Abstract
Androgen receptor (AR) plays a pivotal role in the development of primary as well as advanced castration-resistant prostate cancer. Previous work in our lab identified a novel nuclear export signal (NES) (NESAR) in AR ligand-binding domain essential for AR nucleocytoplasmic trafficking. By characterizing the localization of green fluorescence protein (GFP)-tagged NESAR, we designed and executed a yeast mutagenesis screen and isolated 7 yeast mutants that failed to display the NESAR export function. One of those mutants was identified as the splicing factor pre-mRNA processing factor 8 (Prp8). We further showed that Prp8 could regulate NESAR function using short hairpin RNA knockdown of Prp8 coupled with a rapamycin export assay in mammalian cells and knockdown of Prp8 could induce nuclear accumulation of GFP-tagged AR in PC3 cells. Prp8 expression was decreased in castration-resistant LuCaP35 xenograft tumors as compared with androgen-sensitive xenografts. Laser capture microdissection and quantitative PCR showed Prp8 mRNA levels were decreased in human prostate cancer specimens with high Gleason scores. In prostate cancer cells, coimmunoprecipitation and deletion mutagenesis revealed a physical interaction between Prp8 and AR mainly mediated by NESAR. Luciferase assay with prostate specific antigen promoter-driven reporter demonstrated that Prp8 regulated AR transcription activity in prostate cancer cells. Interestingly, Prp8 knockdown also increased polyubiquitination of endogenous AR. This may be 1 possible mechanism by which it modulates AR activity. These results show that Prp8 is a novel AR cofactor that interacts with NESAR and regulates AR function in prostate cancer cells.
Prostate cancer is the most common cancer and second leading cause of cancer death among men in United States. As a steroid nuclear transcription factor, androgen receptor (AR) regulates the expression of a set of androgen target genes and plays a pivotal role in the development of primary as well as advanced castration-resistant prostate cancer. Androgen deprivation therapy (ADT) has been the standard treatment for advanced prostate cancer since the 1940s. Although ADT is initially effective to control prostate cancer growth by suppression of AR activity, in most patients, these therapies eventually fail and the cancer progresses to be castration resistant (1, 2). In most castration-resistant prostate cancer patients, AR signaling is reactivated or hyperactivated despite the presence of castration levels of androgens. Overexpression of coactivators or loss of corepressors are considered as one of the mechanisms leading to AR reactivation after ADT (3).
In humans, full-length AR is composed of 919 amino acids (a.a.) and is encoded by 8 exons located at the chromosome Xq11–12 (4). Exon 1 encodes the entire NH2-terminal domain (a.a. 1–556), which represents 60% of the AR protein and contains the activation function 1. Exon 2 and 3 encode the 2 zinc fingers in the AR DNA-binding domain (a.a. 556–624), which is responsible for binding to the androgen-responsive element sites in DNA. Exon 4–8 encode the short flexible hinge region and ligand-binding domain (LBD) (a.a. 625–919), which is capable of binding to ligands and harbors activation function 2 (5–7). AR is a ligand-dependent transcription factor and undergoes nuclear translocation in response to testosterone or 5α-dihydrotestosterone. In the absence of androgen, AR becomes inactive as it is exported from nucleus to the cytoplasm (8, 9). Intracellular trafficking is an important step regulating AR activity because nuclear localization is a prerequisite for AR to modulate transcription of AR-target genes and it is regulated by nuclear import (nuclear localization signal [NLS]) and nuclear export signals (NESs) (10).
A novel NES (NESAR) (a.a. 742–817) located in the LBD of AR was identified in 2003 and, subsequently, was found to play a key role in modulating AR polyubiquitination and proteasomal degradation (11, 12). As shown in our previous report, NESAR acts to mediate AR nuclear export in the absence of androgen and is repressed upon androgen binding (12). NESAR nuclear export does not occur via the classical chromosomal maintenance 1 (CRM1)/exportin nuclear export pathway. NESAR neither has the required leucine-rich NES nor is it sensitive to leptomycin B inhibition. Thus, the mechanism of NESAR action is most likely different from that of the classical NES and needs to be determined.
mRNA transcription and processing events are coordinately regulated both temporally and spatially within the nucleus, and splicing factors can bind directly to the C-terminal domain of RNA polymerase II and transcription elongation factors (13). Multiple RNA splicing factors, including the polypyrimidine tract-binding protein-associated splicing factor (PSF), 54 kDa nuclear RNA- and DNA-binding protein (p54nrb), Src associated in mitosis, of 68 kDa (Sam68), and p68 RNA helicase, have been identified as AR cofactors that regulate AR transcriptional activity in prostate cancer (14–17). Pre-mRNA processing factor 8 (Prp8), at the “heart” of the U5 small nuclear ribonucleoprotein (snRNP) particles, can recognize and cross-link to the 5′splice site, the branch point and the 3′splice site in the pre-mRNA as a cofactor in RNA catalysis (18). Prp8 is a highly conserved protein, with human and yeast Prp8 sharing 61% sequence identity but having remarkably low-sequence similarity with other proteins (19). Numerous mutations residing in the C terminus of Prp8 have been found in people with retinitis pigmentosa, a dominantly inherited retinal degeneration disorder (20). U5 snRNP mutations can cause reduced Prp8 stability and result in a genome-wide splicing defect (21). Apoptosis regulated protein 2 (ARP2), a Prp8 fragment protein, has been found to be overexpressed in LNCaP cells upon apoptosis induction, and its overexpression is capable of inducing typical apoptotic morphology changes in oocytes (22).
In this study, splicing factor Prp8 was identified as a regulator of NESAR in the yeast mutagenesis screen. We further found that Prp8 expression was decreased in castration-resistant tumors when compared with androgen-sensitive tumors in the LuCaP35 xenograft tumor model, and laser-capture microdissection and quantitative PCR showed decreased Prp8 mRNA levels in advanced human prostate cancer specimens. Further exploration of the relationship between Prp8 and AR signaling in prostate cancer cells suggested that Prp8 is a novel AR cofactor involved in prostate cancer.
Materials and Methods
Yeast strains, handling, transformations, and localization
For localization studies, BY4741 obtained from the American Type Culture Collection (ATCC) were grown in YPD or Synthetic Defined (SD) media (Clontech) supplemented with appropriate amino acids at 30°C unless otherwise noted. Yeast was transformed by the lithium acetate method (23). For mutagenesis, BY4741 yeast cells were cultured overnight, washed twice with phosphate buffer, and resuspended in sodium phosphate buffer at 2 × 108 cells/mL. Yeast cells were treated with 3% ethyl methanesulfonate at 30°C and subsequently neutralized by washing with 5% sodium thiosulphate. For Hoechst staining of yeast cells, Hoechst 33342 (Molecular Probes) was added to a final concentration of 250nM 45 minutes before visualization. For visualization, 2 μL of yeast culture were spotted on a slide, cover slipped, and protein localization was assessed by fluorescence microscopy with 100× oil immersion lens on the Nikon TE2000 inverted microscope using Metamorph software.
Yeast mating, sporulation, and tetrad analysis
Mutant haploid strain BY4741 was crossed to wild-type haploid BY4741 strain to form diploids. Diploids were then cultured in YPD medium overnight. Diploids were then sporulated by incubating in sporulation medium (SPM) medium. Spores were dissected by micromanipulation using fine glass needle on a YPD plate.
Constructs
All constructs expressed in yeast were cloned into p416 GDP (containing a URA3 marker) yeast expression vector backbone for constitutive expression of the fusion protein (Supplemental Figure 1). To generate the green fluorescence protein (GFP)-tagged simian virus 40 (SV40) NLS (2GFP-NLSSV40) reporter construct, the NLS from SV40 T-antigen (PKKKRKV) was cloned between 2 enhance GFP (EGFP) sequences. For 2GFP-NLSSV40-NESPKI, the NES from protein kinase inhibitor (PKI) (ELALKLAGLDIN) was cloned at the C terminus of the second EGFP sequence of 2GFP-NLSSV40 construct. For 2GFP-NLSSV40-NESAR, the NES from the AR (a.a. 743–817 from AR) (12) was cloned at the C terminus of second EGFP sequence of 2GFP-NLSSV40 construct.
A plasmid vector containing human Prp8 cDNA (NM_006445) was obtained from Origene (sc116070), and it was used as a PCR template to construct Prp8 expression vectors with different epitope-tags by routine molecular cloning techniques. The backbones of Flag-tagged Prp8 expression vector and pIRES2-EGFP Prp8 expression vector were obtained from Clontech. GFP-tagged wild-type AR and various truncated AR expression vectors were cloned as described in previous publications (12, 24). The prostate specific antigen (PSA) luciferase vector containing 6.1-kb PSA promoter region was a generous gift from Dr Marianne Sadar. TK promoter-driven green Renilla luciferase vector was obtained from Thermo Scientific.
Tissue acquisition and laser-capture microdissection
Human prostate cancer specimens were obtained from the Health Sciences Tissue Bank at the University of Pittsburgh Medical Center (UPMC) under approval by the UPMC Institutional Review Board following a standard protocol. All the specimens were from patients with prostate cancer with Gleason score 7 or above. The tissue process for laser-capture microdissection were described previously (25). Laser-capture microdissection was performed using the Leica LMD6000 (Leica Microsystems). Approximately 2000–5000 excised cells were captured into 0.5-mL Eppendorf tube caps. Captured individual tissue specimens were lysed, and RNA isolation, reverse transcription, and real-time PCR were performed using CellsDirect One-Step qRT-PCR kit (Invitrogen). Real-time PCR was performed on ABI Step-One Plus (Applied Biosystems). Real-time PCR data were analyzed by ΔCp (crossing point) method using R = 2[Cp sample −Cp control] to generate the relative expression ratio of Prp8 to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) .
Animals and xenograft tumors
Athymic SCID male mice were obtained from Harlan Labs and were kept in accordance with the National Institutes of Health guidelines under standard animal housing conditions for the care and use of experimental animals. Animal experiments were approved by the Institutional Animal Care Use Committee at the University of Pittsburgh.
LuCaP35 xenografts were maintained in athymic SCID mice through serial transplantation as described (26). To generate xenografts, LuCaP35 tumor bits were implanted in the flank region of mice. Tumors were measured weekly and volume was calculated by the modified ellipsoid formula: length × width2 × 0.52. When the xenograft tumors reached a volume of 200 mm3, transscrotal castration was performed under isoflurane anesthesia with proper aseptic and antiseptic technique as previously described (27). Tumors that shrank in size compared with their volume at the time of castration, within the first 14 days after castration, were considered as androgen-sensitive. Tumors that continued to grow within the 14-day period after castration were considered castration resistant. Castration-resistant tumor specimens were collected when their sizes reached a volume of 2000 mm3 or 45 days after castration. The collected tumors were flash frozen in liquid N2 at the time of collection and stored at −80°C for further use.
Cell culture and transfection
LNCaP cells were obtained from the ATCC. C4–2 cells were generous gift from Dr Leland W. K. Chung. Cells were maintained in RPMI 1640 medium supplied with 10% fetal bovine serum and 1% L-glutamine at 37°C with 5% CO2. For androgen treatment, cells were cultured in RPM1 1640 phenol red-free medium supplemented with 5% dextran-coated charcoal-stripped fetal bovine serum and 1% L-glutamine for 24 hours, and then treated with 1nM methyltrienolone (R1881; PerkinElmer) in above medium. Cell transfection was performed with PolyJet DNA In Vitro Tranfection Reagent (SignaGen Laboratories) according to manufacturer's protocols. Prp8 stable knockdown LNCaP and PC3 cell lines were generated by transduction of scramble short hairpin RNA (shRNA) or shRNA targeting human Prp8 delivered by lentiviral vectors prepared by the Vector Core Facility at University of Pittsburgh Cancer Institute. Puromycin was used to select the Prp8 knockdown cells. To knock down Prp8 by small interfering RNA (siRNA) in C4–2 and LNCaP cells, 50nM Prp8 siRNA (GCAGAUGGAUUGCAGUAUA) was transfected by DharmaFECT1 (T2001; GE Dharmacon) by following manufacturer's protocol.
Rapamycin-mediated nuclear export assay
This export assay was developed based on the study published by Klemm et al (28). In brief, scramble and Prp8 knockdown PC3 cells were cotransfected with red fluorescence protein (RFP)-tagged NLS and Tacrolimus (also FK506)-binding protein 12 (FKBP) (RFP-NLS-FKBP) and GFP-tagged FKBP12-rapamycin-binding domain (FRB) (GFP-FRB) or GFP-NESAR-FRB. Transfected cells were treated with ethanol (EtOH) or rapamycin (ligand; Ariad Pharmaceuticals) at 0.5μM for 30 minutes. Ligand was withdrawn 30 minutes after rapamycin treatment, and cells were cultured for an additional 2 or 18 hours. A total of 50 μg/mL of cycloheximide was added to inhibit protein synthesis in the 18-hour withdrawal experiment. The localization of GFP and RFP fusion proteins was observed by fluorescent microscopy. The experiment was reproduced 3 times.
Western blot analysis
Cultured cells were lysed in modified radioimmunoprecipitation assay buffer containing 10mM Tris-Cl (pH 8.0), 1mM EDTA, 1% Triton X-100, 0.1% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 140mM NaCl, and 1% protease inhibitor cocktail (Sigma-Aldrich). Protein concentration was measured with BCA. Western blotting was conducted using antibodies against GFP (TP401; Chemokine), Flag M2 (F1804; Sigma), AR (sc-816; Santa Cruz Biotechnology, Inc), PSA (sc-7638; Santa Cruz Biotechnology, Inc), Prp8 (MBS120210; MyBiosource), and γ-tubulin (sc-17787; Santa Cruz Biotechnology, Inc), followed by horseradish peroxidase-labeled secondary antibody (sc-2004, sc-2003, and sc-2771; Santa Cruz Biotechnology, Inc). Signals were visualized using chemiluminescence (ECL Western Blotting Detection Reagents; GE Healthcare) and were exposed to x-ray film (Fuji film).
Immunoprecipitation
Immunoprecipitation experiments were performed using similar methods as described previously (29). Briefly, to detect the interaction between Prp8 and AR, cells were lysed with Tergitol-type NP-40 (NP-40) lysis buffer (50mM Tris-HCl, 150mM NaCl, and 1% NP-40; pH 8.0) containing 1% protease inhibitor cocktail, and cell lysates were centrifuged at 12 000g for 10 minutes at 4°C. The supernatants were collected for immunoprecipitation. After preclearing with normal host IgG, AR was immunoprecipitated from the lysates overnight at 4°C with 4-μg antibodies against AR, or normal rabbit IgG as control, followed by precipitation with 60-μL protein A/G Plus-Agarose (Santa Cruz Biotechnology, Inc) for 3 hours at 4°C. GFP-tagged proteins were immunoprecipitated with agarose conjugated with antibody against GFP (D153–8; MBL) 3 hours. The precipitated complexes were washed 3 times with lysis buffer and boiled for 5 minutes in SDS sample buffer, followed by immunoblotting with various antibodies as indicated. In the AR ubiquitination assay, cells were lysed with radioimmunoprecipitation assay buffer, and AR was immunoprecipitated overnight at 4°C with 2-μg antibodies against AR, followed by precipitation with 60-μL protein A/G Plus-Agarose for 3 hours at 4°C.
RT-PCR and real-time PCR
Total RNA from tumor tissues was extracted using the TRIzol RNA isolation system (Invitrogen). The first strand of cDNA was synthesized using 2 μg of RNA in 20 μL of reaction buffer by reverse transcription using AMV-RT (Promega) by following the suggested protocol. The quantitative real-time PCR was performed on ABI PRISM 7000 Sequence Detection System (Applied Biosystems) with 2× SYBR Green PCR Master Mix (Thermo Scientific). The mRNA levels of various genes were calculated after normalized with GAPDH. The primer sequences were as follows: AR, TGGATGGATAGCTACTCCGG and CCCAGAAGCTTCATCTCCAC; Prp8, PSA, AGGCCTTCCCTGTACACCAA and GTCTTGGCCTGGTCATTTCC; and GAPDH, CGACCACTTTGTCAAGCTCA and AGGGGAGATTCAGTGTGGTG.
Luciferase reporter assay
LNCaP cells were transfected with reporter construct PSA-Luc and TK-Renilla-Luc vectors as 10:1 ratio for 48 hours in complete medium. The supernatants of cell lysates were collected for the luciferase assay. Luciferase activity was determined using the Dual Luciferase Assay System kit as described by the manufacturer's protocols (Promega). Relative PSA firefly luciferase activity is normalized with Renilla luciferase activity.
Statistical analysis
Statistical analysis and graphical composition were produced by GraphPad Prism version 6.0 (GraphPad Software, Inc). Data are expressed as the mean ± SEM, and statistical significance was determined by nonpaired t test or Wilcoxon matched-pairs signed rank test as appropriate. P < .05 was considered statistically significant.
Results
Mutation of Prp8 inhibited NESAR function in yeast
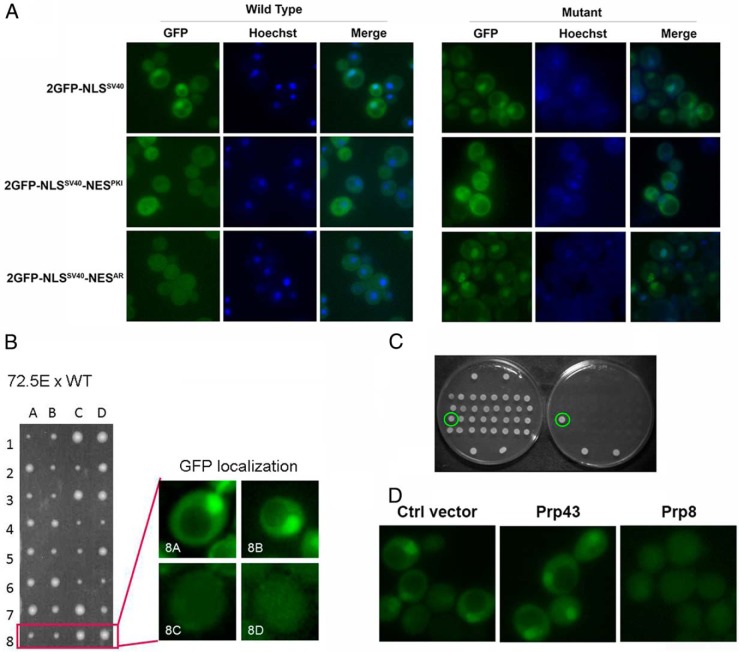
Previous work in our lab demonstrated that the NESAR was necessary and sufficient to result in cytoplasmic localization of AR in cells (12). Furthermore, we have found that NESAR was able to mediate nuclear export in yeast (30). As shown in Figure 1A, left panel, with the addition of the NESAR, GFP-NLSSV40-NESAR was evenly distributed, instead of predominantly in the nucleus as it is GFP-NLSSV40. We performed an ethyl methanesulfonate mutagenesis screen and identified yeast nuclear export mutants that displayed nuclear localization GFP-NLSSV40-NESAR without affecting the subcellular localization of GFP-NLSSV40 and GFP-NLSSV40-NESPKI controls (as shown in Figure 1A, right panel).
Figure 1.
A, All sequence were cloned into yeast p416 GPD expression vector containing a URA3 marker and GPD promoter for constitutive expression. Yeast strain BY4741 was transformed with the indicated constructs, and nuclei were stained with Hochest. The localization of GFP fusion proteins and Hochest staining were visualized by fluorescence microscopy. B, Tetrad analysis of mutant 72.5E. Mutant 72.5E was crossed to wild-type strain BY4741. The diploids were then sporulated, and the resultant haploid spores were separated by microdissection and grown at 30°C for 3 days. Resultant spores showed 2:2 segregation of colony size and GFP nuclear localization. Small colony phenotype segregated with nuclear GFP localization. C, Rescue screen to identify mutated gene. Mutant yeast cells were transformed with wild-type library. Individually transformed cells grown at permissive temperature (30°C, left plate) or restrictive temperature (39°C, right plate). D, The localizations of GFP fusion protein were only rescued by the transformation of wild-type Prp8 vector, but not by wild-type Prp43 or control vector, shown by fluorescence microscopy.
Over 6000 individual clones were screened using fluorescence microscopy and 7 clones displaying strong nuclear localization of GFP-NLSSV40-NESAR fusion protein were isolated. We further performed yeast tetrad analysis on one of the yeast mutants to determine whether the observed phenotype was a result of a single mutation. As shown in Figure 1B, the tetrad spores displayed a 2:2 segregation ratio of the nuclear phenotype, suggesting that the localization phenotype was a result of a mutation in a single gene. Additionally, the spores also displayed 2:2 segregation in relative size (2 small:2 big), suggesting that the mutants also have a growth defect at 30°C, the permissive temperature for yeast growth. When these cells were placed at restrictive temperature (39°C), they failed to grow (data not shown). Furthermore, the small colony size phenotype segregated with the nuclear localization phenotype, suggesting that a mutation in this single gene affected both localization and growth.
To identify the mutated gene, the mutant strain was transformed with a yeast low copy centromeric library (77162; ATCC), and we screened for transformed yeast that could rescue the growth defect. The colonies that were able to grow at restrictive temperature (39°C) after the transformation were assessed for rescue of the GFP localization phenotype (Figure 1C). Library plasmids were recovered from yeast cells and sequenced to identify the genes on the plasmid. In this manner, the mutant was identified as splicing factor Prp8 with single point mutation G5314A, resulting in a change of Gly to Ser at a.a. 1772. To confirm that Prp8 was the gene responsible for the mutant phenotypes, we transformed the mutant strain with a wild-type copy of Prp8. Prp8, but not control vector or Prp43 expression vector, was able to rescue the GFP localization phenotype and the growth defect (Figure 1D).
Prp8 knockdown inhibits NESAR nuclear export in prostate cancer cells
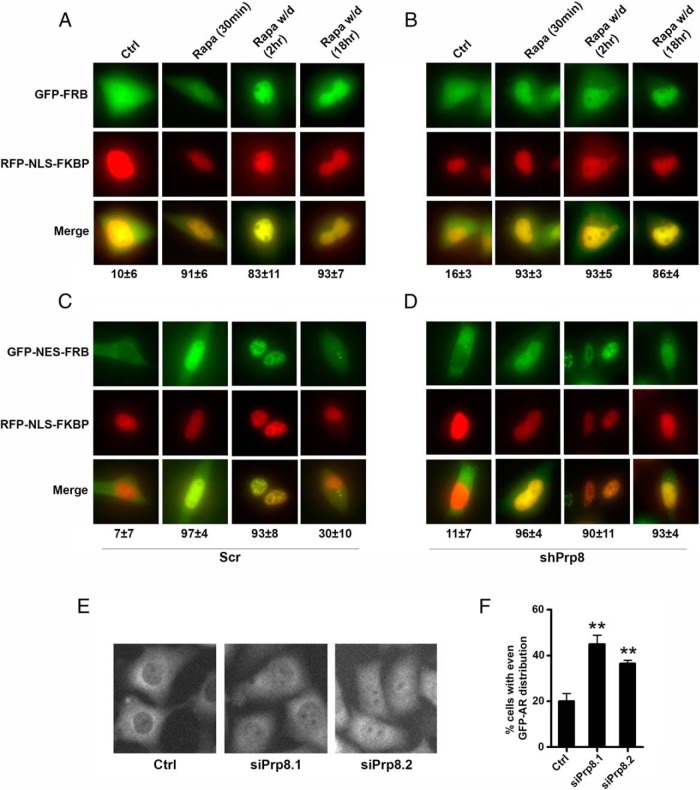
To confirm that Prp8 plays a role in NESAR-mediated nuclear export, we tested the ability of Prp8 knockdown to inhibit NESAR nuclear export. We used the shRNA carried by lentiviral vector to generate the Prp8 knockdown PC3 cells. These cells were then subjected to the modified rapamycin export assay based on a study by Klemm et al (28). GFP-NESAR-FRB or GFP-FRB expression vector was cotransfected with RFP-NLS-FKBP in the Prp8 knockdown cells. The presence of NESAR in GFP-NESAR-FRB facilitates the export of GFP-tagged protein from the nucleus to the cytoplasm. Thus, in the absence of rapamycin, GFP-NESAR-FRB is predominantly localized in the cytoplasm, whereas the control GFP-FRB is evenly distributed (Figure 2, A–D). Upon the addition of rapamycin, both GFP-NESAR-FRB and GFP-FRB accumulated in the nuclei by binding to GFP-NLS-FKBP through AR21967. In the scramble cells, when rapamycin is withdrawn, GFP-NESAR-FRB, but not GFP-FRB, is redistributed to the cytoplasm within 18 hours. However, in the Prp8 knockdown cells, 90% of the cells displayed nuclear localization of GFP-NESAR-FRB signal, which was significantly higher than that seen in PC3 scramble control cells (30%) (Figure 2, A–D).
Figure 2.
Prp8 knockdown inhibited NESAR nuclear export in rapamycin assay. Prp8 was stably knockdown in PC3 cells by shRNA (C and D), and control cells were transfected with scramble shRNA (A and B). Cells were cotransfected with RFP-NLS-FKBP and GFP-FRB (A and C), or cells were cotransfected with RFP-NLS-FKBP and GFP-NESAR-FRB (B and D). Transfected cells were treated with EtOH (control) or rapamycin at 0.5μM for 30 minutes (Ligand). Ligand was withdrawn (W/d) 30 minutes after rapamycin treatment, and cells were cultured for an additional 2 or 18 hours. Cycloheximide (CHX) at 50 μg/mL was added to inhibit protein synthesis in18-hour W/d experiment. The localization of GFP and RFP fusion proteins was observed by fluorescent microscopy. The % of transfected cells exhibiting predominant nuclear localization of GFP-FRB or GFP-NESAR-FRB is indicated. The experiment was reproduced 3 times. E, Localization of GFP-AR in PC3 cells transfected with control siRNA or 2 different siRNAs targeting Prp8. F, Quantitative result shows the percentage of cells with evenly distributed GFP-AR in the cytoplasm and nuclei of PC3 cells.
The localization of GFP-AR was also examined in prostate cancer PC3 cells with Prp8 knockdown. In the control cells, GFP-AR is predominantly localized in the cytoplasm in 80% cells, and 20% cells display evenly distributed GFP-AR. However, in cells with Prp8 knockdown using different siRNAs, 35%–45% cells display evenly distributed GFP-AR, and the difference is statistically significant by comparing with the control cells (Figure 2, E and F). Together, these results suggest that Prp8 plays an important role in NESAR-mediated nuclear export in mammalian cells.
Prp8 expression is down-regulated in castration-resistant xenograft tumors and advanced prostate cancer specimens
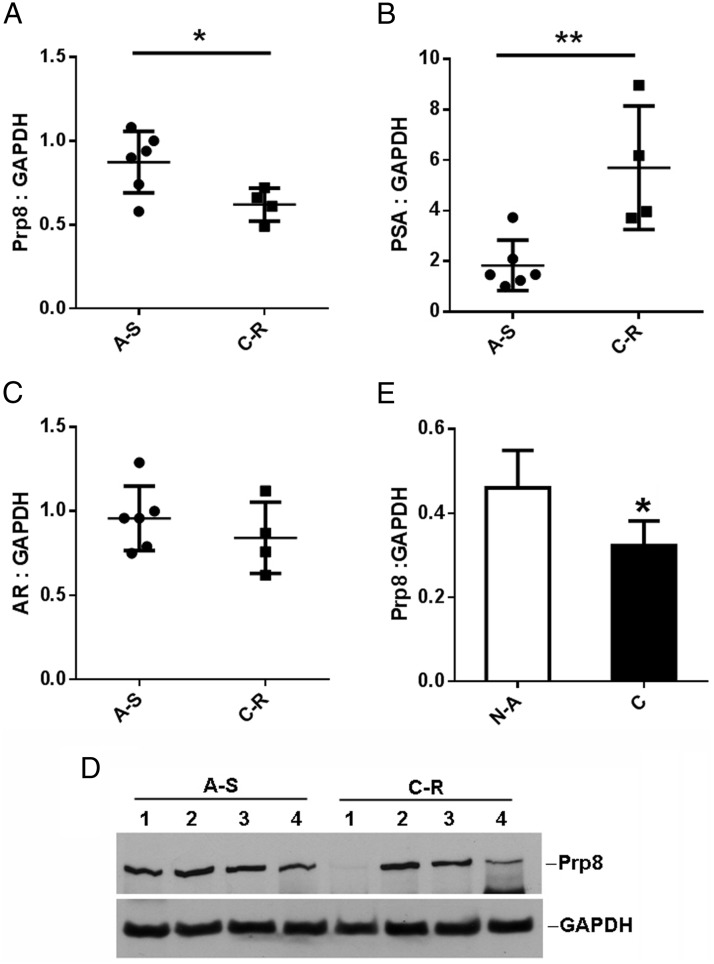
After revealing that splicing factor Prp8 is able to modulate NESAR nuclear export activity in the yeast and mammalian cells, we further examined whether there is any correlation of Prp8 expression level with prostate cancer progression in xenograft tumors and human prostate cancer specimens. LuCaP35 is an androgen-sensitive and PSA-positive prostate cancer xenograft model. LuCaP35 xenograft tumors were established as described in materials and methods in SCID male mice. When the tumors reached to 200 mm3, transscrotal castration was performed. Tumors which responded to castration by decreasing tumor volume significantly compared with intact control animals by week 2 were considered androgen sensitive, whereas those that continued to grow after castration were considered castration resistant (31). To compare Prp8 expression levels, xenograft tumors were collected for quantitative real-time PCR. As shown in Figure 3A, Prp8 mRNA levels were significantly decreased in castration-resistant tumors compared with androgen-sensitive tumors (P < .05). Furthermore, PSA mRNA levels in castration-resistant xenograft tumors were also significantly increased (P < .01). AR expression level in the castration-resistant xenograft tumors did not change as compared with androgen-sensitive xenograft tumors. This indicates that AR hyperactivity in castration-resistant tumors leads to dramatically elevated PSA expression (Figure 3, B and C). Prp8 protein level was examined and compared between 4 androgen-sensitive xenografts and 4 castration-resistant xenografts. The Western blottings showed that Prp8 protein level was decreased in 2 of the 4 castration-resistant xenograft tumors (Figure 3D).
Figure 3.
Prp8 expression is decreased in castration-resistant LuCaP35 xenograft tumors and advanced PCa specimens. Prp8 (A), PSA (B), and AR (C) expression levels in LuCaP35 xenograft tumors were examined by quantitative real-time PCR. D, Prp8 expression levels in LuCaP35 xenograft tumors were examined by Western blottings. A-S, androgen-sensitive tumors showed response to castration by decreased volume; C-R, castration-resistant tumors continued growing after castration and were further transplanted to new castrated mice. E, Human prostate tissues with Gleason score 7+ were acquired (n = 14). Epithelial cells were laser-capture microdissected and used for real-time PCR analysis. Prp8 mRNA levels were quantified and normalized to GAPDH. All data represent matched pairs of cancer (C) and normal adjacent tissue (NA). Wilcoxian rank sum test was performed. *, P < .05.
By searching the Oncomine microarray database, we have found significant down-regulation of Prp8 in prostate cancer when compared with normal prostate in 13 publicly available arrays (32). Also, we have examined Prp8 expression in the published study of MSKCC Prostate Oncogenome Project, which assessed 218 prostate cancer tumors, including 181 primary, 37 metastatic prostate cancer samples, 12 prostate cancer cell lines, and xenografts. Prp8 mRNA was down-regulated in 27% (10 of 37) of metastatic prostate cancer samples and 20% (31 of 157) of all tumor samples by comparing with 149 matched normal tissues obtained from patients treated by radical prostatectomy (33, 34). We also examined Prp8 expression in 14 prostate cancer patient samples with Gleason score 7–10 by comparing them with normal adjacent prostate tissues. Laser capture microdissection coupled with quantitative real-time PCR was performed. As shown in Figure 3E, Prp8 mRNA expression decreased by 30% (P < .05) in prostate cancer specimens compared with normal adjacent specimens. The findings in both a xenograft model and human prostate cancer specimens indicates that the down-regulation of Prp8 expression is associated with prostate cancer progression.
Prp8 interacts with AR
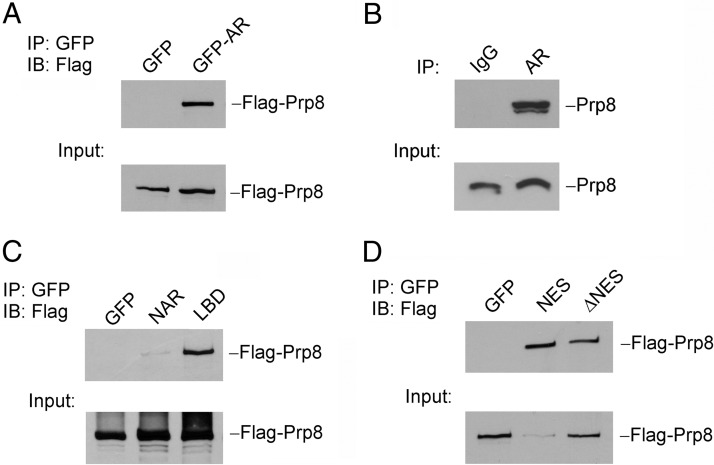
We performed a coimmunoprecipitation assay to determine whether Prp8 physically interacted with AR in prostate cancer cell lines. LNCaP cells were transfected with GFP-tagged AR and Flag-tagged Prp8 expression vectors in complete medium. GFP and Flag-tagged Prp8 expression vectors were transfected as controls. As shown in Figure 4A, Flag-tagged Prp8 was detected in the cell lysates precipitated with GFP antibody-conjugated agarose beads in GFP-tagged AR overexpression sample, which indicates the physical interaction between AR and Prp8. We extended this finding by detection of endogenous interaction between AR and Prp8 using coimmunoprecipitation with anti-AR antibody in LNCaP cells, as shown in Figure 4B.
Figure 4.
Prp8 physically interacts with AR in prostate cancer cells. A, LNCaP cells were cotransfected with Flag-PRP8 and GFP or GFP-AR. Immunoprecipitation was performed with agarose beads conjugated with anti-GFP antibody. Western blottings were performed with anti-Flag antibody. B, LNCaP cell lysates were incubated with anti-AR antibody or normal rabbit IgG overnight followed with incubation with agarose beads for 3 hours. C, LNCaP cells were cotransfected with Flag-Prp8 with GFP, GFP-NAR, or GFP-LBD, respectively. Immunoprecipitation was performed with agarose beads conjugated with anti-GFP antibody. Western blottings were performed with anti-Flag antibody. D, LNCaP cells were cotransfected with Flag-Prp8 with GFP, GFP-NES, or GFP-AR(ΔNES). Immunoprecipitation was performed with agarose beads conjugated with anti-GFP antibody. Western blottings were performed with anti-Flag antibody.
To identify the AR domain(s) that mediates the interaction between AR and Prp8, we generated and tested the interaction of two GFP-tagged AR deletion mutants: GFP-NAR, which contains the AR N-terminal domain, DNA binding region and hinge region, and GFP-LBD, which is able to undergo nuclear translocation in response to androgen treatment in transfected cells (12). As shown in Figure 4C, Flag-tagged Prp8 was precipitated by anti-GFP antibody from the lysate of cells transfected with GFP-tagged LBD but not NAR. In the yeast screen, the mutation of Prp8 was discovered to abolish NESAR export function. Hence, we examined whether the NESAR, which is located in the LBD region, mediates the interaction between AR and Prp8. LNCaP cells were transfected with Flag-tagged Prp8 and GFP-tagged NESAR expression vector. As shown in Figure 4D, Prp8 was shown to bind with GFP-NESAR and GFP-AR with deletion of NESAR, although the interaction between AR(ΔNES) and Prp8 was weaker. In summary, Prp8 is capable of binding to AR in prostate cancer cells and this interaction is mediated through the LBD mainly via the NESAR region in an androgen-repressed manner.
Prp8 expression affects AR transactivation activity
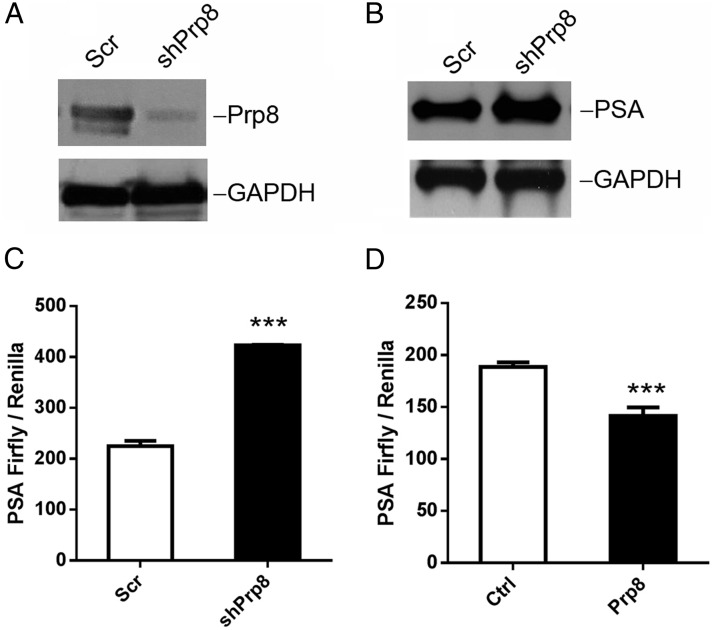
To investigate whether Prp8 regulates AR transactivation activity, we generated a Prp8 stable knockdown LNCaP cell line using lentiviral Prp8 shRNA vectors. Knockdown of Prp8 increased PSA expression compared with scramble control as shown by Western blotting (Figure 5, A and B). To determine whether Prp8 knockdown regulates AR transcriptional activity, luciferase reporter assays were performed with reporter gene constructs containing the 6.1Kb PSA promoter region which includes several well-characterized androgen-responsive elements. Prp8 knockdown caused about 2-fold induction of the PSA-luciferase reporter activity (Figure 5C) as compared with that of the scramble control (P < .01). To confirm the finding that Prp8 expression level was associated with AR transcription activity, LNCaP cells were cotransfected with the Prp8 expression vector and PSA luciferase reporter. Prp8 overexpression inhibited the AR transactivation of the PSA promoter (P < .01), as shown in Figure 5D. Together, these data suggest that the decreased Prp8 expression resulted in increased AR transcriptional activity and led to elevated PSA expression, whereas overexpression of Prp8 attenuates AR transactivation on PSA promoter in prostate cancer cells.
Figure 5.
Prp8 regulates AR transactivation activity. A and B, Prp8 and PSA expression levels were examined by Western blotting of LNCaP cells with a stable knockdown of Prp8. GAPDH served as loading control. C and D, Luciferase reporter assay using the 6.1-kb PSA promoter-driven luciferase reporter in Prp8 knockdown and scramble control LNCaP cells (C) and in Prp8-overexpressing and control LNCaP cells (D). The Renilla luciferase gene was used as an internal control in the luciferase assay. **, P < .01. Experiments were repeated 3 times.
Knockdown of Prp8 increases AR ubiquitination
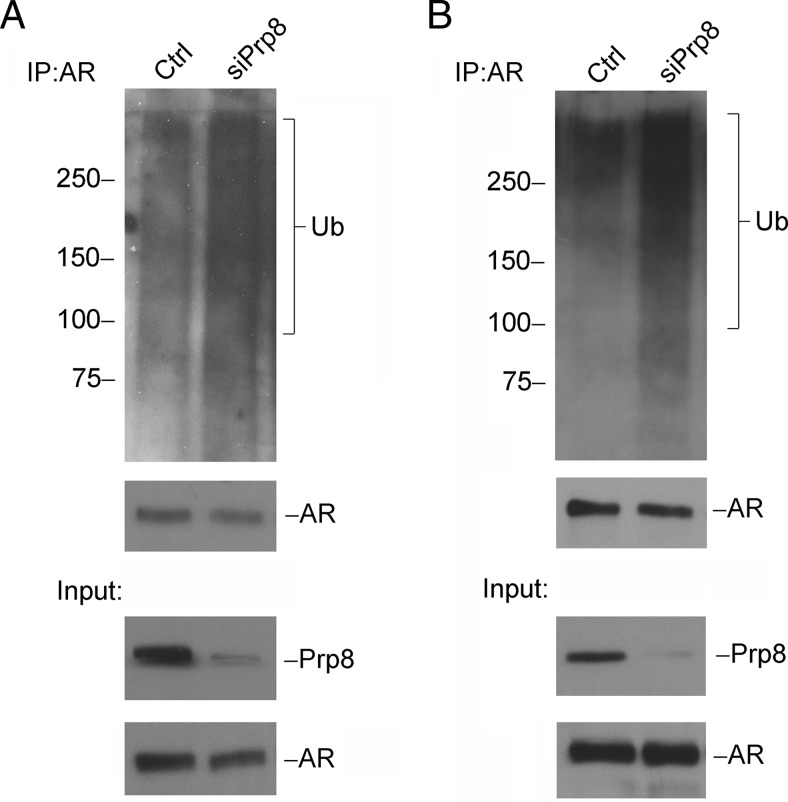
Previous findings in our lab have demonstrated that NESAR, the NES in the LBD of AR, can act as a signal for polyubiquitination for AR degradation (11). Xu et al reported that AR transcription activity is regulated by E3 ligase induced ubiquitination (35). As we observed, Prp8 can interact with NESAR and modulate AR transactivation, hence, we hypothesized that Prp8 regulates AR polyubiquitination. To test our hypothesis, Prp8 was knocked down using siRNA in prostate cancer cell lines LNCaP (Figure 6A) and C4–2 (Figure 6B). After immnunoprecipitation with anti-AR antibody, AR polyubiquitination was detected by Western blotting using an antiubiquitin antibody. Ubiquitination was enhanced in Prp8 knockdown cells when compared with cells transfected with control siRNA. These results suggest that Prp8 can regulate AR ubiquitination.
Figure 6.
Knockdown Prp8 increases AR ubiquitination. Prp8 expression was knocked down in LNCaP (A) and C4–2 (B) cells by siRNA transfection. Cell lysates were incubated with anti-AR antibody overnight followed with incubation with agarose beads for 3 hours. Ubiquitin, AR, and Prp8 expression levels were examined by Western blotting. Experiments were repeated 3 times.
Discussion
To mediate the androgen signaling pathway and regulate its downstream target gene expression, AR undergoes multiple steps, including binding to ligand, conformational change, nuclear translocation, coregulator/transcription factor recruitment, and binding to androgen response element in DNA to initiate gene transcription. In this study, we report the identification of splicing factor Prp8 as an AR coregulator, which regulates NESAR-mediated intracellular trafficking and polyubiquitination. Prp8 was identified in a yeast mutagenesis screen which isolated yeast mutants that failed to display the NESAR export function, followed by a rescue screen to identify the mutated gene. We performed a rapamycin-mediated export assay to confirm that the NESAR-mediated nuclear export was impaired by Prp8 knockdown in mammalian cell lines. We also showed that Prp8 knockdown induced the nuclear accumulation of GFP-AR in PC3 cells. To explore whether Prp8 status is altered in prostate cancers, we compared Prp8 mRNA expression levels and found that PRP8 mRNA levels were decreased in human advanced prostate cancer specimens and in castration-resistant LuCaP35 xenografts. This was consistent with the in silico data available at the Oncomine microarray database and MSKCC Prostate Oncogenome Project. Additionally, we demonstrated that PRP8 coimmunoprecipitated with AR, increased AR transcriptional activity, and plays a role in AR polyubiquitination. Our findings suggest that Prp8 is an important AR coregulator capable of modulating AR function in prostate cancer.
Identification of PRP8 as an AR cofactor that interacts with NESAR through a yeast genetic screen indicates that yeast is an excellent model for the identification of factors regulating NESAR. As the simplest eukaryotic model organism for biomedical research, yeast has been used to study the mechanisms regulating steroid nuclear receptors, including AR, glucocorticoid receptor, estrogen receptor, and mineral corticoid receptor. Yeast appears to have the basic machinery capable of supporting nuclear cytoplasmic trafficking of steroid receptors, including AR (36). Nuclear cytoplasmic trafficking of steroid receptors is mediated through nuclear import and export signals that are regulated by members of karyopherin family in both yeast and mammalian cells. NESAR is active in promoting nuclear export of the protein reporter in the yeast model; however, NESAR function is not regulated by CRM1 and other karyopherins (36). The identification of PRP8 as a key factor required for NESAR function in the yeast model, its subsequent verification of Prp8 interaction with AR, and its regulation of endogenous AR function in mammalian cells suggest that the machinery supporting the function of NESAR is also present in the yeast model and that this pathway is evolutionarily conserved. Thus, yeast appears to be an excellent model to elucidate the mechanism of NESAR action and our strategy of identifying factors required for supporting NESAR activity using yeast genetic screen is feasible.
The finding of Prp8, a key U5 snRNP component involved in mRNA splicing, as an AR cofactor suggests potential cross talk between RNA splicing machinery and AR signaling. This possibility is supported by the reports of other RNA splicing factors, PSF and p54nrb, as AR cofactors (14). These two protein components of the U1 snRNP are capable of linking transcriptional and splicing machinery by interacting with RNA polymerase. The AR N-terminal domain transactivating protein 1 was found to be identical to p102 U5 snRNP-binding protein and capable of enhancing AR transcriptional activity (36). Additionally, the RNA binding and adaptor protein Sam68 was reported to have separate effects on AR-regulated transcriptional activity and alternative splicing (16). The finding of splicing factors interacting with AR provides a mechanism to couple AR-mediated transcription with splicing machinery. The finding of Prp8 as an AR cofactor suggests that Prp8 may work together with other splicing factors to coordinate AR signaling with splicing activity.
Prp8 was identified on the basis of its function to modulate NESAR nuclear export in the yeast model. Prp8 modulation of NESAR export was further supported in a rapamycin-mediated nuclear export assay in mammalian cells. Prp8 also regulated the localization of stably expressed GFP-AR in PC3 cells. However, Prp8 knockdown enhanced endogenous AR nuclear localization in LNCaP prostate cancer cells in some but not other experiments, and the results were inconclusive (data not shown). One potential explanation for the inability of Prp8 knockdown to reproducibly impact AR nuclear cytoplasmic trafficking is that other nuclear import and/or export signals in full-length AR could outweigh the impact of Prp8 on NESAR in LNCaP cells. In addition to NESAR, full-length AR also contains a bipartite NLS in the DNA-binding domain and hinge region. Also, the N-terminal domain of the AR contains regions capable of modulating AR subcellular localization (37). Therefore, the mechanisms regulating intracellular trafficking of full-length AR are complex and knockdown of Prp8 may not be sufficient to significantly impact the overall subcellular localization of full-length endogenous AR.
Although Prp8 knockdown had no significant impact on endogenous AR subcellular localization in LNCaP cells, it significantly enhanced the ubiquitination of endogenous AR in both LNCaP and C4–2 prostate cancer cell lines. The modulation of AR ubiquitination by Prp8 is likely mediated through NESAR because NESAR is a major signal for AR ubiquitination (11). Recent studies have suggested that ubiquitination of AR is associated with inducing transcriptional activity in addition to promoting degradation. The ubiquitin E3 ligase Ring Finger Protein 6 (RNF6) has been shown to enhance AR transcriptional activity by inducing atypical ubiquitination of AR that accumulates without undergoing polyubiquitination-associated proteasomal degradation (35). Another example is the ubiquitin E3 ligase Seven in absentia homolog 2 (Siah2), which can induce the expression of select AR target genes by promoting ubiquitin-dependent degradation of transcriptionally inactive AR (38). Additionally, tumor susceptibility gene 101 was proposed to stimulate AR transcriptional activity by promoting its monoubiquitination and preventing polyubiquitination. Taken together, these studies and ours suggest that the regulation of AR transcriptional activity by Prp8 may be mediated by regulation of ubiquitination of AR through NESAR.
Coimmunoprecipitation demonstrates that the interaction of AR with Prp8 is mainly mediated by NESAR. However, the binding interface of the NESAR with Prp8 has not been explored in the manuscript. In a previous study, we have showed that any point mutations in NESAR region did not change NES AR localization and that NESAR is different from any conserved CRM1 dependent NES. Linker scanning mutagenesis of NESAR showed that all of the NESAR mutants were localized in the cytoplasm (39), indicating no specific amino acid in NESAR is necessary for its export activity. Thus, Prp8 binding is unlikely limited to a few amino acids in NESAR, if the binding to NESAR is critical for NESAR export. Additionally, previous publication reported that endogenous Prp8 is mainly localized in the nuclei (40). AR contains both NLS and NES and shuttles between nuclei and cytoplasm. Therefore, Prp8 binding to AR is likely to occur in the nuclei, potentially blocking AR export. However, more studies will be required to determine the mechanism underlying Prp8 modulation of NESAR activity.
Our studies suggest that PRP8 plays an important role in the progression of prostate cancer, particularly to castration resistance. Down-regulation of Prp8 in advanced human prostate cancer specimens and in castration-resistant xenograft tumors may enhance the activity of AR, driving the growth and oncogenic pathways in prostate cancer cells. Prp8 down-regulation observed in our studies is consistent with the in silico data published in ONCOMINE Microarray Database and MSKCC Prostate Oncogenome Project. We also observed that androgen treatment decreases Prp8 expression in C4–2 cells (data not shown). Therefore, it will be important to further define the role of Prp8 in prostate carcinogenesis and the mechanism of Prp8 modulation of AR signaling.
In summary, our studies identified Prp8 as a novel AR coregulator capable of modulating AR polyubiquitination and transactivation via NESAR, and its down-regulation is associated with prostate cancer progression, particularly to castration resistance. Our study also demonstrated the feasibility of using yeast genetic screen to identify factors involved in the regulation of NESAR. The identification of Prp8 as an AR cofactor provides additional evidence for potential cross talk between AR signaling and splicing machinery. Future studies will be required to define the mechanism by which Prp8 modulates AR ubiquitination and whether Prp8 plays an important role in the production of AR splice variants in the progression of prostate cancer.
Additional material
Supplementary data supplied by authors.
Acknowledgments
We thank Ariad Pharmaceuticals for providing the rapamycin-mediated nuclear export assay system, Aiyuan Zhang for expert technical assistance, Richard Gaber (Northwestern University) for advice on yeast screen, Dr Laura Pascal for critical review, and members of Wang Lab for discussion.
Present address for K.Z.M.: Division of Biotechnology, Sher-e-Kashmir University of Agricultural Sciences and Technology of Kashmir, Shalimar, Srinagar, Jammu and Kashmir 190025, India.
Present address for P.S.: Department of Critical Care Medicine, University of Pittsburgh, Pittsburgh, PA 15261.
Present address for J.A.D.: Central Laboratory, College of Science, King Saud University, Riyadh, Saudia Arabia-11451.
This work was supported by National Institutes of Health (NIH) Grants R01 CA186780 and R01 CA108675, the American Cancer Society (ACS) Grant PF-05-229-01-CSM, and the NIH Training Grant T32 CA080621. D.W. received a postdoctoral fellowship from the Urology Care Foundation of the American Urological Association (AUA) and a fellowship from the Mellam Family Foundation. K.Z.M. was recipient of a Mellam fellowship. This research was also supported by the University of Pittsburgh Cancer Institute (UPCI) Animal Facility, Vector Core, and Tissue and Research Pathology Services funded through NCI Grant CCSG P30CA047904.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- a.a.
- amino acid
- ADT
- androgen deprivation therapy
- AR
- androgen receptor
- ATCC
- American Type Culture Collection
- EGFP
- enhance GFP
- FRB
- FKBP12-rapamycin-binding domain
- GAPDH
- glyceraldehyde 3-phosphate dehydrogenase
- GFP
- green fluorescence protein
- LBD
- ligand-binding domain
- NES
- nuclear export signal
- NLS
- nuclear localization signal
- PKI
- protein kinase inhibitor
- Prp8
- pre-mRNA processing factor 8
- PSA
- prostate specific antigen
- RFP
- red fluorescence protein
- shRNA
- short hairpin RNA
- siRNA
- small interfering RNA
- snRNP
- small nuclear ribonucleoprotein
- SV40
- simian virus 40.
References
- 1. Loblaw DA, Virgo KS, Nam R, et al. Initial hormonal management of androgen-sensitive metastatic, recurrent, or progressive prostate cancer: 2006 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2007;25:1596–1605. [DOI] [PubMed] [Google Scholar]
- 2. Pienta KJ, Bradley D. Mechanisms underlying the development of androgen-independent prostate cancer. Clin Cancer Res. 2006;12:1665–1671. [DOI] [PubMed] [Google Scholar]
- 3. Lonergan PE, Tindall DJ. Androgen receptor signaling in prostate cancer development and progression. J Carcinog. 2011;10:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brown CJ, Goss SJ, Lubahn DB, et al. Androgen receptor locus on the human X chromosome: regional localization to Xq11–12 and description of a DNA polymorphism. Am J Hum Genet. 1989;44:264–269. [PMC free article] [PubMed] [Google Scholar]
- 5. Reid J, Kelly SM, Watt K, Price NC, McEwan IJ. Conformational analysis of the androgen receptor amino-terminal domain involved in transactivation. Influence of structure-stabilizing solutes and protein-protein interactions. J Biol Chem. 2002;277:20079–20086. [DOI] [PubMed] [Google Scholar]
- 6. Shaffer PL, Jivan A, Dollins DE, Claessens F, Gewirth DT. Structural basis of androgen receptor binding to selective androgen response elements. Proc Natl Acad Sci USA. 2004;101:4758–4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. He B, Gampe RT, Jr, Kole AJ, et al. Structural basis for androgen receptor interdomain and coactivator interactions suggests a transition in nuclear receptor activation function dominance. Mol Cell. 2004;16:425–438. [DOI] [PubMed] [Google Scholar]
- 8. Zhou ZX, Sar M, Simental JA, Lane MV, Wilson EM. A ligand-dependent bipartite nuclear targeting signal in the human androgen receptor. Requirement for the DNA-binding domain and modulation by NH2-terminal and carboxyl-terminal sequences. J Biol Chem. 1994;269:13115–13123. [PubMed] [Google Scholar]
- 9. Zhou ZX, Wong CI, Sar M, Wilson EM. The androgen receptor: an overview. Recent Prog Horm Res. 1994;49:249–274. [DOI] [PubMed] [Google Scholar]
- 10. Jenster G, Trapman J, Brinkmann AO. Nuclear import of the human androgen receptor. Biochem J. 1993;293(pt 3):761–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gong Y, Wang D, Dar JA, et al. Nuclear export signal of androgen receptor (NESAR) regulation of androgen receptor level in human prostate cell lines via ubiquitination and proteasome-dependent degradation. Endocrinology. 2012;153:5716–5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Saporita AJ, Zhang Q, Navai N, et al. Identification and characterization of a ligand-regulated nuclear export signal in androgen receptor. J Biol Chem. 2003;278:41998–42005. [DOI] [PubMed] [Google Scholar]
- 13. Braunschweig U, Gueroussov S, Plocik AM, Graveley BR, Blencowe BJ. Dynamic integration of splicing within gene regulatory pathways. Cell. 2013;152:1252–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dong X, Sweet J, Challis JR, Brown T, Lye SJ. Transcriptional activity of androgen receptor is modulated by two RNA splicing factors, PSF and p54nrb. Mol Cell Biol. 2007;27:4863–4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ishitani K, Yoshida T, Kitagawa H, Ohta H, Nozawa S, Kato S. p54nrb acts as a transcriptional coactivator for activation function 1 of the human androgen receptor. Biochem Biophys Res Commun. 2003;306:660–665. [DOI] [PubMed] [Google Scholar]
- 16. Rajan P, Gaughan L, Dalgliesh C, et al. The RNA-binding and adaptor protein Sam68 modulates signal-dependent splicing and transcriptional activity of the androgen receptor. J Pathol. 2008;215:67–77. [DOI] [PubMed] [Google Scholar]
- 17. Clark EL, Coulson A, Dalgliesh C, et al. The RNA helicase p68 is a novel androgen receptor coactivator involved in splicing and is overexpressed in prostate cancer. Cancer Res. 2008;68:7938–7946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grainger RJ, Beggs JD. Prp8 protein: at the heart of the spliceosome. RNA. 2005;11:533–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li X, Zhang W, Xu T, et al. Comprehensive in vivo RNA-binding site analyses reveal a role of Prp8 in spliceosomal assembly. Nucleic Acids Res. 2013;41:3805–3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boon KL, Grainger RJ, Ehsani P, et al. prp8 mutations that cause human retinitis pigmentosa lead to a U5 snRNP maturation defect in yeast. Nat Struct Mol Biol. 2007;14:1077–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kershaw CJ, Barrass JD, Beggs JD, O'Keefe RT. Mutations in the U5 snRNA result in altered splicing of subsets of pre-mRNAs and reduced stability of Prp8. RNA. 2009;15:1292–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tapia-Vieyra JV, Arellano RO, Mas-Oliva J. ARP2 a novel protein involved in apoptosis of LNCaP cells shares a high degree homology with splicing factor Prp8. Mol Cell Biochem. 2005;269:189–201. [DOI] [PubMed] [Google Scholar]
- 23. Gietz RD, Woods RA. Screening for protein-protein interactions in the yeast two-hybrid system. Methods Mol Biol. 2002;185:471–486. [DOI] [PubMed] [Google Scholar]
- 24. Dar JA, Masoodi KZ, Eisermann K, et al. The N-terminal domain of the androgen receptor drives its nuclear localization in castration-resistant prostate cancer cells. J Steroid Biochem Mol Biol. 2014;143:473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. O'Malley KJ, Dhir R, Nelson JB, Bost J, Lin Y, Wang Z. The expression of androgen-responsive genes is up-regulated in the epithelia of benign prostatic hyperplasia. Prostate. 2009;69:1716–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Corey E, Quinn JE, Buhler KR, et al. LuCaP 35: a new model of prostate cancer progression to androgen independence. Prostate. 2003;55:239–246. [DOI] [PubMed] [Google Scholar]
- 27. Wang Y, Gupta S, Hua V, et al. Prolongation of off-cycle interval by finasteride is not associated with survival improvement in intermittent androgen deprivation therapy in LNCaP tumor model. Prostate. 2010;70:147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Klemm JD, Beals CR, Crabtree GR. Rapid targeting of nuclear proteins to the cytoplasm. Curr Biol. 1997;7:638–644. [DOI] [PubMed] [Google Scholar]
- 29. Qiao Z, Wang D, Hahn J, Ai J, Wang Z. Pirin down-regulates the EAF2/U19 protein and alleviates its growth inhibition in prostate cancer cells. Prostate. 2014;74:113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nguyen MM, Harmon RM, Wang Z. Characterization of karyopherins in androgen receptor intracellular trafficking in the yeast model. Int J Clin Exp Pathol. 2014;7:2768–2779. [PMC free article] [PubMed] [Google Scholar]
- 31. Masoodi KZ, Ramos Garcia R, Pascal LE, et al. 5α-reductase inhibition suppresses testosterone-induced initial regrowth of regressed xenograft prostate tumors in animal models. Endocrinology. 2013;154:2296–2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rhodes DR, Kalyana-Sundaram S, Mahavisno V, et al. Oncomine 3.0: genes, pathways, and networks in a collection of 18,000 cancer gene expression profiles. Neoplasia. 2007;9:166–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xu K, Shimelis H, Linn DE, et al. Regulation of androgen receptor transcriptional activity and specificity by RNF6-induced ubiquitination. Cancer Cell. 2009;15:270–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhao Y, Goto K, Saitoh M, et al. Activation function-1 domain of androgen receptor contributes to the interaction between subnuclear splicing factor compartment and nuclear receptor compartment. Identification of the p102 U5 small nuclear ribonucleoprotein particle-binding protein as a coactivator for the receptor. J Biol Chem. 2002;277:30031–30039. [DOI] [PubMed] [Google Scholar]
- 37. Dar JA, Eisermann K, Masoodi KZ, et al. N-terminal domain of the androgen receptor contains a region that can promote cytoplasmic localization. J Steroid Biochem Mol Biol. 2014;139:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Qi J, Tripathi M, Mishra R, et al. The E3 ubiquitin ligase Siah2 contributes to castration-resistant prostate cancer by regulation of androgen receptor transcriptional activity. Cancer Cell. 2013;23:332–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Saporita AJ, University N. Regulation of Androgen Receptor Localization in Prostate Cancer Cells. Chicago, IL: Northwestern University; 2006. [Google Scholar]
- 40. Liu YC, Kuo RL, Lin JY, et al. Cytoplasmic viral RNA-dependent RNA polymerase disrupts the intracellular splicing machinery by entering the nucleus and interfering with Prp8. PLoS Pathog. 2014;10:e1004199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.