Abstract
Suppression of the hypothalamic-pituitary-adrenal (HPA) axis has been shown to occur during cholestatic liver injury. Furthermore, we have demonstrated that in a model of cholestasis, serum bile acids gain entry into the brain via a leaky blood brain barrier and that hypothalamic bile acid content is increased. Therefore, the aim of the current study was to determine the effects of bile acid signaling on the HPA axis. The data presented show that HPA axis suppression during cholestatic liver injury, specifically circulating corticosterone levels and hypothalamic corticotropin releasing hormone (CRH) expression, can be attenuated by administration of the bile acid sequestrant cholestyramine. Secondly, treatment of hypothalamic neurons with various bile acids suppressed CRH expression and secretion in vitro. However, in vivo HPA axis suppression was only evident after the central injection of the bile acids taurocholic acid or glycochenodeoxycholic acid but not the other bile acids studied. Furthermore, we demonstrate that taurocholic acid and glycochenodeoxycholic acid are exerting their effects on hypothalamic CRH expression after their uptake through the apical sodium-dependent bile acid transporter and subsequent activation of the glucocorticoid receptor. Taken together with previous studies, our data support the hypothesis that during cholestatic liver injury, bile acids gain entry into the brain, are transported into neurons through the apical sodium-dependent bile acid transporter and can activate the glucocorticoid receptor to suppress the HPA axis. These data also lend themselves to the broader hypothesis that bile acids may act as central modulators of hypothalamic peptides that may be altered during liver disease.
The hypothalamic-pituitary-adrenal (HPA) axis describes a complex set of positive and negative feedback influences between the hypothalamus, pituitary gland, and adrenal gland (1). These positive and negative feedback mechanisms work in a neuroendocrine manner in order to modulate a number of physiological processes such as immunity (2), digestion (3), and the body's response to stress (3).
The mechanism by which the HPA axis remains in homeostasis depends widely on the release and uptake of several key regulatory molecules. The hypothalamus contains neuroendocrine neurons that secrete corticotropin releasing hormone (CRH). CRH will, in turn, act on the pituitary gland to stimulate the production and release of adrenocorticotropic hormone (ACTH) (4) into the circulation. Circulating ACTH then induces the adrenal gland to synthesize and release corticosteroids such as cortisol and corticosterone. These circulating corticosteroids modulate the vast array of physiological processes influenced by the HPA axis and are also responsible for initiating a negative feedback loop on the HPA axis via activation of the glucocorticoid receptor (GR) in the brain in order to shut down corticosteroid production. Activation of the GR in the hypothalamus has 2 major effects on hypothalamic CRH activity: a fast response, which involves the inhibition of CRH release from the hypothalamus, and a delayed response, which involves the inhibition of CRH synthesis and subsequent release (5).
Suppression of the HPA axis has been associated with cholestatic liver injury (6–8). The link between cholestasis and the HPA axis has been demonstrated clinically, with cholestatic patients often exhibiting clinical features suggestive of adrenal insufficiency such as hypovolemia, hypotension, and renal failure (9, 10). Furthermore, patients with congenital hypopituitarism or glucocorticoid deficiency often exhibit cholestatic hepatitis (11–13). In cirrhosis, adrenal insufficiency is associated with increased mortality and hemodynamic impairment (14, 15). Treatment with low doses of hydrocortisone to cirrhotic patients resolves the hemodynamic impairment and is associated with a higher survival rate (16). In the bile duct ligation (BDL) rat model of cholestasis, there is a general suppression of HPA axis responsiveness to stress (8) as well as a defective CRH-mediated response (7). Furthermore, suppression of the HPA axis contributes to cholangiocyte proliferation observed after BDL and strategies to restore HPA axis function, such as the central administration of recombinant CRH, attenuated BDL-induced cholangiocyte proliferation (6). However, the mechanisms by which cholestatic liver injury leads to a suppression of the HPA axis are poorly understood.
Bile acids are synthesized in the liver from cholesterol and can be conjugated, mainly to glycine or taurine, before being released into the bile to aid in digestion. Conjugated bile acids, which carry a negative charge at physiological pH, require carrier-mediated transport to cross membranes (17). The uptake of bile acids can occur via one of a number of transporters, including the apical sodium-dependent bile acid transporter (ASBT) (18). Once bile acids gain entry into the cell they exert their effects through a number of nuclear receptors, including farnesoid X receptor (19), pregnane X receptor (20), vitamin D receptor (20), and, in certain conditions, the GR (21). Under normal physiological conditions, 95% of the bile acid pool is reabsorbed from the intestine and transported to the liver, which constitutes an inhibitory feedback control on bile acid synthesis. In humans, approximately 90% of the bile acid pool is in the conjugated form, and taurocholic acid (TCA) and glycochenodeoxycholic acid (GCDA) are 2 of the major bile salts in bile (22).
During liver damage there is increased accumulation of bile acids in the liver and a spillover of bile acids into systemic circulation. These elevated serum bile acid levels have been associated with hepatotoxicity (23, 24), hepatic fibrosis (25), pruritus (26), cardiomyopathy (27), and vasodilation (28). We have previously demonstrated that increased serum bile acids gain entry to the brain via a leaky blood brain barrier (29). However, the role of bile acids in the neurological changes associated with liver damage is unknown. Therefore, the aim of the current study was to assess the role of bile acids in the suppression of the HPA axis observed during cholestasis.
Materials and Methods
Materials
Unless otherwise indicated, all chemicals were purchased from Sigma-Aldrich and were of the highest grade available. The fluorescent bile acid derivative, cholyl-lysyl-fluorescein (CLF) was purchased from BD Biosciences. The antibody against ASBT was a kind gift from Dr Paul Dawson (Wake Forest University Health Sciences, Winston Salem, NC) (30). NeuN antibodies were ordered from Millipore. GR and H3 histone antibodies were purchased from Santa Cruz Biotechnology, Inc. All primers and ASBT short hairpin RNA (shRNA) plasmids used in this study were purchased from SABiosciences. The ASBT Vivo-Morpholino (5′-GAACAGACGGAGGAGTTATCCATCA), the ASBT mismatched control sequence (5′-GAAgAGACcGAGcAGTTATgCATgA), the GR Vivo-Morpholino (5′-GGATTCTTTGGAGTCCATTGGCAAA), and the GR mismatched control sequence (5′-GGATTgTTTcGAcTCgATTcGCAAA) were purchased from Gene Tools, LLC. The enzyme immunoassay (EIA) kit for detecting CRH in mouse cell lines was obtained from Phoenix Pharmaceuticals. The EIA for measuring CRH in rat serum was obtained from Cederlane Laboratories. The Corticosterone EIA kit was purchased from Cayman Chemical. The Mouse total bile acid kit was purchased from Crystal Chem, Inc. GR competition assays were performed using the PolarScreen Glucocorticoid Receptor Competitor Assay kit, Green, purchased from Life Technologies.
Animal treatment
Male Sprague Dawley rats (150–175 g) were purchased from Charles River and maintained in a temperature-controlled environment (20°C–22°C) with a 12-hour light, 12-hour dark cycle. Unless otherwise indicated, animals had free access to drinking water and standard rat chow. All animal experiments were performed in accordance with the guidelines of the Baylor Scott & White Health Institutional Animal Care and Use committee. Rats were fed a diet containing 2% cholestyramine or the control diet AIN-93G (Dyets, Inc) for 3 days before either BDL or sham surgeries. Tissue and serum were collected 3 days after surgery between the hours of 8 and 9 am to minimize the circadian variations in glucocorticoid levels (6, 31). In a separate experiment, rats were injected with 20 pmol of the bile acids cholic acid (CA), deoxycholic acid (DCA), chenodeoxycholic acid (CDCA), GCDA, or TCA in the third ventricle (0 mm medial/lateral, −1.8 mm anterior/posterior, +4.5 mm dorsal/ventral from bregma) and serum and tissue were collected 6 hours later. In parallel, rats were infused with 1 mg/kg · d of Vivo-Morpholino sequences into the lateral ventricle at the coordinates (−1.3 mm medial/lateral, −0.2 mm anterior/posterior, +3.5 mm dorsal/ventral from bregma) using the brain infusion kits coupled to subcutaneous implanted minipumps (Alzet) for 3 days before the single GCDA or TCA injection following the method described above. The degree by which the target gene expression was suppressed by Vivo-Morpholino infusion was evaluated by immunofluorescence and immunoblotting as previously described (6, 32, 33).
Cell culture
The mouse hypothalamic cell line MhypoA-21 was purchased from Cedarlane and cultured per the vendor's instructions. The cell line was validated before experimentation by assessing the expression of key hypothalamic neuropeptides and correlating this with the information provided by the vendor. To suppress ASBT expression in hypothalamic neurons, MhypoA-21 cells were transfected with an ASBT shRNA expression vector (Mhypo-ASBT shRNA) conferring resistance to puromycin following the methodology described previously (34). ASBT expression was then assessed in the resulting cell line or the mock-transfected control cell line (Mhypo-puro neg) by real-time PCR, immunofluorescence, and flow cytometry using methodology described previously (32, 35).
Assessment of bile acids, CRH, and corticosterone by EIA in the serum and brain
At the time of terminal tissue collection, rats were anesthetized, blood samples were collected, and then rats were transcardially perfused with ice-cold saline to remove the blood from the brain. Total bile acid content, CRH or corticosterone levels were assessed by EIA in the serum or in hypothalamus tissue homogenates following the manufacturer's instructions. In parallel, hypothalamic cell lines were treated with 10μM of various bile acids in the presence or absence of 100nM of the GR antagonist 11β-(4-Dimethylamino)phenyl-17β-hydroxy-17-(1-propynyl)estra-4,9-dien-3-one (RU486) for 2 hours; CRH mRNA expression from these cell lines was assessed by real-time PCR (6, 33–35). To assess protein secretion from these cell lines, cells were treated with the above-mentioned reagents in phenol red-free, serum-free DMEM media for 24 hours. Conditioned media were collected, and supernatants were concentrated using Centriprep centrifugation tubes with a 10-kDa pore size (Millipore). CRH secretion was assessed in the concentrated conditioned media by EIA.
Immunofluorescence
ASBT immunoreactivity (red) was assessed in the hypothalamus of normal and BDL rats and in MhypoA-21 cells by immunofluorescence using antibodies against the neuron marker, NeuN (green) as a costain using methods described previously (33). Tissue and cells were counterstained with the nuclear dye 4′,6-diamidino-2-phenylindole (DAPI) (blue). Negative controls were stained using preimmune serum in the place of the primary antibody. Images were taken using a Leica TCS SP5-X inverted confocal microscope (Leica Microsystems).
Bile acid uptake assay
Rat hypothalamic cell lines were plated onto coverslips and allowed to adhere overnight. Cells were then treated with the bile acid derivative CLF (5 μg/mL), and after 4 hours, coverslips were washed in cold PBS and fixed in 4% paraformaldehyde (wt/vol). Cells were counterstained with DAPI, and the amount of intracellular fluorescence was visualized using a Leica TCS SP5-X inverted confocal microscope.
GR competitor assay
Bile acids at concentrations of 1nM–1mM were incubated in the presence of native GR protein and a fluorescently tagged GR ligand according to the manufacturer's instructions (Life Technologies). Bile acids able to competitively bind to the GR protein prevented the formation of the fluorescent ligand/GR complex, resulting in lower fluorescence polarization values, which were assayed on a Molecular Devices SpectraMax M5e spectrophotometer.
GR luciferase assay
In addition, the GR transcriptional activity was assessed in hypothalamic neurons using a luciferase reporter construct coupled to a promoter region containing the glucocorticoid response element (GRE) consensus sequence (Panomics) following procedures described previously (36). Neurons were plated onto 96-well plates at a density of 10 000 cells/well and allowed to adhere overnight. Cells were then transfected with the GRE-luciferase reporter construct (0.1-μg DNA/well) with 0.28 μL of TransIT-LT1 transfection reagent (Mirus) overnight at 37°C. After this time, the cells were stimulated with GCDA or TCA (10μM) and were assayed for luciferase activity using the luciferase assay kit (Promega) 24 hours after stimulation. Treatments were done at least in quadruplicate, and results are expressed as the degree of change of luciferase activity per microgram of protein.
GR nuclear translocation
The nuclear translocation of GR was assessed by subcellular fractionation followed by immunoblotting as described previously, or by immunofluorescence as described above. Briefly, MhypoA-21 cells treated for 4 hours with the indicated bile acids (10μM), and the GR nuclear translocation was followed using a specific GR antibody (Santa Cruz Biotechnology, Inc). For the subcellular fractionation experiments, the purity of the fractions was evaluated using β-actin or H3 histone as a cytoplasmic marker or nuclear marker, respectively.
Results
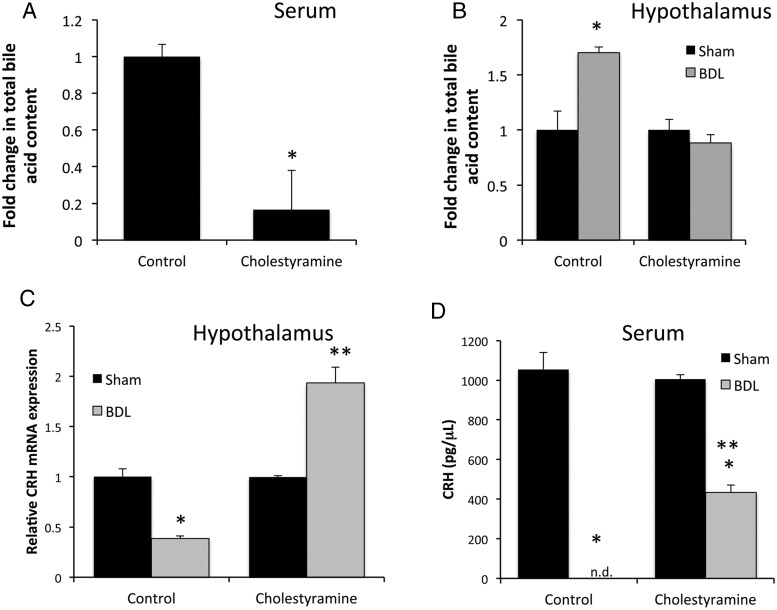
We have previously demonstrated that total bile acid content is increased in both the serum and brain tissue in rats after BDL (29). To assess whether bile acids in the hypothalamus may be responsible for the suppression of the HPA axis observed after BDL (6), rats were fed a diet enriched in the bile acid sequestrant cholestyramine, which reduced serum bile acid concentrations to approximately 16% of the control fed mice (or from 29.44 ± 1.98μM to 4.89 ± 6.28μM) (Figure 1A). The total bile acid content in the hypothalamus was increased after BDL surgery compared with sham surgery, which was attenuated by treatment with cholestyramine (Figure 1B). Once again, similar to our previous data (6), CRH mRNA expression in the hypothalamus was reduced after BDL surgery compared with sham however, cholestyramine feeding reversed the suppressive effects of BDL surgery on hypothalamic CRH mRNA expression (Figure 1C). Similarly, the CRH protein levels in the serum were reduced to below the detectable limits of the assay after BDL surgery, which was partially attenuated with cholestyramine feeding (Figure 1D).
Figure 1.
Cholestyramine feeding reduces bile acid concentrations and CRH expression in the hypothalamus. A, Relative total bile acid concentrations in the serum of rats that were fed a control diet or a cholestyramine-supplemented diet. Data are average ± SEM (n = 6); *, P < .05 compared with control diet. B, Relative total hypothalamic bile acid concentrations in sham- or BDL-operated rats that had consumed a control diet or a cholestyramine-supplemented diet. Data are average ± SEM (n = 6); *, P < .05 compared with sham-operated rats fed a control diet. C, CRH mRNA expression in the hypothalamus of rats with sham or BDL surgery that had been fed a control diet or a cholestryamrine-supplemented diet. Data are average ± SEM (n = 6); *, P < .05 compared with sham-operated rats fed a control diet; **, P < .05 compared with sham-operated rats fed a cholestyramine diet. D, CRH concentrations in the serum of rats that had been fed a control diet or a cholestyramine-supplemented diet after sham or BDL surgery (n.d., not detected). Data are average ± SEM (n = 6); *, P < .05 compared with sham-operated rats fed a control diet; **, P < .05 compared with BDL-operated rats fed a control diet.
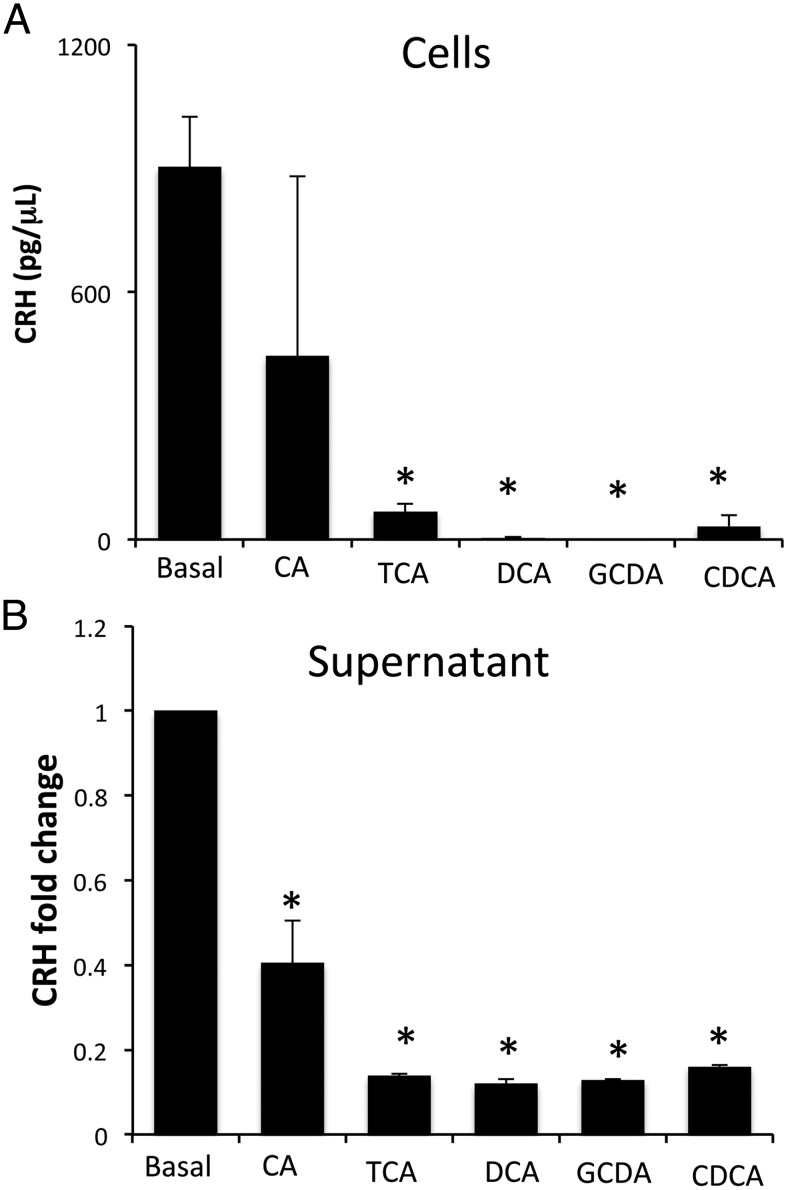
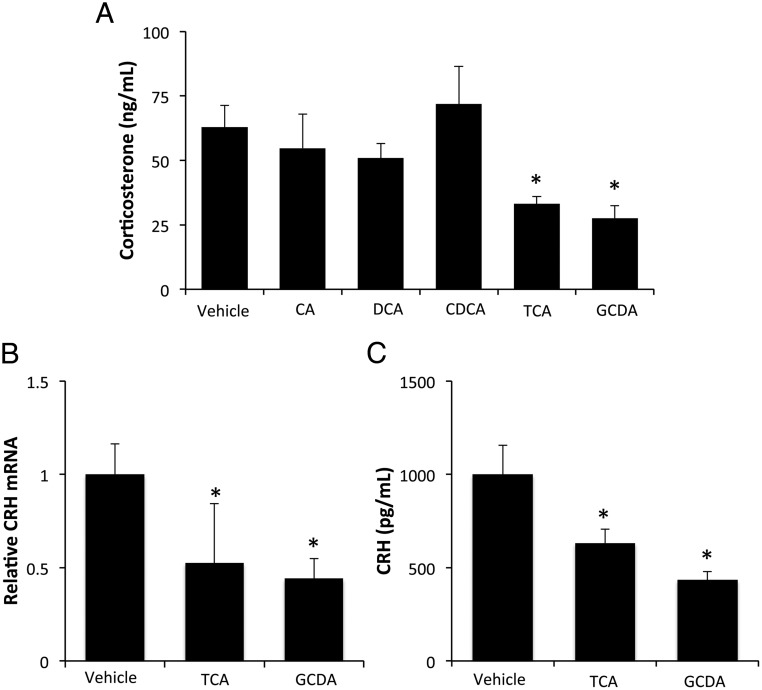
To determine whether bile acids are able to directly regulate hypothalamic CRH expression, cultured hypothalamic cells were treated with various bile acids and CRH content within the cells and supernatants were assessed. All bile acids studied suppressed the cellular content of CRH protein (Figure 2A) with a modest decrease in mRNA expression (data not shown). Furthermore, the bile acids studied decreased the release of CRH into the supernatant (Figure 2B). We then assessed whether direct injection of these selected bile acids into the ventricle of the brain could also suppress the HPA axis in vivo. The suppressive effects of bile acids on the HPA axis were restricted to the conjugated bile acids TCA and GCDA, whereas the unconjugated bile acids CA, DCA, and CDCA had no significant effect on circulating corticosterone levels 6 hours after injection (Figure 3A). In parallel, hypothalamic CRH mRNA expression and circulating CRH protein levels were decreased by TCA and GCDA injection (Figure 3, B and C), whereas the bile acids CA, DCA, and CDCA had no significant effect (data not shown).
Figure 2.
CRH expression and secretion in MhypoA-21 cells is suppressed after treatment with bile acids. MhypoA-21 hypothalamic neurons were treated with 10μM of the bile acids CA, TCA, DCA, GCDA, and CDCA for 24 hours. CRH protein content was assessed in cells (A) and in supernatant (B) by EIA. *, P < .05 compared with basal (n = 5).
Figure 3.
Intracerebroventricular (ICV) injection of TCA and GCDA into normal rats suppresses the HPA axis. Rats were treated with a single injection of the bile acids CA, DCA, CDCA, TCA, and GCDA (20 pmol) into the third ventricle. After 6 hours, serum corticosterone levels were assessed by EIA (A), relative CRH mRNA expression in the hypothalamus of TCA or GCDA-treated rats was assessed by real-time PCR (B), and serum concentrations of CRH were assessed in TCA or GCDA-treated rats by EIA (C). *, P < .05 compared with vehicle (n = 4).
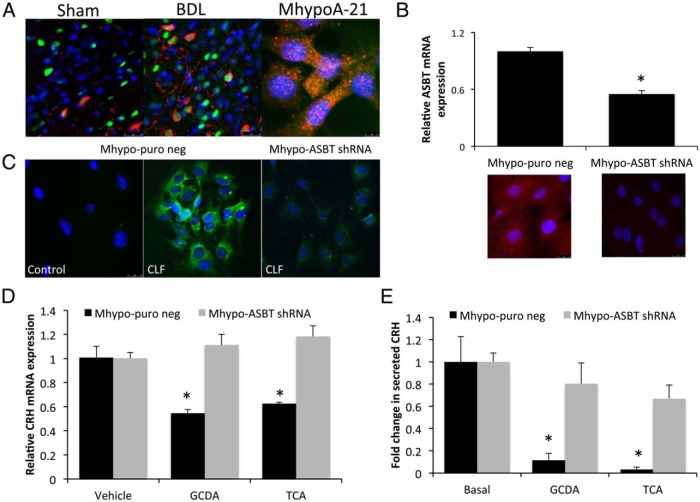
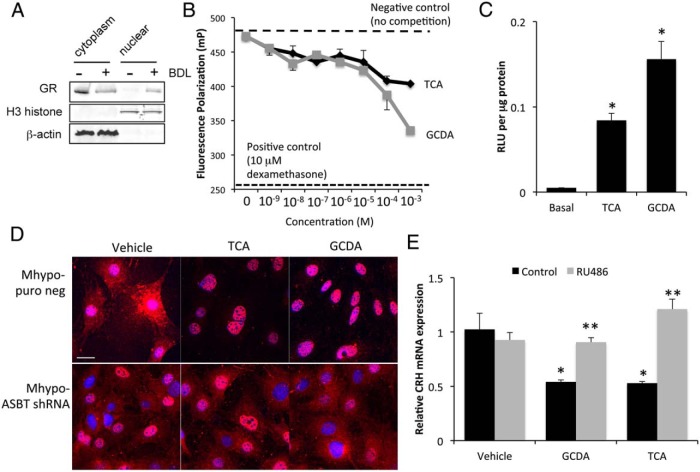
In order for bile acids to exert their effects inside the cell, they need to be transported. The bile acid transporter ASBT (shown in red) is expressed in the hypothalamus and is associated with both cell bodies and cellular processes. The cell body staining colocalizes with the neuron marker NeuN (shown in green; Figure 4A) and is increased after BDL surgery. Furthermore, ASBT is expressed in our hypothalamic cell line (Figure 4A). To assess the involvement of ASBT in the uptake and signaling of bile acids, we established stable-transfected cell lines with an ASBT shRNA plasmid (Mhypo-ASBT shRNA) or an empty vector backbone control (Mhypo-puro neg). The expression of ASBT mRNA was suppressed to approximately 50% in the Mhypo-ASBT shRNA cell line compared with the Mhypo-puro neg control (Figure 4B). Similarly, analysis of ASBT protein expression by flow cytometry indicated that ASBT expression was down-regulated from 30% to 50% of the parental cell line (data not shown). In addition, ASBT immunoreactivity was dramatically reduced in the Mhypo-ASBT shRNA cell line (Figure 4B). The ability for these hypothalamic cells to take up bile acids was assessed using CLF. This fluorescent bile acid derivative is transported with the same kinetics as endogenous bile acids (37, 38). Control hypothalamic cells treated with CLF exhibited intracellular fluorescence that was absent in cells treated with saline (Figure 4C), indicating the ability of these cells to uptake bile acids. The intracellular fluorescence of CLF was reduced in the Mhypo-ASBT shRNA cell line, suggesting that bile acids require ASBT for internalization. Treatment of the control Mhypo-puro neg cells with the bile acids TCA and GCDA suppressed CRH mRNA expression, whereas this effect was absent in the mHypo-ASBT shRNA cell line (Figure 4D). Furthermore, the CRH protein content in the supernatants from Mhypo-puro neg cell lines were decreased after GCDA and TCA treatment, an effect that was once again absent in the mHypo-ASBT cell line (Figure 4E). Taken together, these data suggest that the bile acid-induced down-regulation of CRH expression may be dependent upon ASBT.
Figure 4.
Increased ASBT expression in hypothalamic neurons after BDL promotes conjugated bile acid entry into the cell and subsequent inhibition of CRH release. A, ASBT immunoreactivity (red) was assessed in the hypothalamus of sham- and BDL-operated rats and in the MhypoA-21 hypothalamic cell line. Neurons were counterstained with NeuN (green) and DAPI used as a marker of the nucleus. B, Relative ASBT mRNA expression and immunofluorescence was assessed in MhypoA-21 cells transfected with ASBT shRNA (Mhypo-ASBT shRNA) compared with MhypoA-21 cells transfected with a puromycin negative control vector (Mhypo-puro neg) (n = 4). C, Immunofluorescence images of Mhypo-puro neg and Mhypo-ASBT shRNA cells lines treated with the fluorescent bile acid derivative CLF (5 μg/mL; green) or saline as a control for 4 hours. Nuclei were counterstained with DAPI (blue). D, Relative CRH mRNA expression in Mhypo-puro neg and Mhypo-ASBT shRNA cell lines treated with 10μM of the bile acids GCDA or TCA. *, P < .05 compared with vehicle treatment within each cell line (n = 4). E, The relative amounts of CRH secreted into the supernatant of Mhypo-puro neg and Mhypo-ASBT shRNA cell lines was assessed 24 hours after treatment with 10μM of the bile acids GCDA or TCA by EIA. *, P < .05 compared with vehicle treatment within each cell line (n = 4).
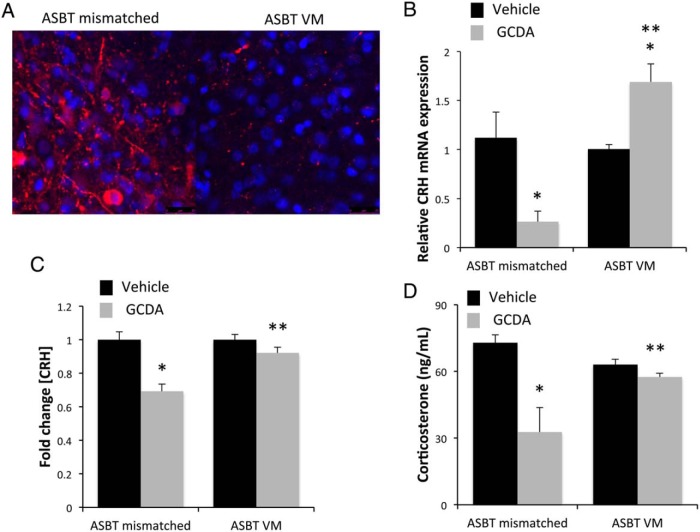
To assess whether the in vivo effect of bile acids was also dependent upon ASBT, we injected an ASBT Vivo-Morpholino or mismatched control sequence into the third ventricle. This significantly suppressed the translation of ASBT protein in the hypothalamic neurons as demonstrated by immunofluorescence (Figure 5A). Direct injection of GCDA into the third ventricle significantly suppressed the hypothalamic CRH mRNA expression, (Figure 5B) protein content (Figure 5C) and circulating corticosterone levels (Figure 5D), an effect that was attenuated or reversed by ASBT Vivo-Morpholino injection.
Figure 5.
Suppression of ASBT protein expression in vivo is able to attenuate bile acid-induced HPA axis suppression. A, ASBT Vivo-Morpholino or ASBT mismatched control sequences were infused ICV (1 mg/kg · d for 3 d), and representative ASBT immunofluorescence (red) are shown with DAPI (blue) used as a nuclear counterstain in the hypothalamus. B, Relative CRH mRNA expression was assessed in the hypothalamus of rats infused with ASBT Vivo-Morpholino or ASBT mismatched sequences and administered an ICV injection of the bile acid GCDA (20 pmol; gray bar) or vehicle (black bar) by real-time PCR. *, P < .05 compared with vehicle injection; **, P < .05 compared with ASBT mismatched Vivo-Morpholino infused, GCDA-injected rats. C, Relative circulating CRH protein concentrations in the serum of rats infused with ASBT Vivo-Morpholino or ASBT mismatched sequences and administered an ICV injection of GCDA (20 pmol; gray bar) or vehicle (black bar) was assessed by EIA. *, P < .05 compared with vehicle injection; **, P < .05 compared with ASBT mismatched Vivo-Morpholino infused, GCDA-injected rats. D, Serum corticosterone concentrations was assessed by EIA in rats infused with either ASBT Vivo-Morpholino or ASBT mismatched sequences and were subsequently administered an ICV injection of GCDA (20 pmol; gray bar) or vehicle (black bar). *, P < .05 compared with vehicle injection; **, P < .05 compared with ASBT mismatched Vivo-Morpholino infused, GCDA-injected rats.
An interaction between bile acids and the GR has previously been suggested (21), given that the activation of the GR by glucocorticoids in hypothalamic neurons is a well-characterized negative feedback mechanism that controls the HPA axis (5). Therefore, we assessed the involvement of the GR in the suppressive effects of TCA and GCDA on the HPA axis. Once activated, the GR is translocated into the nucleus. Nuclear and cytoplasmic fractions were prepared from the hypothalamus of sham and BDL rats. GR protein expression was assessed by immunoblotting and was shown to decrease in the cytoplasmic fraction and increase in the nuclear fraction after BDL compared with sham, indicating GR nuclear translocation after BDL surgery (Figure 6A). In a cell-free competitive binding assay, treatment with the bile acids TCA and GCDA was able to significantly reduce the reporter ligand binding to the GR from 10nM through to 1mM (Figure 6B). Furthermore, treatment of hypothalamic cells with TCA and GCDA resulted in an increase in GR-specific luciferase activity (Figure 6C) and the nuclear translocation of GR immunoreactivity from the cytoplasm to the nucleus, an effect that was not evident in the Mhypo-ASBT shRNA cell line (Figure 6D). Lastly, pretreatment of hypothalamic neurons with the GR antagonist RU486 prevented the bile acid-induced down-regulation of CRH expression (Figure 6E). Taken together, these data suggest that TCA and GCDA are able to weakly bind and cause nuclear translocation of the GR and that blocking GR activity prevented the effects of TCA and GCDA on CRH expression in vitro.
Figure 6.
Nuclear translocation and transcriptional activity of GR can be induced via GCDA and TCA. A, Representative GR immunoblots in cytoplasm and nuclear fractions from sham- and BDL-operated rat hypothalamus. H3 histone is used as a maker for the nuclear fraction, whereas β-actin is used as a marker for the cytoplasmic fraction. B, GR competitor activity assay fluorescence polarization using TCA or GCDA in the indicated doses. Values underneath the dashed line indicate receptor binding and subsequent reduction of GR activity. C, GR transcriptional activity was assessed in hypothalamic neurons following transfection with a GRE-driven luciferase reporter construct. Cells were treated with 10μM of the bile acids TCA or GCDA, and relative luciferase activity was assessed and expressed as relative luminescent units per microgram of protein. *, P < .05 compared with basal treatment (n = 7). D, GR immunoreactivity (red) was assessed in Mhypo-puro neg and Mhypo-ASBT shRNA cell lines treated with 10μM of the bile acids TCA or GCDA with DAPI (blue) used as a nuclear marker. Scale bar, 25 μm. E, Relative CRH mRNA expression in MhypoA-21 cells cotreated with the GR antagonist RU486 (100nM) and the bile acids GCDA or TCA (10μM) or appropriate controls. *, P < .05 compared with control-treated samples; **, P < .05 compared with RU486-treated samples.
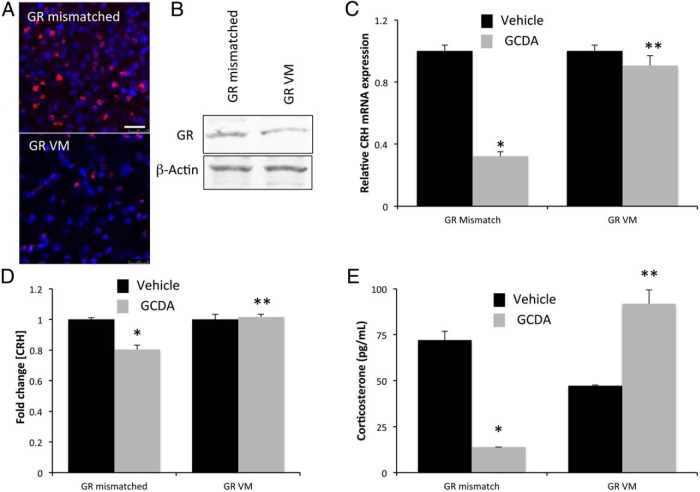
We then assessed whether the ability of bile acids to activate GR is also relevant in vivo. Rats were injected with GR Vivo-Morpholino (or mismatched control sequence) into the lateral ventricle, and the resulting expression of GR in the hypothalamus was assessed by immunofluorescence. As expected, GR immunoreactivity was significantly suppressed after central GR Vivo-Morpholino injection compared with the mismatched control as assessed by immunofluorescence (Figure 7A) and immunoblotting (Figure 7B). Furthermore, subsequent injection of GCDA into the third ventricle significantly suppressed the CRH mRNA expression (Figure 7C), protein content (Figure 7D), and circulating corticosterone levels (Figure 7E), an effect that was attenuated after GR Vivo-Morpholino injection.
Figure 7.
Infusion of GR Vivo-Morpholino sequences attenuates bile acid-induced suppression of the HPA axis. A, GR immunoreactivity (red) was assessed in the hypothalamus of rats infused ICV with GR Vivo-Morpholino or GR mismatched sequences (1 mg/kg · d for 3 d) by immunofluorescence microscopy. Tissue was counterstained with DAPI (blue) as a nuclear marker. Scale bar, 25 μm. B, GR protein expression was assessed in hypothalamic extracts from rats infused ICV with GR Vivo-Morpholino or GR mismatched sequences (1 mg/kg · d for 3 d). β-Actin was used as a loading control. C, Relative CRH mRNA expression was assessed in the hypothalamus of rats infused with GR Vivo-Morpholino or GR mismatched sequences and subsequently injected ICV with 20 pmol of GCDA (gray bars) or control (black bars). D, Relative fold change of CRH protein expression in the serum of rats ICV infused with GR Vivo-Morpholino or GR mismatched sequences and subsequently injected ICV with the bile acid GCDA (gray bars) or control (black bars). E, Corticosterone concentrations in the serum of rats infused with GR Vivo-Morpholino or GR mismatched sequences and subsequently injected with GCDA or control ICV. Where appropriate, *, P < .05 compared with vehicle-injected rats; **, P < .05 compared with GR mismatched infused rats (n = 6).
Discussion
The major findings of this study pertain to the effects of bile acids on the hypothalamus during the course of cholestatic liver injury. A suppression of the HPA axis has previously been demonstrated in models of cholestatic liver injury; the data presented here focus on the mechanism of this suppression. Specifically, we demonstrate that the bile acids TCA and GCDA, the major bile acids in humans, are able to modulate the HPA axis by gaining entry into hypothalamic neurons through ASBT, binding to and activating the GR and suppressing the expression of the HPA axis modulator CRH. This study identifies bile acids act as HPA axis regulators in the hypothalamus.
We have previously demonstrated that the HPA axis is suppressed at every level during the course of cholestasis and that this suppression contributes to biliary proliferation (6). CRH expression was decreased in the hypothalamus, which in turn had an inhibitory effect on ACTH expression in the pituitary, leading to decreased expression of the key steroidogenic enzymes cytochrome p450 11b1 and hydroxysteroid dehydrogenase-3β in the adrenal glands, resulting in decreased circulating glucocorticoids (6). These findings have also been supported by other studies in rodent models of cholestasis (7, 8, 39, 40). As stated above, the link between cholestasis and the HPA axis has been demonstrated clinically, with cholestatic patients often exhibiting clinical features suggestive of adrenal insufficiency such as hypovolemia, hypotension, and renal failure (9, 10). Furthermore, patients with congenital hypopituitarism or glucocorticoid deficiency often exhibit cholestatic hepatitis (11–13). In cirrhosis, adrenal insufficiency is associated with increased mortality and hemodynamic impairment (14, 15). Treatment with low doses of hydrocortisone to cirrhotic patients resolves the hemodynamic impairment and is associated with a higher survival rate (16).
Because we demonstrated that the HPA axis was suppressed at the level of the hypothalamus, we focused our attention to this brain region as the predominant controller of the HPA axis in our model of cholestatic liver injury. A negative feedback loop regulating the HPA axis exists, in which activation of the GR in the hypothalamus leads to the inhibition of the CRH response in 2 phases. The first phase, or fast response, occurs when GR activation inhibits the release of CRH from the hypothalamus, whereas the delayed response involves the inhibition of CRH synthesis and expression (5). We have previously demonstrated that both the expression and release of CRH from the hypothalamus is impaired after BDL (6) and the data presented clearly demonstrate that the bile acids TCA and GCDA may also suppress both the release and synthesis of CRH in vitro and in vivo.
In order for bile acids to modulate the HPA axis at the level of the hypothalamus, they must first be able to cross the blood brain barrier and gain entry to the brain. We have previously demonstrated that there is increased blood brain barrier permeability in the BDL model of cholestasis and that this leakiness allows for an increased influx of radiolabeled TCA to the hypothalamic region (29). We have also previously demonstrated that increased bile acids can be found in other models of liver damage, specifically in a model of drug-induced acute liver failure (41).
Most signaling effects of bile acids are exerted from within the cell. Therefore, once bile acids have gained access to the brain, they must either diffuse or be transported across the cell membrane. Here, we demonstrate that it is predominantly the conjugated bile acids studied that had an effect on CRH expression in vivo. Bile acids can be transported into a cell by the bile acid transporter ASBT, among others (17). Here, we demonstrate the novel finding that ASBT expression in the brain appears to be localized to very discrete neurons in the hypothalamus. These areas are responsible for the production and secretion of many neuroendocrine peptides such as CRH and neuropeptide Y (42). Furthermore, we demonstrated that suppressing ASBT expression in vitro and in vivo attenuated the effects of TCA and GCDA on CRH expression and release in the hypothalamus.
Once inside the cell, bile acids have been shown to exert their effects predominantly via the interaction with nuclear receptors such as the farnesoid X receptor (19), pregnane X receptor (20), and vitamin D receptor (20). Recently, the bile acid, ursodeoxycholic acid has been shown to activate the GR, although the binding efficiency of other bile acids has not yet been studied (21). Here, we present data indicating that TCA and GCDA may also bind to and weakly activate the GR.
To our knowledge, these data represent the first demonstration of a role for bile acids in the central regulation of the HPA axis. Furthermore, the machinery to uptake and respond to bile acids are expressed in nuclei of the hypothalamus. Taken together with previous studies, our data support the hypothesis that during cholestatic liver injury, bile acids are gaining entry into the brain, at least in part through a leaky blood brain barrier, are transported into neurons through ASBT and can activate the GR to bring about a suppression of the HPA axis. These data also lend themselves to the broader hypothesis that bile acids may be capable of acting as central modulators of hypothalamic peptides that may be altered during the course of liver diseases.
Acknowledgments
We thank Stephanie Henderson, Cheryl Galindo, Rachel Petrofes-Chapa, and Carla Khalaf for technical assistance on this project. This material is the result of work supported with resources and the use of facilities at the Central Texas Veterans Health Care System, Temple, TX. The contents do not represent the views of the United States Department of Veterans Affairs of the United States Government.
This work was supported by a National Institutes of Health R01 Award DK082435, a National Institutes of Health K01 Award DK078532, a United States Department of Veterans Affairs Merit Award BX002638-01 from the United States Department of Veterans Affairs Biomedical Laboratory Research and Development Service (to S.D.) and by a Texas A&M Health Science Center College of Medicine summer research program scholarship (to N.P.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ACTH
- adrenocorticotropic hormone
- ASBT
- apical sodium-dependent bile acid transporter
- BDL
- bile duct ligation
- CA
- cholic acid
- CDCA
- chenodeoxycholic acid
- CLF
- cholyl-lysyl-fluorescein
- CRH
- corticotropin releasing hormone
- DAPI
- 4′,6-diamidino-2-phenylindole
- DCA
- deoxycholic acid
- EIA
- enzyme immunoassay
- GCDA
- glycochenodeoxycholic acid
- GR
- glucocorticoid receptor
- GRE
- glucocorticoid response element
- HPA
- hypothalamic-pituitary-adrenal
- ICV
- intracerebroventricular
- RU486
- 11β-(4-Dimethylamino)phenyl-17β-hydroxy-17-(1-propynyl)estra-4,9-dien-3-one
- shRNA
- short hairpin RNA
- TCA
- taurocholic acid.
References
- 1. Jacobson L. Hypothalamic-pituitary-adrenocortical axis regulation. Endocrinol Metab Clin North Am. 2005;34:271–292, vii. [DOI] [PubMed] [Google Scholar]
- 2. Leonard BE. HPA and immune axes in stress: involvement of the serotonergic system. Neuroimmunomodulation. 2006;13:268–276. [DOI] [PubMed] [Google Scholar]
- 3. Koslo RJ, Gmerek DE, Cowan A, Porreca F. Intrathecal bombesin-induced inhibition of gastrointestinal transit: requirement for an intact pituitary-adrenal axis. Regul Pept. 1986;14:237–242. [DOI] [PubMed] [Google Scholar]
- 4. Solomon S. POMC-derived peptides and their biological action. Ann NY Acad Sci. 1999;885:22–40. [DOI] [PubMed] [Google Scholar]
- 5. Jones MT, Hillhouse EW, Burden JL. Dynamics and mechanics of corticosteroid feedback at the hypothalamus and anterior pituitary gland. J Endocrinol. 1977;73:405–417. [DOI] [PubMed] [Google Scholar]
- 6. Quinn M, Ueno Y, Pae HY, et al. Suppression of the HPA axis during extrahepatic biliary obstruction induces cholangiocyte proliferation in the rat. Am J Physiol Gastrointest Liver Physiol. 2012;302:G182–G193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Swain MG, Maric M. Defective corticotropin-releasing hormone mediated neuroendocrine and behavioral responses in cholestatic rats: implications for cholestatic liver disease-related sickness behaviors. Hepatology. 1995;22:1560–1564. [PubMed] [Google Scholar]
- 8. Swain MG, Patchev V, Vergalla J, Chrousos G, Jones EA. Suppression of hypothalamic-pituitary-adrenal axis responsiveness to stress in a rat model of acute cholestasis. J Clin Invest. 1993;91:1903–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Williams RD, Elliott DW, Zollinger RM. The effect of hypotension in obstructive jaundice. Arch Surg. 1960;81:334–340. [DOI] [PubMed] [Google Scholar]
- 10. Zollinger RM, Williams RD. Surgical aspects of jaundice. Surgery. 1956;39:1016–1030. [PubMed] [Google Scholar]
- 11. Binder G, Martin DD, Kanther I, Schwarze CP, Ranke MB. The course of neonatal cholestasis in congenital combined pituitary hormone deficiency. J Pediatr Endocrinol Metab. 2007;20:695–702. [DOI] [PubMed] [Google Scholar]
- 12. Gönç EN, Kandemir N, Andiran N, Ozön A, Yordam N. Cholestatic hepatitis as a result of severe cortisol deficiency in early infancy: report of two cases and review of literature. Turk J Pediatr. 2006;48:376–379. [PubMed] [Google Scholar]
- 13. Karnsakul W, Sawathiparnich P, Nimkarn S, Likitmaskul S, Santiprabhob J, Aanpreung P. Anterior pituitary hormone effects on hepatic functions in infants with congenital hypopituitarism. Ann Hepatol. 2007;6:97–103. [PubMed] [Google Scholar]
- 14. Harry R, Auzinger G, Wendon J. The clinical importance of adrenal insufficiency in acute hepatic dysfunction. Hepatology. 2002;36:395–402. [DOI] [PubMed] [Google Scholar]
- 15. Tsai MH, Peng YS, Chen YC, et al. Adrenal insufficiency in patients with cirrhosis, severe sepsis and septic shock. Hepatology. 2006;43:673–681. [DOI] [PubMed] [Google Scholar]
- 16. Fernández J, Escorsell A, Zabalza M, et al. Adrenal insufficiency in patients with cirrhosis and septic shock: effect of treatment with hydrocortisone on survival. Hepatology. 2006;44:1288–1295. [DOI] [PubMed] [Google Scholar]
- 17. St-Pierre MV, Kullak-Ublick GA, Hagenbuch B, Meier PJ. Transport of bile acids in hepatic and non-hepatic tissues. J Exp Biol. 2001;204:1673–1686. [DOI] [PubMed] [Google Scholar]
- 18. Dawson PA. Role of the intestinal bile acid transporters in bile acid and drug disposition. Handb Exp Pharmacol. 2011:169–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Redinger RN. The role of the enterohepatic circulation of bile salts and nuclear hormone receptors in the regulation of cholesterol homeostasis: bile salts as ligands for nuclear hormone receptors. Can J Gastroenterol. 2003;17:265–271. [DOI] [PubMed] [Google Scholar]
- 20. Zhou H, Hylemon PB. Bile acids are nutrient signaling hormones. Steroids. 2014;86:62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Miura T, Ouchida R, Yoshikawa N, et al. Functional modulation of the glucocorticoid receptor and suppression of NF-κB-dependent transcription by ursodeoxycholic acid. J Biol Chem. 2001;276:47371–47378. [DOI] [PubMed] [Google Scholar]
- 22. Hofmann AF. The continuing importance of bile acids in liver and intestinal disease. Arch Intern Med. 1999;159:2647–2658. [DOI] [PubMed] [Google Scholar]
- 23. Greim H, Trülzsch D, Czygan P, et al. Mechanism of cholestasis. 6. Bile acids in human livers with or without biliary obstruction. Gastroenterology. 1972;63:846–850. [PubMed] [Google Scholar]
- 24. Greim H, Trülzsch D, Roboz J, et al. Mechanism of cholestasis. 5. Bile acids in normal rat livers and in those after bile duct ligation. Gastroenterology. 1972;63:837–845. [PubMed] [Google Scholar]
- 25. Muriel P, Castro V. Dose-response studies of interferon-α 2b on liver fibrosis and cholestasis induced by biliary obstruction in rats. Pharmacology. 1997;54:179–185. [DOI] [PubMed] [Google Scholar]
- 26. Raiford DS. Pruritus of chronic cholestasis. QJM. 1995;88:603–607. [PubMed] [Google Scholar]
- 27. Ma Z, Lee SS, Meddings JB. Effects of altered cardiac membrane fluidity on β-adrenergic receptor signalling in rats with cirrhotic cardiomyopathy. J Hepatol. 1997;26:904–912. [DOI] [PubMed] [Google Scholar]
- 28. Bomzon A, Ljubuncic P. Bile acids as endogenous vasodilators? Biochem Pharmacol. 1995;49:581–589. [DOI] [PubMed] [Google Scholar]
- 29. Quinn M, McMillin M, Galindo C, Frampton G, Pae HY, DeMorrow S. Bile acids permeabilize the blood brain barrier after bile duct ligation in rats via Rac1-dependent mechanisms. Dig Liver Dis. 2014;46:527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shneider BL, Dawson PA, Christie DM, Hardikar W, Wong MH, Suchy FJ. Cloning and molecular characterization of the ontogeny of a rat ileal sodium-dependent bile acid transporter. J Clin Invest. 1995;95:745–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dickmeis T. Glucocorticoids and the circadian clock. J Endocrinol. 2009;200:3–22. [DOI] [PubMed] [Google Scholar]
- 32. McMillin M, Frampton G, Tobin R, et al. TGR5 signaling reduces neuroinflammation during hepatic encephalopathy. J Neurochem. 2015; doi:10.1111/jnc.13243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McMillin M, Galindo C, Pae HY, et al. Gli1 activation and protection against hepatic encephalopathy is suppressed by circulating transforming growth factor β1 in mice. J Hepatol. 2014;61:1260–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. DeMorrow S, Francis H, Gaudio E, et al. The endocannabinoid anandamide inhibits cholangiocarcinoma growth via activation of the noncanonical Wnt signaling pathway. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1150–G1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. DeMorrow S, Glaser S, Francis H, et al. Opposing actions of endocannabinoids on cholangiocarcinoma growth: recruitment of fas and fas ligand to lipid rafts. J Biol Chem. 2007;282:13098–13113. [DOI] [PubMed] [Google Scholar]
- 36. DeMorrow S, Francis H, Gaudio E, et al. Anandamide inhibits cholangiocyte hyperplastic proliferation via activation of thioredoxin 1/redox factor 1 and AP-1 activation. Am J Physiol Gastrointest Liver Physiol. 2008;294:G506–G519. [DOI] [PubMed] [Google Scholar]
- 37. Mills CO, Rahman K, Coleman R, Elias E. Cholyl-lysylfluorescein: synthesis, biliary excretion in vivo and during single-pass perfusion of isolated perfused rat liver. Biochim Biophys Acta. 1991;1115:151–156. [DOI] [PubMed] [Google Scholar]
- 38. de Waart DR, Häusler S, Vlaming ML, et al. Hepatic transport mechanisms of cholyl-L-lysyl-fluorescein. J Pharmacol Exp Ther. 2010;334:78–86. [DOI] [PubMed] [Google Scholar]
- 39. McNeilly AD, Macfarlane DP, O'Flaherty E, et al. Bile acids modulate glucocorticoid metabolism and the hypothalamic-pituitary-adrenal axis in obstructive jaundice. J Hepatol. 2010;52:705–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Swain MG, Maric M, Carter L. Defective interleukin-1-induced ACTH release in cholestatic rats: impaired hypothalamic PGE2 release. Am J Physiol. 1995;268:G404–G409. [DOI] [PubMed] [Google Scholar]
- 41. Ashfaq S, McMillin M, Galindo C, Frampton G, DeMorrow S. Elevated serum bile acids contribute to the neurological impairment following acute liver failure. Gastroenterology. 2014;146:S953–S954. [Google Scholar]
- 42. Williams G, Harrold JA, Cutler DJ. The hypothalamus and the regulation of energy homeostasis: lifting the lid on a black box. Proc Nutr Soc. 2000;59:385–396. [DOI] [PubMed] [Google Scholar]