Abstract
Background
Comparative aging studies, particularly those that include species of exceptional resistance to aging processes, can potentially illuminate novel senescence-retarding mechanisms. In recent years, protein homeostasis (proteostasis) has been implicated in fundamental aging processes. Here we further evaluate the relationship between proteostasis and longevity in a selection of bivalve mollusks and mammals with maximum longevities ranging from 3 to 507 years.
Methods & Results
We experimentally examined proteostasis using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as a reporter, as it is ubiquitously expressed, highly conserved, and conveniently assayed. The ability to maintain this enzymatic function was tested with increasing concentrations of the chaotropic agent urea, revealing a robust relationship with longevity in bivalves and mice. While our shortest-lived mollusk and mouse lost all activity by 2.5 and 3.5 M urea respectively, the longest-lived mollusk species, Arctica islandica, still preserved 45% of its basal function even at 6 M urea. To confirm that GAPDH proteostasis has a broad association with longevity, we also investigated a selection of primate species ranging in maximum longevity from 22 to 122 years. They outperformed the mouse at all concentrations, but among the primates results were variable at low urea doses. Still, at 6 M urea baboon and human samples retained 10% of their activity while both mouse and marmoset samples had no activity.
Mechanism of Exceptional Stress Resistance
To explore possible mechanisms of the exceptional stress resistance of A. islandica GAPDH we enzymatically removed post-translational glycosylation, but observed no decrease in stability. We also removed molecules smaller than 30 kDa, which includes most small heat shock proteins, but again did not compromise the exceptional stress resistance of Arctica GAPDH.
Conclusion
While the mechanism underlying A. islandica’s exceptional stress resistance remains elusive, this research identifies an experimental system that may reveal hitherto unknown mechanisms of protein homeostasis.
Introduction
Exceptionally long-lived species possess a wealth of untapped research potential for gerontology. Novel aging models are emerging with a variety of useful traits, and when combined with the rapid influx of sequencing information these species are an opportunity to elucidate successful aging strategies. Traditional animal models are short-lived and highly susceptible to basic aging processes. They deteriorate and die rapidly which from one perspective enhances their experimental utility. However, humans are already highly resistant to basic aging processes, so the senescence-retarding interventions effective in traditional models may not be as successful at improving and prolonging human health. It is in exceptionally long-lived species that we may discover mechanisms of exceptional senescence-resistance.
Arctica islandica, the ocean quahog, is one such species. With a maximum lifespan of over five hundred years [1,2] it may contain information relevant for combating senescence and age-related diseases. These lifespans can be authenticated in natural populations via schlerochronology–the counting of annual growth rings in the shell [3,4]–a significant advantage of bivalves over many other model organisms. By analyzing these growth rings, a remarkable range of longevities have been determined. The bivalve species utilized in this study have maximum lifespans ranging from 7 to 507 years, yet are all bottom dwelling, filter feeding heterodont bivalves of comparable size. With many ecological similarities it is easier to identify salient differences between these species that may contribute to their variable aging rates. Combined with their low-cost commercial availability and ease of care, bivalves are emerging as an ideal experimental system for comparative analysis of aging processes [5–8].
The progressive loss of protein homeostasis, now called proteostasis, has arisen as a likely contributor to the progressively deteriorating aging phenotype. Proteostasis is the maintenance of a functional proteome through strict regulation of translation, folding, modification, trafficking and degradation. Each of these processes are measurably compromised with age [9–11], leading to cytotoxic aggregates [12], impaired cellular function and contribute to the variety of pathologies we experience with age [13–18]. Notably, neurodegenerative diseases such as Alzheimer's and Parkinson's are characterized by the insoluble accumulation of proteins and are undeniably age-related [19]. Experimental longevity manipulations have further implicated the collapse of proteostasis in senescence processes. Interventions that extend lifespan, such as rapamycin, caloric restriction, and insulin-like signaling pathway manipulation each have a pronounced reduction in protein aggregation [20–23] and oxidative damage markers [24,25] along with increased autophagy [26,27] and molecular chaperone activity [28,29]. Longevity appears to be highly correlated with the effectiveness of the proteostasis network. Indeed, the common daf-2 mutation that extends lifespan in C.elegans absolutely requires HSF1 [30], a master regulator of molecular chaperone expression, key players in proteostasis [31].
We have previously shown that among species of bivalve mollusks, longer-lived species are more resistant to global proteome unfolding and better maintain creatine kinase activity when confronted with misfolding stressors. We also showed that the proteome was dramatically less likely to form large aggregates under heat stress in long-lived species such as A. islandica, compared with shorter-lived species, including the short-lived mouse. We also found that a reporter protein, FITC-tagged bovine serum albumin, was less prone to heat-induced aggregation in tissue lysates of long-lived bivalve species in comparison with shorter-lived bivalves. [5]
In order to extend our investigations of the relation between species longevity and relative proteostatic capability, we exploited glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as a representative protein. GAPDH is an abundant protein commonly known for catalyzing the sixth step of glycolysis, but has been implicated in a range of other cellular functions. It is constitutively expressed, highly conserved, and absolutely essential. It is mostly known for its classical housekeeping role in glycolysis, for which it can be easily assayed. When provided with its substrates glyceraldehyde 3-phosphate and NAD+, GAPDH will catalyze the reaction to D-glycerate 1,3-bisphosphate and NADH. This reaction can be conveniently monitored with a spectrophotometer in real time. However, if the protein has been damaged or unfolded by stressors, this function will be inhibited, reducing the rate of production. We utilized this representative enzymatic activity as an accessible marker of cellular proteostasis to determine each species' relative ability to maintain protein structure and function when stressed. Resisting the deleterious effects of age likely requires the ability to maintain an intact and highly functional proteome, while enduring the variety of internal and external stressors assured with time [9,32].
We assessed GAPDH activity under urea misfolding stress in four species of bivalves ranging in maximum longevity from 7 to 507 years along with the laboratory mouse as a short-lived mammalian standard. In addition, to determine whether the same relation between proteostatic capacity and longevity exists among species more closely related to humans, we performed the same analysis in three species of primates ranging in maximum longevity from 22 to 122 years, again alongside the mouse. We also assessed whether small metabolites, small heat shock proteins, or post-translational glycosylation might play a role in stabilizing GAPDH under stress.
Materials and Methods
Species Included
Four species of bivalve mollusks were included: Ruditapes phillipanarum (maximum reported longevity, Lmax, = 7 years [33]), Callista chione (Lmax = 30 years [34]), Mercenaria mercenaria (Lmax = 106 years [35]) and Arctica islandica (Lmax = 507 years[1,2]). Individuals were collected opportunistically from commercial fishermen working the offshore waters of the state of Washington in the United States (Ruditapes), Croatia (Callista), and the state of Massachusetts (US) and Wales (Mercenaria and Arctica). These species were chosen for their disparate longevities of course, but also for their ecological similarities. They employ similar lifestyles, filter-feeding microorganisms with an extended siphon while their bulk is burrowed beneath the ocean sediment, additionally protected by their thick valves. They are of comparable size, ranging from a maximum shell height of 60 mm (Ruditapes) to 120 mm (Mercenaria) and enjoy overlapping climate preferences of 12-24°C (Ruditapes) to 5–15°C (Arctica). No permissions were required to collective bivalves and their tissues. It should be noted that we are testing for phylogenetic independent effects, as there is no obvious phylogenetic relation to longevity; the shortest-lived and second to longest-lived are the closest relatives[5]. In order to bring our results a medically relevant perspective we also tested a trio of primates with disparate longevities: common marmoset (Callithrix jacchus, Lmax = 22 years [36]) baboon (Papio hamadryas, L max = 38 years [37]) and human (Homo sapiens, Lmax = 122 years). Additionally, we included the common aging model C57BL/6 mouse (Mus musculus, Lmax = 3 years). We are thus investigating our hypothesis in both bivalves and mammals, but the chance to compare the most successful aging model against the most common aging model should not be understated. While such a comparison across phyla is of questionable relevance, placing each group on the same proteostasis scale could be enlightening.
Ethics Statement
All procedures and protocols used in this entire study were approved by their respective animal or human use committee. In addition, all mammalian tissues were obtained from samples taken for purposes unrelated to this study. Specifically, human muscle tissue was obtained from volunteers in a diabetes study (samples were from controls) with full written informed consent, approved by the University of Texas Health Science Center San Antonio Institutional Review Board. Mouse and marmoset muscle tissue were obtained from captive colonies used in aging research at the University of Texas Health Science Center San Antonio and the Texas Biomedical Research Institute, respectively. Baboon tissue was obtained from the captive colonies at the Southwest National Primate Research Center, within the Texas Biomedical Research Institute. Euthanasia methods were those approved by the American Veterinary Medical Association for these species. Each protocol was approved either by the University of Texas Health Science Center San Antonio Institutional Animal Care and Use Committee (mice), and the use of baboon tissue was approved by the Texas Biomedical Research Institute Institutional Animal Care and Use Committee.
Tissues Used
For the bivalves, adductor muscle samples were obtained from wild caught individuals provided by collaborators at the Virginia Institute of Marine Science and the School of Ocean Sciences in Bangor, Wales. The adductor muscle holds the valves closed, maintaining the bivalve's only defense against the ocean's host of predators. This function must be preserved for successful aging as a prey animal. Individual ages were determined by our collaborators via counting annual growth rings in the shell [3,4] and young adults were chosen from each species. Young adults should have minimal age-related degeneration yet prevent any developmental stage dependent results between species; the longer-lived species have not reached sexual maturity by the time the shorter-lived species have died. Thus we used individuals of approximately 3, 10, 20 and 35 years of age for Ruditapes, Callista, Mercenaria and Arctica respectively, based on availability and maturation age. They were of unknown sex. Unfortunately, young adult samples for the three primates were not available and older individuals had to be incorporated into the study. The marmoset and baboon samples were approximately 7 and 17 years of age respectively and of mixed sex. The human samples were of unknown age and unknown sex. For the mammals, skeletal muscle was chosen as the functionally equivalent tissue to the bivalve adductor. Age related decreases in mammalian muscle function are well-documented [38] and, like the bivalves, impairing this tissue would be an obvious detriment to survival and longevity in the wild. In order to delay these consequences, exceptionally long-lived species likely utilize superior proteostasis to prevent or ameliorate these age-related declines in function and its consequent increase in mortality.
GAPDH Activity
Common enzymatic activities can be employed as convenient representatives of overall proteome homeostasis. Many essential enzymes have well defined substrates, active sites and overall functions which are easily probed. These catalysts are completely dependent on their complex three dimensional structure, which if disrupted, will cause a commensurate decrease in activity. We utilized urea as chaotropic agent, disrupting hydrogen bonding and weakening the hydration shell critical for protein folding [39]. At low concentrations, active sites and overall conformation will be mildly disrupted. High concentrations can denature the structure completely. We probed each species' ability to resist these disruptions and maintain enzymatic activity as a marker for their relative proteostasis. GAPDH function was measured and represented as the fold change in activity from basal levels to stressed.
In brief, adductor muscle (bivalves) or skeletal muscle (mammals) was homogenized in 15mM sodium pyrophosphate buffer containing 30mM sodium arsenate at pH 8.5, with a protease inhibitor cocktail to maintain sample integrity after homogenization. Ultracentrifugation at 100,000g for one hour was used to isolate the cytosolic fraction. From this, equal amounts of protein, as determined by BCA, were loaded into reaction mixtures containing the following: 0.3mM NAD+, 0.3mM D-glyceraldehyde-3-phosphate, 4mM DTT, and 3.5ug/uL lysate. Cuvettes were read in a spectrophotometer and the ΔA340/minute corresponding with the GAPDH dependent reduction of NAD+ to NADH is determined from the initial linear response. This is a conventional GAPDH enzyme activity assay [40,41]. The conditions described yielded measurable baseline activity to which stressed levels could be compared. When stressors were included, they were added twenty minutes prior to the addition of D-glyceraldehyde-3-phosphate, effectively pre-stressing the enzyme in that species' lysate before the activity was assayed.
To narrow and identify potential facilitators of GAPDH stability the samples were rinsed through a centricon filter six times, isolating the representative enzyme from anything less than 30kDa. This small molecule depleted lysate was then utilized as previously described, but with the small molecules lacking, their influence on GAPDH stability could be analyzed. The effects of glycosylation post-translational modification (PTMs) were also of interest, as these are well known to enhance protein stability [42,43]. Samples were incubated in a digestive enzyme cocktail (Sigma-Aldrich: EDEGLY-KT) designed to remove all N-linked and simple O-linked carbohydrates from the sample prior to the stressed GAPDH activity assay.
Statistical Analysis
Statistics were performed using JMP 9.0 software (SAS, Cary, NC, USA). Data are visualized as means, error bars are +/- 1 SEM. Differences among species were assessed by ANOVA. Comparisons notated with asterisks are statistically different by post hoc testing using Tukey’s HSD, p < 0.05 (*), 0.001 (**), and 0.0001(***). Raw data for all figures found in S1 Table.
Results
GAPDH Activity
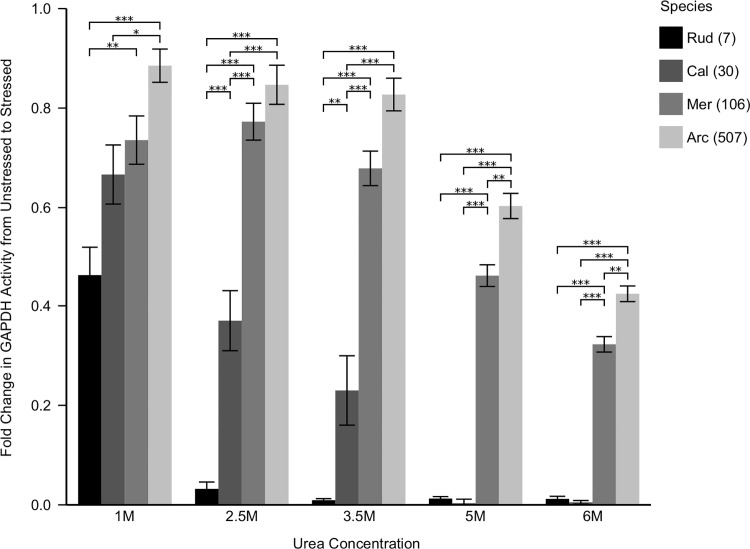
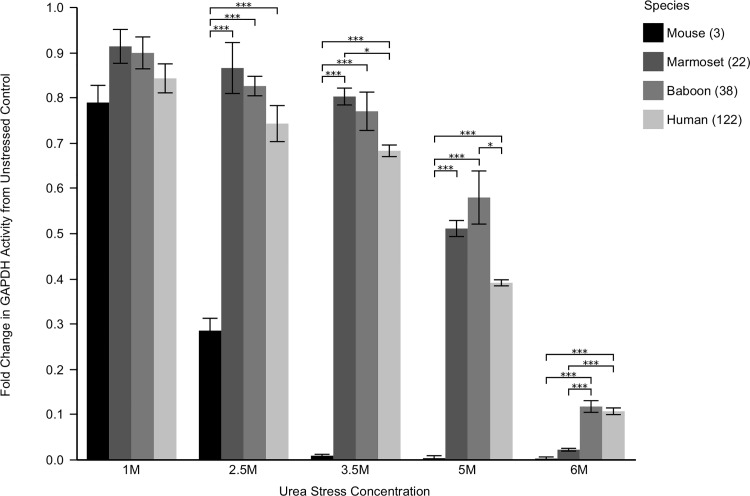
In order to assess each species' relative proteostasis potential, GAPDH activity was monitored under increasingly stressed conditions and quantified as the fold change from basal levels. As a representative of global proteome stability, protection of GAPDH activity was robustly correlated with longevity in both bivalves and mammals. While GAPDH in short-lived Ruditapes lost all activity by 2.5 M urea, and mouse showed negligible activity by 3.5 M urea, the exceptionally long-lived Arctica maintained 45% of its basal activity in 6 M urea (Fig 1). Long-lived mammals also performed well, with human and baboon samples maintaining 10% of their basal activity at 6 M urea (Fig 2), significantly outperforming the other, shorter-lived mammals. However, at lower doses, the shorter-lived primate's GAPDH was more stable than the human samples. At all doses the commonly used but short-lived C57Bl6 was the least stable mammal, and lost all activity by 3.5 M urea. It should be noted that the long-lived bivalves retained greater GAPDH activity relative to unstressed controls than even the human sample. This suggests that long-lived bivalves may possess more effective proteostasis mechanisms than even the longest-lived mammals.
Fig 1. GAPDH Activity with Increasing Urea Stress in Bivalves.
Lysates from each species were pre-stressed for twenty minutes in the noted urea concentrations. Glyceraldehyde 3-phosphate was then added, and the activity of endogenous GAPDH was monitored as ΔA340/minute, corresponding to the reduction of NAD+ to NADH. Data is reported as the fold change from unstressed activity. Differences among species were assessed by two way analysis of variance indicating a significant main effect of species (F3,60 = 23.7, p < 0.0001) stress (F4,60 = 30.9, p < 0.0001), as well as the interaction between species and stress (F12,60 = 10.2, p < 0.0001). Long-lived species maintain GAPDH function at all doses tested, while shorter-lived species were dramatically compromised. Asterisks indicate significant individual differences as assessed post hoc with Tukey's HSD, p < 0.05 (*), 0.001 (**), and 0.0001 (***), respectively. Rud = Ruditapes, Cal = Callista, Mer = Mercenaria, Arc = Arctica. Numbers in parentheses are maximum species longevity in years.
Fig 2. GAPDH Activity with Increasing Urea Stress in Mammals.
Lysates from each species were pre-stressed for twenty minutes in the noted urea concentrations. Glyceraldehyde 3-phosphate was then added, and the activity of endogenous GAPDH was monitored as ΔA340/minute, corresponding to the reduction of NAD+ to NADH. Data is reported as the fold change from unstressed activity. Differences among species were assessed by two way analysis of variance indicating a significant main effect of species (F3,60 = 222.5, p < 0.0001) stress (F4,60 = 429.9, p < 0.0001), as well as the interaction between species and stress (F12,60 = 26.1, p < 0.0001). Long-lived species maintain GAPDH function at all doses tested, while shorter-lived species were dramatically compromised. Asterisks indicate significant individual differences as assessed post hoc with Tukey's HSD, p < 0.05 (*), 0.001 (**), and 0.0001(***), respectively. Numbers in parenthesis are maximum species longevity in years.
Small Molecule and Glycosylation Removal
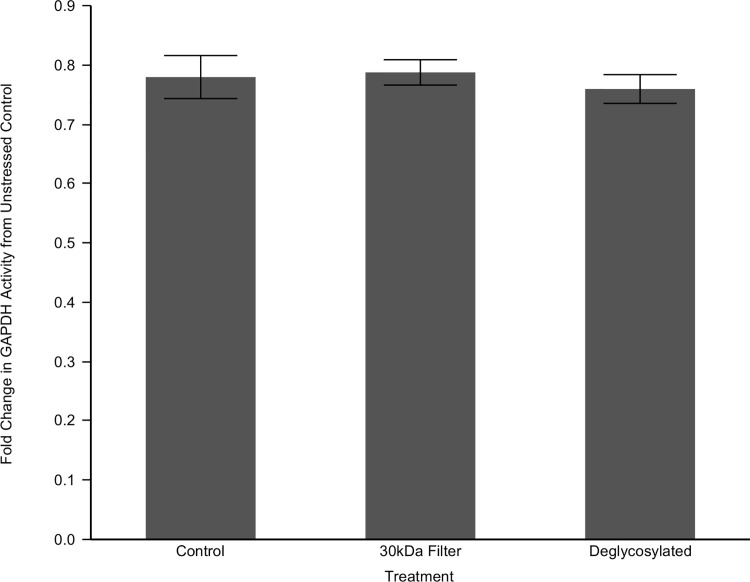
The remarkable protein stability demonstrated by muscle lysate of Arctica raises the obvious question of what stabilizing components they employ that shorter-lived species lack. Two possibilities are small heat shock proteins and/or small metabolites may contribute to this stability. In order to examine these possibilities, we isolated them from the system by running the lysate through a 30kDa centricon, removing cellular components less than 30kDa in size. This should remove stabilizing metabolites and monomers of small heat shock proteins. This depleted fraction was utilized for the GAPDH assay as before, comparing 3.5 M urea stressed activity to basal levels. We focused our attention on A. islandica as it exhibited the most robust result in response to the urea. If small proteins or other molecules are major contributors to this result, their removal should increase GAPDH's susceptibility to urea stress. Surprisingly, there was no significant difference in Arctica's GAPDH stability under this urea stress when depleted of components less than 30kDa in size (Fig 3).
Fig 3. Small Molecule and Glycosylation Effects on GAPDH Stability in Arctica islandica.
Lysates from Arctica islandica were filtered through a 30kDa centricon, isolating GAPDH from any potential small stabilizers. Another sample was treated with a deglycosylation kit to remove N-linked and O-linked carbohydrate modifications. These depleted lysates were pre-stressed for twenty minutes in 3.5 M urea. Glyceraldehyde 3-phosphate was then added, and the activity of endogenous GAPDH was monitored as ΔA340/minute, corresponding to the reduction of NAD+ to NADH. Data is reported as the fold change from unstressed activity. GAPDH stability was not further compromised by deglycosylation (p = 0.62) or the removal of small molecules (p = 0.85) as compared to the unmodified control.
Considering the lack of an effect from small molecules, we next hypothesized that long-lived species may employ certain post-translational modifications to stabilize protein structure and maintain function. Glycosylation modifications have a variety of structural consequences depending on their type and location, but can include improved tertiary stability. We applied a commercial deglycosylation kit to globally remove all N-linked and O-linked carbohydrate post-translational modifications. This enzyme suite was applied to the lysate at manufacturer specifications and the experiment re-run with 3.5 M urea stress as before. Again, there was no significant difference in Arctica's GAPDH stability when compared to the unmodified lysate (Fig 3).
Discussion
We have shown that the exceptionally long-lived bivalve mollusk species, Arctica islandica, is exceptionally successful at preserving its GAPDH activity in muscle compared with shorter-lived species of bivalve mollusks, mice and primates. Combined with our previous work demonstrating that its proteome is less subject to misfolding, aggregation and also that it preserves the activity of creatine kinase as compared with shorter-lived species [5], we can conclude that Arctica has evolved exceptionally effective mechanisms for protecting its proteome from stress. If we can identify the mechanism(s) by underlying this stability, it could have dramatic implications for interventions in human protein folding diseases and also potentially for aging itself.
One obvious mechanism of superior proteome protection would be a superior system of molecular chaperones, which are key components of the proteostasis network [31]. A robust and adaptive suite of chaperones present in lysates from long-lived species' muscle could bind and stabilize GAPDH to maintain functional conformation in the presence of misfolding stress. However, the large chaperone families that would be expected to mediate this effect–heat shock protein 60 (HSP60), HSP70, and HSP90 –are ATP-dependent [44]. ATP was not included in our reaction mixtures, making this scenario unlikely unless long-lived bivalves possess unique large chaperones not dependent on ATP. Provocatively, the one bivalve species with a high quality whole genome sequence–the Pacific oyster (Crassostrea gigas)–has 88 HSP70’s compared with ~17 in humans [45]. However, as no large HSPs that do not require ATP are known from other organisms, this possibility should be considered highly remote. Additionally, the possibility of any adaptive stress responses, including all forms of protein turnover such as enhanced autophagy, proteasome activity or accelerated de novo translation, should have been eliminated by our homogenization and centrifugation procedures. The 100,000g soluble fraction used in our study represents a “snapshot” of the proteome and its stability without adaptive elements. Within these confines, our previous results indicated this stability is not unique to a single protein. Our long-lived species' entire proteome demonstrated resistance to urea induced unfolding and temperature induced aggregation [5]. The protective mechanism must preserve the proteome indiscriminately.
Our experimental system eliminates many of the most obvious candidates for proteome stabilization but a few remain. Small heat shock proteins (sHSPs) are molecular chaperones [46] that function independent of ATP [47] yet can bind and stabilize misfolded proteins. Indeed, HSP18.1 in peas was found to dodecomerize and bind to GAPDH under heat stress, preventing its aggregation [48]. Additionally, a variety of osmolytes are known to stabilize protein structure [49–51] counteracting hydrostatic pressure, increasing thermostability and even combating denaturization by urea [52]. We evaluated the possibility that similar sHSPsor osmolytes may have been responsible for Arctica's high GAPDH stability by running our samples through a 30kDa centricon filter to remove any such small stabilizing molecules. This removal did not affect GAPDH stability, suggesting something larger than 30kDa is the key stabilizer. While many potentially stabilizing metabolites were removed, this data should not be regarded as conclusive evidence of sHSP non-involvement; they may oligomerize beyond the 30kDa filter threshold or may be bound strongly enough to prevent their removal by filtration. We also tested the possible role of glycosylation post-translational modifications, as they have a demonstrated influence on protein stability [42, 43] and GAPDH has a known modification site at Th227 [53]. As there may be novel sites, we removed all N-linked and O-linked glycosylations enzymatically, but did not yield a reduction of GAPDH activity. Other modifications were not probed and could play an important role.
GAPDH stability may be interesting in its own right and not just as a representative for the rest of the proteome. While the phylogenetic distance between bivalves and mammals is great, GAPDH is exceedingly well conserved with ~75% perfect identities between human and invertebrate GAPDH, likely due to its essential role in glycolysis. Beyond this classic housekeeping role, GAPDH has been implicated in a variety of other cellular processes, including cytoskeletal organization, organelle biogenesis and autophagy. Importantly, single nucleotide polymorphisms in the GAPDH gene have been associated with late onset Alzheimer's disease [54]. GAPDH is involved in neuronal apoptosis [55,56] and known to bind both amyloid beta [57] and its precursor protein [58]. As Alzheimer's disease is characterized by the collapse of proteostasis and GAPDH is involved, discovering novel stabilizing components could provide therapeutic targets for further research. These targets could be affirmed in conventional mouse models. Their poor protein stability is an opportunity for intervention and enhancement by translating the superior proteostasis of long-lived models like Arctica islandica. Combined with our previous work and other comparative studies, long-lived species' general resilience against protein aggregation is a salient factor of successful aging and is notably lacking in more common models.
While the relationship between proteostasis and longevity was robust in the bivalves, it should be noted that the trio of primates do not fit our hypothesis at all doses. The mammals are firmly rooted by the poor C57BL6 performance, but the marmoset, baboon and human results are inconsistent at lower urea concentrations. At 3.5 M urea, GAPDH activity from human muscle is actually significantly lower than marmoset (p < 0.05) and at 5 M urea baboon GAPDH has greater activity than the other mammals. It is only at 6 M urea, that human and baboon are significantly superior to marmoset. These results are puzzling. We should note that unlike our bivalve samples, our primate samples were acquired from any source (and any muscle) we could find. Consequently, the samples were taken from different muscle groups (mouse and marmoset: gastrocnemius, baboon: masseter, human: vastus lateralis). These different muscle groups are composed of different mixtures of fiber types. Also, unlike our bivalve samples, we were unable to control the relative ages of the individuals from which the samples were obtained. Together, these factors could confound direct comparisons among the primate species. The hypothesized trend of greater GAPDH activity in response to misfolding stress only appears among primates at the highest dose tested.
In sum, we have shown that stress resistance of the proteome is reliably associated with longevity in a selection of bivalve mollusk species ranging over nearly two orders of magnitude. As protein homeostasis is emerging as a key player in the aging process, the success in maintaining proteostasis shown by our longest-lived species, A. islandica, suggests that it may have evolved proteome stabilizing mechanisms of relevance to developing interventions that slow the aging process in humans.
Supporting Information
Species are Ruditapes = Ruditapes phillipanarum, Callista = Callista chione, Mercenaria = Mercenaria mercenaria, Arctica = Arctica islandica.
(XLSX)
Acknowledgments
This study was supported by the National Institutes of Health grants P30 AG13319, R01 AG035327, and T32 AG021890 and the Barshop Institute for Longevity and Aging Studies. We thank Mark Luckenbach, Iain Ridgway, Chris Richardson, Suzette Tardif, Michelle Zavala and Nicolas Musi for supplying tissue samples.
Data Availability
All data are contained within the supporting information.
Funding Statement
This study was supported by the National Institutes of Health (http://www.nia.nih.gov/) (grants P30 AG13319, [Center Grant, SNA Core Director, R01 AG035327 (SNA), and T32 AG021890 (SNA)]) and the Barshop Institute for Longevity and Aging Studies. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Schöne BR, Fiebig J, Pfeiffer M, Gleβ R, Hickson J, Johnson ALA, et al. Climate records from a bivalved Methuselah (Arctica islandica, Mollusca; Iceland). Palaeogeogr Palaeoclimatol Palaeoecol. 2005;228: 130–148. 10.1016/j.palaeo.2005.03.049 [DOI] [Google Scholar]
- 2. Wanamaker AD, Heinemeier J, Scourse JD, Richardson CA, Butler PG, Eiriksson J, et al. Very Long-Lived Mollusks Confirm 17th Century AD Tephra-Based Radiocarbon Reservoir Ages for North Icelandic Shelf Waters. Radiocarbon. 2008;50: 399–412. 10.2458/azu_js_rc.v50i3.3222 [DOI] [Google Scholar]
- 3. Richardson CA. Molluscs as archives of environnmental change. Oceanogr Mar Biol Annu Rev. 2001;39: 103–164. [Google Scholar]
- 4. Richardson CA, Walker P. The age structure of a population of the Hard-Shell clam Mercenaria mercenaria from Southhampton Water, England, derived from acetate peel replicas of shell sections. ICES J Mar Sci. 1991;48: 229–236. [Google Scholar]
- 5. Treaster SB, Ridgway ID, Richardson CA, Gaspar MB, Chaudhuri AR, Austad SN. Superior proteome stability in the longest lived animal. Age Dordr Neth. 2014;36: 9597 10.1007/s11357-013-9597-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Philipp E, Brey T, Pörtner H-O, Abele D. Chronological and physiological ageing in a polar and a temperate mud clam. Mech Ageing Dev. 2005;126: 598–609. 10.1016/j.mad.2004.12.003 [DOI] [PubMed] [Google Scholar]
- 7. Strahl J, Philipp E, Brey T, Broeg K, Abele D. Physiological aging in the Icelandic population of the ocean quahog Arctica islandica. Aquat Biol. 2007;1: 77–83. 10.3354/ab00008 [DOI] [Google Scholar]
- 8. Abele D, Strahl J, Brey T, Philipp EE. Imperceptible senescence: Ageing in the ocean quahog Arctica islandica. Free Radic Res. 2008;42: 474–480. 10.1080/10715760802108849 [DOI] [PubMed] [Google Scholar]
- 9. Koga H, Kaushik S, Cuervo AM. Protein homeostasis and aging: The importance of exquisite quality control. Ageing Res Rev. 2011;10: 205–215. 10.1016/j.arr.2010.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morimoto RI, Cuervo AM. Protein Homeostasis and Aging: Taking Care of Proteins From the Cradle to the Grave. J Gerontol A Biol Sci Med Sci. 2009;64A: 167–170. 10.1093/gerona/gln071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Beckman KB, Ames BN. The Free Radical Theory of Aging Matures. Physiol Rev. 1998;78: 547–581. [DOI] [PubMed] [Google Scholar]
- 12. David DC, Ollikainen N, Trinidad JC, Cary MP, Burlingame AL, Kenyon C. Widespread protein aggregation as an inherent part of aging in C. elegans. PLoS Biol. 2010;8: e1000450 10.1371/journal.pbio.1000450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bokov A, Chaudhuri A, Richardson A. The role of oxidative damage and stress in aging. Mech Ageing Dev. 2004;125: 811–826. 10.1016/j.mad.2004.07.009 [DOI] [PubMed] [Google Scholar]
- 14. Martin GM, Austad SN, Johnson TE. Genetic analysis of ageing: role of oxidative damage and environmental stresses. Nat Genet. 1996;13: 25–34. 10.1038/ng0596-25 [DOI] [PubMed] [Google Scholar]
- 15. Nyström T. Role of oxidative carbonylation in protein quality control and senescence. EMBO J. 2005;24: 1311–1317. 10.1038/sj.emboj.7600599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morimoto RI. Proteotoxic stress and inducible chaperone networks in neurodegenerative disease and aging. Genes Dev. 2008;22: 1427–1438. 10.1101/gad.1657108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rana A, Rera M, Walker DW. Parkin overexpression during aging reduces proteotoxicity, alters mitochondrial dynamics, and extends lifespan. Proc Natl Acad Sci U S A. 2013;110: 8638–8643. 10.1073/pnas.1216197110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reis-Rodrigues P, Czerwieniec G, Peters TW, Evani US, Alavez S, Gaman EA, et al. Proteomic analysis of age-dependent changes in protein solubility identifies genes that modulate lifespan. Aging Cell. 2012;11: 120–127. 10.1111/j.1474-9726.2011.00765.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Qiu C, Kivipelto M, von Strauss E. Epidemiology of Alzheimer’s disease: occurrence, determinants, and strategies toward intervention. Dialogues Clin Neurosci. 2009;11: 111–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Berger Z, Ravikumar B, Menzies FM, Oroz LG, Underwood BR, Pangalos MN, et al. Rapamycin alleviates toxicity of different aggregate-prone proteins. Hum Mol Genet. 2006;15: 433–442. 10.1093/hmg/ddi458 [DOI] [PubMed] [Google Scholar]
- 21. Selsby JT, Judge AR, Yimlamai T, Leeuwenburgh C, Dodd SL. Life long calorie restriction increases heat shock proteins and proteasome activity in soleus muscles of Fisher 344 rats. Exp Gerontol. 2005;40: 37–42. 10.1016/j.exger.2004.08.012 [DOI] [PubMed] [Google Scholar]
- 22. Walker GA, Lithgow GJ. Lifespan extension in C. elegans by a molecular chaperone dependent upon insulin-like signals. Aging Cell. 2003;2: 131–139. 10.1046/j.1474-9728.2003.00045.x [DOI] [PubMed] [Google Scholar]
- 23. Cohen E, Bieschke J, Perciavalle RM, Kelly JW, Dillin A. Opposing activities protect against age-onset proteotoxicity. Science. 2006;313: 1604–1610. 10.1126/science.1124646 [DOI] [PubMed] [Google Scholar]
- 24. Dubey A, Forster MJ, Lal H, Sohal RS. Effect of age and caloric intake on protein oxidation in different brain regions and on behavioral functions of the mouse. Arch Biochem Biophys. 1996;333: 189–197. 10.1006/abbi.1996.0380 [DOI] [PubMed] [Google Scholar]
- 25. Sohal RS, Weindruch R. Oxidative Stress, Caloric Restriction, and Aging. Science. 1996;273: 59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chong ZZ, Shang YC, Wang S, Maiese K. Shedding New Light on Neurodegenerative Diseases Through the Mammalian Target of Rapamycin. Prog Neurobiol. 2012;99: 128–148. 10.1016/j.pneurobio.2012.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Graziotto JJ, Cao K, Collins FS, Krainc D. Rapamycin activates autophagy in Hutchinson-Gilford progeria syndrome. Autophagy. 2012;8: 147–151. 10.4161/auto.8.1.18331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moore SA, Lopez A, Richardson A, Pahlavani MA. Effect of age and dietary restriction on expression of heat shock protein 70 in rat alveolar macrophages. Mech Ageing Dev. 1998;104: 59–73. 10.1016/S0047-6374(98)00052-9 [DOI] [PubMed] [Google Scholar]
- 29. Steinkraus KA, Smith ED, Davis C, Carr D, Pendergrass WR, Sutphin GL, et al. Dietary restriction suppresses proteotoxicity and enhances longevity by an hsf-1-dependent mechanism in Caenorhabditis elegans. Aging Cell. 2008;7: 394–404. 10.1111/j.1474-9726.2008.00385.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hsu A-L, Murphy CT, Kenyon C. Regulation of Aging and Age-Related Disease by DAF-16 and Heat-Shock Factor. Science. 2003;300: 1142–1145. 10.1126/science.1083701 [DOI] [PubMed] [Google Scholar]
- 31. Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319: 916–919. 10.1126/science.1141448 [DOI] [PubMed] [Google Scholar]
- 32. Kikis EA, Gidalevitz T, Morimoto RI. Protein homeostasis in models of aging and age-related conformational disease. Adv Exp Med Biol. 2010;694: 138–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ponurovskii SK. Population structure and growth of the Japanese littleneck clam Ruditapes philippinarum in Amursky Bay, Sea of Japan. Russ J Mar Biol. 2008;34: 329–332. [Google Scholar]
- 34. Powell EN, Cummins H. Are molluscan maximum life spans determined by long-term cycles in benthic benthos? Oecologia. 1985;67: 177–182. [DOI] [PubMed] [Google Scholar]
- 35. Ungvari Z, Ridgway I, Philipp EE, Campbell CM, McQuary P, Chow T, et al. Extreme Longevity Is Associated With Increased Resistance to Oxidative Stress in Arctica islandica, the Longest-Living Non-Colonial Animal. J Gerontol A Biol Sci Med Sci. 2011;66A: 741–750. 10.1093/gerona/glr044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nishijima K, Saitoh R, Tanaka S, Ohsato-Suzuki M, Ohno T, Kitajima S. Life span of common marmoset (Callithrix jacchus) at CLEA Japan breeding colony. Biogerontology. 2012;13: 439–443. 10.1007/s10522-012-9388-1 [DOI] [PubMed] [Google Scholar]
- 37. Nowak RM. Walker’s Mammals of the World. JHU Press; 1999. [Google Scholar]
- 38. Doran P, Donoghue P, O’Connell K, Gannon J, Ohlendieck K. Proteomics of skeletal muscle aging. Proteomics. 2009;9: 989–1003. 10.1002/pmic.200800365 [DOI] [PubMed] [Google Scholar]
- 39. Bennion BJ, Daggett V. The molecular basis for the chemical denaturation of proteins by urea. Proc Natl Acad Sci U S A. 2003;100: 5142–5147. 10.1073/pnas.0930122100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Krebs E. Glyceraldehyde-3-Phosphate Dehydrogenase from Yeast. Methods Enzymol. 1955;1. [DOI] [PubMed] [Google Scholar]
- 41. Velick S. Glyceraldehyde-3-Phosphate Dehydrogenase from Muscle. Methods Enzymol. 1955;1. [Google Scholar]
- 42. Solá RJ, Griebenow K. Effects of Glycosylation on the Stability of Protein Pharmaceuticals. J Pharm Sci. 2009;98: 1223–1245. 10.1002/jps.21504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vegarud G, Christnsen TB. Glycosylation of Proteins: a new method of enzyme stabilization. Biotechnol Bioeng. 1975;17: 1391–1397. 10.1002/bit.260170918 [DOI] [PubMed] [Google Scholar]
- 44. M B J B. How chaperones fold proteins. Biol Chem. 1998;379: 245–259. [PubMed] [Google Scholar]
- 45. Zhang G, Fang X, Gui X, Li L, Luo R, Xu F, et al. The oyster genome reveals stress adaptation and complexity of shell formation. Nature 2012; 490:49–54. 10.1038/nature11413 [DOI] [PubMed] [Google Scholar]
- 46. Jakob U, Gaestel M, Engel K, Buchner J. Small heat shock proteins are molecular chaperones. J Biol Chem. 1993;268: 1517–1520. [PubMed] [Google Scholar]
- 47. Ehrnsperger M, Gräber S, Gaestel M, Buchner J. Binding of non-native protein to Hsp25 during heat shock creates a reservoir of folding intermediates for reactivation. EMBO J. 1997;16: 221–229. 10.1093/emboj/16.2.221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lee GJ, Roseman AM, Saibil HR, Vierling E. A small heat shock protein stably binds heat-denatured model substrates and can maintain a substrate in a folding-competent state. EMBO J. 1997;16: 659–671. 10.1093/emboj/16.3.659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Arakawa T, Timasheff SN. The stabilization of proteins by osmolytes. Biophys J. 1985;47: 411–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bolen DW. Effects of naturally occurring osmolytes on protein stability and solubility: issues important in protein crystallization. Methods San Diego Calif. 2004;34: 312–322. 10.1016/j.ymeth.2004.03.022 [DOI] [PubMed] [Google Scholar]
- 51. Yancey PH. Water Stress, Osmolytes and Proteins. Am Zool. 2001;41: 699–709. 10.1093/icb/41.4.699 [DOI] [Google Scholar]
- 52. Yancey PH. Organic osmolytes as compatible, metabolic and counteracting cytoprotectants in high osmolarity and other stresses. J Exp Biol. 2005;208: 2819–2830. 10.1242/jeb.01730 [DOI] [PubMed] [Google Scholar]
- 53. Park J, Han D, Kim K, Kang Y, Kim Y. O-GlcNAcylation disrupts glyceraldehyde-3-phosphate dehydrogenase homo-tetramer formation and mediates its nuclear translocation. Biochim Biophys Acta BBA—Proteins Proteomics. 2009;1794: 254–262. 10.1016/j.bbapap.2008.10.003 [DOI] [PubMed] [Google Scholar]
- 54. Li Y, Nowotny P, Holmans P, Smemo S, Kauwe JSK, Hinrichs AL, et al. Association of late-onset Alzheimer’s disease with genetic variation in multiple members of the GAPD gene family. Proc Natl Acad Sci U S A. 2004;101: 15688–15693. 10.1073/pnas.0403535101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ishitani R, Chuang DM. Glyceraldehyde-3-phosphate dehydrogenase antisense oligodeoxynucleotides protect against cytosine arabinonucleoside-induced apoptosis in cultured cerebellar neurons. Proc Natl Acad Sci U S A. 1996;93: 9937–9941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sawa A, Khan AA, Hester LD, Snyder SH. Glyceraldehyde-3-phosphate dehydrogenase: nuclear translocation participates in neuronal and nonneuronal cell death. Proc Natl Acad Sci U S A. 1997;94: 11669–11674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Oyama R, Yamamoto H, Titani K. Glutamine synthetase, hemoglobin alpha-chain, and macrophage migration inhibitory factor binding to amyloid beta-protein: their identification in rat brain by a novel affinity chromatography and in Alzheimer’s disease brain by immunoprecipitation. Biochim Biophys Acta. 2000;1479: 91–102. [DOI] [PubMed] [Google Scholar]
- 58. Schulze H, Schuler A, Stüber D, Döbeli H, Langen H, Huber G. Rat brain glyceraldehyde-3-phosphate dehydrogenase interacts with the recombinant cytoplasmic domain of Alzheimer’s beta-amyloid precursor protein. J Neurochem. 1993;60: 1915–1922. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Species are Ruditapes = Ruditapes phillipanarum, Callista = Callista chione, Mercenaria = Mercenaria mercenaria, Arctica = Arctica islandica.
(XLSX)
Data Availability Statement
All data are contained within the supporting information.