Significance
Many viruses use phosphatidylserine (PS) receptors to gain entry into target cells, and phagocytes use these receptors to clear apoptotic cells. PS receptors mediate these activities by binding PS on the viruses or apoptotic cells. We demonstrate here that phosphatidylethanolamine (PE) is also a ligand for PS receptors, and that PE plays a key role in apoptotic cell clearance and infection of various pathogenic viruses, including West Nile, dengue, and Ebola viruses. This finding provides significant insights; it clarifies how PS receptors promote viral infection, suggests PE is a useful broad-spectrum antiviral target, and deepens our understanding of apoptosis and the process by which apoptotic cells are cleared.
Keywords: apoptotic mimicry, phosphatidylserine receptors, phosphatidylethanolamine, T-cell immunoglobulin mucin domain proteins, virus entry
Abstract
Phosphatidylserine (PS) receptors contribute to two crucial biological processes: apoptotic clearance and entry of many enveloped viruses. In both cases, they recognize PS exposed on the plasma membrane. Here we demonstrate that phosphatidylethanolamine (PE) is also a ligand for PS receptors and that this phospholipid mediates phagocytosis and viral entry. We show that a subset of PS receptors, including T-cell immunoglobulin (Ig) mucin domain protein 1 (TIM1), efficiently bind PE. We further show that PE is present in the virions of flaviviruses and filoviruses, and that the PE-specific cyclic peptide lantibiotic agent Duramycin efficiently inhibits the entry of West Nile, dengue, and Ebola viruses. The inhibitory effect of Duramycin is specific: it inhibits TIM1-mediated, but not L-SIGN-mediated, virus infection, and it does so by blocking virus attachment to TIM1. We further demonstrate that PE is exposed on the surface of apoptotic cells, and promotes their phagocytic uptake by TIM1-expressing cells. Together, our data show that PE plays a key role in TIM1-mediated virus entry, suggest that disrupting PE association with PS receptors is a promising broad-spectrum antiviral strategy, and deepen our understanding of the process by which apoptotic cells are cleared.
Members of the filovirus and flavivirus families are the causative agents of life-threatening diseases. Ebola virus (EBOV), a filovirus, causes hemorrhagic fever with an average case fatality rate as high as 65% (1). Although there are EBOV vaccine candidates (2, 3), there is currently no licensed vaccine or treatment. Dengue virus (DENV) and West Nile virus (WNV) belong to the flavivirus family. Both are transmitted to humans through mosquito bites and can cause lethal hemorrhagic fever (in the case of DENV) or severe neurological diseases (in the case of WNV) (4, 5). Flaviviruses are emerging as major health concerns in tropical and subtropical areas worldwide. More than one third of the world’s population is estimated to be at risk for DENV infection, with approximately 400 million people infected yearly (6). There are currently no approved vaccines or therapeutic agents against DENV or WNV.
Virus entry into host cells typically initiates with the interaction between viral entry glycoproteins (GPs) and a receptor or coreceptor expressed at the surface of the target cell. Viruses also use less specific mechanisms to localize to target cell membranes, for example through GP association with various attachment factors (7). During the past few years, it has been increasingly recognized that many viruses also use a strategy known as apoptotic mimicry to promote their association with, and internalization into their target cells (8). Receptors for phospholipids, specifically phosphatidylserine (PS), normally involved in the clearance of apoptotic cells, markedly enhance the infection of a number of enveloped viruses. These PS receptors are presumed to engage PS on the virion membrane rather than the viral entry protein (9, 10). Enveloped viruses acquire their lipid membrane from the cells from which they bud. Consistent with the concept of apoptotic mimicry, the presence of PS in viral membranes has been reported for several viruses, including Pichinde virus, vesicular stomatitis virus (VSV), vaccinia virus, and DENV (9, 11, 12). Although phosphatidylethanolamine (PE) and PS are restricted to the inner leaflet in eukaryotic membranes, they are believed to redistribute to both leaflets of the viral membrane as a result of the absence of flippases to maintain membrane asymmetry (13, 14).
T-cell Ig mucin domain (TIM) proteins are PS receptors, with three members in humans: TIM1, TIM3, and TIM4. These proteins are type I cell-surface GPs consisting of four major domains: an Ig variable (IgV)-like N-terminal domain containing a high-affinity binding site for PS, a heavily O-glycosylated mucin-like domain, a transmembrane domain, and a cytoplasmic domain. They have established roles in PS-dependent phagocytosis of apoptotic cells (15, 16) and viral entry (9, 10, 17–20). The latter function was indicated for the first time when TIM1 was suggested to be a receptor for EBOV and Marburg virus (17). Subsequent reports determined that virion lipids, rather than the entry protein, were responsible for binding to TIM1 (10, 18). Additional studies identified TIM1 and TIM4 as potent enhancers of the entry of a wide range of enveloped viruses, including DENV and WNV (9, 10).
Here we demonstrate that the phospholipid PE is a ligand for human TIM (hTIM) proteins, especially TIM1. We also show that PE is present at the surface of enveloped viruses and promotes TIM1-mediated entry of replication-competent DENV type 2 (DENV2), WNV virus-like particles (VLPs), and EBOV VLPs in cells engineered to express TIM1 but also in those naturally expressing PS receptors. Moreover, virus entry promoted by TIM1 is abrogated by Duramycin, a PE-specific lantibiotic agent (Fig. S1). The role of PE in TIM biological functions is not limited to viral entry, as we show that PE also contributes to the uptake of apoptotic cells by TIM1-expressing cells. Collectively, our results show that PE makes an important contribution to PS receptor-mediated internalization of EBOV, DENV, and WNV, and suggest that virion-associated PE is a potential target for the design of broad-spectrum antiviral compounds.
Results
PE Is a Ligand for TIM1.
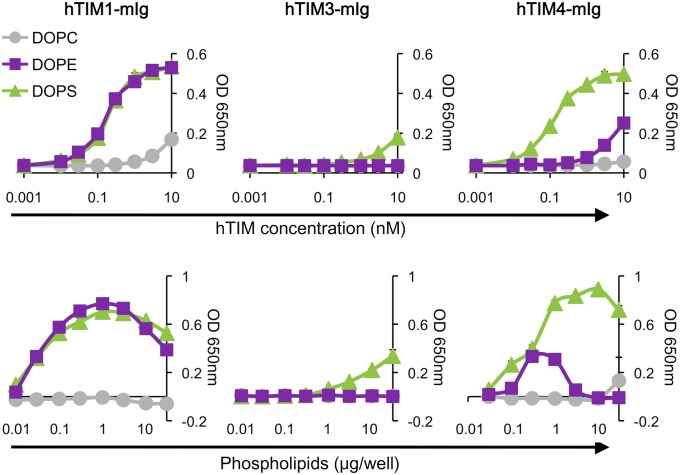
We and others have reported that PS receptors promote the entry of viruses by binding PS present in the viral envelope (9, 10, 18, 19, 21). However, we also observed that TIM1-mediated entry of various viruses, including replication-competent DENV2, EBOV VLPs, and WNV VLPs, was efficiently inhibited by PE liposomes in addition to PS liposomes (Fig. S2) (10), implying that PE may also be involved in TIM1-mediated viral entry. Therefore, we first assessed the binding of TIM proteins to synthetic 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), [1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) and 1,2-dioleoyl-sn-glycero-3-phospho-l-serine (DOPS)] by ELISA, using constructs in which the extracellular domain of hTIM1, hTIM3, or hTIM4 was fused to the Fc domain of murine IgG2a (hTIM1-mIg, hTIM3-mIg, and hTIM4-mIg, respectively). As shown in Fig. 1, hTIM1- and hTIM4-mIg efficiently bound DOPS, with detectable binding observed at a concentration as low as 0.1 nM. On the contrary, we could detect only marginal binding of hTIM3-mIg to DOPS. Supporting our hypothesis, hTIM1- and hTIM4-mIg, but not hTIM3-mIg, indeed bound DOPE. As TIM proteins contain a metal ion-dependent ligand binding site contributing to PS binding (22–24), we assessed the effect of Ca2+ on TIM binding to PE or PS. Fig. S3A shows that the absence of Ca2+ slightly lower binding of hTIM1-mIg to PE or PS. However, hTIM4-mIg binding to PS, and more so to PE, was markedly reduced, suggesting that hTIM4 relies on metal ions more strongly to bind PE and PS. To ensure that TIM protein binding to PE was not affected by the source of phospholipids, we compared synthetic phospholipids to phospholipids extracted from mammalian tissues for their binding to TIM1. Identical results were obtained with both types of phospholipids (Fig. S3B). Together, these data show that PE is an effective ligand for TIM1.
Fig. 1.
PE is a ligand for TIM1. (Upper) A total of 1 μg per well of phospholipids was dried out on ELISA plates and incubated with hTIM1-, hTIM3-, or hTIM4-mIg. (Lower) Same as upper panels except that increasing amounts of phospholipids and 1 nM hTIM-mIg proteins were used. Data, expressed as arbitrary units of absorbance at OD650, are shown as mean ± SD of duplicates and are representative of at least three independent experiments.
Duramycin Binds PE in Viral Membranes.
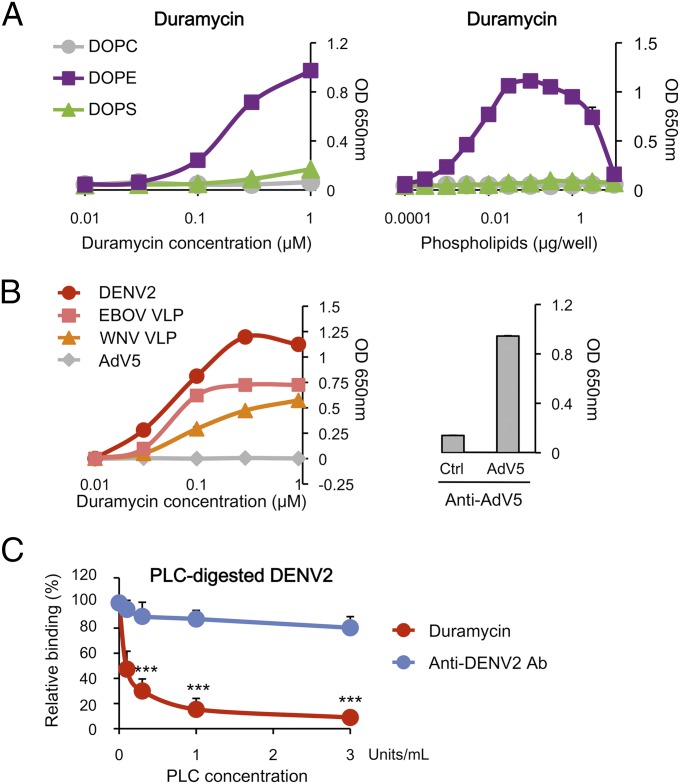
To further investigate the role of PE in TIM1-mediated viral entry, we used a small, PE-specific molecule, Duramycin, a 19-aa lantibiotic agent (Fig. S1) that has been described to interact with PE with high specificity (25). Although Duramycin was reported to lyse red blood cells at high concentrations, its derivative, biotin-Duramycin, was shown to be substantially less hemolytic while retaining its affinity and specificity for PE (26, 27). We thus used biotin-Duramycin throughout these studies. We first confirmed by ELISA that Duramycin interacted only with DOPE. No signal was detected with DOPC or DOPS, even when high concentrations of Duramycin or phospholipids were used (Fig. 2A). We then assessed Duramycin binding to antibody-captured enveloped viruses, including DENV2, WNV VLPs, and EBOV VLPs. Nonenveloped adenovirus type 5 (AdV5) was used as a control. As shown in Fig. 2B, Duramycin efficiently bound to DENV2, EBOV VLPs, and WNV VLPs, but not to AdV5. To ensure that the lack of Duramycin binding to AdV5 was not a result of the absence of the virus, we used a second AdV5-specific antibody that demonstrated the presence of the antibody-captured virus. To further verify that Duramycin binds PE and not any other viral components, antibody-captured DENV2 was digested with phospholipase C (PLC), which removes the head group of phospholipids. We observed a significant and dose-dependent decrease of Duramycin binding to PLC-digested DENV2 (Fig. 2C). A sandwich ELISA specific for DENV2 showed that the amount of virus was similar in all of the wells, regardless of the concentrations of PLC used, verifying that PLC digestion did not cause the virus to dissociate from the capturing antibody. Together, these results show that Duramycin specifically binds PE on EBOV, DENV2, and WNV, indicating that PE is exposed at their surface and could therefore be engaged in viral entry.
Fig. 2.
Duramycin binds PE in viral membranes. (A) Duramycin specifically binds PE. (Left) A total of 1 μg per well of phospholipids was dried out on ELISA plates and incubated with increasing concentrations of biotin-Duramycin. (Right) Same as left panel except that increasing amounts of lipids and 100 nM of biotin-Duramycin were used. (B) Duramycin binds enveloped viruses. ELISA plates coated with antibodies directed against DENV2, EBOV, WNV, or AdV5 were incubated with these viruses or VLPs, followed by biotin-Duramycin (Left). Proper capture of AdV5 was verified by sandwich ELISA (Right). (A and B) Data, expressed as arbitrary units of absorbance at OD650, are shown as mean ± SD of duplicates and are representative of three independent experiments. (C) PLC digestion eliminates Duramycin binding to virions. ELISA plates coated with anti-DENV2 antibody were incubated with DENV2, followed by PLC and biotin-Duramycin. A sandwich ELISA specific for DENV2 was performed to verify that PLC digestion did not cause the virus to detach. Duramycin and anti-DENV2 antibody binding to PLC-treated DENV2 were normalized to those of untreated DENV2. The average ± SD of five experiments is shown (***P < 0.0001).
PE Contributes to hTIM1-Mediated Viral Entry of EBOV, DENV2, and WNV.
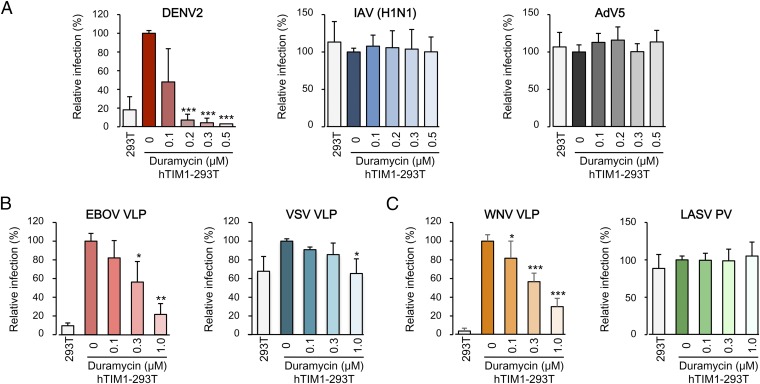
To assess whether the ability of TIM1 to bind PE is important for its function as a mediator of viral entry, we used Duramycin as an inhibitor in infection assays. DENV2, EBOV VLPs, and WNV VLPs were preincubated with increasing concentrations of Duramycin and used to infect 293T cells or 293T cells expressing hTIM1 (hTIM1-293T; Fig. S2A). As expected, hTIM1 expression markedly increased infection of the cells by DENV2, EBOV VLPs, and WNV VLPs. We observed that Duramycin drastically decreased TIM1-mediated entry, with 75% of inhibition for EBOV VLPs and more than 90% for DENV2 at 0.3 μM (Fig. 3 A and B and Fig. S4 A and B). Although less sensitive, hTIM1-mediated entry of WNV VLPs was completely abolished by 1 μM of Duramycin (Fig. 3C and Fig. S4C). Replication-competent influenza A virus (IAV) H1N1, which does not use TIM1 to enter cells (10), and nonenveloped virus AdV5 were used as controls for DENV2 (Fig. 3A and Fig. S4A). As expected, neither hTIM1 expression nor Duramycin affected the infection of hTIM1-293T with these viruses. VSV VLPs, generated by pseudotyping EBOV VP40 matrix protein fused to β-lactamase (Bla) with VSV GP, was used as a control for EBOV VLPs (Fig. 3B and Fig. S4B). The moderate enhancement of VSV VLP entry into hTIM1-293T cells correlated with a moderate inhibitory effect of Duramycin treatment. MLV-based pseudoviruses (PVs) bearing the entry protein of Lassa virus (LASV), which does not use hTIM1 or any other PS receptors (10), was used as an additional control (Fig. 3C and Fig. S4C). LASV PV entry was not affected by hTIM1 expression or Duramycin treatment.
Fig. 3.
PE contributes to hTIM1-mediated viral entry of EBOV, DENV2, and WNV. (A–C) DENV2, IAV (H1N1), and AdV5 (A); or EBOV and VSV VLPs (B); or WNV VLPs and LASV PVs (C) were preincubated with biotin-Duramycin and used to infect hTIM1-293T cells. Parental 293T cells were also infected to show TIM1 use of these viruses. Infection levels are normalized to that of hTIM1-293T cells infected in the absence of Duramycin. The average ± SD of three independent duplicated experiments is shown (*P < 0.01, **P < 0.001, and ***P < 0.0001). Representative experiments without normalization are shown in Fig. S4.
To exclude the possibility that the inhibition of infection by Duramycin was caused by any cytotoxic effect, we measured the leakage of the cytosolic lactate dehydrogenase (LDH) into the culture medium. Fig. S5A shows that Duramycin had no cytotoxic effect in hTIM1-293T cells at concentrations as high as 1 µM, the highest concentration used in these studies. To demonstrate that Duramycin also has no virolytic activity on TIM1-using viruses, WNV VLPs preincubated with Duramycin were used to infect hTIM1- or hL-SIGN-293T cells. As shown in Fig. S5B, WNV VLP infection into hLSIGN-293T cells was not affected by Duramycin, whereas entry into hTIM1-293T cells was inhibited. Thus, Duramycin did not specifically inactivate TIM1-using viruses. Further, Duramycin’s inhibitory effect was not observed with TIM1-independent viruses. Although antibody-captured IAV (H1N1) efficiently bound Duramycin (Fig. S5C), it did not inhibit IAV (H1N1) entry into hTIM1-293T cells (Fig. 3A). These results are consistent with the fact that IAV does not use PS receptors (10), and indicate that Duramycin-mediated inhibition is specific for TIM1-using viruses.
To examine Duramycin’s mode of action, we performed time-of-addition experiments. When Duramycin was added to cells postinfection, it showed no effect (Fig. S6A, Right), indicating that Duramycin works at early steps of virus infection. Moreover, Fig. S6B shows that Duramycin inhibits DENV2 association with hTIM-293T cells, whereas binding of the same virus to hL-SIGN-293T cells is not affected. Taken together, our results show that Duramycin potently inhibits TIM1-mediated virus infection by blocking virus association with TIM1, and confirm that virion PE plays a crucial role in this process.
Contribution of PE in PS Receptor-Mediated Viral Entry Is Physiological.
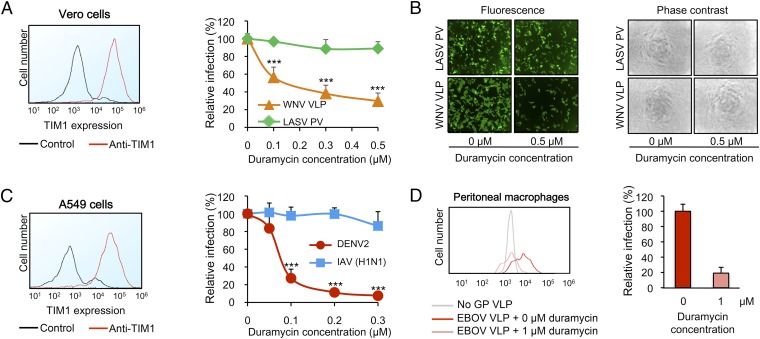
We further investigated the involvement of PE in virus entry into cells naturally expressing TIM1, such as Vero cells and A549 cells (Fig. 4 A and C, Left). When Vero cells were infected with WNV VLPs or LASV PVs preincubated with Duramycin, WNV VLP entry was substantially decreased in a dose-dependent manner, but that of LASV PVs was not (Fig. 4A). Selected fluorographs are shown in Fig. 4B, Left: a strong effect of Duramycin on WNV VLP entry is evident at 0.5 μM. Similarly, in A549 cells, Duramycin dramatically decreased DENV2 infection, whereas IAV (H1N1) infection remained unaffected (Fig. 4C). As Fig. S7 shows, the inhibition of infection in Vero and A549 cells is not caused by any cytotoxic effect of Duramycin, and is consistent with the fact that WNV and DENV2 use PS receptors whereas LASV PV and IAV (H1N1) do not (10). We then assessed the contribution of PE to EBOV VLP entry into primary cells. Naïve mouse peritoneal macrophages, which are known to express PS receptors (15), were infected with EBOV VLPs either preincubated with Duramycin or not. Fig. 4D shows that 1 μM of Duramycin nearly completely abolished the infection of the macrophages by EBOV VLPs. These results demonstrate that PE is a key player in virus entry into cells naturally expressing PS receptors.
Fig. 4.
Contribution of PE in PS receptor-mediated viral entry is physiological. (A, Left) Vero cells were stained for TIM1 expression. Rabbit IgG was used as a control. (Right) WNV VLPs and LASV PVs were preincubated with biotin-Duramycin and used to infect cells. Infection levels are normalized to the level without Duramycin treatment. The average ± SD of three duplicated experiments is shown (***P < 0.0001). (B) Selected fluorographs (Left) and phase-contrast micrographs (Right) from an experiment shown in A. (C) Same as A except that A549 cells and DENV2 were used. IAV (H1N1) was used as a control. The average ± SD of three duplicated experiments is shown (***P < 0.0001). (D) EBOV VLPs were either preincubated or not with biotin-Duramycin and used to infect naïve peritoneal macrophages. VP40-Bla VLPs without GP (No GP VLP) were used as a negative control. Infection levels were normalized to those macrophages infected with untreated EBOV VLPs. (Left) Infection levels of one experiment are shown as histograms. (Right) Average of relative infection ± SD of two different batches of EBOV VLPs tested in the same preparation of macrophages.
PE Is Exposed on Apoptotic Cells and Promotes TIM1-Mediated Phagocytosis.
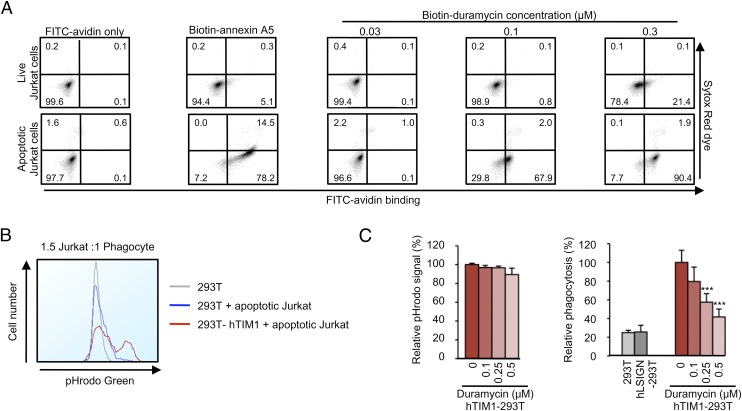
TIM1 is known to promote phagocytosis by binding PS at the surface of apoptotic cells (15). To further verify that the ability of TIM1 to bind PE contributes to its physiological functions, we investigated the role of PE in TIM1-mediated phagocytosis. Like PS, PE is mostly confined to the inner leaflet of the plasma membrane (28, 29). To assess whether PE is exposed on the surface of apoptotic cells, we induced apoptosis in Jurkat cells by using the transcription inhibitor actinomycin D, and stained them with biotin-annexin A5 or biotin-Duramycin. As shown in Fig. 5A, Duramycin bound the majority of these cells, confirming that PE exposure is a physiological feature of apoptosis. Annexin A5, used as a positive control, strongly bound actinomycin D-treated cells. Live Jurkat cells remained negative for Duramycin and annexin A5 binding, although, at the highest concentration (0.3 µM), Duramycin showed a low level of binding. To test the role of PE in phagocytosis, apoptotic Jurkat cells were loaded with the pH-sensitive dye pHrodo and incubated with increasing amounts of Duramycin. hTIM1-293T cells were then cocultured with these cells and washed extensively before analysis to remove the Jurkat cells that were not engulfed. hL-SIGN-293T cells were also used to ensure that protein overexpression at the surface of the cells did not trigger any nonspecific engulfment. Because pHrodo emits brighter fluorescence in acidic environments, only Jurkat cells that have been engulfed and have reached a low pH compartment are scored. Expression of hTIM1 in 293T cells resulted in approximately a fivefold increase in phagocytosis compared with parental 293T and hL-SIGN-293T cells (Fig. 5B). Preincubation of apoptotic Jurkat cells with Duramycin significantly decreased their uptake, with 80% inhibition of TIM1-mediated phagocytosis at 0.5 μM Duramycin (Fig. 5C). Taken together, our results demonstrate that PE becomes exposed at the cell surface during apoptosis and plays an important role in TIM1-mediated phagocytic clearance of apoptotic cells.
Fig. 5.
PE is exposed on apoptotic cells and promotes TIM1-mediated phagocytosis. (A) Duramycin binds apoptotic cells. DMSO- (live) or actinomycin D-treated (apoptotic) Jurkat cells were stained with nothing, biotin-annexin A5, or biotin-Duramycin, followed by FITC-avidin. Cells were loaded with SYTOX Red dye for 10 min before analysis to distinguish necrotic cells from apoptotic cells. (B) Phagocytosis of apoptotic Jurkat cells by hTIM1-293T cells. pHrodo green-loaded, apoptotic Jurkat cells were cocultured with hTIM1-293T cells, and analyzed by flow cytometry to detect engulfed Jurkat cells. (C) Duramycin inhibits hTIM1-mediated phagocytosis. Similar experiments as in B except that pHrodo green-loaded apoptotic Jurkat cells were incubated with biotin-Duramycin before coculture with 293T, hLSIGN-, or hTIM1-293T cells. (Left) Leakage of pHrodo dye was assessed before phagocytosis assay. Results were normalized to pHrodo fluorescence in untreated Jurkat cells and expressed as relative pHrodo signal. (Right) Jurkat cells were cocultured with 293T, hLSIGN-, or hTIM1-293T cells at a 1.5:1 ratio of Jurkat cells to phagocytes. Results were normalized to the phagocytosis in hTIM1-293T cells without Duramycin treatment, and expressed as relative phagocytosis. The average ± SD of four duplicated experiments is shown (***P < 0.0001).
Discussion
We show here that PE is a ligand for TIM proteins, especially for TIM1, and other PS-binding proteins (Fig. 1 and Figs. S3 and S8). We also demonstrate that PE is present on the virions of enveloped viruses, including EBOV, DENV2, and WNV (Fig. 2B), and that virion-associated PE promotes virus entry into cells exogenously or naturally expressing TIM1 (Figs. 3 and 4 and Fig. S4). By using PE-specific Duramycin, we show that PE on the virion membrane mediates virus attachment to TIM1 (Fig. S6B). We further show that PE is exposed at the surface of apoptotic cells and promotes TIM1-mediated phagocytosis of those cells (Fig. 5), showing that PE is also involved in the physiological activities of PS receptors.
The ability of TIM-family proteins to bind PE and its important role in viral entry have not been described thus far to our knowledge. In 2007, Kobayashi et al. showed that human and murine TIM proteins bound PS, but not PE (15). However, by using similar methods (ELISA) and constructs (TIM extracellular domains fused to mIg), we demonstrate here that PE is an unambiguous ligand for hTIM1 (Fig. 1 and Fig. S3). This discrepancy could be explained by the use of two different cell lines, hamster CHO cells and human 293T cells, to produce the proteins. Thus, glycosylation differences in the IgV head domain, which contains a PS and PE binding site, may have altered the specificity of TIM1. Also, at high concentrations of PE and PS, hTIM-mIg binding is rather decreased (Fig. 1 and Fig. S3), a possible consequence of micelle formation at high concentrations. This effect is more pronounced for PE than PS, perhaps explaining why some investigators have overlooked the role of PE (15, 16). Other groups have previously reported that other so-called PS-binding proteins also bind PE. When the milk fat globule-EGF factor 8 (MFG-E8) protein was shown to promote phagocytosis, it was observed that it bound PE in addition to PS (30). Similarly, annexin A5 was previously shown to bind PE (31, 32). However, these observations were overlooked, and such proteins were subsequently described to exclusively bind PS (33, 34). We therefore assessed the phospholipid binding profile of MFG-E8 and annexin A5 by ELISA and confirmed that PE is a ligand for both proteins (Fig. S8). The ability to bind PE is therefore not exclusive to TIM proteins, but rather a common feature of at least a subset of PS-binding proteins. Because the ability to bind PE varies among PS receptors, it is tempting to speculate that cells bearing these receptors can detect varying PE/PS ratios. Similarly, PS receptors vary dramatically in their dependence on Ca2+ concentrations (Fig. 1 and Fig. S3A). Levels of PE and perhaps Ca2+ may therefore regulate processes mediated by PS receptors such as phagocytosis, as well as innate and adaptive immunity (35, 36).
It is noteworthy that Duramycin-mediated inhibition is almost complete for all viruses tested (Fig. 3), although PS is also present on the virions. A simple explanation is that Duramycin molecules bound to PE on the virions occlude access of TIM1, whose head domain consists of ∼100 aa, to neighboring PS. Nonetheless, efficient PE liposome blocking of infection (Fig. S2) and TIM1/PE ELISA data (Fig. 1) indicate that most of the Duramycin-mediated infection inhibition seems to result from direct binding of PE to TIM1.
PS receptors may be useful to viruses because these proteins are redundantly expressed in a wide range of cells, including macrophages and dendritic cells that are often the primary target cells for many viruses (37–40). EBOV buds from PS-rich microdomains (41), which may ensure abundant PS on the virion to amplify PS receptor utilization. PS receptors may also be especially beneficial to viruses that cycle between evolutionarily distant hosts, such as mosquitoes and mammals, because PS-binding proteins are functionally conserved. The subset of PS receptors that bind PE in addition to PS might be of special interest to viruses because PE content in mammalian membranes (15–25% of total phospholipids; as much as 45% in the brain) is much higher than that of PS (5–10%) (42, 43). Thus, viral envelopes are likely to contain more PE than PS. With PE content in insect cells being even higher (as much as 46–58%) (44–48), it is not surprising that arboviruses, such as flavi- and alphaviruses, evolved to use PE-binding receptors like TIM1 with high efficiency (9, 10).
For viruses such as DENV, vaccine design is extremely challenging. Effective vaccines or immunotherapies are complex to develop as a result of the severe complications caused by cross-reactive antibodies (49, 50). Thus, a broad antiviral strategy might be one of the few viable options to control dengue hemorrhagic fever. Targeting PS on virions has previously been suggested as a broad antiviral strategy (12), but proven difficult. Soares et al. showed that guinea pigs survived a lethal Pichinde virus infection when treated with an antibody originally described as PS-specific (51). However, this antibody primarily recognizes β-2 GP I and cardiolipin (52). Similarly, another anti-PS antibody (clone 4B6) we tested did not bind PS but rather bound PE (Fig. S9). We have shown here that the PE-specific antibiotic agent Duramycin effectively inhibits the infection of many pathogenic viruses without any approved vaccines or treatments. Given the importance of PE in viral entry processes, the presumed high abundance of PE on the virions, the fact that it is not exposed on the surface of healthy cells (29), Duramycin and similar PE-binding compounds may make useful antiviral agents. Although Duramycin itself contains lanthionine and methyllantionine bridges, making it expensive to synthesize and difficult to improve, its ability to control the replication of major pathogens such as EBOV, DENV, and WNV suggests that PE is a promising target for the design of broad-spectrum antiviral therapies.
Materials and Methods
ELISAs.
For protein and Duramycin binding to phospholipids, phospholipids were dried on ELISA plates and incubated with the indicated molecules. Binding was detected by using HRP-conjugated anti-mouse antibody for mIg fusion proteins and HRP-conjugated streptavidin for biotin-Duramycin and biotin-annexin A5. For Duramycin binding to viruses and to PLC-digested DENV2 virions, ELISA plates coated with virus-specific antibodies were incubated with virus, followed by incubation with PLC when indicated, then Duramycin and HRP-conjugated streptavidin to detect the binding.
Duramycin Inhibition of Infection.
Replication-competent viruses, VLPs, or PVs were preincubated with biotin-Duramycin for 1 h at room temperature and used to infect cells for 1 h [DENV2, IAV (H1N1), AdV5, WNV VLPs, and LASV PVs] or 3 h (EBOV and VSV VLPs) at 37 °C. Cells were then analyzed by flow cytometry.
Annexin A5 or Duramycin Binding to Apoptotic Cells.
Jurkat cells were incubated with 2 µM of actinomycin D (Cayman Chemical) or DMSO as a control for 16 h at 37 °C and incubated for 25 min on ice with biotin-Duramycin or biotin-annexin A5, followed by FITC-conjugated avidin and flow cytometry analysis.
Phagocytosis Assays.
Apoptotic Jurkat cells were loaded with pH-sensitive pHrodo green dye, preincubated or not with Duramycin for 30 min on ice, and cocultured with 293T, h-L-SIGN-, or hTIM1-293T cells for 4 h at 37 °C. The fluorescence of the phagocytes was analyzed by flow cytometry.
Animal Studies.
Experiments involving mice have been performed according to the guidelines established by Scripps Florida Institutional Animal Care and Use Committee (protocol 14–007).
Supplementary Material
Acknowledgments
We are grateful to Kimberly Schmitt for critical reading of this manuscript, as well as Bivian Torres from the flow cytometry core and Luise Angelini from the animal resources facility of the Scripps Research Institute for their help. This work was supported by National Institutes of Health Grant AI110692 (to H.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1508095112/-/DCSupplemental.
References
- 1.Lefebvre A, et al. Case fatality rates of Ebola virus diseases: A meta-analysis of World Health Organization data. Med Mal Infect. 2014;44(9):412–416. doi: 10.1016/j.medmal.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Hoenen T, Groseth A, Feldmann H. Current ebola vaccines. Expert Opin Biol Ther. 2012;12(7):859–872. doi: 10.1517/14712598.2012.685152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martínez-Romero C, García-Sastre A. June 6, 2015. Against the clock towards new Ebola virus therapies. Virus Res, 10.1016/j.virusres.2015.05.025.
- 4.Sejvar JJ. Clinical manifestations and outcomes of West Nile virus infection. Viruses. 2014;6(2):606–623. doi: 10.3390/v6020606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis LE, et al. West Nile virus neuroinvasive disease. Ann Neurol. 2006;60(3):286–300. doi: 10.1002/ana.20959. [DOI] [PubMed] [Google Scholar]
- 6.Moureau G, et al. New insights into flavivirus evolution, taxonomy and biogeographic history, extended by analysis of canonical and alternative coding sequences. PLoS One. 2015;10(2):e0117849. doi: 10.1371/journal.pone.0117849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jolly CL, Sattentau QJ. Attachment factors. Adv Exp Med Biol. 2013;790:1–23. doi: 10.1007/978-1-4614-7651-1_1. [DOI] [PubMed] [Google Scholar]
- 8.Amara A, Mercer J. Viral apoptotic mimicry. Nat Rev Microbiol. 2015;13(8):461–469. doi: 10.1038/nrmicro3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meertens L, et al. The TIM and TAM families of phosphatidylserine receptors mediate dengue virus entry. Cell Host Microbe. 2012;12(4):544–557. doi: 10.1016/j.chom.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jemielity S, et al. TIM-family proteins promote infection of multiple enveloped viruses through virion-associated phosphatidylserine. PLoS Pathog. 2013;9(3):e1003232. doi: 10.1371/journal.ppat.1003232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mercer J, Helenius A. Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells. Science. 2008;320(5875):531–535. doi: 10.1126/science.1155164. [DOI] [PubMed] [Google Scholar]
- 12.Soares MM, King SW, Thorpe PE. Targeting inside-out phosphatidylserine as a therapeutic strategy for viral diseases. Nat Med. 2008;14(12):1357–1362. doi: 10.1038/nm.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanaka K, Fujimura-Kamada K, Yamamoto T. Functions of phospholipid flippases. J Biochem. 2011;149(2):131–143. doi: 10.1093/jb/mvq140. [DOI] [PubMed] [Google Scholar]
- 14.Sebastian TT, Baldridge RD, Xu P, Graham TR. Phospholipid flippases: building asymmetric membranes and transport vesicles. Biochim Biophys Acta. 2012;1821(8):1068–1077. doi: 10.1016/j.bbalip.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobayashi N, et al. TIM-1 and TIM-4 glycoproteins bind phosphatidylserine and mediate uptake of apoptotic cells. Immunity. 2007;27(6):927–940. doi: 10.1016/j.immuni.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyanishi M, et al. Identification of Tim4 as a phosphatidylserine receptor. Nature. 2007;450(7168):435–439. doi: 10.1038/nature06307. [DOI] [PubMed] [Google Scholar]
- 17.Kondratowicz AS, et al. T-cell immunoglobulin and mucin domain 1 (TIM-1) is a receptor for Zaire Ebolavirus and Lake Victoria Marburg virus. Proc Natl Acad Sci USA. 2011;108(20):8426–8431. doi: 10.1073/pnas.1019030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moller-Tank S, Kondratowicz AS, Davey RA, Rennert PD, Maury W. Role of the phosphatidylserine receptor TIM-1 in enveloped-virus entry. J Virol. 2013;87(15):8327–8341. doi: 10.1128/JVI.01025-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morizono K, Chen IS. Role of phosphatidylserine receptors in enveloped virus infection. J Virol. 2014;88(8):4275–4290. doi: 10.1128/JVI.03287-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moller-Tank S, Maury W. Phosphatidylserine receptors: enhancers of enveloped virus entry and infection. Virology. 2014;468-470:565–580. doi: 10.1016/j.virol.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morizono K, et al. The soluble serum protein Gas6 bridges virion envelope phosphatidylserine to the TAM receptor tyrosine kinase Axl to mediate viral entry. Cell Host Microbe. 2011;9(4):286–298. doi: 10.1016/j.chom.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson AC, Xiao S, Kuchroo VK. Tim protein structures reveal a unique face for ligand binding. Immunity. 2007;26(3):273–275. doi: 10.1016/j.immuni.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santiago C, et al. Structures of T cell immunoglobulin mucin protein 4 show a metal-ion-dependent ligand binding site where phosphatidylserine binds. Immunity. 2007;27(6):941–951. doi: 10.1016/j.immuni.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santiago C, et al. Structures of T Cell immunoglobulin mucin receptors 1 and 2 reveal mechanisms for regulation of immune responses by the TIM receptor family. Immunity. 2007;26(3):299–310. doi: 10.1016/j.immuni.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwamoto K, et al. Curvature-dependent recognition of ethanolamine phospholipids by Duramycin and cinnamycin. Biophys J. 2007;93(5):1608–1619. doi: 10.1529/biophysj.106.101584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hou S, Johnson SE, Zhao M. A one-step staining probe for phosphatidylethanolamine. Chembiochem. 2015;16(13):1955–1960. doi: 10.1002/cbic.201500127. [DOI] [PubMed] [Google Scholar]
- 27.Stafford JH, Thorpe PE. Increased exposure of phosphatidylethanolamine on the surface of tumor vascular endothelium. Neoplasia. 2011;13(4):299–308. doi: 10.1593/neo.101366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vance JE. Phosphatidylserine and phosphatidylethanolamine in mammalian cells: Two metabolically related aminophospholipids. J Lipid Res. 2008;49(7):1377–1387. doi: 10.1194/jlr.R700020-JLR200. [DOI] [PubMed] [Google Scholar]
- 29.Murate M, et al. Transbilayer lipid distribution in nano scale. J Cell Sci. 2015;128(8):1627–1638. doi: 10.1242/jcs.163105. [DOI] [PubMed] [Google Scholar]
- 30.Hanayama R, et al. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417(6885):182–187. doi: 10.1038/417182a. [DOI] [PubMed] [Google Scholar]
- 31.Swairjo MA, Concha NO, Kaetzel MA, Dedman JR, Seaton BA. Ca(2+)-bridging mechanism and phospholipid head group recognition in the membrane-binding protein annexin V. Nat Struct Biol. 1995;2(11):968–974. doi: 10.1038/nsb1195-968. [DOI] [PubMed] [Google Scholar]
- 32.Stuart MC, Reutelingsperger CP, Frederik PM. Binding of annexin V to bilayers with various phospholipid compositions using glass beads in a flow cytometer. Cytometry. 1998;33(4):414–419. [PubMed] [Google Scholar]
- 33.Borisenko GG, Iverson SL, Ahlberg S, Kagan VE, Fadeel B. Milk fat globule epidermal growth factor 8 (MFG-E8) binds to oxidized phosphatidylserine: Implications for macrophage clearance of apoptotic cells. Cell Death Differ. 2004;11(8):943–945. doi: 10.1038/sj.cdd.4401421. [DOI] [PubMed] [Google Scholar]
- 34.Simhadri VR, et al. Human CD300a binds to phosphatidylethanolamine and phosphatidylserine, and modulates the phagocytosis of dead cells. Blood. 2012;119(12):2799–2809. doi: 10.1182/blood-2011-08-372425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Freeman GJ, Casasnovas JM, Umetsu DT, DeKruyffRH TIM genes: A family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunol Rev. 2010;235(1):172–189. doi: 10.1111/j.0105-2896.2010.00903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez-Manzanet R, DeKruyff R, Kuchroo VK, Umetsu DT. The costimulatory role of TIM molecules. Immunol Rev. 2009;229(1):259–270. doi: 10.1111/j.1600-065X.2009.00772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geisbert TW, et al. Pathogenesis of Ebola hemorrhagic fever in cynomolgus macaques: Evidence that dendritic cells are early and sustained targets of infection. Am J Pathol. 2003;163(6):2347–2370. doi: 10.1016/S0002-9440(10)63591-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kyle JL, Beatty PR, Harris E. Dengue virus infects macrophages and dendritic cells in a mouse model of infection. J Infect Dis. 2007;195(12):1808–1817. doi: 10.1086/518007. [DOI] [PubMed] [Google Scholar]
- 39.Hensley LE, et al. Pathogenesis of Marburg hemorrhagic fever in cynomolgus macaques. J Infect Dis. 2011;204(suppl 3):S1021–S1031. doi: 10.1093/infdis/jir339. [DOI] [PubMed] [Google Scholar]
- 40.Mercer J, Greber UF. Virus interactions with endocytic pathways in macrophages and dendritic cells. Trends Microbiol. 2013;21(8):380–388. doi: 10.1016/j.tim.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 41.Soni SP, Stahelin RV. The Ebola virus matrix protein VP40 selectively induces vesiculation from phosphatidylserine-enriched membranes. J BiolChem. 2014;289(48):33590–33597. doi: 10.1074/jbc.M114.586396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9(2):112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vance JE, Tasseva G. Formation and function of phosphatidylserine and phosphatidylethanolamine in mammalian cells. Biochim Biophys Acta. 2013;1831(3):543–554. doi: 10.1016/j.bbalip.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 44.Luukkonen A, Brummer-Korvenkontio M, Renkonen O. Lipids of cultured mosquito cells (Aedes albopictus). Comparison with cultured mammalian fibroblasts (BHK 21 cells) Biochim Biophys Acta. 1973;326(2):256–261. doi: 10.1016/0005-2760(73)90251-8. [DOI] [PubMed] [Google Scholar]
- 45.Butters TD, Hughes RC. Isolation and characterization of mosquito cell membrane glycoproteins. Biochim Biophys Acta. 1981;640(3):655–671. doi: 10.1016/0005-2736(81)90096-1. [DOI] [PubMed] [Google Scholar]
- 46.Marheineke K, Grünewald S, Christie W, Reiländer H. Lipid composition of Spodoptera frugiperda (Sf9) and Trichoplusiani (Tn) insect cells used for baculovirus infection. FEBS Lett. 1998;441(1):49–52. doi: 10.1016/s0014-5793(98)01523-3. [DOI] [PubMed] [Google Scholar]
- 47.Luukkonen A, Kaariainen L, Renkonen O. Phospholipids of Semliki Forest virus grown in cultured mosquito cells. Biochim Biophys Acta. 1976;450(2):109–120. doi: 10.1016/0005-2760(76)90082-5. [DOI] [PubMed] [Google Scholar]
- 48.Fast PG. A comparative study of the phospholipids and fatty acids of some insects. Lipids. 1966;1(3):209–215. doi: 10.1007/BF02531874. [DOI] [PubMed] [Google Scholar]
- 49.Halstead SB. Neutralization and antibody-dependent enhancement of dengue viruses. Adv Virus Res. 2003;60:421–467. doi: 10.1016/s0065-3527(03)60011-4. [DOI] [PubMed] [Google Scholar]
- 50.Guzman MG, Alvarez M, Halstead SB. Secondary infection as a risk factor for dengue hemorrhagic fever/dengue shock syndrome: An historical perspective and role of antibody-dependent enhancement of infection. Arch Virol. 2013;158(7):1445–1459. doi: 10.1007/s00705-013-1645-3. [DOI] [PubMed] [Google Scholar]
- 51.Ran S, Huang X, Downes A, Thorpe PE. Evaluation of novel antimouse VEGFR2 antibodies as potential antiangiogenic or vascular targeting agents for tumor therapy. Neoplasia. 2003;5(4):297–307. doi: 10.1016/S1476-5586(03)80023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luster TA, et al. Plasma protein beta-2-glycoprotein 1 mediates interaction between the anti-tumor monoclonal antibody 3G4 and anionic phospholipids on endothelial cells. J BiolChem. 2006;281(40):29863–29871. doi: 10.1074/jbc.M605252200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.