Abstract
Immunotherapy has great potential to treat cancer and prevent future relapse by activating the immune system to recognize and kill cancer cells. A variety of strategies are continuing to evolve in the laboratory and in the clinic, including therapeutic noncellular (vector-based or subunit) cancer vaccines, dendritic cell vaccines, engineered T cells, and immune checkpoint blockade. Despite their promise, much more research is needed to understand how and why certain cancers fail to respond to immunotherapy and to predict which therapeutic strategies, or combinations thereof, are most appropriate for each patient. Underlying these challenges are technological needs, including methods to rapidly and thoroughly characterize the immune microenvironment of tumors, predictive tools to screen potential therapies in patient-specific ways, and sensitive, information-rich assays that allow patient monitoring of immune responses, tumor regression, and tumor dissemination during and after therapy. The newly emerging field of immunoengineering is addressing some of these challenges, and there is ample opportunity for engineers to contribute their approaches and tools to further facilitate the clinical translation of immunotherapy. Here we highlight recent technological advances in the diagnosis, therapy, and monitoring of cancer in the context of immunotherapy, as well as ongoing challenges.
Keywords: immunoengineering, cancer vaccine, adoptive T-cell therapy, diagnostic tools, checkpoint blockade
Cancer immunotherapy harnesses the patient’s immune system to kill tumor cells and prevent future relapse. Research focused on tumor immunology and translational immunotherapy has been bolstered by recent successes in clinical trials (1–4), and the pharmaceutical industry is pursuing immunological targets with unprecedented energy. However, major challenges still exist in translating these promising approaches to practical, clinically feasible therapies that can treat a larger range of cancer types, including those that are most difficult to treat with chemotherapy. Although the genetic signatures of individual tumors can now be determined rapidly, which can help identify small-molecule targets for specifically killing these cells, successful immunotherapy depends on the host immune cells, the tumor microenvironment, and many other features that are not necessarily directly reflected in the tumor’s genetic signature (Fig. 1). New enabling technologies are needed to support, facilitate, and accelerate the clinical translation of immunotherapy. Specifically, the technological needs include the following:
-
•
Rapid characterization of the tumor and its immune microenvironment at the time of diagnosis: Revealing the type of immune defense mechanisms that the tumor has created will help predict how the tumor will respond to immunotherapy and thus help guide patient-specific therapeutic strategies.
-
•
Predictive models of therapeutic outcome: In vitro patient-derived tumor models that faithfully recapitulate key features of the tumor microenvironment will allow rapid and high-throughput screening of potential therapies including drug and immunotherapy combinations to guide treatment decisions for each individual patient.
-
•
New tools for treatment: New methods to target specific tissues, cells, and intracellular processes in precise ways will allow fine-tuning of desired immune responses, improve the efficacy of existing and new immunotherapies, and reduce toxicity and side effects. A wide variety of technologies are being explored that enable cellular therapies, vaccines, antibodies, and small molecules in clinical oncology.
-
•
Comprehensive assays for patient monitoring: Sensitive, accurate, and information-rich assays that determine the presence of cancer byproducts and host immune responses will allow clinicians to more effectively determine therapeutic response and allow treatment strategies to be fine-tuned and adapted during and after treatment. Reliable and ultrasensitive assays that detect the presence of micrometastases (e.g., circulating tumor cells, exosomes, and DNA) will allow routine and frequent testing after treatment and during remission, because metastatic relapse is the main cause of cancer mortality. Inexpensive, noninvasive, and easy-to-use assays will allow more frequent monitoring after therapy as well as more frequent cancer screening for the general population.
Fig. 1.
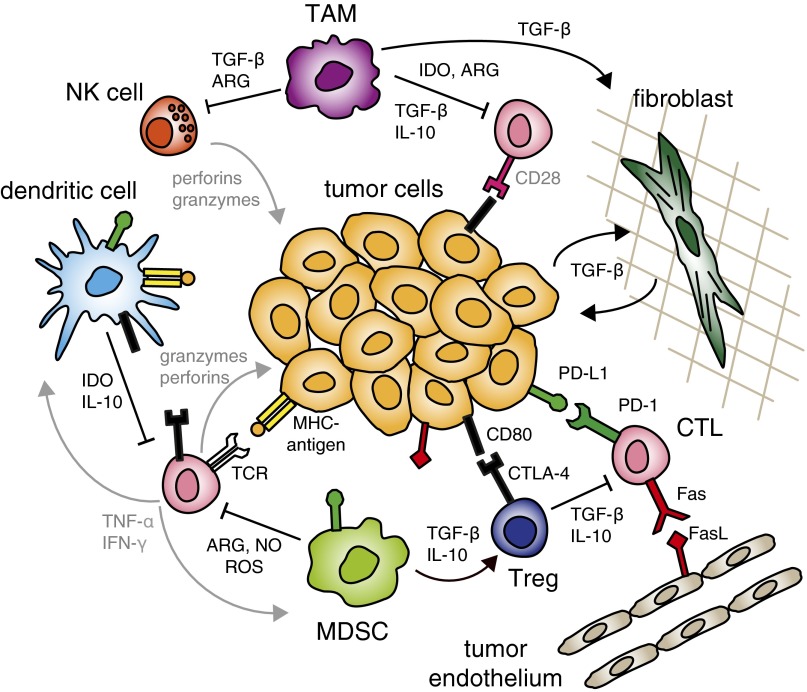
Features of the tumor microenvironment that hinder immunotherapy. To dampen the killing functions of cytotoxic T lymphocytes (CTLs), regulatory T cells (Tregs) secrete TGF-β and IL-10, whereas myeloid-derived suppressor cells (MDSCs) secrete arginase (ARG), nitric oxide (NO), and reactive oxygen species (ROS). Immature or regulatory dendritic cells secrete IL-10 and indoleamine 2,3-dioxygenase (IDO) and can further present antigens to T cells in ways that drive anergy or tolerance, including through overexpression of ligands for the checkpoint molecules PD-1 and CTLA-4. Tumor-associated macrophages (TAMs) also secrete immune suppressive factors and cytokines to block the activity of natural killer (NK) cells and CTLs. Tumor cells and host stromal cells (including fibroblasts and blood and lymphatic endothelium) can also express TGF-β, checkpoint ligands, and FasL to directly cause T-cell apoptosis.
These technological challenges present important opportunities for engineers. In nearly all areas of basic science, engineers have helped translate discoveries into useful technologies. In the biomedical sector, they have devised imaging technologies for early cancer diagnosis, polymers to deliver drugs to specific tissues, and diagnostics that can rapidly detect minute levels of a specific protein. In clinical cancer immunology, engineers may soon help translate the promise of immunotherapy into reality by developing the ultrasensitive, reliable assays needed for effective patient monitoring, the tissue engineered models needed for rapidly determining patient-specific therapeutic strategies, and the protein engineering approaches to achieve in situ what is currently only done with expensive cellular harvesting and manipulation ex vivo, to name a few. This nascent field is often referred to as immunoengineering, and we will focus this review on the major challenges and opportunities that exist in facilitating and enabling the translation of promising new immunotherapeutic strategies into the clinic.
This perspective will not attempt to exhaustively review the field of cancer immunotherapy (1, 5–10) or the field of nanomaterial vaccines (11–16), topics that are well covered in recent reviews. Instead we will focus on the technical challenges that exist in translating the principles of cancer immunotherapy into clinical reality: what approaches are being used, and where opportunities exist for new ideas and development. Overcoming these challenges will enable us to learn more from long, expensive clinical trials, to better predict how each individual patient should be treated, and to allow immunotherapy to reach a greater number of patients.
Strategies for Cancer Immunotherapy
With traditional preventive vaccines against viral diseases like polio or influenza, the live or attenuated virus is injected with an adjuvant, or danger signal, which activates local dendritic cells (DCs) to (i) take up and process the viral antigens, (ii) mature and migrate to the draining lymph node (LN) via lymphatic vessels, (iii) present various peptide antigens on MHC molecules, and (iv) engage with and activate T cells specific for these antigenic epitopes. The T cells, in turn, expand into short-lived effector and long-lived memory populations, poised to rapidly respond on later antigen re-encounter. The vast success of such vaccines depends in part on the foreign nature of the viral antigens to which the host immune system is completely naïve before vaccination. Preventive vaccines for cancers caused by chronic viral infections, such as cervical and hepatocellular carcinoma (caused by human papilloma virus and hepatitis B virus, respectively), work this way: by preventing the viral infection from taking hold and becoming chronic in the first place, they indirectly prevent those cancers (2, 17).
On the other hand, therapeutic cancer vaccination faces a number of challenges compared with preventive vaccines against infectious agents, due to the complex coevolution of the tumor and the host immune response. Of course, cancer cells are mutated “self,” and although the immune system can recognize the abundant mutated proteins common in cancer cells, often those with the highest avidity T-cell receptors (TCRs) have been deleted or compromised by central and peripheral tolerance mechanisms. Furthermore, those tumors that do become recognized by the host immune system can build up a number of defense mechanisms that suppress effector functions of T cells and natural killer (NK) cells (Fig. 1).
However, there are several different approaches that have been developed to turn the immune system against the tumor and activate potent T-cell immunity. Vector-based or subunit vaccines are similar to traditional vaccines in that they target DCs, but can involve specifically defined antigens (or even whole tumor lysate) and may also be coupled to delivery strategies like polymeric nanoparticles. DC vaccines bypass the first step of targeting these cells in vivo and instead educate the DCs ex vivo in controlled settings before reinjecting them into the patient. T-cell therapies go one step further and bypass the need for DCs altogether; T cells are expanded ex vivo, stimulated and genetically modified to be highly aggressive, and then reinfused into the patient to home to the tumor. Finally, checkpoint blockade therapy works when antitumor T cells are already present but exhausted or anergic; these cells are reawakened in situ through molecular blockade of mechanisms that inhibit their function. A handful of cancer immunotherapies have recently been approved and many more are currently in clinical trials. Some immunotherapies are antigen specific and require foreknowledge of relevant cancer antigens to which the patient would be sensitive; for these, a major challenge in the design of cancer immunotherapies is thus finding appropriate antigens. Other immunotherapies, such as checkpoint blockade, do not require foreknowledge of the tumor antigens, because processes are triggered in vivo to endogenous antigens.
Vaccines and Antigen Selection
Characterizing the antigenic repertoire for each tumor is a major challenge for developing personalized vaccines. Most tumors are very heterogeneous in nature, due to high rates of mutation from genomic instability along with numerous environmental selection pressures, including those resulting from the patient’s past treatments and immune history.
Most known and defined tumor antigens are self-antigens, which include (i) tissue differentiation antigens such as TRP-2, gp100, and Melan-A, which are present in all melanocytes and thus in melanoma cells; (ii) overexpressed antigens such as survivin, which is expressed in a number of cancers at a much higher level than in noncancerous cells; and (iii) cancer-testis antigens such as NY-ESO-1, which is expressed in a number of cancer cells but is normally expressed only in germ-line cells and not normal somatic cells. However, all of these self-antigens carry risks of off-target effects because other nontumor cells can express them; for example, vaccination against TRP-2 in melanoma frequently results in vitiligo, i.e., autoimmunity of normal melanocytes in the skin (18, 19).
The selection of antigens for tumor immunotherapy is further complicated by the tumor’s response to therapy. Antigen-specific immunotherapy places selective pressure on the tumor, which in turn can down-regulate expression of those antigens (or even of MHC I) during immunotherapy. To avoid antigen selection, vaccines can contain multiple defined epitopes that induce broader T-cell immunity; several multipeptide vaccines have already shown promising clinical results (20, 21). Furthermore, because central tolerance mechanisms often destroy those T cells with the highest avidity to such self-antigens, vaccine-induced effector CD8+ T-cell responses against self tumor antigens are often weak at best (22).
As an alternative, neoantigens represent a new class of unique tumor-specific antigens that lack tolerance against them and that can be recognized by tumor-infiltrating CD4+ helper (23) and CD8+ cytotoxic T cells (24–26). Neoantigens arise from somatic mutations that make up the tumor mutanome (or cancer genome) and can be identified in tumor biopsies by cancer exome sequencing and bioinformatics analysis (27). These neoantigen sequences can then be synthesized and incorporated in the design of patient-specific vaccines (25, 28). On the other hand, identifying neoantigens and developing them into personalized vaccines is expensive and time-consuming, and such vaccines must still overcome the various mechanisms of immune suppression that coevolve with the tumor (Fig. 1).
Many therapeutic cancer vaccines in current trials include tumor-associated antigens in the form of short peptides containing epitopes that bind to MHC I, which prime CD8+ T cells to become cytotoxic and are codelivered with different adjuvant formulations. More recently, long synthetic peptides (LSPs) are being developed; these comprise both MHC I and MHC II epitopes to additionally prime CD4+ T helper cells and yield broader, more effective immunity. For example, LSPs of HPV16 proteins E6 and E7 were included in the design of cancer vaccine trials for cervical cancer (17). Such studies have shown promise and clinical trials are now underway.
Some vaccine strategies avoid the need to define or isolate tumor antigens altogether. One such strategy involves the delivery of oncolytic viruses, which are viruses designed to specifically infect tumor cells and lead to immunogenic cell death and subsequent release of both the (viral) antigens, which are expressed only in the tumor cells, as well as antigens enriched in tumor cells (29). In this way, the immune response to the viral protein creates immunity that can spread to tumor-specific antigens even in tumor cells that have not been infected by the virus. Such strategies are being pursued in preclinical studies and in clinical trials for a variety of tumor types (30).
Engineers are contributing to vaccine design through development of nanomaterials and other biomolecular complexes to target DCs in specific locations as well as to direct antigen processing within the cell to induce strong CD8+ T-cell immunity, leading to generation of abundant cytotoxic T lymphocyte (CTL) responses. They are also using such nanomaterials to blunt immune suppressive mechanisms in the tumor, for example, by targeting myeloid-derived suppressor cells (MDSCs) (31), which down-regulate the activity of CTLs and provide a mechanism of tumor tolerance.
Vaccines have traditionally been delivered as intramuscular depots, at least in prophylactic vaccines for infectious diseases. However, considerable work in cancer vaccines has focused on targeting immature DCs that are resident in the LN, because the LN serves as an anatomical site for T-cell priming by DCs (12, 16). Although vaccines may be injected within the LN with beneficial effect (32), this is technically challenging and may damage the LN. As an alternative, nanocarriers can be designed in such a way to target DCs directly in LNs, both skin-draining and tumor-draining LNs, depending on their size: larger particles (>200 nm) are hindered by the interstitial space after injection and thus associate more with skin-resident DCs that then migrate to draining LNs, whereas smaller particles (10–100 nm) flow directly into lymphatic vessels and drain directly into the LN (33, 34), where they are taken up by LN-resident DCs. Free proteins and peptides are less efficient in LN targeting because of lower retention in the LN. Thus, coupling antigen and/or adjuvant to nanomaterials, virus-like particles, or even targeting endogenous albumin (35) are effective means to optimize LN targeting of vaccine components.
Not all LNs are equivalent in their immunological potential. For example, the tumor-draining LN has more suppressive cytokines and cells than a noninvolved LN due to the multitude of factors released by the tumor and its microenvironment; on the other hand, it has been exposed over time to antigens shed by the tumor. Our laboratory has used very small nanoparticles (NPs) in melanoma and lymphoma models to show that the tumor-draining LN was a particularly opportune site for cancer vaccination due to its antigen experience (36–38).
To stimulate CTL responses against exogenous antigen, it is important to deliver antigen in such a way that it can be efficiently cross-presented on MHC I, and nanocarriers can be engineered to help direct intracellular antigen trafficking. Our laboratory has explored different chemical schemes to achieve this and has shown that conjugation of antigen on the surface of the NP via a reduction-sensitive link, such as a disulfide, allows antigen release in the early endosome (a reductive environment) to promote cross-presentation onto MHC I and therefore CD8+ T-cell activation (39). On the other hand, when antigen liberation occurs later in processing, such as in the lysosome (an oxidative environment), more substantial presentation on MHC II occurred with corresponding stimulation of CD4+ T-cell immunity (40, 41).
Dendritic Cell Vaccines
The choice of tumor antigens is also a key factor in another class of immunotherapy, namely DC vaccines, in which DCs are generated ex vivo from peripheral blood monocytes, pulsed with tumor-derived peptides or proteins along with costimulatory molecules and cytokines, and then reinjected into the patient where they home to secondary lymphoid tissues to activate T cells there. Sipuleucel-T (Provenge) was the first US Food and Drug Administration (FDA)-approved therapeutic DC vaccine for use in castration-resistant prostate cancer patients (42), and since then, many more DC vaccines have been explored (7, 43, 44). Challenges in DC vaccine efficacy include the small fraction of transferred DCs that migrate to the LN after injection and their relatively short time of action. DC vaccines also face the same challenges with antigen choice described above, but because DC stimulation occurs ex vivo, cell lysate from tumor biopsies can be effectively used as a broad antigen pool. Furthermore, if lysate is created in a particularly immunogenic manner it can also serve as adjuvant. For example, treating ovarian tumor cells with hypochlorous acid before lysing was shown to be particularly effective (45) and is now being investigated in recurrent ovarian cancer patients (46).
As an alternative to activating DCs ex vivo, which involves expensive and specialized procedures, others have explored the approach of creating in vivo depots of antigens and chemokines into which DCs can migrate and become activated. Macroporous matrices composed of poly(lactide-coglycolide), a common biodegradable medical polymer, have been loaded with tumor cell lysate and GM-CSF to enhance infiltration of DCs and their monocyte precursors. These materials are implanted s.c.; their efficacy has been demonstrated in the B16F10 murine melanoma model (47, 48) and are currently in clinical trials to test their safety in humans.
T-Cell Therapies
Another approach is to bypass the reliance on DCs to activate T cells and instead modify the T cells directly. Tremendous effort has been put into the advancement of T-cell therapies, which include expanded autologous T cells and T cells with engineered TCRs and chimeric antigen receptors (CARs). In its simplest form, tumor-infiltrating lymphocytes (TILs) are isolated from tumor biopsies and expanded before being reinfused into the patient, based on the premise that these TILs are tumor cell specific (5). For gene-modified T-cell therapy, circulating T cells are isolated by apheresis and transduced with a specific CAR, composed of an intracellular antibody-binding domain specific for tumor cell markers (49) or fused to a cytoplasmic domain based on native TCRs, or with a TCR specific for defined tumor antigens before being transferred back to the patient. Such engineered T cells were developed to overcome immune tolerance and to include mutated tumor-specific T cells, which are otherwise not present in patients. CAR- and TCR-engineered T cells are currently receiving extensive attention with the recent denomination of “breakthrough status” from the FDA. Most T-cell therapies explored in clinical trials currently focus on leukemia and melanoma patients and include αβ T cells, but other T cells, including γδ and NK T cells, are being investigated as well (5).
Challenges in T-cell therapies include all of the technical and manufacturing challenges inherent to any cell therapy, as well as the same issues described above related to vaccine therapies. When the domain recognized is specific to a particular cell type from which cancer cells derive, then the therapy can kill both cancer and normal cells. Such is the case with CAR T-cell therapies targeting CD19 in acute lymphoblastic leukemia, a receptor that is present on both leukemic cells and normal B cells (49, 50). Ongoing advances in designing CARs include modifications in their extracellular domain to recognize tumor cells and in their cytoplasmic domain to enhance both efficacy through potent signaling and safety through inclusion of regulatory domains (50).
Engineering technologies have been developed to enhance T-cell therapies. T cells harbor free thiols on their surface, which can be exploited for nanocarrier conjugation. For example, maleimide-functionalized lipid NPs have been coupled onto the surface of T cells before adoptive transfer to enable direct delivery of phosphatase inhibitors to the synapse of T cells; this led to dramatic T-cell expansion at the tumor site and enhanced survival of mice with prostate tumors (51). T cells have also been used to carry drugs to the sites of tumors in directed chemotherapy: specifically, polyclonal T cells have been loaded with chemotherapeutics and after reinjection have been shown to home to the LNs, which harbor tumor cells in situations such as lymphoma (52). Finally, universal and chemo-resistant T cells are being developed to avoid host-versus-graft disease (53).
Immune Checkpoint Blockade Therapy
In addition to vaccines and cellular therapies, immune checkpoint blockade therapy has led to exciting recent clinical advances in cancer immunotherapy (3, 4). It works by blocking inhibitory pathways that would normally dampen the activity of effector T cells such as cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), which interferes with costimulation (54–56), and programmed cell death protein 1 (PD-1), which dampens signaling mediated by the TCR (6) and negatively regulates antigen responsiveness (57). Ipilimumab, an anti–CTLA-4 monoclonal antibody, was the first immune checkpoint inhibitor to be approved in 2011; two blocking antibodies against PD-1 (Nivolumab and Pembrolizumab) were approved in 2014 for use in melanoma patients, and other antibodies for checkpoint blockade are in the pipeline (6). Other T-cell inhibitory receptors that are being explored as immunotherapy targets include T-cell immunoglobulin domain and mucin domain-3 (TIM-3), lymphocyte-activation gene 3 (LAG-3), V-domain immunoglobulin suppressor of T-cell activation (VISTA), and B- and T-lymphocyte attenuator (BTLA) (58, 59). Furthermore, in addition to blocking checkpoint molecules on T cells, blocking of their inhibitory ligands (e.g., PD-L1) on tumor cells, stromal cells, and tumor-associated macrophages and DCs is also being pursued (60).
Challenges for improving checkpoint blockade immunotherapy derive from the key underlying function of these pathways in maintaining immune regulation, because blockade of these regulatory pathways is not antigen specific. As such, patients undergoing checkpoint blockade immunotherapy are prone to developing autoimmune reactions, such as dermatitis, enterocolitis, and uveitis with CTLA-4 therapy (61–64). To date, engineering approaches to these challenges are lacking, but the opportunities are abundant. For example, antibody engineering and targeted delivery technologies could be used to develop strategies for local rather than systemic checkpoint blockade, to avoid overstimulating tumor-irrelevant, self-reactive T cells and thus limit autoimmunity-related side effects. Furthermore, patient-derived tissue engineered models that faithfully recapitulate the tumor-immune microenvironment could be used to screen potential checkpoint blockade therapies and their combinations with drugs targeting the immune suppressive pathways in the tumor stroma. To date, although a plethora of in vitro patient-derived tumor models have been developed, very few address the immune compartment (65).
Tumor Characterization
In the standard of practice, cancer diagnosis is usually based on imaging, histopathology and immunohistochemistry from biopsies as well as genotyping in cancers where specific mutations have been identified and drugs developed against those mutations. In most cases, therapies are chosen based on specific targets identified in the tumor biopsies. However, factors that affect the efficacy of immunotherapy include much more than the genomic makeup of the tumors, and characterization of the tumor immune microenvironment can help guide therapy (Fig. 1). In addition, evidence suggests that many other factors including age, sex, metabolic state, immune history, and even the gut microbiota can exert major influences on the immune system (66) and thus affect immunotherapy outcome. Here we will focus on technological needs for more comprehensive analysis of tumors and their microenvironment that can guide therapy among the rapidly growing repertoire of immunotherapeutic strategies.
The presence of TILs signals that the host immune system has recognized the cancer, and generally implies that the tumor has built up mechanisms to counteract host immunity, including attracting immune suppressive cells, remodeling the extracellular matrix, inducing lymphangiogenesis, down-regulating antigenic proteins and MHC I, and secreting suppressive factors (67–69). However, because tumor-specific T cells are present (although suppressed), highly infiltrated tumors may be particularly responsive to checkpoint blockade (6, 70). Furthermore, expression of PD-1 ligands by tumor cells, stromal cells, and leukocytes can also serve as useful prognostic indicators and has been shown to correlate with responsiveness to checkpoint blockade (64, 71). Conversely, the lack of immune cell infiltrates often implies that the tumor has escaped immune detection and these tumors are likely to respond poorly to checkpoint blockade (6, 70).
Antigen specificity of TILs is an important diagnostic metric as well, and mutational load (i.e., the number of neoantigens harbored by tumor cells) has been shown to correlate with clinical response to CTLA-4 blockade (72). The presence of tumor-specific T cells and their phenotype can be measured in tumor biopsies and in the blood of patients with various assays, including tetramer staining of TCRs, restimulation of T cells with tumor antigens followed by quantification of proliferation or cytokine production, and recently tools to monitor neoantigen-specific T cells with two-color coded multimer staining (73).
The tumor stroma and vasculature surrounding the tumor are important features of the tumor microenvironment that affect immunotherapy efficacy. Tumor vasculature can dictate trafficking and homing of small molecules and chemotherapeutics drugs, and more importantly, it can modulate homing of T cells to the tumor bed. For example, it was recently shown that tumor endothelium can express FasL, which binds Fas (CD95) to induce cell death. Because cytotoxic CD8+ T cells have higher Fas expression than T regulatory cells (Tregs), FasL expression in the tumor endothelium was inversely correlated with CTL infiltration; such tumors respond better to cancer immunotherapy when used in combination with inhibitors of FasL (74). Thus, characterizing the tumor microenvironment is important for choosing the most promising immunotherapeutic strategies.
When accessible and relevant, tumor-draining LNs are biopsied as well to diagnose metastatic disease. The tumor-draining LN is an immune-suppressed site (75) “educated” by the upstream tumor, for example, via exosomes released by melanoma cells preceding metastasis (76). Tumor-draining LNs contain both tumor antigen-primed T cells with up-regulated checkpoint molecules and immune cells with up-regulated checkpoint ligands (36). LN-resident DCs can induce tolerance in CD8+ T cells, and LN stromal cells can play similar roles (77–80). These factors suggest that the tumor-draining LN may contain important biomarkers relevant to diagnosis. On the other hand, the difficulty in obtaining lymph samples is likely to limit its potential clinical use.
Cancer Diagnosis and Monitoring
Characterizing features of the immune microenvironment requires access to the tumor; however, not all tumors are surgically accessible, and thus developing new means to detect and measure these characteristics is an important technological need. Engineering tools are being developed to help identify noninvasive ways to detect and characterize tumors, as well as improve sensitivity to detect relapse or metastasis earlier. In some cancers, tumor markers may be detected in the blood, such as CA125 in ovarian cancer or more generally noncoding micro-RNAs (miRNAs). A novel microfluidic-based multiplex quantitative RT-PCR can identify the miRNA signature in the serum of prostate cancer patients, which may in the near future be used diagnostically (81). Urine can also be monitored; for example, Kwong et al. developed synthetic biomarkers composed of mass-encoded peptides linked to NPs with protease-cleavable linkers such that tumor-derived proteases liberate the peptides into the urine of patients (82). Similarly, engineered iron oxide “nanoworms” conjugated with a peptide linked to a reporter can also be proteolytically cleaved into the urine, which can then be read on a low-cost paper lateral flow assay (83). Finally, Danino et al. engineered an Escherichia coli-based diagnostic tool that selectively delivers gene products to metastatic cells in the liver, yielding detectable signals in the urine of mice (84). These approaches are among many new approaches being developed for noninvasive diagnostics, which may someday allow routine screening for a wide range of cancers and have enough sensitivity to detect microscopic metastases.
A key focus of diagnostic development is in identifying and capturing circulating tumor cells (CTCs), which can signal relapse and metastasis. Although much development has occurred in the last few years, there is still great need for effective, sensitive, and easy-to-use methods to capture and characterize these cells. Many microfluidic approaches are being developed, such as a high-throughput capture chip based on intertial focusing that enables sorting of magnetically labeled rare CTCs from the blood and of subsequent RNA-based single-cell characterization (85). Standard flow cytometry can also be used with fluorescence labels, but the relative scarcity of such cells makes detection difficult; approaches to overcome that includes an imaging flow cytometer that images streaks by adjusting exposure time and that allows very rare cells to be detected (86). Label-free methods to detect CTCs from patient samples are also under development. Deformability cytometry is a method that identifies cancer cells by their altered mechanical properties, measured as they deform under compressive and shear forces passing through microfluidic channels (87). Acoustic-based microfluidic separation, which is contact-free and preserves cell integrity, was developed to sort tumor cells from blood in breast cancer patients, and showed high sensitivity (as few as 100 cells/mL could be detected) (88). Although still in experimental development, these examples are among the many exciting new methods for detecting CTCs.
In conclusion, there are many technological needs in the detection, characterization, treatment, and monitoring of cancer in the context of immunotherapy, as well as many exciting new ideas and approaches to address those needs. These developments are due in part to the rapidly increasing numbers of engineers, from a wide range of fields that are turning to immunology-related problems and forming a new field of immunoengineering. As an increasing fraction of the translational cancer research community is turning toward immunotherapy, we expect that the fraction of bioengineers addressing problems in immunology, including clinical cancer immunotherapy, to rapidly grow in the coming years.
Acknowledgments
We thank the European Research Council, the Swiss National Science Foundation, Swiss Transmed, and Oncosuisse for funding.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article is part of the special series of PNAS 100th Anniversary articles to commemorate exceptional research published in PNAS over the last century.
References
- 1.Drake CG, Lipson EJ, Brahmer JR. Breathing new life into immunotherapy: Review of melanoma, lung and kidney cancer. Nat Rev Clin Oncol. 2014;11(1):24–37. doi: 10.1038/nrclinonc.2013.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Melero I, et al. Therapeutic vaccines for cancer: An overview of clinical trials. Nat Rev Clin Oncol. 2014;11(9):509–524. doi: 10.1038/nrclinonc.2014.111. [DOI] [PubMed] [Google Scholar]
- 3.Hamid O, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369(2):134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolchok JD, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.June CH, Riddell SR, Schumacher TN. Adoptive cellular therapy: A race to the finish line. Sci Transl Med. 2015;7(280):280ps7. doi: 10.1126/scitranslmed.aaa3643. [DOI] [PubMed] [Google Scholar]
- 6.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348(6230):56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 7.Palucka K, Banchereau J. Dendritic-cell-based therapeutic cancer vaccines. Immunity. 2013;39(1):38–48. doi: 10.1016/j.immuni.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maus MV, et al. Adoptive immunotherapy for cancer or viruses. Annu Rev Immunol. 2014;32:189–225. doi: 10.1146/annurev-immunol-032713-120136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiang CLL, Balint K, Coukos G, Kandalaft LE. Potential approaches for more successful dendritic cell-based immunotherapy. Expert Opin Biol Ther. 2015;15(4):569–582. doi: 10.1517/14712598.2015.1000298. [DOI] [PubMed] [Google Scholar]
- 10.Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12(4):237–251. doi: 10.1038/nrc3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Irvine DJ, Swartz MA, Szeto GL. Engineering synthetic vaccines using cues from natural immunity. Nat Mater. 2013;12(11):978–990. doi: 10.1038/nmat3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swartz MA, Hirosue S, Hubbell JA. Engineering approaches to immunotherapy. Sci Transl Med. 2012;4(148):148rv9. doi: 10.1126/scitranslmed.3003763. [DOI] [PubMed] [Google Scholar]
- 13.Li WA, Mooney DJ. Materials based tumor immunotherapy vaccines. Curr Opin Immunol. 2013;25(2):238–245. doi: 10.1016/j.coi.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wen Y, Collier JH. Supramolecular peptide vaccines: Tuning adaptive immunity. Curr Opin Immunol. 2015;35:73–79. doi: 10.1016/j.coi.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith DM, Simon JK, Baker JR., Jr Applications of nanotechnology for immunology. Nat Rev Immunol. 2013;13(8):592–605. doi: 10.1038/nri3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu H, Irvine DJ. Guiding principles in the design of molecular bioconjugates for vaccine applications. Bioconjug Chem. 2015;26(5):791–801. doi: 10.1021/acs.bioconjchem.5b00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Burg SH, Melief CJ. Therapeutic vaccination against human papilloma virus induced malignancies. Curr Opin Immunol. 2011;23(2):252–257. doi: 10.1016/j.coi.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 18.Bronte V, et al. Genetic vaccination with “self” tyrosinase-related protein 2 causes melanoma eradication but not vitiligo. Cancer Res. 2000;60(2):253–258. [PMC free article] [PubMed] [Google Scholar]
- 19.Overwijk WW, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003;198(4):569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suzuki H, et al. Multiple therapeutic peptide vaccines consisting of combined novel cancer testis antigens and anti-angiogenic peptides for patients with non-small cell lung cancer. J Transl Med. 2013;11(1):97. doi: 10.1186/1479-5876-11-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walter S, et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med. 2012;18(8):1254–1261. doi: 10.1038/nm.2883. [DOI] [PubMed] [Google Scholar]
- 22.Pedersen SR, Sørensen MR, Buus S, Christensen JP, Thomsen AR. Comparison of vaccine-induced effector CD8 T cell responses directed against self- and non-self-tumor antigens: Implications for cancer immunotherapy. J Immunol. 2013;191(7):3955–3967. doi: 10.4049/jimmunol.1300555. [DOI] [PubMed] [Google Scholar]
- 23.Linnemann C, et al. High-throughput epitope discovery reveals frequent recognition of neo-antigens by CD4+ T cells in human melanoma. Nat Med. 2015;21(1):81–85. doi: 10.1038/nm.3773. [DOI] [PubMed] [Google Scholar]
- 24.Matsushita H, et al. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature. 2012;482(7385):400–404. doi: 10.1038/nature10755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gubin MM, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515(7528):577–581. doi: 10.1038/nature13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell. 2015;160(1-2):48–61. doi: 10.1016/j.cell.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348(6230):69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 28.Castle JC, et al. Exploiting the mutanome for tumor vaccination. Cancer Res. 2012;72(5):1081–1091. doi: 10.1158/0008-5472.CAN-11-3722. [DOI] [PubMed] [Google Scholar]
- 29.Lichty BD, Breitbach CJ, Stojdl DF, Bell JC. Going viral with cancer immunotherapy. Nat Rev Cancer. 2014;14(8):559–567. doi: 10.1038/nrc3770. [DOI] [PubMed] [Google Scholar]
- 30.Zamarin D, et al. Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Sci Transl Med. 2014;6(226):226ra32. doi: 10.1126/scitranslmed.3008095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeanbart L, Kourtis IC, van der Vlies AJ, Swartz MA, Hubbell JA. 6-Thioguanine-loaded polymeric micelles deplete myeloid-derived suppressor cells and enhance the efficacy of T cell immunotherapy in tumor-bearing mice. Cancer Immunol Immunother. 2015;64(8):1033–1046. doi: 10.1007/s00262-015-1702-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jewell CM, López SCB, Irvine DJ. In situ engineering of the lymph node microenvironment via intranodal injection of adjuvant-releasing polymer particles. Proc Natl Acad Sci USA. 2011;108(38):15745–15750. doi: 10.1073/pnas.1105200108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manolova V, et al. Nanoparticles target distinct dendritic cell populations according to their size. Eur J Immunol. 2008;38(5):1404–1413. doi: 10.1002/eji.200737984. [DOI] [PubMed] [Google Scholar]
- 34.Reddy ST, et al. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat Biotechnol. 2007;25(10):1159–1164. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- 35.Liu H, et al. Structure-based programming of lymph-node targeting in molecular vaccines. Nature. 2014;507(7493):519–522. doi: 10.1038/nature12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jeanbart L, et al. Enhancing efficacy of anticancer vaccines by targeted delivery to tumor-draining lymph nodes. Cancer Immunol Res. 2014;2(5):436–447. doi: 10.1158/2326-6066.CIR-14-0019-T. [DOI] [PubMed] [Google Scholar]
- 37.Kourtis IC, et al. Peripherally administered nanoparticles target monocytic myeloid cells, secondary lymphoid organs and tumors in mice. PLoS One. 2013;8(4):e61646. doi: 10.1371/journal.pone.0061646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomas SN, Vokali E, Lund AW, Hubbell JA, Swartz MA. Targeting the tumor-draining lymph node with adjuvanted nanoparticles reshapes the anti-tumor immune response. Biomaterials. 2014;35(2):814–824. doi: 10.1016/j.biomaterials.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 39.Hirosue S, Kourtis IC, van der Vlies AJ, Hubbell JA, Swartz MA. Antigen delivery to dendritic cells by poly(propylene sulfide) nanoparticles with disulfide conjugated peptides: Cross-presentation and T cell activation. Vaccine. 2010;28(50):7897–7906. doi: 10.1016/j.vaccine.2010.09.077. [DOI] [PubMed] [Google Scholar]
- 40.Scott EA, et al. Dendritic cell activation and T cell priming with adjuvant- and antigen-loaded oxidation-sensitive polymersomes. Biomaterials. 2012;33(26):6211–6219. doi: 10.1016/j.biomaterials.2012.04.060. [DOI] [PubMed] [Google Scholar]
- 41.Stano A, Scott EA, Dane KY, Swartz MA, Hubbell JA. Tunable T cell immunity towards a protein antigen using polymersomes vs. solid-core nanoparticles. Biomaterials. 2013;34(17):4339–4346. doi: 10.1016/j.biomaterials.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 42.Cheever MA, Higano CS. PROVENGE (Sipuleucel-T) in prostate cancer: The first FDA-approved therapeutic cancer vaccine. Clin Cancer Res. 2011;17(11):3520–3526. doi: 10.1158/1078-0432.CCR-10-3126. [DOI] [PubMed] [Google Scholar]
- 43.Yu JS, et al. Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res. 2004;64(14):4973–4979. doi: 10.1158/0008-5472.CAN-03-3505. [DOI] [PubMed] [Google Scholar]
- 44.Trepiakas R, et al. Vaccination with autologous dendritic cells pulsed with multiple tumor antigens for treatment of patients with malignant melanoma: Results from a phase I/II trial. Cytotherapy. 2010;12(6):721–734. doi: 10.3109/14653241003774045. [DOI] [PubMed] [Google Scholar]
- 45.Chiang CLL, Ledermann JA, Rad AN, Katz DR, Chain BM. Hypochlorous acid enhances immunogenicity and uptake of allogeneic ovarian tumor cells by dendritic cells to cross-prime tumor-specific T cells. Cancer Immunol Immunother. 2006;55(11):1384–1395. doi: 10.1007/s00262-006-0127-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kandalaft LE, et al. Autologous lysate-pulsed dendritic cell vaccination followed by adoptive transfer of vaccine-primed ex vivo co-stimulated T cells in recurrent ovarian cancer. OncoImmunology. 2013;2(1):e22664. doi: 10.4161/onci.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ali OA, Emerich D, Dranoff G, Mooney DJ. In situ regulation of DC subsets and T cells mediates tumor regression in mice. Sci Transl Med. 2009;1(8):8ra19. doi: 10.1126/scitranslmed.3000359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ali OA, et al. Identification of immune factors regulating antitumor immunity using polymeric vaccines with multiple adjuvants. Cancer Res. 2014;74(6):1670–1681. doi: 10.1158/0008-5472.CAN-13-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kershaw MH, Westwood JA, Darcy PK. Gene-engineered T cells for cancer therapy. Nat Rev Cancer. 2013;13(8):525–541. doi: 10.1038/nrc3565. [DOI] [PubMed] [Google Scholar]
- 50.Gill S, June CH. Going viral: Chimeric antigen receptor T-cell therapy for hematological malignancies. Immunol Rev. 2015;263(1):68–89. doi: 10.1111/imr.12243. [DOI] [PubMed] [Google Scholar]
- 51.Stephan MT, Stephan SB, Bak P, Chen J, Irvine DJ. Synapse-directed delivery of immunomodulators using T-cell-conjugated nanoparticles. Biomaterials. 2012;33(23):5776–5787. doi: 10.1016/j.biomaterials.2012.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang B, et al. Active targeting of chemotherapy to disseminated tumors using nanoparticle-carrying T cells. Sci Transl Med. 2015;7(291):291ra94. doi: 10.1126/scitranslmed.aaa5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Valton J, et al. A multidrug resistant engineered CAR T cell for allogeneic combination immunotherapy. Mol Ther. 2015;23(9):1507–1518. doi: 10.1038/mt.2015.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci USA. 2010;107(9):4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pentcheva-Hoang T, Simpson TR, Montalvo-Ortiz W, Allison JP. Cytotoxic T lymphocyte antigen-4 blockade enhances antitumor immunity by stimulating melanoma-specific T-cell motility. Cancer Immunol Res. 2014;2(10):970–980. doi: 10.1158/2326-6066.CIR-14-0104. [DOI] [PubMed] [Google Scholar]
- 56.Twyman-Saint Victor C, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520(7547):373–377. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Honda T, et al. Tuning of antigen sensitivity by T cell receptor-dependent negative feedback controls T cell effector function in inflamed tissues. Immunity. 2014;40(2):235–247. doi: 10.1016/j.immuni.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Woo SR, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72(4):917–927. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Matsuzaki J, et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci USA. 2010;107(17):7875–7880. doi: 10.1073/pnas.1003345107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Powles T, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515(7528):558–562. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 61.Phan GQ, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci USA. 2003;100(14):8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Attia P, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23(25):6043–6053. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: A common denominator approach to cancer therapy. Cancer Cell. 2015;27(4):450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Topalian SL, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hirt C, et al. “In vitro” 3D models of tumor-immune system interaction. Adv Drug Deliv Rev. 2014;79-80:145–154. doi: 10.1016/j.addr.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 66.Pulendran B. Systems vaccinology: Probing humanity’s diverse immune systems with vaccines. Proc Natl Acad Sci USA. 2014;111(34):12300–12306. doi: 10.1073/pnas.1400476111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19(11):1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5(4):263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 69.Gajewski TF, Schreiber H, Fu Y-X. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14(10):1014–1022. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Teng MWL, Ngiow SF, Ribas A, Smyth MJ. Classifying cancers based on T-cell infiltration and PD-L1. Cancer Res. 2015;75(11):2139–2145. doi: 10.1158/0008-5472.CAN-15-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Taube JM, et al. Differential expression of immune-regulatory genes associated with PD-L1 display in melanoma: Implications for PD-1 pathway blockade. Clin Cancer Res. 2015;21(17):3969–3976. doi: 10.1158/1078-0432.CCR-15-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Snyder A, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371(23):2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Andersen RS, et al. Parallel detection of antigen-specific T cell responses by combinatorial encoding of MHC multimers. Nat Protoc. 2012;7(5):891–902. doi: 10.1038/nprot.2012.037. [DOI] [PubMed] [Google Scholar]
- 74.Motz GT, et al. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat Med. 2014;20(6):607–615. doi: 10.1038/nm.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Munn DH, Mellor AL. The tumor-draining lymph node as an immune-privileged site. Immunol Rev. 2006;213(1):146–158. doi: 10.1111/j.1600-065X.2006.00444.x. [DOI] [PubMed] [Google Scholar]
- 76.Hood JL, San RS, Wickline SA. Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res. 2011;71(11):3792–3801. doi: 10.1158/0008-5472.CAN-10-4455. [DOI] [PubMed] [Google Scholar]
- 77.Dubrot J, et al. Lymph node stromal cells acquire peptide-MHCII complexes from dendritic cells and induce antigen-specific CD4⁺ T cell tolerance. J Exp Med. 2014;211(6):1153–1166. doi: 10.1084/jem.20132000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fletcher AL, et al. Lymph node fibroblastic reticular cells directly present peripheral tissue antigen under steady-state and inflammatory conditions. J Exp Med. 2010;207(4):689–697. doi: 10.1084/jem.20092642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hirosue S, et al. Steady-state antigen scavenging, cross-presentation, and CD8+ T cell priming: A new role for lymphatic endothelial cells. J Immunol. 2014;192(11):5002–5011. doi: 10.4049/jimmunol.1302492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lund AW, et al. VEGF-C promotes immune tolerance in B16 melanomas and cross-presentation of tumor antigen by lymph node lymphatics. Cell Reports. 2012;1(3):191–199. doi: 10.1016/j.celrep.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 81.Moltzahn F, et al. Microfluidic-based multiplex qRT-PCR identifies diagnostic and prognostic microRNA signatures in the sera of prostate cancer patients. Cancer Res. 2011;71(2):550–560. doi: 10.1158/0008-5472.CAN-10-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kwong GA, et al. Mass-encoded synthetic biomarkers for multiplexed urinary monitoring of disease. Nat Biotechnol. 2013;31(1):63–70. doi: 10.1038/nbt.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Warren AD, Kwong GA, Wood DK, Lin KY, Bhatia SN. Point-of-care diagnostics for noncommunicable diseases using synthetic urinary biomarkers and paper microfluidics. Proc Natl Acad Sci USA. 2014;111(10):3671–3676. doi: 10.1073/pnas.1314651111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Danino T, et al. Programmable probiotics for detection of cancer in urine. Sci Transl Med. 2015;7(289):289ra84. doi: 10.1126/scitranslmed.aaa3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ozkumur E, et al. Inertial focusing for tumor antigen-dependent and -independent sorting of rare circulating tumor cells. Sci Transl Med. 2013;5(179):179ra47. doi: 10.1126/scitranslmed.3005616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Balsam J, Bruck HA, Rasooly A. Cell streak imaging cytometry for rare cell detection. Biosens Bioelectron. 2015;64(c):154–160. doi: 10.1016/j.bios.2014.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tse HTK, et al. Quantitative diagnosis of malignant pleural effusions by single-cell mechanophenotyping. Sci Transl Med. 2013;5(212):212ra163. doi: 10.1126/scitranslmed.3006559. [DOI] [PubMed] [Google Scholar]
- 88.Li P, et al. Acoustic separation of circulating tumor cells. Proc Natl Acad Sci USA. 2015;112(16):4970–4975. doi: 10.1073/pnas.1504484112. [DOI] [PMC free article] [PubMed] [Google Scholar]