTT associations are specific to a particular sub-set of Rab-containing vesicles.
Reduction of HTT affects the sub-cellular distribution of axonal Rab8 during axonal transport
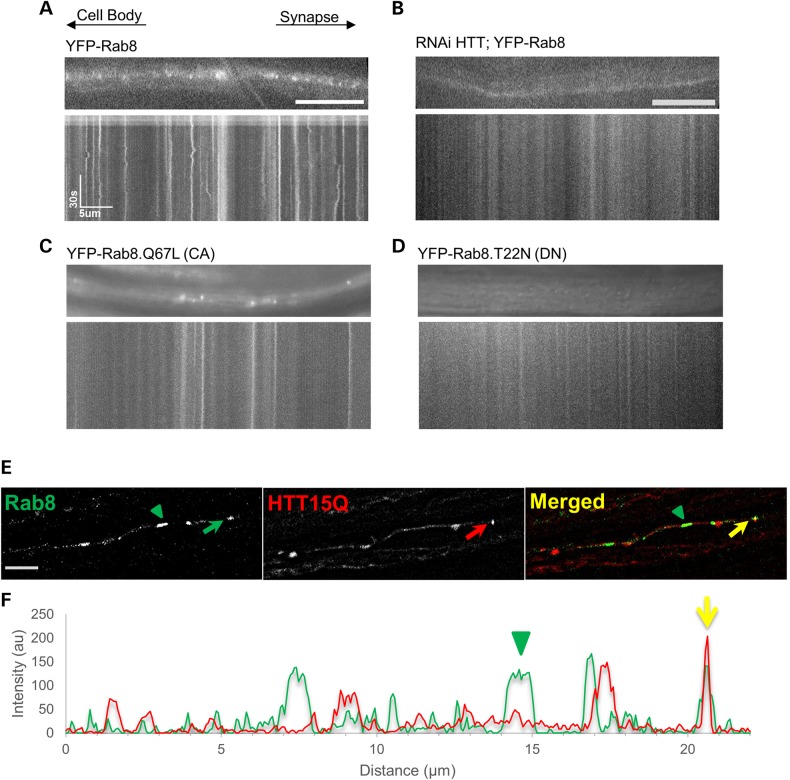
Rab8 was suggested to localize to recycling endosomes and synaptic vesicles based on partial co-localization in immunofluorescence experiments using antibodies against Rab11 and the synaptic vesicle marker cysteine string protein (CSP) (25). Similar to what was seen in cultured epithelial cells (40), some Rab8 positive cargoes displayed a tubular localization pattern during axonal transport (Fig. 5A and E). Interestingly, the motility of Rab8 within axons was slower than most of the other Rab proteins we analyzed with average anterograde and retrograde velocities of 0.348 and 0.287 μm/s2, respectively (Supplementary Material, Table S1). HTT reduction dramatically changed the sub-cellular distribution pattern of Rab8 from a more tubular or membrane localization to a highly diffused cytoplasmic localization (Fig. 5B). We note that we were unable to analyze these movies using our particle tracker program as there was no motility observed in these movies. Interestingly, expression of a constitutively active (CA) form of Rab8 (Rab8.Q67L, a point mutation in the GTP-binding site that results in a permanently GTP-bound state) revealed a tubular or membrane localization pattern for Rab8 within axons (Fig. 5C), suggesting that the GTP-bound state is required for membrane binding as previously shown (12,41). Expression of a constitutively inactive form of Rab8 (Rab8.T22N, a point mutation that results in a permanently GDP-bound state) showed a diffused cytoplasmic localization pattern for Rab8 (Fig. 5C) similar to what we observed with reduction of HTT in the context of Rab8 (Fig. 5B). Perhaps HTT may normally function to regulate the membrane localization of Rab8 via an action on GTP hydrolysis and loss of HTT may release Rab8 from membranes; halting motility.
Figure 5.
Reduction of HTT influences the sub-cellular distribution of Rab 8 within axons and HTT co-localizes with Rab8-containing puncta in axons. Representative images of larval segmental nerve axons and corresponding kymographs of YFP-Rab8 in the context of 100% (A) or with 30% HTT (RNAi HTT;YFP-Rab8, (B). Note that reduction of HTT changes the sub-cellular localization of Rab8 within larval axons from a vesicular localization (A) to a more diffused cytoplasmic localization. (B) Representative images and kymographs taken from a larvae expressing a CA form (Rab8.Q67L) (C) and a dominant negative (DN) form of Rab8 (YFP-Rab8 T22N). (D) Note the vesicular localization of Rab8-CA (C) compared with the more diffused cytoplasmic localization of Rab8-DN (D). (E) Representative image of a segmental nerve from larvae expressing both YFP-Rab8 and HTT15Q-mRFP. Some puncta exhibit co-localization in axons (arrows) while some YFP-Rab8 are not co-localized with HTT15Q (arrowhead). Scale bar = 5 µm. (F) The intensity plot shows the extent of co-localization between Rab8 (green) and HTT (red, yellow arrows). Y axis = intensity in arbitrary unites (au) and X axis = distance in microns (µm). Ten movies from 5 larvae were analyzed for each genotype.
To further evaluate whether Rab8 and HTT are present together on the same tubular/membrane compartment during axonal transport, we expressed both YFP-Rab8 and HTT-mRFP together in the same larval segmental nerves. Using dual-view in vivo imaging, we found that some puncta contained both Rab8 and HTT (Fig. 5E and F). However, not all the Rab8 puncta co-localized with HTT (green arrowhead in Fig. 5E and F). Collectively, our analysis suggests that HTT and Rab8 are likely on the same vesicular compartment during axonal transport where HTT may locally regulate GTP hydrolysis on Rab8-containing vesicles during axonal transport.
Discussion
We have identified a novel role for HTT in the regulation of the axonal transport of a particular sub-set of Rab-containing vesicles under physiological conditions. Our in vivo observations led us to two major findings; (1) HTT differentially regulates the movement of a specific sub-set of Rab-containing vesicles within axons, and (2) HTT is present on these Rab-containing vesicles during axonal transport. At least two possible mechanisms could exist by which HTT exerts a differential control on Rab motility, (1) by associations with specific Rab-containing vesicles, and/or (2) by regulating the motors on moving Rab-containing vesicles, although these two pathways may not be mutually exclusive (Supplementary Material, Fig. S7). Collectively, our findings provide new insight into the normal physiological role of HTT which, when disrupted, may contribute to disease pathology observed in HD.
Differential regulation of Rab protein motility: distinct associations between HTT, Rab and/or linker proteins
Different Rab-containing vesicles, even those within the same sub-cellular compartment, move at varying velocities (Supplementary Material, Table S2), suggesting that Rab proteins found in the same compartment are differentially regulated. Indeed, different regulatory mechanisms could exist as Rab proteins control trafficking in both the secretory and endocytic pathways (17,18). Some Rab proteins are in distinct sub-sets of neurons suggesting that Rabs have roles in diversely regulated mechanisms (25), and HTT may function to differentially regulate the motility of these neuronal Rab proteins via Rab protein specific associations. Previously, we found that reduction of HTT perturbed the motility of Rab11-containing vesicles but not Rab5-containing vesicles (12). Since Rab11 is a marker for recycling endosomes and Rab5 is a marker for early endosomes, we hypothesized that HTT influences the axonal motility of all recycling endosomes. Contrary to this, however, our systematic in vivo analysis found that HTT does not influence the motility of all Rab proteins found in recycling endosomes, but rather, HTT only affects the movement of particular Rab proteins located in many different compartments. Reduction of HTT function perturbed the bi-directional motility of Rab19, a recycling endosomal Rab, while no effect was seen in the motility of other recycling endosomal Rab proteins (Table 1 and Supplementary Material, Table S1). Additionally, reduction of HTT perturbed the bi-directional motility of Rab3, a Rab protein known to be present in synaptic vesicles (42). Intriguingly, reduction of HTT perturbed the retrograde movement of Rab7 (present on late endosomes), while the anterograde movement of Rab2 (present in ER-Golgi associated compartments, 32) was stimulated by reduction of HTT. Perhaps this differential regulation that is observed with reduction of HTT may be caused by the existence of different HTT-Rab-containing motor complexes. Either several HTT-Rab-containing vesicle complexes may exist or alternatively more than one Rab could be present with a single HTT-Rab-containing vesicle (Supplementary Material, Fig. S7A and B). Perhaps, during long distance transport within axons, HTT-mediated regulation of specific Rab-containing vesicles is required for particular functions at the synapse. Indeed, similar to many Rab proteins, roles for HTT in endocytosis, intracellular trafficking and membrane recycling have also been proposed (43,44).
Specific associations between HTT, Rab proteins and linker proteins could perhaps dictate one potential mechanism by which HTT-mediated differential regulation of Rab-containing vesicle motility occurs. It is thought that associations between HTT and motors are facilitated by HTT associated proteins (HAPs) (7,8,44,45). Pal et al. showed that HTT can mediate the transport of a Rab5-HAP40-HTT-containing early endosome on actin filaments via associations with myosin, the actin motor (46). HTT can also interact with myosin via optineurin. Optineurin is a binding partner that links both myosin and HTT to the Golgi network via Rab8 for ER-Golgi trafficking in the secretory pathway (47). HTT can also associate with Rab8 through FIP-2 for regulated cell polarization and morphogenesis (48). Since reduction of HTT altered the sub-cellular localization of Rab8 (Fig. 5B) our observations suggest that HTT can play a role in linking Rab8 to vesicles; enabling associations with MT motors during axonal transport. While the involvement of optineurin or FIP-2 in the association of HTT and Rab8 in the context of axonal movement is still unclear, what is clear is that HTT is likely required for the membrane-bound state of Rab8 during axonal transport under physiological conditions (Supplementary Material, Fig. S7C).
We previously proposed that a HTT-Rab11-motor complex likely exists during axonal transport (12). The motility of Rab11-containing vesicles was perturbed with reduction of HTT. Both kinesin-1 and dynein motors were required for MT motility of Rab11. Additionally, membrane binding of Rab11 was decreased in HD knock-in mice (44), suggesting that similar to Rab8, HTT is also likely required for the membrane-bound state of Rab 11. The Rab11 effector Rip11 regulates the endocytic recycling pathway by forming a complex with Rab11 and kinesin II (49). Rip11 is also important for the trafficking of Rab11 from apical recycling endosomes to the apical membrane (50). Perhaps Rip11 may act as a linker that connects Rab11 and HTT similar to optineurin linking Rab8 and HTT. Rabphilin-3A, a Rab3 effector molecule may link Rab3 vesicles to HTT during axonal transport. Studies have shown that Rab3 and Rabphilin-3A are both transported by fast anterograde transport and associate with synaptic vesicles (42). Thus, although further study is needed, Rab-associated proteins could aid in linking specific Rab-containing vesicles with HTT during axonal transport.
Differential regulation of Rab protein motility: HTT as a molecular regulator of motors
Alternatively, HTT-mediated differential regulation of Rab protein motility could result due to changes in motor protein regulation (Supplementary Material, Fig. S7D). Indeed, previous work postulated HTT as a molecular switch that determines the direction of movement during axonal transport (51). HTT is phosphorylated by Akt (protein kinase B) (a serine-threonine kinase) at serine 421. Constitutively phosphorylated (S421D) HTT can recruit kinesin-1 to the dynactin complex to facilitate anterograde transport while disruption of phosphorylation at S421 (S421A) prevents kinesin association with HTT and the motor complex, enabling retrograde transport (51). Perhaps HTT's role as a molecular regulator during axonal transport could result in the HTT-mediated motility changes we observe, since reduction of HTT not only perturbed the bi-directional motility of Rab3 and 19, and the retrograde motility of Rab7, but also stimulated the anterograde motility of Rab2, via specific changes to motility parameters; vesicle velocities, pause duration/frequencies and run lengths (Figs 1 and 2, Supplementary Material, Figs S3 and S4). While the functional significance of the differential regulation of Rab motility and the mechanistic steps of how HTT controls motors in the context of the different Rab-containing complexes are still unclear, perhaps particular Rab proteins could also exert a regulatory function during vesicle motility by affecting the phosphorylation state of HTT and changing the direction of vesicle movement. Interestingly, several Rabs have been shown to be effectors of kinases. Rab5 and Rab7 are thought to be effectors of PI3K, which is an upstream activator of Akt (52,53). Additionally, Zala et al. showed that HTT can act as a scaffold to transport glycolytic machinery down the axon that is required for vesicular motility (54). Reduction of HTT could decrease glycolysis disrupting the motility of Rab-containing vesicles. Further experiments will be needed to dissect the mechanistic steps involved in the differential regulation of these different HTT-Rab-containing complexes during axonal transport under physiological conditions.
Unidirectional HTT-Rab complexes during axonal transport
An intriguing result from our analysis was that reduction of HTT influenced the retrograde transport of Rab7 (Fig. 2C and D), although Rab7-containing vesicles moved bi-directionally (Fig. 2C). Previous work has implicated Rab7 in neurotrophin receptor trafficking, particularly in the retrograde transport of TrkB/p75NTR-positive signaling endosomes in motor neurons (55). Consistent with this, CMT2B Rab7 mutants altered trafficking and signaling of retrograde NGF/TrkA trophic signals (36). Thus, since HTT and Rab7 co-localize on moving vesicles during axonal transport (Fig. 4B) a HTT-Rab7-containing signaling endosome could exist during axonal transport. Alternatively, since Rab7 is a marker for late endosomal and lysosomal compartments, and HTT and dynein were found to be required for the perinuclear positioning of lysosomes (56), perhaps a HTT-Rab7-containing lysosome could exist during axonal transport. Work has also shown that Rab7 and LC3 (a marker for autophagosomes) are together during the transport of autophagosomes at growth cones (57) and that the retrograde movement of autophagosomes is required for their maturation (58). Interestingly Rab7 interacting lysosomal protein (RILP) was shown to control lysosomal transport by recruiting dynein-dynactin to Rab7-containing late endosomes/lysosomes (59). The FYVE (Fab1-YotB-Vac1p-EEA1) and coiled-coil domain-containing 1 protein (FYCO1) was found to function as an adaptor to link autophagosomes to kinesin via Rab7 (60). Additionally, both HTT and HAP1 were identified as regulators of autophagosome transport in neurons (35). Thus, our results are consistent with these observations and suggest that perhaps a HTT-Rab7-authophagosome complex and/or a HTT-Rab7-signaling endosomal complex could exist under physiological conditions.
Surprisingly our analysis also revealed that reduction of HTT stimulated the anterograde velocity of Rab2, although Rab2-containing vesicles moved bi-directionally (Fig. 2A). Rab2 is known to regulate the anterograde and retrograde trafficking of vesicles between the Golgi, the ER-Golgi intermediate compartment and the ER (29). Rab2 was also one of the Rab proteins that showed the most neuronal sub-cellular localization behaviors: synaptic enrichment with expression of a CA form and loss of synaptic localization with the dominant negative form (25), suggesting a role for Rab2 at the synapse. While roles for HTT at synapses have been documented (5,61–63), perhaps HTT may function to regulate the anterograde motility of a Rab2-containing complex, although the functional significance for this complex at the synapse is still unknown.
Defects in the axonal transport of HTT-Rab complexes during disease
Rab dysfunction has been implicated in many neuronal diseases. For example, a missense mutation in Rab7 was demonstrated in the myelin and axonal disorder Charcot-Marie Tooth disease Type 2B (64). Altered expression of Rab1, Rab8, and Rab2 was shown to cause Golgi fragmentation in Parkinson's disease (65). Expansion of a hexanucleotide repeat in C9ORF72, a Rab-associated GEF, was seen in both Amyotrophic Lateral Sclerosis (ALS) and Fronto-Temporal Dementia (FTD), suggesting that this mutant form of the GEF may contribute to the physiology of the disease through Rab dysfunction (66). Defects in the recycling of Rab7 from lysosomes to early endosomes impaired the transport and degradation of amyloid beta (Aβ) in Alzheimer's disease (AD) (67). Rab6 was shown to modulate the unfolded protein response due to ER stress in AD (68). Interestingly, defects in Rab11 function were recently observed in HD. Expression of Rab11 was decreased in HD mouse models, and Rab11 activation was impaired by mutant HTT (69,70). Over expression of Rab11 rescued neurodegeneration, dendritic spine loss, synaptic defects and behavioral defects in HD models in both mice and Drosophila (61,62). Perhaps defects to HTT-mediated axonal transport of a specific sub-set of Rab-containing vesicles could contribute to neurodegeneration and synaptic defects observed in HD. Thus our work could highlight a potential novel therapeutic pathway for early treatment of HD pathology.
Materials and Methods
Drosophila genetics
Generation of the Drosophila htt (dhtt) RNAi (UAS-RNAi-HTT) line has been previously described (10). For tissue specific knockdown of dhtt, the pan-neuronal GAL4 driver Appl-GAL4 was used. To screen neuronal Drosophila Rabs that were affected by reduction of HTT, the UAS-YFP-Rab transgenic lines (28) were used for in vivo imaging experiments. UAS-YFP-Rab males were first crossed to APPL-GAL4;T(2:3) CyO TM6B, Tb/Pin88K virgin females to obtain APPL-GAL4/y;UAS-YFP-Rab/ T(2:3) CyO TM6B, Tb males. The chromosome carrying T(2:3) CyO TM6B, Tb is referred to as B3 and carries the dominant markers, Hu, Tb and CyO. The larval Tb (tubby) marker is used to select larvae. The APPL-GAL4/y;UAS-YFP-Rab/B3 males were crossed to UAS-RNAi-HTT virgin females and non-tubby female larvae were dissected for in vivo imaging.
For simultaneous; dual color image analysis, from the human HTT line, UAS-hHTT.15Q-mRFP males were crossed to APPL-GAL4;B3/Pin88K female virgins. APPL-GAL4;UAS-hHTT.15Q-mRFP/B3 males were then crossed to UAS-YFP-Rab2, UAS-YFP-Rab3, UAS-Rab7-GFP, UAS-YFP-Rab19 or UAS-YFP-Rab26 virgin females. Non-tubby female 3rd instar larvae were then dissected and used for in vivo imaging. To visualize the motility of human HTT with normal polyQ repeats UAS-HTTexon1-15Q-mRFP males were crossed with APP-GAL4 virgin females.
In-vivo analysis of vesicle motility within whole mount larval axons
Larvae were dissected and immediately imaged under physiological conditions as previously detailed in (71). The motility of YFP-Rab-containing vesicles was visualized within living larval segmental nerves using a Nikon Eclipse TE 2000U microscope using the ×100 objective (Nikon, Melville, NY, USA). From each larva four sets of movies at a imaging window frame size of 90 microns at 150 frames were taken from the mid-region of the larva at an exposure of 500 ms using a Cool Snap HQ cooled CCD camera (Photometrics, Tucson, AZ, USA) and the Metamorph imaging system (Molecular Devices, Sunnyvale, CA, USA). Kymographs were generated in Metamorph using the kymograph stack tool. From a total of 10 larvae a set of 40 movies were imaged for each genotype at a spatial resolution of 0.126 micron/pixel. Movies were analyzed using a MATLAB-based particle tracker program as previously detailed (72). The four movies, each lasting 1.5 min span a total time of 6 min. Since most of the vesicles take <1 min to move they will have moved out of the 90 micron imaging window by the end of the first movie since each time frame for each movie lasts 1.5 min. Vesicle trajectories were analyzed to obtain the overall distribution of cargo populations and individual vesicle movement behaviors (velocities, pause frequencies/durations, run lengths). Duration-weighted segmental velocity evaluates the average velocity behavior that vesicles exhibit per time spent moving. For simultaneous dual-view imaging, a GFP/mRFP filter cassette was added to the Cool Snap HQ camera. Movies were taken in Dual-View mode using the split view software in Metamorph at 150 frames from the mid-region of the larva at an exposure of 500 ms to simultaneously image RFP and GFP tagged vesicles. The Cool Snap HQ camera Dual-View mode was aligned using Metamorph software (Split-View settings) before each imaging session. Movies were split by wavelength and each kymograph for each split movie was created, merged and analyzed for co-localization as detailed below.
Statistical analysis
For all analysis, a stringent significance threshold of P < 0.01 (99% confidence) was used to determine significance of HTT effects on Rab protein transport. Statistical significance of mean differences in percent of cargo population was calculated in EXCEL (Microsoft Corp.) using a two-tailed Student's t-test since these data tend to follow normal distributions. Duration-weighted segmental velocity distributions often followed a mixture of normal distributions or a single normal distribution. To select the appropriate statistical test, these velocity distributions were first checked for normality using the nortest package of R: the Lilliefors test and Anderson–Darling test. Statistical significance of normal distributions were calculated by a two-sample two-tailed Student's t-test while the non-normal segmental velocity distributions were compared using the non-parametric Wilcoxon–Mann–Whitney rank sum test in EXCEL and SPSS Statistics 20 (IBM Corp.). All velocity distributions were found to be non-normal. Distributions of corrected pause frequency, normalized pause duration and run length follow non-normal distributions and, thus, the Wilcoxon–Mann–Whitney rank sum test was used to calculate significance. For velocity quantification, all duration-weighted segmental velocities from each segment from all 10 larvae were pooled together before statistical analysis. This allows each particle to be considered an independent sample in the analysis to account for possible unique motor configurations on each particle (72–74).
Two-color kymograph analysis
Trajectories of vesicles with co-localized signals were identified manually from kymographs using Metamorph software. For each fluorescence channel, a kymograph was generated using Metamorph as previously done (73). Briefly, after selecting the first channel, all frames within the time-lapse image sequence of this channel were added together to produce a sum image. Then a polyline was generated on an axon. After generation of the polyline, the kymograph was created using Metamorph software. The polyline was then copied from one fluorescent channel to the other and used to create a kymograph for the other fluorescent channel. To identify vesicles with co-localized signals from both channels, the kymographs were colored in red and green, respectively, and combined into a single RGB kymograph. Non-stationary vesicles with co-localized signals were identified and counted by their yellow color in the combined kymograph. Note that to differentiate meaningful co-localization we evaluated the co-localization of the entire trajectory of a moving particle during the entire time frame of the movie. Therefore only particles containing the same trajectory in both red and green would show co-localization in yellow when merged and spurious co-localization observed in one time frame would be avoided. The total number of co-localized full trajectories in 10 kymographs from five larvae was counted for each genotype.
Supplementary Material
Funding
This work was supported by funds from NIH/NINDS (R03-NS084386-01) and the John R. Oishei Foundation to S.G. K.Z. was supported by a University at Buffalo (UB) SMURI Summer Research Award from SUNY Central. K.H.Z. was supported by a UB Center for Undergraduate Research and Creative Activities fellowship.
Supplementary Material
Acknowledgements
We thank the members of the Gunawardena laboratory for constructive discussions, the Bloomington Drosophila Stock Center for all Rab lines, Dr Norbert Perrimon for HTT-eGFP lines, Dr John Littleton for the HTT-mRFP lines, Shruthi Srinivasan for initial experiments and Tenzin Kelsang for help with data analysis. S.G. thanks Priyantha Karunaratne for constant support.
Conflict of Interest statement. None declared.
References
- 1.Sharp A.H., Loev S.J., Schilling G., Li S.H., Li X.J., Bao J., Wagster M.V., Kotzuk J.A., Steiner J.P., Lo A. et al. (1995) Widespread expression of Huntington's disease gene (IT15) protein product. Neuron, 14, 1065–1074. [DOI] [PubMed] [Google Scholar]
- 2.Nasir J., Floresco S.B., O'Kusky J.R., Diewert V.M., Richman J.M., Zeisler J., Borowski A., Marth J.D., Phillips A.G., Hayden M.R. (1995) Targeted disruption of the Huntington's disease gene results in embryonic lethality and behavioral and morphological changes in heterozygotes. Cell, 81, 811–823. [DOI] [PubMed] [Google Scholar]
- 3.Li X.J., Li S.H., Sharp A.H., Nucifora F.C. Jr, Schilling G., Lanahan A., Worley P., Snyder S.H., Ross C.A. (1995) A huntingtin-associated protein enriched in brain with implications for pathology. Nature, 378, 398–402. [DOI] [PubMed] [Google Scholar]
- 4.Lee W.C., Yoshihara M., Littleton J.T. (2004) Cytoplasmic aggregates trap polyglutamine-containing proteins and block axonal transport in a Drosophila model of Huntington's disease. Proc. Natl. Acad. Sci. USA, 101, 3224–3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zala D., Hinckelmann M-V., Saudou F. (2013) Huntingtin's function in axonal transport is conserved in Drosophila melanogaster. PLoS ONE, 8, e60162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caviston J.P., Ross J.L., Antony S.M., Tokito M., Holzbaur E.L. (2007) Huntingtin facilitates dynein/dynactin-mediated vesicle transport. Proc. Natl. Acad. Sci. USA, 104, 10045–10050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engelender S., Sharp A.H., Colomer V., Tokito M.K., Lanahan A., Worley P., Holzbaur E.L., Ross C.A. (1997) Huntingtin-associated protein 1 (HAP1) interacts with the p150Glued subunit of dynactin. Hum. Mol. Genet., 6, 2205–2212. [DOI] [PubMed] [Google Scholar]
- 8.Li S.H., Hosseini S.H., Gutekunst C.A., Hersch S.M., Ferrante R.J., Li X.J. (1998) A human HAP1 homologue. Cloning, expression, and interaction with huntingtin. J. Biol. Chem., 273, 19220–19227. [DOI] [PubMed] [Google Scholar]
- 9.Mcguire J.R., Rong J., Li S.H., Li X.J. (2006) Interaction of Huntingtin-associated protein-1 with kinesin light chain: implications in intracellular trafficking in neurons. J. Biol. Chem., 281, 3552–3559. [DOI] [PubMed] [Google Scholar]
- 10.Gunawardena S., Her L.S., Brusch R.G., Laymon R.A., Niesman I.R., Gordesky-Gold B., Sintasath L., Bonini N.M., Goldstein L.S. (2003) Disruption of axonal transport by loss of huntingtin or expression of pathogenic polyQ proteins in Drosophila. Neuron, 40, 25–40. [DOI] [PubMed] [Google Scholar]
- 11.Gauthier L.R., Charrin B.C., Borrell-Pagès M., Dompierre J.P., Rangone H., Cordelières F.P., De Mey J., MacDonald M.E., Lessmann V., Humbert S. et al. (2004) Huntingtin controls neurotrophic support and survival of neurons by enhancing BDNF vesicular transport along microtubules. Cell, 118, 127–138. [DOI] [PubMed] [Google Scholar]
- 12.Power D., Srinivasan S., Gunawardena S. (2012) In-vivo evidence for the disruption of Rab11 vesicle transport by loss of huntingtin. Neuroreport, 23, 970–977. [DOI] [PubMed] [Google Scholar]
- 13.Allaire P.D., Marat A.L., Dall'Armi C., Di Paolo G., McPherson P.S., Ritter B. (2010) The connecdenn DENN domain: a GEF for Rab35 mediating cargo-specific exit from early endosomes. Mol. Cell., 37, 370–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bacon R.A., Salminen A., Ruohola H., Novick P., Ferro-Novick S. (1989) The GTP-binding protein Ypt1 is required for transport in vitro: the Golgi apparatus is defective in ypt1 mutants. J. Cell. Biol., 109, 1015–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giannakouros T., Magee A.I. (1992) Protein prenylation and associated modifications. In Schlesinger M.J. (ed.), Lipid Modifications of Proteins. CRC Press Inc., Boca Raton, Florida, USA, pp. 133–160. [Google Scholar]
- 16.Seabra M.C., Goldstein J.L., Südhof T.C., Brown M.S. (1992) Rab geranylgeranyl transferase. A multisubunit enzyme that prenylates GTP-binding proteins terminating in Cys-X-Cys or Cys-Cys. J. Biol. Chem., 267, 14497–14503. [PubMed] [Google Scholar]
- 17.Bourne H.R. (1988) Do GTPases direct membrane traffic in secretion? Cell, 53, 669–671. [DOI] [PubMed] [Google Scholar]
- 18.Chavrier P., Parton R.G., Hauri H.P., Simons K., Zerial M. (1990) Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell, 62, 317–329. [DOI] [PubMed] [Google Scholar]
- 19.Bonifacino J.S., Glick B.S. (2004) The mechanisms of vesicle budding and fusion. Cell, 116, 153–166. [DOI] [PubMed] [Google Scholar]
- 20.Zerial M., McBride H. (2001) Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Bio., 2, 107–117. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi H., Fukuda M. (2012) Rab35 regulates Arf6 activity through centaurin-β2 (ACAP2) during neurite outgrowth. J. Cell Sci., 125, 2235–2243. [DOI] [PubMed] [Google Scholar]
- 22.Villarroel-Campos D., Gastaldi L., Conde C., Caceres A., Gonzalez-Billault C. (2014) Rab-mediated trafficking role in neurite formation. J. Neurochem., 129, 240–248. [DOI] [PubMed] [Google Scholar]
- 23.Horgan C.P., Mccaffrey M.W. (2011) Rab GTPases and microtubule motors. Biochem. Soc. Trans., 39, 1202–1206. [DOI] [PubMed] [Google Scholar]
- 24.Stenmark H. (2009) Rab GTPases as coordinators of vesicle traffic. Nat. Rev. Mol. Cell Biol., 10, 513–525. [DOI] [PubMed] [Google Scholar]
- 25.Chan C.C., Scoggin S., Wang D., Cherry S., Dembo T., Greenberg B., Jin E.J., Kuey C., Lopez A., Mehta S.Q. et al. (2011) Systematic discovery of Rab GTPases with synaptic functions in Drosophila. Curr Biol., 21, 1704–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang J., Schulze K.L., Hiesinger P.R., Suyama K., Wang S., Fish M., Acar M., Hoskins R.A., Bellen H.J., Scott M.P. (2007) Thirty-one flavors of Drosophila Rab proteins. Genetics, 176, 1307–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krench M., Littleton J.T. (2013) Modeling Huntington disease in Drosophila. Insights into axonal transport defects and modifiers of toxicity. Fly, 7, 229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nuoffer C., Davidson H.W., Matteson J., Meinkoth J., Balch W.E. (1994) A GDP-bound of Rab1 inhibits protein export from the endoplasmic reticulum and transport between Golgi compartments. J. Cell Biol., 125, 225–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tisdale E.J., Bourne J.R., Khosravi-far R., Der C.J., Balch W.E. (1992) GTP-binding mutants of Rab1 and Rab2 are potent inhibitors of vesicular transport from the endoplasmic reticulum to the Golgi complex. J. Cell Biol., 119, 749–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Junutula J.R., De Maziére A.M., Peden A.A., Ervin K.E., Advani R.J., van Dijk S.M., Klumperman J., Scheller R.H. (2004) Rab14 is involved in membrane trafficking between the Golgi complex and endosomes. Mol. Biol. Cell., 15, 2218–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.English A.R., Voeltz G.K. (2013) Rab10 GTPase regulates ER dynamics and morphology. Nat. Cell Biol., 15, 169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gerondopoulos A., Bastos R.N., Yoshimura S., Anderson R., Carpanini S., Aligianis I., Handley M.T., Barr F.A. (2014) Rab18 and a Rab18 GEF complex are required for normal ER structure. J. Cell Biol., 205, 707–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harris K.P., Littleton J.T. (2011) Vesicle trafficking: a Rab family profile. Curr Biol., 21, 841–843. [DOI] [PubMed] [Google Scholar]
- 34.Bucci C., Thomsen P., Nicoziani P., McCarthy J., van Deurs B. (2000) Rab7: a key to lysosome biogenesis. Mol. Biol. Cell., 11, 467–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wong Y.C., Holzbaur E.L. (2014) The regulation of autophagosome dynamics by huntingtin and HAP1 is disrupted by expression of mutant huntingtin, leading to defective cargo degradation. J. Neurosci., 34, 1293–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang K., Fishel Ben Kenan R., Osakada Y., Xu W., Sinit R.S., Chen L., Zhao X., Chen J.Y., Cui B., Wu C. (2013) Defective axonal transport of Rab7 GTPase results in dysregulated trophic signaling. J. Neurosci., 33, 7451–7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Broadwell R.D., Cataldo A.M. (1984) The neuronal endoplasmic reticulum: its cytochemistry and contribution to the endomembrane system. II. Axons and terminals. J. Comp. Neurol., 230, 231–248. [DOI] [PubMed] [Google Scholar]
- 38.Kanaseki T., Ikeuchi Y., Tashiro Y. (1998) Rough surfaced smooth endoplasmic reticulum in rat and mouse cerebellar Purkinje cells visualized by quick-freezing techniques. Cell Struct. Funct., 23, 373–387. [DOI] [PubMed] [Google Scholar]
- 39.O'Sullian N.C., Jahn T.R., Reid E., O'Kane C.J. (2012) Reticulon-like-1, the Drosophila orthologue of the hereditary spastic paraplegia gene reticulon 2, is required for organization of endoplasmic reticulum and of distal motor axons. Hum. Mol. Genet., 21, 3356–3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hattula K., Peränen J. (2005) Purification and functional properties of a Rab8-specific GEF (Rabin3) in action remodeling and polarized transport. Meth. Enzymol., 403, 284–295. [DOI] [PubMed] [Google Scholar]
- 41.Calero M., Chen C.Z., Zhu W., Winand N., Havas K.A., Gilbert P.M., Collins R.N. (2003) Dual prenylation is required for Rab protein localization and function. Mol. Biol. Cell, 14, 1852–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanaka M., Miyoshi J., Ishizaki H., Togawa A., Ohnishi K., Endo K., Matsubara K., Mizoguchi A., Nagano T., Sato M. et al. (2001) Role of Rab3 GDP/GTP exchange protein in synaptic vesicle trafficking at the mouse neuromuscular junction. Mol. Biol. Cell., 12, 1421–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trushina E., Singh R.D., Dyer R.B., Cao S., Shah V.H., Parton R.G., Pagano R.E., McMurray C.T. (2006) Mutant huntingtin inhibits clathrin-independent endocytosis and causes accumulation of cholesterol in vitro and in vivo. Hum. Mol. Genet., 15, 3578–3591. [DOI] [PubMed] [Google Scholar]
- 44.Li X., Standley C., Sapp E., Valencia A., Qin Z.H., Kegel K.B., Yoder J., Comer-Tierney L.A., Esteves M., Chase K. et al. (2009) Mutant huntingtin impairs vesicle formation from recycling endosomes by interfering with Rab11 activity. Mol. Cell Biol., 29, 6106–6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li H., Li H.F., Felder R.A., Periasamy A., Jose P.A. (2008) Rab4 and Rab11 coordinately regulate the recycling of angiotensin II type I receptor as demonstrated by fluorescence resonance energy transfer microscopy. J. Biomed. Opt., 13, 031206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pal A., Severin F., Lommer B., Shevchenko A., Zerial M. (2006) Huntingtin-HAP40 complex is a novel Rab5 effector that regulates early endosome motility and is up-regulated in Huntington's disease. J. Cell Biol., 172, 605–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sahlender D.A., Roberts R.C., Arden S.D., Spudich G., Taylor M.J., Luzio J.P., Kendrick-Jones J., Buss F. (2005) Optineurin links myosin VI to the Golgi complex and is involved in Golgi organization and exocytosis. J. Cell Biol., 169, 285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hattula K., Peränen J. (2000) FIP-2, a coiled-coil protein, links Huntingtin to Rab8 and modulates cellular morphogenesis. Curr. Biol., 10, 1603–1606. [DOI] [PubMed] [Google Scholar]
- 49.Schonteich E., Wilson G.M., Burden J., Hopkins C.R., Anderson K., Goldenring J.R., Prekeris R. (2008) The Rip11/Rab11-FIP5 and kinesin II complex regulates endocytic protein recycling. J. Cell Sci., 121, 3824–3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prekeris R., Klumperman J., Scheller R.H. (2000) A Rab11/Rip11 protein complex regulates apical membrane trafficking via recycling endosomes. Mol. Cell., 6, 1437–1448. [DOI] [PubMed] [Google Scholar]
- 51.Colin E., Zala D., Liot G., Rangone H., Borrell-Pagès M., Li X.-J., Humbert S. (2008) Huntingtin phosphorylation acts as a molecular switch for anterograde/retrograde transport in neurons. EMBO J., 27, 2124–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murray J.T., Panaretou C., Stenmark H., Miaczynska M., Backer J.M. (2002) Role of Rab5 in the recruitment of hVps34/p150 to the early endosome. Traffic, 3, 416–427. [DOI] [PubMed] [Google Scholar]
- 53.Stein M.P., Feng Y., Cooper K.L., Welford A.M., Wandinger-Ness A. (2003) Human VPS34 and p150 are Rab7 interacting partners. Traffic, 4, 754–771. [DOI] [PubMed] [Google Scholar]
- 54.Zala D., Hinckelmann M.V., Yu H., Lyra da Cunha M.M., Liot G., Cordelières F.P., Marco S., Saudou F. (2013) Vesicular glycolysis provides on-board energy for fast axonal transport. Cell, 152, 479–491. [DOI] [PubMed] [Google Scholar]
- 55.Deinhardt K., Salinas S., Verastegui C., Watson R., Worth D., Hanrahan S., Bucci C., Schiavo G. (2006) Rab5 and Rab7 control endocytic sorting along the axonal retrograde transport pathway. Neuron, 52, 293–305. [DOI] [PubMed] [Google Scholar]
- 56.Caviston J.P., Zajac A.L., Tokito M., Holzbaur E.L. (2011) Huntingtin coordinates the dynein-mediated dynamic positioning of endosomes and lysosomes. Mol. Biol. Cell., 22, 478–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fu M.M., Nirschl J.J., Holzbaur E.L. (2014) LC3 binding to the scaffolding protein JIP1 regulates processive dynein-driven transport of autophagosomes. Dev. Cell., 29, 577–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maday S., Wallace K.E., Holzbaur E.L. (2012) Autophagosomes initiate distally and mature during transport toward the cell soma in primary neurons. J. Cell Biol., 196, 407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jordens I., Fernandez-Borja M., Marsman M., Dusseljee S., Janssen L., Calafat J., Janssen H., Wubbolts R., Neefjes J. (2001) The Rab7 effector protein RILP controls lysosomal transport by inducing the recruitment of dynein-dynactin motors. Curr. Bio., 11, 1680–1685. [DOI] [PubMed] [Google Scholar]
- 60.Pankiv S., Alemu E.A., Brech A., Bruun J.A., Lamark T., Overvatn A., Bjørkøy G., Johansen T. (2010) FYCO1 is a Rab7 effector that binds to LC3 and PI3P to mediate microtubule plus end-directed vesicle transport. J. Cell Biol., 188, 253–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Richards P., Didszun C., Campesan S., Simpson A., Horley B., Young K.W., Glynn P., Cain K., Kyriacou C.P., Giorgini F. et al. (2011) Dendritic spine loss and neurodegeneration is rescued by Rab11 in models of Huntington's disease . Cell Death Differ., 18, 191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Steinert J.R., Campesan S., Richards P., Kyriacou C.P., Forsythe I.D., Giorgini F. (2012) Rab11 rescues synaptic dysfunction and behavioural deficits in a Drosophila model of Huntington's disease. Hum. Mol. Genet., 21, 2912–2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kang M.J., Hansen T.J., Mickiewicz M., Kaczynski T.J., Fye S., Gunawardena S. (2014) Disruption of axonal transport perturbs bone morphogenetic protein (BMP)—signaling and contributes to synaptic abnormalities in two neurodegenerative diseases. PLoS One, 15, e104617 doi:10.1371/journal.pone.0104617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Spinosa M.R., Progida C., De luca A., Colucci A.M., Alifano P., Bucci C. (2008) Functional characterization of Rab7 mutant proteins associated with Charcot-Marie-Tooth type 2B disease. J. Neurosci., 28, 1640–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rendón W.O., Martínez-alonso E., Tomás M., Martínez-martínez N., Martínez-menárguez J.A. (2013) Golgi fragmentation is Rab and SNARE dependent in cellular models of Parkinson's disease. Histochem. Cell Biol., 139, 671–684. [DOI] [PubMed] [Google Scholar]
- 66.Zhang D., Iyer L.M., He F., Aravind L. (2012) Discovery of novel DENN proteins: implications for the evolution of eukaryotic intracellular membrane structures and human disease. Front. Genet., 3, 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee C.Y., Tse W., Smith J.D., Landreth G.E. (2012) Apolipoprotein E promotes β-amyloid trafficking and degradation by modulating microglial cholesterol levels. J. Biol. Chem., 287, 2032–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Elfrink H.L., Zwart R., Cavanillas M.L., Schindler A.J., Baas F., Scheper W. (2012) Rab6 is a modulator of the unfolded protein response: implications for Alzheimer's disease. J. Alzheimers Dis., 28, 917–929. [DOI] [PubMed] [Google Scholar]
- 69.Li X., Valencia A., Sapp E., Masso N., Alexander J., Reeves P., Kegel K.B., Aronin N., Difiglia M. (2010) Aberrant Rab11-dependent trafficking of the neuronal glutamate transporter EAAC1 causes oxidative stress and cell death in Huntington's disease. J. Neurosci., 30, 4552–4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li X., DiFigurelia M. (2012) The recycling endosome and its role in neurological disorders. Prog. Neurobiol., 97, 127–141. [DOI] [PubMed] [Google Scholar]
- 71.Kuznicki M.L., Gunawardena S. (2010) In vivo visualization of synaptic vesicles within Drosophila larval segmental axons. J. Vis. Exp., 44, 2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weaver C., Leidel C., Szpankowski L., Farley N.M., Shubeita G.T., Goldstein L.S. (2013) Endogenous GSK-3/shaggy regulates bidirectional axonal transport of the amyloid precursor protein. Traffic, 14, 295–308. [DOI] [PubMed] [Google Scholar]
- 73.Reis G.F., Yang G., Szpankowski L., Weaver C., Shah S.B., Robinson J.T., Hays T.S., Danuser G., Goldstein L.S. (2012) Molecular motor function in axonal transport in vivo probed by genetic and computational analysis in Drosophila. Mol. Biol. Cell, 23, 1700–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gunawardena S., Yang G., Goldstein L.S. (2013) Presenilin controls kinesin-1 and dynein function during APP-vesicle transport in vivo. Hum. Mol. Genet., 22, 3828–3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.