Significance
Dynamic changes of cortical excitability are relevant in both healthy and pathological network dynamics. In epilepsy, pathological changes in excitability commonly underlie the initiation and spread of seizures. Accordingly, the ability to monitor excitability and control its degree is important for adequate clinical care and treatment because classic EEG markers found in epilepsy such as interictal spikes do not reflect seizure propensity and thus excitability. Here, we identify excitability markers and test them on long-term electrocorticogram and EEG recordings. We show that they correlate with more direct excitability measures using external stimulation and allow for real-time excitability monitoring. Our results provide evidence that excitability of cortical networks is reduced by antiepileptic drugs and increases as a function of time awake.
Keywords: excitability, epilepsy, sleep
Abstract
Pathological changes in excitability of cortical tissue commonly underlie the initiation and spread of seizure activity in patients suffering from epilepsy. Accordingly, monitoring excitability and controlling its degree using antiepileptic drugs (AEDs) is of prime importance for clinical care and treatment. To date, adequate measures of excitability and action of AEDs have been difficult to identify. Recent insights into ongoing cortical activity have identified global levels of phase synchronization as measures that characterize normal levels of excitability and quantify any deviation therefrom. Here, we explore the usefulness of these intrinsic measures to quantify cortical excitability in humans. First, we observe a correlation of such markers with stimulation-evoked responses suggesting them to be viable excitability measures based on ongoing activity. Second, we report a significant covariation with the level of AED load and a wake-dependent modulation. Our results indicate that excitability in epileptic networks is effectively reduced by AEDs and suggest the proposed markers as useful candidates to quantify excitability in routine clinical conditions overcoming the limitations of electrical or magnetic stimulation. The wake-dependent time course of these metrics suggests a homeostatic role of sleep, to rebalance cortical excitability.
Normal functioning of cortical networks critically depends on a finely tuned level of excitability, the transient or steady-state response in which the brain reacts to a stimulus. Whereas small, local responses indicate a comparably small excitability, large and global responses consequently suggest excitability to be high. The importance of adequate excitability levels is highlighted by the pathological consequences and impaired performance resulting from aberrant network excitability. In epilepsy, changes in cortical network excitability are believed to be an important cause underlying the initiation and spread of seizures, that is, the large nonphysiological neuronal activity events across time and space (1–3). Evidence for changes of excitability in brain networks affected in epilepsy has come from a variety of observations. To assess the level of excitability under in vivo experimental conditions one usually measures the size of the evoked cortical activity to external perturbations, typically either by transcranial magnetic stimulation (TMS) or, in the case of epilepsy patients, by electrical stimulation. Studies of electrical stimulation with subdural electrodes during interictal periods found the evoked potentials to be larger in regions related to the cortical onset region of epileptiform activity (4–6). The enhanced responses to electrical stimulation in epileptogenic cortex were taken as indication of an increased excitability of neural tissue in these areas. Similarly, studies using TMS consistently reported a hyperexcitability in focal epilepsies (7–10). Consequently, changes in excitability have been helpful in identifying the seizure focus before resective surgery. The insight that epilepsy is related to hyperexcitability is also at the basis of pharmacological treatment options for patients. Most antiepileptic drugs (AEDs) aim to reduce the excitability in neural tissue by reducing the excitability of individual neurons through selective ion channel blockers, enhancing inhibitory synaptic transmission or inhibiting excitatory synaptic transmission (11). Robust markers of excitability could therefore potentially be useful to guide the treatment with AEDs by giving feedback of their effect and efficiency on epileptic networks.
Apart from aberrant pathological deviations, changes in cortical excitability are believed to play a role in normal conditions during the course of wake and sleep. A perturbational approach to study excitability in human cortex found increased responses after a period of sustained wakefulness that were rebalanced after sleep (12, 13). Such findings suggest that excitability could increase during wake, which might result in suboptimal information processing in cortical networks (14, 15) and point to a pivotal role of sleep in rebalancing the level of excitability. However, a continuous measurement of excitability during the wake/sleep cycle supporting this hypothesis is hard to obtain with current measures.
A thorough understanding of excitability and how to monitor it in brain networks is therefore highly desirable for an understanding of both normal as well as pathological brain function. Cortical excitability is generally a product of both excitation and inhibition in a network. Changes in excitability can consequently arise from changes in excitation and/or changes in inhibition. To date, most approaches to quantify excitability have focused on perturbational approaches by electrical or magnetic stimulation where evoked responses to a brief electrical or magnetic stimulation are plotted over time (5, 6, 16, 17). A disadvantage of these methods, however, is their complex design, which limits regular clinical use and continued monitoring of the time course over extended periods of time. Even more so, the fact that such perturbations can induce seizures constitutes a considerable limitation for its application in patients suffering from epilepsy (18). For these various reasons, intrinsic excitability measures (IEMs) based on ongoing activity without the need of external perturbations would be preferable and likely to provide novel insights because of a broader applicability due to their noninvasive nature.
Recent insights into cortical activity from computational modeling (19, 20), experiments in cultures in vitro (21), rodents (22, 23), and also human magnetoencephalography and EEG (14, 24) have pointed to the ability of global synchronization measures to characterize physiological cortical dynamics. It was shown that phase synchronization of ongoing cortical activity exhibits moderate mean over a broad range of frequency bands under normal conditions. The ability to pharmacologically control the excitation/inhibition (E/I) axis in cortical in vitro preparations and rodents in vivo revealed the E/I level to be a crucial parameter affecting cortex activity to which synchronization markers are sensitive. Specifically, the E/I balance or ratio can be modulated toward a more inhibited system by selectively blocking excitatory glutamatergic synaptic transmission. Conversely, inhibition can be reduced by pharmacological application of inhibitory gabaergic synaptic transmission blockers, leading to an overall disinhibited state. Experimental approaches in which the E/I axis was altered in this way have shown that networks, which transition from inhibited to normal to disinhibited states by pharmacological manipulation, exhibit spontaneous dynamics characterized by a progressive increase in phase synchronization and a peak in its variability at normal, unchanged E/I levels. These synchronization changes have been shown to occur between cell assemblies measured by microelectrode arrays and can be observed over a broad frequency range (21). The distinct changes from cell assemblies in vitro and in rodents suggest global synchronization measures to be potentially valuable IEMs in human cortical networks.
Here, we explore the use of IEMs as a method to monitor excitability in clinical settings. To this end we first compare intrinsic measures to more direct measures of excitability using a perturbational approach. Second, we analyze large-scale invasive EEG (iEEG) datasets obtained during presurgical monitoring of epilepsy patients as well as scalp EEG data of healthy participants during sustained wakefulness. During the multiday, continuous iEEG recordings, AEDs were typically tapered off to increase the likelihood for epileptic seizures and identify their onset zones (25). These datasets are therefore ideally suited to study electrophysiological markers of excitability in clinical settings under changing levels of E/I balance due to variations in AED dosage.
Results
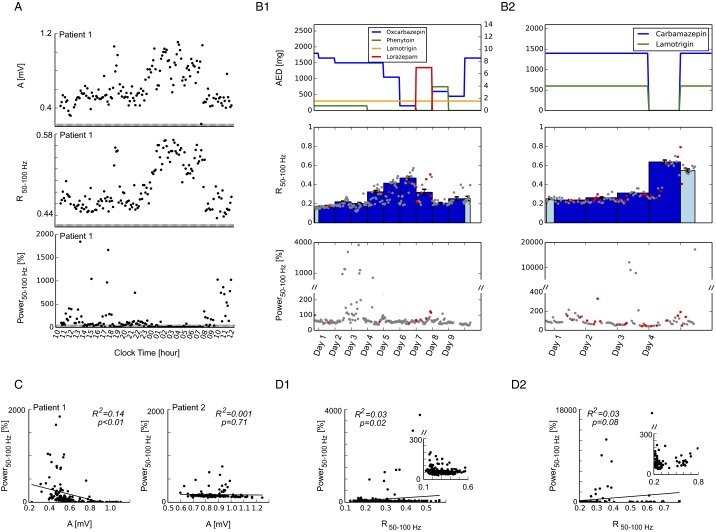
In light of the characteristic changes observed in cortical cultures in vitro and rodents in vivo, we focused on the mean of phase synchronization dynamics as a potential IEM in cortex in humans. Specifically, we hypothesized that the mean levels of phase synchronization should positively correlate with cortical excitability. To evaluate whether such a direct correlation exists we probed cortical excitability in human electrocorticogram (ECoG) by electrical stimulation. Previous work has shown that the amplitude of evoked cortical potentials by short pulses of electrical stimulation is a direct measure of cortical excitability: Whereas small amplitudes indicate a comparably small excitability, large responses suggest excitability to be high (5, 6, 26). We designed a stimulation protocol that allowed us to measure electrical stimulation-evoked responses as a direct marker of excitability, as well as phase synchronization of ongoing activity as a potential IEM over long periods of time within individual patients. Fig. 1A shows a typical evoked response in one channel. The amplitude A of evoked potentials, measured from highest peak to lowest trough, exhibited considerable variation (Fig. 1B), indicating varying levels of excitability over the course of hours. Unperturbed time segments before each stimulus (Fig. 1A, gray bar) were used to calculate phase synchronization in different frequency bands. We observed that mean synchronization levels R followed a very similar time course (Fig. 1B) that was reflected in high correlation values between amplitude A and synchronization R (Fig. 1C). This significant correlation was observed across a broad range of frequencies from 50 to 400 Hz and in n = 2 patients under investigation (Fig. 1D). Throughout the paper, we will focus on IEM in this frequency range (i.e., the bands 50–100 Hz, 100–200 Hz, and 200–400 Hz). Conversely, absolute power averaged over all channels in these frequency bands did not exhibit a positive correlation with stimulation-evoked responses (Fig. S1 A and C). These results provide an indication that mean levels of phase synchronization are related to cortical excitability in humans and consequently suggest them as valid indicators of excitability based on ongoing cortical activity.
Fig. 1.
Intrinsic measures of synchronization correlate with the size of stimulation-evoked responses. (A) Evoked responses to subdural electrical stimulation were used to directly infer cortical excitability. The plot shows a representative example of the mean evoked response from one electrode of patient 1. Excitability is reflected by the size of the evoked potential and was quantified as the absolute difference A between positive and negative maxima of the evoked response. Electrical stimulation was continuously applied (approx. 0.3 Hz) for multiple hours, allowing continuous measurement of cortical excitability. Unperturbed segments before the stimulation (gray bar) were used to calculate synchronization R across different electrodes. (B) Time course of stimulation-evoked response A and synchronization R of ongoing cortical activity over multiple hours in patient 1. Each black dot corresponds to a stimulation block (i.e., 10 min). (C) Evoked response amplitude A and synchronization R are highly correlated across broad frequency bands. Black line shows linear regression, reflects the goodness of fit. (D) Summary results of linear regression analysis ( values, **P ≤ 0.001) for different frequency ranges of patient 1 and patient 2.
Fig. S1.
Comparison of synchronization R and signal power. (A) Stimulation response A (Top) is plotted together with synchronization R (Middle) and mean power across all channels (Bottom) in the frequency band 50–100 Hz over multiple hours of recording. Power is given in percent of the first measurement. (B, 1 and 2) Level of medication (Top), synchronization R (Middle), and signal power (Bottom, in percent of first recording value) of two representative patients during multiday recordings with varying AED load. Dots correspond to 1-h measurements (red dots signify that at least one epileptic seizure occurred during this 1 h; gray dots signify no seizures), and bars correspond to averages over 1 d. Light colors were used when recordings did not encompass a full 24-h day. Time is labeled in days where each day starts at midnight. (C and D, 1 and 2) Scatter plots of stimulation response A versus power (C) and synchronization R versus power (D, 1 and 2, corresponding to patients in B, 1 and 2), respectively. R2 and P values refer to the linear regression analysis (black line).
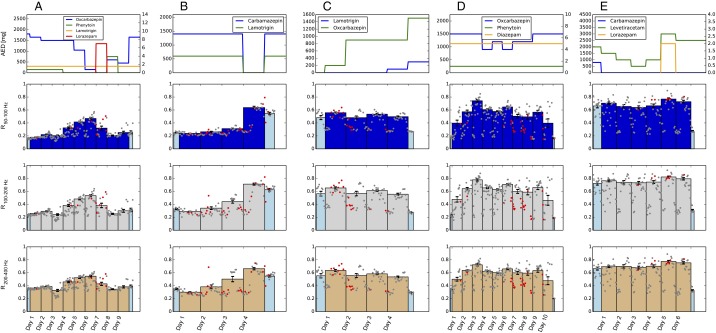
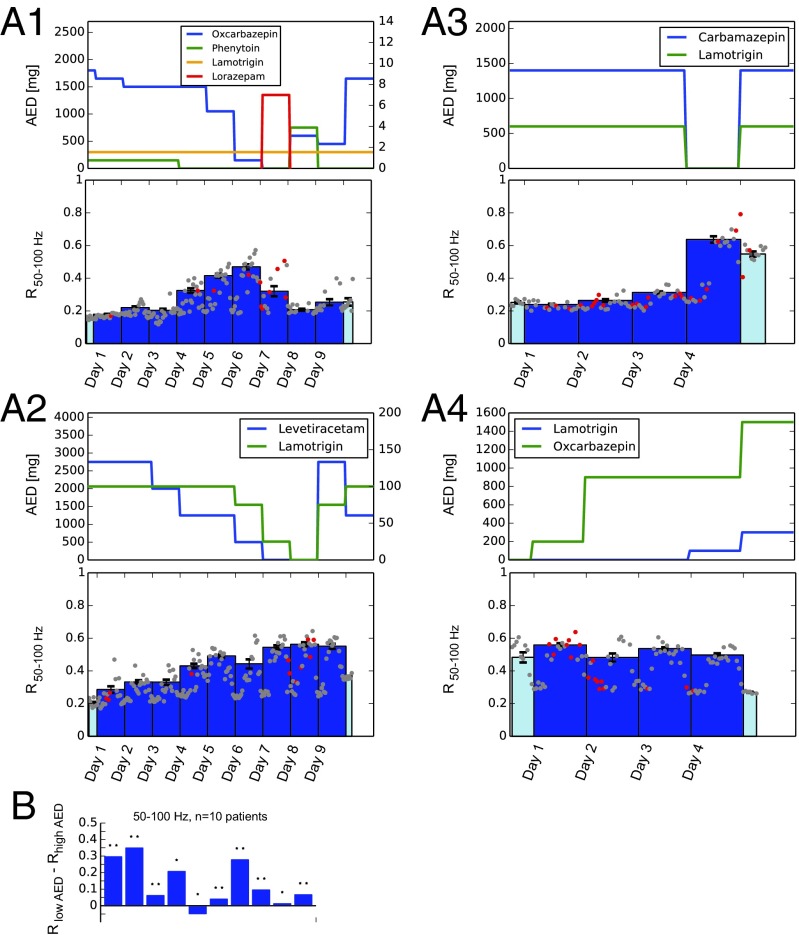
Next, we analyzed invasive EEG from 10 patients undergoing presurgical monitoring during which antiepileptic medication had been varied. No stimulation was performed in these patients. The type of AEDs used during this time, their dosages, and the time course by which they were tapered off were solely determined by clinical considerations and varied between patients. We were interested whether synchronization measures would, analogously to in vitro analyses under pharmacological manipulation, exhibit an AED-dependent trajectory that would be consistent with the hypothesis of a change in E/I balance. We thereby focused particularly on mean levels of synchronization R in the frequency range of 50–100 Hz because stimulation analysis had revealed a good correlation with evoked responses in this frequency band and because this frequency range could also be resolved in datasets recorded with lower sampling rates. In the following we will therefore use as the primary IEM, although results were generally robust over a broader range of frequencies (Fig. S2 and see Fig. 3). Mean synchronization R exhibited considerable variability during the multiday recordings in each patient. Fig. 2A shows time courses for four representative patients. Typically, R was low during days with high AED load and increased when AEDs were reduced. The time course of R thereby closely followed an inverse relation with AED load. Because especially the highest R values showed a strong dependence on AED, we averaged over each day’s highest 12 h to determine a daily mean (Fig. 2A, solid bars). Absolute signal power in these frequency bands did not, similar to stimulation results, correlate as well with AED load (Fig. S1 B and D). To quantify the visually observed dependence of R on AED dosage (Fig. 2A), we compared R values from one day of highest AED load (high AED) to the day with the lowest dose of AED (low AED) in each patient. The day with high AED load was usually the first full day of recording. When there was more than 1 d with the same amount of low or no AED, we chose the one furthest away from the high-AED day. Statistical analysis revealed a significant increase in R from high to low levels of AED for the majority of patients (Fig. 2B, two-sided paired sample t test).
Fig. S2.
Intrinsic measures of synchronization during multiday recordings show similar behavior across different frequency bands. (A–E) Synchronization and level of medication of all five patients with data recorded at 1,024 Hz. (Top) AED dosage. (Bottom) Changes in mean phase synchronization (R) of frequency bands 100–200 Hz (third row) and 200–400 Hz (fourth row). Dots correspond to 1-h measurements (red dots signify that at least one epileptic seizure occurred during this 1 h; gray dots signify no seizures), and bars correspond to averages over 1 d. Light blue colors were used when recordings did not encompass a full 24-h day. Time is labeled in days, where each day starts at midnight.
Fig. 3.
AEDs shift network dynamics from moderate synchrony to states with low synchrony. (A) Combined data from 10 patients and different frequency bands. Left vertical axis: Variability (H) as a function of mean synchronization (R, gray dots). Right vertical axis: Histogram of time (hours) spent at different levels of mean synchronization. Without AEDs (black histogram bars), network dynamics predominantly settles at . With AEDs (blue histogram bars), network dynamics spends more time in low synchrony states. (B) Averages of mean (R, left plot) and variability (H, right plot) of synchronization for hours without (Left) and with (Right) AEDs. *P ≤ 0.05, **P ≤ 0.001, two-sided independent sample t test. (C) Illustration of the behavior of measures R and H as a function of the ratio of excitation and inhibition (E/I) or, more generally, network excitability. The decrease of R and H in our data suggests that AEDs drive the network toward a more disfacilitated/inhibited state (blue arrow).
Fig. 2.
Intrinsic measures of synchronization track AED action during multiday recordings. (A, 1–4) Markers and level of medication of four patients. (Top) AED dosage. (Bottom) Changes in mean phase synchronization (R) for the frequency band 50–100 Hz. Dots correspond to 1-h measurements (red dots signify that at least one epileptic seizure occurred during this 1 h; gray dots signify no seizures). Daily averages were taken over the 12 highest hours of each day and are plotted as bars. Light colors were used when recordings did not encompass a full 24-h day. Error bars on each solid bar indicate SEM. Time on the x axis is labled in days, where each day starts at midnight. (B) Differences between full days of low and high AED levels for all 10 patients. *P ≤ 0.05, **P ≤ 0.001, two-sided paired sample t test. For complete time courses of the other high-frequency bands, see Fig. S2.
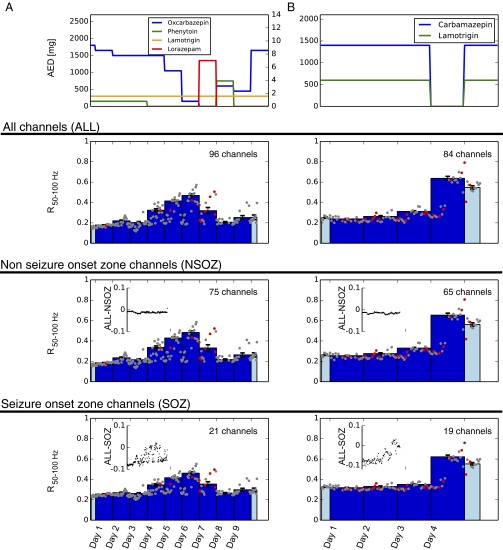
As a test whether the observed changes in R were only driven by a subset of channels we repeated the synchronization analysis for channels in the seizure onset zone only as well as for the channels outside the seizure onset zone. As channels that were part of the seizure onset zone we considered those that had been defined by an experienced epileptologist as channels from which at least one seizure had originated. The remaining channels were consequently defined as those outside of the seizure onset zone. Fig. S3 shows results from two representative patients; the AED-dependent changes in R are similarly observed in networks encompassing all channels, only channels of the seizure onset zone, and only channels outside the seizure onset zone. Our results are therefore consistent with the hypothesis that AEDs reduce the E/I balance and decrease excitability in widespread cortical networks.
Fig. S3.
Covariation of synchrony R with AED load is independent whether R is measured within the seizure onset zone or outside the seizure onset zone. (A and B) The two columns show results from two representative patients. Level of medication (AED) is plotted on top. Mean synchronization R was calculated from all channels (second row, ALL), from channels outside of the seizure onset zone only (third row, NSOZ), and from channels within the seizure onset zone only (fourth row, SOZ). As channels of the seizure onset zone (SOZ) we considered those channels that had been defined by an experienced epileptologist as channels from which at least one seizure had originated. Consequently, non-seizure-onset zone channels (NSOZ) were defined as the complement of SOZ (i.e., all channels except those that had been defined as the origin of at least one seizure). In patient A, this classification resulted in 21 of the 96 to be considered as part of the seizure onset zone (SOZ) and, consequently, the remaining 75 channels to be considered NSOZ. In patient B, 19 channels were defined as SOZ, the remaining 65 as NSOZ. The time course of R50–100 showed a similar behavior independent whether all (ALL, second row), channels outside the seizure onset zone (NSOZ, third row) or channels within the seizure onset zone (SOZ, fourth row) were considered in the analysis. In fact, hourly values look very similar but are not exactly the same, as can be seen more easily from the difference plots comparing NSOZ and SOZ to all channels (Insets).
To gain better insight into the function of AEDs on network dynamics, we separated days on which no AED had been given from days where AED had been applied. We observed that cortical network activity without AED typically settled at R levels in the vicinity of (Fig. 3A, black bars in histogram). Previous in vitro studies had suggested that normal cortical dynamics under a physiological E/I balance is, besides moderate levels of the mean, characterized by a maximum in variability of synchronization (21). In our case, we observed that peak variability H was found at (Fig. 3A, gray circles). For a measure like R bounded between [0,1] a peak variability occurring near is not surprising because any fluctuations will be increasingly cut off the closer the mean gets to either of the boundaries, resulting in decreases of variability there. During times when AEDs had been administered, we observed that markedly more time was spent at lower R values, as is evident by the left shift visible in the histogram (Fig. 3A, blue bars in histogram). Comparison between “no AED” and “AED” hours revealed a significant decrease from to lower values across a broad range of frequencies along with a drop in variability H (Fig. 3B, two-sided independent sample t test). Fig. 3C schematically summarizes the qualitative behavior of IEMs as a function of the ratio of excitation and inhibition (E/I) or, more generally, network excitability as observed in our data. As excitability is pharmacologically increased from disfacilitated to disinhibited dynamics, R increases. In our data we observed an increase of R when AED load was reduced. This is in line with observations in cortex cultures and provides strong indication for an increase in excitability in cortical networks when AEDs are tapered off. These analogies suggest that excitability is effectively reduced by AEDs (Fig. 3C, blue arrow) and, together with the correlation to stimulation-evoked responses reported above, provide further support for R as a measure of cortical excitability. An advantage of excitability measures based on ongoing activity such as R is their ability to be used for continuous monitoring over extended periods of time. In the following we will further explore how this continual measurement can uncover insights into the modulation of excitability.
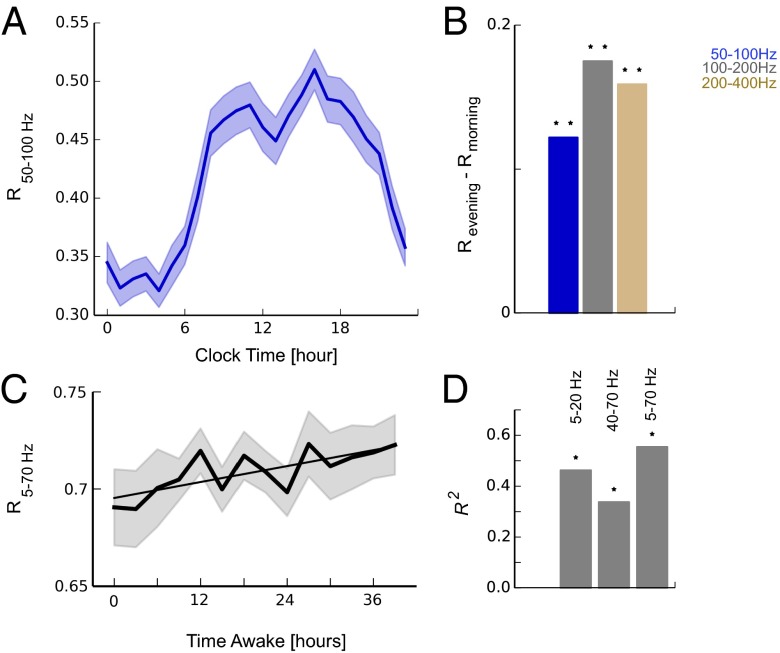
Apart from the dependency on AED load, we observed that R exhibited considerable variability within a day (Fig. 2A, gray markers). To further investigate these modulations we averaged the daily time course of all 10 subjects and over all 70 d. Values of R showed a characteristic modulation over the course of a day: an increase and high levels from morning to evening hours followed by a decrease and low levels during the night (Fig. 4A and Fig. S4). The increase from morning hours (hours 5–7) to evening hours (hours 17–19) was significant over a broad frequency range (Fig. 4B, two-sided paired sample t test) and could suggest distinct changes in cortical excitability over the 24-h time course. Because, on average, patients were more likely to be awake during daytime hours and more likely to sleep during nighttime hours, we hypothesized that this modulation of R could be related to changing levels of vigilance and be a function of time awake. Although the average sleep pattern of patients provides some indication for such a vigilance-dependent modulation, further proof is needed because a rigorous control of vigilance across time for all patients was not feasible in a clinical setting. To further investigate the relationship between R and vigilance we therefore extended our analysis to a dataset of eight healthy subjects where vigilance had been rigorously monitored and controlled. There, scalp EEG had been recorded every 3 h over a period of 40 h of sustained wakefulness (14, 27). During this period of wakefulness we observed an increase of R that was significantly correlated to the time awake (Fig. 4 C and D and Fig. S4). In light of the ability of R to closely track excitability in electrical stimulation and AED conditions, the results from invasive and noninvasive scalp EEG recordings are in line with the hypothesis of an increasing excitability as a function of time awake.
Fig. 4.
IEMs increase as a function of time awake. (A) Mean synchronization R from n = 10 patients during iEEG monitoring over a total of n = 70 d exhibits an increase during daytime hours and a decrease during the night (mean ± SEM) in different frequency bands. (B) The increase during day quantified as the difference between morning (hours 5–7) and evening hours (hours 17–19) of different frequency bands. *P ≤ 0.05, **P ≤ 0.001, two-sided paired sample t test. (C) Increase of R as a function of time awake during a 40-h period of total sleep deprivation where scalp EEGs were recorded every 3 h (n = 8 healthy subjects; straight line shows linear regression). (D) Summary results of linear regression analysis ( values, *P ≤ 0.05) of sleep deprivation EEG data; an increase in synchronization R was observed consistently in different frequency bands.
Fig. S4.
Change of R during day and as a function of time awake. (A) Modulation of R from all 70 nights of iEEG monitoring for the frequency bands 100–200 Hz and 200–400 Hz. (B) Increase of R as a function of time awake during a 40-h period of sustained wakefulness (sleep deprivation). The plots show the time courses of R in the additional frequency bands for which summary statistics and significance values are provided in Fig. 4 C and D. Straight line shows linear regression.
Discussion
In the present work we propose IEMs, that is, measures that quantify cortical network excitability based on intrinsic ongoing activity. The use of such IEMs is motivated by theoretical considerations suggesting cortical networks reside near a synchronization transition (19, 20) along with experimental studies illustrating their sensitivity to changes in the E/I axis in cortical in vitro preparations and rodents (21–23,). Our main finding is that these measures correlate well with AED load in patients, which suggests that they are viable markers of cortical excitability in humans. This is further supported by their correlation with stimulation-evoked responses—the current gold standard for excitability measurements—in human subdural recordings. We uncover a characteristic modulation over 24 h in line with the hypothesis of a progressively increasing excitability during wakefulness that is rebalanced during sleep.
To date, reliable measures of cortical excitability based on ongoing activity have been difficult to obtain. In particular, classic EEG markers found in epilepsy such as interictal spikes have been shown not to reflect seizure propensity and thus excitability (28). Instead, excitability is usually measured as the response to electrical or magnetic stimulation. Such perturbational approaches allow measurements to be well-controlled in space and time. However, the invasiveness and relative technical complexity in setup and analysis limit the practicability and its applicability for long-term and continuous monitoring. Furthermore, individual responses to perturbations will naturally exhibit a high variability due to the dependence of the induced activity on initial conditions (29), which requires a high number of trials to converge to a robust measure. With respect to a potentially broad applicability in epilepsy patients perturbational approaches by electric pulses or TMS can be problematic due to their ability to induce seizures. IEMs as proposed in the present study naturally circumvent these problems.
One of the advantages of IEMs is their ability to passively monitor excitability over long continuous time periods; in contrast, perturbational approaches classically considered to measure excitability are limited in this regard. Consequently, the investigation of changes in human cortical excitability during the sleep–wake cycle has so far been limited to only a few measurements based on TMS responses (13). There, increased responses as a function of time awake were found and taken as indication of an increasing cortical excitability. A TMS study on epilepsy patients, too, suggested increased excitability during sleep deprivation (12). Similarly, a recent study using an implantable brain stimulation and sensing system in canines reported characteristic changes in excitability following a circadian profile and a dependency on AEDs (30). The continuous iEEG measurement of IEMs over combined 70 d of 10 subjects and in sleep deprivation data reported here revealed changes in R including elevated levels in daytime hours and an increase during wake which are in line with these studies indicating an increase in excitability as a function of time awake. The combined analysis from all 70 nights investigated here also robustly showed a decrease of R during nighttime even though one of the two stimulation patients (patient 1) temporarily exhibited high values of synchronization R and evoked potential amplitude A during the night. A potential explanation for the increased stimulation responses could be the intrinsic tendency of cortical neurons to fall into a down state after a transient activation during non-rapid eye movement sleep, as recently discussed in ref. 31. The overall steady levels of cortical stimulation markers in the long term observed here as well as in ref. 32 do not suggest stimulation itself to be the cause for an increasing excitability, in our opinion. With more datasets including both ongoing and stimulation activity becoming available it will be important to further explore the relation between A and R beyond the two patient datasets presented in this study.
The observed dependence of IEMs on time awake and sleep could indicate a close relation to the homeostatic function of sleep (33). A current hypothesis states that synaptic homeostasis is underlying sleep homeostasis: Net synaptic strength increases during waking due to plastic processes leading to a larger excitability and decreases by means of synaptic downscaling during sleep (34, 35). Observations of changes in synaptic strength in Drosophila (36) support the hypothesis by providing evidence of structural changes occurring during waking and their reorganization during sleep. Such changes could underlie an increase in excitability during time awake and would be in line with the interpretation of R as a measure of it. Besides a growth in net synaptic strength other mechanisms are also conceivable to govern an increase in network excitability. For example, a relative reduction of inhibition during time awake would similarly lead to an increase in the E/I balance. On a neuronal network level such structural wake-dependent alterations have been discussed to drive networks to states suboptimal for information processing (14). Despite the possibility that increasing synchrony R indicates an increase in excitability during wake, it is also conceivable that mechanisms other than increasing excitability are underlying the observed changes in R. For example, one could speculate that the elevated likelihood of neurons to go “offline” together, as observed in sleep-deprived rats (37), could manifest itself in increased synchronization levels measured from larger-scale data such as EEG.
Although perturbational approaches to measure excitability by electrical or transcranial magnetic stimulation have proven to be a valuable research tool, they are currently unsuitable for regular clinical monitoring. It has been shown that long-term continuous monitoring can reveal important insights into seizure dynamics and its predictions (38). For these kinds of clinical settings passive measures of excitability by IEMs are preferable. Quantitative markers of excitability can potentially guide more objective and individualized treatment approaches in epilepsy patients. Although IEMs cannot directly show changes in neuronal excitability, their change as a function of AED load reported here is consistent with the hypothesis of an effective reduction of cortical excitability by AEDs and provides a further indirect validation for IEMs besides their correlation with more direct measurements of excitability by stimulation. Synchronization R changed similarly as a function of AED load across all frequency bands investigated from 50 to 400 Hz. It is possible that these changes are to some extent correlated with the appearance of ripples or fast ripples in epilepsy patients. The similar trajectories observed within and outside the seizure onset zone as well as across frequencies, however, make a sole dependence of measure R on fast ripples less likely. A reliable biomarker of excitability stands the chance to monitor the therapeutic effectiveness of newly given AEDs. The observation of a robust value of in iEEG characterizing physiological network synchronization levels without AEDs could, if further validated, provide an absolute reference point with which drug effects on network dynamics could be compared. In scalp EEG recording analyzed here, we observed overall higher values of R (Fig. 4), which might be due to higher volume conduction compared with ECoG. In such a case, relative changes in R within a subject could be used to monitor excitability under different conditions and time. Regarding a potential reference point of , one should also keep in mind the possibility that epilepsy patients could present a slightly different R value, either a bit higher or lower than healthy subjects, even when untreated.
The moderate and similar levels of R across patients and frequency bands observed here under normal, no-AED conditions posits cortical networks to spend most of the time at the transition from low to high synchronization. Previous theoretical work has shown that self-organization to such a synchronization transition is conceivable under realistic assumptions (19, 20) and that, more generally, being positioned near a transition can support optimal information processing capabilities (39). With respect to the use of AEDs, the markers proposed here can potentially provide a complementary tool in adjusting AED dosages to optimal levels for a successful treatment on one side and controlling adverse drug effects on the other side. This is particularly important in light of common side effects of AEDs on cognition such as cognitive slowing, sedation, somnolence, and distractibility (40). Although it may be good for epilepsy patients to have a lower R value corresponding to a lower excitability to prevent the likelihood of seizures, the concomitant decrease in the variability of synchronization might be disadvantageous for optimal brain network functioning. Previous studies using in vitro preparations have shown that the IEMs similar to the ones used here are closely related to certain capabilities in information processing (41–44). IEMs could therefore be potential candidates to quantify the neural correlates of such cognitive deficits in humans.
Materials and Methods
Preprocessing of ECoG Data.
We analyzed two datasets of ECoG data: (i) data from n = 2 patients where regularly recurring electrical stimuli had been applied over at least a day while stimulation responses as well as unperturbed activity in between stimulations had been recorded and (ii) multiday recordings of ongoing activity (i.e., without electrical stimulation) from n = 10 patients under varying levels of AEDs (see Supporting Information for details). Patients gave informed consent. Ethics approval for dataset (i) was obtained from St. Vincent's Human Research Ethics Committee.
EEG Recordings During Prolonged Wakefulness.
We analyzed waking EEG recordings of eight healthy, young, right-handed males (23.0 ± 0.46 y; mean ± SEM) during 40 h of sustained wakefulness (data from a previous study; for details see ref. 27). During this time, participants were under constant surveillance. The waking EEG was recorded in 14 sessions at 3-h intervals starting at 0700 hours. Sessions consisted of a first 5-min eyes-open period, followed by a 4- to 5-min eyes-closed period, and a second 5-min eyes-open period. Twenty-seven EEG derivations (extended 10–20 system; n = 27 electrodes; reference electrode 5% rostral to Cz) were sampled with 256 Hz (high-pass filter at 0.16 Hz; anti-aliasing low-pass filter at 70 Hz). Artifacts including eye blinks were marked by visual inspection. We analyzed artifact-free segments of 4-s duration during the eyes-open condition. Each 4-s segment was filtered in the frequency band of interest (phase neutral filter by applying a second-order Butterworth filter in both directions) to derive synchrony measures.
SI Materials and Methods
Preprocessing of ECoG Data.
The stimulation dataset (i) was used to directly measure cortical excitability based on activity responses following electrical stimulation and investigate its relationship to IEMs. The long duration over which electrical stimulation was applied in these recordings allowed us to correlate the response to stimulation, which is often taken as a direct measurement of cortical excitability, to the synchronization measures derived from ongoing activity. Data were collected from two patients suffering from focal epilepsies undergoing evaluation for the surgical resection of epileptic foci at St. Vincent’s Hospital in Melbourne, Australia. Intracranial depths electrodes (n = 24 electrodes, patient 1) as well as subdural grid and strip electrodes (n = 93 electrodes, patient 2) were used. Data were sampled at 5,000 Hz. The stimulation protocol has been described previously (32) and was followed here closely. The stimulation protocol consisted of blocks of stimulations with biphasic electrical pulses of 3-mA amplitude and 0.5-ms pulse width that were delivered to two electrodes in each patient with a stimulation frequency of ∼0.3 Hz (every 15,050 sampling points) and referenced against a third electrode. Each stimulation block consisted of 100 stimulations and was repeated every 10 min over at least 24 h in each patient during which data were continuously recorded for offline analysis. For further offline analysis, the two stimulation electrodes, the reference electrode, and other electrodes showing signs of large stimulation artifacts or epileptic discharges were excluded. Stimulation-evoked potentials in each channel were derived by averaging the responses in each stimulation block (100 stimulations) time-locked to the onset of stimulation and applying a band-pass filter (third-order Butterworth filter; 0.01–100 Hz in patient 1, 1–100 Hz in patient 2 due to sometimes-appearing slow current transients) in the reverse time direction so that potential stimulation artifacts or ringing would end up before the stimulation. In each patient, two channels showing strong stimulation-evoked responses were chosen and their mean amplitude, A, defined as the distance from peak to trough in each channel (Fig. 1A), was taken as a measure of excitability (6). Mean synchronization, R, was calculated across channels from 950-ms-long segments preceding and leaving a 25-ms distance to the stimulation onset (−975 to −25 ms from stimulation onset). These segments were first filtered in the frequency band of choice using a phase-neutral filter by applying a second-order Butterworth filter in both directions. A notch filter to eliminate line noise was applied subsequently. In patient 2, some stimulation blocks had to be removed from the analysis due to high-frequency noise levels (approximately 1,000–2,000 Hz) occurring predominantly at the beginning of the stimulation protocol. AEDs were kept constant during the stimulation period.
The second ECoG dataset (ii) consisted of multiday recordings from 10 patients undergoing presurgical monitoring at the Epilepsy Center of the University Hospital of Freiburg, Germany (25). No stimulation was performed in these patients. All patients suffered from focal epilepsies. The number of electrodes varied between patients (from n = 30 to n = 114) and included both surface and intracranial depths electrodes and their placement was solely determined by clinical considerations. The invasive EEG data were sampled at either 256 (n = 4 patients), 512 (n = 1 patient), or 1,024 Hz (n = 5 patients). To capture dynamic changes in our markers, EEG data were analyzed in segments of 10-min duration. To eliminate possible line noise and low-frequency components, the EEG data were preprocessed by a 50-Hz notch filter and a phase-neutral band-pass filter in the frequency band of choice (phase-neutral filter by applying a second-order Butterworth filter in both directions). A small number of 10-min segments were excluded from the analysis due to artifacts presenting as high noise levels in the approximately 25- to 30-Hz frequency range across electrodes.
Synchrony and Variability Measures.
We derived estimates of mean phase synchronization for band-pass-filtered data in all three datasets. After filtering the data in the respective frequency band, we first obtained a phase trace θi(t) from each ECoG or EEG trace Fi(t) by applying its Hilbert transform H[Fi(t)]:
Next, we quantified the mean synchrony R in each ECoG or EEG segment by
where L is the length of the data segment in samples and r(t) is the Kuramoto order parameter
which was used as a time-dependent measure of phase synchrony. Here, N is the number of ECoG or EEG channels in the data segment (see description of datasets). The length of the segment in samples L is the product of the time segment considered and the sampling frequency. As such it ranged from 4,750 samples for the 950-ms segments used in dataset (i) to the 10-min intervals multiplied by the respective sampling frequencies in dataset (ii). When checking results for different interval lengths in dataset (ii), we observed results to be robust for different interval lengths.
As a measure for the variability of synchronization we derived the entropy of r(t) in each segment by
where we estimated a probability distribution of r(t) by binning values into intervals. pi is then the probability that r(t) falls into a range bi < r(t)≤ bi+1. Similar to refs. 14 and 21, we found results to be robust over a broad range for the number of bins B used. We applied B = 24 bins in the current analysis.
Power Density Spectra.
Invasive EEG power density spectra were computed for all channels of the same epochs used to derive synchronization measures (950-ms duration in dataset (i), 10-min duration in dataset (ii); FFT routine; Hanning window). Power was summed across the frequency band of interest and averaged over all channels.
Acknowledgments
This study was supported by the Intramural Research Program of the National Institute of Mental Health (ZIAMH002797). This study used the high-performance computational capabilities of the Biowulf Linux cluster at the National Institutes of Health. A.S.-B. would like to acknowledge support by the Cluster of Excellence BrainLinks-BrainTools (EXC-1086).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1513716112/-/DCSupplemental.
References
- 1.Stafstrom CE. Epilepsy: A review of selected clinical syndromes and advances in basic science. J Cereb Blood Flow Metab. 2006;26(8):983–1004. doi: 10.1038/sj.jcbfm.9600265. [DOI] [PubMed] [Google Scholar]
- 2.Bazhenov M, Timofeev I, Fröhlich F, Sejnowski TJ. Cellular and network mechanisms of electrographic seizures. Drug Discov Today Dis Models. 2008;5(1):45–57. doi: 10.1016/j.ddmod.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trevelyan AJ, Schevon CA. How inhibition influences seizure propagation. Neuropharmacology. 2013;69:45–54. doi: 10.1016/j.neuropharm.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 4.Valentín A, et al. Responses to single pulse electrical stimulation identify epileptogenesis in the human brain in vivo. Brain. 2002;125(Pt 8):1709–1718. doi: 10.1093/brain/awf187. [DOI] [PubMed] [Google Scholar]
- 5.Valentín A, et al. Single-pulse electrical stimulation identifies epileptogenic frontal cortex in the human brain. Neurology. 2005;65(3):426–435. doi: 10.1212/01.wnl.0000171340.73078.c1. [DOI] [PubMed] [Google Scholar]
- 6.Matsumoto R, et al. In vivo epileptogenicity of focal cortical dysplasia: A direct cortical paired stimulation study. Epilepsia. 2005;46(11):1744–1749. doi: 10.1111/j.1528-1167.2005.00284.x. [DOI] [PubMed] [Google Scholar]
- 7.Cantello R, et al. Cortical excitability in cryptogenic localization-related epilepsy: Interictal transcranial magnetic stimulation studies. Epilepsia. 2000;41(6):694–704. doi: 10.1111/j.1528-1157.2000.tb00230.x. [DOI] [PubMed] [Google Scholar]
- 8.Werhahn KJ, Lieber J, Classen J, Noachtar S. Motor cortex excitability in patients with focal epilepsy. Epilepsy Res. 2000;41(2):179–189. doi: 10.1016/s0920-1211(00)00136-4. [DOI] [PubMed] [Google Scholar]
- 9.Hamer HM, et al. Motor cortex excitability in focal epilepsies not including the primary motor area--a TMS study. Brain. 2005;128(Pt 4):811–818. doi: 10.1093/brain/awh398. [DOI] [PubMed] [Google Scholar]
- 10.Badawy RA, Vogrin SJ, Lai A, Cook MJ. The cortical excitability profile of temporal lobe epilepsy. Epilepsia. 2013;54(11):1942–1949. doi: 10.1111/epi.12374. [DOI] [PubMed] [Google Scholar]
- 11.Bialer M, White HS. Key factors in the discovery and development of new antiepileptic drugs. Nat Rev Drug Discov. 2010;9(1):68–82. doi: 10.1038/nrd2997. [DOI] [PubMed] [Google Scholar]
- 12.Badawy RA, Curatolo JM, Newton M, Berkovic SF, Macdonell RA. Sleep deprivation increases cortical excitability in epilepsy: Syndrome-specific effects. Neurology. 2006;67(6):1018–1022. doi: 10.1212/01.wnl.0000237392.64230.f7. [DOI] [PubMed] [Google Scholar]
- 13.Huber R, et al. Human cortical excitability increases with time awake. Cereb Cortex. 2013;23(2):332–338. doi: 10.1093/cercor/bhs014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meisel C, Olbrich E, Shriki O, Achermann P. Fading signatures of critical brain dynamics during sustained wakefulness in humans. J Neurosci. 2013;33(44):17363–17372. doi: 10.1523/JNEUROSCI.1516-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tononi G, Cirelli C. Sleep and the price of plasticity: From synaptic and cellular homeostasis to memory consolidation and integration. Neuron. 2014;81(1):12–34. doi: 10.1016/j.neuron.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rothwell JC. Techniques and mechanisms of action of transcranial stimulation of the human motor cortex. J Neurosci Methods. 1997;74(2):113–122. doi: 10.1016/s0165-0270(97)02242-5. [DOI] [PubMed] [Google Scholar]
- 17.Rossini PM, Rossi S. Transcranial magnetic stimulation: Diagnostic, therapeutic, and research potential. Neurology. 2007;68(7):484–488. doi: 10.1212/01.wnl.0000250268.13789.b2. [DOI] [PubMed] [Google Scholar]
- 18.Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety of TMS Consensus Group Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120(12):2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meisel C, Gross T. Adaptive self-organization in a realistic neural network model. Phys Rev E Stat Nonlin Soft Matter Phys. 2009;80(6 Pt 1):061917. doi: 10.1103/PhysRevE.80.061917. [DOI] [PubMed] [Google Scholar]
- 20.Meisel C, Storch A, Hallmeyer-Elgner S, Bullmore E, Gross T. Failure of adaptive self-organized criticality during epileptic seizure attacks. PLOS Comput Biol. 2012;8(1):e1002312. doi: 10.1371/journal.pcbi.1002312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang H, Shew WL, Roy R, Plenz D. Maximal variability of phase synchrony in cortical networks with neuronal avalanches. J Neurosci. 2012;32(3):1061–1072. doi: 10.1523/JNEUROSCI.2771-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castro-Alamancos MA. Origin of synchronized oscillations induced by neocortical disinhibition in vivo. J Neurosci. 2000;20(24):9195–9206. doi: 10.1523/JNEUROSCI.20-24-09195.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castro-Alamancos MA, Rigas P. Synchronized oscillations caused by disinhibition in rodent neocortex are generated by recurrent synaptic activity mediated by AMPA receptors. J Physiol. 2002;542(Pt 2):567–581. doi: 10.1113/jphysiol.2002.019059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kitzbichler MG, Smith ML, Christensen SR, Bullmore E. Broadband criticality of human brain network synchronization. PLOS Comput Biol. 2009;5(3):e1000314. doi: 10.1371/journal.pcbi.1000314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ihle M, et al. EPILEPSIAE - a European epilepsy database. Comput Methods Programs Biomed. 2012;106(3):127–138. doi: 10.1016/j.cmpb.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 26.Enatsu R, et al. Cortical excitability varies upon ictal onset patterns in neocortical epilepsy: A cortico-cortical evoked potential study. Clin Neurophysiol. 2012;123(2):252–260. doi: 10.1016/j.clinph.2011.06.030. [DOI] [PubMed] [Google Scholar]
- 27.Finelli LA, Baumann H, Borbély AA, Achermann P. Dual electroencephalogram markers of human sleep homeostasis: Correlation between theta activity in waking and slow-wave activity in sleep. Neuroscience. 2000;101(3):523–529. doi: 10.1016/s0306-4522(00)00409-7. [DOI] [PubMed] [Google Scholar]
- 28.Stacey W, Le Van Quyen M, Mormann F, Schulze-Bonhage A. What is the present-day EEG evidence for a preictal state? Epilepsy Res. 2011;97(3):243–251. doi: 10.1016/j.eplepsyres.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 29.Arieli A, Sterkin A, Grinvald A, Aertsen A. Dynamics of ongoing activity: Explanation of the large variability in evoked cortical responses. Science. 1996;273(5283):1868–1871. doi: 10.1126/science.273.5283.1868. [DOI] [PubMed] [Google Scholar]
- 30.Freestone DR, et al. A method for actively tracking excitability of brain networks using a fully implantable monitoring system. Conf Proc IEEE Eng Med Biol Soc. 2013;2013:6151–6154. doi: 10.1109/EMBC.2013.6610957. [DOI] [PubMed] [Google Scholar]
- 31.Pigorini A, et al. Bistability breaks-off deterministic responses to intracortical stimulation during non-REM sleep. Neuroimage. 2015;112:105–113. doi: 10.1016/j.neuroimage.2015.02.056. [DOI] [PubMed] [Google Scholar]
- 32.Freestone DR, et al. Electrical probing of cortical excitability in patients with epilepsy. Epilepsy Behav. 2011;22(Suppl 1):S110–S118. doi: 10.1016/j.yebeh.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 33.Achermann P, Borbély AA. 2011. Sleep homeostasis and models of sleep regulation. Principles and Practice of Sleep Medicine, eds Kryger MH, Roth T, Dement WC (Elsevier, St. Louis), 5th Ed, pp 431–444.
- 34.Tononi G, Cirelli C. Sleep and synaptic homeostasis: A hypothesis. Brain Res Bull. 2003;62(2):143–150. doi: 10.1016/j.brainresbull.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 35.Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10(1):49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 36.Bushey D, Tononi G, Cirelli C. Sleep and synaptic homeostasis: Structural evidence in Drosophila. Science. 2011;332(6037):1576–1581. doi: 10.1126/science.1202839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vyazovskiy VV, et al. Local sleep in awake rats. Nature. 2011;472(7344):443–447. doi: 10.1038/nature10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cook MJ, et al. Prediction of seizure likelihood with a long-term, implanted seizure advisory system in patients with drug-resistant epilepsy: A first-in-man study. Lancet Neurol. 2013;12(6):563–571. doi: 10.1016/S1474-4422(13)70075-9. [DOI] [PubMed] [Google Scholar]
- 39.Langton CG. Computation at the edge of chaos: Phase transitions and emergent computation. Physica D. 1990;42(1–3):12–37. [Google Scholar]
- 40.Ortinski P, Meador KJ. Cognitive side effects of antiepileptic drugs. Epilepsy Behav. 2004;5(Suppl 1):S60–S65. doi: 10.1016/j.yebeh.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 41.Beggs JM, Plenz D. Neuronal avalanches in neocortical circuits. J Neurosci. 2003;23(35):11167–11177. doi: 10.1523/JNEUROSCI.23-35-11167.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haldeman C, Beggs JM. Critical branching captures activity in living neural networks and maximizes the number of metastable states. Phys Rev Lett. 2005;94(5):058101. doi: 10.1103/PhysRevLett.94.058101. [DOI] [PubMed] [Google Scholar]
- 43.Shew WL, Yang H, Petermann T, Roy R, Plenz D. Neuronal avalanches imply maximum dynamic range in cortical networks at criticality. J Neurosci. 2009;29(49):15595–15600. doi: 10.1523/JNEUROSCI.3864-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shew WL, Yang H, Yu S, Roy R, Plenz D. Information capacity and transmission are maximized in balanced cortical networks with neuronal avalanches. J Neurosci. 2011;31(1):55–63. doi: 10.1523/JNEUROSCI.4637-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]