Abstract
Vector borne diseases are susceptible to climate change because distributions and densities of many vectors are climate driven. The Amazon region is endemic for cutaneous leishmaniasis and is predicted to be severely impacted by climate change. Recent records suggest that the distributions of Lutzomyia (Nyssomyia) flaviscutellata and the parasite it transmits, Leishmania (Leishmania) amazonensis, are expanding southward, possibly due to climate change, and sometimes associated with new human infection cases. We define the vector’s climatic niche and explore future projections under climate change scenarios. Vector occurrence records were compiled from the literature, museum collections and Brazilian Health Departments. Six bioclimatic variables were used as predictors in six ecological niche model algorithms (BIOCLIM, DOMAIN, MaxEnt, GARP, logistic regression and Random Forest). Projections for 2050 used 17 general circulation models in two greenhouse gas representative concentration pathways: “stabilization” and “high increase”. Ensemble models and consensus maps were produced by overlapping binary predictions. Final model outputs showed good performance and significance. The use of species absence data substantially improved model performance. Currently, L. flaviscutellata is widely distributed in the Amazon region, with records in the Atlantic Forest and savannah regions of Central Brazil. Future projections indicate expansion of the climatically suitable area for the vector in both scenarios, towards higher latitudes and elevations. L. flaviscutellata is likely to find increasingly suitable conditions for its expansion into areas where human population size and density are much larger than they are in its current locations. If environmental conditions change as predicted, the range of the vector is likely to expand to southeastern and central-southern Brazil, eastern Paraguay and further into the Amazonian areas of Bolivia, Peru, Ecuador, Colombia and Venezuela. These areas will only become endemic for L. amazonensis, however, if they have competent reservoir hosts and transmission dynamics matching those in the Amazon region.
Introduction
The latest report of the Intergovernmental Panel on Climate Change (IPCC) states that climate change will affect human health through exacerbation of health problems that already exist [1,2]. Vector borne diseases are particularly susceptible to climate change because the distributions of the species involved in the complex transmission cycles are highly related to climatic variables. Under the assumption that species occupy only climatically suitable areas, changes in the geographical distribution of infectious diseases vectors are expected [3–5]. Leishmaniases are climate-sensitive diseases, not least because the distribution and behaviour of their sand fly vectors are strongly affected by rainfall, temperature and humidity [6,7]. The current report investigates the potential effects of climate change on the spatial distribution of Lutzomyia (Nyssomyia) flaviscutellata (Mangabeira) (Diptera, Psychodidae), a phlebotomine sand fly vector [8] of the parasitic protozoan Leishmania (Leishmania) amazonensis Lainson & Shaw (Kinetoplastida, Trypanosomatidae), a causative agent of zoonotic cutaneous leishmaniasis (ZCL) throughout much of tropical South America [9,10].
Leishmaniases are among the world’s six most neglected diseases, affecting men, women and children. The World Health Organization estimates the yearly occurrence as about 200,000 to 400,000 human cases of visceral leishmaniasis and 700,000 to 1.2 million human cases of cutaneous leishmaniasis distributed in 98 countries. In the American continent, Brazil is the country with the highest estimated incidences of both visceral and cutaneous leishmaniases [11]. During the past decades, human migrations have resulted in major deforestation and unplanned settlements in Brazil. This has led to the emergence of new transmission profiles of ZCL, driven mostly by human-made environmental changes [12].
In Brazil, seven Leishmania species are involved in ZCL transmission [13]. The most widely distributed is Leishmania (Viannia) braziliensis (Vianna), recorded in every Brazilian state and causative agent of mucocutaneous leishmaniasis. Leishmania (Viannia) guyanensis Floch is also noteworthy, because of its characteristic clinical manifestation with multiple skin lesions. Leishmania amazonensis is mainly distributed in the Amazon region. This parasite, when infecting humans, can cause localized cutaneous lesions and eventually develop a more severe clinical form, diffuse cutaneous leishmaniasis (DCL). This clinical form is rare, with chronic development, where the immunodepressed patient shows frequent relapses and insufficient responses to available therapies. Human cases of DCL caused by L. amazonensis are recorded sporadically in Amazon areas of Brazil, Venezuela, Colombia, Bolivia and Peru. In Brazil, DCL is recorded in North, Northeast, Central West and Southeast regions [14, 15].
Lutzomyia flaviscutellata has been incriminated as the vector of L. amazonensis in Amazonian Brazil [16,17]. It is a sylvatic sand fly that feeds at ground level on a variety of animals including marsupials and birds, but it is most strongly attracted to rodents [18]. In addition to L. flaviscutellata, there may be as many as five other taxa in the “L. flaviscutellata complex” [19,20], which share morphological and behavioural characteristics and are implicated in the transmission of L. amazonensis or closely related Leishmania species [9]. Lutzomyia (Nyssomyia) olmeca olmeca (Vargas & Nájera) is restricted to Central America; L. (N.) olmeca bicolor Fairchild & Theodor is found in Central America and northern South America; L. (N.) olmeca nociva Young & Arias is restricted to western Amazonian Brazil; L. (N.) reducta Feliciangeli, Ramirez Pérez & Ramirez is restricted to the western Amazon region; and L. (N.) inornata Martins, Falcão & Silva can be considered a synonym of L. flaviscutellata [20]. In contrast, Galati [21] treated the L. flaviscutellata complex as the genus Bichromomyia.
There is evidence that the distribution and population ecology of L. flaviscutellata are influenced by climate, particularly by seasonal precipitation. In eastern Amazon, for example, this vector was more abundant during the dry season in flooded Igapó forests but during the wet season in secondary Capoeira forests [22,23]. Its distribution stretches to forest patches and riverine gallery forests in the Brazilian savannah (the Cerrado biome) and also in the coastal Atlantic forest [20,21].
Future projections from General Circulation Models (GCMs, models that simulate energy transfer in the atmosphere) indicate that the Amazon region will become progressively drier through strengthening and lengthening of the dry season [24] and the precipitation variability associated with the El Niño-Southern Oscillation (ENSO) will likely intensify [25]. In the last eight years, there have been reports of the first human infected by L. amazonensis in Rio de Janeiro State, Brazil [26], together with more widespread captures of L. flaviscutellata to the south of the Amazon region, namely in the Atlantic forest [27,28] and in the Cerrado [29–32]. This has prompted the hypothesis that this vector could be expanding its geographical distribution. Ecological niche modelling (ENM) provides a way of exploring the environmental requirements of L. flaviscutellata and how its distribution might change in response to climate change.
The known occurrences of species can be linked to environmental variation across landscapes in order to estimate ecological niches and geographic distributions. Ecological niche modelling has been widely used in ecology, biogeography and conservation studies, with many published reviews on general applications and specific steps of model development [33–35]. In these models, an algorithm is used to calculate the relationship between species’ occurrence records and environmental variables, in order to create a surface of environmental suitability or probability of species occurrence [36,37]. In climate change studies, after an ENM is critically tested and validated, it can be projected in different time or space, allowing the examination of possible range expansions, contractions or shifts. Discussions of future projections of species’ distributions have to account for variability between different GCMs. Although the use of different GCMs and climate change scenarios can be a great source of variation in ENM, comparative studies demonstrate that most uncertainty is caused by the use of different ENM algorithms [38,39]. Recent comparisons of several niche modelling algorithms concluded that there is not a single approach recommended for every study, and therefore a suite of algorithms should be tested for predictive ability before answering particular questions regarding species niches [40]. In addition to the importance of testing the use of different algorithms, the type and quality of species data strongly influence model outputs. Most ENMs of disease vectors are based on accessible species presence datasets and randomly generated pseudo-absences, instead of carefully selecting absence data in order to significantly improve model performance [41]. The use of species absence data tends to produce better model outputs, which are closely fitted to input data because they can more effectively detect the environmental features discriminating between species presence and absence [42].
Few published studies assessed current and future projections of ecological niches of sand flies using different methods [43–45]. Among South American species, three ZCL vectors in central and southern Brazil–L. (N.) whitmani (Antunes & Coutinho), L. (N.) intermedia (Lutz & Neiva) and L. migonei (França)—were modelled and the results showed that each should find improving climatic conditions in the future, with L. whitmani having the largest predicted range expansion [46]. These three sand fly species are involved mostly in the transmission of Leishmania (Viannia) braziliensis in long-colonized regions, a parasite species with wider distribution and different epidemiology than L. amazonensis [10]. Most transmission of L. amazonensis occurs in the lessdeforested Amazon region. Therefore, it cannot be assumed that L. flaviscutellata will expand into southeastern Brazil, the most populous region of the country [47], in the same way as predicted for L. whitmani [46]. The aims of the current study, therefore, were to define the climate niche of L. flaviscutellata and to use it to explore future projections under climate change scenarios.
Materials and Methods
Review of the current distribution
We performed an extensive literature review to compile occurrence records of L. flaviscutellata. We searched three online databases (PubMed, http://www.ncbi.nlm.nih.gov/pubmed; ISI Web of Knowledge, http://apps.webofknowledge.com and SCOPUS, http://www.scopus.com) during October 2014 using different combinations of the keywords “Psychodidae”, “Lutzomyia” and “flaviscutellata”. We considered as valid records the following species names: Lutzomyia (Nyssomyia) flaviscutellata, Bichromomyia flaviscutellata, Phlebotomus flaviscutellatus, Flebotomus flaviscutellatus, Psychodopygus flaviscutellatus and Phlebotomus apicalis. We also gathered unpublished records from the Health Departments of Brazil and from major Brazilian sand fly collections (Centro de Pesquisas Rene Rachou—FIOCRUZ, Instituto Evandro Chagas—IEC and Faculdade de Saude Publica—USP).
Prior to the descriptions of L. olmeca, L. olmeca bicolor and L. olmeca nociva, all morphologically similar sand flies were identified as L. flaviscutellata. Articles published up to 1980, therefore, were more carefully reviewed and excluded from the database if cited as having identification errors in later reviews [20,48]. In addition, L. flaviscutellata only occurs in South America, whereas the range of L. olmeca stretches northward into Central America and up to Mexico [20,49].
For the ENMs, we also inferred absence records from the literature. The vast majority of the reviewed studies used light traps to capture sand flies. This lowers detectability because L. flaviscutellata is not as highly attracted to light as many sand fly vectors. The most effective traps for L. flaviscutellata are rodent-baited “Disney”-like traps [18,50]. Nonetheless, it can be captured in light traps if the local abundance is high enough–usually in the Amazon forest [51,52]–or in long-term monitoring studies in other biomes [28,53]. Therefore, only localities with at least one year of monthly sand fly sampling with no record of L. flaviscutellata, regardless of the capture method, were considered as absence records.
Species occurrence datasets from secondary data tend to be spatially biased, especially in the Amazon, with records following access points such as roads or rivers [54,55]. Because this could hinder model accuracy, we refined our dataset by removing duplicate records. First, all unique presence and absence points were classified in three categories according to their spatial precision (High: geographical coordinates of capture site given in the reference; Medium: geographical coordinates approximated according to description of capture site; Low: only district or municipality level information, S1 Fig). Then a 20 km buffer was set around each record–if multiple records fell inside the same buffer zone, we retained only the one with the higher spatial precision. The final occurrence dataset used to run the models was composed of 199 presence and 86 absence points (S2 Fig).
Climate variables
WorldClim (http://www.worldclim.org) provides 19 bioclimatic variables derived from monthly averages of temperature and precipitation [56]. We used a subset of these variables as predictors in the current (average for 1950–2000) and 2050 (average for 2041–2060) projections of L. flaviscutellata climate suitability. For future conditions we used downscaled and calibrated projections of 17 GCMs (S2 Table) from the fifth assessment report of the International Panel on Climate Change [25], under two different greenhouse gas concentration pathways: “stabilization” (RCP 4.5) and “high increase” (RCP 8.5). These were chosen to represent contrasting scenarios of 21st century climate policies, where radiative forcing of greenhouse gas stabilizes by 2100 (in RCP4.5) or keeps rising after 2100 (in RCP8.5) [25, 57]. All climate data were at the spatial resolution of 10 arc-minutes (ca. 344 km2 at the equator). This coarse resolution is compatible with the spatial precision of our L. flaviscutellata occurrence data. Furthermore, climatic effects on species distributions are better perceived at coarser spatial resolutions [33,58].
To reduce collinearity in the bioclimatic dataset, we selected a subset of less correlated variables. We generated a Pearson correlation matrix (S3 Fig) from the bioclimatic values of L. flaviscutellata’s records using the package corrplot in the software R (version 0.73 [59]) and within each pair or group of highly correlated variables (r > 0.6) all but one was removed, with the selection criteria being ecological relevance to the vector. The final set of climate predictors used to run the models consisted of: annual mean temperature (BIO1), mean diurnal range (BIO2), temperature seasonality (BIO4), annual precipitation (BIO12), precipitation seasonality (BIO15) and precipitation of warmest quarter (BIO18).
Ecological Niche Models
A critical step is the selection of the modelling algorithm, because the use of different methods can lead to different results [40,42,60]. We modelled using two different algorithms for each type of species dataset: presence only (BIOCLIM and DOMAIN), presence/background (MaxEnt and GARP) and presence/absence (GLM and Random Forests). These six algorithms represent different modelling approaches: climate envelope (BIOCLIM), environmental distance (DOMAIN), statistical adjustment (GLM) and machine learning (MaxEnt, GARP and Random Forests).
For the models produced by BIOCLIM and DOMAIN, only the set of 199 presence records of L. flaviscutellata was used. These presence-only models are developed by constraining the range of environmental predictors to either the minimum and maximum values assigned to all presence records, as in BIOCLIM [61] or by multivariate metrics in environmental space, as in DOMAIN [62].
Presence/background methods estimate potential distributions by comparing the environmental characteristics at sites where the species has been recorded (presence) with those throughout the study region (background). We used MaxEnt, a machine learning algorithm based on maximum entropy [63] and GARP, the genetic algorithm for rule set prediction [64]. For these models, we used the 199 presence records with 10,000 randomly generated background points throughout the study area (South America).
Statistical adjustment and classification algorithms are often used when absence data are available. We used logistic regression, the most common type of Generalized Linear Models (GLM) used in ENM [65] and Random Forests, a machine learning algorithm based on classification of regression trees [66]. GLM and Random Forests models used the full set of 199 presence and 86 absence records.
Most models were developed using the package dismo (version 1.0–5 [67]) in the software R (version 3.1.1 [68]). GARP models were run in OpenModeller (version 1.1.0 [69]), using its “Best Subsets” new implementation [70]. For every model, we used 10-fold cross validation in order to use the whole set of presence/absence points for both model training and testing. In each model run, 10% of points were randomly selected for model testing. Sixty model runs were performed (10 runs for each one of the six algorithms).
We restricted the model outputs to historically accessible areas to the species via dispersal (M area in the BAM diagram framework [71, 72]). We hypothesized the accessible area of L. flaviscutellata based on the ecoregions and elevation where it occurs (using data from WWF [73] and WorldClim [56]) and excluding known areas of the vector absence due to major dispersion barriers, such as the Andes [20,49].
The outputs of the algorithms were mapped as continuous values per pixel representing climate suitability. We used standard deviation to compare results from different algorithms and map uncertainty. As the range of values is different for each algorithm, outputs were converted to binary (0 and 1) by applying a threshold, in order to create ensemble maps. We tested two different threshold rules: i) maximization of sensitivity (true positive rate) and specificity (true negative rate), which performs well in evaluations of climate change impacts on species’ ranges [74,75] and ii) zero omission, a more conservative approach which fully maximizes sensitivity while decreasing specificity. We also masked out the predictor values outside the ranges of the input data to avoid strict model extrapolation in the binary predictions of each algorithm, because, for instance, this could include the consideration of high suitability under extreme values unlikely to be biologically realistic [76]. Model significance was evaluated by binomial probabilities calculated over binary outputs, whereas model performance was assessed by True Skill Statistics (TSS) and Cohen’s kappa. Both TSS and kappa range from -1 to +1, where +1 indicates perfect agreement and values of zero or less indicate a performance no better than random [77].
We then produced ensemble maps overlapping the binary projections of the six models with highest TSS and kappa values for each algorithm. Only the areas of agreement of at least four models were retained in the final maps, following the majority ensemble rule [78]. As we opted to include variability of all 17 GCMs, we summed their projection maps for each algorithm. Final consensus maps of current and future predictions were overlapped to visualize expansion and contraction areas of climate suitability in both climate change scenarios.
All binary output maps were projected in the Albers Equal Area Conic coordinate system using the software ArcGIS 10.1. We then calculated the total predicted area of climate suitability for L. flaviscutellata from the final consensus maps and the changes between current and future predictions. The elevation range of the whole climatically suitable area from the current and future consensus maps was calculated using the Digital Elevation Model available from WorldClim. We sampled elevation values from 10,000 randomly generated points inside the predicted climatically suitable areas, and produced kernel density plots to compare the elevation profiles under current and future scenarios. Wilcoxon rank sum tests were performed to assess statistical differences between each future scenario and the current prediction. Graphics and statistical tests were developed in the software R.
Results
The complete occurrence database of L. flaviscutellata included 342 presence records. Most of them are from Brazil (277), but other South American countries with records of the species include French Guiana (17), Suriname (15), Colombia (11), Peru (10), Trinidad and Tobago (4), Venezuela (4), Bolivia (2) and Ecuador (2) (S1 Fig, see S1 Table for the gazetteer of occurrence records).
Lutzomyia flaviscutellata occurs in areas where the annual mean temperature ranges from 21 to 27.6°C and the annual precipitation varies between 1,139 and 3,843 mm (Table 1). Its current elevation range stretches between 4 and 1,539 m (Table 1).
Table 1. Bioclimatic and elevation ranges of occurrence records of Lutzomyia flaviscutellata.
| Min. | Median | Mean | Max. | |
|---|---|---|---|---|
| Annual Mean Temperature (°C) | 21 | 26.1 | 25.6 | 27.6 |
| Mean Diurnal Range (°C) | 6.4 | 9.8 | 10.21 | 15.5 |
| Temperature Seasonality (standard deviation) | 2.35 | 5.53 | 7.203 | 28.69 |
| Annual Precipitation (mm) | 1139 | 2109 | 2089 | 3843 |
| Precipitation Seasonality (coefficient of variation) | 15 | 58 | 56.98 | 94 |
| Precipitation of Warmest Quarter (mm) | 19 | 318 | 354.2 | 1034 |
| Elevation | 4 | 134 | 200.3 | 1539 |
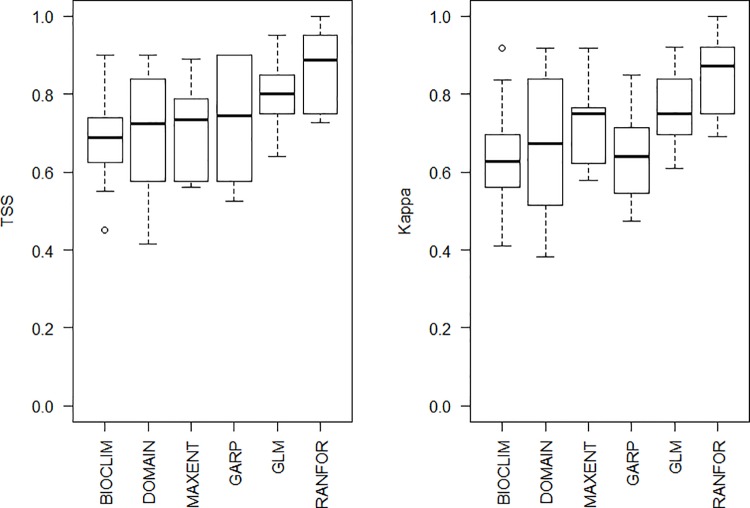
Model performance ranged from fair to excellent (0.4 < TSS > 1; 0.3 < kappa > 1, Fig 1). Outputs with higher values of both TSS and kappa were selected to produce the ensemble models; all of them were significantly better than random predictions (binary probabilities, p< 0.001). For predictions under current climatic conditions, the different algorithms showed a common general pattern with some regional variation (Fig 2). Testing different threshold rule methods showed differences in binary outputs, more evidently in DOMAIN and GLM, while in Random Forests the difference could barely be noticed (S4 Fig). Masking out the predictor values outside the ranges of the input variables showed that models produced by all algorithms had little to no areas of model extrapolation (S5 Fig).
Fig 1. Performance of models produced by the different algorithms according to TSS and Cohen’s kappa.
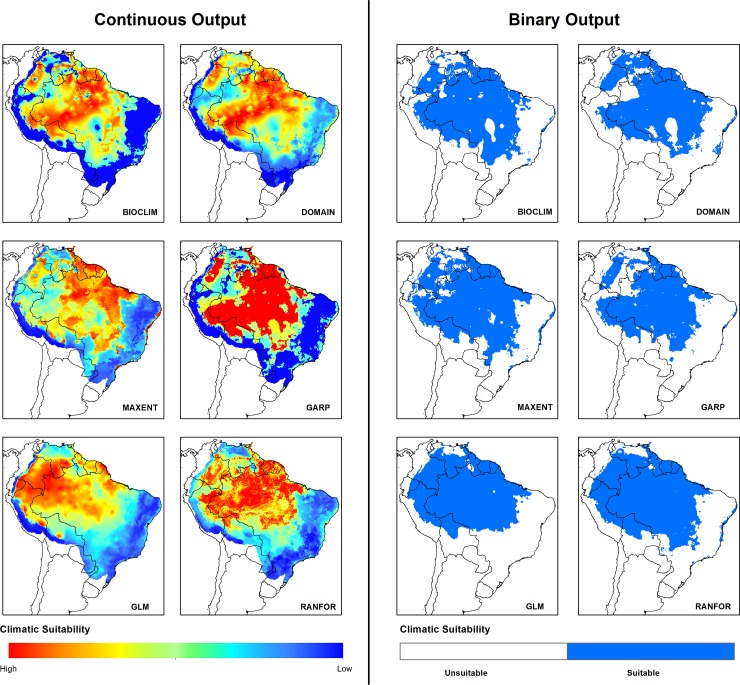
Fig 2. Climate suitability for Lutzomyia flaviscutellata in South America under current conditions from six modelling algorithms.
Continuous output: stretched values of climate suitability. Binary output: suitable areas after the application of the threshold that maximizes model sensitivity and specificity.
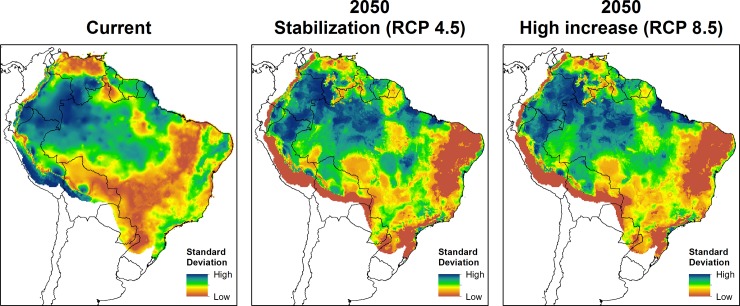
Under current climate conditions, all six algorithms predict most of the Amazon as climatically suitable (Fig 2). Mapping the uncertainty between models (Fig 3) showed that the northwestern region of the continent (most of Colombia, southern Venezuela, northern Peru and northwestern Brazil) were areas of disagreement between models. This becomes clearer in the ensemble outputs, where lighter shades of blue and red indicate fewer consensus between models (Fig 4).
Fig 3. Uncertainty mapping for ecological niche models of L. flaviscutellata.
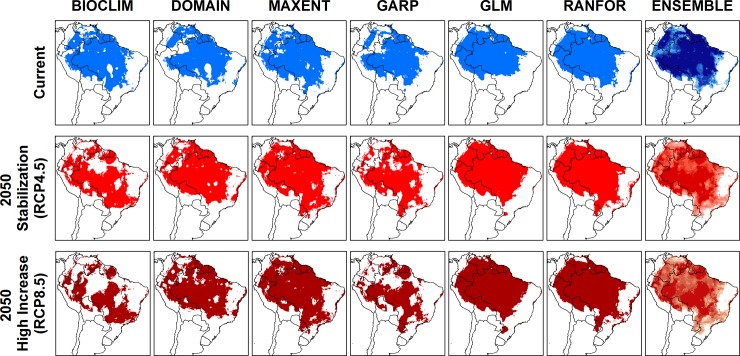
Fig 4. Current and future climate suitability for Lutzomyia flaviscutellata from six modelling algorithms and ensemble maps.
Projections under climate change conditions showed quite different results for each of the 17 GCMs, although there was more variation between different ENM algorithms than between different GCMs (S6, S7, S8 and S9 Figs). When combined, however, most algorithms predicted an expansion of the total area of climate suitability of L. flaviscutellata (Fig 4). All models agree that the species should find improving climate conditions towards the southern limits of its distribution, especially in the “high increase” scenario (RCP 8.5).
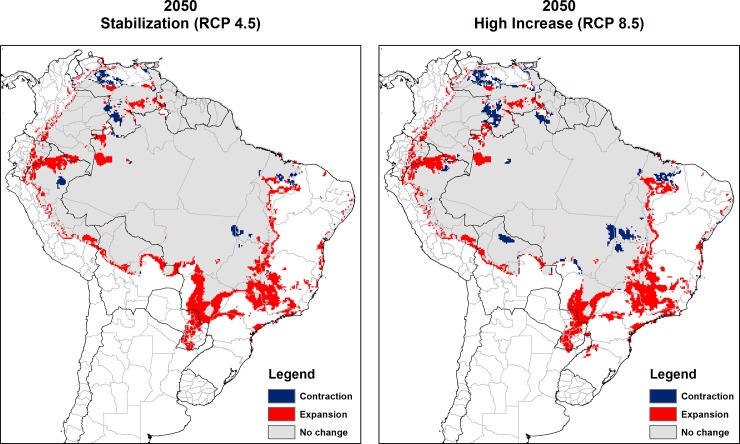
The final consensus maps for both scenarios predict the expansion of the southern limits of the climatically suitable area for L. flaviscutellata (Fig 5), including most notably the Brazilian states of Minas Gerais, Mato Grosso do Sul, São Paulo, Amazonas and Maranhão. Other major expansion areas include eastern Paraguay and Loreto Department in Peru. Some minor contraction is also projected in specific areas of central Brazil, Venezuela and Peru.
Fig 5. Consensus maps of predicted future climate suitability of Lutzomyia flaviscutellata.
Left: Stabilization climate scenario (RCP4.5); right: High increase climate scenario (RCP8.5). Future expansion areas (red), future contraction (blue) and no change between current and future climate suitability (grey).
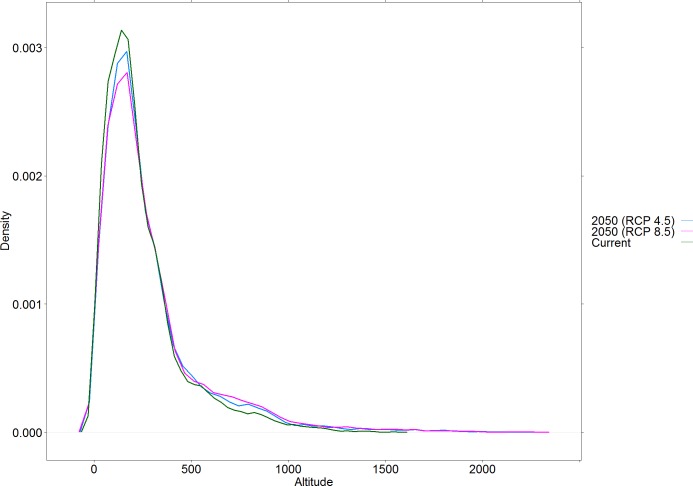
The final predicted climate suitability area for L. flaviscutellata increased by 12.8% in the “stabilization” scenario and by 10.7% in the “high increase” scenario when compared with current predictions (Table 2). There were significant changes in the predicted elevation profile of the species (Fig 6), with the maximum elevation value increasing from 1,545 m to 2,213 m in the “stabilization” scenario and to 2,265 m in the “high increase” scenario (Table 2).
Table 2. Predicted area of climate suitability and elevation ranges of Lutzomyia flaviscutellata calculated from binary predictions of final consensus maps.
| Area (km²) | Elevation (m) | ||||||
|---|---|---|---|---|---|---|---|
| Total | Difference | Min. | Median | Mean | Max. | Difference* | |
| Current | 8,126,549 | - | 0 | 185 | 242.9 | 1,545 | - |
| 2050 (“stabilization” scenario) | 9,165,933 | +12.8% | 0 | 197 | 277.6 | 2,213 | W = 47,022,356 p<0.001 |
| 2050 (“high increase” scenario) | 8,991,938 | +10.7% | 0 | 202 | 287.7 | 2,265 | W = 46,296,853 p<0.001 |
*Statistical significance in elevation difference defined from Wilcoxon rank sum tests between each future scenario and the current prediction.
Fig 6. Elevation profiles of current and future projections of climate suitability of Lutzomyia flaviscutellata.
Discussion
Model predictions and uncertainty
The performance testing of different algorithms followed by selection of the best ones in a consensus is becoming the norm [40,78,79], because the use of different modelling methods may lead to contrasting results [38,39,80]. Presence only models are more mathematically simple and therefore our models produced by BIOCLIM and DOMAIN showed the widest variation in predictive performance. Presence/background methods MaxEnt and GARP had good and similar performances, although MaxEnt had a shorter TSS variability between its outputs. Therefore, MaxEnt would be the algorithm with the best results if we did not have any information on absence records of L. flaviscutellata. This agrees with a comparative study of the predictive power of several modelling algorithms, where MaxEnt was one of the best among methods that do not use absence data [80]. MaxEnt has been a popular method in recent years [43–45,81–83], possibly because of its easy interface and good performance. Random Forests models had the best performance, which agrees with a modelling exercise of Culicoides imicola Kieffer, vector of Bluetongue virus in Spain, where it outperformed GLM and discriminant analysis [41]. Our results showed that the inclusion of species absence data greatly improves model performance, which is in accordance with recent ENM studies [41, 42]. Absence data, however, can also be a source of bias in models if not treated correctly. An absence record may be false if the studied species has low detectability, which could happen for reasons such as low abundance, marked seasonality or lack of an optimal capture method. Even if a species is really absent from a surveyed region, this might be explained by reasons other than lack of environmental suitability, such as dispersal limitations, historical factors or biotic interactions [84]. This is why we used a very conservative criterion for selecting absence records of L. flaviscutellata. Several locations were surveyed for sand flies without the detection of L. flaviscutellata, but they were not used for modelling because, being mostly sporadic captures, they did not provide the sampling effort needed to detect the fly’s presence.
Variation from different GCMs were expected because there is an inherent uncertainty in forecasting anthropogenic climate change [38,85]. However, comparative studies demonstrate that the use of different ENM algorithms, rather than different GCMs, causes most model uncertainty [38,39]. This was shown in individual predictions by ENM algorithms and GCMs (S6, S7, S8 and S9 Figs), where outputs from the same algorithms are more similar to each other than outputs from different ones. The 17 GCMs used here are the most up-to-date, from the phase 5 of the Coupled Model Intercomparison Project [25]. For earlier sets of GCMs, comparative studies demonstrated that HadCM3 had very good representation of the South American climate [86]. However, the latest GCMs have only recently become available and their regional variation is yet to be fully explored. Future attempts to improve projections of species distributions based on climate models would benefit from assessments of regional performance of GCMs.
Future projections from our models indicate that the climatically suitable area for L. flaviscutellata should expand mainly southeastward and southward towards higher latitudes. Other ZCL vectors from South America showed similar results, including L. whitmani [46], as well as other sand flies from Central and North America [43,45]. Both climate change scenarios also indicate that some parts of the Amazon (mainly west and central) should become less climatically suitable for L. flaviscutellata, which might be associated with the region’s predicted reduced precipitation in future decades [24]. ENMs combined with climate change predictions also demonstrated some loss of suitable environments in the Amazon for L. whitmani and L. intermedia [46]. However, these two species occur mainly in the Brazilian savannah and the Atlantic forest, and they are not as widespread in the Amazon as L. flaviscutellata.
In the “high increase” scenario, we expected the total area of climate suitability to be higher than in the “stabilization” scenario, but we found the opposite result (Table 2). This might be an indication that moderate changes in precipitation and temperature may be beneficial to L. flaviscutellata, whereas strong changes would be harmful. This type of response to different scenarios of climate change was observed for other ecological systems, such as the effect of tree species composition on forest net primary production [87]. The increase in the upper elevation bound predicted by both climate change scenarios (Table 2) suggests that the species could shift its elevation range upwards. This was empirically demonstrated for Phlebotomus ariasi Tonnoir from the Madrid region, Spain [88,89]. Future elevation shifts were also predicted for Lutzomyia (Lutzomyia) longipalpis (Lutz & Neiva) and L. evansi (Nuñez-Tovar), vectors of visceral leishmaniasis in Colombia [90].
Public health priorities and future research
Knowledge about vector distributions is essential for understanding leishmaniasis eco-epidemiology and for the success of control and surveillance activities. Our results include the updated geographical distribution of L. flaviscutellata, main incriminated vector of L. amazonensis in South America. This new list of occurrence locations (S1 Table) can support Health Departments for the planning of surveillance activities. The updated distribution, however, does not substantially change the previously known country range of the species according to Young & Duncan [20]. A few recent records of L. flaviscutellata are outside the boundaries of the previously known distribution, such as in Mato Grosso do Sul, Brazil [91,92], in Cusco, Peru [93] and in Orellana, Ecuador [94]. The species has been recorded in almost every South American country north of the Equator except Guyana, although it is likely to occur there based on all model outputs (Fig 2). In Guyana and elsewhere in South America, the detection of L. amazonensis in human cases should ideally be followed up by vector surveys using rodent-baited traps, such as modified Disney traps [50].
There are recent records of L. flaviscutellata in southeastern Brazil [27,28,53,95]. It is nearly impossible, however, to test the hypothesis of a recent expansion of L. flaviscutellata distribution associated with climate change, because of insufficient earlier sampling to demonstrate the species’ prior absence in some regions. Both the coastal Atlantic forest and savannah regions in Southeast and Central Brazil are already climatically suitable for L. flaviscutellata according to our models for current conditions. This result could be interpreted as a refutation of the hypothesis of a recent distribution expansion associated with climate change. However, the recent records of the species in the Atlantic forest and Brazilian savannah biomes were incorporated into the model, assuming that they represent part of the historical distribution of the species. Because these records were included in model building, the classification of the region where they occur as climatically suitable under current conditions was expected, and could be a methodological artefact. However, the emergence of transmission of L. amazonensis could be a proxy for the vector’s expansion, such as the recent records in Paraty, southeast Brazil [26] and in Serra da Bodoquena region, central-west Brazil [91,96]. At the same time, there is growing evidence that L. flaviscutellata can be found near human dwellings in rural areas. The species has been recently captured in peridomestic areas outside the Amazon forest [28,32,97] and even in peri-urban areas [29,30,95,98]. The ability of L. flaviscutellata to survive deforestation and rapidly colonize secondary habitats has been demonstrated near the Brazilian city of Belém [22] as well as in plantations of exotic trees in the eastern Amazon [23]. This suggests that, even if L. flaviscutellata does not fully expand its distribution to the predicted future areas of climate suitability, it may colonize areas of recent deforestation at a local scale and thus increase the local risk of human exposure to L. amazonensis. The inclusion of land cover variables in our models would likely have reduced the biotic uncertainty of our predictions at a local scale. Nevertheless, the decision to use only climate variables was justified because of the continental scale and relatively low resolution of the current study.
Our models do not include information on the occurrence of the parasite L. amazonensis. Some correlations between ENSO and increases in leishmaniasis have been demonstrated [99–102]. Because future forecasts suggest an intensification of ENSO-related precipitation variability [25], ZCL transmission in the Amazon might increase due to climate change, regardless of the likely changes in the distribution of its vectors. Surveillance for infections of L. amazonensis is difficult, because identification of the parasite to species is not routine. Inclusion of data on parasite occurrence would improve our ability to predict risk areas for human infection, for which information on the distribution of competent reservoir hosts would also be required, as well as a mechanistic, process-based modelling of the transmission dynamics [8,103].
The current results raise awareness of the predicted expansion of L. flaviscutellata near the borders of the Amazon–in Bolivia, Peru, Colombia and Venezuela–as well as many parts of Minas Gerais and São Paulo states, in Southeast Brazil (Fig 5). The resident population of these two states is approximately 60.8 million people, more than twice the 25.4 million people living in all the Amazonian states of Brazil [47], where most recorded transmission of L. amazonensis currently occurs. In fact, there are two relatively recent records of L. amazonensis infections in domestic dogs in both states, Minas Gerais [104] and São Paulo [105]. The predicted expansion of the area of climate suitable for L. flaviscutellata in Maranhão state has the potential to significantly increase the prevalence of DCL caused by L. amazonensis, because this form of the disease is associated with the state [14]. This parasite species has also been recorded sporadically in Paraguay, but not L. flaviscutellata [9,106]. The elevation range of L. flaviscutellata could increase as predicted, although vector abundances might well remain too low to permit establishment of new L. amazonensis transmission cycles. At high elevation, such as the Andes region, sand fly diversity is lower and leishmaniasis transmission is sustained by a few dominant vectors [107,108]. If transmission cycles of L. amazonensis driven by the dispersion of the vector L. flaviscutellata establish in these regions, more people will be at risk of acquiring the disease.
Our large-scale study serves as a base for future studies exploring factors that constrain the distribution of L. flaviscutellata at finer scales, which is a necessary contribution to Public Health research and interventions aimed at reducing the disease burden. We conclude that this vector might well find improving climate conditions for its expansion in the approaching decades, although these new areas will only become endemic for the transmission of L. amazonensis, if reservoir host populations are present and transmission dynamics are sufficient. In Southeast Brazil, at least, this is already happening [26,104,105, 109].
Supporting Information
High: geographical coordinates of capture site given in the published article; Medium: geographical coordinates approximated according to description of capture site; Low: only district or municipality level information.
(TIF)
(TIF)
bio1: annual mean temperature; bio2: mean diurnal range; bio3: isothermality; bio4: temperature seasonality; bio5: max temperature of warmest month; bio6: min temperature of coldest month; bio7: temperature annual range; bio8: mean temperature of wettest quarter; bio9: mean temperature of driest quarter; bio10: mean temperature of warmest quarter; bio11: mean temperature of coldest quarter; bio12: annual precipitation; bio13: precipitation of wettest month; bio14: precipitation of driest month; bio15: precipitation seasonality; bio16: precipitation of wettest quarter; bio17: precipitation of driest quarter; bio18: precipitation of warmest quarter; bio19: precipitation of coldest quarter.
(TIFF)
(TIF)
(TIF)
Each map shows binary model outputs. Future projections include the percentage of area lost or gain in comparison with current predictions. For names of each General Circulation Model, see S2 Table.
(TIF)
Each map shows binary model outputs. Future projections include the percentage of area lost or gain in comparison with current predictions. For names of each General Circulation Model, see S2 Table.
(TIF)
Each map shows binary model outputs. Future projections include the percentage of area lost or gain in comparison with current predictions. For names of each General Circulation Model, see S2 Table.
(TIF)
Each map shows binary model outputs. Future projections include the percentage of area lost or gain in comparison with current predictions. For names of each General Circulation Model, see S2 Table.
(TIF)
(XLSX)
(XLSX)
Acknowledgments
The authors thank the curators of the sand fly collections of Centro de Pesquisas René Rachou–FIOCRUZ, Instituto Evandro Chagas–IEC, and Faculdade de Saúde Pública–USP, also Thiago Vasconcelos dos Santos, Andrey Andrade and Maurício Luiz Vilela for providing species records. We also thank A. Townsend Peterson and two anonymous reviewers who gave substantial contributions to this manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was supported by Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro, FAPERJ (http://www.faperj.br; Grants E-26/101.450/2012, BMC; E-26/111.618/2011, EFR; E-26/111.577/2014, MMV); Conselho Nacional de Desenvolvimento Científico e Tecnológico, CNPq (http://www.cnpq.br; Grants 203165/2014-4, BMC; 400113/2011-2, EFR; 34/2012, MMV; 477524/2012-2, MMV; 444704/2014, MMV; 550022/2014-7, MMV); Financiadora de Estudos e Projetos, FINEP (http://www.finep.gov.br; Grant 01.13.0353.00, MMV); Instituto Oswaldo Cruz, IOC/FIOCRUZ (http://www.ioc.fiocruz.br; EFR), and National Science Foundation (http://www.nsf.gov; Grants #DBI-1300426, PDR). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Woodward A, Smith KR, Campbell-Lendrum D, Chadee DD, Honda Y, Liu Q, et al. Climate change and health: on the latest IPCC report. Lancet. 2014; 383: 1185–1189. 10.1016/S0140-6736(14)60576-6 [DOI] [PubMed] [Google Scholar]
- 2. IPCC. Summary for Policymakers In: Field CB, Barros VR, Dokken DJ, Mach KJ, Mastrandrea MD, Bilir TE, et al. , editors. Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change Cambridge: Cambridge University Press; 2014. p. 1–32. [Google Scholar]
- 3. Kovats RS, Campbell-Lendrum DH, McMichael AJ, Woodward A, Cox JH. Early effects of climate change: do they include changes in vector-borne diseases? Philos Trans R Soc Lond B. 2001; 356: 1057–1068. 10.1098/rstb.2001.0894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rosenthal J. Climate change and the geographical distribution of infectious diseases. Ecohealth. 2009; 189–495. 10.1007/s10393-010-0314-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Parham PE, Waldock J, Christophides GK, Hemming D, Agusto F, Evans KJ, et al. Climate, environmental and socio-economic change: weighing up the balance in vector-borne disease transmission. Philos Trans R Soc B. 2015; 370: 20130551 10.1098/rstb.2013.0551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ready PD. Leishmaniasis emergence and climate change. Rev Sci Tech. 2008; 27: 399–412. [PubMed] [Google Scholar]
- 7.WHO. World Health Organization. Control of the leishmaniases: report of a meeting of the WHO Expert Committee on the Control of Leishmaniases, Geneva, 22–26 March 2010. WHO Technical Report Series, no. 949. Geneva: WHO Press; 2010.
- 8. Ready PD. Biology of phlebotomine sand flies as vectors of disease agents. Annu Rev Entomol. 2013; 58: 227–250. 10.1146/annurev-ento-120811-153557 [DOI] [PubMed] [Google Scholar]
- 9. Lainson R, Shaw JJ, New World Leishmaniasis In: Collier L, Balows A, Sussman M, editors. Topley & Wilson’s Microbiology and Microbial Infections, 10th ed., Vol 5: Parasitology London: Hodder Arnold; 2005. p. 313–349. [Google Scholar]
- 10. Rangel EF, Lainson R. Proven and putative vectors of American cutaneous leishmaniasis in Brazil: aspects of their biology and vectorial competence. Mem Inst Oswaldo Cruz. 2009; 104(7): 937–954. 10.1590/S0074-02762009000700001 [DOI] [PubMed] [Google Scholar]
- 11. Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE. 2012; 7(5): e35671 10.1371/journal.pone.0035671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rangel EF, Costa SM, Carvalho BM. Environmental changes and the geographic spreading of American cutaneous leishmaniasis in Brazil In: Claborn D, editor. Leishmaniasis–Trends in Epidemiology, Diagnosis and Treatment. Rijeka: InTech; 2014. 10.5772/57207 [DOI] [Google Scholar]
- 13. Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde Manual de Vigilância da Leishmaniose Tegumentar Americana, 2a ed. Brasília: Editora do Ministério da Saúde; 2007. Available: http://bvsms.saude.gov.br/bvs/publicacoes/manual_vigilancia_leishmaniose_2ed.pdf. [Google Scholar]
- 14. Costa JA, Costa AAUML, Elkhoury AN, Bezerril ACR, Barral A, Saldanha ACR. Leishmaniose cutânea difusa (LCD) no Brasil após 60 anos de sua primeira descrição. Gazeta Médica da Bahia. 2009; 79(Supl.3): 16–24. [Google Scholar]
- 15. Zerpa O, Convit J. Leishmaniasis cutánea en Venezuela. Gazeta Médica da Bahia. 2009; 79(Supl.3): 30–34. [Google Scholar]
- 16. Lainson R, Shaw JJ. Leishmaniasis in Brazil I. Observations on enzootic rodent leishmaniasis—Incrimination of Lutzomyia flaviscutellata (Mangabeira) as the vector in the lower Amazonian Basin. Trans R Soc Trop Med Hyg. 1968; 62: 385–395. 10.1016/0035-9203(68)90090-4 [DOI] [PubMed] [Google Scholar]
- 17. Lainson R, Shaw JJ, Silveira FT, de Souza AA, Braga RR, Ishikawa EA. The dermal leishmaniases of Brazil, with special reference to the eco-epidemiology of the disease in Amazonia. Mem Inst Oswaldo Cruz. 1994; 89(3): 435–443. 10.1590/S0074-02761994000300027 [DOI] [PubMed] [Google Scholar]
- 18. Shaw JJ, Lainson R. Leishmaniasis in Brazil: II. Observations on enzootic rodent leishmaniasis in the lower Amazon region–the feeding habits of the vector, Lutzomyia flaviscutellata, in reference to man, rodents and other animals. Trans R Soc Trop Med Hyg. 1968; 62(3): 396–405. 10.1016/0035-9203(68)90091-6 [DOI] [PubMed] [Google Scholar]
- 19. Lewis DJ. The Lutzomyia flaviscutellata complex (Diptera: Psychodidae). J Med Entomol. 1975; 12(3): 363–368. 10.1093/jmedent/12.3.363 [DOI] [PubMed] [Google Scholar]
- 20. Young DG, Duncan NA. Guide to the identification and geographic distribution of Lutzomyia sand flies in Mexico, the West Indies, Central and South America (Diptera: Psychodidae). Memoirs of the American Entomological Institute. 1994; 54: 1–881. [Google Scholar]
- 21. Galati EAB. Classificação de Phlebotominae In: Rangel EF, Lainson R., editors. Flebotomíneos do Brasil. Rio de Janeiro: Editora Fiocruz; 2003. p. 23–52. [Google Scholar]
- 22. Shaw JJ, Lainson R. Leishmaniasis in Brazil: VI. Observations on the seasonal variations of Lutzomyia flaviscutellata in different types of forest and its relationship to enzootic rodent leishmaniasis (Leishmania mexicana amazonensis). Trans R Soc Trop Med Hyg. 1972; 66(5): 709–717. 10.1016/0035-9203(72)90084-3 [DOI] [PubMed] [Google Scholar]
- 23. Ready PD, Lainson R, Shaw JJ. Leishmaniasis in Brazil. XX: Prevalence of “enzootic rodent leishmaniasis” (Leishmania mexicana amazonensis) and apparent absence of pian-bois (Le. braziliensis guyanensis), in plantations of introduced tree species and in other non-climax forests in eastern Amazonia. Trans R Soc Trop Med Hyg. 1983; 77: 775–785. 10.1016/0035-9203(83)90288-2 [DOI] [PubMed] [Google Scholar]
- 24. Joetzjer E, Douville H, Delire C, Ciais P. Present-day and future Amazonian precipitation in global climate models: CMIP5 versus CMIP3. Clim Dyn. 2013; 41(11–12): 2921–2936. 10.1007/s00382-012-1644-1 [DOI] [Google Scholar]
- 25. IPCC. Summary for Policymakers In: Stocker TF, Qin D, Plattner G-K, Tignor M, Allen SK, Boschung J, et al. , editors. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change Cambridge: Cambridge University Press; 2013. p. 1–27. [Google Scholar]
- 26. Azeredo-Coutinho RBG, Conceição-Silva F, Schubach A, Cupolillo E, Quintela LP, Madeira MF, et al. First report of diffuse cutaneous leishmaniasis and Leishmania amazonensis infection in Rio de Janeiro State, Brazil. Trans R Soc Trop Med Hyg. 2007; 101: 735–737. 10.1016/j.trstmh.2007.01.005 [DOI] [PubMed] [Google Scholar]
- 27. Pinto IS, dos Santos CB, Ferreira AL, Falqueto A. Richness and diversity of sand flies (Diptera, Psychodidae) in an Atlantic rainforest reserve in southeastern Brazil. J Vector Ecol. 2010; 35(2): 325–332. 10.1111/j.1948-7134.2010.00090.x [DOI] [PubMed] [Google Scholar]
- 28. Carvalho BM, Maximo M, Costa WA, Santana ALF, Costa SM, Rego TANC, et al. Leishmaniasis transmission in an ecotourism area: potential vectors in Ilha Grande, Rio de Janeiro State, Brazil. Parasit Vectors. 2013; 6: 325 10.1186/1756-3305-6-325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nunes VLB, Galati EAB, Cardozo C, Rocca MEG, de Andrade ARO, Santos MFC, et al. Estudo de flebotomíneos (Diptera, Psychodidae) em área urbana do município de Bonito, Mato Grosso do Sul, Brasil. Rev Bras Entomol. 2008; 52(3): 446–451. 10.1590/S0085-56262008000300019 [DOI] [Google Scholar]
- 30. Vilela ML, Azevedo CG, Carvalho BM, Rangel EF. Phlebotomine fauna (Diptera: Psychodidae) and putative vectors of leishmaniases in impacted area by hydroelectric plant, state of Tocantins, Brazil. PLoS One. 2011; 6(12): e27721 10.1371/journal.pone.0027721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vilela ML, Pita-Pereira D, Azevedo CG, Godoy RE, Britto C, Rangel EF. The phlebotomine fauna (Diptera: Psychodidae) of Guaraí, state of Tocantins, with an emphasis on the putative vectors of American cutaneous leishmaniasis in rural settlement and periurban areas. Mem Inst Oswaldo Cruz. 2013; 108(5): 578–585. 10.1590/S0074-02762013000500007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brito VN, de Almeida ABPF, Nakazato L, Duarte R, Souza CO, Sousa VRF. Phlebotomine fauna, natural infection rate and feeding habits of Lutzomyia cruzi in Jaciara, state of Mato Grosso, Brazil. Mem Inst Oswaldo Cruz. 2014; 109(7): 899–904. 10.1590/0074-0276140112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guisan A, Zimmermann NE. Predictive habitat distribution models in ecology. Ecol Modell. 2000; 135: 147–186. 10.1016/S0304-3800(00)00354-9 [DOI] [Google Scholar]
- 34. Guisan A, Thuiller W. Predicting species distribution: offering more than simple habitat models. Ecol Lett. 2005; 8: 993–1009. 10.1111/j.1461-0248.2005.00792.x [DOI] [PubMed] [Google Scholar]
- 35. Elith J, Leathwick JR. Species distribution models: ecological explanation and prediction across space and time. Annual Review of Ecology, Evolution and Systematics. 2009; 40: 677–697. 10.1146/annurev.ecolsys.110308.120159 [DOI] [Google Scholar]
- 36. Franklin J. Mapping species distributions Spatial inference and prediction. Cambridge: Cambridge University Press; 2010. [Google Scholar]
- 37. Peterson AT, Soberón J, Pearson RG, Anderson RP, Martínez-Meyer E, Nakamura M, et al. Ecological niches and geographic distributions Monographs in Population Biology 49 New Jersey: Princeton University Press; 2011. [Google Scholar]
- 38. Pearson RG, Thuiller W, Araújo MB, Martinez-Meyer E, Brotons L, McClean C, et al. Model-based uncertainty in species range prediction. J Biogeogr. 2006; 33: 1704–1711. 10.1111/j.1365-2699.2006.01460.x [DOI] [Google Scholar]
- 39. Diniz-Filho JAF, Bini LM, Rangel TF, Loyola RD, Hof C, Nogués-Bravo D, et al. Partitioning and mapping uncertainties in ensembles of forecasts of species under climate change. Ecography. 2009; 32: 897–906. 10.1111/j.1600-0587.2009.06196.x [DOI] [Google Scholar]
- 40. Qiao H, Soberón J, Peterson AT. No silver bullets in correlative ecological niche modeling: Insights from testing among many potential algorithms for niche estimation. Methods Ecol Evol. [Google Scholar]
- 41. Peters J, De Baets B, Van doninck J, Calvete C, Lucientes J, De Clercq EM, et al. Absence reduction in entomological surveillance data to improve niche-based distribution models for Culicoides imicola . Prev Vet Med. 2011; 100: 15–28. 10.1016/j.prevetmed.2011.03.004 [DOI] [PubMed] [Google Scholar]
- 42. Li X, Wang Y. Applying various algorithms for species distribution modelling. Integr Zool. 2013; 8: 124–135. 10.111/1749-4877.12000 [DOI] [PubMed] [Google Scholar]
- 43. González C, Wang O, Strutz SE, González-Salazar C, Sánchez-Cordero V, Sarkar S. Climate change and risk of leishmaniasis in North America: predictions from ecological niche models of vector and reservoir species. PLoS Negl Trop Dis. 2010; 4(1): e585 10.1371/journal.pntd.0000585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fischer D, Moeller P, Thomas SM, Naucke TJ, Beierkuhnlein C. Combining climatic projections and dispersal ability: a method for estimating the responses of sandfly vector species to climate change. PLoS Negl Trop Dis. 2011; 5(11): e1407 10.1371/journal.pntd.0001407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Moo-Llanes D, Ibarra-Cerdeña CN, Rebollar-Téllez EA, Ibáñez-Bernal S, González C, Ramsey JM. Current and future niche of North and Central American sand flies (Diptera: Psychodidae) in climate change scenarios. PLoS Negl Trop Dis. 2013; 7(9): e2421 10.1371/journal.pntd.0002421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Peterson AT, Shaw JJ. Lutzomyia vectors for cutaneous leishmaniasis in Southern Brazil: ecological niche models, predicted geographic distributions, and climate change effects. Int J Parasitol. 2003; 33: 919–931. 10.1016/S0020-7519(03)00094-8 [DOI] [PubMed] [Google Scholar]
- 47.IBGE. Censo 2010. 2010. Available: http://www.censo2010.ibge.gov.br.
- 48. Fairchild GB, Theodor O. On Lutzomyia flaviscutellata (Mangabeira) and L. olmeca (Vargas and Diaz-Najera) (Diptera: Psychodidae). J Med Entomol. 1971; 8(2): 153–159. 10.1093/jmedent/8.2.153 [DOI] [PubMed] [Google Scholar]
- 49. Forattini OP. Entomologia médica 4° Volume: Psychodidae Phlebotominae. Leishmanioses. Bartonelose São Paulo: Edgard Blücher; 1973. [Google Scholar]
- 50. Dorval MEC, Alves TP, Oliveira AG, Brazil RP, Galati EAB, Cunha RV. Modification of Disney trap for capture of sand flies (Diptera: Psychodidae: Phlebotominae). Mem Inst Oswaldo Cruz. 2007; 102(7): 877–878. 10.1590/S0074-02762007005000111 [DOI] [PubMed] [Google Scholar]
- 51. Alves VR, Freitas RA, Santos FL, Oliveira AFJ, Barrett TV, Shimabukuro PHF. Sand flies (Diptera, Psychodidae, Phlebotominae) from Central Amazonia and four new records for the Amazonas state, Brazil. Rev Bras Entomol. 2012; 56(2): 220–227. 10.1590/S0085-56262012005000020 [DOI] [Google Scholar]
- 52. Ferreira JVS, dos Santos TV, dos Santos EM, Gorayeb IS. Phlebotomine sand flies (Diptera: Psychodidae) in forest fragments of Belém metropolitan area, Pará State, Brazil, with considerations on vectors of American cutaneous leishmaniasis agents. Revista Pan-Amazônica de Saúde. 2014; 5(2): 29–35. 10.5123/S2176-62232014000200004 [DOI] [Google Scholar]
- 53. Gomes AC, Galati EAB, de Paula MB, Mucci LF. Phlebotomines in the area adjacent to the Porto Primavera dam, between São Paulo and Mato Grosso do Sul States, Brazil. Revista de Patologia Tropical. 2012; 41(2): 215–221. 10.5216/rpt.v41i2.19323 [DOI] [Google Scholar]
- 54. Phillips SJ, Dudík M, Elith J, Graham CH, Lehmann A, Leathwick J, et al. Sample selection bias and presence-only distribution models: implications for background and pseudo-absence data. Ecol Appl. 2009; 19: 181–197. 10.1890/07-2153.1 [DOI] [PubMed] [Google Scholar]
- 55. Vale MM, Jenkins CN. Across-taxa incongruence in patterns of collecting bias. J Biogeogr. 2012; 39(9): 1744–1748. 10.1111/j.1365-2699.2012.02750.x [DOI] [Google Scholar]
- 56. Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology. 2005; 25: 1965–1978. 10.1002/joc.1276 [DOI] [Google Scholar]
- 57. Moss RH, Edmonds JA, Hibbard KA, Manning MR, Rose SK, van Vuuren DP, et al. The next generation of scenarios for climate change research and assessment. Nature. 2010; 463: 747–756. 10.1038/nature08823 [DOI] [PubMed] [Google Scholar]
- 58. Pearson RG, Dawson TP. Predicting the impacts of climate change on the distribution of species: are bioclimate envelope models useful? Glob Ecol Biogeogr. 2003; 12: 361–371. 10.1046/j.1466-822X.2003.00042.x [DOI] [Google Scholar]
- 59.Wei T. corrplot: visualization of a correlation matrix. R package version 0.73. 2013. Available: http://CRAN.R-project.org/package=corrplot.
- 60. Elith J, Graham CH. Do they? How do they? WHY do they differ? On finding reasons for differing performances of species distribution models. Ecography. 2009; 32(1), 66–77. 10.1111/j.1600-0587.2008.05505.x [DOI] [Google Scholar]
- 61. Booth TH, Nix HA, Busby JR, Hutchinson MF. BIOCLIM: the first species distribution modelling package, its early applications and relevance to most current MaxEnt studies. Divers Distrib. 2014; 20: 1–9. 10.1111/ddi.12144 [DOI] [Google Scholar]
- 62. Carpenter G, Gillison AN, Winter J. DOMAIN: a flexible modeling procedure for mapping potential distributions of plants and animals. Biodivers Conserv. 1993; 2: 667–680. 10.1007/BF00051966 [DOI] [Google Scholar]
- 63. Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modeling of species geographic distributions. Ecol Modell. 2006; 190: 231–259. 10.1016/j.ecolmodel.2005.03.026 [DOI] [Google Scholar]
- 64. Stockwell D. The GARP modelling system: problems and solutions to automated spatial prediction, Int J Geogr Inf Sci. 1999; 13(2): 143–158. 10.1080/136588199241391 [DOI] [Google Scholar]
- 65. Guisan A, Edwards TC Jr, Hastie T. Generalized linear and generalized additive models in studies of species distributions: setting the scene. Ecol Modell. 2002; 157: 89–100. 10.1016/S0304-3800(02)00204-1 [DOI] [Google Scholar]
- 66. Breiman L. Random forests. Mach Learn. 2001; 45: 15–32. 10.1023/A:1010933404324 [DOI] [Google Scholar]
- 67.Hijmans RJ, Phillips S, Leathwick J, Elith J. dismo: Species distribution modeling. R package version 1.0–5. 2014. Available: http://CRAN.R-project.org/package=dismo.
- 68. R Core Team. R: A language and environment for statistical computing R foundation for Statistical Computing, Vienna, Austria: 2014. Available: http://www.R-project.org/. [Google Scholar]
- 69. Muñoz MES, Giovanni R, Siqueira MF, Sutton T, Brewer P, Pereira RS, et al. "openModeller: a generic approach to species' potential distribution modelling". Geoinformatica. 2011; 15(1): 111–135. 10.1007/s10707-009-0090-7 [DOI] [Google Scholar]
- 70. Anderson RP, Lew D, Peterson AT. Evaluating predictive models of species' distributions: criteria for selecting optimal models. Ecol Modell. 2003; 162: 211–232. 10.1016/S0304-3800(02)00349-6 [DOI] [Google Scholar]
- 71. Soberón J, Peterson AT. Interpretation of models of fundamental ecological niches and species’ distributional areas. Biodiversity Informatics. 2005; 2: 1–10. 10.17161/bi.v2i0.4 [DOI] [Google Scholar]
- 72. Barve N, Barve V, Jiménez-Valverde A, Lira-Noriega A, Maher SP, Peterson AT, et al. The crucial role of the accessible area in ecological niche modeling and species distribution modeling. Ecol Modell. 2011; 222: 1810–1819. 10.1016/j.ecolmodel.2011.02.011 [DOI] [Google Scholar]
- 73. Olson DM, Dinerstein E, Wikramanayake ED, Burgess ND, Powell GVN, Underwood EC, et al. Terrestrial ecoregions of the world: a new map of life on Earth. BioScience. 2001; 51(11): 933–938. 10.1641/0006-3568(2001)051[0933:TEOTWA]2.0.CO;2 [DOI] [Google Scholar]
- 74. Liu C, Berry PM, Dawson TP, Pearson RG. Selecting thresholds of occurrence in the prediction of species distributions. Ecography. 2005; 28: 385–393. 10.1111/j.0906-7590.2005.03957.x [DOI] [Google Scholar]
- 75. Liu C, White M, Newell G. Selecting thresholds for the prediction of species occurrence with presence-only data. J Biogeogr. 2013; 40: 778–789. 10.1111/jbi.12058 [DOI] [Google Scholar]
- 76. Owens HL, Campbell LP, Dornak L, Saupe EE, Barve N, Soberón J, et al. Constraints on interpretation of ecological niche models by limited environmental ranges on calibration areas. Ecol Modell. 2013; 263: 10–18. 10.1016/j.ecolmodel.2013.04.011 [DOI] [Google Scholar]
- 77. Allouche O, Tsoar A, Kadmon R. Assessing the accuracy of species distribution models: prevalence, kappa and the true skill statistics (TSS). J Appl Ecol. 2006; 43: 1223–1232. 10.1111/j.1365-2664.2006.01214.x [DOI] [Google Scholar]
- 78. Araújo M, New M. Ensemble forecasting of species distributions. Trends Ecol Evol. 2007; 22(1): 42–47. 10.1016/j.tree.2006.09.010 [DOI] [PubMed] [Google Scholar]
- 79. Guillera-Arroita G, Lahoz-Monfort JJ, Elith J, Gordon A, Kujala H, Lentini PE. Is my species distribution model fit for purpose? Matching data and models to applications. Global Ecol Biogeogr. 2015; 24: 276–292. 10.1111/geb.12268 [DOI] [Google Scholar]
- 80. Elith J, Graham CH, Anderson RP, Dudík M, Ferrier S, Guisan A, et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography. 2006; 29: 129–151. 10.1111/j.2006.0906-7590.04596.x [DOI] [Google Scholar]
- 81. Fischer D, Thomas SM, Niemitz F, Reineking B, Beierkuhnlein C. Projection of climatic suitability for Aedes albopictus Skuse (Culicidae) in Europe under climate change conditions. Glob Planet Change. 2011; 78: 54–64. 10.1016/j.gloplacha.2011.05.008 [DOI] [Google Scholar]
- 82. Fuller DO, Parenti MS, Hassan AN, Beier J. Linking land cover and species distribution models to project potential ranges of malaria vectors: an example using Anopheles arabiensis in Sudan and Upper Egypt. Malar J. 2012; 11: 264 10.1186/1475-2875-11-264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Porretta D, Mastrantonio V, Amendolia S, Gaiarsa, Epis S, Genchi C, et al. Effects of global changes on the climatic niche of the tick Ixodes ricinus inferred by species distribution modelling. Parasit Vect. 2013; 6: 271 10.1186/1756-3305-6-271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lobo JM, Jiménez-Valverde A, Joaquín H. The uncertain nature of absences and their importance in species distribution modelling. Ecography. 2010; 33: 103–114. 10.1111/j.1600-0587.2009.06039.x [DOI] [Google Scholar]
- 85. Thuiller W. Patterns and uncertainties of species’ range shifts under climate change. Glob Chang Biol. 2004; 10: 2020–2027. 10.1111/j.1365-2486.2004.00859.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Marengo JA. Mudanças Climáticas Globais e seus Efeitos sobre a Biodiversidade 2a Ed. Ministério do Meio Ambiente Brasília: MMA; 2007. [Google Scholar]
- 87. Chiang JM, Iverson LR, Prasad A, Brown KL. Effects of climate change and shifts in forest composition on forest net primary production. J Integr Plant Biol. 2008; 50: 1426–1439. 10.1111/j.1744-7909.2008.00749.x [DOI] [PubMed] [Google Scholar]
- 88. Gálvez R, Descalzo MA, Miró G, Jiménez MI, Martín O, Sandos-Brandao F, et al. Seasonal trends and spatial relations between environmental/meteorological factors and leishmaniosis sand fly vector abundances in Central Spain. Acta Trop. 2010; 115: 95–102. 10.1016/j.actatropica.2010.02.009 [DOI] [PubMed] [Google Scholar]
- 89. Gálvez R, Descalzo MA, Guerrero I, Miró G, Molina R. Mapping the current distribution and predicted spread of the leishmaniosis sand fly vector in the Madrid region (Spain) based on environmental variables and expected climate change. Vector Borne Zoonotic Dis. 2011; 11(7): 799–806. 10.1089/vbz.2010.0109 [DOI] [PubMed] [Google Scholar]
- 90. González C, Paz A, Ferro C. Predicted altitudinal shifts and reduced spatial distribution of Leishmania infantum vector species under climate change scenarios in Colombia. Acta Trop. 2014; 129: 83–90. 10.1016/j.actatropica.2013.08.014 [DOI] [PubMed] [Google Scholar]
- 91. Dorval MEC, Alves TP, Cristaldo G, da Rocha HC, Alves MA, Oshiro ET, et al. Sand fly captures with Disney traps in area of occurrence of Leishmania (Leishmania) amazonensis in the State of Mato Grosso do Sul, mid-western Brazil. Rev Soc Bras Med Trop. 2010; 43(5): 491–495. 10.1590/S0037-86822010000500003 [DOI] [PubMed] [Google Scholar]
- 92. Dorval MEC, Cristaldo G, Rocha HC, Alves TP, Alves MA, Oshiro ET, et al. Phlebotomine fauna (Diptera: Psychodidae) of an American cutaneous leishmaniasis endemic area in the state of Mato Grosso do Sul, Brazil. Mem Inst Oswaldo Cruz. 2009; 104(5): 695–702. 10.1590/S0074-02762009000500005 [DOI] [PubMed] [Google Scholar]
- 93.Rado Covarrubias CD. Estudos sobre os flebotomíneos (Diptera: Psychodidae: Phlebotominae) e os potenciais vetores de Leishmania spp. na província de La Convención, Cusco, Perú. M.Sc. Thesis, Instituto Oswaldo Cruz. 2011. Available: http://www.arca.fiocruz.br.
- 94. Kato H, Calvopiña M, Criollo H, Hashiguchi Y. First human cases of Leishmania (Viannia) naiffi infection in Ecuador and identification of its suspected vector species. Acta Trop. 2013; 128: 710–713. 10.1016/j.actatropica.2013.09.001 [DOI] [PubMed] [Google Scholar]
- 95. Rodrigues EAZ, Andrade Filho JD, Limongi JE, Paula MBC. Sandfly fauna (Diptera: Psychodidae) in Parque do Sabiá complex, Uberlândia, Minas Gerais, Brazil. Rev Inst Med Trop Sao Paulo. 2011; 53(5): 255–258. 10.1590/S0036-46652011000500003 [DOI] [PubMed] [Google Scholar]
- 96. Galati EAB, Nunes VLB, Boggiani PC, Dorval MEC, Cristaldo G, Rocha HC, et al. Phlebotomines (Diptera: Psychodidae) in forested areas of the Serra da Bodoquena, state of Mato Grosso do Sul, Brazil. Mem Inst Oswaldo Cruz. 2006; 101(2): 175–193. 10.1590/S0074-02762006000200010 [DOI] [PubMed] [Google Scholar]
- 97. Queiroz MFM, Varjão JR, de Moraes SC, Salcedo GE. Analysis of sandflies (Diptera: Psychodidae) in Barra do Garças, State of Mato Grosso, Brazil, and the influence of environmental variables on the vector density of Lutzomyia longipalpis (Lutz & Neiva, 1912). Rev Soc Bras Med Trop. 2012; 45(3):313–317. 10.1590/S0037-86822012000300007 [DOI] [PubMed] [Google Scholar]
- 98. Oliveira AG, Andrade Filho JD, Falcão AL, Brazil RP. Estudo de flebotomíneos (Diptera, Psychodidae, Phlebotominae) na zona urbana da Cidade de Campo Grande, Mato Grosso do Sul, Brasil, 1999–2000. Cad Saude Publica. 2003; 19(4): 933–944. 10.1590/S0102-311X2003000400016 [DOI] [PubMed] [Google Scholar]
- 99. Franke CR, Ziller M, Staubach C, Latif M. Impact of the El Niño/Southern Oscillation on visceral leishmaniasis, Brazil. Emerg Infect Dis. 2002; 8(9): 914–917. 10.3201/eid0809.010523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Chaves LF, Pascual M. Climate cycles and forecasts of cutaneous leishmaniasis, a nonstationary vector-borne disease. PLoS Med. 2006; 3(8): e295 10.1371/journal.pmed.0030295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Cardenas R, Sandoval CM, Rodríguez-Morales AJ, Franco-Paredes C. Impact of climate variability in the occurrence of leishmaniasis in northeastern Colombia. Am J Trop Med Hyg. 2006; 75(2): 273–277. [PubMed] [Google Scholar]
- 102. Cardenas R, Sandoval CM, Rodríguez-Morales AJ, Vivas P. Zoonoses and climate variability. The example of leishmaniasis in Southern departments of Colombia. Ann N Y Acad Sci. 2008; 1149: 326–330. 10.1196/annals.1428.094 [DOI] [PubMed] [Google Scholar]
- 103. Bates PA, Depaquit J, Galati EAB, Kamhawi S, Maroli M, McDowell MA, et al. Recent advances in phlebotomine sand fly research related to leishmaniasis control. Parasit Vectors. 2015; 8:131 10.1186/s13071-015-0712-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Dias ES, Regina-Silva S, França-Silva JC, Paz GF, Michalsky EM, Araújo SC, et al. Eco-epidemiology of visceral leishmaniasis in the urban area of Paracatu, state of Minas Gerais, Brazil. Vet Parasitol. 2011; 176: 101–111. 10.1016/j.vetpar.2010.11.014 [DOI] [PubMed] [Google Scholar]
- 105. Tolezano JE, Uliana SRB, Taniguchi HH, Araújo MFL, Barbosa JAR, Barbosa JER, et al. The first records of Leishmania (Leishmania) amazonensis in dogs (Canis familiaris) diagnosed clinically as having canine visceral leishmaniasis from Araçatuba County, São Paulo State, Brazil. Vet Parasitol. 2007; 149: 280–284. 10.1016/j.vetpar.2007.07.008 [DOI] [PubMed] [Google Scholar]
- 106. Hashiguchi Y, Chiller T, Inchausti A, Arias A, Kawabata M, Alexander B. Phlebotomine sandfly species in Paraguay and their infection with Leishmania . Ann Trop Med Parasitol. 1992; 86(2): 175–180. [DOI] [PubMed] [Google Scholar]
- 107. Perez JE, Veland N, Espinosa D, Torres K, Ogusuku E, Llanos-Cuentas A, et al. Isolation and molecular identification of Leishmania (Viannia) peruviana from naturally infected Lutzomyia peruensis (Diptera: Psychodidae) in the Peruvian Andes. Mem Inst Oswaldo Cruz. 2007; 102(6): 655–658. 10.1590/S0074-02762007005000077 [DOI] [PubMed] [Google Scholar]
- 108. Gomez EA, Kato H, Mimori T, Hashigichi Y. Distribution of Lutzomyia ayacuchensis, the vector of Andean-type cutaneous leishmaniasis, at different altitudes on the Andean slope of Ecuador. Acta Trop. 2014; 137: 118–122. 10.1016/j.actatropica.2014.05.006 [DOI] [PubMed] [Google Scholar]
- 109. Carvalho BM, Dias CMG, Rangel EFR. Phlebotomine sand flies (Diptera, Psychodidae) from Rio de Janeiro State, Brazil: Species distribution and potential vectors of leishmaniases. Rev Bras Entomol. 2014; 58(1): 77–87. 10.1590/S0085-56262014000100013 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
High: geographical coordinates of capture site given in the published article; Medium: geographical coordinates approximated according to description of capture site; Low: only district or municipality level information.
(TIF)
(TIF)
bio1: annual mean temperature; bio2: mean diurnal range; bio3: isothermality; bio4: temperature seasonality; bio5: max temperature of warmest month; bio6: min temperature of coldest month; bio7: temperature annual range; bio8: mean temperature of wettest quarter; bio9: mean temperature of driest quarter; bio10: mean temperature of warmest quarter; bio11: mean temperature of coldest quarter; bio12: annual precipitation; bio13: precipitation of wettest month; bio14: precipitation of driest month; bio15: precipitation seasonality; bio16: precipitation of wettest quarter; bio17: precipitation of driest quarter; bio18: precipitation of warmest quarter; bio19: precipitation of coldest quarter.
(TIFF)
(TIF)
(TIF)
Each map shows binary model outputs. Future projections include the percentage of area lost or gain in comparison with current predictions. For names of each General Circulation Model, see S2 Table.
(TIF)
Each map shows binary model outputs. Future projections include the percentage of area lost or gain in comparison with current predictions. For names of each General Circulation Model, see S2 Table.
(TIF)
Each map shows binary model outputs. Future projections include the percentage of area lost or gain in comparison with current predictions. For names of each General Circulation Model, see S2 Table.
(TIF)
Each map shows binary model outputs. Future projections include the percentage of area lost or gain in comparison with current predictions. For names of each General Circulation Model, see S2 Table.
(TIF)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.