Abstract
Organ and tissue loss through disease and injury motivate the development of therapies that can regenerate tissues and decrease reliance on transplantations. Regenerative medicine, an interdisciplinary field that applies engineering and life science principles to promote regeneration, can potentially restore diseased and injured tissues and whole organs. Since the inception of the field several decades ago, a number of regenerative medicine therapies, including those designed for wound healing and orthopedics applications, have received Food and Drug Administration (FDA) approval and are now commercially available. These therapies and other regenerative medicine approaches currently being studied in preclinical and clinical settings will be covered in this review. Specifically, developments in fabricating sophisticated grafts and tissue mimics and technologies for integrating grafts with host vasculature will be discussed. Enhancing the intrinsic regenerative capacity of the host by altering its environment, whether with cell injections or immune modulation, will be addressed, as well as methods for exploiting recently developed cell sources. Finally, we propose directions for current and future regenerative medicine therapies.
Keywords: regenerative medicine, tissue engineering, biomaterials, review
Regenerative medicine has the potential to heal or replace tissues and organs damaged by age, disease, or trauma, as well as to normalize congenital defects. Promising preclinical and clinical data to date support the possibility for treating both chronic diseases and acute insults, and for regenerative medicine to abet maladies occurring across a wide array of organ systems and contexts, including dermal wounds, cardiovascular diseases and traumas, treatments for certain types of cancer, and more (1–3). The current therapy of transplantation of intact organs and tissues to treat organ and tissue failures and loss suffers from limited donor supply and often severe immune complications, but these obstacles may potentially be bypassed through the use of regenerative medicine strategies (4).
The field of regenerative medicine encompasses numerous strategies, including the use of materials and de novo generated cells, as well as various combinations thereof, to take the place of missing tissue, effectively replacing it both structurally and functionally, or to contribute to tissue healing (5). The body's innate healing response may also be leveraged to promote regeneration, although adult humans possess limited regenerative capacity in comparison with lower vertebrates (6). This review will first discuss regenerative medicine therapies that have reached the market. Preclinical and early clinical work to alter the physiological environment of the patient by the introduction of materials, living cells, or growth factors either to replace lost tissue or to enhance the body's innate healing and repair mechanisms will then be reviewed. Strategies for improving the structural sophistication of implantable grafts and effectively using recently developed cell sources will also be discussed. Finally, potential future directions in the field will be proposed. Due to the considerable overlap in how researchers use the terms regenerative medicine and tissue engineering, we group these activities together in this review under the heading of regenerative medicine.
Therapies in the Market
Since tissue engineering and regenerative medicine emerged as an industry about two decades ago, a number of therapies have received Food and Drug Administration (FDA) clearance or approval and are commercially available (Table 1). The delivery of therapeutic cells that directly contribute to the structure and function of new tissues is a principle paradigm of regenerative medicine to date (7, 8). The cells used in these therapies are either autologous or allogeneic and are typically differentiated cells that still maintain proliferative capacity. For example, Carticel, the first FDA-approved biologic product in the orthopedic field, uses autologous chondrocytes for the treatment of focal articular cartilage defects. Here, autologous chondrocytes are harvested from articular cartilage, expanded ex vivo, and implanted at the site of injury, resulting in recovery comparable with that observed using microfracture and mosaicplasty techniques (9). Other examples include laViv, which involves the injection of autologous fibroblasts to improve the appearance of nasolabial fold wrinkles; Celution, a medical device that extracts cells from adipose tissue derived from liposuction; Epicel, autologous keratinocytes for severe burn wounds; and the harvest of cord blood to obtain hematopoietic progenitor and stem cells. Autologous cells require harvest of a patient's tissue, typically creating a new wound site, and their use often necessitates a delay before treatment as the cells are culture-expanded. Allogeneic cell sources with low antigenicity [for example, human foreskin fibroblasts used in the fabrication of wound-healing grafts (GINTUIT, Apligraf) (10)] allow off-the-shelf tissues to be mass produced, while also diminishing the risk of an adverse immune reaction.
Table 1.
Regenerative medicine FDA-approved products
| Category | Name | Biological agent | Approved use |
| Biologics | laViv | Autologous fibroblasts | Improving nasolabial fold appearance |
| Carticel | Autologous chondrocytes | Cartilage defects from acute or repetitive trauma | |
| Apligraf, GINTUIT | Allogeneic cultured keratinocytes and fibroblasts in bovine collagen | Topical mucogingival conditions, leg and diabetic foot ulcers | |
| Cord blood | Hematopoietic stem and progenitor cells | Hematopoietic and immunological reconstitution after myeloablative treatment | |
| Cell-based medical devices | Dermagraft | Allogenic fibroblasts | Diabetic foot ulcer |
| Celution | Cell extraction | Transfer of autologous adipose stem cells | |
| Biopharmaceuticals | GEM 125 | PDGF-BB, tricalcium phosphate | Periodontal defects |
| Regranex | PDGF-BB | Lower extremity diabetic ulcers | |
| Infuse, Infuse bone graft, Inductos | BMP-2 | Tibia fracture and nonunion, and lower spine fusion | |
| Osteogenic protein-1 | BMP-7 | Tibia nonunion |
Materials are often an important component of current regenerative medicine strategies because the material can mimic the native extracellular matrix (ECM) of tissues and direct cell behavior, contribute to the structure and function of new tissue, and locally present growth factors (11). For example, 3D polymer scaffolds are used to promote expansion of chondrocytes in cartilage repair [e.g., matrix-induced autologous chondrocyte implantation (MACI)] and provide a scaffold for fibroblasts in the treatment of venous ulcers (Dermagraft) (12). Decellularized donor tissues are also used to promote wound healing (Dermapure, a variety of proprietary bone allografts) (13) or as tissue substitutes (CryoLife and Toronto's heart valve substitutes and cardiac patches) (14). A material alone can sometimes provide cues for regeneration and graft or implant integration, as in the case of bioglass-based grafts that permit fusion with bone (15). Incorporation of growth factors that promote healing or regeneration into biomaterials can provide a local and sustained presentation of these factors, and this approach has been exploited to promote wound healing by delivery of platelet derived growth factor (PDGF) (Regranex) and bone formation via delivery of bone morphogenic proteins 2 and 7 (Infuse, Stryker's OP-1) (16). However, complications can arise with these strategies (Infuse, Regranex black box warning) (17, 18), likely due to the poor control over factor release kinetics with the currently used materials.
The efficacies of regenerative medicine products that have been cleared or approved by the FDA to date vary but are generally better or at least comparable with preexisting products (9). They provide benefit in terms of healing and regeneration but are unable to fully resolve injuries or diseases (19–21). Introducing new products to the market is made difficult by the large time and monetary investments required to earn FDA approval in this field. For drugs and biologics, the progression from concept to market involves numerous phases of clinical testing, can require more than a dozen years of development and testing, and entails an average cost ranging from $802 million to $2.6 billion per drug (22, 23). In contrast, medical devices, a broad category that includes noncellular products, such as acellular matrices, generally reach the market after only 3–7 years of development and may undergo an expedited process if they are demonstrated to be similar to preexisting devices (24). As such, acellular products may be preferable from a regulatory and development perspective, compared with cell-based products, due to the less arduous approval process.
Therapies at the Preclinical Stage and in Clinical Testing
A broad range of strategies at both the preclinical and clinical stages of investigation are currently being explored. The subsequent subsections will overview these different strategies, which have been broken up into three broad categories: (i) recapitulating organ and tissue structure via scaffold fabrication, 3D bioprinting, and self assembly; (ii) integrating grafts with the host via vascularization and innervation; and (iii) altering the host environment to induce therapeutic responses, particularly through cell infusion and modulating the immune system. Finally, methods for exploiting recently identified and developed cell sources for regenerative medicine will be mentioned.
Recapitulating Tissue and Organ Structure.
Because tissue and organ architecture is deeply connected with function, the ability to recreate structure is typically believed to be essential for successful recapitulation of healthy tissue (25). One strategy to capture organ structure and material composition in engineered tissues is to decellularize organs and to recellularize before transplantation. Decellularization removes immunogenic cells and molecules, while theoretically retaining structure as well as the mechanical properties and material composition of the native extracellular matrix (26, 27). This approach has been executed in conjunction with bioreactors and used in animal models of disease with lungs, kidneys, liver, pancreas, and heart (25, 28–31). Decellularized tissues, without the recellularization step, have also reached the market as medical devices, as noted above, and have been used to repair large muscle defects in a human patient (32). A variation on this approach involves the engineering of blood vessels in vitro and their subsequent decellularization before placement in patients requiring kidney dialysis (33). Despite these successes, a number of challenges remain. Mechanical properties of tissues and organs may be affected by the decellularization process, the process may remove various types and amounts of ECM-associated signaling molecules, and the processed tissue may degrade over time after transplantation without commensurate replacement by host cells (34, 35). The detergents and procedures used to strip cells and other immunogenic components from donor organs and techniques to recellularize stripped tissue before implantation are actively being optimized.
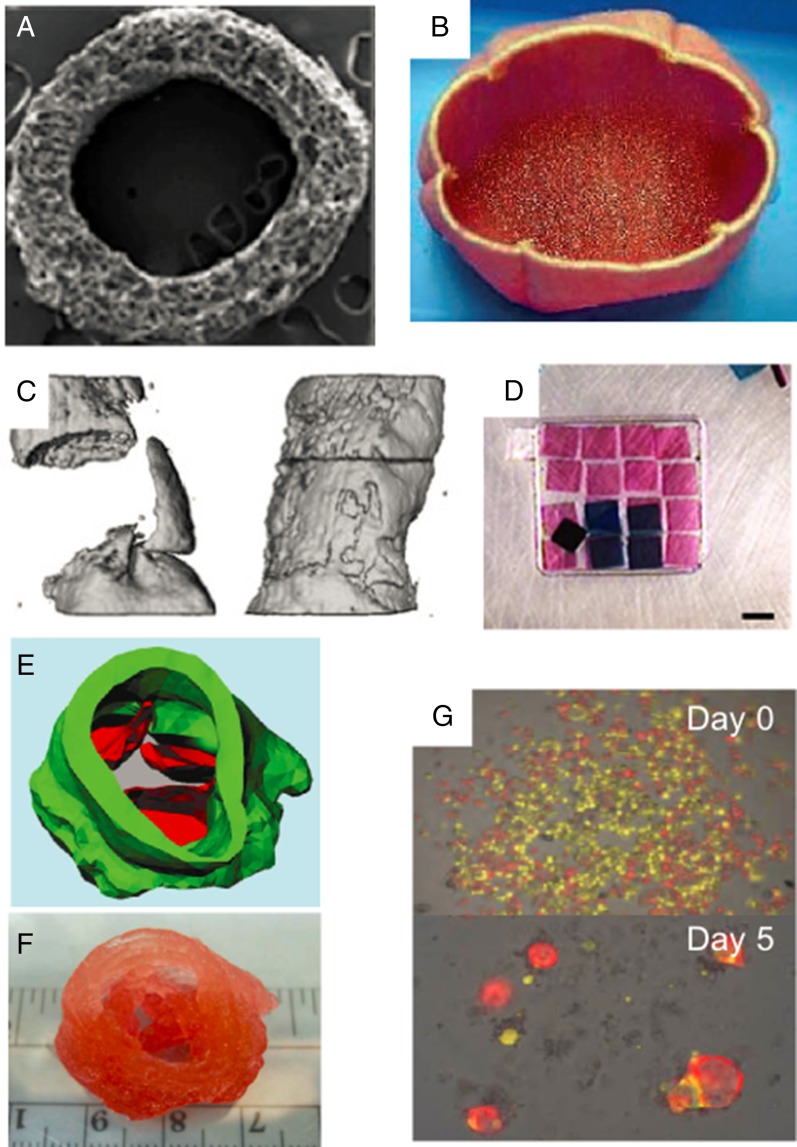
Synthetic scaffolds may also be fabricated that possess at least some aspects of the material properties and structure of target tissue (36). Scaffolds have been fabricated from naturally derived materials, such as purified extracellular matrix components or algae-derived alginate, or from synthetic polymers, such as poly(lactide-coglycolide) and poly(ethylene glycol); hydrogels are composed largely of water and are often used to form scaffolds due to their compositional similarity to tissue (37, 38). These polymers can be engineered to be biodegradable, enabling gradual replacement of the scaffold by the cells seeded in the graft as well as by host cells (39). For example, this approach was used to fabricate tissue-engineered vascular grafts (TEVGs), which have entered clinical trials, for treating congenital heart defects in both pediatric and adult patients (40) (Fig. 1 A and B). It was found using animal models that the seeded cells in TEVGs did not contribute structurally to the graft once in the host, but rather orchestrated the inflammatory response that aided in host vascular cells populating the graft to form the new blood vessel (41, 42). Biodegradable vascular grafts seeded with cells, cultured so that the cells produced extracellular matrix and subsequently decellularized, are undergoing clinical trials in the context of end-stage renal failure (Humacyte) (33). Scaffolds that encompass a wide spectrum of mechanical properties have been engineered both to provide bulk mechanical support to the forming tissue and to provide instructive cues to adherent cells (11). For example, soft fibrin–collagen hydrogels have been explored as lymph node mimics (43) whereas more rapidly degrading alginate hydrogels improved regeneration of critical defects in bone (44). In some cases, the polymer's mechanical properties alone are believed to produce a therapeutic effect. For example, injection of alginate hydrogels to the left ventricle reduced the progression of heart failure in models of dilated cardiomyopathy (45) and is currently undergoing clinical trials (Algisyl). Combining materials with different properties can enhance scaffold performance, as was the case of composite polyglycolide and collagen scaffolds that were seeded with cells and served as bladder replacements for human patients (46). In another example, an electrospun nanofiber mesh combined with peptide-modified alginate hydrogel and loaded with bone morphogenic protein 2 improved bone formation in critically sized defects (47). Medical imaging technologies such as computed tomography (CT) and magnetic resonance imaging (MRI) can be used to create 3D images of replacement tissues, sometimes based on the patient's own body (48, 49) (Fig. 1C). These 3D images can then be used as molds to fabricate scaffolds that are tailored specifically for the patient. For example, CT images of a patient were used for fabricating polyurethane and polyethylene-based synthetic trachea, which were then seeded with cells (50). Small building blocks, often consisting of cells embedded in a small volume of hydrogel, can also be assembled into tissue-like structures with defined architectures and cell patterning using a variety of recently developed techniques (51, 52) (Fig. 1D).
Fig. 1.
Regenerative medicine strategies that recapitulate tissue and organ structure. (A) Scanning electron microscopy image of a TEVG cross-section. Reproduced with permission from ref. 41. (B) Engineered bladder consisting of a polyglycolide and collagen composite scaffold, fabricated based on CT image of patient and seeded with cells. Reproduced with permission from ref. 46. (C) CT image of bone regeneration in critically sized defects without (Left) and with (Right) nanofiber mesh and alginate scaffold loaded with growth factor. Reproduced with permission from ref. 47. (D) Small hydrogel building blocks are assembled into tissue-like structures with microrobots. Reproduced from ref. 52, with permission from Nature Communications. (E) Blueprint for 3D bioprinting of a heart valve using microextrusion printing, with different colors representing different cell types. (F) Printed product. Reproduced with permission from ref. 59. (G) Intestinal crypt stem cells seeded with supporting Paneth cells self-assemble into organoids in culture. Reproduced from ref. 67, with permission from Nature.
Although cell placement within scaffolds is generally poor controlled, 3D bioprinting can create structures that combine high resolution control over material and cell placement within engineered constructs (53). Two of the most commonly used bioprinting strategies are inkjet and microextrusion (54). Inkjet bioprinting uses pressure pulses, created by brief electrical heating or acoustic waves, to create droplets of ink that contains cells at the nozzle (55, 56). Microextrusion bioprinting dispenses a continuous stream of ink onto a stage (57). Both are being actively used to fabricate a wide range of tissues. For example, inkjet bioprinting has been used to engineer cartilage by alternating layer-by-layer depositions of electrospun polycaprolactone fibers and chondrocytes suspended in a fibrin–collagen matrix. Cells deposited this way were found to produce collagen II and glycosaminoglycans after implantation (58). Microextrusion printing has been used to fabricate aortic valve replacements using cells embedded in an alginate/gelatin hydrogel mixture. Two cell types, smooth muscle cells and interstitial cells, were printed into two separate regions, comprising the valve root and leaflets, respectively (59) (Fig. 1 E and F). Microextrusion printing of inks with different gelation temperatures has been used to print complex 3D tubular networks, which were then seeded with endothelial cells to mimic vasculature (60). Several 3D bioprinting machines are commercially available and offer different capabilities and bioprinting strategies (54). Although extremely promising, bioprinting strategies often suffer trade-offs in terms of feature resolution, cell viability, and printing resolution, and developing bioprinting technologies that excel in all three aspects is an important area of research in this field (54).
In some situations, it may be possible to engineer new tissues with scaffold-free approaches. Cell sheet technology relies on the retrieval of a confluent sheet of cells from a temperature-responsive substrate, which allows cell–cell adhesion and signaling molecules, as well as ECM molecules deposited by the cells themselves, to remain intact (61, 62). Successive sheets can be layered to produce thicker constructs (63). This approach has been explored in a variety of contexts, including corneal reconstruction (64). Autologous oral mucosal cells have been grown into sheets, harvested, and implanted, resulting in reepithelialization of human corneas (64). Autonomous cellular self-assembly may also be used to create tissues and be used to complement bioprinting. For example, vascular cells aggregated into multicellular spheroids were printed in layer-by-layer fashion, using microextrusion, alongside agarose rods; hollow and branching structures that resembled a vascular network resulted after physical removal of the agarose once the cells formed a continuous structure (65). Given the appropriate cues and initial cell composition, even complex structures may form autonomously (66). For example, intestinal crypt-like structures can be grown from a single crypt base columnar stem cell in 3D culture in conjunction with augmented Wnt signaling (67) (Fig. 1G). Understanding the biological processes that drive and direct self-assembly will aid in fully taking advantage of this approach. The ability to induce autonomous self-assembly of the modular components of organs, such as intestinal crypts, kidney nephrons, and lung alveoli, could be especially powerful for the construction of organs with complex structures.
Integrating Graft Tissue by Inducing Vascularization and Innervation.
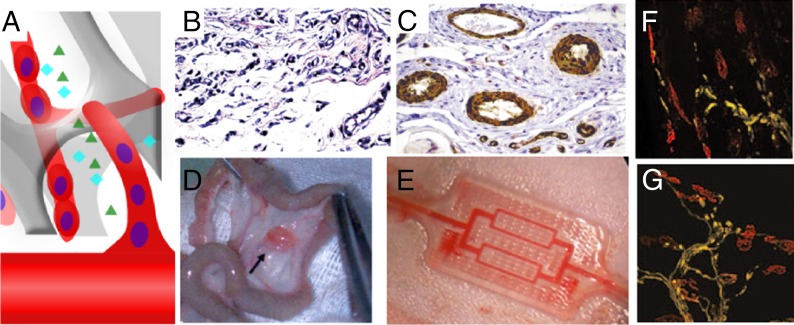
To contribute functionally and structurally to the body, implanted grafts need to be properly integrated with the body. For cell-based implants, integration with host vasculature is of primary importance for graft success (Fig. 2A) (68). Most cells in the body are located within 100 μm from the nearest capillary, the distance within which nutrient exchange and oxygen diffusion from the bloodstream can effectively occur (68). To vascularize engineered tissues, the body's own angiogenic response may be exploited via the presentation of angiogenic growth factors (69). A variety of growth factors have been implicated in angiogenesis, including vascular endothelial growth factor (VEGF), angiopoietin (Ang), platelet-derived growth factor (PDGF), and basic fibroblast growth factor (bFGF) (70, 71). However, application of growth factors may not be effectual without proper delivery modality, due to their short half-life in vivo and the potential toxicity and systemic effects of bolus delivery (45). Sustained release of VEGF, bFGF, Ang, and PDGF leads to robust angiogenic responses and can rescue ischemic limbs from necrosis (45, 72, 73). Providing a sequence of angiogenic factors that first initiate and then promote maturation of newly formed vessels can yield more functional networks (74) (Fig. 2 B and C), and mimicking development via delivery of both promoters and inhibitors of angiogenesis from distinct spatial locations can create tightly defined angiogenic zones (75).
Fig. 2.
Strategies for vascularizing and innervating tissue-engineered graft. (A) Tissue-engineered graft may be vascularized before implantation: for example, by self-assembly of seeded endothelial cells or by host blood vessels in a process mediated by growth factor release. Compared with bolus injection of VEGF and PDGF (B), sustained release of the same growth factors from a polymeric scaffold (C) led to a higher density of vessels and formation of larger and thicker vessels. Reproduced from ref. 74, with permission from Nature Biotechnology. (D) Scaffold vascularized by being implanted in the omentum before implantation at the injury site. Reproduced with permission from ref. 83. (E) Biodegradable microfluidic device surgically connected to vasculature. Reproduced with permission from ref. 85. Compared with blank scaffold (F), scaffolds delivering VEGF (G) increase innervation of injured skeletal muscle. Reproduced from ref. 97, with permission from Molecular Therapy.
Another approach to promote graft vascularization at the target site is to prevascularize the graft or target site before implantation. Endothelial cells and their progenitors can self-organize into vascular networks when transplanted on an appropriate scaffold (76–79). Combining endothelial cells with tissue-specific cells on a scaffold before transplantation can yield tissues that are both better vascularized and possess tissue-specific function (80). It is also possible to create a vascular pedicle for an engineered tissue that facilitates subsequent transplantation; this approach has been demonstrated in the context of both bone and cardiac patches by first placing a scaffold around a large host vessel or on richly vascularized tissue, and then moving the engineered tissue to its final anatomic location once it becomes vascularized at the original site (81–83) (Fig. 2D). This strategy was successfully used to vascularize an entire mandible replacement, which was later engrafted in a human patient (84). Microfluidic and micropatterning techniques are currently being explored to engineer vascular networks that can be anastomosed to the femoral artery (85, 86) (Fig. 2E). The site for cell delivery may also be prevascularized to enhance cell survival and function, as in a recent report demonstrating that placement of a catheter device allowed the site to become vascularized due to the host foreign body response to the material; this device significantly improved the efficacy of pancreatic cells subsequently injected into the device (87).
Innervation by the host will also be required for proper function and full integration of many tissues (88, 89), and is particularly important in tissues where motor control, as in skeletal tissue, or sensation, as in the epidermis, provides a key function (90, 91). Innervation of engineered tissues may be induced by growth factors, as has been shown in the induction of nerve growth from mouse embryonic dorsal root ganglia to epithelial tissue in an in vitro model (92). Hydrogels patterned with channels that are subsequently loaded with appropriate extracellular matrices and growth factors can guide nerve growth upon implantation, and this approach has been used to support nerve regeneration after injury (93, 94). Angiogenesis and nerve growth are known to share certain signaling pathways (95), and this connection has been exploited via the controlled delivery of VEGF using biomaterials to promote axon regrowth in regenerating skeletal muscle (96, 97) (Fig. 2 F and G).
Altering the Host Environment: Cell Infusions and Modulating the Immune System.
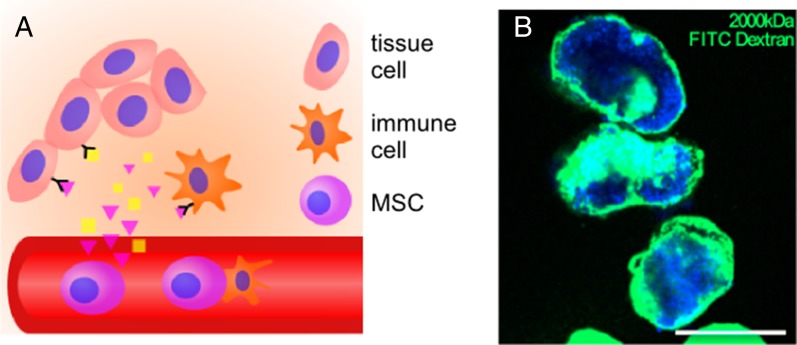
Administration of cells can induce therapeutic responses by indirect means, such as secretion of growth factors and interaction with host cells, without significant incorporation of the cells into the host or having the transplanted cells form a bulk tissue (98). For example, infusion of human umbilical cord blood cells can aid in stroke recovery due to enhanced angiogenesis (99), which in turn may have induced neuroblast migration to the site of injury. Similarly, transplanted macrophages can promote liver repair by activating hepatic progenitor cells (100). Transplanted cells can also normalize the injured or diseased environment, by altering the ECM, and improve tissue regeneration via this mechanism. For example, some types of epidermolysis bullosa (EB), a rare genetic skin blistering disorder, are associated with a failure of type VII collagen deposition in the basement membrane. Allogeneic injected fibroblasts were found to deposit type VII collagen deposition, thereby temporarily correcting disease morphology (101). A prototypical example of transplanted cells inducing a regenerative effect is the administration of mesenchymal stem cells (MSCs), which are being widely explored both preclinically and clinically to improve cardiac regeneration after infarction, and to treat graft-versus-host disease, multiple sclerosis, and brain trauma (2, 102) (Fig. 3A). Positive effects of MSC therapy are observed, despite the MSCs being concentrated with some methods of application in the lungs and poor MSC engraftment in the diseased tissue (103). This finding suggests that a systemic paracrine modality is sufficient to produce a therapeutic response in some situations. In other situations, cell–cell contact may be required. For example, MSCs can inhibit T-cell proliferation and dampen inflammation, and this effect is believed to at least partially depend on direct contact of the transplanted MSCs with host immune cells (104). Cells are often infused, typically intravenously, in current clinical trials, but cells administered in this manner often experience rapid clearance, which may explain their limited efficacy (105). Immunocloaking strategies, such as with hydrogel encapsulation of both cell suspensions and small cell clusters and hydrogel cloaking of whole organs, can lead to increased cell residency time and delayed allograft rejection (106, 107) (Fig. 3B). Coating infused cells with targeting antibodies and peptides, sometimes in conjunction with lipidation strategies, known as “cell painting,” has been shown to improve residency time at target tissue site (108). Infused cells can also be modified genetically to express a targeting ligand to control their biodistribution (109).
Fig. 3.
Illustrations of regenerative medicine therapies that modulate host environment. (A) Injected cells, such as MSCs, can release cytokines and interact with host cells to induce a regenerative response. (B) Polyethylene glycol hydrogel (green) conformally coating pancreatic islets (blue) can support islets after injection. (Scale bar: 200 μm.) Reproduced with permission from ref. 107.
Although the goal of regenerative medicine has long been to avoid rejection of the new tissue by the host immune system, it is becoming increasingly clear that the immune system also plays a major role in regulating regeneration, both impairing and contributing to the healing process and engraftment (110, 111). At the extreme end of immune reactions is immune rejection, which is a serious obstacle to the integration of grafts created with allogeneic cells. Immune engineering approaches have shown promise in inducing allograft tolerance: for example, by engineering the responses of immune cells such as dendritic cells and regulatory T cells (112, 113). Changing the properties of implanted scaffolds can also reduce the inflammation that accompanies implantation of a foreign object. For example, decreasing scaffold hydrophobicity and the availability of adhesion ligands can reduce inflammatory responses, and scaffolds with aligned fibrous topography experience less fibrous encapsulation upon implantation (114). Adaptive immune cells may actively inhibit even endogenous regeneration, as shown when depletion of CD8 T cells improved bone fracture healing in a preclinical model (115). Engineering the local immune response may thus allow active promotion of regeneration. For example, the release of cytokines to polarize macrophages to M2 phenotypes, which are considered to be antiinflammatory and proregeneration, was found to increase Schwann cell infiltration and axonal growth in a nerve gap model (116).
Existing and New Cell Sources.
Most regenerative medicine strategies rely on an ample cell source, but identifying and obtaining sufficient numbers of therapeutic cells is often a challenge. Stem, progenitor, and differentiated cells derived from both adult and embryonic tissues are widely being explored in regenerative medicine although adult tissue-derived cells are the dominant cell type used clinically to date due to both their ready availability and perceived safety (8). All FDA-approved regenerative medicine therapies to date and the vast majority of strategies explored in the clinic use adult tissue-derived cells. There is great interest in obtaining greater numbers of stem cells from adult tissues and in identifying stem cell populations suitable for therapeutic use in tissues historically thought not to harbor stem cells (117). Basic studies aiming to understand the processes that control stem cell renewal are being leveraged for both purposes, with the prototypical example being studies with hematopoietic stem cells (HSCs) (3). For example, exposure of HSCs in vitro to cytokines that are present in the HSC niche leads to significant HSC expansion, but this increase in number is accompanied by a loss of repopulation potential (118, 119). Coculture of HSCs with cells implicated in the HSC niche and in microenvironments engineered to mimic native bone marrow may improve maintenance of HSC stemness during expansion, enhancing stem cell numbers for transplantation. For example, direct contact of HSCs with MSCs grown in a 3D environment induces greater CD34+ expansion than with MSCs grown on 2D substrate (120). Another example is that culture of skeletal muscle stem cells on substrates with mechanical properties similar to normal muscle leads to greater stem cell expansion (121) and can even rescue impaired proliferative ability in stem cells from aged animals (122).
Embryonic stem (ES) cells and induced pluripotent stem (iPS) cells represent potentially infinite sources of cells for regeneration and are moving toward clinical use (123, 124). ES cells are derived from blastocyst-stage embryos and have been shown to be pluripotent, giving rise to tissues from all three germ layers (125). Several phase I clinical trials using ES cells have been completed, without reports of safety concerns (Geron, Advanced Cell Technology, Viacyte). iPS cells are formed from differentiated somatic cells exposed to a suitable set of transcription factors that induce pluripotency (126). iPS cells are an attractive cell source because they can be generated from a patient's own cells, thus potentially circumventing the ethical issues of ES and rejection of the transplanted cells (127, 128). Although iPS cells are typically created by first dedifferentiating adult cells to an ES-like state, strategies that induce reprogramming without entering a pluripotent stage have attracted attention due to their quicker action and anticipation of a reduced risk for tumor formation (129). Direct reprogramming in vivo by retroviral injection has been reported to result in greater efficiency of conversion, compared with ex vivo manipulation, and allows in vitro culture and transplantation to be bypassed (130). Strategies developed for controlled release of morphogens that direct regeneration could potentially be adapted for controlling delivery of new genetic information to target cells in vivo, to improve direct reprogramming. Cells resulting from both direct reprogramming and iPS cell differentiation methods have been explored for generating cells relevant to a variety of tissues, including cardiomyocytes, vascular and hematopoietic cells, hepatocytes, pancreatic cells, and neural cells (131). Because ES and iPS cells can form tumors, a tight level of control over the fate of each cell is crucial for their safe application. High-throughput screens of iPS cells can determine the optimal dosages of developmental factors to achieve lineage specification and minimize persistence of pluripotent cells (132). High-throughput screens have also been useful for discovering synthetic materials for iPS culture, which would allow culture in defined, xenogen-free conditions (133). In addition, the same principles used to engineer cellular grafts from differentiated cells are being leveraged to create appropriate microenvironments for reprogramming. For example, culture on polyacrylamide gel substrates with elastic moduli similar to the heart was found to enable longer term survival of iPS-derived cardiomyocytes, compared with other moduli (134). In another study, culture of iPS cell-derived cardiac tissue in hydrogels with aligned fibers, and in the presence of electrical stimulation, enhanced expression of genes associated with cardiac maturation (135).
Conclusion
To date, regenerative medicine has led to new, FDA-approved therapies being used to treat a number of pathologies. Considerable research has enabled the fabrication of sophisticated grafts that exploit properties of scaffolding materials and cell manipulation technologies for controlling cell behavior and repairing tissue. These scaffolds can be molded to fit the patient's anatomy and be fabricated with substantial control over spatial positioning of cells. Strategies are being developed to improve graft integration with the host vasculature and nervous system, particularly through controlled release of growth factors and vascular cell seeding, and the body's healing response can be elicited and augmented in a variety of ways, including immune system modulation. New cell sources for transplantation that address the limited cell supply that hampered many past efforts are also being developed.
A number of issues will be important for the advancement of regenerative medicine as a field. First, stem cells, whether isolated from adult tissue or induced, will often require tight control over their behavior to increase their safety profile and efficacy after transplantation. The creation of microenvironments, often modeled on various stem cell niches that provide specific cues, including morphogens and physical properties, or have the capacity to genetically manipulate target cells, will likely be key to promoting optimal regenerative responses from therapeutic cells. Second, the creation of large engineered replacement tissues will require technologies that enable fully vascularized grafts to be anastomosed with host vessels at the time of transplant, allowing for graft survival. Thirdly, creating a proregeneration environment within the patient may dramatically improve outcomes of regenerative medicine strategies in general. An improved understanding of the immune system's role in regeneration may aid this goal, as would technologies that promote a desirable immune response. A better understanding of how age, disease state, and the microbiome of the patient affect regeneration will likely also be important for advancing the field in many situations (136–138). Finally, 3D human tissue culture models of disease may allow testing of regenerative medicine approaches in human biology, as contrasted to the animal models currently used in preclinical studies. Increased accuracy of disease models may improve the efficacy of regenerative medicine strategies and enhance the translation to the clinic of promising approaches (139).
Acknowledgments
This work was supported by National Institutes of Health Grant RO1EB014703 (to D.J.M.) and the National Science Foundation Graduate Research Fellowship Program (A.S.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article is part of the special series of PNAS 100th Anniversary articles to commemorate exceptional research published in PNAS over the last century.
References
- 1.Jaklenec A, Stamp A, Deweerd E, Sherwin A, Langer R. Progress in the tissue engineering and stem cell industry “are we there yet?”. Tissue Eng Part B Rev. 2012;18(3):155–166. doi: 10.1089/ten.TEB.2011.0553. [DOI] [PubMed] [Google Scholar]
- 2.Bailey AM, Mendicino M, Au P. An FDA perspective on preclinical development of cell-based regenerative medicine products. Nat Biotechnol. 2014;32(8):721–723. doi: 10.1038/nbt.2971. [DOI] [PubMed] [Google Scholar]
- 3.Mendelson A, Frenette PS. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat Med. 2014;20(8):833–846. doi: 10.1038/nm.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vacanti JP, Otte J-B, Wertheim JA. In: Introduction: Regenerative medicine and solid organ transplantation from a historical perspective. Regenerative Medicine Applications in Organ Transplantation. Orlando G, Lerut J, Soker S, Stratta RJ, editors. Elsevier; London: 2014. pp. 1–15. [Google Scholar]
- 5.Bajaj P, Schweller RM, Khademhosseini A, West JL, Bashir R. 3D biofabrication strategies for tissue engineering and regenerative medicine. Annu Rev Biomed Eng. 2014;16:247–276. doi: 10.1146/annurev-bioeng-071813-105155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kami D, Gojo S. Tuning cell fate: From insights to vertebrate regeneration. Organogenesis. 2014;10(2):231–240. doi: 10.4161/org.28816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buckler L. Opportunities in regenerative medicine. Bioprocess Int. 2011;2011(March):14–18. [Google Scholar]
- 8.Fisher MB, Mauck RL. Tissue engineering and regenerative medicine: Recent innovations and the transition to translation. Tissue Eng Part B Rev. 2013;19(1):1–13. doi: 10.1089/ten.teb.2012.0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dewan AK, Gibson MA, Elisseeff JH, Trice ME. Evolution of autologous chondrocyte repair and comparison to other cartilage repair techniques. BioMed Res Int. 2014;2014:272481. doi: 10.1155/2014/272481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Falanga V, Sabolinski M. A bilayered living skin construct (APLIGRAF) accelerates complete closure of hard-to-heal venous ulcers. Wound Repair Regen. 1999;7(4):201–207. doi: 10.1046/j.1524-475x.1999.00201.x. [DOI] [PubMed] [Google Scholar]
- 11.Huebsch N, Mooney DJ. Inspiration and application in the evolution of biomaterials. Nature. 2009;462(7272):426–432. doi: 10.1038/nature08601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harding K, Sumner M, Cardinal M. A prospective, multicentre, randomised controlled study of human fibroblast-derived dermal substitute (Dermagraft) in patients with venous leg ulcers. Int Wound J. 2013;10(2):132–137. doi: 10.1111/iwj.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song JJ, Ott HC. Organ engineering based on decellularized matrix scaffolds. Trends Mol Med. 2011;17(8):424–432. doi: 10.1016/j.molmed.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Chambers JB, Rimington HM, Rajani R, Hodson F, Shabbo F. A randomized comparison of the Cryolife O’Brien and Toronto stentless replacement aortic valves. J Thorac Cardiovasc Surg. 2007;133(4):1045–1050. doi: 10.1016/j.jtcvs.2006.10.071. [DOI] [PubMed] [Google Scholar]
- 15.Oryan A, Alidadi S, Moshiri A, Maffulli N. Bone regenerative medicine: Classic options, novel strategies, and future directions. J Orthop Surg. 2014;9(1):18. doi: 10.1186/1749-799X-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walsh G. Biopharmaceutical benchmarks 2010. Nat Biotechnol. 2010;28(9):917–924. doi: 10.1038/nbt0910-917. [DOI] [PubMed] [Google Scholar]
- 17.Papanas N, Maltezos E. Becaplermin gel in the treatment of diabetic neuropathic foot ulcers. Clin Interv Aging. 2008;3(2):233–240. doi: 10.2147/cia.s1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Epstein NE. Basic science and spine literature document bone morphogenetic protein increases cancer risk. Surg Neurol Int. 2014;5(Suppl 15):S552–S560. doi: 10.4103/2152-7806.148039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim PJ, Dybowski KS, Steinberg JS. A closer look at bioengineered alternative tissues. Podiatry Today. 2006;19(7):1–9. [Google Scholar]
- 20.Supp DM, Boyce ST. Engineered skin substitutes: Practices and potentials. Clin Dermatol. 2005;23(4):403–412. doi: 10.1016/j.clindermatol.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 21.O’Brien T, Barry FP. Stem cell therapy and regenerative medicine. Mayo Clin Proc. 2009;84(10):859–861. doi: 10.4065/84.10.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: New estimates of drug development costs. J Health Econ. 2003;22(2):151–185. doi: 10.1016/S0167-6296(02)00126-1. [DOI] [PubMed] [Google Scholar]
- 23.Avorn J. The $2.6 billion pill: Methodologic and policy considerations. N Engl J Med. 2015;372(20):1877–1879. doi: 10.1056/NEJMp1500848. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan AV, et al. Medical device development: From prototype to regulatory approval. Circulation. 2004;109(25):3068–3072. doi: 10.1161/01.CIR.0000134695.65733.64. [DOI] [PubMed] [Google Scholar]
- 25.Nelson CM, Bissell MJ. Of extracellular matrix, scaffolds, and signaling: Tissue architecture regulates development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2006;22:287–309. doi: 10.1146/annurev.cellbio.22.010305.104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32(12):3233–3243. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Macchiarini P, et al. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372(9655):2023–2030. doi: 10.1016/S0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]
- 28.Petersen TH, et al. Tissue-engineered lungs for in vivo implantation. Science. 2010;329(5991):538–541. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uygun BE, et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med. 2010;16(7):814–820. doi: 10.1038/nm.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakayama KH, Batchelder CA, Lee CI, Tarantal AF. Decellularized rhesus monkey kidney as a three-dimensional scaffold for renal tissue engineering. Tissue Eng Part A. 2010;16(7):2207–2216. doi: 10.1089/ten.tea.2009.0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goh SK, et al. Perfusion-decellularized pancreas as a natural 3D scaffold for pancreatic tissue and whole organ engineering. Biomaterials. 2013;34(28):6760–6772. doi: 10.1016/j.biomaterials.2013.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mase VJ, Jr, et al. Clinical application of an acellular biologic scaffold for surgical repair of a large, traumatic quadriceps femoris muscle defect. Orthopedics. 2010;33(7):511. doi: 10.3928/01477447-20100526-24. [DOI] [PubMed] [Google Scholar]
- 33.Dahl SL, et al. Readily available tissue-engineered vascular grafts. Sci Transl Med. 2011;3(68):68ra9. doi: 10.1126/scitranslmed.3001426. [DOI] [PubMed] [Google Scholar]
- 34.Fishman JM, et al. Airway tissue engineering: An update. Expert Opin Biol Ther. 2014;14(10):1477–1491. doi: 10.1517/14712598.2014.938631. [DOI] [PubMed] [Google Scholar]
- 35.Badylak SF, Taylor D, Uygun K. Whole-organ tissue engineering: Decellularization and recellularization of three-dimensional matrix scaffolds. Annu Rev Biomed Eng. 2011;13:27–53. doi: 10.1146/annurev-bioeng-071910-124743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang S, Leong KF, Du Z, Chua CK. The design of scaffolds for use in tissue engineering. Part I. Traditional factors. Tissue Eng. 2001;7(6):679–689. doi: 10.1089/107632701753337645. [DOI] [PubMed] [Google Scholar]
- 37.Kim BS, Mooney DJ. Development of biocompatible synthetic extracellular matrices for tissue engineering. Trends Biotechnol. 1998;16(5):224–230. doi: 10.1016/s0167-7799(98)01191-3. [DOI] [PubMed] [Google Scholar]
- 38.Drury JL, Mooney DJ. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials. 2003;24(24):4337–4351. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 39.Wong T, et al. Potential of fibroblast cell therapy for recessive dystrophic epidermolysis bullosa. J Invest Dermatol. 2008;128(9):2179–2189. doi: 10.1038/jid.2008.78. [DOI] [PubMed] [Google Scholar]
- 40.Patterson JT, et al. Tissue-engineered vascular grafts for use in the treatment of congenital heart disease: From the bench to the clinic and back again. Regen Med. 2012;7(3):409–419. doi: 10.2217/rme.12.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roh JD, et al. Tissue-engineered vascular grafts transform into mature blood vessels via an inflammation-mediated process of vascular remodeling. Proc Natl Acad Sci USA. 2010;107(10):4669–4674. doi: 10.1073/pnas.0911465107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hibino N, et al. Tissue-engineered vascular grafts form neovessels that arise from regeneration of the adjacent blood vessel. FASEB J. 2011;25(8):2731–2739. doi: 10.1096/fj.11-182246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cupedo T, Stroock A, Coles M. Application of tissue engineering to the immune system: Development of artificial lymph nodes. Front Immunol. 2012;3:343. doi: 10.3389/fimmu.2012.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simmons CA, Alsberg E, Hsiong S, Kim WJ, Mooney DJ. Dual growth factor delivery and controlled scaffold degradation enhance in vivo bone formation by transplanted bone marrow stromal cells. Bone. 2004;35(2):562–569. doi: 10.1016/j.bone.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 45.Lee K, Silva EA, Mooney DJ. Growth factor delivery-based tissue engineering: General approaches and a review of recent developments. J R Soc Interface. 2011;8(55):153–170. doi: 10.1098/rsif.2010.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367(9518):1241–1246. doi: 10.1016/S0140-6736(06)68438-9. [DOI] [PubMed] [Google Scholar]
- 47.Kolambkar YM, et al. An alginate-based hybrid system for growth factor delivery in the functional repair of large bone defects. Biomaterials. 2011;32(1):65–74. doi: 10.1016/j.biomaterials.2010.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ballyns JJ, et al. Image-guided tissue engineering of anatomically shaped implants via MRI and micro-CT using injection molding. Tissue Eng Part A. 2008;14(7):1195–1202. doi: 10.1089/ten.tea.2007.0186. [DOI] [PubMed] [Google Scholar]
- 49.Appel AA, Anastasio MA, Larson JC, Brey EM. Imaging challenges in biomaterials and tissue engineering. Biomaterials. 2013;34(28):6615–6630. doi: 10.1016/j.biomaterials.2013.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ajalloueian F, et al. Biomechanical and biocompatibility characteristics of electrospun polymeric tracheal scaffolds. Biomaterials. 2014;35(20):5307–5315. doi: 10.1016/j.biomaterials.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 51.Guven S, et al. Multiscale assembly for tissue engineering and regenerative medicine. Trends Biotechnol. 2015;33(5):269–279. doi: 10.1016/j.tibtech.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tasoglu S, Diller E, Guven S, Sitti M, Demirci U. Untethered micro-robotic coding of three-dimensional material composition. Nat Commun. 2014;5:3124. doi: 10.1038/ncomms4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ozbolat IT. Bioprinting scale-up tissue and organ constructs for transplantation. Trends Biotechnol. 2015;33(7):395–400. doi: 10.1016/j.tibtech.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 54.Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32(8):773–785. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 55.Tekin E, Smith PJ, Schubert US. Inkjet printing as a deposition and patterning tool for polymers and inorganic particles. Soft Matter. 2008;4(4):703. doi: 10.1039/b711984d. [DOI] [PubMed] [Google Scholar]
- 56.Cui X, Boland T, D’Lima DD, Lotz MK. Thermal inkjet printing in tissue engineering and regenerative medicine. Recent Pat Drug Deliv Formul. 2012;6(2):149–155. doi: 10.2174/187221112800672949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Derby B. Printing and prototyping of tissues and scaffolds. Science. 2012;338(6109):921–926. doi: 10.1126/science.1226340. [DOI] [PubMed] [Google Scholar]
- 58.Xu T, et al. Hybrid printing of mechanically and biologically improved constructs for cartilage tissue engineering applications. Biofabrication. 2013;5(1):015001. doi: 10.1088/1758-5082/5/1/015001. [DOI] [PubMed] [Google Scholar]
- 59.Duan B, Hockaday LA, Kang KH, Butcher JT. 3D bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J Biomed Mater Res A. 2013;101(5):1255–1264. doi: 10.1002/jbm.a.34420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kolesky DB, et al. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv Mater. 2014;26(19):3124–3130. doi: 10.1002/adma.201305506. [DOI] [PubMed] [Google Scholar]
- 61.Okano T, Yamada N, Sakai H, Sakurai Y. A novel recovery system for cultured cells using plasma-treated polystyrene dishes grafted with poly(N-isopropylacrylamide) J Biomed Mater Res. 1993;27(10):1243–1251. doi: 10.1002/jbm.820271005. [DOI] [PubMed] [Google Scholar]
- 62.Nakajima K, et al. Intact microglia are cultured and non-invasively harvested without pathological activation using a novel cultured cell recovery method. Biomaterials. 2001;22(11):1213–1223. doi: 10.1016/s0142-9612(00)00270-2. [DOI] [PubMed] [Google Scholar]
- 63.Yamato M, Okano T. Cell sheet engineering. Mater Today. 2004;7(5):42–47. [Google Scholar]
- 64.Nishida K, et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med. 2004;351:1187–1196. doi: 10.1056/NEJMoa040455. [DOI] [PubMed] [Google Scholar]
- 65.Norotte C, Marga FS, Niklason LE, Forgacs G. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials. 2009;30(30):5910–5917. doi: 10.1016/j.biomaterials.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sasai Y. Next-generation regenerative medicine: Organogenesis from stem cells in 3D culture. Cell Stem Cell. 2013;12(5):520–530. doi: 10.1016/j.stem.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 67.Sato T, et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469(7330):415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lovett M, Lee K, Edwards A, Kaplan DL. Vascularization strategies for tissue engineering. Tissue Eng Part B Rev. 2009;15(3):353–370. doi: 10.1089/ten.teb.2009.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Battler A, et al. Intracoronary injection of basic fibroblast growth factor enhances angiogenesis in infarcted swine myocardium. J Am Coll Cardiol. 1993;22(7):2001–2006. doi: 10.1016/0735-1097(93)90790-8. [DOI] [PubMed] [Google Scholar]
- 70.Darland DC, D’Amore PA. Blood vessel maturation: Vascular development comes of age. J Clin Invest. 1999;103(2):157–158. doi: 10.1172/JCI6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Conway EM, Collen D, Carmeliet P. Molecular mechanisms of blood vessel growth. Cardiovasc Res. 2001;49(3):507–521. doi: 10.1016/s0008-6363(00)00281-9. [DOI] [PubMed] [Google Scholar]
- 72.Silva EA, Mooney DJ. Spatiotemporal control of vascular endothelial growth factor delivery from injectable hydrogels enhances angiogenesis. J Thromb Haemost. 2007;5(3):590–598. doi: 10.1111/j.1538-7836.2007.02386.x. [DOI] [PubMed] [Google Scholar]
- 73.Hea L. The effect of the controlled release of basic fibroblast growth factor from ionic gelatin-based hydrogels on angiogenesis in a murine critical limb ischemia model. Biomaterials. 2007;28(16):8. doi: 10.1016/j.biomaterials.2007.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19(11):1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 75.Yuen WW, Du NR, Chan CH, Silva EA, Mooney DJ. Mimicking nature by codelivery of stimulant and inhibitor to create temporally stable and spatially restricted angiogenic zones. Proc Natl Acad Sci USA. 2010;107(42):17933–17938. doi: 10.1073/pnas.1001192107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nör JE, et al. Engineering and characterization of functional human microvessels in immunodeficient mice. Lab Invest. 2001;81(4):453–463. doi: 10.1038/labinvest.3780253. [DOI] [PubMed] [Google Scholar]
- 77.Chen X, et al. Prevascularization of a fibrin-based tissue construct accelerates the formation of functional anastomosis with host vasculature. Tissue Eng Part A. 2009;15(6):1363–1371. doi: 10.1089/ten.tea.2008.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fedorovich NE, Haverslag RT, Dhert WJ, Alblas J. The role of endothelial progenitor cells in prevascularized bone tissue engineering: Development of heterogeneous constructs. Tissue Eng Part A. 2010;16(7):2355–2367. doi: 10.1089/ten.TEA.2009.0603. [DOI] [PubMed] [Google Scholar]
- 79.Montaño I, et al. Formation of human capillaries in vitro: The engineering of prevascularized matrices. Tissue Eng Part A. 2010;16(1):269–282. doi: 10.1089/ten.TEA.2008.0550. [DOI] [PubMed] [Google Scholar]
- 80.Lesman A, et al. Engineering vessel-like networks within multicellular fibrin-based constructs. Biomaterials. 2011;32(31):7856–7869. doi: 10.1016/j.biomaterials.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 81.Mikos AG, et al. Prevascularization of porous biodegradable polymers. Biotechnol Bioeng. 1993;42(6):716–723. doi: 10.1002/bit.260420606. [DOI] [PubMed] [Google Scholar]
- 82.Levenberg S, et al. Engineering vascularized skeletal muscle tissue. Nat Biotechnol. 2005;23(7):879–884. doi: 10.1038/nbt1109. [DOI] [PubMed] [Google Scholar]
- 83.Dvir T, et al. Prevascularization of cardiac patch on the omentum improves its therapeutic outcome. Proc Natl Acad Sci USA. 2009;106(35):14990–14995. doi: 10.1073/pnas.0812242106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Warnke PH, et al. Growth and transplantation of a custom vascularised bone graft in a man. Lancet. 2004;364(9436):766–770. doi: 10.1016/S0140-6736(04)16935-3. [DOI] [PubMed] [Google Scholar]
- 85.Zhang B, et al. 2013. Microfluidic tissue: A biodegradable scaffold with built-in vasculature for cardiac tissue vascularization and surgical vascular anastomosis. Proceedings of the Seventeenth International Conference on Miniaturized Systems for Chemistry and Life Sciences (Chemical and Biological Microsystems Society, San Diego), pp 2019–2021.
- 86.Hasan A, et al. Microfluidic techniques for development of 3D vascularized tissue. Biomaterials. 2014;35(26):7308–7325. doi: 10.1016/j.biomaterials.2014.04.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pepper AR, et al. A prevascularized subcutaneous device-less site for islet and cellular transplantation. Nat Biotechnol. 2015;33(5):518–523. doi: 10.1038/nbt.3211. [DOI] [PubMed] [Google Scholar]
- 88.Anand P, et al. The role of endogenous nerve growth factor in human diabetic neuropathy. Nat Med. 1996;2(6):703–707. doi: 10.1038/nm0696-703. [DOI] [PubMed] [Google Scholar]
- 89.Tuszynski MH, Steward O. Concepts and methods for the study of axonal regeneration in the CNS. Neuron. 2012;74(5):777–791. doi: 10.1016/j.neuron.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Griffith M, et al. Artificial corneas: A regenerative medicine approach. Eye (Lond) 2009;23(10):1985–1989. doi: 10.1038/eye.2008.409. [DOI] [PubMed] [Google Scholar]
- 91.Cezar CA, Mooney DJ. Biomaterial-based delivery for skeletal muscle repair. Adv Drug Deliv Rev. 2015;84:188–197. doi: 10.1016/j.addr.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Suuronen EJ, et al. Functional innervation in tissue engineered models for in vitro study and testing purposes. Toxicol Sci. 2004;82(2):525–533. doi: 10.1093/toxsci/kfh270. [DOI] [PubMed] [Google Scholar]
- 93.Midha R, Munro CA, Dalton PD, Tator CH, Shoichet MS. Growth factor enhancement of peripheral nerve regeneration through a novel synthetic hydrogel tube. J Neurosurg. 2003;99(3):555–565. doi: 10.3171/jns.2003.99.3.0555. [DOI] [PubMed] [Google Scholar]
- 94.Tsai EC, Dalton PD, Shoichet MS, Tator CH. Matrix inclusion within synthetic hydrogel guidance channels improves specific supraspinal and local axonal regeneration after complete spinal cord transection. Biomaterials. 2006;27(3):519–533. doi: 10.1016/j.biomaterials.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 95.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438(7070):932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 96.Borselli C, et al. Functional muscle regeneration with combined delivery of angiogenesis and myogenesis factors. Proc Natl Acad Sci USA. 2010;107(8):3287–3292. doi: 10.1073/pnas.0903875106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shvartsman D, et al. Sustained delivery of VEGF maintains innervation and promotes reperfusion in ischemic skeletal muscles via NGF/GDNF signaling. Mol Ther. 2014;22(7):1243–1253. doi: 10.1038/mt.2014.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Forbes SJ, Rosenthal N. Preparing the ground for tissue regeneration: From mechanism to therapy. Nat Med. 2014;20(8):857–869. doi: 10.1038/nm.3653. [DOI] [PubMed] [Google Scholar]
- 99.Taguchi A, et al. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest. 2004;114(3):330–338. doi: 10.1172/JCI20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bird TG, et al. Bone marrow injection stimulates hepatic ductular reactions in the absence of injury via macrophage-mediated TWEAK signaling. Proc Natl Acad Sci USA. 2013;110(16):6542–6547. doi: 10.1073/pnas.1302168110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Thangapazham RL, Darling TN, Meyerle J. Alteration of skin properties with autologous dermal fibroblasts. Int J Mol Sci. 2014;15(5):8407–8427. doi: 10.3390/ijms15058407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: Environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 2013;45:e54. doi: 10.1038/emm.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee RH, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5(1):54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Krampera M, et al. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24(2):386–398. doi: 10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]
- 105.Kean TJ, Lin P, Caplan AI, Dennis JE. MSCs: Delivery routes and engraftment, cell-targeting strategies, and immune modulation. Stem Cells Int. 2013;2013:732742. doi: 10.1155/2013/732742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Brasile L. In: Immunocloaking. Regenerative Medicine Applications in Organ Transplantation. Orlando G, Lerut J, Soker S, Stratta RJ, editors. Elsevier; London: 2014. pp. 919–933. [Google Scholar]
- 107.Tomei AA, et al. Device design and materials optimization of conformal coating for islets of Langerhans. Proc Natl Acad Sci USA. 2014;111(29):10514–10519. doi: 10.1073/pnas.1402216111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dennis JE, Cohen N, Goldberg VM, Caplan AI. Targeted delivery of progenitor cells for cartilage repair. J Orthop Res. 2004;22(4):735–741. doi: 10.1016/j.orthres.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 109.Cheng Z, et al. Targeted migration of mesenchymal stem cells modified with CXCR4 gene to infarcted myocardium improves cardiac performance. Mol Ther. 2008;16(3):571–579. doi: 10.1038/sj.mt.6300374. [DOI] [PubMed] [Google Scholar]
- 110.Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: Molecular and cellular mechanisms. J Invest Dermatol. 2007;127(3):514–525. doi: 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
- 111.Schmidt-Bleek K, Kwee BJ, Mooney DJ, Duda GN. Boon and bane of inflammation in bone tissue regeneration and its link with angiogenesis. Tissue Eng Part B Rev. 2015;21(4):354–364. doi: 10.1089/ten.teb.2014.0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zakrzewski JL, van den Brink MR, Hubbell JA. Overcoming immunological barriers in regenerative medicine. Nat Biotechnol. 2014;32(8):786–794. doi: 10.1038/nbt.2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sicard A, Koenig A, Morelon E, Defrance T, Thaunat O. Cell therapy to induce allograft tolerance: Time to switch to plan B? Front Immunol. 2015;6:149. doi: 10.3389/fimmu.2015.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Boehler RM, Graham JG, Shea LD. Tissue engineering tools for modulation of the immune response. Biotechniques. 2011;51(4):239–240, 242, 244 passim. doi: 10.2144/000113754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Reinke S, et al. Terminally differentiated CD8⁺ T cells negatively affect bone regeneration in humans. Sci Transl Med. 2013;5(177):177ra36. doi: 10.1126/scitranslmed.3004754. [DOI] [PubMed] [Google Scholar]
- 116.Mokarram N, Merchant A, Mukhatyar V, Patel G, Bellamkonda RV. Effect of modulating macrophage phenotype on peripheral nerve repair. Biomaterials. 2012;33(34):8793–8801. doi: 10.1016/j.biomaterials.2012.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lane SW, Williams DA, Watt FM. Modulating the stem cell niche for tissue regeneration. Nat Biotechnol. 2014;32(8):795–803. doi: 10.1038/nbt.2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang CC, Lodish HF. Murine hematopoietic stem cells change their surface phenotype during ex vivo expansion. Blood. 2005;105(11):4314–4320. doi: 10.1182/blood-2004-11-4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Walasek MA, van Os R, de Haan G. Hematopoietic stem cell expansion: Challenges and opportunities. Ann N Y Acad Sci. 2012;1266:138–150. doi: 10.1111/j.1749-6632.2012.06549.x. [DOI] [PubMed] [Google Scholar]
- 120.Zhang Y, Chai C, Jiang XS, Teoh SH, Leong KW. Co-culture of umbilical cord blood CD34+ cells with human mesenchymal stem cells. Tissue Eng. 2006;12(8):2161–2170. doi: 10.1089/ten.2006.12.2161. [DOI] [PubMed] [Google Scholar]
- 121.Kim BS, Mooney DJ. Scaffolds for engineering smooth muscle under cyclic mechanical strain conditions. J Biomech Eng. 2000;122(3):210–215. doi: 10.1115/1.429651. [DOI] [PubMed] [Google Scholar]
- 122.Cosgrove BD, et al. Rejuvenation of the muscle stem cell population restores strength to injured aged muscles. Nat Med. 2014;20(3):255–264. doi: 10.1038/nm.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Evans M. Discovering pluripotency: 30 years of mouse embryonic stem cells. Nat Rev Mol Cell Biol. 2011;12(10):680–686. doi: 10.1038/nrm3190. [DOI] [PubMed] [Google Scholar]
- 124.Harrison RH, St-Pierre JP, Stevens MM. Tissue engineering and regenerative medicine: A year in review. Tissue Eng Part B Rev. 2014;20(1):1–16. doi: 10.1089/ten.TEB.2013.0668. [DOI] [PubMed] [Google Scholar]
- 125.Keller G. Embryonic stem cell differentiation: Emergence of a new era in biology and medicine. Genes Dev. 2005;19(10):1129–1155. doi: 10.1101/gad.1303605. [DOI] [PubMed] [Google Scholar]
- 126.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 127.Hirschi KK, Li S, Roy K. Induced pluripotent stem cells for regenerative medicine. Annu Rev Biomed Eng. 2014;16:277–294. doi: 10.1146/annurev-bioeng-071813-105108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Araki R, et al. Negligible immunogenicity of terminally differentiated cells derived from induced pluripotent or embryonic stem cells. Nature. 2013;494(7435):100–104. doi: 10.1038/nature11807. [DOI] [PubMed] [Google Scholar]
- 129.Sancho-Martinez I, Baek SH, Izpisua Belmonte JC. Lineage conversion methodologies meet the reprogramming toolbox. Nat Cell Biol. 2012;14(9):892–899. doi: 10.1038/ncb2567. [DOI] [PubMed] [Google Scholar]
- 130.Qian L, et al. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012;485(7400):593–598. doi: 10.1038/nature11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sadahiro T, Yamanaka S, Ieda M. Direct cardiac reprogramming: Progress and challenges in basic biology and clinical applications. Circ Res. 2015;116(8):1378–1391. doi: 10.1161/CIRCRESAHA.116.305374. [DOI] [PubMed] [Google Scholar]
- 132.Nazareth EJ, et al. High-throughput fingerprinting of human pluripotent stem cell fate responses and lineage bias. Nat Methods. 2013;10(12):1225–1231. doi: 10.1038/nmeth.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Celiz AD, et al. Materials for stem cell factories of the future. Nat Mater. 2014;13(6):570–579. doi: 10.1038/nmat3972. [DOI] [PubMed] [Google Scholar]
- 134.Heras-Bautista CO, et al. The influence of physiological matrix conditions on permanent culture of induced pluripotent stem cell-derived cardiomyocytes. Biomaterials. 2014;35(26):7374–7385. doi: 10.1016/j.biomaterials.2014.05.027. [DOI] [PubMed] [Google Scholar]
- 135.Thavandiran N, et al. Design and formulation of functional pluripotent stem cell-derived cardiac microtissues. Proc Natl Acad Sci USA. 2013;110(49):E4698–E4707. doi: 10.1073/pnas.1311120110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Oh J, Lee YD, Wagers AJ. Stem cell aging: Mechanisms, regulators and therapeutic opportunities. Nat Med. 2014;20(8):870–880. doi: 10.1038/nm.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci Transl Med. 2014;6(265):265sr6. doi: 10.1126/scitranslmed.3009337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Scales BS, Huffnagle GB. The microbiome in wound repair and tissue fibrosis. J Pathol. 2013;229(2):323–331. doi: 10.1002/path.4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bhatia SN, Ingber DE. Microfluidic organs-on-chips. Nat Biotechnol. 2014;32(8):760–772. doi: 10.1038/nbt.2989. [DOI] [PubMed] [Google Scholar]