The anatomy, pathology, and imaging of anal and rectal cancers are discussed, with special emphasis on distinguishing factors that affect use of radiation therapy for these conditions.
Abstract
Although rectal and anal cancers are anatomically close, they are distinct entities with different histologic features, risk factors, staging systems, and treatment pathways. Imaging is at the core of initial clinical staging of these cancers and most commonly includes magnetic resonance imaging for local-regional staging and computed tomography for evaluation of metastatic disease. The details of the primary tumor and involvement of regional lymph nodes are crucial in determining if and how radiation therapy should be used in treatment of these cancers. Unfortunately, available imaging modalities have been shown to have imperfect accuracy for identification of nodal metastases and imaging features other than size. Staging of nonmetastatic rectal cancers is dependent on the depth of invasion (T stage) and the number of involved regional lymph nodes (N stage). Staging of nonmetastatic anal cancers is determined according to the size of the primary mass and the combination of regional nodal sites involved; the number of positive nodes at each site is not a consideration for staging. Patients with T3 rectal tumors and/or involvement of perirectal, mesenteric, and internal iliac lymph nodes receive radiation therapy. Almost all anal cancers warrant use of radiation therapy, but the extent and dose of the radiation fields is altered on the basis of both the size of the primary lesion and the presence and extent of nodal involvement. The radiologist must recognize and report these critical anatomic and staging distinctions, which affect use of radiation therapy in patients with anal and rectal cancers.
©RSNA, 2015
SA-CME LEARNING OBJECTIVES
After completing this journal-based SA-CME activity, participants will be able to:
■ Identify important anatomic and staging distinctions between rectal and anal cancers.
■ Describe the roles and limitations of various imaging modalities in workup of anorectal cancers.
■ Recognize critical findings in radiologic workup and staging of anorectal cancers that affect use of radiation therapy.
Introduction
Colorectal cancer has remained the third most common cancer in the United States for the past 30 years, with an estimated 96,830 new cases and an estimated 50,310 deaths in 2014 (1). Of the new cases per year, 40,000 are determined to be rectal cancer. Anal cancer is much less common, representing only 7210 new cases per year (1). Although the anatomic locations are close and often overlap, rectal cancer and anal cancer are distinct entities with different inherent risk factors, histologic features, and patterns of spread, resulting in different workup and treatment approaches. Although radiation therapy is an important part of treatment of both cancers, the treatment algorithm differs on the basis of both the type and stage of cancer. Radiologists play a vital role in reporting the extent of primary tumor and regional nodal disease, both of which are critical to planning and delivery of radiation therapy.
We first review the anatomy and histology of the anorectal region. We then discuss the epidemiology, pathologic classification, and staging of anal and rectal cancers. In the final section, we address use of radiation therapy in these entities, including the key areas in which imaging findings affect treatment decisions. Throughout this article, we highlight the critical distinctions between these two cancers that are relevant to radiologic staging and treatment planning.
General Anatomy, Histology, and Pathology
Gross Anatomy of the Anorectum
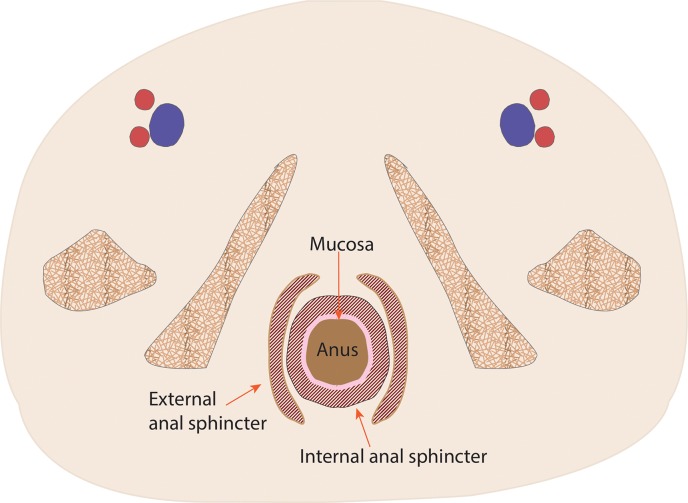
The rectum is located near the midline of the pelvis and measures approximately 15 cm in length. The rectosigmoid junction is variably defined in the literature. The American Joint Committee on Cancer (AJCC) defines it as at the level of confluence of the taeniae coli, but others define it with regard to the shape of the colon, the anterior deflection of the colon away from the sacrum, or the distance from the anal verge (2,3). The rectum extends distally to the proximal anorectal sphincter, as defined by the palpable upper border of the puborectalis muscle (Fig 1) (4–7). The proximal rectum is covered by peritoneum anteriorly and laterally, and the mid rectum is only partially covered anteriorly (8,9). The distal rectum is entirely extraperitoneal and is surrounded by perirectal fat, mesorectal lymph nodes, and vessels, all of which are encased by the mesorectal fascia (9,10). The mesorectal fascia tapers inferiorly and fuses with the anal sphincter (Fig 2) (8–11).
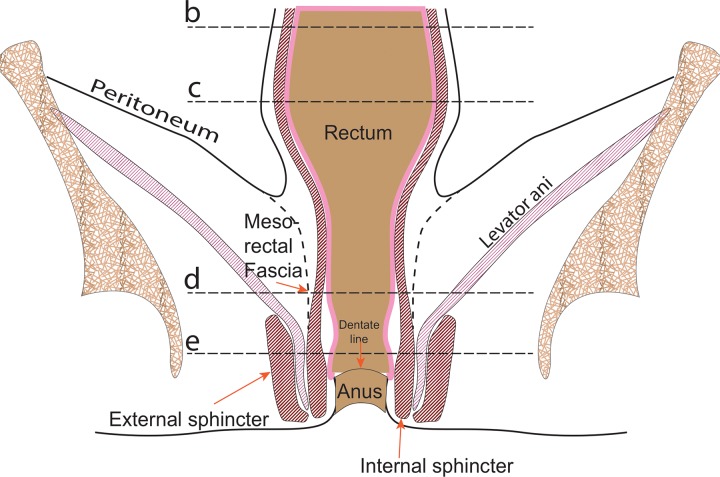
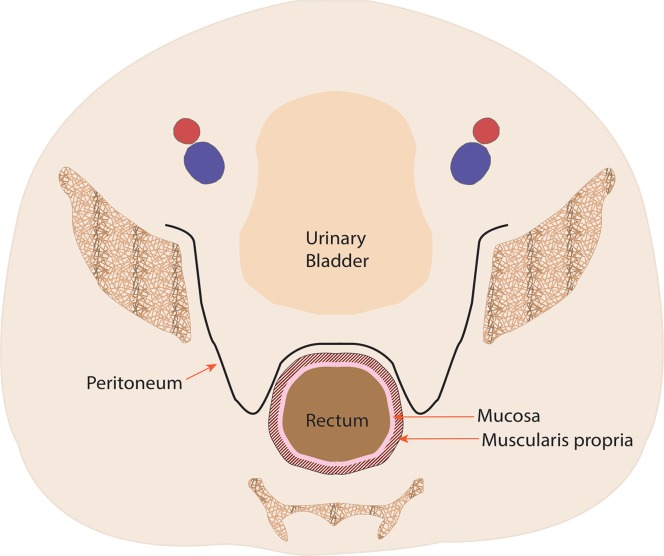
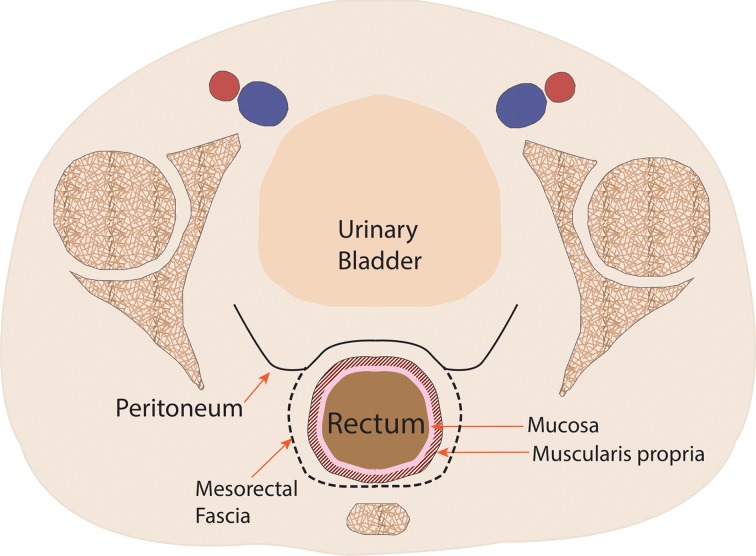
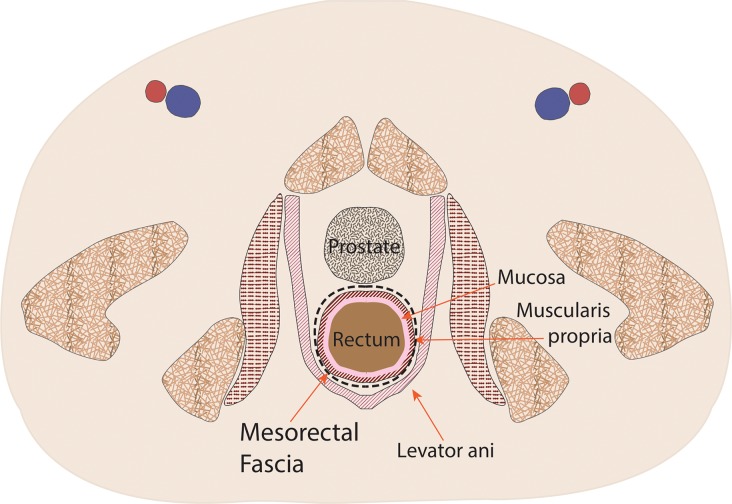
Figure 1a.
Coronal (a) and axial multilevel (b–e) drawings show the anatomy of the rectum and anus. The horizontal dashed lines in a correspond to the levels shown in b–e. In b–d, the red and blue circles represent the external iliac arteries and veins; in e, they represent the femoral arteries and veins.
Figure 2a.
Key anatomic features of the rectum. (a, b) Axial computed tomographic (CT) images obtained at different levels in a patient with ascites show a peritoneal layer (arrow) covering the anterior and lateral upper rectum and a purely anterior peritoneal covering at the mid rectum. (c, d) In a different patient, axial T2-weighted magnetic resonance (MR) image (c) shows (from outside to inside) the mesorectal fascia (solid arrow), mesorectal fat, muscularis propria (arrowhead), submucosa, and mucosa (dashed arrow). Sagittal T2-weighted MR image (d) shows the relationship between the rectum and adjacent structures. The layers of the anus and rectum are also seen.
Figure 1b.
Coronal (a) and axial multilevel (b–e) drawings show the anatomy of the rectum and anus. The horizontal dashed lines in a correspond to the levels shown in b–e. In b–d, the red and blue circles represent the external iliac arteries and veins; in e, they represent the femoral arteries and veins.
Figure 1c.
Coronal (a) and axial multilevel (b–e) drawings show the anatomy of the rectum and anus. The horizontal dashed lines in a correspond to the levels shown in b–e. In b–d, the red and blue circles represent the external iliac arteries and veins; in e, they represent the femoral arteries and veins.
Figure 1d.
Coronal (a) and axial multilevel (b–e) drawings show the anatomy of the rectum and anus. The horizontal dashed lines in a correspond to the levels shown in b–e. In b–d, the red and blue circles represent the external iliac arteries and veins; in e, they represent the femoral arteries and veins.
Figure 1e.
Coronal (a) and axial multilevel (b–e) drawings show the anatomy of the rectum and anus. The horizontal dashed lines in a correspond to the levels shown in b–e. In b–d, the red and blue circles represent the external iliac arteries and veins; in e, they represent the femoral arteries and veins.
Figure 2b.
Key anatomic features of the rectum. (a, b) Axial computed tomographic (CT) images obtained at different levels in a patient with ascites show a peritoneal layer (arrow) covering the anterior and lateral upper rectum and a purely anterior peritoneal covering at the mid rectum. (c, d) In a different patient, axial T2-weighted magnetic resonance (MR) image (c) shows (from outside to inside) the mesorectal fascia (solid arrow), mesorectal fat, muscularis propria (arrowhead), submucosa, and mucosa (dashed arrow). Sagittal T2-weighted MR image (d) shows the relationship between the rectum and adjacent structures. The layers of the anus and rectum are also seen.
Figure 2c.
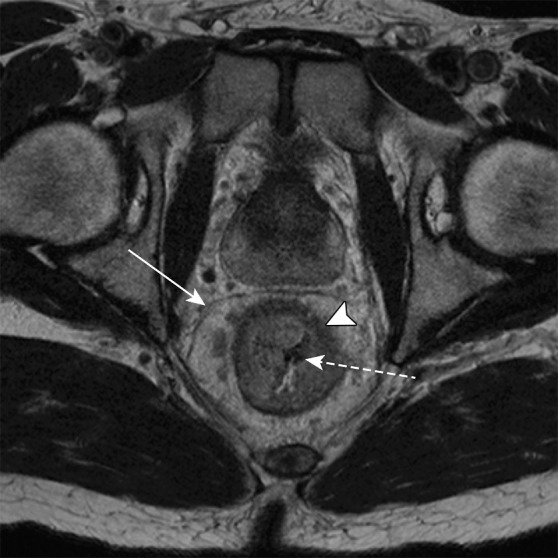
Key anatomic features of the rectum. (a, b) Axial computed tomographic (CT) images obtained at different levels in a patient with ascites show a peritoneal layer (arrow) covering the anterior and lateral upper rectum and a purely anterior peritoneal covering at the mid rectum. (c, d) In a different patient, axial T2-weighted magnetic resonance (MR) image (c) shows (from outside to inside) the mesorectal fascia (solid arrow), mesorectal fat, muscularis propria (arrowhead), submucosa, and mucosa (dashed arrow). Sagittal T2-weighted MR image (d) shows the relationship between the rectum and adjacent structures. The layers of the anus and rectum are also seen.
Figure 2d.
Key anatomic features of the rectum. (a, b) Axial computed tomographic (CT) images obtained at different levels in a patient with ascites show a peritoneal layer (arrow) covering the anterior and lateral upper rectum and a purely anterior peritoneal covering at the mid rectum. (c, d) In a different patient, axial T2-weighted magnetic resonance (MR) image (c) shows (from outside to inside) the mesorectal fascia (solid arrow), mesorectal fat, muscularis propria (arrowhead), submucosa, and mucosa (dashed arrow). Sagittal T2-weighted MR image (d) shows the relationship between the rectum and adjacent structures. The layers of the anus and rectum are also seen.
The anal sphincter comprises two components, an internal sphincter and an external sphincter complex, which are made of smooth muscle and skeletal muscle, respectively (4,8). The internal sphincter is simply the distal continuation of the inner circular muscular layer of the rectum, while the external sphincter complex is composed of several parts, including the inferior confluence of the levator ani muscle; the puborectalis sling; and the deep, subcutaneous, superficial external sphincter muscles (Fig 3).
Figure 3a.
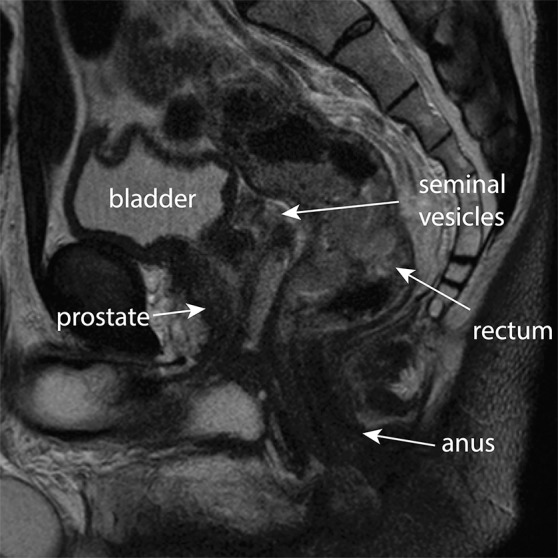
Key anatomic features of the anus. (a) Coronal CT image shows tapering of the mesorectal fascia around the anorectal junction at the pelvic floor. A thin fat plane is visible between the inner and outer sphincter muscles (arrows). (b) Axial T2-weighted MR image shows the internal sphincter (dashed arrow) and external sphincter (solid arrow). (c) Coronal T2-weighted MR image shows the confluence of the levator ani muscle (solid arrow) with the external anal sphincter (dashed arrow). The internal sphincter is contiguous with the rectal muscularis propria (arrowheads). (d) Sagittal CT image shows the relationship (anterior to posterior) of the bladder, seminal vesicles, rectum, and presacral fat. The anatomic rectosigmoid junction (bracket) is often defined at the sacral promontory or S3 to aid in treatment planning. The structure of the anal sphincter is poorly delineated on the CT image.
Figure 3b.
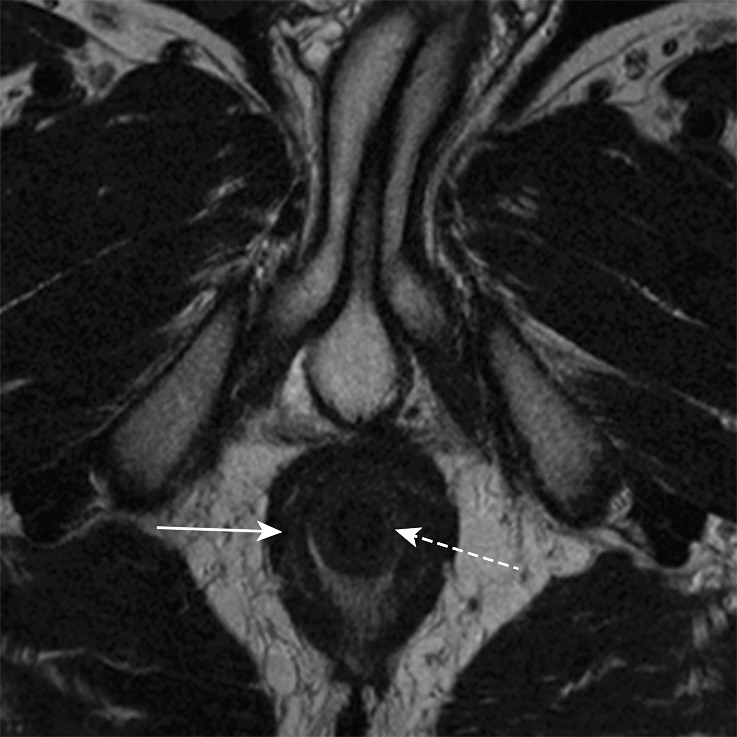
Key anatomic features of the anus. (a) Coronal CT image shows tapering of the mesorectal fascia around the anorectal junction at the pelvic floor. A thin fat plane is visible between the inner and outer sphincter muscles (arrows). (b) Axial T2-weighted MR image shows the internal sphincter (dashed arrow) and external sphincter (solid arrow). (c) Coronal T2-weighted MR image shows the confluence of the levator ani muscle (solid arrow) with the external anal sphincter (dashed arrow). The internal sphincter is contiguous with the rectal muscularis propria (arrowheads). (d) Sagittal CT image shows the relationship (anterior to posterior) of the bladder, seminal vesicles, rectum, and presacral fat. The anatomic rectosigmoid junction (bracket) is often defined at the sacral promontory or S3 to aid in treatment planning. The structure of the anal sphincter is poorly delineated on the CT image.
Figure 3c.
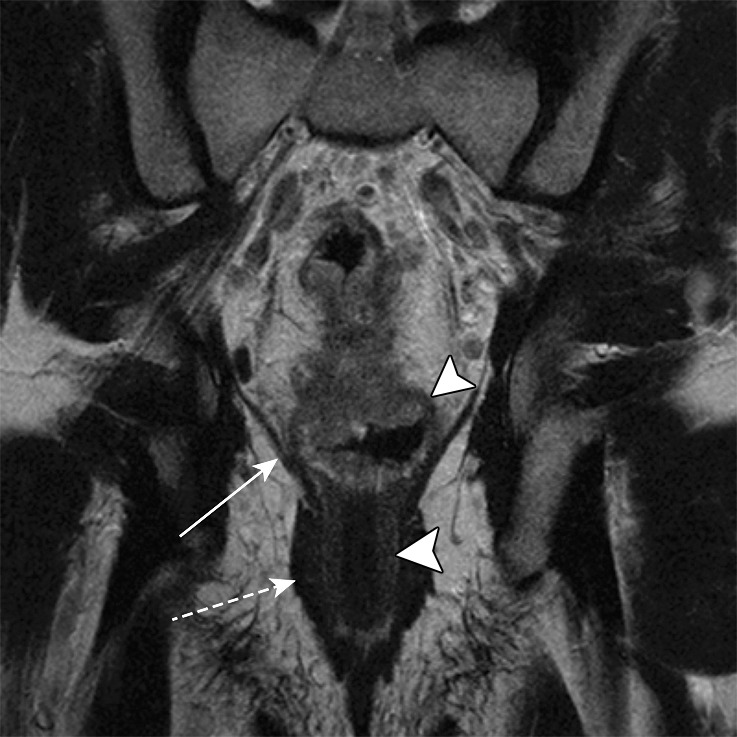
Key anatomic features of the anus. (a) Coronal CT image shows tapering of the mesorectal fascia around the anorectal junction at the pelvic floor. A thin fat plane is visible between the inner and outer sphincter muscles (arrows). (b) Axial T2-weighted MR image shows the internal sphincter (dashed arrow) and external sphincter (solid arrow). (c) Coronal T2-weighted MR image shows the confluence of the levator ani muscle (solid arrow) with the external anal sphincter (dashed arrow). The internal sphincter is contiguous with the rectal muscularis propria (arrowheads). (d) Sagittal CT image shows the relationship (anterior to posterior) of the bladder, seminal vesicles, rectum, and presacral fat. The anatomic rectosigmoid junction (bracket) is often defined at the sacral promontory or S3 to aid in treatment planning. The structure of the anal sphincter is poorly delineated on the CT image.
Figure 3d.
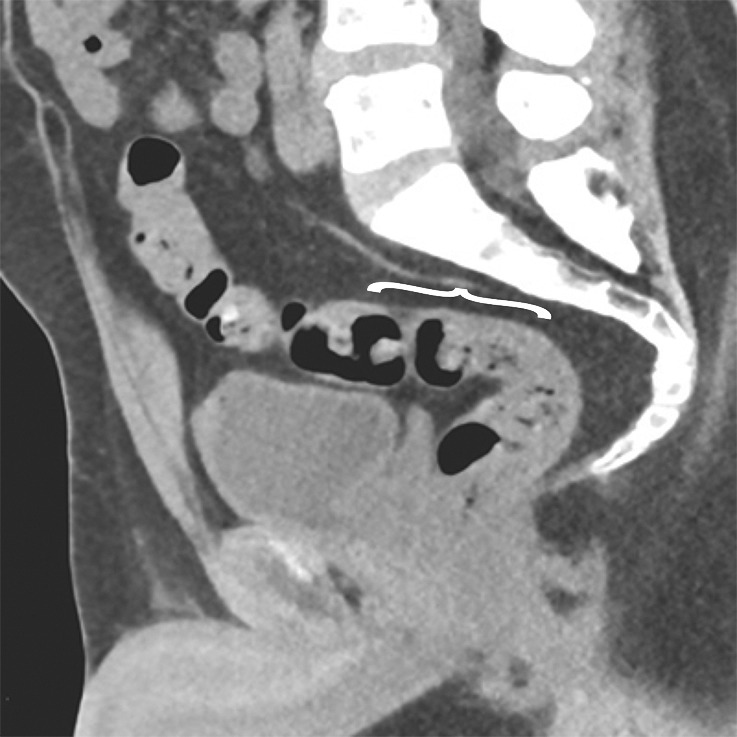
Key anatomic features of the anus. (a) Coronal CT image shows tapering of the mesorectal fascia around the anorectal junction at the pelvic floor. A thin fat plane is visible between the inner and outer sphincter muscles (arrows). (b) Axial T2-weighted MR image shows the internal sphincter (dashed arrow) and external sphincter (solid arrow). (c) Coronal T2-weighted MR image shows the confluence of the levator ani muscle (solid arrow) with the external anal sphincter (dashed arrow). The internal sphincter is contiguous with the rectal muscularis propria (arrowheads). (d) Sagittal CT image shows the relationship (anterior to posterior) of the bladder, seminal vesicles, rectum, and presacral fat. The anatomic rectosigmoid junction (bracket) is often defined at the sacral promontory or S3 to aid in treatment planning. The structure of the anal sphincter is poorly delineated on the CT image.
The anal canal extends from the anorectal sphincter to the anal verge, usually 3–6 cm in length (4,6,7,9). The anal margin extends from the anal verge (or the introitus of the anal orifice) and extends in a 5–6-cm radius across the external skin-covered region.
Lymphatic Drainage Pathways
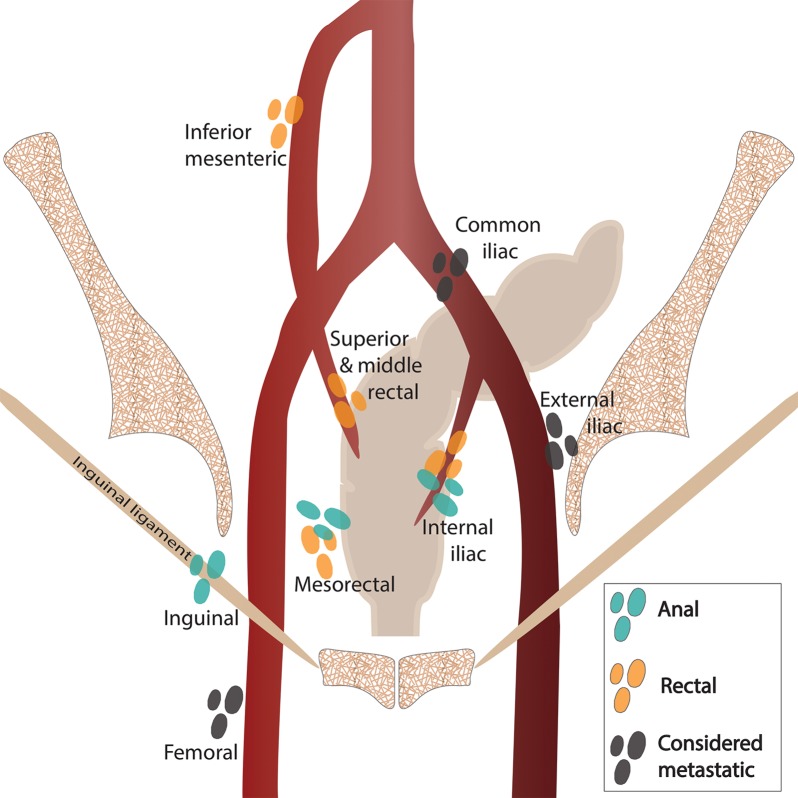
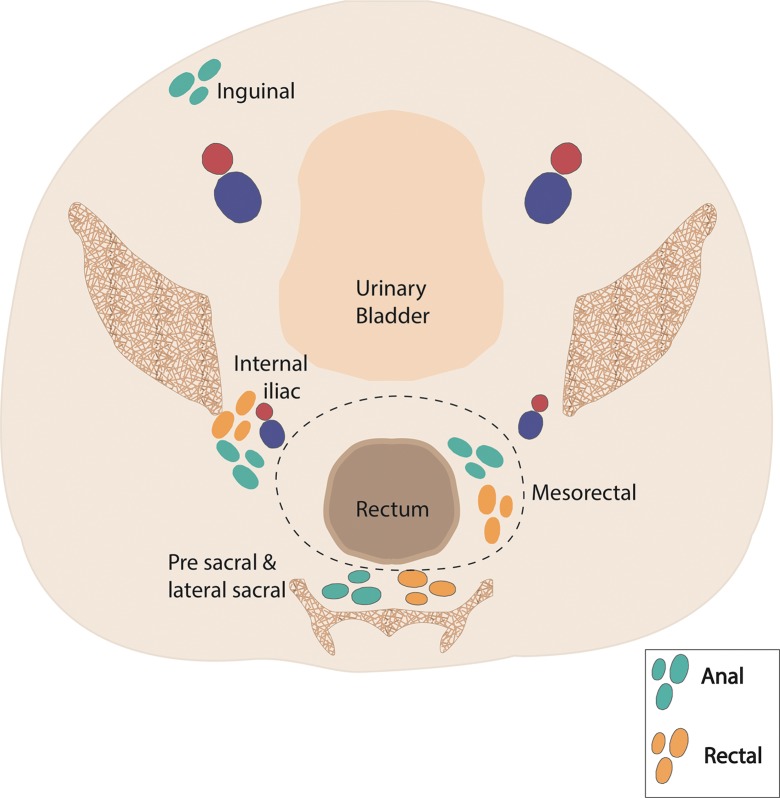
The lymphatic drainage pathways of the rectum and anus include both inguinal and mesenteric components. As a result, patterns of nodal and systemic metastasis can vary significantly on the basis of the location of the primary tumor. The drainage pathways are as follows: (a) upper rectum: through the superior rectal nodes to the inferior mesenteric nodes, (b) lower rectum: from the lower rectum through the middle rectal nodes to the internal iliac nodes, and (c) anal canal: through the mesorectal (also known as inferior rectal) nodes to the inguinal and femoral nodes.
As a result of these drainage pathways, proximal rectal cancers are more likely to produce mesenteric adenopathy, and distal rectal cancers can produce internal iliac adenopathy. In comparison, anal cancers are more likely to produce inguinal adenopathy, with internal iliac adenopathy also occurring when the tissues near the anorectal junction are involved. The differences in these drainage pathways and resulting patterns of nodal metastases are reflected in the selection of fields for radiation therapy, as we discuss in subsequent sections.
Although there is some overlap in the definition of regional lymph nodes in patients with rectal and anal cancers, there are important distinctions between the two. These distinctions are shown in Figure 4 and discussed in the section on nodal staging.
Figure 4a.
Coronal (a) and axial (b) drawings show the nodal stations relevant to anal and rectal cancers. Regional nodal stations are shown for anal cancers (blue-green) and rectal cancers (orange). Nodal stations considered metastatic for both anal and rectal cancer are shaded dark gray. Red and blue circles in b = external and internal iliac arteries and veins.
Figure 4b.
Coronal (a) and axial (b) drawings show the nodal stations relevant to anal and rectal cancers. Regional nodal stations are shown for anal cancers (blue-green) and rectal cancers (orange). Nodal stations considered metastatic for both anal and rectal cancer are shaded dark gray. Red and blue circles in b = external and internal iliac arteries and veins.
Normal Histology of the Anorectum
Histologically, rectal mucosa consists of a columnar crypt-forming epithelium comprising absorptive cells, goblet cells, and endocrine cells and transitions to anal squamous mucosa at the level of the dentate line (Fig 5) (6,12). The dentate line is not only a microscopic landmark but can be seen macroscopically as well, appearing as mucosal undulations created by anal glands and vertical columns of Morgagni (rectal mucosa). The dentate line is not visualized with radiologic imaging techniques (13). The actual transition between rectal and anal mucosa is varied among patients and may be smooth and abrupt or irregular, with intervening urothelial epithelium (14,15). This landmark is important because of the pattern of lymphatic drainage in this region; tumors located above the dentate line drain to mesorectal, presacral, and internal iliac nodal stations, while tumors below the dentate line drain to inguinal and femoral nodal stations (6,15).
Figure 5.
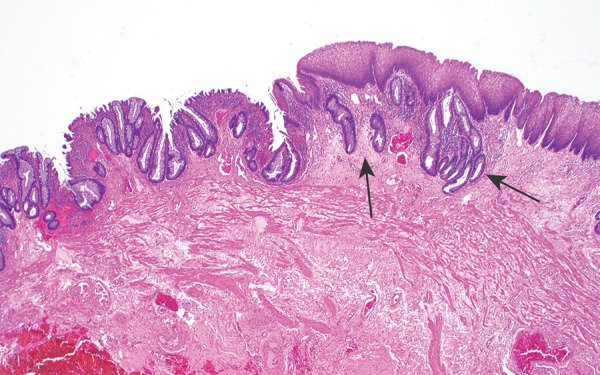
Photomicrograph shows the anorectal junctional mucosa, with the transition from the columnar mucinous epithelium of the rectum (left) to the simple squamous epithelium of the anal canal (right); the transition is often somewhat irregular, and the rectal columnar epithelium can extend under the squamous mucosa for a short distance in this region (arrows). (Original magnification, ×40; hematoxylin-eosin [H-E] stain.)
The layers of the rectal wall extending outward are the mucosa, which includes the lamina propria and the muscularis mucosa; the submucosa; the muscularis propria; and in some portions of the rectum, the serosa. In the rectum, the muscularis propria is formed by the inner circular layer and the outer longitudinal layer. The inner circular muscle continues distally to form the internal anal sphincter, while the outer longitudinal muscle layer forms the plane between the internal and external sphincters (intersphincteric plane).
The anal canal epithelium is composed of two distinct histologic regions: the proximal, squamous-lined mucosa of the anal canal, which lacks a stratum corneum and is therefore nonkeratinized and also lacks adnexal structures in the subepithelial layer, and the distal, squamous-lined epidermis of the anal margin, which has a stratum corneum, shows keratinization, and contains adnexal structures such as hair follicles and sweat glands in the underlying dermis (6). Cancers arising from the anal margin are categorized differently, in that T1 lesions are treated as skin cancers and thus will not be discussed here; lesions greater than T1 are treated similarly to anal canal cancers.
Pathologic Classification of Anorectal Cancers
Because of the glandular nature of rectal mucosa, 95%–97% of rectal cancers are adenocarcinomas, with neuroendocrine tumors being the second most common histologic type, representing less than 2% of malignant tumors of the rectum (16,17). Adenocarcinomas are distinguished by the presence of a neoplastic columnar epithelium invading the lamina propria or beyond and show either a pure gland-forming growth pattern (Fig 6a) or a mixed growth pattern with glandular, solid, and cribriform areas. Variable amounts of extracellular mucin production are seen in patients with adenocarcinoma, and an associated adenomatous precursor lesion is usually identified. Tumor cells are positive for broad-spectrum cytokeratins, cytokeratin 20, and the more-specific intestinal markers CDX2 and SATB2. The presence of squamous cell carcinoma in the rectum is usually due to proximal extension of an anal primary tumor. The remaining small fraction of histologic subtypes includes lymphoma, melanoma, and sarcoma.
Figure 6a.
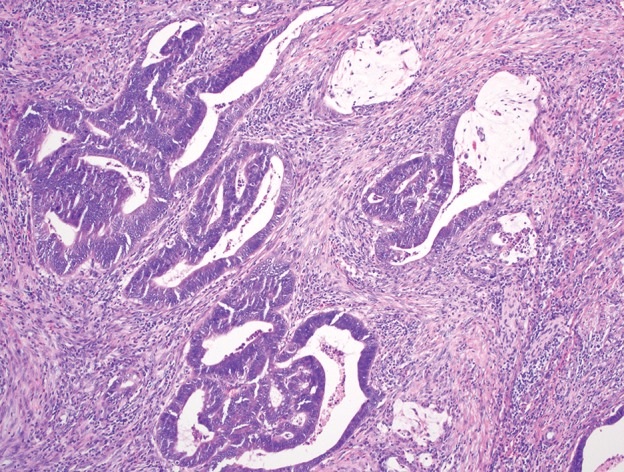
Carcinomas of the anorectal region. (a) Photomicrograph shows adenocarcinoma of the rectum composed of infiltrating malignant glands, with associated desmoplastic stroma and mucin production. (Original magnification, ×100; H-E stain.) (b) Photomicrograph shows squamous cell carcinoma of the proximal anal canal, with predominantly basaloid morphology and foci of keratinization. (Original magnification, ×200; H-E stain.)
Most anal cancers are squamous cell carcinomas (Fig 6b) (18). The dentate line, which corresponds to the transition between rectal and anal mucosa, also represents a landmark for differentiating histologic subtypes of squamous cell carcinoma arising in and around this region. Tumors originating in the transitional zone itself are frequently basaloid squamous cell carcinomas, composed of nests of tumor cells with a high nuclear-to-cytoplasmic ratio and small amounts of cytoplasm, which imparts a more poorly differentiated appearance, and these tumors may or may not show keratinization; those originating distal to the dentate line are more often well-differentiated keratinizing squamous cell carcinomas (4,6). There is no significant difference in the behavior and prognosis of either histologic subtype. Squamous cell carcinomas express broad-spectrum cytokeratins and p63 but are negative for cytokeratin 20 and the intestinal markers CDX2 and SATB2. Much less commonly occurring anal cancers include adenocarcinoma originating in the anal glands, melanoma, and small cell carcinoma (which usually arises in association with squamous cell carcinoma), which will not be discussed here (4).
Figure 6b.
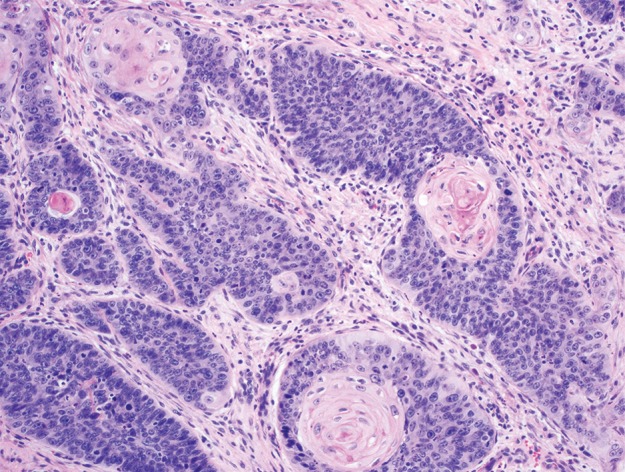
Carcinomas of the anorectal region. (a) Photomicrograph shows adenocarcinoma of the rectum composed of infiltrating malignant glands, with associated desmoplastic stroma and mucin production. (Original magnification, ×100; H-E stain.) (b) Photomicrograph shows squamous cell carcinoma of the proximal anal canal, with predominantly basaloid morphology and foci of keratinization. (Original magnification, ×200; H-E stain.)
Ultimately, given the variability in anatomic and histologic transition between the rectum and anus among patients, for tumors occurring in the anorectal region, it is the pathologic classification that determines diagnosis, staging, and treatment of that cancer. However, the anatomic location of the primary tumor may have implications for local extension and lymph node drainage, which can affect staging and treatment decisions.
Epidemiology
Rectal Cancer
Traditionally, noninherited risk factors for colorectal cancer were thought to include age greater than 50 years, obesity, physical inactivity, consumption of red or processed meat, alcohol consumption, and smoking (Table 1) (1). More recently, authors of studies have investigated whether the risk factors for colon cancer and rectal cancer differ when each primary site is considered individually. Wei et al (19) performed a large prospective cohort study of more than 134,000 men and women that suggested that family history and physical inactivity may not increase likelihood of rectal cancer as previously thought and that these two factors principally influence the risk of colon cancer only.
Table 1:
Risk Factors for Rectal and Anal Cancer
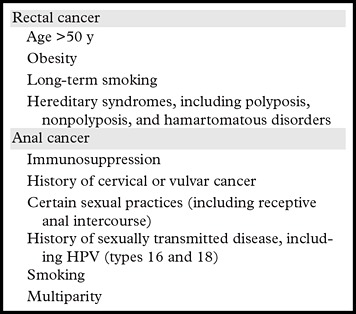
Note.—HPV = human papillomavirus.
Several well-known genetic predispositions to rectal cancer include nonpolyposis disorders such as Lynch syndrome; several polyposis disorders, including familial adenomatous polyposis and Turcot syndrome; and hamartomatous disorders such as Peutz-Jeghers syndrome and Cowden syndrome (17,20).
Anal Cancer
Historically, it was thought that recurrent inflammation such as hemorrhoids, fistulas, or even inflammatory bowel disease increased the likelihood of anal carcinoma, although this has since been refuted (6). HPV infection has now emerged as the key risk factor for anal cancers. HPV infection is associated with certain sexual practices, including receptive anal intercourse, history of sexually transmitted infections, and having more than 10 sexual partners (6,12,14,21–25). Certain strains of HPV are considered high risk for dysplasia and malignancy, the most common of which are HPV 16 and 18 (24). Infection by one of these strains, especially with several additional environmental risk factors such as cigarette smoking, multiparity, and long-term use of contraceptives, confers an increased risk for development of anal cancer (21). Prognosis is unrelated to HPV status (6,24).
Immunosuppression, particularly human immunodeficiency virus (HIV), also increases risk for anal cancer, independent of sexual risk factors or use of highly active antiretroviral therapy (6,21). Immunosuppressed patients who have undergone organ transplantation also have increased risk for anal cancer (21).
Initial Assessment
Rectal Cancer
Staging.—Most patients with nonmetastatic rectal cancer undergo surgical treatment, and therefore their cancers ultimately are staged at pathologic examination during surgery. However, the treatment pathway before surgery is dependent on accurate clinical staging, which is primarily determined on the basis of radiologic imaging (Table 2) (26).
Table 2:
AJCC TNM Stage and Simplified Treatment Algorithm for Rectal Cancer
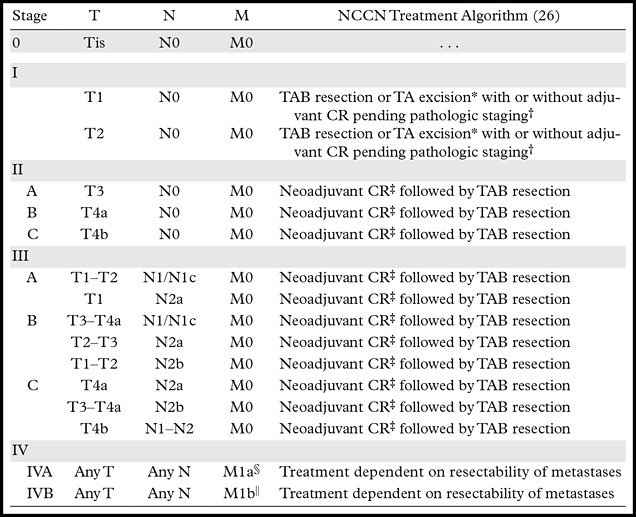
Note.—Adapted and reprinted, with permission, from reference 3. CR = chemotherapy and radiation therapy, NCCN = National Comprehensive Cancer Network, TA = transanal, TAB = transabdominal.
*T1 disease may be treated with either TA excision or TAB resection. If a lesion has high-risk features or is restaged to T2, TAB resection is recommended. T2 disease is treated with TAB resection.
†If tumor is upstaged after resection, treatment with “sandwich regimen” of adjuvant chemotherapy, followed by CR, followed by chemotherapy.
‡Neoadjuvant treatment may be CR versus chemotherapy followed by CR followed by TAB resection.
§M1a is considered metastasis confined to one organ or site, excluding the peritoneum.
||M1b is considered metastasis in more than one organ or site or in the peritoneum.
According to AJCC staging and the TNM definitions, T staging in rectal cancer is related to depth of invasion, with particular attention to tumor extension through the submucosa and muscularis propria (Fig 7) (3). Tumor invasion into the submucosa is considered T1, invasion through the submucosa into the muscularis propria is T2, and extension through the muscularis propria into the perirectal tissues is T3 (3,8,11).
Figure 7a.
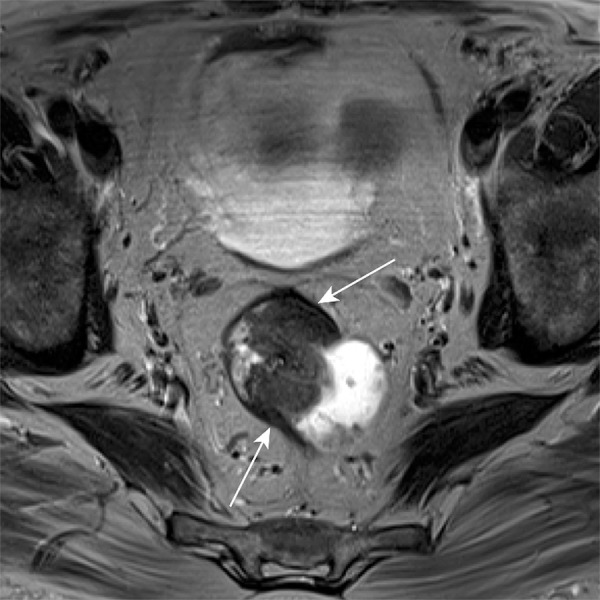
T staging of rectal cancer according to depth of tumor invasion as seen on axial T2-weighted MR images. (a) T1 cancers invade the submucosa (arrows) but do not involve the muscularis propria. (b) T2 cancers invade the muscularis propria but do not reach the subserosa (arrow). (c) T3 cancers invade beyond the muscularis propria (arrow). (d) T4 cancers invade through the serosal covering (T4a) or another fascial plane (T4b) and/or involve an adjacent organ (T4b) (arrows).
Figure 7b.
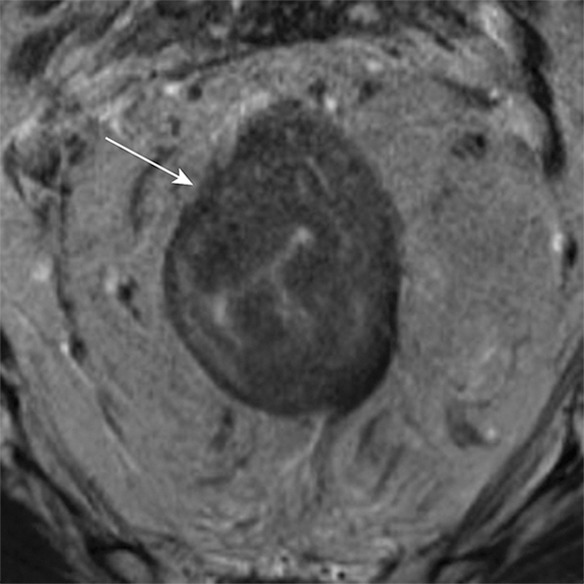
T staging of rectal cancer according to depth of tumor invasion as seen on axial T2-weighted MR images. (a) T1 cancers invade the submucosa (arrows) but do not involve the muscularis propria. (b) T2 cancers invade the muscularis propria but do not reach the subserosa (arrow). (c) T3 cancers invade beyond the muscularis propria (arrow). (d) T4 cancers invade through the serosal covering (T4a) or another fascial plane (T4b) and/or involve an adjacent organ (T4b) (arrows).
Figure 7c.
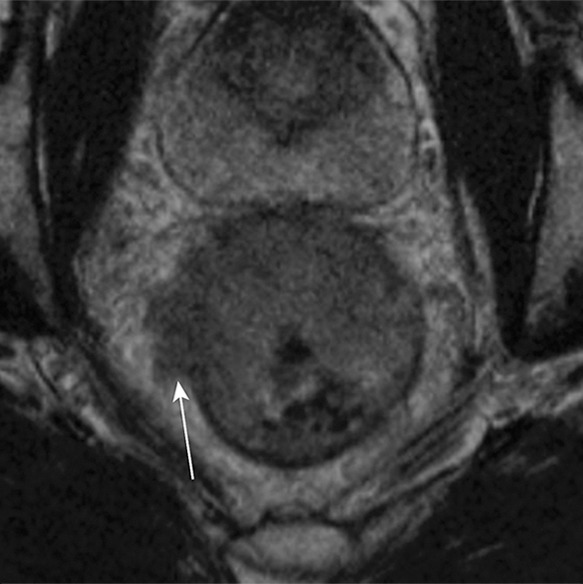
T staging of rectal cancer according to depth of tumor invasion as seen on axial T2-weighted MR images. (a) T1 cancers invade the submucosa (arrows) but do not involve the muscularis propria. (b) T2 cancers invade the muscularis propria but do not reach the subserosa (arrow). (c) T3 cancers invade beyond the muscularis propria (arrow). (d) T4 cancers invade through the serosal covering (T4a) or another fascial plane (T4b) and/or involve an adjacent organ (T4b) (arrows).
Figure 7d.
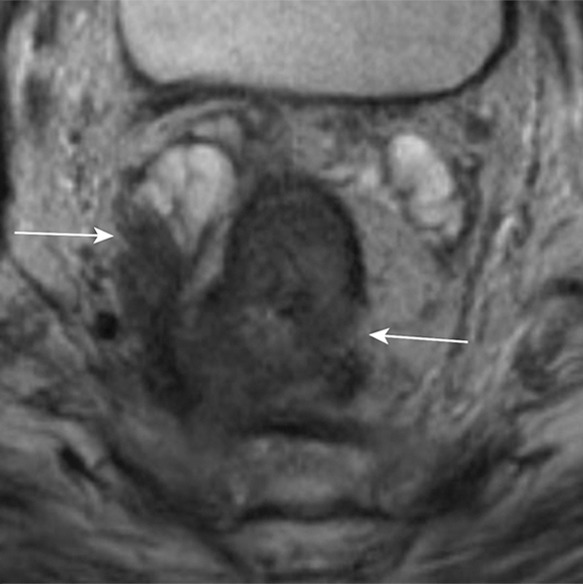
T staging of rectal cancer according to depth of tumor invasion as seen on axial T2-weighted MR images. (a) T1 cancers invade the submucosa (arrows) but do not involve the muscularis propria. (b) T2 cancers invade the muscularis propria but do not reach the subserosa (arrow). (c) T3 cancers invade beyond the muscularis propria (arrow). (d) T4 cancers invade through the serosal covering (T4a) or another fascial plane (T4b) and/or involve an adjacent organ (T4b) (arrows).
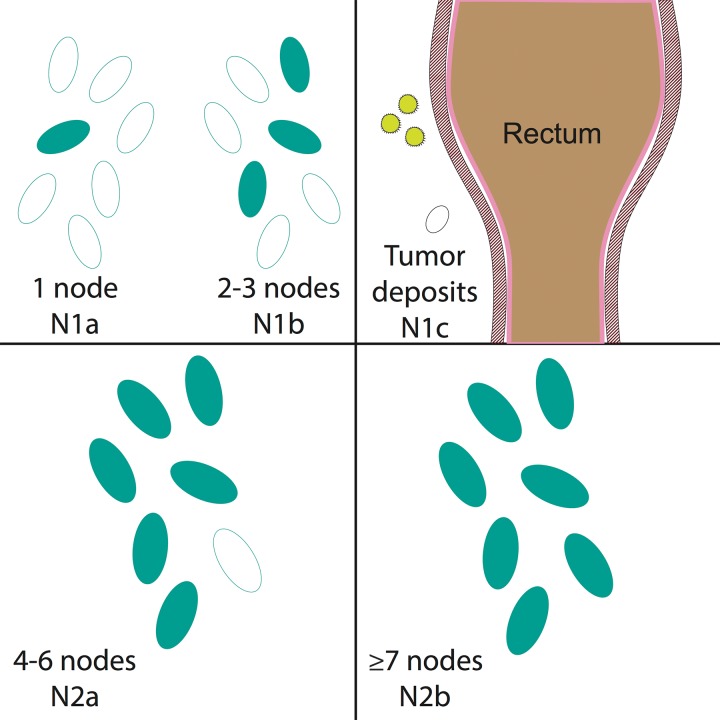
N staging is based on the number of involved regional lymph nodes, regardless of which regional lymph node stations are affected (Fig 8). The following lymph node stations are considered regional for rectal cancer by the AJCC: inferior mesenteric, superior rectal, middle rectal, mesorectal (inferior rectal), and internal iliac (Fig 4). Presacral lymph nodes are primarily part of the internal iliac drainage pathway and are considered regional nodes. The seventh edition of the AJCC staging manual (3) introduced the N1c nodal stage for tumor deposits that are found within perirectal tissues without regional nodal metastasis. Metastatic disease is subcategorized on the basis of whether the metastases are confined to one organ (M1a) or are found in more than one organ or the peritoneum (M1b).
Figure 8.
N staging of rectal cancer according to the number of involved regional lymph nodes. Drawings show that N1 rectal cancers have metastases in one (N1a) or two to three (N1b) regional lymph nodes. N1c rectal cancers have perirectal tumor deposits outside the lymph nodes without direct nodal involvement. N2a rectal cancers involve four to six regional lymph nodes. N2b rectal cancers involve seven or more regional lymph nodes.
Initial Evaluation.—Initial evaluation of rectal adenocarcinoma on the basis of biopsy results includes measurement of carcinoembryonic antigen level, complete colonoscopy to assess for synchronous tumors, and rigid proctoscopy to assess the exact distance of the rectal mass from the anal verge. The prostate-specific antigen level is measured in men to assess for prostate involvement. Radiologic assessments include endorectal ultrasonography (US) and/or pelvic MR imaging for evaluation of local extent of tumor and regional lymph nodes (T and N staging), and contrast material–enhanced CT of the chest, abdomen, and pelvis for detection of both regional and metastatic disease (N and M staging) (Table 3) (26,28).
Table 3:
Comparison of NCCN and American College of Radiology Strategies for Initial Radiologic Staging of Rectal and Anal Cancers
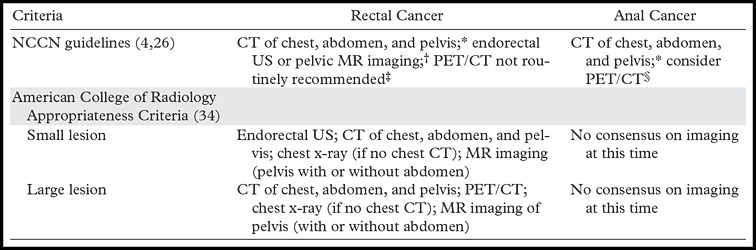
Note.—PET = positron emission tomography.
*CT with both intravenous and oral contrast agent administration.
†Endorectal US suggested for superficial tumors; limited by high rectal cancers and obstructing cancers.
‡PET/CT may be used to troubleshoot an equivocal finding or if there is a contraindication to contrast agent administration at CT.
§PET/CT may be useful for higher T stage (T2–T4) tumors or if there are any positive nodes (N positive).
There is ongoing debate regarding the relative roles of MR imaging and endorectal US for assessment of local-regional spread. Results of numerous studies have shown relatively equivalent performance for assessment of T stage, with slightly increased accuracy of endorectal US in evaluation of small superficial T1 and T2 tumors (29–32). Some pitfalls of transrectal US include the inability to use it when patients have bulky, obstructive tumors; small field of view, which limits evaluation of deep perirectal tissue and lymph node stations; inability to evaluate the mesorectal fascia; and operator dependency (9,11,33). The American College of Radiology appropriateness criteria currently recommend endorectal US over MR imaging for use in evaluation of small or superficial tumors, and MR imaging over endorectal US for larger lesions (34).
Because of its superior characterization of soft tissue and relatively large field of view, which can include the entire pelvis, MR imaging may be used for both T and N staging (8,11,29,30). Standard imaging protocols have been detailed extensively in the literature (8). High-spatial-resolution T2-weighted sequences are particularly useful for evaluation of tumor depth (T stage) because they allow visualization of the hyperintense submucosa, outer hypointense muscularis propria, and hypointense mesorectal fascia (Fig 9). The mesorectal fascia is an important landmark because it represents the potential surgical margin of a total mesorectal excision (also called the circumferential resection margin), which is an en bloc resection. Achieving a mesorectal excision with negative margins portends a better prognosis with improved overall survival and decreased risk for local recurrence (35,36). Radiologists must indicate the extent of tumor spread into the perirectal fat and any suspicious perirectal lymph nodes or tumor deposits that might threaten the ability to attain a mesorectal excision with negative margins.
Figure 9a.
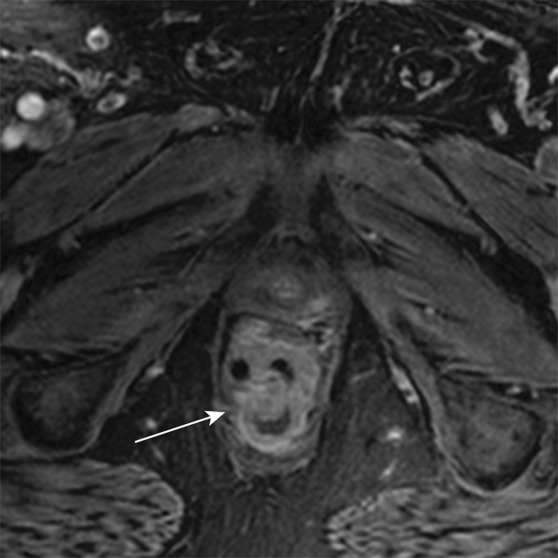
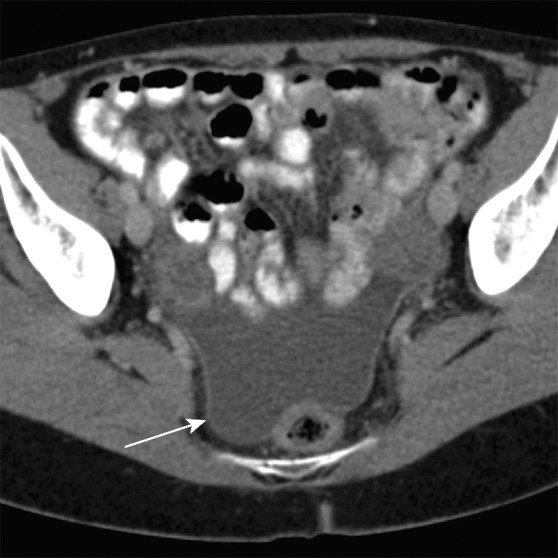
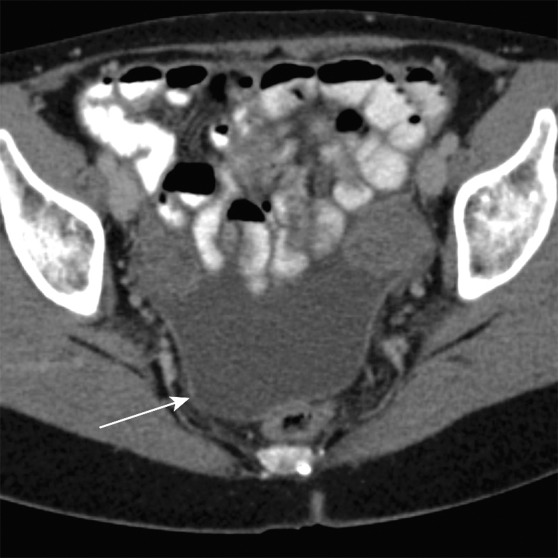
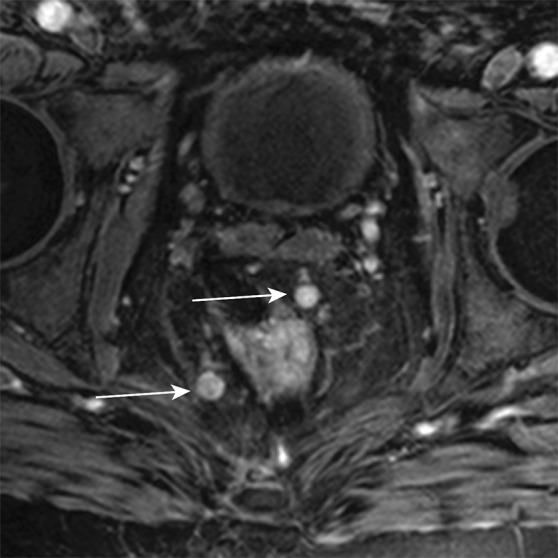
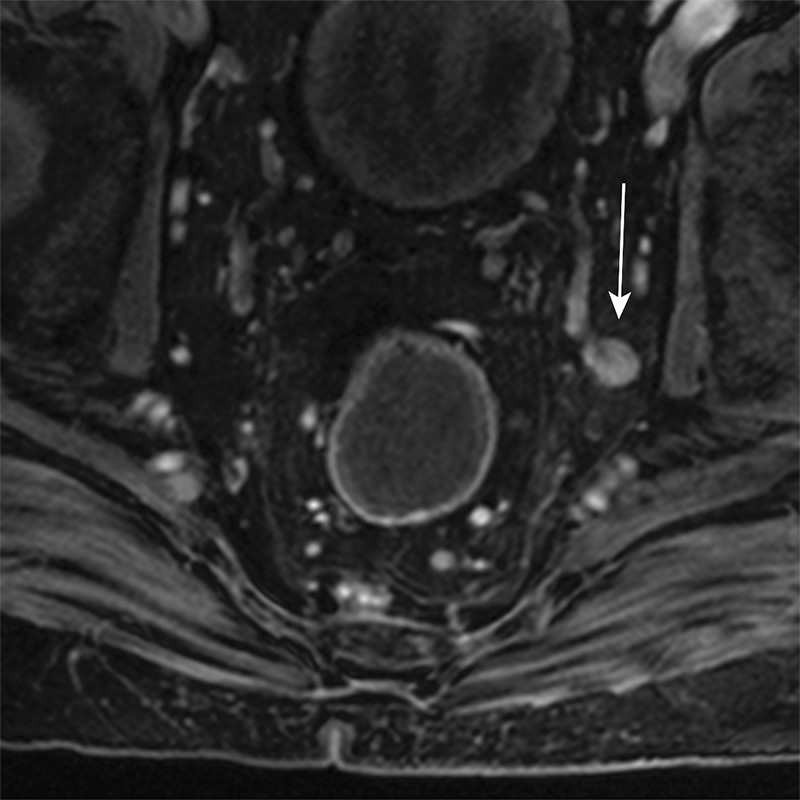
Axial contrast-enhanced MR images in a patient with stage IIIB (cT3N2M0) adenocarcinoma of the rectum. (a) Image shows a low rectal mass with a small focus of extension into the adjacent mesorectal fat (arrow) (T3 disease). (b, c) Multiple enhancing subcentimeter mesorectal lymph nodes (arrows in b) and a left internal iliac lymph node (arrow in c) suggest N2 disease on the basis of the number of potentially involved lymph nodes. The patient subsequently underwent neoadjuvant chemotherapy and radiation therapy and resection.
Figure 9b.
Axial contrast-enhanced MR images in a patient with stage IIIB (cT3N2M0) adenocarcinoma of the rectum. (a) Image shows a low rectal mass with a small focus of extension into the adjacent mesorectal fat (arrow) (T3 disease). (b, c) Multiple enhancing subcentimeter mesorectal lymph nodes (arrows in b) and a left internal iliac lymph node (arrow in c) suggest N2 disease on the basis of the number of potentially involved lymph nodes. The patient subsequently underwent neoadjuvant chemotherapy and radiation therapy and resection.
Figure 9c.
Axial contrast-enhanced MR images in a patient with stage IIIB (cT3N2M0) adenocarcinoma of the rectum. (a) Image shows a low rectal mass with a small focus of extension into the adjacent mesorectal fat (arrow) (T3 disease). (b, c) Multiple enhancing subcentimeter mesorectal lymph nodes (arrows in b) and a left internal iliac lymph node (arrow in c) suggest N2 disease on the basis of the number of potentially involved lymph nodes. The patient subsequently underwent neoadjuvant chemotherapy and radiation therapy and resection.
Radiologists also can identify T4 disease, defined as invasion by tumor into the visceral peritoneum (T4a) or adjacent organs such as the vagina, urethra, prostate, or bladder (T4b) at MR imaging. Although the peritoneum itself can be difficult to assess directly by means of imaging, knowledge of which portions of the rectum are covered by peritoneum is essential. In addition, one should evaluate for secondary signs that suggest the presence of T4a disease, including tumor growth into structures known to be covered by peritoneum, nodularity of the adjacent peritoneal surface, or increased peritoneal fluid without cause (37).
MR imaging also is used to delineate the relationship between the primary tumor and the anal sphincter, best seen on coronal images. It is important to report the distance of the tumor from the upper border of the anal sphincter (specifically, the upper margin of the puborectalis muscle) because this relationship is implicated in treatment planning for both radiation and surgery (8,9).
CT shows poor soft-tissue contrast and therefore is not optimal for use in T staging. However, enteric and intravenous contrast-enhanced CT can be used as an adjunct to MR imaging for N staging and is currently the primary modality used to evaluate for the presence of distant metastases, which most commonly occur in the liver, lungs, brain, and bones (38). Liver metastases, found in approximately 24% of newly diagnosed cancers, are predominantly low-attenuating lesions, best seen during portal venous phase imaging (9). If intravenous iodinated contrast material cannot be used, abdominal and pelvic MR imaging with or without contrast enhancement and nonenhanced chest CT can be substituted (26).
Rectal adenocarcinoma shows fluorodeoxyglucose (FDG) uptake at FDG PET. Although at this time, FDG PET/CT is not recommended for first-line imaging in staging of all rectal cancers, it can be used in certain situations, for troubleshooting, and most importantly, for treatment planning. PET/CT is currently recommended by the American College of Radiology for staging of large rectal cancers because results of studies have shown that use of this modality may accurately change the staging or influence treatment planning for this subset of patients (34,39). Results of several meta-analyses have shown PET/CT to be superior to CT for use in detection of hepatic metastases (40,41). One scenario in particular that may be amenable to PET/CT evaluation is exclusion of occult metastases in patients being considered for liver metastectomy with curative intent (26). In a recent randomized controlled trial (42) in which the effect of preoperative PET/CT was compared with that of standard CT in surgical treatment of resectable hepatic metastases, the authors found that 8% of patients had a change in surgical treatment plan after FDG PET, but the effect on overall survival was not statistically significant. PET/CT also outperforms contrast-enhanced CT for detection of extrahepatic metastatic disease (43). More research is needed to establish a role for PET/CT in staging of rectal cancer.
PET/CT is increasingly important in radiation treatment planning. In a small study of 34 patients with T3–T4, N0–N1, and M0–M1 disease, Whaley et al (44) found that PET/CT altered staging in 18% of patients and improved interobserver concordance in contouring boost treatment volumes compared with CT alone. This may be due to the superior ability of PET/CT to delineate tumor volume, which serves to minimize treatment margins and exclude nondiseased tissue (40).
Anal Cancer
Staging.—Unlike patients with rectal cancer, most patients with anal cancer do not undergo surgical resection, and thus, staging is purely clinical and is based on a combination of physical examination and imaging (Table 4). Although the T stage in patients with rectal cancer relates to depth of invasion, in the AJCC guidelines for TNM staging of anal cancer, the T stage corresponds to the size of the primary tumor, evaluated by means of clinical examination with additional information obtained at MR imaging (Fig 10) (3). Unlike N staging for rectal cancer, the location of nodal stations involved (not the number of positive nodes) determines the N stage for anal cancer (Fig 11). The following lymph node stations are considered regional in anal cancer: mesorectal (inferior rectal), internal iliac, and inguinal. The presence of common iliac or retroperitoneal adenopathy is considered metastatic disease in anal cancer (Fig 12).
Table 4:
AJCC TNM Stage and Simplified Treatment Algorithm of Anal Cancer
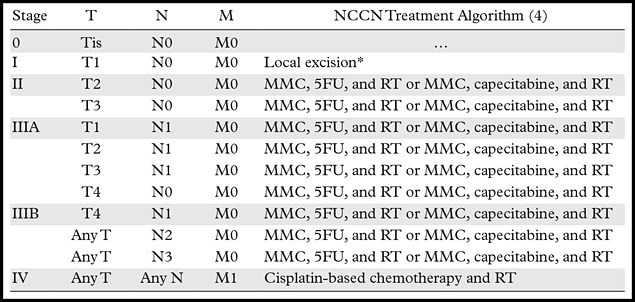
Note.—Adapted and reprinted, with permission, from reference 3. 5FU = fluorouracil, MMC = mitomycin C, RT = radiation therapy.
*If inadequate margins, re-excision is preferred over chemotherapy and radiation therapy.
Figure 10.
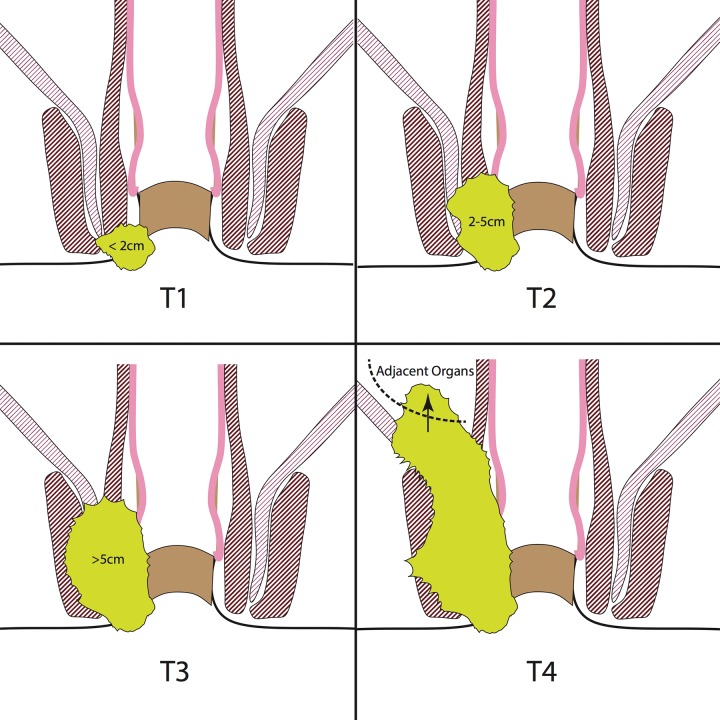
T staging of anal cancer according to tumor size. T1 anal cancers measure less than 2 cm in greatest dimension, T2 anal cancers measure 2–5 cm, T3 anal cancers measure greater than 5 cm, and T4 anal cancers invade adjacent organs (eg, vagina or bladder). Yellow-green = cancer.
Figure 11.
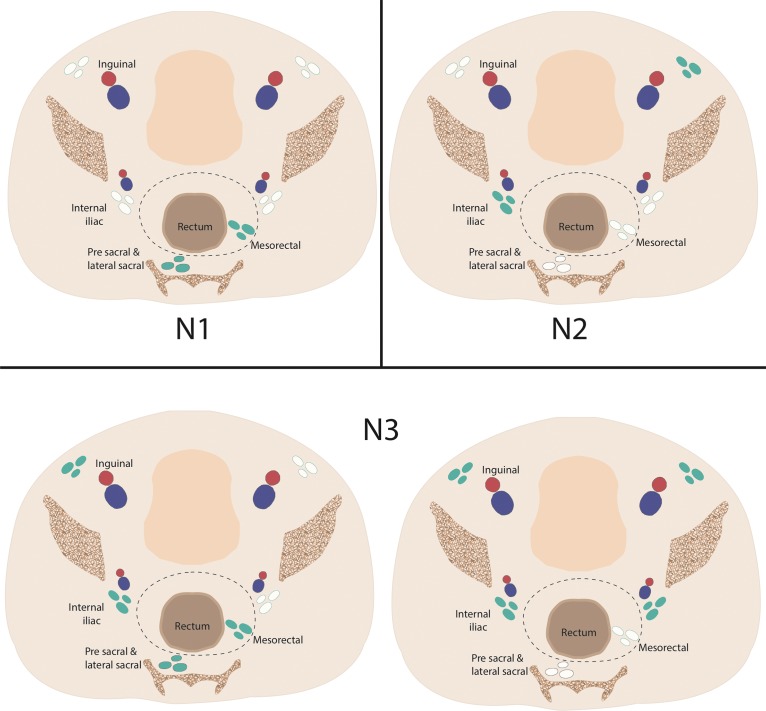
N staging of anal cancer according to the locations of involved regional lymph nodes. N1 anal cancers have metastases in perianorectal lymph nodes only. N2 anal cancers have metastases in unilateral internal iliac and/or inguinal lymph nodes. N3 anal cancers have metastases in mesorectal and internal iliac and/or inguinal nodes or in bilateral internal iliac and/or inguinal nodes. Blue-green = involved lymph nodes, red and blue = external and internal iliac arteries and veins.
Figure 12a.
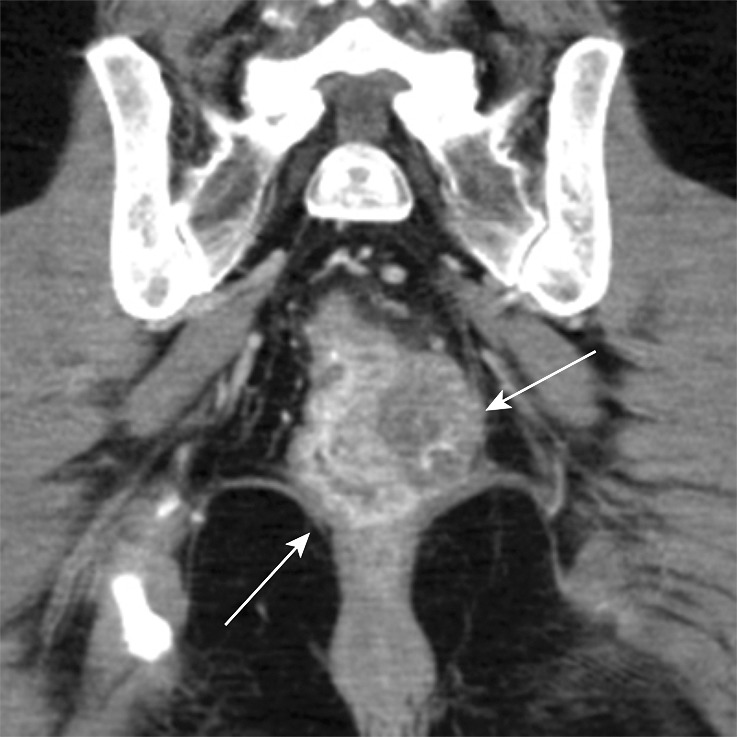
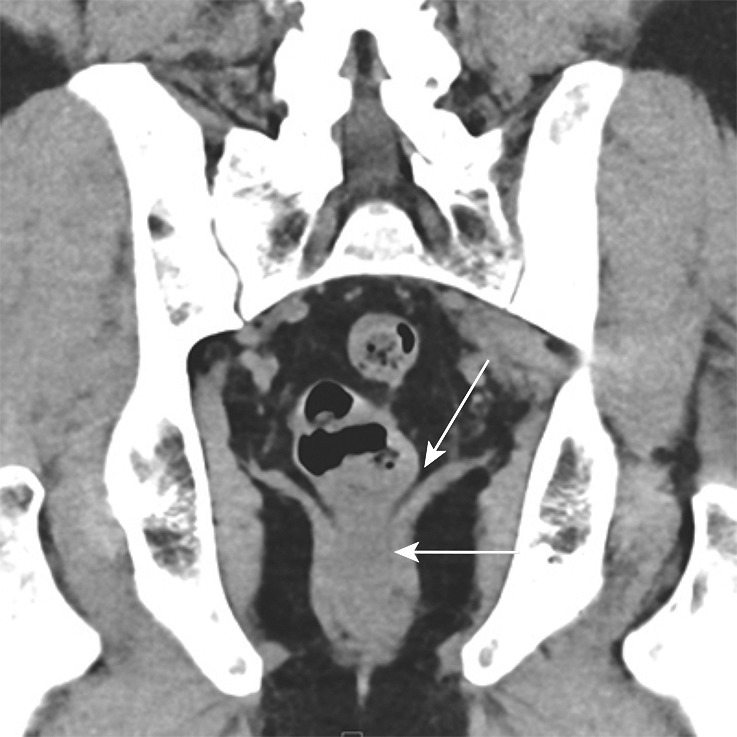
Stage IIIB (cT3N2M0) squamous cell carcinoma of the anus. (a, b) Coronal CT images obtained at different levels show a locally extensive mass extending superiorly from the anorectal junction (arrows in a). Pathologic analysis demonstrated squamous cell carcinoma consistent with anal cancer, despite the relatively superior location. A prominent left internal iliac lymph node is also seen (arrow in b). (c, d) Coronal CT images obtained at different levels 3 months after completion of definitive chemoradiation therapy show a partial radiographic response of both the primary mass and the left internal iliac lymph node.
Figure 12b.
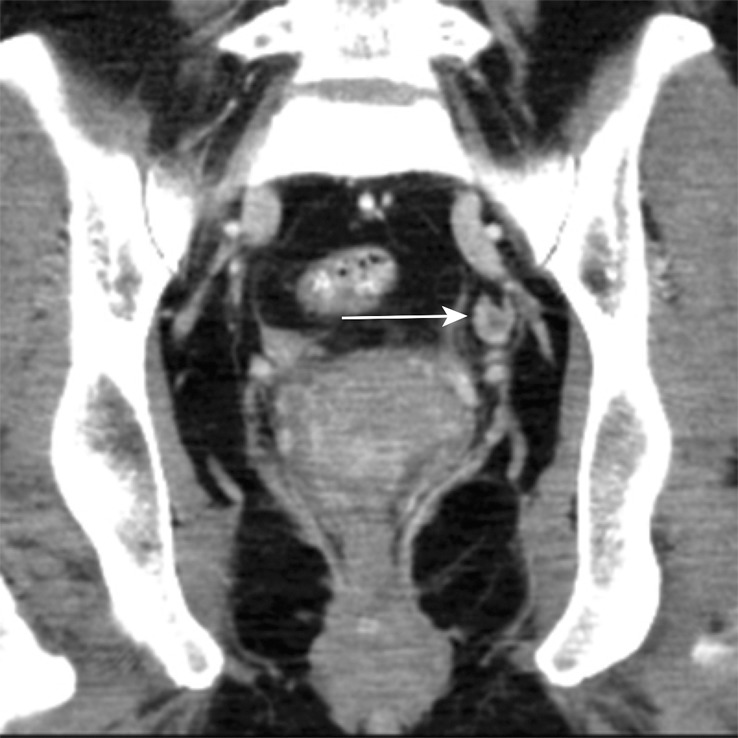
Stage IIIB (cT3N2M0) squamous cell carcinoma of the anus. (a, b) Coronal CT images obtained at different levels show a locally extensive mass extending superiorly from the anorectal junction (arrows in a). Pathologic analysis demonstrated squamous cell carcinoma consistent with anal cancer, despite the relatively superior location. A prominent left internal iliac lymph node is also seen (arrow in b). (c, d) Coronal CT images obtained at different levels 3 months after completion of definitive chemoradiation therapy show a partial radiographic response of both the primary mass and the left internal iliac lymph node.
Figure 12c.
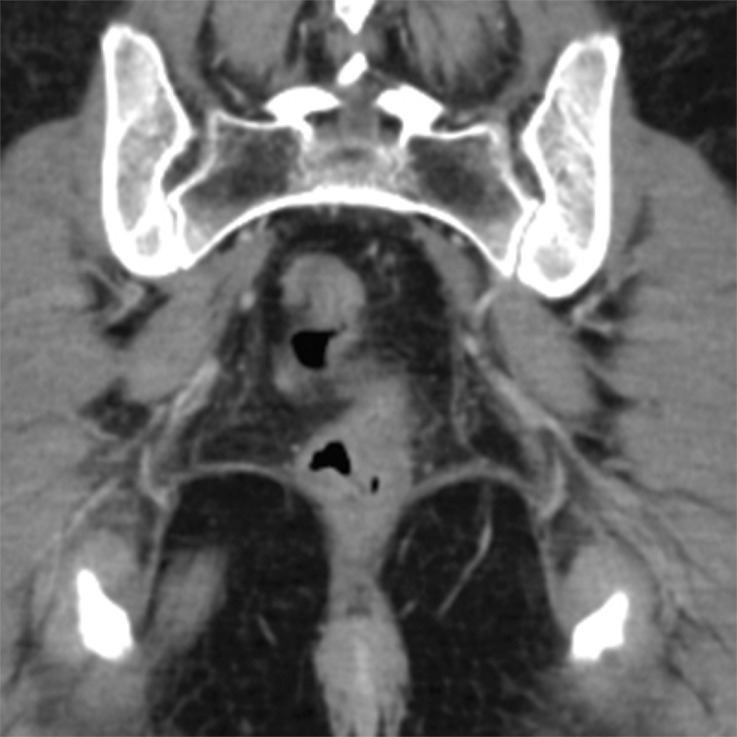
Stage IIIB (cT3N2M0) squamous cell carcinoma of the anus. (a, b) Coronal CT images obtained at different levels show a locally extensive mass extending superiorly from the anorectal junction (arrows in a). Pathologic analysis demonstrated squamous cell carcinoma consistent with anal cancer, despite the relatively superior location. A prominent left internal iliac lymph node is also seen (arrow in b). (c, d) Coronal CT images obtained at different levels 3 months after completion of definitive chemoradiation therapy show a partial radiographic response of both the primary mass and the left internal iliac lymph node.
Figure 12d.
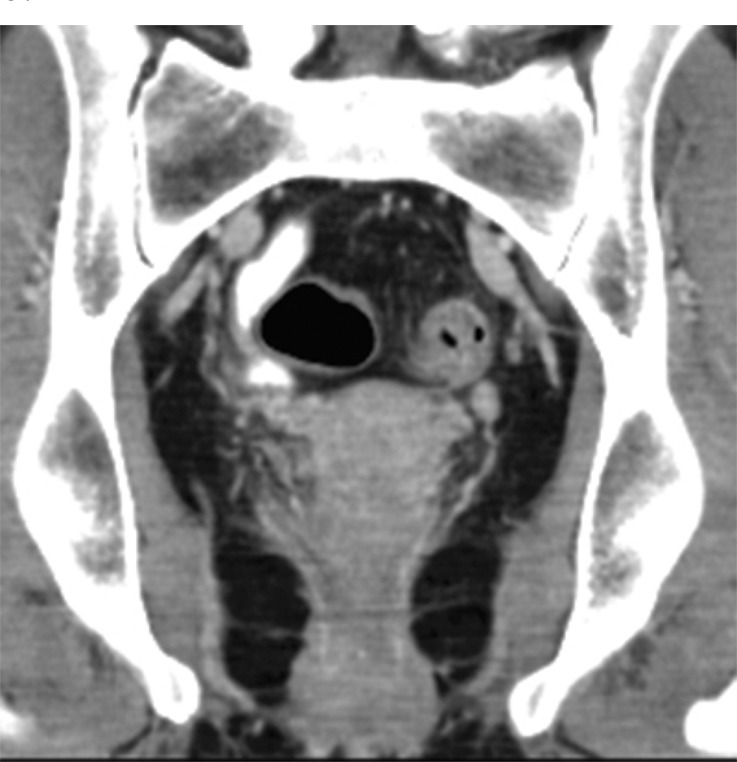
Stage IIIB (cT3N2M0) squamous cell carcinoma of the anus. (a, b) Coronal CT images obtained at different levels show a locally extensive mass extending superiorly from the anorectal junction (arrows in a). Pathologic analysis demonstrated squamous cell carcinoma consistent with anal cancer, despite the relatively superior location. A prominent left internal iliac lymph node is also seen (arrow in b). (c, d) Coronal CT images obtained at different levels 3 months after completion of definitive chemoradiation therapy show a partial radiographic response of both the primary mass and the left internal iliac lymph node.
Initial Evaluation.—When primary anal cancer is diagnosed on the basis of biopsy results, standard workup includes a digital rectal examination and anoscopy; inguinal lymph node assessment, with biopsy if indicated; and systemic radiologic evaluation (Table 3) (4,45). A prostate-specific antigen level is obtained in men to assess for prostate involvement, as is done in initial evaluation for rectal cancers. Women should undergo a gynecologic examination to evaluate for invasion into the posterior wall of the vagina and to test for concomitant cervical cancer, given the high association with HPV infection (4,6,46). HIV testing is usually performed.
Recommended radiologic imaging per the NCCN guidelines includes MR imaging to evaluate the extent of local disease (T and N staging), CT of the abdomen and pelvis, and chest x-ray or chest CT to assess for the presence of metastatic disease (M staging). The most common sites of anal cancer metastasis, which occurs in less than 10% of cases, are the liver and lungs (4,5,7,45,47).
High-spatial-resolution multiplanar MR imaging of the anal canal, sphincter complex, surrounding structures, and regional lymph nodes can be performed with or without an endoanal coil (5). Cancers usually show intermediate to low signal intensity on T1-weighted images and intermediate to high signal intensity on T2-weighted images. Potential drawbacks of MR imaging are high cost, length of examination, and potential contraindications in patients with pacemakers or other non–MR-compatible devices.
Endoanal US has shown comparable performance to that of MR imaging for assessment of local tumor extent, but it does not provide data on many of the regional nodal stations for anal cancer (13,15,29,48). In addition, the local stage of anal cancer is based purely on size, so the benefit of fine anatomic distinctions is less substantial than in rectal cancers. As a result of these factors, the NCCN does not recommend use of endoanal US at this time (4).
Approximately 98% of anal cancers are FDG avid, which aids in identification of the primary tumor and any nodal or metastatic sites. Similar to its use in rectal cancer, FDG PET/CT may be considered as an adjunct to contrast-enhanced CT in patients with T2–T4 N0 disease, or any T-stage N-positive disease (4). Results of several studies (49–53) have shown increased sensitivity of FDG PET/CT compared with contrast-enhanced CT alone for detection of abnormal lymph nodes, especially for advanced T2 tumors. This ultimately may lead to a change in treatment intent or altered radiation treatment fields (53). These studies remain limited because histologic confirmation of FDG-avid nodes is not part of the standard of care for anal cancer, leaving ambiguity about the distinction between tumor involvement and reactive changes in these nodes (52). Further research is needed to validate the use of FDG PET in patients with anal cancer.
Controversies in Nodal Staging of Rectal and Anal Cancers
An important issue in rectal and anal cancer staging is that the nodal staging systems are largely based on data from analyses of surgical specimens, whereas the initial clinical staging is done on the basis of a combination of imaging and biopsy results. All available imaging modalities have been shown to have imperfect accuracy for identification of rectal nodal metastases, and nodal size alone is not a reliable indicator of metastatic involvement; nonetheless, radiologists must give a reasonable assessment of the likelihood of nodal involvement on the basis of the available imaging features (10,29,30,37,54,55). Several different criteria exist to assist in identifying involved lymph nodes, including round shape, heterogeneity of appearance, irregular border, presence of mucin and/or calcifications, loss of the normal fatty hilum, and increased short-axis diameter (37). Although size was historically the most widely used criterion, results of studies (4,55) have shown that a large proportion of cancer-containing mesorectal lymph nodes from dissection specimens measure less than 0.5 cm in short-axis diameter, which suggests that additional criteria are crucial to accurate diagnosis of cancer of the lymph nodes on the basis of imaging.
Brown and colleagues (55) examined 437 surgically resected lymph nodes from 42 patients, all of whom underwent preoperative high-spatial-resolution MR imaging. Regarding size, they found that the sizes of negative lymph nodes at pathologic examination were 2–10 mm, while positive lymph nodes were 3–15 mm, which suggests that there is a considerable overlap in size between benign and malignant lymph nodes. With the criteria of either mixed signal intensity or irregular borders, sensitivity was 85% and specificity was 95%. Brown et al (55) found that including size criteria does not significantly change the number of false-positive or false-negative nodes. Alternative imaging methods to address this concern, including diffusion-weighted imaging, are a topic of ongoing research, and new intravenous agents are being investigated for use in differentiating benign from malignant nodes (56,57).
Inguinal nodes are important to staging of both rectal and anal cancers, although for different reasons. In patients with rectal cancer, generally speaking, inguinal nodes are considered nonregional, constituting M1 disease (3). The exception to this rule is that, in rectal cancers extending into the anal canal, inguinal lymph nodes may be considered regional, and patients are treated with curative intent. In patients with anal cancer, inguinal nodes are considered regional nodes, are included in the initial radiation field, and receive a boost dose if there is evidence of gross nodal disease (3,4,45).
Role of Radiation Treatment
Radiation therapy is an integral part of treatment of anal and rectal cancer. The dose, timing of treatment, target volumes, and intent of treatment—neoadjuvant, adjuvant, definitive, or palliative—often differ considerably between anal and rectal cancer and can also differ according to stage and histologic findings. The previously described imaging features are important for selection of an appropriate treatment plan for each patient. Radiation therapy is integral to primary treatment of all but the smallest anal cancers, but it is used more selectively in treatment of rectal cancers. General concepts in radiation therapy, as well as important distinctions, are discussed in the following sections.
Rectal Cancer
The treatment algorithm for nonmetastatic rectal cancer often includes trimodality therapy (chemotherapy, radiation, and surgery) and is highly dependent on accurate clinical staging (26). Patients with clinical stage I rectal cancers often undergo surgery first, and adjuvant chemotherapy and radiation therapy may be added if the cancers are upstaged at pathologic analysis to stage II or III (eg, T3, node-positive, positive or close margins). Adjuvant therapy is strongly considered for positive or close margins.
Rectal cancers with an initial clinical stage of II or III are often treated with trimodality therapy consisting of neoadjuvant chemotherapy and radiation therapy followed by surgical resection. Preliminary data from small studies suggest that patients with nonmetastatic rectal cancer who achieve a clinically complete response to neoadjuvant chemotherapy and radiation therapy may be able to forgo surgery and can be followed closely with clinical, radiologic, and endoscopic examinations, although surgery after neoadjuvant therapy remains the standard of care (58). Another experimental approach currently under investigation is the use of preoperative chemotherapy with a combination of folinic acid, fluorouracil, and oxaliplatin, without routine use of radiation (59). Further validation of these techniques is needed.
According to the NCCN guidelines, radiation therapy for rectal cancer should include 45–50 Gy in 25–28 increments given by using a multifield technique (26). Multifield external beam radiation involves multiple overlapping radiation beams at different orientations that are individually shaped to produce the desired dose field. Standard radiation fields include the tumor (or tumor bed, if previously resected) with a 2–5-cm margin and the presacral and internal iliac nodes (Fig 13). According to the NCCN, the external iliac nodes should be included in the radiation field for T4 tumors that invade anterior structures. It is imperative that radiologists be precise in describing anterior extension of rectal cancer into the urogenital structures of the pelvis because involvement of these structures will alter the radiation treatment plan. In addition, if the primary tumor is close to the anal sphincter complex (within 2 cm), many radiation oncologists would consider treating the inguinal lymph nodes. If the patient is receiving postoperative radiation after an abdominal-perineal resection, the perineum also should be treated.
Figure 13a.
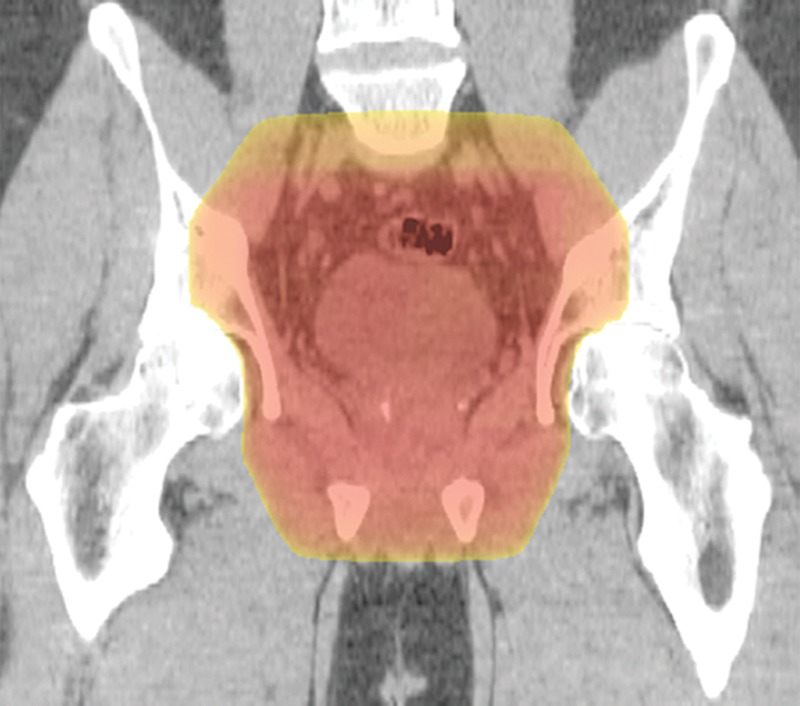
Example of a standard radiation treatment plan for rectal carcinoma. Coronal (a) and axial (b) CT images (obtained with prone patient position per radiation therapy standards) with superimposed radiation treatment plans show inclusion of the primary tumor bed and the presacral and internal iliac nodes. Higher-dose areas are more red; lower-dose areas are yellow. The external iliac nodes (not shown) were not included because the tumor did not invade anterior pelvic structures.
Figure 13b.
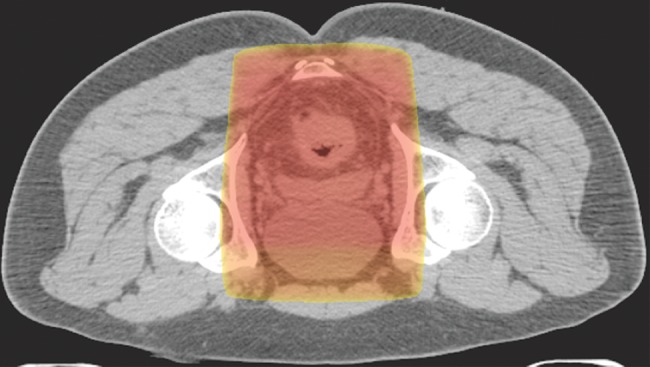
Example of a standard radiation treatment plan for rectal carcinoma. Coronal (a) and axial (b) CT images (obtained with prone patient position per radiation therapy standards) with superimposed radiation treatment plans show inclusion of the primary tumor bed and the presacral and internal iliac nodes. Higher-dose areas are more red; lower-dose areas are yellow. The external iliac nodes (not shown) were not included because the tumor did not invade anterior pelvic structures.
Anal Cancer
For much of the mid-20th century, abdominal-perineal resection was the mainstay in treatment of anal canal carcinomas. This extensive surgery not only resulted in considerable morbidity, including a permanent colostomy, but also afforded only a 40%–70% 5-year survival rate (6,12). In 1974, Nigro and colleagues (60) demonstrated the potential of combined radiation therapy and chemotherapy in three patients, all of whom showed no evidence of disease at short-interval follow-up. Decades later, numerous nonrandomized and randomized trials continue to support use of radiation therapy and chemotherapy with fluorouracil and mitomycin C as the standard of care for all anal canal carcinomas, with abdominal-perineal resection used only for salvage in patients with locally recurrent disease (4,46). Metastatic disease is considered incurable, and patients instead receive palliative treatment (3). Small anal margin cancers may be treated by means of excision alone, but larger (T2 positive) or margin-positive tumors warrant radiation therapy per the NCCN guidelines.
According to the NCCN guidelines, radiation therapy for anal cancer should include a minimum dose of 45 Gy in 1.8-Gy increments for gross disease by using the previously described multifield technique. The standard radiation field includes the pelvis, anus, perineum, and inguinal nodes (Fig 14) (4). Dose is adjusted on the basis of additional factors; for example, the lateral field is reduced if the inguinal nodes are negative for cancer, while a boost dose is delivered to stage T3 or T4 or node-positive cancers. Effort is made to reduce the dose to the femoral heads to avoid avascular necrosis. An alternative treatment approach includes intensity-modulated radiation therapy, which involves use of a complex and dynamic beam and collimator geometry with computer-based dose painting to achieve comparable doses for the primary tumor and regional lymph nodes (61).
Figure 14a.
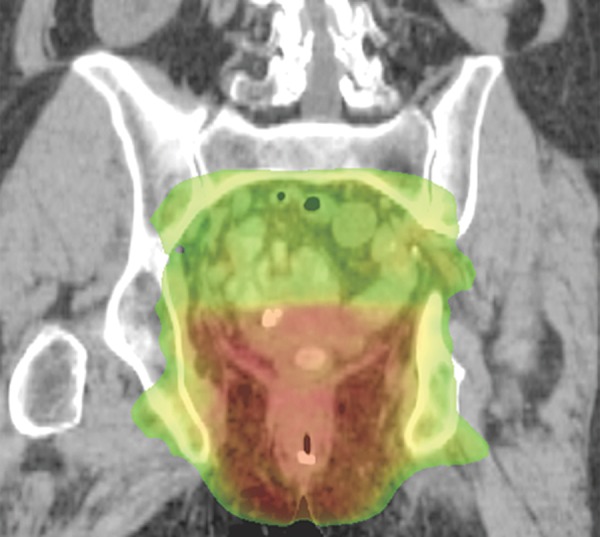
Representative example of a standard radiation treatment plan for anal carcinoma. Coronal (a) and axial (b) CT images show inclusion of the tumor, anus, perineum, and inguinal nodes. Higher-dose areas are more red, and lower-dose areas are more yellow-green. Note the attempt to spare radiation dose to the femoral heads.
Figure 14b.
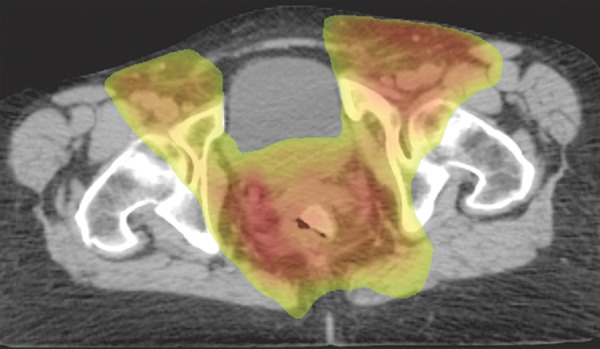
Representative example of a standard radiation treatment plan for anal carcinoma. Coronal (a) and axial (b) CT images show inclusion of the tumor, anus, perineum, and inguinal nodes. Higher-dose areas are more red, and lower-dose areas are more yellow-green. Note the attempt to spare radiation dose to the femoral heads.
Critical Imaging Distinctions for Radiation Planning
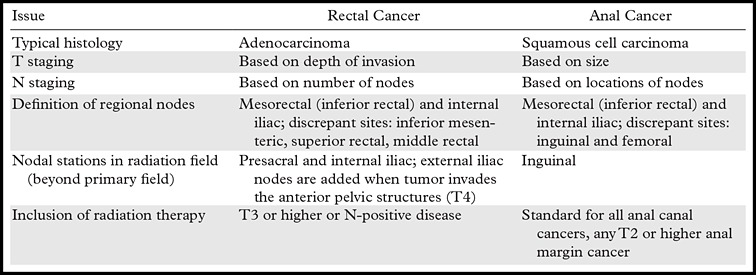
Table 5 summarizes the key distinctions that distinguish imaging, staging, and radiation therapy for anal and rectal cancers. As shown in this table, radiologists should take special care to tailor their reporting of the features of the primary tumor and sites of nodal disease on the basis of the origin of the patient’s cancer. Seemingly small differences in terminology for lymph nodes, such as external versus internal iliac, can fundamentally alter treatment of anal and rectal cancers.
Table 5:
Summary of Key Distinctions between Anal and Rectal Cancers
Conclusion
Rectal and anal cancers are distinct entities with different pathologic features, risk factors, staging, and treatment algorithms. Radiologists must understand these critical distinctions, and with this knowledge, accurately report the local-regional extent of the tumor, with special attention to the sphincter complex, mesorectal fascia, peritoneum, adjacent organs, and relevant lymph node stations. The radiologist must accurately assess each of these key areas of concern to ensure that our radiation oncology colleagues have the best possible information available when making their radiation therapy treatment plan.
Presented as an education exhibit at the 2014 RSNA Annual Meeting.
For this journal-based SA-CME activity, the author H.J.M. has provided disclosures (see “Disclosures of Conflicts of Interest”); all other authors, the editor, and the reviewers have disclosed no relevant relationships.
Funding: C.S.F. supported by the National Institutes of Health [grant numbers 5P50CA127003, R01CA149222, R01CA118553] and the National Cancer Institute [grant number 5P30CA06516].
Disclosures of Conflicts of Interest.—: H.J.M. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: honorarium from UptoDate as coauthor of textbook chapters, honorarium from Cerulean Pharmaceuticals for workshop participation. Other activities: disclosed no relevant relationships.
Abbreviations:
- AJCC
- American Joint Committee on Cancer
- FDG
- fluorodeoxyglucose
- H-E
- hematoxylin-eosin
- HIV
- human immunodeficiency virus
- HPV
- human papillomavirus
- NCCN
- National Comprehensive Cancer Network
References
- 1.American Cancer Society . Cancer facts and figures 2014. Atlanta, Ga: American Cancer Society, 2014. [Google Scholar]
- 2.Gay HA, Barthold HJ, O’Meara E, et al. Pelvic normal tissue contouring guidelines for radiation therapy: a Radiation Therapy Oncology Group consensus panel atlas. Int J Radiat Oncol Biol Phys 2012;83(3):e353–e362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edge SB, Byrd DR, Compton CC, Fritz AG, Green FL, Trotti A. AJCC cancer staging manual. 7th ed. New York, NY: Springer, 2010. [Google Scholar]
- 4.Engstrom PF, Arnoletti JP, Benson AB, 3rd, et al. NCCN clinical practice guidelines in oncology: anal carcinoma. J Natl Compr Canc Netw 2010;8(1):106–120. [DOI] [PubMed] [Google Scholar]
- 5.Hussain SM, Stoker J, Laméris JS. Anal sphincter complex: endoanal MR imaging of normal anatomy. Radiology 1995;197(3):671–677. [DOI] [PubMed] [Google Scholar]
- 6.Ryan DP, Compton CC, Mayer RJ. Carcinoma of the anal canal. N Engl J Med 2000;342(11):792–800. [DOI] [PubMed] [Google Scholar]
- 7.Scherrer A, Reboul F, Martin D, Dupuy JC, Menu Y. CT of malignant anal canal tumors. RadioGraphics 1990;10(3): 433–453. [DOI] [PubMed] [Google Scholar]
- 8.Kaur H, Choi H, You YN, et al. MR imaging for preoperative evaluation of primary rectal cancer: practical considerations. RadioGraphics 2012;32(2):389–409. [DOI] [PubMed] [Google Scholar]
- 9.Liang TY, Anil G, Ang BW. Imaging paradigms in assessment of rectal carcinoma: loco-regional and distant staging. Cancer Imaging 2012;12:290–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iafrate F, Laghi A, Paolantonio P, et al. Preoperative staging of rectal cancer with MR imaging: correlation with surgical and histopathologic findings. RadioGraphics 2006;26(3):701–714. [DOI] [PubMed] [Google Scholar]
- 11.Beaumont C, Pandey T, Gaines Fricke R, Laryea J, Jambhekar K. MR evaluation of rectal cancer: current concepts. Curr Probl Diagn Radiol 2013;42(3):99–112. [DOI] [PubMed] [Google Scholar]
- 12.Ryan DP, Mayer RJ. Anal carcinoma: histology, staging, epidemiology, treatment. Curr Opin Oncol 2000;12(4): 345–352. [DOI] [PubMed] [Google Scholar]
- 13.Parikh J, Shaw A, Grant LA, et al. Anal carcinomas: the role of endoanal ultrasound and magnetic resonance imaging in staging, response evaluation and follow-up. Eur Radiol 2011;21(4):776–785. [DOI] [PubMed] [Google Scholar]
- 14.Bendell JC, Ryan DP. Current perspectives on anal cancer. Oncology (Williston Park) 2003;17(4):492–497, 502–503; discussion 503, 507–509. [PubMed] [Google Scholar]
- 15.Kochhar R, Plumb AA, Carrington BM, Saunders M. Imaging of anal carcinoma. AJR Am J Roentgenol 2012;199(3):W335–W344. [DOI] [PubMed] [Google Scholar]
- 16.Kang H, O’Connell JB, Leonardi MJ, Maggard MA, McGory ML, Ko CY. Rare tumors of the colon and rectum: a national review. Int J Colorectal Dis 2007;22(2):183–189. [DOI] [PubMed] [Google Scholar]
- 17.National Cancer Institute . Rectal cancer treatment: for health professionals (PDQ). http://www.cancer.gov/types/colorectal/hp/rectal-treatment-pdq. Updated 2015. Accessed January 2, 2015.
- 18.National Cancer Institute . Anal cancer treatment: for health professionals (PDQ). http://www.cancer.gov/types/anal/hp/anal-treatment-pdq. Updated 2015. Accessed January 2, 2015.
- 19.Wei EK, Giovannucci E, Wu K, et al. Comparison of risk factors for colon and rectal cancer. Int J Cancer 2004;108(3):433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yurgelun MB. Next-generation strategies for hereditary colorectal cancer risk assessment. J Clin Oncol 2015;33(5):388–393. [DOI] [PubMed] [Google Scholar]
- 21.Assi R, Reddy V, Einarsdottir H, Longo WE. Anorectal human papillomavirus: current concepts. Yale J Biol Med 2014;87(4):537–547. [PMC free article] [PubMed] [Google Scholar]
- 22.Holly EA, Whittemore AS, Aston DA, Ahn DK, Nickoloff BJ, Kristiansen JJ. Anal cancer incidence: genital warts, anal fissure or fistula, hemorrhoids, and smoking. J Natl Cancer Inst 1989;81(22):1726–1731. [DOI] [PubMed] [Google Scholar]
- 23.Daling JR, Weiss NS, Hislop TG, et al. Sexual practices, sexually transmitted diseases, and the incidence of anal cancer. N Engl J Med 1987;317(16):973–977. [DOI] [PubMed] [Google Scholar]
- 24.Williams GR, Lu QL, Love SB, Talbot IC, Northover JM. Properties of HPV-positive and HPV-negative anal carcinomas. J Pathol 1996;180(4):378–382. [DOI] [PubMed] [Google Scholar]
- 25.Hoots BE, Palefsky JM, Pimenta JM, Smith JS. Human papillomavirus type distribution in anal cancer and anal intraepithelial lesions. Int J Cancer 2009;124(10): 2375–2383. [DOI] [PubMed] [Google Scholar]
- 26.Engstrom PF, Arnoletti JP, Benson AB, 3rd, et al. NCCN clinical practice guidelines in oncology: rectal cancer. J Natl Compr Canc Netw 2009;7(8):838–881. [DOI] [PubMed] [Google Scholar]
- 27.Edge SB, Byrd DR, Compton CC, Fritz AG, Green FL, Trotti A. AJCC cancer staging manual. 7th ed. New York, NY: Springer, 2010. [Google Scholar]
- 28.Jones WE, 3rd, Thomas CR, Jr, Herman JM, et al. ACR Appropriateness Criteria: resectable rectal cancer. Radiat Oncol 2012;7:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bipat S, Glas AS, Slors FJ, Zwinderman AH, Bossuyt PM, Stoker J. Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging—a meta-analysis. Radiology 2004;232(3):773–783. [DOI] [PubMed] [Google Scholar]
- 30.Blomqvist L, Machado M, Rubio C, et al. Rectal tumour staging: MR imaging using pelvic phased-array and endorectal coils vs endoscopic ultrasonography. Eur Radiol 2000;10(4):653–660. [DOI] [PubMed] [Google Scholar]
- 31.Lahaye MJ, Engelen SM, Nelemans PJ, et al. Imaging for predicting the risk factors—the circumferential resection margin and nodal disease—of local recurrence in rectal cancer: a meta-analysis. Semin Ultrasound CT MR 2005;26(4):259–268. [DOI] [PubMed] [Google Scholar]
- 32.McMahon CJ, Smith MP. Magnetic resonance imaging in locoregional staging of rectal adenocarcinoma. Semin Ultrasound CT MR 2008;29(6):433–453. [DOI] [PubMed] [Google Scholar]
- 33.Brown G, Richards CJ, Newcombe RG, et al. Rectal carcinoma: thin-section MR imaging for staging in 28 patients. Radiology 1999;211(1):215–222. [DOI] [PubMed] [Google Scholar]
- 34.Dewhurst C, Rosen MP, Blake MA, et al. ACR Appropriateness Criteria: pretreatment staging of colorectal cancer. J Am Coll Radiol 2012;9(11):775–781. [DOI] [PubMed] [Google Scholar]
- 35.Glynne-Jones R, Mawdsley S, Novell JR. The clinical significance of the circumferential resection margin following preoperative pelvic chemo-radiotherapy in rectal cancer: why we need a common language. Colorectal Dis 2006;8(9): 800–807. [DOI] [PubMed] [Google Scholar]
- 36.Nagtegaal ID, Quirke P. What is the role for the circumferential margin in the modern treatment of rectal cancer? J Clin Oncol 2008;26(2):303–312. [DOI] [PubMed] [Google Scholar]
- 37.Torkzad MR, Kamel I, Halappa VG, Beets-Tan RG. Magnetic resonance imaging of rectal and anal cancer. Magn Reson Imaging Clin N Am 2014;22(1):85–112. [DOI] [PubMed] [Google Scholar]
- 38.Lincender-Cvijetić L, Banjin-Čardžić M, Vegar-Zubović S, Vrcić D. Radiological imaging of rectal cancer. Acta Med Acad 2012;41(2):199–209. [DOI] [PubMed] [Google Scholar]
- 39.Heriot AG, Hicks RJ, Drummond EG, et al. Does positron emission tomography change management in primary rectal cancer? A prospective assessment. Dis Colon Rectum 2004;47(4):451–458. [DOI] [PubMed] [Google Scholar]
- 40.Agarwal A, Marcus C, Xiao J, Nene P, Kachnic LA, Subramaniam RM. FDG PET/CT in the management of colorectal and anal cancers. AJR Am J Roentgenol 2014;203(5):1109–1119. [DOI] [PubMed] [Google Scholar]
- 41.Bipat S, van Leeuwen MS, Comans EF, et al. Colorectal liver metastases: CT, MR imaging, and PET for diagnosis—meta-analysis. Radiology 2005;237(1):123–131. [DOI] [PubMed] [Google Scholar]
- 42.Moulton CA, Gu CS, Law CH, et al. Effect of PET before liver resection on surgical management for colorectal adenocarcinoma metastases: a randomized clinical trial. JAMA 2014;311(18):1863–1869. [DOI] [PubMed] [Google Scholar]
- 43.Selzner M, Hany TF, Wildbrett P, McCormack L, Kadry Z, Clavien PA. Does the novel PET/CT imaging modality impact on the treatment of patients with metastatic colorectal cancer of the liver? Ann Surg 2004;240(6):1027–1034; discussion 1035–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whaley JT, Fernandes AT, Sackmann R, et al. Clinical utility of integrated positron emission tomography/computed tomography imaging in the clinical management and radiation treatment planning of locally advanced rectal cancer. Pract Radiat Oncol 2014;4(4):226–232. [DOI] [PubMed] [Google Scholar]
- 45.Benson AB, 3rd, Arnoletti JP, Bekaii-Saab T, et al. Anal carcinoma, version 2.2012: featured updates to the NCCN guidelines. J Natl Compr Canc Netw 2012;10(4):449–454. [DOI] [PubMed] [Google Scholar]
- 46.Chin JY, Hong TS, Ryan DP. Mitomycin in anal cancer: still the standard of care. J Clin Oncol 2012;30(35): 4297–4301. [DOI] [PubMed] [Google Scholar]
- 47.Expert Panel on Radiation Oncology–Rectal/Anal Cancer , Hong TS, Pretz JL, et al. ACR Appropriateness Criteria: anal cancer. Gastrointest Cancer Res 2014;7(1):4–14. [PMC free article] [PubMed] [Google Scholar]
- 48.Otto SD, Lee L, Buhr HJ, Frericks B, Höcht S, Kroesen AJ. Staging anal cancer: prospective comparison of transanal endoscopic ultrasound and magnetic resonance imaging. J Gastrointest Surg 2009;13(7):1292–1298. [DOI] [PubMed] [Google Scholar]
- 49.Bhuva NJ, Glynne-Jones R, Sonoda L, Wong WL, Harrison MK. To PET or not to PET? That is the question: staging in anal cancer. Ann Oncol 2012;23(8):2078–2082. [DOI] [PubMed] [Google Scholar]
- 50.Cotter SE, Grigsby PW, Siegel BA, et al. FDG-PET/CT in the evaluation of anal carcinoma. Int J Radiat Oncol Biol Phys 2006;65(3):720–725. [DOI] [PubMed] [Google Scholar]
- 51.Mistrangelo M, Pelosi E, Bellò M, et al. Role of positron emission tomography–computed tomography in the management of anal cancer. Int J Radiat Oncol Biol Phys 2012;84(1):66–72. [DOI] [PubMed] [Google Scholar]
- 52.Nguyen BT, Joon DL, Khoo V, et al. Assessing the impact of FDG-PET in the management of anal cancer. Radiother Oncol 2008;87(3):376–382. [DOI] [PubMed] [Google Scholar]
- 53.Winton Ed, Heriot AG, Ng M, et al. The impact of 18-fluorodeoxyglucose positron emission tomography on the staging, management and outcome of anal cancer. Br J Cancer 2009;100(5):693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim JH, Beets GL, Kim MJ, Kessels AG, Beets-Tan RG. High-resolution MR imaging for nodal staging in rectal cancer: are there any criteria in addition to the size? Eur J Radiol 2004;52(1):78–83. [DOI] [PubMed] [Google Scholar]
- 55.Brown G, Richards CJ, Bourne MW, et al. Morphologic predictors of lymph node status in rectal cancer with use of high-spatial-resolution MR imaging with histopathologic comparison. Radiology 2003;227(2):371–377. [DOI] [PubMed] [Google Scholar]
- 56.Sinha R, Rajiah P, Ramachandran I, Sanders S, Murphy PD. Diffusion-weighted MR imaging of the gastrointestinal tract: technique, indications, and imaging findings. RadioGraphics 2013;33(3):655–676; discussion 676–680. [DOI] [PubMed] [Google Scholar]
- 57.Koh DM, Brown G, Temple L, et al. Rectal cancer: mesorectal lymph nodes at MR imaging with USPIO versus histopathologic findings—initial observations. Radiology 2004;231(1):91–99. [DOI] [PubMed] [Google Scholar]
- 58.Smith JD, Ruby JA, Goodman KA, et al. Nonoperative management of rectal cancer with complete clinical response after neoadjuvant therapy. Ann Surg 2012;256(6): 965–972. [DOI] [PubMed] [Google Scholar]
- 59.Schrag D, Weiser MR, Goodman KA, et al. Neoadjuvant chemotherapy without routine use of radiation therapy for patients with locally advanced rectal cancer: a pilot trial. J Clin Oncol 2014;32(6):513–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nigro ND, Vaitkevicius VK, Considine B, Jr. Combined therapy for cancer of the anal canal: a preliminary report. Dis Colon Rectum 1974;17(3):354–356. [DOI] [PubMed] [Google Scholar]
- 61.Kachnic LA, Winter K, Myerson RJ, et al. RTOG 0529: a phase 2 evaluation of dose-painted intensity modulated radiation therapy in combination with 5-fluorouracil and mitomycin-C for the reduction of acute morbidity in carcinoma of the anal canal. Int J Radiat Oncol Biol Phys 2013;86(1):27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]