Significance
The massive cell death, associated with a heart attack, is mainly due to disruption of mitochondrial membrane integrity upon activation of the mitochondrial permeability transition pore. Thus, it is important to understand how this pore is regulated to prevent cardiac cell death. In this study, we reported that hematopoietic-substrate-1 associated protein X-1 (HAX-1) is an inhibitor of the pore and promotes cell survival. HAX-1 works through recruitment of a chaperone protein called Hsp90 from cyclophilin-D, a major component of the pore. Displacement of Hsp90 from cyclophilin-D promotes cyclophilin-D degradation, resulting in inhibition of pore opening and cell death. Given that the opening of the mitochondrial permeability transition pore contributes to various diseases, our findings have broader applications reaching beyond the heart.
Keywords: HAX-1, necrosis, mitochondrial permeability transition, cyclophilin-D, heat shock protein-90
Abstract
The major underpinning of massive cell death associated with myocardial infarction involves opening of the mitochondrial permeability transition pore (mPTP), resulting in disruption of mitochondria membrane integrity and programmed necrosis. Studies in human lymphocytes suggested that the hematopoietic-substrate-1 associated protein X-1 (HAX-1) is linked to regulation of mitochondrial membrane function, but its role in controlling mPTP activity remains obscure. Herein we used models with altered HAX-1 expression levels in the heart and uncovered an unexpected role of HAX-1 in regulation of mPTP and cardiomyocyte survival. Cardiac-specific HAX-1 overexpression was associated with resistance against loss of mitochondrial membrane potential, induced by oxidative stress, whereas HAX-1 heterozygous deficiency exacerbated vulnerability. The protective effects of HAX-1 were attributed to specific down-regulation of cyclophilin-D levels leading to reduction in mPTP activation. Accordingly, cyclophilin-D and mPTP were increased in heterozygous hearts, but genetic ablation of cyclophilin-D in these hearts significantly alleviated their susceptibility to ischemia/reperfusion injury. Mechanistically, alterations in cyclophilin-D levels by HAX-1 were contributed by the ubiquitin-proteosomal degradation pathway. HAX-1 overexpression enhanced cyclophilin-D ubiquitination, whereas proteosomal inhibition restored cyclophilin-D levels. The regulatory effects of HAX-1 were mediated through interference of cyclophilin-D binding to heat shock protein-90 (Hsp90) in mitochondria, rendering it susceptible to degradation. Accordingly, enhanced Hsp90 expression in HAX-1 overexpressing cardiomyocytes increased cyclophilin-D levels, as well as mPTP activation upon oxidative stress. Taken together, our findings reveal the role of HAX-1 in regulating cyclophilin-D levels via an Hsp90-dependent mechanism, resulting in protection against activation of mPTP and subsequent cell death responses.
The dynamics of contraction and relaxation in the heart are highly regulated by a finely tuned cross-talk between demand by the periphery and energy production by the mitochondria. The heart relies on mitochondria for fueling ATP on a beat-to-beat basis and for adjusting mitochondrial metabolism to meet the increases in cardiac output during exercise and flight-or-fight responses (1, 2). Mitochondria are also essential in controlling cell death through apoptosis, necrosis, and autophagy (3, 4). Indeed, disruption of mitochondrial integrity underlies cardiomyocyte death associated with myocardial infarction (5) that attributes to one of every six deaths in the United States (6).
The mitochondrial hematopoietic-substrate-1 associated protein X-1 (HAX-1), a 35-kDa protein has recently emerged as a critical regulator of cell survival in various tissues (7–9). Indeed, global ablation of HAX-1 was shown to result in death by 14 wk of age due to neuronal degeneration from excessive cell death (10). In addition, a recent study demonstrated that degradation of HAX-1 triggers cell death in human B-cell lymphoma, further supporting the pivotal role of HAX-1 in dictating cell survival (11). Although HAX-1 has been studied in various cell types since its discovery in 1997 (7), its presence in cardiomyocytes was only recently identified. Interestingly, cardiac HAX-1 localizes not only to mitochondria but also the endo/sarcoplasmic reticulum (ER/SR) and it binds to phospholamban, increasing inhibition of the SR calcium transport ATPase and reducing SR calcium cycling. As cardiomyocyte contraction-relaxation is coupled to calcium oscillation, HAX-1 is an important regulator of cardiomyocyte contractility (12, 13). HAX-1 can also modulate ER stress responses by inhibiting the inositol requiring enzyme-1 (IRE-1) signaling arm, which diminishes cell death through reduction of proapoptotic transcription factor expression and caspase activation in an ischemic insult, leading to improved contractile recovery during reperfusion (14). However, HAX-1 also localizes to mitochondria in the heart (13), but its role in this organelle is unknown. Importantly, human mutations in HAX-1 have been linked to mitochondrial function. These mutations, resulting in loss of protein or activity, were discovered in a subgroup of severe neutropenia patients (8, 15, 16) and they were associated with loss of mitochondrial membrane potential in the neutrophils of the human carriers (16). Thus, HAX-1 deficiency may cause a defect in the mitochondrial membrane.
One of the mechanisms that regulate mitochondrial membrane integrity is the mitochondria permeability transition pore (mPTP), a large conductance channel that spans through the outer and inner membranes of mitochondria and becomes activated upon oxidative stress, mitochondrial calcium overload, or alkaline intracellular environment. The opening of this pore during an ischemia/reperfusion insult underlies the etiology of cardiac injury (5), because it allows solutes and ions that should be excluded from mitochondria to infuse into their matrix. Concomitantly, influx of water results in swelling of the originally compacted cristae structure, leading to complete mitochondria membrane failure and subsequent activation of massive necrotic cell death and fibrosis (5, 17). This injury is irreversible, posing greater damage to organs that possess limited regenerative capacity, such as the heart (5). Although the mitochondrial permeability transition pore was first described in 1976 (18), the full identity of its protein components remains obscure. Several constituents have been proposed (17), but only cyclophilin-D (Cyp-D), a peptidyl-prolylcis-trans isomerase (PPI) in the mitochondrial matrix, has been a genetically proven regulator of mPTP (19). Ablation of this protein by gene targeting or pharmacological inhibition by cyclosporine A can effectively prevent the opening of the pore and inhibit the activation of cell death during ischemia/reperfusion injury (17, 19), demonstrating the crucial role of Cyp-D function in cell survival. Furthermore, Cyp-D activity in cancer cells can be regulated by a mitochondrial chaperone network that involves the cytosolic heat shock protein-90 (Hsp90) and its mitochondria analogs, which are crucial in inducing cancer cell death (20).
Based on our recent observations that Hsp90 is a newly identified binding partner of HAX-1 (14), it was intriguing to speculate that the Hsp90/HAX-1 interaction may be also involved in regulation of Cyp-D function in the heart. Thus, the current study was designed to address this hypothesis by using mouse models with alterations in HAX-1 levels. Our findings indicate that HAX-1 could specifically reduce Cyp-D levels by enhancing its ubiquitination and proteosomal degradation, which, in turn, protected mitochondria from oxidative stress and calcium overload. These beneficial effects of HAX-1 were mediated through Hsp90, which appeared to stabilize Cyp-D protein in cardiomyocytes. Taken together, our results reveal a novel mechanism by which the HAX-1/Hsp90 complex may inhibit activation of the mPTP via regulation of Cyp-D levels, and promote cardiomyocyte survival upon oxidative stress and calcium overload in ischemia/reperfusion injury.
Results
HAX-1 Preserves Mitochondria Membrane Integrity and Inhibits Cell Death.
The integrity of the mitochondrial membrane is crucial in maintaining cardiomyocyte function and survival. Increases in the generation of reactive oxygen species or mitochondrial calcium overload during cardiac stress, such as ischemia/reperfusion injury, can disrupt mitochondrial membrane integrity, dissipate membrane potential for ATP production, and cause massive cell death (5). HAX-1 has recently emerged as a regulator of cardioprotection, and we have shown that it localizes to mitochondria besides ER/SR (13). To further address its localization, we isolated mitochondria (Fig. S1) and subjected them to protease accession. HAX-1 was present in the inner mitochondrial membrane (Fig. S2), consistent with previous reports (10, 21). Thus, we sought to investigate whether HAX-1 may regulate mitochondria membrane potential and control activation of cell death. To examine this hypothesis, cardiomyocytes were isolated from HAX-1 overexpressing (HAX-OE, 2.5-fold expression; Fig. 1A) (13), wild-type (WT), and HAX-1 heterozygous knockout (HAX+/−, 40% protein expression; Fig. 1A) (13) mice, and they were loaded with a mitochondrial localized florescent membrane potential indicator, TMRE, allowing us to monitor the membrane potential before and after hydrogen peroxide challenge, using confocal microscopy. Under basal conditions, the percentage of TMRE-positive cardiomyocytes was similar among the three groups (Fig. 1 B and C). However, after administration of 2 mM hydrogen peroxide to induce oxidative challenge, both WT and HAX heterozygous cardiomyocytes lost some florescence, and by 20 min, only 30% of the heterozygous cells maintained the TMRE signal, compared with 50% of WT. In contrast, more than 70% of the HAX-overexpressing cardiomyocytes were resistant to 20 min of oxidative challenge (Fig. 1 B and C). These findings suggest a protective role of HAX-1 in maintaining mitochondrial membrane potential during oxidative stress.
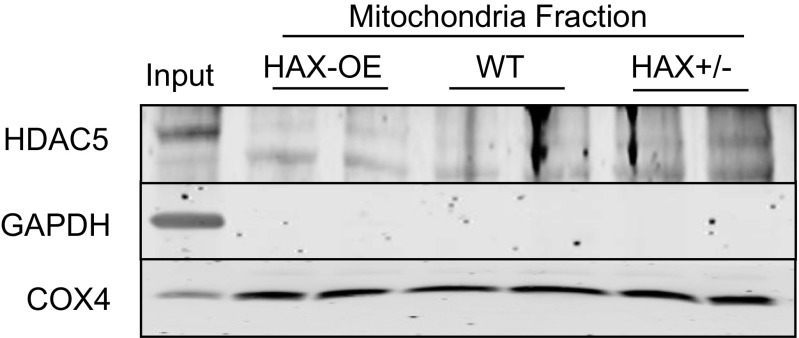
Fig. S1.
The purity of the mitochondrial fraction. Based on the use of HDAC5, GAPDH, and COX4 as nuclear, cytosolic and mitochondrial markers, respectively, the purity of mitochondrial fraction appeared high.
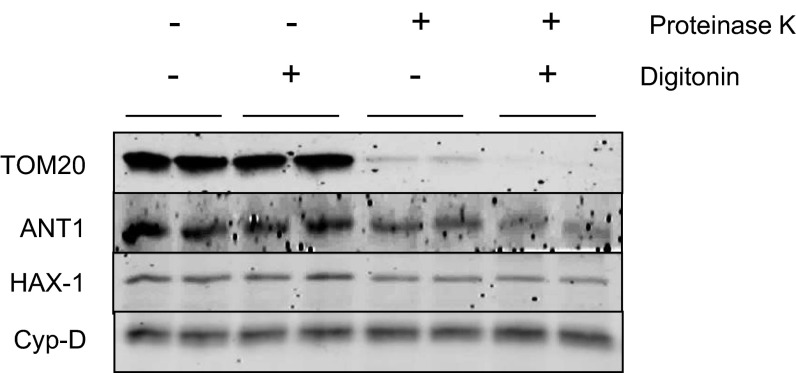
Fig. S2.
HAX-1 is localized to mitochondria. Isolated WT mouse mitochondria were subjected to protein digestion by proteinase K in the presence or absence of the outer membrane disruptor, digitonin. Translocase of the outer membrane 20 Homolog Type (TOM20), adenine nucleotide translocase 1 (ANT1), and cyclophilin-D (Cyp-D) were used as markers for mitochondrial outer membrane, inner membrane, and matrix, respectively. Proteinase K can only digest proteins that are accessible and not protected by the mitochondrial membrane. HAX-1 was not digested by proteinase K when the outer membrane was disrupted by digitonin, suggesting that it is localized to the inner membrane of mitochondria.
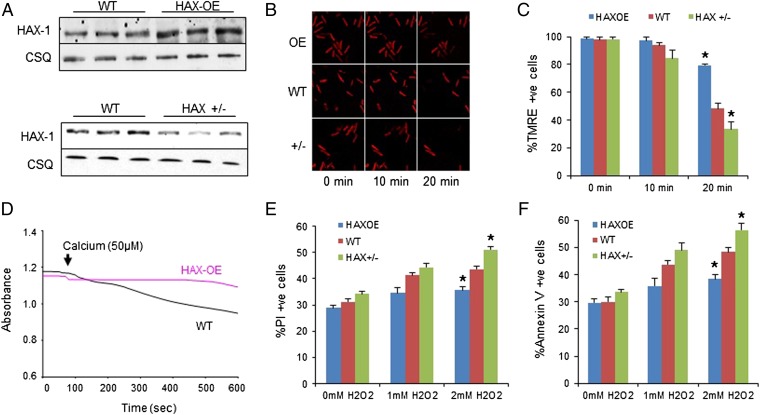
Fig. 1.
Overexpression of HAX-1 protects mitochondrial membrane integrity against oxidative stress and calcium overload, whereas heterozygous ablation of HAX-1 has opposite effects. (A) HAX-1 protein expression in HAX-OE and HAX+/− hearts. CSQ was used as loading control. (B and C) Isolated WT, HAX-OE, and HAX+/− cardiomyocytes were loaded with mitochondrial membrane potential fluorescent dye, TMRE. Upon 2 mM H2O2 administration for 20 min, membrane potential was diminished in all groups, but this effect was most pronounced in the heterozygous cells. HAX-OE exhibited more than 80% preserved TMRE signals. n = 7 hearts for HAX-OE, 16 hearts for WT, and 9 hearts for HAX+/− (>8 cells per heart); *P < 0.05 vs. WT at 20 min. (D) HAX-1 overexpressing mitochondria demonstrated resistance to swelling induced by 50 μM calcium. n = 3 hearts for each group. (E and F) HAX-1 overexpression in cardiomyocytes prevented cell death due to loss of plasma membrane integrity (E) and apoptosis (F) after 20 min of 2 mM H2O2 treatment, whereas HAX-1 heterozygous ablation increased cell death. n = 8 hearts for HAX-OE, 12 hearts for WT, and 13 hearts for HAX+/− (>10,000 cells per heart); *P < 0.05 vs. WT at 2 mM H2O2. Data are expressed as mean ± SEM.
The protective effects of HAX-1 on mitochondrial integrity were also evidenced upon calcium overload, a situation that induces the opening of the permeability transition pore (mPTP), leading to failure of osmotic pressure control, water influx, and swelling of mitochondria (17). Isolated WT cardiac mitochondria demonstrated a steady loss of light scattered by the mitochondrial membrane structure after the addition of 50 mM calcium. However, HAX-1 overexpressing mitochondria were resistant under the same conditions and showed no sign of swelling (Fig. 1D). In contrast, isolated HAX-1 heterozygous-deficient mitochondria exhibited significant swelling under basal conditions. These findings together with the results from the hydrogen peroxide treatment indicate that HAX-1 may protect the mitochondrial membrane integrity.
To determine whether the protection of mitochondrial membrane by HAX-1 would translate to improved cell survival, we used isolated cardiomyocytes and assessed the degree of propidium iodide (PI) inclusion as an indicator of necrotic cell death. Treatment of cells with either 1 or 2 mM hydrogen peroxide for 20 min resulted in increases in Propidium iodide-positive cells. Notably, 2 mM hydrogen peroxide, which was associated with loss of mitochondria membrane integrity (Fig. 1C), resulted in a 15% increase in PI-positive WT cardiomyocytes, but this increase was significantly blunted in HAX-1 overexpressing cells. However, heterozygous loss of HAX-1 exacerbated the increase in cells with propidium iodide inclusion (Fig. 1E), consistent with the findings in the TMRE experiments. Similar results were obtained, when the expression of cell surface annexin V was quantified, as a marker of apoptotic cell death. Increasing HAX-1 levels effectively reduced apoptosis, whereas loss of HAX-1 elevated its activation under hydrogen peroxide stress (Fig. 1F). Thus, our data suggest that HAX-1 maintains the mitochondrial membrane potential and protects membrane integrity, resulting in reduced apoptosis and necrotic cardiomyocyte death.
HAX-1 Regulates the Opening of Mitochondrial Permeability Transition Pore.
It has been demonstrated that mitochondrial membrane integrity highly depends on the function of mPTP. The opening of this pore is a key mediator of cardiac ischemia/reperfusion injury because it facilitates loss of mitochondrial membrane potential, induces swelling, and leads to the rupture of mitochondrial membrane and activation of cell death (5). To determine whether the HAX-1 regulatory effects in mitochondria are mediated through an mPTP-dependent mechanism, we assessed the effects of mPTP inhibition in cardiomyocytes. To this end, we treated cardiomyocytes with cyclosporine A (CsA), an mPTP inhibitor (17). CsA resulted in preservation of mitochondrial membrane potential in both WT and HAX-1 heterozygous cardiomyocytes exposed to hydrogen peroxide (Fig. 2 A and B). Importantly, the levels of TMRE in these treated groups were similar to those in HAX-1 overexpressing cells (Fig. 1B), suggesting that the protective effects of HAX-1 may be mediated through the mPTP. To further confirm that the observed protection against loss of mitochondrial membrane potential by HAX-1 overexpression was not due to impaired function of the mPTP, we used a high calcium concentration (375 mM) in the swelling assay to induce mPTP opening in isolated mitochondria. Under these conditions, the swelling response of the HAX-1 overexpressing mitochondria was similar to that of WTs (Fig. 2C), suggesting that HAX-1 regulates the sensitivity of the mPTP opening. To further address this hypothesis, we determined the dose of cyclosporine A required for complete inhibition of mitochondrial swelling, induced by 375 mM calcium. Both WT and HAX-1 overexpressing mitochondria were resistant to swelling in the presence of 1 μM cyclosporine A. However, upon dose reduction to 0.5 μM, WT mitochondria exhibited swelling in a time-dependent manner, whereas HAX-1 overexpressing mitochondria maintained their resistance (Fig. 2D). These findings further suggest that overexpression of HAX-1 reduces the sensitivity of mPTP opening in cardiomyocytes.
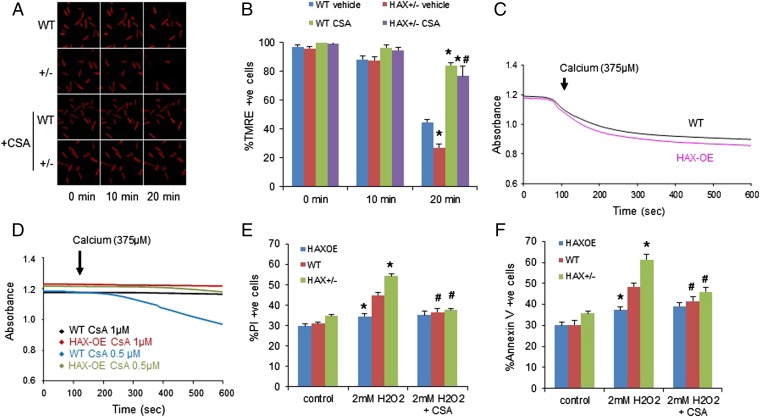
Fig. 2.
HAX-1 regulates the mitochondrial permeability transition pore. (A and B) Cyclosporine A abrogated the detrimental effect of HAX-1 heterozygous ablation on mitochondrial membrane potential after H2O2 treatment for 20 min. n = 4–8 hearts (>12 cells per heart); *P < 0.05 vs. WT with vehicle (DMSO) at 20 min; #P < 0.05 vs. HAX+/− with vehicle at 20 min. (C) Swelling induced by high calcium concentration in HAX-OE and WT mitochondria. n = 3 hearts for each group. (D) Inhibition of mitochondrial swelling required a lower cyclosporine A dose in HAX-1 overexpressing mitochondria. n = 3 hearts for each group. (E and F) Cyclosporine A abrogated the effect of HAX-1 on necrotic (E) and apoptotic (F) cell death. n = 8–15 hearts (>10,000 cells per heart); *P < 0.05 vs. WT at 2 mM H2O2; #P < 0.05 vs. the respective untreated group at 2 mM H2O2. Data are expressed as mean ± SEM.
We also applied cyclosporine A before hydrogen peroxide challenge and assessed the degree of cell death activation in isolated cardiomyocytes to confirm that the prosurvival effects of HAX-1 under the hydrogen peroxide treatment depended on the opening of mPTP. Indeed, this pretreatment completely abolished the differences between the three groups regarding the number of cells with reduced plasma membrane integrity (Fig. 2E). Accordingly, apoptotic cell death was also reduced in WT and HAX-1 heterozygous cardiomyocytes (Fig. 2F) by CsA inhibition of mPTP, and the values in the three groups were similar. Thus, regulation of mPTP activity appears to play a key role in the cell survival mechanisms mediated by HAX-1.
HAX-1 Regulates Cardiac Cyclophilin-D Protein Levels.
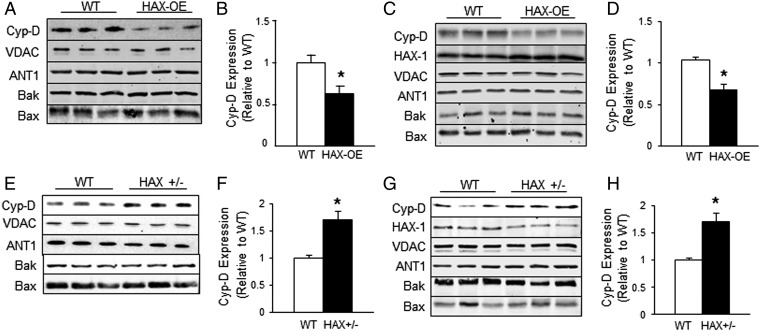
Because cyclosporine A is a direct pharmacological inhibitor of cyclophilin-D (17), an important regulator of the permeability transition pore, we hypothesized that the reduced dose of CsA required for complete blockade of swelling in HAX-1 overexpressing mitochondria may be associated with decreases in Cyp-D level or activity. Indeed, quantitative immunoblotting indicated that the Cyp-D levels in HAX-1 overexpressing hearts were reduced by 40% compared with WTs (Fig. 3 A and B). Interestingly, the effects of HAX-1 appeared specific for Cyp-D, because the expression levels of other proposed mPTP components, namely the voltage-dependent anion channel (VDAC) and adenine nucleotide translocase (ANT1), were not altered (Fig. 3A). Furthermore, the expression levels of Bax and Bak, which were recently suggested to govern the porosity of the mitochondrial outer membrane (22), were not altered by HAX-1 overexpression either. These findings in cardiac homogenates were also similar to those obtained in isolated mitochondria (Fig. 3 C and D). Thus, we speculated that if HAX-1 can negatively regulate Cyp-D expression, then loss of HAX-1 should have a positive effect on Cyp-D levels. Indeed, heterozygous-deficient hearts with 60% loss of HAX-1 (13) exhibited a 70% increase in Cyp-D levels (Fig. 3 E and F). Similar findings were observed with isolated mitochondria (Fig. 3 G and H). There were no alterations in the levels of VDAC, ANT1, Bax, and Bak (Fig. 3 E and G), similar to HAX-1 overexpression. Thus, the protective effect of HAX-1 on mPTP opening may be attributed to the reduction of Cyp-D expression in the mitochondria.
Fig. 3.
HAX-1 specifically alters cyclophilin-D levels in the heart. (A and B) Cyp-D levels were reduced in HAX-OE cardiac homogenates. n = 4 hearts for each group. (C and D) Cyp-D levels were reduced in HAX-OE cardiac mitochondrial fraction. n = 5 hearts for HAX-OE, 6 hearts for WT. (E and F) Cyp-D levels were increased in HAX +/− cardiac homogenates. n = 6 hearts for each group. (G and H) Cyp-D levels were increased in the HAX+/− mitochondrial fraction. n = 9 hearts for each group. *P < 0.05 vs. WT. Data are expressed as mean ± SEM.
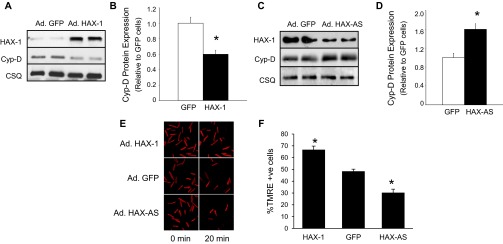
To exclude the possibility that reduction of Cyp-D level was an indirect compensatory response to chronic HAX-1 overexpression in vivo, we used adenoviral delivery to acutely increase HAX-1 levels in cardiomyocytes. At 24 h after infection, HAX-1 overexpression (15-fold increase in HAX-1 levels) was associated with a 40% decrease in Cyp-D levels (Fig. S3 A and B). Accordingly, acute down-regulation of HAX-1 by 55%, using an antisense approach, led to a 60% increase in Cyp-D expression levels in cardiomyocytes (Fig. S3 C and D). The acute alterations in Cyp-D levels reflected parallel changes in the TMRE responses upon hydrogen peroxide challenge. Cardiomyocytes with increased HAX-1 levels showed enhanced resistance to loss of mitochondria membrane potential, whereas down-regulation of HAX-1 led to increased dissipation of proton gradient (Fig. S3 E and F). These findings coupled with the ones above in animal models suggest that HAX-1 may be a regulator of cardiac Cyp-D expression levels.
Fig. S3.
HAX-1 alters cyclophilin-D levels in infected cardiomyocytes. (A and B) Cyclophilin-D levels were reduced when HAX-1 was acutely overexpressed in rat cardiomyocytes. n = 4; *P < 0.05 vs. GFP. (C and D) Cyclophilin-D levels were increased when HAX-1 was acutely down-regulated in rat cardiomyocytes. n = 4; *P < 0.05 vs. GFP. (E and F) Mitochondrial membrane potential was protected with increased HAX-1 expression levels. n = 6; *P < 0.05 vs. GFP.
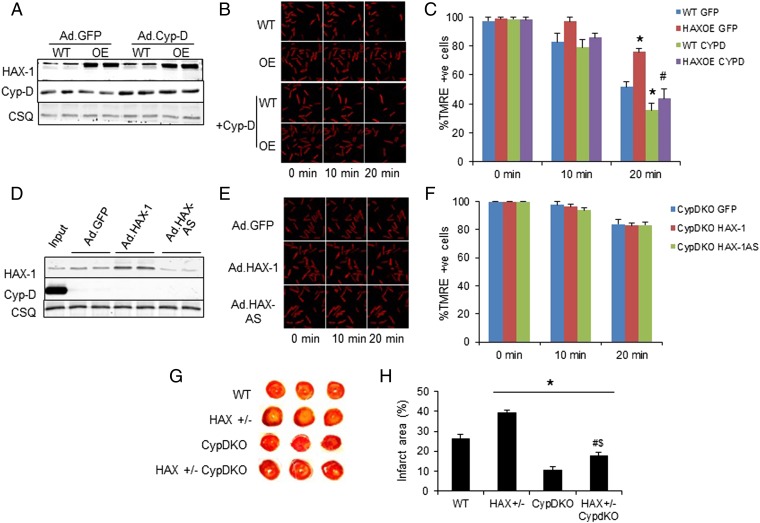
To further delineate the contribution of reduced Cyp-D levels in the protective effects of HAX-1 on mPTP opening and membrane potential, we used two complementary approaches and examined the effects of altered Cyp-D expression on the TMRE responses upon hydrogen peroxide challenge. First, we infected adult mouse WT and HAX-1 overexpressing cardiomyocytes with an adenovirus-encoding Cyp-D cDNA. Increased Cyp-D protein levels to a similar extent between WT and transgenic cells (Fig. 4A) effectively abolished the protective effects of HAX-1 overexpression on mPTP opening (Fig. 4 B and C). Second, we isolated cardiomyocytes from the Cyp-D knockout (or PPIF null) mouse and infected them with adenoviruses carrying HAX-1 sense or antisense cDNA (Ad.HAX-1 or Ad.HAX-AS) to alter HAX-1 expression levels (Fig. 4D). In the absence of Cyp-D, the alterations in HAX-1 levels did not affect the TMRE response to hydrogen peroxide challenge (Fig. 4 E and F). Collectively, the regulatory effects of HAX-1 on mPTP sensitivity appear to be mediated through alterations of Cyp-D levels.
Fig. 4.
The effects of HAX-1 are mainly mediated by cyclophilin-D. (A) Cyp-D expression was increased to similar levels in WT and HAX-OE cells after adenoviral infection of adult rat cardiomyocytes. (B and C) Adenoviral delivery of Cyp-D removed the protection mediated by HAX-1 overexpression upon 2 mM hydrogen peroxide administration. CSQ was used as a loading control. n = 4–6 hearts (>12 cells per heart); *P < 0.05 vs. WT GFP at 2 min; #P < 0.05 vs. HAX-OE GFP at 20 min. (D) HAX-1 levels after adenoviral delivery of Ad.GFP, Ad.HAX-1, and Ad.HAX-AS in CypD-KO cells. WT cardiac homogenate was loaded to serve as input. (E and F) Cyp-D ablation abolished the effect of different HAX-1 levels on the maintenance of mitochondrial membrane potential upon 2 mM hydrogen peroxide challenge. CSQ was used as a loading control. n = 4–5 hearts (>16 cells per heart). (G and H) Cyp-D ablation significantly reduced infarct size in HAX-1 heterozygous knockout hearts. n = 4 for each group; *P < 0.05 vs. WT; #P < 0.05 vs. HAX+/−; $P < 0.05 vs. CypD-KO. Data are expressed as mean ± SEM.
We previously reported that reduction in HAX-1 levels have detrimental effects in the heart, when subjected to ischemia/reperfusion injury. Because mPTP has also been shown to play an important role in this injury, we examined whether the effects of HAX-1 may be mediated through Cyp-D. To this end, we crossed the Cyp-D knockout mouse with the HAX+/− model (CypD-KO/HAX+/−) and obtained HAX-1 heterozygous mice that were also deficient in Cyp-D. Hearts from the WT, HAX+/−, CypD-KO, and the cross model were then subjected to global no-flow ischemia (40 min), followed by reperfusion (60 min) in the Langendorff perfusion mode. This protocol induced a 26% infarct size in WT hearts (Fig. 4 G and H), consistent with previous studies (14). Heterozygous loss of HAX-1 increased myocardial infarction to 40%, whereas ablation of Cyp-D significantly diminished it to 11% (Fig. 4 G and H). Importantly, ablation of Cyp-D in HAX+/− hearts resulted in significant reduction of infarct size from 40 to 18% (Fig. 4 G and H), suggesting that mPTP plays a critical role in the protective effects of HAX-1 in cardiac ischemia/reperfusion injury.
HAX-1 Regulates Cyclophilin-D Levels Through Hsp90.
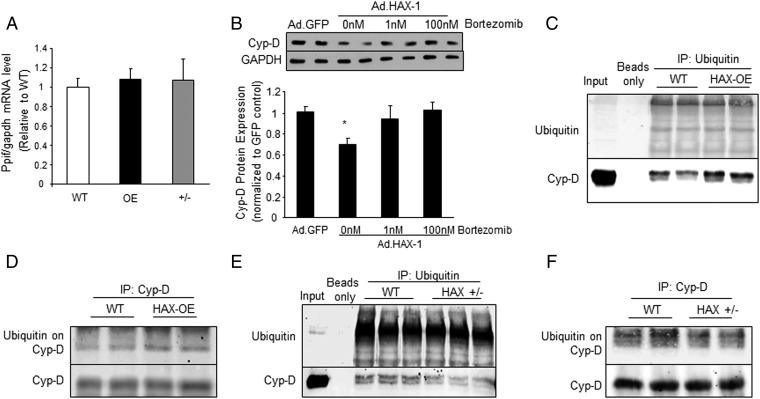
To determine whether the effects of HAX-1 were mediated at the transcriptional level, we assessed cyclophilin-D (or ppif) mRNA expression in WT, HAX-OE, and HAX+/− cardiac homogenates (Fig. 5A). There were no alterations among the three groups, indicating that HAX-1 does not regulate Cyp-D transcription. We then hypothesized that HAX-1 modulates the Cyp-D protein levels specifically through its degradation by the proteosomal pathway. To test this hypothesis, we acutely increased HAX-1 expression in adult rat cardiomyocytes by adenoviral delivery (Ad.HAX-1) and subsequently treated the cells with bortezomib, the well-recognized proteosomal inhibitor in cancer cells (23, 24). HAX-1 overexpression decreased Cyp-D levels as expected, but the effect was abolished by bortezomib, suggesting that HAX-1 regulates proteosomal degradation of Cyp-D (Fig. 5B). Because protein degradation is known to be controlled by ubiquitination (25), we sought to examine whether HAX-1 may affect Cyp-D ubiquitination. Thus, HAX-OE or HAX+/− cardiac homogenates were subjected to immunoprecipitation studies, using either ubiquitin or Cyp-D as a bait. Indeed, HAX-1 overexpression markedly increased Cyp-D ubiquitination (Fig. 5 C and D), whereas heterozygous ablation of HAX-1 resulted in less ubiquitinated Cyp-D, compared with WTs (Fig. 5 E and F). These findings indicate that HAX-1 regulates the degradation of Cyp-D protein through the ubiquitination-proteosomal pathway.
Fig. 5.
HAX-1 enhances the degradation of cyclophilin-D. (A) Cyp-D mRNA transcript, or ppif levels were similar in WT, HAX-OE, and HAX+/− hearts. n = 4 hearts for WT, and 5 hearts for each of the HAX-OE and HAX+/−. (B) Proteasomal inhibitor, Bortezomib, restored Cyp-D levels in HAX-1 overexpressing cells. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a loading control. n = 4 hearts for each group; *P < 0.05 vs. Ad.GFP. (C and D) Ubiquitination of Cyp-D was enhanced in HAX-OE hearts. Three independent experiments were performed. n = 4 hearts for each group. (E and F) Ubiquitination of Cyp-D was reduced in HAX+/− hearts. Three independent experiments were performed. n = 4 hearts for each group. Data are expressed as mean ± SEM.
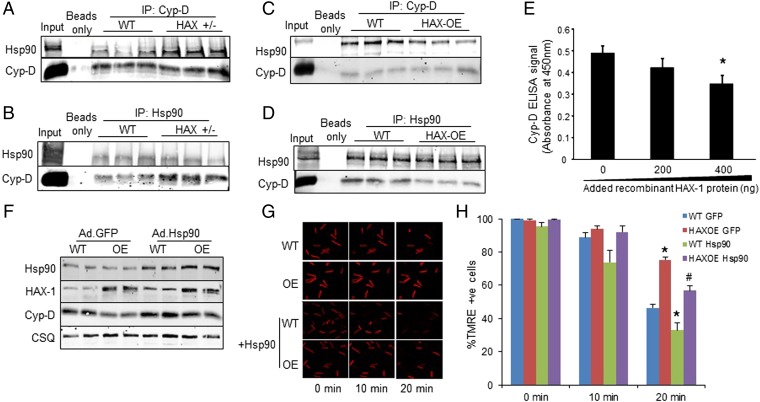
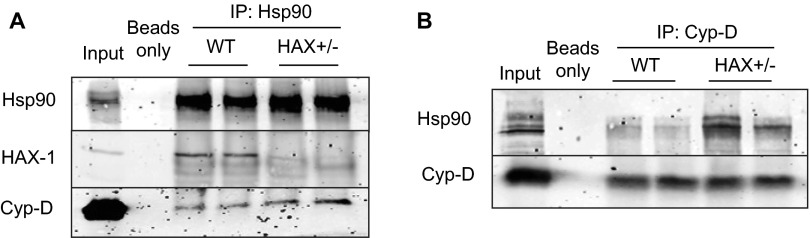
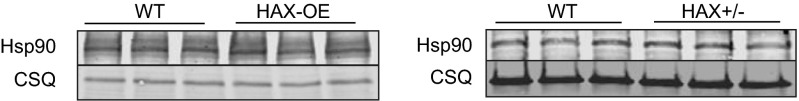
Previous studies in mitochondria isolated from various cell types have shown that Cyp-D interacts with the heat shock protein 90 (Hsp90) in the matrix (20). Interestingly, we also recently found that Hsp90 is a binding partner of HAX-1 in the heart (14). These findings prompted us to investigate whether the observed effects of HAX-1 on Cyp-D levels may involve Hsp90. Because the role of Hsp90 on Cyp-D is not known, we acutely overexpressed Hsp90 in adult cardiomyocytes and determined the expressions of Cyp-D. We observed a significant increase in Cyp-D protein levels (Fig. S4) and reduction in its ubiquitination (Fig. S5), suggesting that Hsp90 may serve as a chaperone for Cyp-D and prevent its degradation. This notion was further supported by immunoprecipitation studies in cardiac homogenates from HAX-1 heterozygous-deficient hearts, which exhibit increases in Cyp-D levels. Using Cyp-D as bait, there was an increase in the amount of Hsp90 associated with Cyp-D, compared with WTs (Fig. 6A). A similar increase in the Cyp-D levels was observed when Hsp90 was used as bait (Fig. 6B). On the contrary, overexpression of HAX-1 resulted in reduced Hsp90/Cyp-D association, using either Hsp90 or Cyp-D as bait in the cardiac homogenates (Fig. 6 C and D). These alterations appear to occur in mitochondria, because similar findings were observed when isolated mitochondrial fractions from HAX-OE and HAX+/− hearts were used for immunoprecipitation experiments (Figs. S6 and S7). Notably, the changes in Hsp90/Cyp-D complex formation were not associated with alterations in cardiac or mitochondrial Hsp90 levels in either HAX-1 overexpressing or heterozygous-deficient hearts (Figs. S8 and S9). Taken together, these findings suggest that HAX-1 may directly displace Cyp-D from binding to Hsp90 in the mitochondria. To urther examine whether HAX-1 physically hinders the interaction of Cyp-D with Hsp90, we performed an in vitro competitive binding ELISA, using recombinant proteins. We observed a progressive reduction of the Cyp-D/Hsp90 binding in the presence of increasing HAX-1 protein levels (Fig. 6E). Thus, increases in HAX-1 appear to reduce the levels of mitochondrial Hsp90 that bind to Cyp-D, leading to degradation of Cyp-D.
Fig. S4.
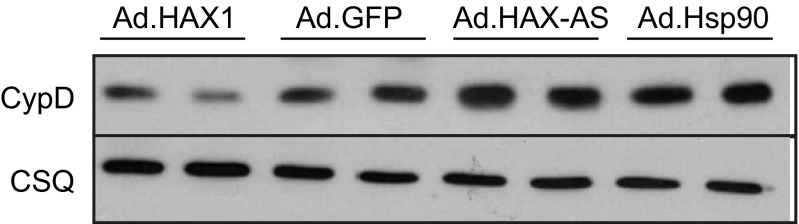
Adenoviral delivery of Hsp90 results in increased Cyp-D levels in rat cardiomyocytes. Rat cardiomyocytes were isolated, cultured and infected with Ad.HAX-1, Ad.GFP, Ad.HAX-AS (HAX-1 antisense), or Ad.Hsp90 for 24 h. CypD levels were increased in Ad.Hsp90 cells to a comparable level as in Ad.HAX-AS cells.
Fig. S5.
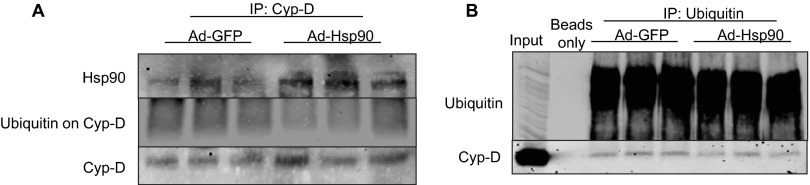
Adenoviral delivery of Hsp90 results in reduced Cyp-D ubiquitination in rat cardiomyocytes. Rat cardiomyocytes were isolated, cultured, and infected with Ad.GFP or Ad.Hsp90 for 24 h. Cells were then collected and lysed for immunoprecipitation experiments. Using either Cyp-D (A) or Ubiquitin (B) as bait, ubiquitination of Cyp-D was reduced when Hsp90 levels were enhanced.
Fig. 6.
HAX-1 displaces Hsp90 from cyclophilin-D, rendering it susceptible to degradation. (A and B) Immunoprecipitations using Cyp-D (A) or Hsp90 (B) as a bait, demonstrated that the Cyp-D/Hsp90 association was enhanced in HAX+/− hearts. WT cardiac homogenate served as input. (C and D) Immunoprecipitations, using Cyp-D (C) or Hsp90 (D) as a bait, demonstrated that the Cyp-D/Hsp90 association was reduced in HAX-OE hearts. WT cardiac homogenate served as input. (E) The Cyp-D/Hsp90 interaction was examined in the presence of increasing levels of HAX-1, using recombinant proteins. ELISA signals were calculated after subtraction of the mean value of controls, represented by GST-coated wells (n = 25 wells). (F) Increases in Hsp90 levels by adenoviral infection of cultured adult WT and HAX-OE cardiomyocytes enhanced Cyp-D protein expression. CSQ was used as a loading control. (G and H) Hsp90 overexpression exacerbated the loss of mitochondrial membrane potential in both WT and HAX-OE cells. n = 4–7 hearts (>10 cells per heart); *P < 0.05 vs. WT GFP at 20 min; #P < 0.05 vs. HAX-OE GFP at 20 min. Data are expressed as mean ± SEM.
Fig. S6.
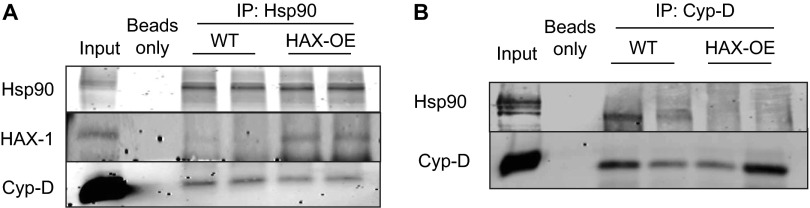
HAX-1 overexpression reduced mitochondrial Hsp90/Cyp-D association. Using either Hsp90 (A) or Cyp-D (B) as bait, a reduction of Hsp90/Cyp-D binding was observed in HAX-OE mitochondrial fraction.
Fig. S7.
Heterozygous ablation of HAX-1 enhanced mitochondrial Hsp90/Cyp-D association. Using either Hsp90 (A) or Cyp-D (B) as bait, an increase of Hsp90/Cyp-D binding was observed in HAX+/− mitochondrial fraction.
Fig. S8.
Expression of Hsp90 in cardiac homogenates. Hsp90 expression was not altered by overexpression or heterozygous deficiency of HAX-1 in cardiac homogenates.
Fig. S9.
Expression of Hsp90 in cardiac mitochondrial fraction. Hsp90 expression was not altered by HAX-1 overexpression or heterozygous deficiency in cardiac mitochondrial fraction.
To further test whether the effect of HAX-1 overexpression on decreasing the Cyp-D levels is mediated through reduction of the Hsp90/Cyp-D association, we acutely overexpressed Hsp90 to restore complex formation. Indeed, Hsp90 overexpression in the setting of HAX-1 overexpression resulted in markedly enhanced Cyp-D levels (Fig. 6F), abolishing the protective effects of HAX-1 and exacerbating loss of membrane potential upon hydrogen peroxide treatment (Fig. 6 G and H). These data provide further evidence that the cardioprotective effects of HAX-1 are mediated through regulation of mPTP function and specifically through recruitment of Hsp90 from the Hsp90/Cyp-D complex, contributing to degradation of Cyp-D protein and increased survival.
Discussion
In this study, we identified HAX-1 as a regulator of mPTP and mitochondrial membrane integrity, impacting cell survival in the heart. Increases in HAX-1 expression protected mitochondrial membrane from damage associated with oxidative stress and calcium overload, whereas decreases in HAX-1 levels exacerbated loss of membrane integrity. The underlying mechanisms involved the ability of HAX-1 to sequester Hsp90 from Cyp-D, which then rendered Cyp-D prone to ubiquitination and degradation. Thus, HAX-1 inhibits the opening of mPTP and prevents loss of membrane integrity and cell death activation during oxidative stress or mitochondrial calcium overload. These findings, coupled with our recent observations, indicating that HAX-1 can suppress ER stress-induced apoptosis (14), reveal a previously unidentified paradigm of cell death regulation by HAX-1 in two different cellular organelles.
Growing evidence demonstrates an axiomatic prosurvival role of HAX-1, and human mutations of this protein have been shown to associate with diseases characterized of altered cell viability, such as congenital neutropenia (15), neurodevelopmental retardation (26), and mantle cell lymphoma (11). Although HAX-1 has been known to predominantly localize to mitochondria in various tissues and cell types (8), and mitochondria are known for their gate-keeping role in cell death activation, it is surprising that there are only a few studies addressing the function of HAX-1 in regulation of cell death by this organelle. One study reported that mitochondrial HAX-1 interacts with the high temperature requirement protein-2 (HtrA2) and abrogation of this complex by HAX-1 ablation in mice contributes to Bax accumulation, neurodegeneration, and a short lifespan (14 wk). However, ablation of Bax in HAX-1–deficient mice could only prolong survival by 9 wk without fully rescuing the phenotype (10), suggesting that the HtrA2/Bax regulation is not the sole antiapoptotic mechanism mediated by HAX-1. Our findings herein suggest that mitochondrial localized HAX-1 has a direct protective effect on the membrane integrity of this subcellular organelle by regulating the mPTP opening.
We previously showed that cardiac ischemia/reperfusion injury is associated with loss of HAX-1 and overexpression of this protein conferred protection by reducing infarct size, limiting troponin release and inhibiting DNA fragmentation. On the contrary, heterozygous loss of HAX-1 led to exacerbated cardiac injury (14). Particularly, leakage of intracellular content, such as troponin proteins, is a sign of plasma membrane rupture and necrotic cell death (27, 28), which is mainly caused by failure to maintain plasma membrane potential due to loss of ATP supply (3, 4). The diminished energy production reflects compromised mitochondrial function associated with mPTP opening during ischemia/reperfusion injury (5). Although we previously showed that HAX-1 inhibits apoptosis mediated by the ER stress response, this effect would not account for the reduction in cardiac troponin I release during ischemia/reperfusion injury. Thus, the current identification of HAX-1 as a regulator of Cyp-D levels and an inhibitor of mPTP activation provides additional insights to its cardioprotective mechanisms. The role of Cyp-D was further elucidated by generation of a cross model with ablation of this protein in the HAX-1 heterozygous the deficient hearts, which were characterized by increased levels of this protein and mPTP activity. The cross model exhibited diminished myocardial infarction, although its infarct size was not reduced to the level of Cyp-D knockout hearts, suggesting that inhibition of both mPTP and ER stress contributes to the cardioprotective effects of HAX-1. Given that myocardial infarction is characterized by a continuum of apoptosis and necrosis, our data demonstrate that HAX-1 orchestrates a dual protective mechanism by inhibiting a mitochondrial-based necrotic activation, in addition to the previously reported ER-based apoptotic cell death program.
The activation of mitochondrial permeability transition pore has been shown to be a main driver in the development of myocardial infarction upon ischemia/reperfusion injury (5). Although the genetic identity of the pore is unknown, it has been suggested to consist of two complexes located on the outer and inner mitochondrial membrane. Although it was originally indicated that VDAC constitutes the outer membrane portion, a recent report suggested that VDAC is dispensable in mPTP activation (29) and outer membrane porosity is induced by the oligomerization of Bax/Bak (22). Furthermore, HAX-1 homozygous ablation was shown to facilitate Bax accumulation and cause neurodegeneration (10). However, in both HAX-1 overexpressing and heterozygous-deficient hearts, the levels of Bax and Bak were not altered in either the homogenates or mitochondrial fractions, suggesting that the alterations of mPTP in our models were independent of Bax/Bak accumulation.
The only genetically proven regulator of the mPTP is Cyp-D, which is located within the matrix of the mitochondria. Either ablation or pharmacological inhibition of this protein results in significant reduction of infarct size after ischemia/reperfusion injury (17, 19). However, increasing the expression of Cyp-D results in enhanced mPTP activity and mitochondrial swelling (19, 30). Thus, regulation of Cyp-D expression level becomes of paramount importance in the control of cell survival. To this end, our study presents the first evidence to our knowledge that Cyp-D levels can be posttranslationally regulated by HAX-1 and the chaperone protein Hsp90. Chaperone proteins have been well recognized to work coordinately with cochaperones and regulate protein turnover, directing unfolded or unwanted proteins to the ubiquitination/proteosomal degradation pathway. We previously demonstrated that HAX-1 works with Hsp90 to control the activity of IRE-1, one of the ER stress response signaling components, suggesting that HAX-1 may function as a cochaperone protein for Hsp90 (14). This chaperone/cochaperone regulation appears to exist also in mitochondria, controlling the ubiquitination and degradation of Cyp-D. Increases in HAX-1 expression can sequester Hsp90 from the Cyp-D/Hsp90 complex, promoting Cyp-D ubiquitination and degradation. In turn, the reduced Cyp-D levels confer resistance to mPTP opening and subsequent membrane potential loss upon stress stimuli. These findings are consistent with previous observations in breast carcinoma, colon cancer, and HEK293 cell lines, which showed that cleavage of HAX-1 by granzyme B leads to loss of mitochondrial membrane potential, and this loss can be prevented by introduction of Cyp-D siRNA (31).
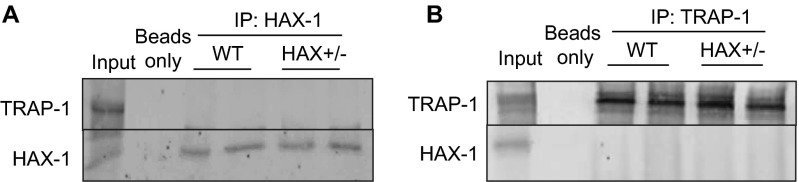
Recently, several breakthroughs have been made regarding regulation of Cyp-D function and necrotic cell death. In tumor cells, it was reported that the Cyp-D activity is inhibited by a mitochondrial Hsp90 chaperone network that contains cytosolic Hsp90, a mitochondrial Hsp90 analog, the tumor necrosis factor receptor associated protein-1 (TRAP-1), and several cochaperone proteins. Inclusion of a mitochondrial-directed inhibitor, targeting the ATPase activity of both Hsp90 analogs, was able to increase the opening of mPTP upon oxidative stress (20). Thus, it was proposed that both Hsp90 and TRAP-1 serve to antagonize Cyp-D function. Based on this hypothesis, it is expected that displacement of Hsp90 from Cyp-D by HAX-1 overexpression would increase Cyp-D activity and promote mPTP opening. However, we did not observe a reduction of mPTP activation when HAX-1 was overexpressed. Moreover, we found that overexpression of Hsp90 in either WT or HAX-OE cardiomyocytes resulted in increased Cyp-D expression (Fig. 6F) and exacerbated loss of mitochondrial membrane potential after hydrogen peroxide challenge (Fig. 6 G and H). These results suggest that Hsp90 promotes Cyp-D function in cardiac cells. The apparent discrepancy between our current results and previous reports in tumor cell lines may be due to the high dependency of Hsp90 function on the availability and combination of its cochaperone proteins, which may differ between cell types. It is likely that tumor cells have a remodeled mitochondrial Hsp90 chaperone network to enhance their survival and proliferation. In support of this notion, we failed to observe any significant association between HAX-1/TRAP-1 (Fig. S10), suggesting that the Hsp90 interaction with HAX-1 in cardiac cells may not be associated with TRAP-1. Indeed, mitochondrial targeted Hsp90 inhibitors have been shown to exhibit excellent specificity toward tumor cells, but not normal cells (20, 32). These reports support our results and point to a complex regulation of Hsp90 function by its cochaperones and the intracellular environment.
Fig. S10.
HAX-1 does not appear to associate with TRAP-1 in mouse cardiac homogenates. Using either HAX-1 (A) or TRAP-1 (B) as bait, we did not detect HAX-1/TRAP-1 association in cardiac homogenate.
Our findings on the newly discovered function of HAX-1 in controlling Cyp-D levels and activation of the mitochondrial permeability transition pore are not only critical in heart function and survival, but also provide significant insights in other diseases, such as immunodeficiency, tumorgenesis, and neurodegeneration. Studies in patients with HAX-1 mutation, exhibiting severe congenital neutropenia, showed that their neutrophils had low mitochondrial membrane potential compared with the healthy controls (16). However, elevated HAX-1 levels are associated with various types of cancer (8), where the mPTP activity can be inhibited for prosurvival purposes (33).
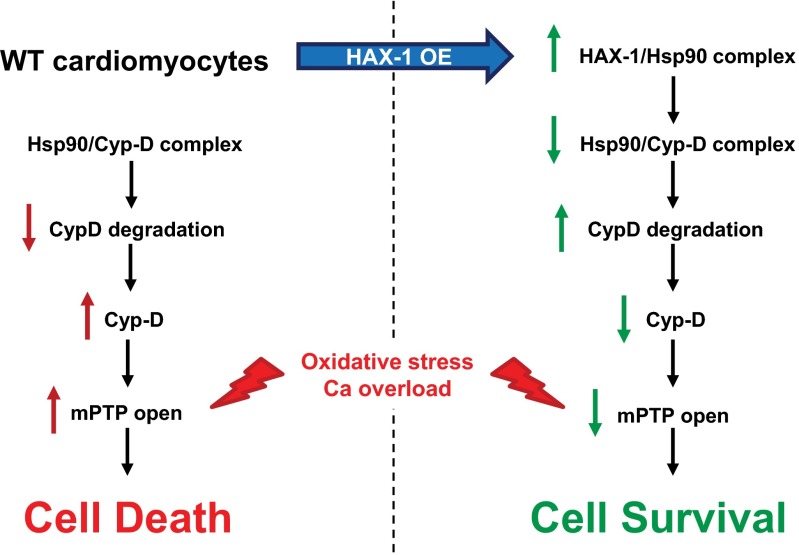
In summary, this is the first study to our knowledge to demonstrate that HAX-1 and specifically the HAX-1/Hsp90 complex promote Cyp-D ubiquitination and degradation. This regulatory mechanism confers resistance to mPTP opening and prevents cell death against oxidative stress and mitochondrial calcium overload (Fig. S11). Thus, HAX-1 and Hsp90, which are ubiquitously expressed, serve as common nodal points in both apoptotic and necrotic cell death. Because mPTP opening is implicated in the pathogenesis of various diseases, our findings suggest that the HAX-1/Hsp90 complex may be potentially exploited as a feasible therapeutic target.
Fig. S11.
Proposed HAX-1 protective mechanism against the opening of mitochondrial permeability transition pore in cardiomyocytes.
Materials and Methods
Animal Models.
HAX-1 transgenic (HAX-OE, FVB/N) (13), HAX-1 knockout heterozygous (HAX+/−, FVB/N) (13), Cyp-D knockout (B6129s) (19), the cross of CypD-KO/HAX+/− (B6129s) mice, and their wild-type littermates were used in this study. To avoid the complication of gender differences, only male mice at 3 mo of age were used for these studies, according to the National Institutes of Health Publication No. 8523: Guide for the Care and Use of Laboratory Animals (34).
Mouse Cardiomyocyte Isolation and Virus Infection.
Mouse myocytes from WT and HAX-1 OE hearts were isolated as described (14). Briefly, adult mouse hearts were excised following mouse anesthesia with sodium pentobarbital (70 mg/kg, i.p.) and cannulated on a Langendorff system. Ca-free Tyrode solution (113 nmol/L NaCl, 4.7 mmol/L KCl, 0.6 mmol/L KH2PO4, 0.6 mmol/L Na2HPO4, 1.2 mmol/L MgSO4⋅7H2O, 12 mmol/L NaHCO3, 10 mmol/L KHCO3, 5 mmol/L Hepes, 3 0 mmol/L taurine, 10 mmol/L 2,3-butanedione monoxime, and 5.5 mmol/L glucose, pH 7.4) was used to perfuse the heart for 3 min at 37 °C. The perfusion was then switched to a digestion solution containing 0.25 g/L liberase blendzyme (Roche). Following digestion, the left ventricular tissue was excised, minced, and dissociated into a cell suspension. Calcium was serially added to the cellular suspension until final calcium concentration in the Tyrode solution was 1.2 mmol/L. Cardiomyocytes were pelleted by gravity, resuspended in plating media [DMEM with 5% (vol/vol) FBS, 10 mmol/L 2,3-butanedione monoxime, 100 units/mL penicillin streptomycin and 2 mL of l-glutamine] and placed on a six-well plate containing laminin-coated coverslips for 1 h. Supplementing the media with 5% (vol/vol) FBS allows optimal cardiomyocyte attachment. Once the cells attached to the coverslip, the medium was replaced with infection medium (plating medium containing no FBS). Adenoviruses (Ad.GFP, Ad.HAX-1, Ad.HAX-AS, Ad.Cyp-D, and Ad.Hsp90) were applied at 500 multiplicity of infection (MOI). After 3 h of incubation at 37 °C, medium was removed and replaced with culture medium, which is DMEM containing 5 mg/L ITS (bovine insulin, human transferrin and sodium selenite; Sigma), 100 units/mL penicillin streptomycin, 2 mmol/L l-glutamine, 4 mmol/L NaHCO3, 10 mmol/L Hepes, 0.2% BSA, and 25 μmol/L blebbistatin (Cayman Chemical). The cell phenotype and morphology remained similar among noninfected and adenoviral-infected groups after 24 h of infection.
Mitochondrial Membrane Potential.
At 24 h after adenoviral infection, mouse cardiomyocytes were stained with TMRE dye (eBioscience) for 15 min at room temperature. Fluorescence signals from TMRE excited at 560 nm were collected at emission of 610 nm, using live-cell confocal imaging. To induce mitochondrial membrane potential transition, stained mouse cardiomyocytes were treated with 2 mM hydrogen peroxide, in the absence or presence of cyclosporin A (Sigma). The TMRE signal was recorded every minute for a course of 20 min to calculate the percentage of cells with preserved mitochondrial membrane potential before and after oxidative challenge. There was no significant loss of mitochondrial membrane observed until the 15th–18th minute of the treatment, suggesting that primary cardiomyocytes are resistant to cell death initiation.
Mitochondria Isolation and Swelling.
Mouse heart mitochondria were isolated by homogenization followed by different centrifugation. Briefly, mouse hearts were homogenized in Precellys 24 Lysis and homogenization machine at 5,000 cpm for 10 s with 750 µL of 2.3-nm Zirconia/silica beads and an equal amount of homogenization buffer containing 250 mM sucrose, 10 mM Tris at pH 7.4, and 1 mM EDTA. The homogenates were spun at 1,300 × g for 10 min at 4 °C to pellet nuclei and cell debris. The supernatant was then spun at 12,000 × g for 30 min at 4 °C to pellet the mitochondria. After washing twice in homogenization buffer (minus EDTA), the mitochondria were resuspended in buffer containing 120 mM KCl, 10 mM Tris at pH 7.4, and 5 mM KH2PO4 for the swelling assay. Mitochondrial swelling was induced by 50 or 375 mM, where the light-scattering of 250 μg of mitochondria in a 1-mL volume was measured at 540 nm for 10 min.
Propidium Iodide and Annexin V Assays.
Cells were gently washed once and stained with annexin V-specific APC dye (eBioscience) for 20 min. Various concentrations of hydrogen peroxide were applied at the beginning of annexin V treatment. Stained cardiomyocytes were then washed gently once and resuspended in solution containing propidium iodide (eBioscience) for exclusion of cells that had lost their membrane integrity. Fluorescence signals from APC and propidium iodide, excited at 633 nm and 488 nm, were collected at emission of 660 nm and 610 nm, respectively, in >10,000 cardiomyocytes per sample group. FlowJo software (Tree Star) was used to generate the diagram of cell distribution according to fluorescence intensity and to calculate the percent of Annexin V-positive population as an indication of apoptosis.
Quantitative Immunoblot Analysis.
Hearts were snap frozen in liquid nitrogen at the end of the Langendorff perfusion period and homogenized in 1× Cell Lysis Buffer (Cell Signaling Technology) supplemented with 1 mM PMSF and complete protease inhibitor mixture (Roche Applied Science). For each protein, equal amounts of samples (5–120 μg) from each heart were analyzed by SDS/PAGE, as described (13). After transfer to membranes, immunoblotting analysis was performed with the corresponding primary antibodies (Bax, Bak, and ubiquitin from Cell Signaling; Cyp-D, COX4 VDAC, ANT1, Hsp90, and TRAP-1 from Abcam; HAX-1 from BD Biosciences; calsequestrin (CSQ) and GAPDH from Thermo Scientific). This step was followed by incubation with secondary antibody conjugated with horseradish peroxidase at a 1:5,000 dilution. Visualization was achieved by using SuperSignal West Pico chemiluminescent substrate (Pierce) or ECLPLUS Western Blotting Detection kit (Amersham Pharmacia Biotech). The intensities of bands were determined by the AlphaEaseFC software. For each protein, the densitometric values from pre-I/R WT controls were arbitrarily converted to 1.0, and the values of samples from the other groups were normalized accordingly.
Global Ischemia/Reperfusion Injury.
Myocardial infarction was induced by using an isolated perfused heart model, as described (14). Briefly, hearts were mounted on a Langendorff apparatus, and perfused with Krebs–Henseleit buffer (KHB). Temperature was maintained constant at 37 °C by water-jacketed glassware for the heart chamber, buffer reservoirs, and perfusion lines. In addition, an overhead light source was used to ensure maintenance of temperature during ischemia, which was monitored by a thermometer placed close to the perfused heart in the glass chamber. A water-filled balloon made of plastic film was inserted into the left ventricle and adjusted to achieve a left ventricular end-diastolic pressure of 5–10 mmHg. The distal end of the catheter was connected to a Heart Performance Analyzer (Micro-Med) via a pressure transducer. Hearts were paced at 400 bpm except during ischemia, and pacing was reinitiated 2 min after reperfusion. After a 20-min equilibration period, hearts were subjected to 40 min of no flow global ischemia, followed by 60 min of reperfusion. Maximum rate of contraction (+dP/dt) and maximum rate of relaxation (−dP/dt) were monitored during this process. After reperfusion, the heart was perfused with 1% 2,3,5-triphenyltetrazolium chloride and incubated at 37 °C for 10 min. The heart was perfused and rinsed with PBS solution, followed by 10% (vol/vol) formalin tissue fixation. Five or six transverse slices were cut and weighed after 1 h of fixation. The images of the slices were digitally analyzed for infarct area and total ventricular area, using NIH image software.
Quantitative Real-Time PCR.
Total RNA was extracted and purified from heart tissue with miRNeasy Mini Kit (QIAGEN 133220834). The first-strand cDNA were generated from total RNA (1 μg) with reverse transcriptase kit (Invitrogen catalog no.11752). PCR was then performed with Bio-Rad real-time thermal cycler by using the following specific primer sequences: Mouse mitochondria cyclophilin-D (Ppif): (Forward) 5′-TGGCTCTCAGTTCTTTATCTGC-3′, (Reverse) 5′-ACATCCATGCCCTCTTTGAC-3′, and mouse GAPDH (Forward) 5′-TCAACAGC AACTCCCACTCTT-3′, (Reverse) 5′-ACCCTGTTGCTGTAGCCGTATTCA-3′. Rat Ppif: Forward: 5′-TGG AGT TAA AGG CAG ATG TCG-3′, Reverse: 5′-CAT TGT GAT TGG TGA AGT CGC-3′; Rat GAPDH Forward: 5′- CCTAAATGATACCCCACCGTG-3′, Reverse: 5′-TGT CAC AAG AGA AGG CAG C-3′. The values were normalized to those obtained with GAPDH.
Rat Myocyte Isolation and Virus Infection.
Adult male Sprague–Dawley rats (8-10 wk, 250–350 g) were anesthetized by pentobarbital (100 mg/kg) and supplemented with heparin (5000 U/kg). Hearts were quickly removed and the aorta was cannulated by the Langendorff mode and perfused with modified KHB: 118 mM NaCl, 4.8 mM KCl, 25 mM Hepes, 1.25 mM K2HPO4, 1.25 mM MgSO4, 11 mM glucose, 5 mM taurine, and 10 mM butanedione monoxime; pH 7.4) for 5–10 min. Subsequently, hearts were perfused with an enzyme solution [KHB containing 0.7 mg/mL collagenase type II (278 U/mg), 0.2 mg/mL hyaluronidase, 0.1% BSA, and 25 µM Ca] for 15 min. Then, 25 µM Ca was added to the perfusion buffer, and hearts were perfused for another 5 min. The Ca concentration in the perfusion buffer was raised to 100 µM and perfusion continued for 5 min. Once the hearts became flaccid, the left ventricular tissue was excised, minced, and filtered through a 240-µm screen. Cells were quickly centrifuged at low speed (200 × g for 5 min), harvested, and resuspended in 25 mL of 100 µM Ca-KHB with 1% BSA. The cells were allowed to settle automatically, and the Ca concentration was gradually raised to 1.0 mM. Afterward, the cells were washed two times with DMEM supplemented with ATCC (2 mg/mL BSA, 2 mM l-carnitine, 5 mM creatine, 5 mM taurine, 100 IU penicillin, and 100 µg/mL streptomycin). Cells were then counted and plated on laminin-coated dishes. After 2 h of plating, isolated rat cardiomyocytes were infected with Ad.Hsp90, Ad.HAX-1, Ad.HAX-AS viruses, or control virus Ad.GFP at an MOI of 500 for 2 h, before addition of a suitable volume of complete DMEM (DMEM containing 2 mg/mL BSA, 2 mM l-carnitine, 5 mM creatine, 5 mM taurine, 100 IU/mL penicillin and 100 µg/mL streptomycin), as described previously (14). The efficiency of adenoviral gene transfer was evaluated in cultured adult rat myocytes with the expression of GFP. Nearly 100% of myocytes appeared infected at 500 MOI by 24–48 h. The cell phenotype and morphology remained similar among noninfected and adenoviral-infected groups after 24 h of infection. The myocytes were washed with PBS and harvested for quantitative immunoblotting, or used in the experiments outlined in the results.
Coimmunoprecipitation.
Hearts from WT, HAX-OE, and HAX+/− mice were homogenized with 1× cell lysis buffer (Cell Signaling Technology), supplemented with protease inhibitor mixture and phosphatase inhibitor mixture. The homogenate was centrifuged at 9,000 × g for 30 min at 4 °C. The centrifuged homogenate was diluted to 1 µg/µl (1 mL of final volume) and incubated with either anti-HAX-1 (BD Biosciences), anti-Hsp90 (Abcam), anti-Cyp-D (Abcam), or anti-ubiquitin (Abcam) at 4 °C overnight with rotation. One hundred microliters of protein G PLUS agarose beads (Santa Cruz Biotechnology) were added into the mixture and incubated for an additional 5 h. Agarose beads were sedimented and washed six times with the cell lysis buffer. Beads-bound proteins were dissociated in 2× SDS at room temperature for 30 min with vortexing at 5-min intervals. The identification of the associated proteins was detected by Western blots. Homogenates from WT hearts were used as positive controls.
Competitive Protein Binding ELISA.
Competitive binding assay using recombinant proteins was performed as described (35). Briefly, each well on a 96-well high affinity binding plate (BD Falcon catalog no. 351172) was coated with 200 ng of recombinant Hsp90β protein (StressMarq) or GST protein (Novus Biologicals, as control) in 100 µL of coating buffer (85 mmol/L NaHCO3 and 15 mmol/L Na2CO3, pH 9.5) overnight at 4 °C. After five washes with PBS and 0.05% Tween-20 (PBS-T), blocking solution (PBS containing 1% BSA) was added for 1 h at room temperature. Following another five PBS-T washes, 400 ng of Cyp-D protein (Abnova) was added along with 400 ng of “competition protein mixture.” This mixture contained various amounts of HAX-1 (Abnova) supplemented with GST to reach a total of 400 ng of protein/reaction. Incubation proceeded for 2 h at room temperature. Each well was then washed with PBS-T six times, and the HAX-1 antibody, which was dissolved in blocking solution, was applied for 2 h at room temperature. After six washes with PBS-T, the secondary antibody conjugated with horseradish peroxidase was added for 1 h at room temperature. Following eight PBS-T washes, TMB substrate reagent (BD Biosciences) was applied to develop signal in blue color. After color was developed, 1 mol/L HCl was added to stop the reaction. The colorimetric intensity of each well was read at 450 nm with a microplate reader.
Statistical Analysis.
Data were expressed as the mean ± SEM. Comparisons between the means of two groups were performed by unpaired Student’s t test. Multiple groups were analyzed by using one-way ANOVA with a Bonferroni test for post hoc analysis. Results were considered statistically significant at P < 0.05.
Acknowledgments
We thank Dr. J. Molkentin for providing the Ad.Cyp-D virus; Dr. W. Sessa for providing the Ad.Hsp90 plasmid; Drs. A. Maloyan and J. Robbins for assistance with the mitochondrial swelling assay; and Drs. J. Molkentin and J. Karch for helpful discussions.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1508760112/-/DCSupplemental.
References
- 1.Carley AN, Taegtmeyer H, Lewandowski ED. Matrix revisited: Mechanisms linking energy substrate metabolism to the function of the heart. Circ Res. 2014;114(4):717–729. doi: 10.1161/CIRCRESAHA.114.301863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen L, Knowlton AA. Mitochondria and heart failure: New insights into an energetic problem. Minerva Cardioangiol. 2010;58(2):213–229. [PMC free article] [PubMed] [Google Scholar]
- 3.Galluzzi L, Kepp O, Trojel-Hansen C, Kroemer G. Mitochondrial control of cellular life, stress, and death. Circ Res. 2012;111(9):1198–1207. doi: 10.1161/CIRCRESAHA.112.268946. [DOI] [PubMed] [Google Scholar]
- 4.Chiong M, et al. Cardiomyocyte death: Mechanisms and translational implications. Cell Death Dis. 2011;2:e244. doi: 10.1038/cddis.2011.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev. 2008;88(2):581–609. doi: 10.1152/physrev.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mozaffarian D, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics--2015 update: A report from the American Heart Association. Circulation. 2015;131(4):e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki Y, et al. HAX-1, a novel intracellular protein, localized on mitochondria, directly associates with HS1, a substrate of Src family tyrosine kinases. J Immunol. 1997;158(6):2736–2744. [PubMed] [Google Scholar]
- 8.Fadeel B, Grzybowska E. HAX-1: A multifunctional protein with emerging roles in human disease. Biochim Biophys Acta. 2009;1790(10):1139–1148. doi: 10.1016/j.bbagen.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Yap SV, Koontz JM, Kontrogianni-Konstantopoulos A. HAX-1: A family of apoptotic regulators in health and disease. J Cell Physiol. 2011;226(11):2752–2761. doi: 10.1002/jcp.22638. [DOI] [PubMed] [Google Scholar]
- 10.Chao JR, et al. Hax1-mediated processing of HtrA2 by Parl allows survival of lymphocytes and neurons. Nature. 2008;452(7183):98–102. doi: 10.1038/nature06604. [DOI] [PubMed] [Google Scholar]
- 11.Baumann U, et al. Disruption of the PRKCD-FBXO25-HAX-1 axis attenuates the apoptotic response and drives lymphomagenesis. Nat Med. 2014;20(12):1401–1409. doi: 10.1038/nm.3740. [DOI] [PubMed] [Google Scholar]
- 12.Vafiadaki E, et al. Phospholamban interacts with HAX-1, a mitochondrial protein with anti-apoptotic function. J Mol Biol. 2007;367(1):65–79. doi: 10.1016/j.jmb.2006.10.057. [DOI] [PubMed] [Google Scholar]
- 13.Zhao W, et al. The anti-apoptotic protein HAX-1 is a regulator of cardiac function. Proc Natl Acad Sci USA. 2009;106(49):20776–20781. doi: 10.1073/pnas.0906998106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lam CK, et al. Novel role of HAX-1 in ischemic injury protection involvement of heat shock protein 90. Circ Res. 2013;112(1):79–89. doi: 10.1161/CIRCRESAHA.112.279935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein C, et al. HAX1 deficiency causes autosomal recessive severe congenital neutropenia (Kostmann disease) Nat Genet. 2007;39(1):86–92. doi: 10.1038/ng1940. [DOI] [PubMed] [Google Scholar]
- 16.Faiyaz-Ul-Haque M, et al. A novel HAX1 gene mutation in severe congenital neutropenia (SCN) associated with neurological manifestations. Eur J Pediatr. 2010;169(6):661–666. doi: 10.1007/s00431-010-1150-6. [DOI] [PubMed] [Google Scholar]
- 17.Elrod JW, Molkentin JD. Physiologic functions of cyclophilin D and the mitochondrial permeability transition pore. Circ J. 2013;77(5):1111–1122. doi: 10.1253/circj.cj-13-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunter DR, Haworth RA, Southard JH. Relationship between configuration, function, and permeability in calcium-treated mitochondria. J Biol Chem. 1976;251(16):5069–5077. [PubMed] [Google Scholar]
- 19.Baines CP, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434(7033):658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 20.Kang BH, et al. Regulation of tumor cell mitochondrial homeostasis by an organelle-specific Hsp90 chaperone network. Cell. 2007;131(2):257–270. doi: 10.1016/j.cell.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 21.Yap SV, Vafiadaki E, Strong J, Kontrogianni-Konstantopoulos A. HAX-1: A multifaceted antiapoptotic protein localizing in the mitochondria and the sarcoplasmic reticulum of striated muscle cells. J Mol Cell Cardiol. 2010;48(6):1266–1279. doi: 10.1016/j.yjmcc.2009.10.028. [DOI] [PubMed] [Google Scholar]
- 22.Karch J, et al. Bax and Bak function as the outer membrane component of the mitochondrial permeability pore in regulating necrotic cell death in mice. eLife. 2013;2:e00772. doi: 10.7554/eLife.00772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen D, Frezza M, Schmitt S, Kanwar J, Dou QP. Bortezomib as the first proteasome inhibitor anticancer drug: Current status and future perspectives. Curr Cancer Drug Targets. 2011;11(3):239–253. doi: 10.2174/156800911794519752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mato AR, Feldman T, Goy A. Proteasome inhibition and combination therapy for non-Hodgkin’s lymphoma: From bench to bedside. Oncologist. 2012;17(5):694–707. doi: 10.1634/theoncologist.2011-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pagan J, Seto T, Pagano M, Cittadini A. Role of the ubiquitin proteasome system in the heart. Circ Res. 2013;112(7):1046–1058. doi: 10.1161/CIRCRESAHA.112.300521. [DOI] [PubMed] [Google Scholar]
- 26.Lanciotti M, et al. Novel HAX1 gene mutations associated to neurodevelopment abnormalities in two Italian patients with severe congenital neutropenia. Haematologica. 2010;95(1):168–169. doi: 10.3324/haematol.2009.015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morrow DA, et al. National Academy of Clinical Biochemistry National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: Clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Clin Chem. 2007;53(4):552–574. doi: 10.1373/clinchem.2006.084194. [DOI] [PubMed] [Google Scholar]
- 28.Kurz K, Giannitsis E, Zehelein J, Katus HA. Highly sensitive cardiac troponin T values remain constant after brief exercise- or pharmacologic-induced reversible myocardial ischemia. Clin Chem. 2008;54(7):1234–1238. doi: 10.1373/clinchem.2007.097865. [DOI] [PubMed] [Google Scholar]
- 29.Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol. 2007;9(5):550–555. doi: 10.1038/ncb1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matas J, et al. Increased expression and intramitochondrial translocation of cyclophilin-D associates with increased vulnerability of the permeability transition pore to stress-induced opening during compensated ventricular hypertrophy. J Mol Cell Cardiol. 2009;46(3):420–430. doi: 10.1016/j.yjmcc.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 31.Han J, et al. Deregulation of mitochondrial membrane potential by mitochondrial insertion of granzyme B and direct Hax-1 cleavage. J Biol Chem. 2010;285(29):22461–22472. doi: 10.1074/jbc.M109.086587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siegelin MD. Inhibition of the mitochondrial Hsp90 chaperone network: A novel, efficient treatment strategy for cancer? Cancer Lett. 2013;333(2):133–146. doi: 10.1016/j.canlet.2013.01.045. [DOI] [PubMed] [Google Scholar]
- 33.Rasola A, Bernardi P. The mitochondrial permeability transition pore and its adaptive responses in tumor cells. Cell Calcium. 2014;56(6):437–445. doi: 10.1016/j.ceca.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Committee on Care and Use of Laboratory Animals (1996) Guide for the Care and Use of Laboratory Animals (Natl Inst Health, Bethesda), DHHS Publ No (NIH) 85-23.
- 35.Zhang X, et al. Hsp20 functions as a novel cardiokine in promoting angiogenesis via activation of VEGFR2. PLoS One. 2012;7(3):e32765. doi: 10.1371/journal.pone.0032765. [DOI] [PMC free article] [PubMed] [Google Scholar]