Abstract
Nanoparticle technologies intended for human administration must be designed to interact with, and ideally leverage, a living host environment. Here, we describe smart nanosystems classified in two categories: (i) those that sense the host environment and respond and (ii) those that first prime the host environment to interact with engineered nanoparticles. Smart nanosystems have the potential to produce personalized diagnostic and therapeutic schema by using the local environment to drive material behavior and ultimately improve human health.
Keywords: nanosystems, smart nanomaterials, environment-responsive, host priming, cancer nanotechnology
Nanomedicine seeks to exploit the convergence of two phenomena that emerge at the nanometer length scale. On the one hand, material properties change at the nanoscale to give rise to novel optical, electronic, and magnetic properties (1–3). On the other hand, biological properties also change at the nanoscale, including cellular trafficking, systemic biodistribution, and mechanisms of biodegradation (4–6). The first generation of nanomaterials that have been clinically translated has already leveraged such nanoscale properties to achieve improved diagnostic or therapeutic capabilities. For example, superparamagnetic iron oxide nanoparticles are used as a contrast agent to identify tumors in MRI (7), and the formulation of drugs into nanometer-sized particles alter their systemic and cellular trafficking, leading to decreased off-target toxicity, improved patient tolerability, and an increased therapeutic index (8). These technologies exert their function based on passive physical properties, such as nanoparticle size and surface chemistry, but cannot actively engage the host environment.
In nature, there is a diversity of agents that coevolved with their host organism to gain advanced capabilities to exploit their environment. For example, viruses are nanoscale pathogens that exhibit exquisite targeting specificity, sense their surroundings, and use escape mechanisms to evade the host defense to deliver nucleic acid cargo (9). Engineers have been inspired by nature to evolve the next generation of smart nanosystems that interrogate the host environment and react autonomously to yield a diagnosis and/or treatment of disease. These technologies have been endowed with the ability to translate changes in the environment into nanoparticle behaviors, such as degradation, swelling, or recruitment of biological cascades. In next generation nanomaterials, this paradigm can be extended further to develop systems where the host microenvironment can be primed to enable complex nanoparticle behaviors.
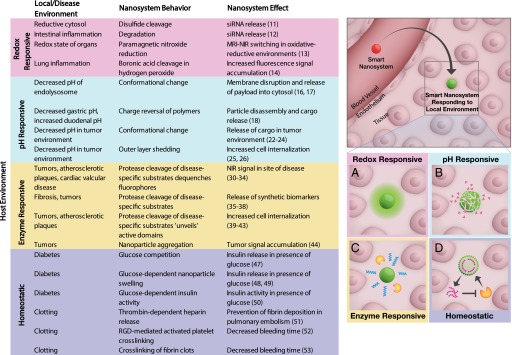
We will describe two classes of smart nanoparticle systems that interact with the living host (Figs. 1 and 2). The first class encompasses nanoparticles that sense and subsequently respond to their environment (Fig. 1). There are several biological compartments and disease conditions that exhibit altered biochemical properties (redox potential, pH, enzymatic activity, homeostatic pathways), and these traits can be leveraged to mobilize nanoparticle systems that are administered in these preexisting contexts. The second class is defined by an emerging paradigm of nanoparticle systems, such that the host environment is manipulated by an external influence to enable desired host-nanoparticle and nanoparticle-nanoparticle interactions, such as communication, recruitment, or amplification. Modifications to the host that achieve this primed environment can be accomplished by administering energy (X-rays, infrared light, heat), drugs, or nanoparticles themselves.
Fig. 1.
Smart nanosystems in circulation (red) can sense their local environment and are responsive (green) to (A) redox, (B) pH, (C) enzymes, or (D) homeostatic regulation.
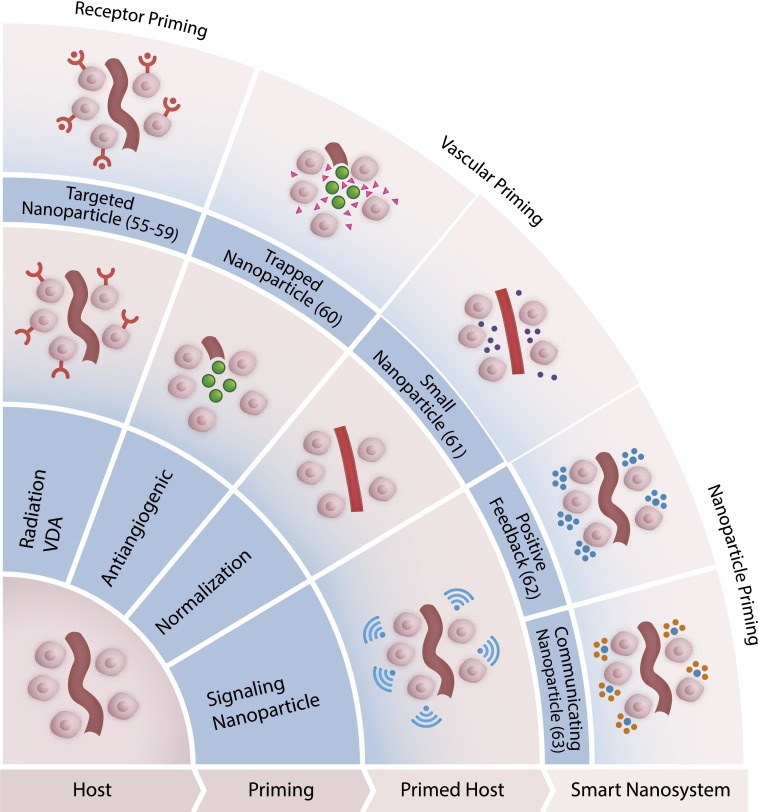
Fig. 2.
The host environment can be primed for the reception of nanoparticles using energy (radiation), drugs (VDA or anti-VEGF antibody), or nanoparticles themselves.
Environment-Responsive Nanosystems
Redox Responsive.
Redox potential has been leveraged in the design of several nanotechnologies for diagnostics and therapeutics (Fig. 1A, red). Biological host environments may exist in altered redox states that result from accumulation of reducing molecules (e.g., glutathione, NAD+, and NADP+) or free radicals [e.g., H2O2, reactive oxygen species (ROS)]. Reducing environments can occur in subcellular compartments, such as the cytosol of mammalian cells or the periplasm in gram-negative bacteria. Imbalances can also occur at the tissue level in disease contexts, such as sepsis, inflammation, and hypoxia. In nature, the pathogen HIV uses reduction of disulfide bonds to interact with host target cells (10). One way redox energy has been captured in nanoparticle design is by engineering systems with disulfide bonds to tether cargo. This principle is demonstrated with a nanoparticle platform for siRNA delivery (11), in which silencing activity is only observed when siRNA is tethered to the surface of quantum dots using a disulfide labile cross-linker. In another siRNA delivery platform, Wilson and colleagues use a polymer delivery system that releases siRNA in response to ROS in a model of intestinal inflammation (12). In this application, polymer nanoparticles composed of ROS-sensitive thioketal linkages respond to the 10- to 100-fold increase in mucosal ROS concentrations of inflamed intestine. On oral administration, these particles release their siRNA payload only in tissue regions with high ROS concentrations, resulting in the delivery of siRNA against the proinflammatory cytokine TNF-α.
Responsiveness to redox environments may also be used to design strategies to detect sites of disease. In one example, a nanoscale diagnostic system for MRI and fluorescence was able to map the redox state of a live animal (13). In this study, a brush polymer is decorated with two imaging probes: paramagnetic nitroxides as an MRI contrast agent and a near-infrared (NIR) dye for fluorescence imaging. Nanoscale materials allow for configurations in which nitroxides are proximal to a fluorophore, causing nitroxides to quench excited singlet states of nearby fluorophores. By contrast, in reducing states, nitroxides become diamagnetic, resulting in fluorescence signal enhancement. This compensatory imaging probe achieves high MRI contrast in oxidating conditions and a high NIR signal in reducing conditions, thus enabling delineation of the redox state in a live animal. In another example of ROS detection, the Tsien group deploys a boronic acid-cleavable linker to sense and react to hydrogen peroxide (14), aberrant levels of which occur in several disease states such as cancer, diabetes, and cardiovascular disease. This nanosystem is composed of a cationic cell penetrating peptide (d-Arg9) masked by a polyanionic sequence (d-Glu9) covalently attached by the H2O2-cleavable boronic acid linker. To monitor cleavage, the two peptides also have a FRET acceptor-donor pair conjugated to each half of the sensor. In this configuration, H2O2 levels are measured based on the relative levels of fluorescence signal derived from the cleaved donor fluorophore. This system was used to locate the presence of H2O2 in a lipopolysaccharide-induced model of lung inflammation.
pH Responsive.
Local reduction in pH can occur on a cellular or tissue level and has been exploited in several nanoparticle systems, where acidity can trigger conformational and/or solubility changes, binding affinity of receptor-ligand pairs, or the hydrolysis of acid-sensitive bonds (Fig. 1B, blue). Within a cell, the contents of endocytic vesicles are typically trafficked to the early endosome and can be directed either to the recycling endosome or to the endolysosomal pathway. After internalization, many nanomaterials are trafficked to the endolysosomal pathway, where there is a gradual decrease in pH by the action of proton pumps and fusion with acidic lysosomes. As an example of biological inspiration, the decreased pH of maturing endosomes has been taken advantage of by influenza virus, where protonation of key residues triggers conformational changes in the coat protein, exposing domains that facilitate escape into the cytosol (15). pH-sensitive systems are particularly advantageous in nucleic acid delivery applications, due to the large macromolecular cargo that must be localized to the cytosol to mediate its activity but is often trapped in endosomes in the absence of an active release mechanism. The Stayton group has developed a suite of polymers that are tuned to transition from micelles to unimers that have membrane-active properties over physiological pH ranges and that mediate endosomal release of a wide range of cargo. In one recent iteration, a pH-sensitive targeted micelle was loaded with a designer protein inhibitor for treatment of an Epstein–Barr virus-positive lymphoma model (16). A smaller peptide payload was also delivered for application in a model of human B-cell lymphoma (17).
Differential pH also occurs at the tissue level. The stomach and the vagina are both naturally low pH environments. Several technologies have been designed for oral delivery, where the pH (as low as ∼1 in the stomach and slightly basic in the small intestine) can trigger activation and transport of drugs. Self-assembled nanoparticles made from chitosan and poly-γ-glutamic acid (pγGA) disassemble at pH lower than the pKa of the carboxylic acid groups of pγGA (pH 2.5) or higher than the pKa of the amino groups on chitosan (pH 6.6) due to the loss of electrostatic force (18). The mucoadhesive property of chitosan can extend the residence time of nanoparticles in the gastrointestinal tract (19). This nanoparticle system is loaded with insulin for protein delivery through oral administration, wherein the increased pH of the small intestine dismantles the particle to release free chitosan and insulin. The release of chitosan has an added biological function: disassembling the tight junctions between cells to promote paracellular delivery of insulin. In another example of environmentally-controlled “falling apart,” Almutairi and coworkers constructed AND gated polymer nanoparticles that require both redox and pH changes to degrade rapidly and have been used in diagnostic applications (20).
The decreased pH present in tumor environments has also been harnessed in a multitude of nanoparticle systems. Poor access to vasculature combined with inadequate lymphatic drainage in tumors cause dysregulated metabolism in tumors, leading to the accumulation of acidic metabolites (21). The overall effect is a decrease in extracellular pH in the tumor interstitium. To exploit the decreased pH in the tumor, groups have developed pH-sensitive systems for targeted release of chemotherapeutics. The Langer group tuned poly(ethylene oxide)-modified poly(b-amino ester) (PEO-PbAE) polymeric nanoparticles to have pH-dependent solubility characteristics that sharply transition between pH 6.5 and 7.4. This response leads to rapid and complete release of cargo once the pH decreases (22). Nanoparticles made from PEO-PbAE are able to deliver paclitaxel to the cytosol of cells (23) and to tumors (24) at higher concentrations compared with a pH-insensitive formulation. In another method of pH-driven nanoparticle disassembly in the tumor, researchers in the Hammond group have used acidic pH in the tumor microenvironment to drive shedding in layer-by-layer (LbL) nanoparticle assemblies. In one such technology, a hydrophilic PEG coating was attached on top of a cationic core in a LbL assembly using a pH-sensitive neutravidin-iminobiotin bond to take advantage of both the benefits of a neutral hydrophilic nanoparticle in systemic circulation and a positive-charged surface in the acidic environment of the tumor (25). The authors observe similar levels of accumulation in pH-dependent and pH-independent formulations of these nanoparticles at shorter time points (8 h), but an increase in retention in pH-dependent PEG sheddable nanoparticles at longer time points (24–48 h) due to the improved internalization of cationic nanoparticles. In another iteration of a pH-sensitive LbL system that actively targets the tumor environment, hydrophilic hyaluronic acid-coated nanoparticles circulate and home to tumors via CD44 binding and disassemble in the acidic environment of the tumor to reveal a cationic core that engages with cells (26).
Enzyme Responsive.
Dysregulated enzyme expression is a common feature of many disease processes; thus, the ability of a nanomaterial to sense enzymatic cues in the host environment is a powerful tool for both diagnostic and therapeutic applications. Proteases, encompassing more than 500 distinct enzymes that cleave other peptides and proteins, are of particular interest in the diagnosis of disease because of their role as markers and agents of diverse pathologic processes including malignancy and metastasis, inflammatory conditions, atherosclerosis, and blood clotting disorders (27, 28). Some viruses hijack host proteases to trigger their own pathogenic pathways; for example, dengue virus is cleaved by furin in the endolysosome to reach full maturation (29). Engineering smart nanomaterials that can sense and respond to proteolytic activity has been bolstered by progress in identifying peptide substrates and their corresponding specificity for different enzymes. Researchers have used creative methods to use these substrates to lend insight into disease progression and localization (Fig. 1C, orange).
One approach to capturing the diagnostic potential of protease profiling is to create nanomaterials with protease-sensitive domains whose cleavage causes unquenching of fluorogenic probes for imaging applications. For example, the Weissleder group has developed nanoscale imaging diagnostics based on a family of NIR fluorescence probes constructed as a poly-l-lysine backbone substituted with PEG and adjacent homoquenched dyes (30). In ProSense probes, the poly-l-lysine sequence is susceptible to cleavage by cysteine proteases such as cathepsins, thus unquenching fluorophores flanking the cleavage site. Alternatively, by tethering the NIR dyes via protease-cleavable substrates, it is possible to make the probes specific for alternate proteases such as urokinase plasminogen activator (31) or MMP2/9 (32). Such probes have been useful in imaging tumors (30), atherosclerotic plaques (33), and cardiac valvular disease in the setting of hypercholesterolemia (34). The coadministration of these protease-sensitive fluorescent probes with other platforms such as targeted magnetofluorescent nanoparticles enables powerful multimodality imaging to study the biology and pathophysiology of complex inflammatory processes.
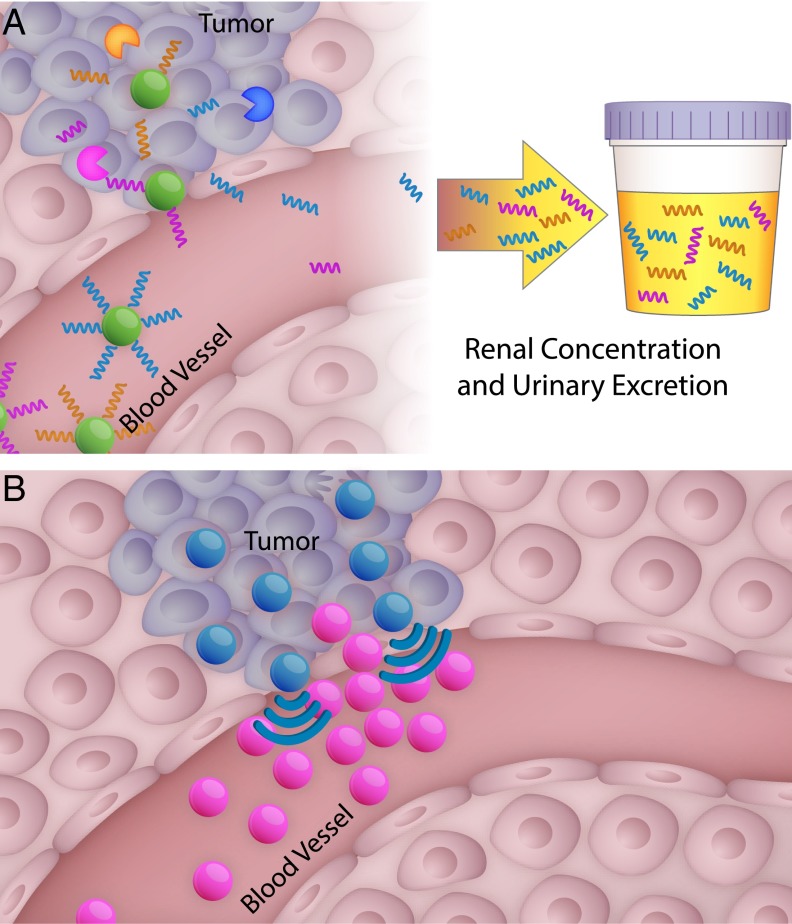
Protease-sensitive nanomaterials can also be engineered for diagnostic applications through the collection and analysis of cleavage fragments that serve as highly sensitive and specific markers of protease activity in disease. This concept is the basis of the synthetic biomarker platform developed in our research group: nanoparticle cores are decorated with multiplexed probes linked by protease-cleavable substrates, such that only probes liberated by proteases are small enough to be excreted in the urine (Fig. 3A). Traditional endogenous biomarkers for disease diagnosis are hampered by dilution in the blood, chemical instability, highly variable expression, and their presence at basal levels in benign conditions. In contrast, synthetic biomarker probes take advantage of the catalytic nature of proteases for signal amplification, as each enzyme may liberate up to 1,000 probe fragments per hour; downstream, the signal is further amplified by renal concentration of urine. Synthetic urinary biomarkers may be readily detected by a wide range of existing analytical techniques, including mass spectrometry (35), immunoassays (36), paper-based microfluidics (37), and ultra-sensitive single molecule techniques (38). We demonstrated the diagnostic potential of synthetic biomarkers in many diseases associated with protease activity: a thrombin-sensitive synthetic biomarker has allowed urinary detection of thrombosis in a model of pulmonary embolism (36), and a set of probes has been shown to be more sensitive and specific for detecting tumors in a model of colorectal cancer compared with a standard blood biomarker, carcinoembryonic antigen (CEA) (35). The platform can be multiplexed by using multiple substrates paired with unique mass-encoded probes, creating a panel of 10 different markers that can profile protease signatures of colorectal cancer and liver fibrosis disease states. This approach has been applied to detect signatures of progressive liver fibrosis and its resolution (35).
Fig. 3.
Two examples of environment-responsive nanosystems. (A) Multiplexed synthetic biomarkers are shed in response to protease cleavage in the tumor and are then concentrated in the urine for noninvasive disease monitoring (35). (B) Amplified nanoparticle accumulation can be achieved by cooperative systems (63).
In addition to harnessing the passive properties of proteolytically cleavable materials for noninvasive diagnostics such as imaging and urinary analysis, researchers are exploring materials that gain active functions following protease cleavage. The Tsien group developed activatable cell-penetrating peptides (ACPPs) sensitive to proteases such as MMP2/9 (39, 40) and thrombin (41). These peptides consist of fluorescently tagged polyarginine fused to an anionic polyglutamate inhibitory domain via a protease-cleavable linker that only dissociates following a proteolytic encounter (39). Thus, the cell-internalizing function of polyarginine is only activated if the appropriate protease signal is present in the environment. MMP2/9-sensitive ACPPs have been shown to preferentially internalize into tumor cells, allowing in vivo imaging of a variety of tumor types (40); similarly, thrombin-sensitive ACPPs enable fluorescent in vivo imaging of atherosclerotic plaques (41). In another example of protease-activated functionality, our group has designed stealth dextran-coated iron oxide nanoparticles decorated with a brush layer of PEG linked via MMP2/9-cleavable substrates. Such nanoparticles only unveil a surface of multivalent polyarginine moieties under the PEG brush layer after cleavage by MMPs in the tumor environment, thus leading to an increased interaction and internalization into tumor cells compared with particles that could not be unveiled (42). In the Torchilin lab, the concept of PEG unveiling has also been extended to targeted systems for chemotherapeutic delivery of a liposomal formulation of paclitaxel and leads to significantly decreased tumor growth compared with when liposomes were formulated with uncleavable PEG (43). It is also possible to harness enzyme-mediated cleavage to cause structural changes in the sensing material. The Rao laboratory has devised small caspase-sensitive nanoaggregation fluorescent probes (C-SNAFs), in which caspase cleavage of a peptide cap promotes cyclization of the C-SNAF molecule through a condensation reaction, and these cyclized forms in turn self-assemble into nanoaggregates (44). Thus, in apoptotic cells permissive to entry of C-SNAFs, this caspase-triggered aggregation leads to a robust fluorescent signal; indeed, C-SNAFs enabled visualization of apoptotic regions in xenograft tumor models treated with doxorubicin. In summary, smart nanomaterials that sense protease activity can be engineered to provide spatial and kinetic snapshots of many key disease processes in the host, and furthermore can enable dynamic behaviors in response to detection of protease cleavage events at the sites of interest.
Homeostatic.
A wide range of physiological functions are regulated via homeostatic mechanisms, and this natural sensing paradigm has inspired a class of closed-loop nanotechnology platforms. Homeostatic nanoparticle systems have been designed by incorporating a sensor, an effector, and a negative feedback system. This combination results in particles that both surveil and react at a commensurate level to the sensor signal. The basis of reaction can be either direct competitive binding of the detected ligand or, in many cases, utilization of the same reactive principles described above (pH, enzymatic activity) to drive release of the effector (Fig. 1D, purple).
One area where homeostatic systems have been applied is insulin delivery for the regulation of blood glucose. In diabetes mellitus, patients experience hyperglycemia due to either insufficient production of insulin or insulin resistance and are treated by the delivery of exogenous insulin. Glycemic control is imperative in diabetes patients, with both hyper- and hypoglycemia leading to negative outcomes (45, 46). Currently, patients regulate their blood glucose by making an exogenous measurement and taking either oral or s.c. insulin, resulting in large fluctuations in glucose levels due to the time lag between the bolus delivery of insulin and a glucose response. Diabetes management could benefit from a method of insulin delivery that responds to glucose levels without outside input, resulting in the capacity to both sense glucose and release insulin without intervention. Toward this goal, several groups have developed nanomaterials that can both sense glucose and deliver insulin in response to endogenous glucose levels. A commercially developed nanotechnology, “Smart Insulin,” is a nanoparticle formulation that releases insulin based on lectin-mediated glucose binding (47). Insulin release can also be achieved through an enzymatic mediator: Gu and colleagues used the enzyme glucose oxidase as a sensor to trigger a pH-responsive material coloaded with insulin (48, 49). Glucose oxidase converts glucose to gluconic acid, leading to a local pH change, swelling of the encapsulating pH-responsive polymer, and subsequent release of insulin. As the level of glucose decreases, the pH returns to physiological levels, decreasing the release of insulin. These technologies are able to tune the blood glucose levels to desirable levels for longer periods of time relative to nonresponsive insulin delivery in a diabetic mouse model (48, 49). Finally, insulin itself has been engineered with a glucose-binding switch, allowing for glucose-mediated activation of insulin in the blood in a single molecule formulation (50).
The process of blood clotting is another tightly regulated homeostatic system, in which positive and negative feedback cycles maintain the balance between procoagulant and anticoagulant factors. Pathologic processes that disrupt this balance in either direction pose significant risk of morbidity or mortality, with hypercoaguability resulting in life-threatening thromboses or emboli and hypocoaguability resulting in life-threatening bleeds. Unfortunately, the vast majority of therapeutics available for the correction of clotting diseases are unimodal, blunt instruments that operate “open loop,” without responding to relevant host clotting cues. Thus, anticoagulants can easily be overdosed, pushing the hypercoagulable patient into coagulopathy. Newer anticoagulant classes have more predictable pharmacological effects, but lack both antidotes and a mechanism of self-regulation. Furthermore, a single patient can be at risk for both thrombosis and bleeding in different sites in the body, and no anticoagulants exist that are able to navigate such a scenario. Smart nanosystems have the potential to provide anticoagulation or procoagulation specifically when and where it is needed through interaction with local signals such as thrombin activity or the altered surface properties of activated platelets.
Toward this goal, our group devised nanoparticle-based self-titrating anticoagulants (nanoSTATs) (51), comprised of PEGylated thrombin-sensitive synthetic peptides encapsulating heparin, a common anticoagulant that inhibits thrombin and other serine proteases via antithrombin III. Unlike conventional free heparin, which constitutively and globally antagonizes coagulation enzymes, these nanoparticles release heparin in response and in proportion to local thrombin activity, which is typically higher in the setting of thrombosis compared with normal hemostasis. Accordingly, nanoSTATs are able to acutely prevent fibrin deposition in a model of pulmonary embolism while maintaining a normal bleeding time in a tail bleeding assay. In the opposite scenario, an ideal procoagulant rapidly promotes clotting specifically at a site of uncontrolled bleeding, without globally increasing the risk of thrombosis. One design of an i.v. hemostat developed in the Lavik group consists of PLGA-PLL nanoparticle cores displaying multiple PEG chains terminated in RGD motifs (52). The specificity of RGD for integrins expressed on activated platelets allows these particles to act as multivalent cross-linkers only when clotting is initiated naturally. In a femoral artery injury model of trauma, these intravenously-delivered hemostats cut the bleeding time in half while showing no thrombotic complications in healthy animals. In a polymeric mimic of factor XIIIa, polymeric hemostats (polySTATs) cross-link fibrin to enhance clotting rates and create more robust clot integrity while also possessing a long clearance half-life of ∼14 h. In a femoral artery injury model, polySTAT-treated mice showed ∼11-fold reductions in blood volume loss and required less i.v. fluids to maintain blood pressure (53). These examples underscore the potential therapeutic benefit of developing smart materials that interface with and respond to the host to create nuanced and sophisticated behaviors, enforcing the homeostatic regulation of clotting.
Environment-Primed Nanosystems
The smart nanosystems described above are responsive to preexisting conditions in the host environment. However, in some scenarios, the manifestation of disease may not culminate in changes that can be targeted by smart nanosystems. We dedicate the second part of this review to an emerging paradigm of nanosystems that primes the host environment to become receptive to administered, engineered nanoparticles (Fig. 2). This phenomenon of potentiating changes in the host to prime recruitment of effector entities is exemplified by the mammalian immune system, in which a subset of “sensor” cells orchestrate the recruitment of “effector” cells to the site of disease by releasing cytokine signals to broadcast their location (54). In engineered systems, the transformation of the host environment can be achieved by several inputs, such as energy, small molecules, or even nanoparticles themselves, that are delivered in a multistage treatment, or as part of a self-regulating cycle. The most developed examples of this concept to date have been tailored for use in cancer therapy, due to the relatively extensive characterization of the tumor environment combined with the large armamentarium of cancer-targeted nanotechnologies. We envision that a parallel approach can be adopted for a broad range of disease settings.
One such environment priming approach uses radiation to induce expression changes in tumor cells that can be exploited by the smart nanosystem. Radiation is a common treatment for cancer patients in the clinic, allowing a radiation-responsive nanoparticle technology to easily fit within existing clinical regimes. Radiation as part of cancer therapy is known to elicit expression pattern changes in the tumor microvasculature (55). Phage display techniques have been used to probe irradiated tumors and identify peptides that bind to newly expressed, tumor-specific neoantigens for diagnostic purposes (56) or to target nanoparticle therapeutics (57, 58). When peptide-targeted doxorubicin liposomes are delivered, irradiated tumors accumulate more drug than nonirradiated tumors after 3 d in an s.c. model of lung cancer in mice (57). Moreover, the peptide sequences identified by phage panning are tumor type specific, such that different peptide sequences can be retrieved to target irradiated gliomas (58). The binding partner of this glioma-specific peptide is significantly enriched in irradiated tumors and targetable by polymer nanoparticles loaded with paclitaxel. However, the use of energy to induce priming of the host environment requires a priori knowledge of tumor location.
Small molecule therapeutics and antibody conjugates are the standard in today’s treatment arena. A valuable asset that can be gleaned from the vast array of small molecules investigated in cell lines, animals, and humans is the pattern of downstream changes that can occur in the tumor microenvironment in response to these agonists/antagonists. These data could inform decisions for combination therapy with a nanoparticle delivery system to elicit synergistic effects or to rejuvenate orphan small molecules that failed to demonstrate clinical efficacy when used as a single agent, yet were shown to be safe. There are several approaches that modify the vasculature of tumors for the purpose of improving nanoparticle delivery or retention. In our group, a vascular disrupting agent (VDA), ombrabulin, was applied to selectively increase the expression of p32, a stress-related protein, on the surface of tumor cells for subsequent active targeting by a nanoparticle (59). When either diagnostic or therapeutic nanoparticles decorated with p32-targeting peptides were administered 24 h after VDA treatment, the recruitment of nanoparticles into the tumor tissue increased significantly over untreated controls. The combination of the VDA with targeted liposomes encapsulating a chemotherapeutic led to decreased tumor sizes compared untargeted liposomes or when the vasculature was not primed with the VDA. In another technology engineered in the Saskisekharan lab, the “nanocell” is preferentially taken up by the tumor based on passive targeting, and first releases an antiangiogenic agent to cause vascular shutdown, followed temporally by release of a chemotherapeutic (60). In this case, the nanoparticle carrying chemotherapy is effectively trapped inside the tumor environment, achieving focal delivery and reduced off-target toxicity. The Jain group, in contrast, has approached dysregulated angiogenesis from the opposite direction, and has developed agents to normalize tumor vasculature to reduce interstitial pressure and homogenize vascular pore sizes. They discovered that antagonism of angiogenesis via a VEGF-2 blocking antibody causes increase flux of small- but not large-diameter nanoparticles (12 vs. 125 nm) (61). This principle was evaluated with two clinically approved nanoparticles, Abraxane (10 nm) and Doxil (100 nm), and resulted in VEGF-2 blocking antibody-treated mice having decreased tumor doubling time in response to Abraxane but not Doxil. It is interesting to note that modifying tumor vasculature to become either more normalized or more abnormal can result in local environment changes (improved pressure profiles or increased receptor expression, respectively) that can be exploited by nanoparticles.
In addition to using energy or small molecule agents to induce a primed environment, nanoparticles themselves can become agents that mediate conditioning in the host. In one example of a self-amplifying cascade, the Ruoslahti group, together with our group and the Sailor group, built a nanoparticle system that exhibits an affinity for tumor tissue through binding to clotting proteins found naturally in the tumor environment. Accumulation of these nanoparticles induces further clotting and subsequent recruitment of more nanoparticles, initiating a positive feedback loop (62). This nanosystem relies on both the nanoparticle system that homes to clotting proteins and the host’s own clotting machinery to amplify the signal and recruit more nanoparticles to the site of the tumor. In our group, the harnessing of the coagulation pathway was extended to a two nanoparticle system as a means to improve the control of signal transduction (Fig. 3B) (63). In the two-particle nanosystem, the first signaling nanoparticle targets the tumor and is activated to initiate a local coagulation response that broadcasts the tumor location to downstream receiving nanoparticles. The signaling component can be generated in two different modes of signaling and receiving systems. One mode is to use tumor-targeted tissue factor, an engineered protein that activates coagulation pathways in the presence of antigenic tumor receptors. In the second mode, gold nanorods, which home to tumors based on their physicochemical properties, are remotely activated using NIR light, based on unique plasmonic properties that emerge when gold is configured into rods at the nanoscale, to generate heat and cause disruption of tumor vessels. The resulting natural clotting signal is transmitted to receiving nanoparticles that home to fibrin clots. These signaling-receiving nanosystems carry either diagnostic or therapeutic cargo to the tumors, and the final tumor accumulation is elevated 40-fold over noncommunicating systems. The collective behavior that arises from nanoparticle communication paradigms can be extended to other ensemble systems, such as swarming systems, to achieve desired cooperative strategies (64).
Conclusion
We discussed several smart nanosystems that are defined by their ability to sense the host environment after systemic administration and to respond based on the local information they sense to achieve both diagnostic and therapeutic goals. These smart nanosystems can recapitulate a variety of complex biological behaviors and require host participation to be fully effective. We furthermore outline a paradigm in which leveraging of the host can be amplified by priming unique microenvironments that can be exploited by cleverly designed nanomaterials. Timing is an important factor in all these systems, such that the eventual outcome mediated by the nanosystem is predicated by an induction-dependent change to the target environment that must be harnessed within a limited time window. The capacity to leverage a primed host environment broadens the applicability of nanoscale technologies to larger subsets of disease, because this paradigm results in an amplified signal that is broadcast to engage surveilling nanoparticles. Translational challenges include manufacturing and regulatory hurdles in addition to the unanticipated complexity of how nanoparticulate materials can interact with the host architecture and biology. The first targeted nanomedicines [CALAA-01 (65); BIND-014 (66)] and smart probes [Lumicell (67)] are now entering human studies, demonstrating feasibility, and will inform design criteria for future generation of nanosystems as the pipeline matures. In an era of precision medicine, in which there is an increasing emphasis on disease stratification for personalized therapeutics, we envision that smart, environmentally responsive therapeutics may offer a complementary approach that instead collapses the treatment of distinct subtypes to a single formulation that is responsive to an individual’s disease.
Acknowledgments
Due to space limitations, we restricted our discussion to the last 10 y of nanoscale systems that have been evaluated in animal models. We gratefully acknowledge the entire community of investigators that have built the foundations of this evolving field, but whom we were unable to cite individually. We thank Dr. Heather Fleming (MIT) for critical reading of the manuscript and a longstanding nanomedicine collaboration with Drs. Erkki Ruoslahti and Michael Sailor. This work has been funded in part by Koch Institute Support Grant P30-CA14051 from the National Cancer Institute, Core Center Grant P30-ES002109 from the National Institute of Environmental Health Sciences, an Amar G. Bose research grant, the Marie-D. & Pierre Casimir-Lambert Fund, and the MIT-Harvard Center of Cancer Nanotechnology Excellence (National Institutes of Health Grant U54CA151884). This work was also supported by the Defense Advanced Research Projects Agency under Cooperative Agreement HR0011-13-2-0017. The content of the information within this document does not necessarily reflect the position or the policy of the Government.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article is part of the special series of PNAS 100th Anniversary articles to commemorate exceptional research published in PNAS over the last century.
References
- 1.Anglin EJ, Cheng L, Freeman WR, Sailor MJ. Porous silicon in drug delivery devices and materials. Adv Drug Deliv Rev. 2008;60(11):1266–1277. doi: 10.1016/j.addr.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weissleder R, et al. Ultrasmall superparamagnetic iron oxide: Characterization of a new class of contrast agents for MR imaging. Radiology. 1990;175(2):489–493. doi: 10.1148/radiology.175.2.2326474. [DOI] [PubMed] [Google Scholar]
- 3.Wang F, Banerjee D, Liu Y, Chen X, Liu X. Upconversion nanoparticles in biological labeling, imaging, and therapy. Analyst (Lond) 2010;135(8):1839–1854. doi: 10.1039/c0an00144a. [DOI] [PubMed] [Google Scholar]
- 4.Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov. 2010;9(8):615–627. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- 5.Cheng CJ, Tietjen GT, Saucier-Sawyer JK, Saltzman WM. A holistic approach to targeting disease with polymeric nanoparticles. Nat Rev Drug Discov. 2015;14(4):239–247. doi: 10.1038/nrd4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: An emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7(9):771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 7.Harisinghani MG, et al. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N Engl J Med. 2003;348(25):2491–2499. doi: 10.1056/NEJMoa022749. [DOI] [PubMed] [Google Scholar]
- 8.Jain RK, Stylianopoulos T. Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol. 2010;7(11):653–664. doi: 10.1038/nrclinonc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schrauwen EJA, Fouchier RAM. Host adaptation and transmission of influenza A viruses in mammals. Emerg Microbes Infec. 2014 doi: 10.1038/emi.2014.9. 3, e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryser HJ, Levy EM, Mandel R, DiSciullo GJ. Inhibition of human immunodeficiency virus infection by agents that interfere with thiol-disulfide interchange upon virus-receptor interaction. Proc Natl Acad Sci USA. 1994;91(10):4559–4563. doi: 10.1073/pnas.91.10.4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh N, Agrawal A, Leung AK, Sharp PA, Bhatia SN. Effect of nanoparticle conjugation on gene silencing by RNA interference. J Am Chem Soc. 2010;132(24):8241–8243. doi: 10.1021/ja102132e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson DS, et al. Orally delivered thioketal nanoparticles loaded with TNF-α-siRNA target inflammation and inhibit gene expression in the intestines. Nat Mater. 2010;9(11):923–928. doi: 10.1038/nmat2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sowers MA, et al. Redox-responsive branched-bottlebrush polymers for in vivo MRI and fluorescence imaging. Nat Commun. 2014;5:5460. doi: 10.1038/ncomms6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinstain R, Savariar EN, Felsen CN, Tsien RY. In vivo targeting of hydrogen peroxide by activatable cell-penetrating peptides. J Am Chem Soc. 2014;136(3):874–877. doi: 10.1021/ja411547j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harrison SC. Viral membrane fusion. Nat Struct Mol Biol. 2008;15(7):690–698. doi: 10.1038/nsmb.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Procko E, et al. A computationally designed inhibitor of an Epstein-Barr viral Bcl-2 protein induces apoptosis in infected cells. Cell. 2014;157(7):1644–1656. doi: 10.1016/j.cell.2014.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berguig GY, et al. Intracellular delivery system for antibody-peptide drug conjugates. Molec Therap. 2015;23(5):907–917. doi: 10.1038/mt.2015.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sonaje K, et al. Self-assembled pH-sensitive nanoparticles: A platform for oral delivery of protein drugs. Adv Funct Mater. 2010;20(21):3695–3700. [Google Scholar]
- 19.Bowman K, Leong KW. Chitosan nanoparticles for oral drug and gene delivery. Int J Nanomedicine. 2006;1(2):117–128. doi: 10.2147/nano.2006.1.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joshi-Barr S, de Gracia Lux C, Mahmoud E, Almutairi A. Exploiting oxidative microenvironments in the body as triggers for drug delivery systems. Antioxid Redox Signal. 2014;21(5):730–754. doi: 10.1089/ars.2013.5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin GR, Jain RK. Noninvasive measurement of interstitial pH profiles in normal and neoplastic tissue using fluorescence ratio imaging microscopy. Cancer Res. 1994;54(21):5670–5674. [PubMed] [Google Scholar]
- 22.Lynn DM, Amiji MM, Langer R. pH-responsive polymer microspheres: Rapid release of encapsulated material within the range of intracellular pH. Angew Chem Int Ed Engl. 2001;40(9):1707–1710. [PubMed] [Google Scholar]
- 23.Shenoy D, Little S, Langer R, Amiji M. Poly(ethylene oxide)-modified poly(beta-amino ester) nanoparticles as a pH-sensitive system for tumor-targeted delivery of hydrophobic drugs. 1. In vitro evaluations. Mol Pharm. 2005;2(5):357–366. doi: 10.1021/mp0500420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shenoy D, Little S, Langer R, Amiji M. Poly(ethylene oxide)-modified poly(beta-amino ester) nanoparticles as a pH-sensitive system for tumor-targeted delivery of hydrophobic drugs: Part 2. In vivo distribution and tumor localization studies. Pharm Res. 2005;22(12):2107–2114. doi: 10.1007/s11095-005-8343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poon Z, Chang D, Zhao X, Hammond PT. Layer-by-layer nanoparticles with a pH-sheddable layer for in vivo targeting of tumor hypoxia. ACS Nano. 2011;5(6):4284–4292. doi: 10.1021/nn200876f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dreaden EC, et al. Bimodal tumor-targeting from microenvironment responsive hyaluronan layer-by-layer (LbL) nanoparticles. ACS Nano. 2014;8(8):8374–8382. doi: 10.1021/nn502861t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.López-Otín C, Hunter T. The regulatory crosstalk between kinases and proteases in cancer. Nat Rev Cancer. 2010;10(4):278–292. doi: 10.1038/nrc2823. [DOI] [PubMed] [Google Scholar]
- 28.Turk B. Targeting proteases: Successes, failures and future prospects. Nat Rev Drug Discov. 2006;5(9):785–799. doi: 10.1038/nrd2092. [DOI] [PubMed] [Google Scholar]
- 29.Yu IM, et al. Structure of the immature dengue virus at low pH primes proteolytic maturation. Science. 2008;319(5871):1834–1837. doi: 10.1126/science.1153264. [DOI] [PubMed] [Google Scholar]
- 30.Weissleder R, Tung CH, Mahmood U, Bogdanov A., Jr In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nat Biotechnol. 1999;17(4):375–378. doi: 10.1038/7933. [DOI] [PubMed] [Google Scholar]
- 31.Law B, Curino A, Bugge TH, Weissleder R, Tung C-H. Design, synthesis, and characterization of urokinase plasminogen-activator-sensitive near-infrared reporter. Chem Biol. 2004;11(1):99–106. doi: 10.1016/j.chembiol.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 32.Chen J, et al. Near-infrared fluorescent imaging of matrix metalloproteinase activity after myocardial infarction. Circulation. 2005;111(14):1800–1805. doi: 10.1161/01.CIR.0000160936.91849.9F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jaffer FA, Libby P, Weissleder R. Optical and multimodality molecular imaging: Insights into atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29(7):1017–1024. doi: 10.1161/ATVBAHA.108.165530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aikawa E, et al. Multimodality molecular imaging identifies proteolytic and osteogenic activities in early aortic valve disease. Circulation. 2007;115(3):377–386. doi: 10.1161/CIRCULATIONAHA.106.654913. [DOI] [PubMed] [Google Scholar]
- 35.Kwong GA, et al. Mass-encoded synthetic biomarkers for multiplexed urinary monitoring of disease. Nat Biotechnol. 2013;31(1):63–70. doi: 10.1038/nbt.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin KY, Kwong GA, Warren AD, Wood DK, Bhatia SN. Nanoparticles that sense thrombin activity as synthetic urinary biomarkers of thrombosis. ACS Nano. 2013;7(10):9001–9009. doi: 10.1021/nn403550c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Warren AD, Kwong GA, Wood DK, Lin KY, Bhatia SN. Point-of-care diagnostics for noncommunicable diseases using synthetic urinary biomarkers and paper microfluidics. Proc Natl Acad Sci USA. 2014;111(10):3671–3676. doi: 10.1073/pnas.1314651111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Warren AD, et al. Disease detection by ultrasensitive quantification of microdosed synthetic urinary biomarkers. J Am Chem Soc. 2014;136(39):13709–13714. doi: 10.1021/ja505676h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang T, et al. Tumor imaging by means of proteolytic activation of cell-penetrating peptides. Proc Natl Acad Sci USA. 2004;101(51):17867–17872. doi: 10.1073/pnas.0408191101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olson ES, et al. In vivo characterization of activatable cell penetrating peptides for targeting protease activity in cancer. Integr Biol. 2009;1(5-6):382–393. doi: 10.1039/b904890a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olson ES, et al. In vivo fluorescence imaging of atherosclerotic plaques with activatable cell-penetrating peptides targeting thrombin activity. Integr Biol. 2012;4(6):595–605. doi: 10.1039/c2ib00161f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harris TJ, et al. Protease-triggered unveiling of bioactive nanoparticles. Small. 2008;4(9):1307–1312. doi: 10.1002/smll.200701319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu L, Wang T, Perche F, Taigind A, Torchilin VP. Enhanced anticancer activity of nanopreparation containing an MMP2-sensitive PEG-drug conjugate and cell-penetrating moiety. Proc Natl Acad Sci USA. 2013;110(42):17047–17052. doi: 10.1073/pnas.1304987110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ye D, et al. Bioorthogonal cyclization-mediated in situ self-assembly of small-molecule probes for imaging caspase activity in vivo. Nat Chem. 2014;6(6):519–526. doi: 10.1038/nchem.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabetic Med. 1998;15(7):539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 46.Cryer PE, Davis SN, Shamoon H. Hypoglycemia in diabetes. Diabetes Care. 2003;26(6):1902–1912. doi: 10.2337/diacare.26.6.1902. [DOI] [PubMed] [Google Scholar]
- 47.Veiseh O, Tang BC, Whitehead KA, Anderson DG, Langer R. Managing diabetes with nanomedicine: challenges and opportunities. Nat Rev Drug Discov. 2015;14(1):45–57. doi: 10.1038/nrd4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gu Z, et al. Injectable nano-network for glucose-mediated insulin delivery. ACS Nano. 2013;7(5):4194–4201. doi: 10.1021/nn400630x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gu Z, et al. Glucose-responsive microgels integrated with enzyme nanocapsules for closed-loop insulin delivery. ACS Nano. 2013;7(8):6758–6766. doi: 10.1021/nn401617u. [DOI] [PubMed] [Google Scholar]
- 50.Chou DH, et al. Glucose-responsive insulin activity by covalent modification with aliphatic phenylboronic acid conjugates. Proc Natl Acad Sci USA. 2015;112(8):2401–2406. doi: 10.1073/pnas.1424684112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin KY, Lo JH, Consul N, Kwong GA, Bhatia SN. Self-titrating anticoagulant nanocomplexes that restore homeostatic regulation of the coagulation cascade. ACS Nano. 2014;8(9):8776–8785. doi: 10.1021/nn501129q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bertram JP, et al. Intravenous hemostat: Nanotechnology to halt bleeding. Sci Transl Med. 2009;1(11):11ra22. doi: 10.1126/scitranslmed.3000397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chan LW, et al. A synthetic fibrin cross-linking polymer for modulating clot properties and inducing hemostasis. Sci Transl Med. 2015;7(277):277ra29. doi: 10.1126/scitranslmed.3010383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Iwasaki A, Medzhitov R. Control of adaptive immunity by the innate immune system. Nat Immunol. 2015;16(4):343–353. doi: 10.1038/ni.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hallahan D, et al. Integrin-mediated targeting of drug delivery to irradiated tumor blood vessels. Cancer Cell. 2003;3(1):63–74. doi: 10.1016/s1535-6108(02)00238-6. [DOI] [PubMed] [Google Scholar]
- 56.Han Z, et al. Noninvasive assessment of cancer response to therapy. Nat Med. 2008;14(3):343–349. doi: 10.1038/nm1691. [DOI] [PubMed] [Google Scholar]
- 57.Lowery A, Onishko H, Hallahan DE, Han Z. Tumor-targeted delivery of liposome-encapsulated doxorubicin by use of a peptide that selectively binds to irradiated tumors. J Control Release. 2011;150(1):117–124. doi: 10.1016/j.jconrel.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Passarella RJ, et al. Targeted nanoparticles that deliver a sustained, specific release of Paclitaxel to irradiated tumors. Cancer Res. 2010;70(11):4550–4559. doi: 10.1158/0008-5472.CAN-10-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lin KY, Kwon EJ, Lo JH, Bhatia SN. Drug-induced amplification of nanoparticle targeting to tumors. Nano Today. 2014;9(5):550–559. doi: 10.1016/j.nantod.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sengupta S, et al. Temporal targeting of tumour cells and neovasculature with a nanoscale delivery system. Nature. 2005;436(7050):568–572. doi: 10.1038/nature03794. [DOI] [PubMed] [Google Scholar]
- 61.Chauhan VP, et al. Normalization of tumour blood vessels improves the delivery of nanomedicines in a size-dependent manner. Nat Nanotechnol. 2012;7(6):383–388. doi: 10.1038/nnano.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Simberg D, et al. Biomimetic amplification of nanoparticle homing to tumors. Proc Natl Acad Sci USA. 2007;104(3):932–936. doi: 10.1073/pnas.0610298104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.von Maltzahn G, et al. Nanoparticles that communicate in vivo to amplify tumour targeting. Nat Mater. 2011;10(7):545–552. doi: 10.1038/nmat3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hauert S, Bhatia SN. Mechanisms of cooperation in cancer nanomedicine: Towards systems nanotechnology. Trends Biotechnol. 2014;32(9):448–455. doi: 10.1016/j.tibtech.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Davis ME, et al. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464(7291):1067–1070. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hrkach J, et al. Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Sci Transl Med. 2012;4(128):128ra39. doi: 10.1126/scitranslmed.3003651. [DOI] [PubMed] [Google Scholar]
- 67.Mito JK, et al. Intraoperative detection and removal of microscopic residual sarcoma using wide-field imaging. Cancer. 2012;118(21):5320–5330. doi: 10.1002/cncr.27458. [DOI] [PMC free article] [PubMed] [Google Scholar]