Significance
Signaling through antigen receptors is essential for lymphocyte development, survival, and function. The receptor-like tyrosine phosphatase CD45 plays a critical role in these processes by regulating antigen receptor signaling via its cytosolic phosphatase domain. Despite its abundance, the function of the large, alternatively spliced extracellular domain of CD45 has remained elusive. We used CD45 transgenes either incorporating a phosphatase-inactivating point mutation or lacking the cytoplasmic domain to uncouple the enzymatic and noncatalytic functions of CD45. Both transgenes partially rescue the phenotype of CD45-deficient B cells by modulating the B cell inhibitory surface coreceptor CD22. These data demonstrate an in vivo function for the extracellular domain of CD45 in restraining CD22 inhibitory function to maintain tonic B-cell antigen receptor signaling.
Keywords: CD45, CD22, BCR signaling, B-cell development, TCR signaling
Abstract
The receptor-like tyrosine phosphatase CD45 regulates antigen receptor signaling by dephosphorylating the C-terminal inhibitory tyrosine of the src family kinases. However, despite its abundance, the function of the large, alternatively spliced extracellular domain of CD45 has remained elusive. We used normally spliced CD45 transgenes either incorporating a phosphatase-inactivating point mutation or lacking the cytoplasmic domain to uncouple the enzymatic and noncatalytic functions of CD45 in lymphocytes. Although these transgenes did not alter T-cell signaling or development irrespective of endogenous CD45 expression, both partially rescued the phenotype of CD45-deficient B cells. We identify a noncatalytic role for CD45 in regulating tonic, but not antigen-mediated, B-cell antigen receptor (BCR) signaling through modulation of the function of the inhibitory coreceptor CD22. This finding has important implications for understanding how naïve B cells maintain tonic BCR signaling while restraining inappropriate antigen-dependent activation to preserve clonal “ignorance.”
B lymphocytes face the daunting tasks of distinguishing between benign self-antigens and pathogenic foreign epitopes and of mounting a robust immune response to the latter, but not the former. Although extremely autoreactive B-cell antigen receptors (BCRs) are removed from the repertoire during B-cell development, a large fraction of mature naïve B cells express mildly autoreactive BCRs (1). That these specificities pose a threat to immune homeostasis is evidenced by the extensive network of inhibitory coreceptors and effector phosphatases expressed in naïve B cells that serve to suppress BCR signaling (2). Defects in these pathways result in autoantibody production and frank autoimmune disease in mice and humans (2, 3). However, “tonic” signaling downstream of the BCR is essential for B-cell survival (4). How B cells balance the requirement for tonic BCR signaling with the demands of so-called “clonal ignorance” is not well understood.
The receptor-like tyrosine phosphatase CD45 serves as a critical positive regulator of antigen receptor (AgR) signaling by constitutively dephosphorylating the C-terminal inhibitory tyrosine of the src family kinases (SFKs) (5, 6). This function is counteracted by the kinase Csk, which stabilizes a “closed” autoinhibited conformation of the SFKs by phosphorylating this site. Active SFKs in turn phosphorylate tyrosines within cytoplasmic immunoreceptor tyrosine-based activation motifs (ITAMs) of the AgRs that are essential for recruitment of the Syk family kinases and downstream signaling (7). Consistent with this model, the inhibitory tyrosine (Y505) of the T-cell-specific SFK Lck is hyperphosphorylated in CD45-deficient primary T cells (6, 8). Consequently, T-cell receptor (TCR) signaling and T-cell development are severely impaired in mice deficient for CD45 (8–11). BCR signaling and B-cell development are also disrupted in CD45-deficient mice, but this phenotype is much milder than that of T cells because of the expression of a partially redundant tyrosine phosphatase, CD148 (12).
CD45 consists of a large, heavily glycosylated extracellular (EC) domain, a single transmembrane (TM) domain, and a cytoplasmic segment that contains tandem protein tyrosine phosphatase domains, the more membrane-distal of which is enzymatically inactive. Although CD45 is the most abundantly expressed protein on the surface of nucleated hematopoietic cells, the function of its EC domain remains unknown. Alternative splicing of this domain generates multiple CD45 isoforms whose expression is tightly regulated in a cell-type, activation, and developmental stage-specific manner (6, 13). A genetic polymorphism that alters splicing, but not amino acid sequence, is associated with human autoimmune disease (14). These data strongly suggest that the EC domain of CD45 serves an important role, yet a bona fide ligand has not been identified, despite great effort. Postulated functions for this domain have included homodimerization-induced inhibition of CD45 phosphatase activity, association with cis- and trans-interacting partners such as the CD4 coreceptor and the inhibitory B-cell coreceptor CD22, and a role in kinetic segregation (6, 15–17).
CD22 is a B-cell-specific member of the sialic acid-binding Ig-like lectin (Siglec) family that exerts its inhibitory effect by virtue of three tandem immunoreceptor tyrosine inhibitory motifs (ITIMs) in its cytosolic segment (18). BCR cross-linking increases CD22 ITIM phosphorylation by the tyrosine kinase Lyn, facilitating recruitment of SHP-1 and SHIP1 phosphatases, which in turn restrain AgR signaling (18, 19). CD22 has been shown to interact directly with IgM (20, 21), an interaction that serves to recruit SHP-1 to the immediate proximity of the BCR (18). CD22-deficient B cells exhibit enhanced intracellular calcium responses to BCR ligation and decreased marginal zone (MZ) B-cell development in the spleen (22–25). The function of CD22 is partially dependent upon binding to α-2,6 sialic acids (sia), and disruption of this activity through mutation of the sia-binding domain (CD22 R130E mutant) or impaired sia generation (ST6galI enzyme deficiency) results in dampened BCR calcium signaling, suggesting that sia binding serves to restrain the inhibitory function of CD22 (18, 26, 27). It has been proposed that sia-dependent CD22 oligomerization sequesters CD22 away from the BCR, thus dampening its inhibitory function (28).
Defects in the CD22-dependent inhibitory pathway are associated with lupus-like disease in mice, and common variants are linked to disease in humans (2, 3, 29). Rare mutations in the enzyme sialic acid esterase, which generates ligands for CD22, are associated with up to an eightfold increased risk of autoimmunity (2). Conversely, activation of this inhibitory pathway through copresentation of antigen and sia ligands can induce B-cell tolerance (30). However, how CD22 activity is differentially regulated in the resting, basal state of B cells and upon antigen-receptor signaling is not well understood.
To uncouple the enzymatic and noncatalytic functions of CD45 in lymphocytes, we take advantage of two highly expressed and normally spliced CD45 transgenes that either harbor a phosphatase-inactive version of full length CD45 (C817S or “CS” Tg) or lack the cytoplasmic domain entirely (“ΔC” Tg). Although these transgenes fail to alter T-cell signaling and development irrespective of endogenous CD45 expression, both transgenes partially rescue the phenotype of CD45-deficient B cells in a manner that is independent of antigen, and completely dependent upon expression of the inhibitory coreceptor CD22. Because endogenous CD45 expression is dispensable for this phenotype, our data argue against a dominant negative function for the transgenes. We identify a role for the EC domain of CD45 in regulating tonic, but not antigen-mediated, BCR signaling, exclusively through modulation of CD22 function. These data demonstrate an in vivo function for the EC domain of CD45 and have important implications for understanding how naïve B cells maintain tonic BCR signaling while restraining inappropriate antigen-dependent activation to maintain clonal ignorance.
Results
CS and ΔC Transgenes Are Highly Expressed and Normally Spliced.
We have previously described an allelic series of mice in which CD45 splicing is intact, but expression is varied across a broad range (0–180%, relative to WT, in B cells) (31, 32). In T and B cells, increasing CD45 expression across this series reduces phosphorylation of the inhibitory tyrosines of Lck and Lyn, respectively (31, 32). Titration of CD45 results in a range of developmental and AgR-dependent signaling phenotypes in T and B cells. However, it remains unknown which, if any, of these phenotypes is due to the phosphatase activity of CD45 and which might be due to a noncatalytic function of the protein.
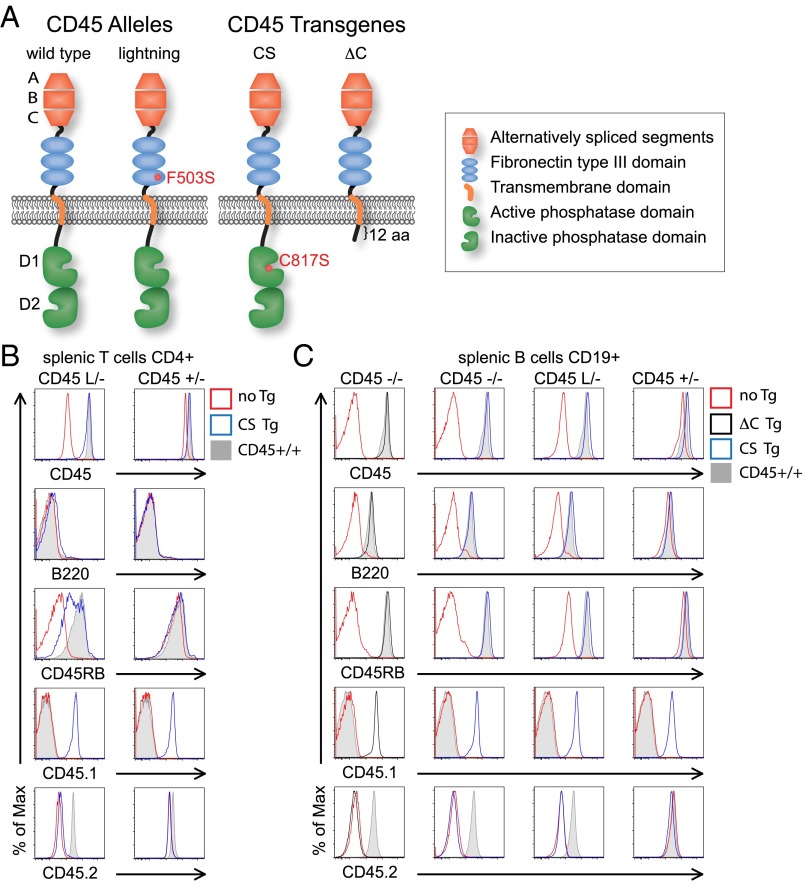
To uncouple expression of the CD45 protein from its catalytic function, we took advantage of two normally spliced transgenes of CD45 under the control of the LFA1 promoter to drive expression comparable to endogenous levels in hematopoietic lineages (Fig. 1A). The first of these, CS Tg, drives expression of a full-length CD45 protein harboring a single point mutation in the catalytic cysteine of the phosphatase domain (C817S) that renders the protein enzymatically inactive (33, 34). The second line, ΔC Tg, encodes CD45 protein-truncated 12 amino acids carboxyl relative to the TM domain, thereby eliminating the cytosolic domain (Fig. 1A).
Fig. 1.
CS and ΔC transgenes are highly expressed and normally spliced. (A) Schematic of endogenous CD45 wild-type and lightning (L) alleles and CD45 transgene constructs (CS and ΔC). (B and C) CD4 T and CD19+ B cells from allelic series mice (CD45−/−, L/−, and +/−) with or without CD45 Tgs were stained to detect total CD45 and isoforms of CD45 (B220/CD45RABC, CD45RB), as well as CD45 allotypes (CD45.1 to detect Tgs and CD45.2 to detect endogenous CD45). Data are representative of at least three independent experiments.
CS and ΔC transgenes were superimposed upon a background of varying amounts of endogenous functional CD45 by using our published allelic series of mouse lines (31). The allelic series incorporates, in addition to wild-type and null CD45 alleles, an N-ethyl-N-nitrosourea–generated point mutant of CD45, Lightning (F503S; “L”) that is characterized by low surface expression (L/− expresses only 7% of normal CD45 levels) with preserved phosphatase activity and alternative splicing (Fig. 1A). We expressed single copies of either of the two transgenes in the context of endogenous CD45−/−, L/−, or +/− alleles. By comparing these allelic series mice in the presence or absence of the CS and ΔC Tgs, we uncoupled phosphatase activity from extracatalytic function of CD45, but importantly, do not generate substantially supraphysiologic CD45 expression (Fig. 1 B and C and Fig. S1A). Of note, in B, but not T, cells, expression of these transgenes slightly reduced endogenous CD45 expression in the CD45+/− genetic background. Importantly, as described in studies of an analogously generated CD45 minigene (35), splicing of the EC domain of CD45 is intact in these transgenes. This conclusion is supported by expression of the unspliced large B220/RABC form of CD45 only in B cells from both CS and ΔC Tg mice, suggesting that regulated splicing is triggered appropriately in all other hematopoietic lineages (Fig. 1 B and C and Fig. S1A).
Fig. S1.
CS transgene does not alter T-cell development or thymocyte signaling. (A) Thymocytes from allelic series mice (CD45−/−, L/−, and +/−) with or without CD45 Tgs were stained to detect total CD45, isoforms of CD45 (B220/CD45RABC, CD45RB), as well as CD45 allotypes (CD45.1 to detect Tgs and CD45.2 to detect endogenous CD45). Data are representative of at least three independent experiments. (B) Thymocytes from allelic series mice (CD45−/−, CD45+/−, CD45+/+) with or without a single copy of the CS Tg were stained with CD4, CD8, CD5, and TCRβ to identify T-cell subsets. Upper represents total thymocytes and Lower represents DP thymocytes with gate drawn to identify postselection DP thymocytes. (C) DP thymocytes from allelic series mice (CD45−/−, CD45+/−, CD45+/+) with or without CS Tg expression were stimulated with 10 μg/mL of anti-CD3ε (2χ11), fixed, permeabilized, and stained to detect pErk. (D) Thymocytes from CD45+/− mice expressing the OT1 TCR transgene in the presence or absence of CS Tg were stained with CD4, CD8, and Vα2 to identify T-cell subsets (Upper) and Vα2+ SP8 thymocytes (Lower). (E) DP or SP4 thymocytes from CD45+/− mice with (blue) or without (red) CS Tg were loaded with indo-1 to detect intracellular calcium, stimulated with varying doses of anti-CD3ε or with ionomycin, and assess via flow cytometry. (F) DP thymocytes from allelic series mice with or without CS Tg expression were stimulated with varying doses of anti-CD3ε, fixed, permeabilized, and stained to detect pErk. Data are representative of at least two independent experiments.
CS and ΔC Transgenes Do Not Alter T-Cell Development, Signaling, or Activation.
CD45-deficient (Ptprc−/−) thymocytes exhibit a severe block in TCR signal transduction and consequently at the thymic positive selection checkpoint (8). Transition from double-positive (DP) to postselection DP and single-positive (SP) thymocytes was not rescued by the CS Tg, confirming via this sensitive in vivo assay that the C817S point mutation indeed abolishes phosphatase activity (Fig. 2 A and B and Fig. S1B). Consistently, anti-CD3–induced Erk-phosphorylation was not rescued in CD45−/− thymocytes by CS Tg expression (Fig. S1C).
Fig. 2.
CS and ΔC transgenes do not alter T-cell development, signaling, or activation. (A and B) Thymocytes (A) and lymph node cells (B) from allelic series mice (CD45−/−, CD45 L/−, CD45+/−) with or without a single copy of the CS Tg were stained with CD4 and CD8 to identify T-cell subsets. (C) Histograms represent allelic series DP thymocytes as gated in A stained to detect surface expression of CD45, CD5, and TCR-β. WT cells (gray histogram) are overlaid as a reference. Each column represents an allelic series genotype with (blue) or without (red) CS Tg expression. (D) Thymocytes (Upper) and lymph node cells (Lower) from CD45+/− mice expressing the OT2 TCR transgene in the presence or absence of CS Tg were stained with CD4 and CD8 to identify T-cell subsets. (E) Graph represents percent SP4 thymocytes as gated in D (± SEM) from three biological replicates. (F) Histograms represent thymic DP, thymic SP4, or LN CD4 T cells as gated in D stained to detect surface expression of CD5, TCR-β, and CD69 from CD45+/− mice expressing the OT2 TCR transgene in the presence (blue) or absence (red) of CS Tg. (G) Blots represent unstimulated thymocytes from allelic series mice with or without CS Tg expression probed to detect total Erk1/2, total Lck, and Lck pY505. Data are representative of at least three independent experiments.
T-cell development requires CD45 phosphatase activity, but a single lightning allele (L/−) rescues thymic development in an otherwise CD45-null background, permitting the study of mature T cells highly deficient in phosphatase-active CD45. This strategy also permitted us to search for a putative dominant-negative function of CD45 by overexpressing catalytically inactive CD45 relative to functional endogenous CD45 (Fig. 1B and Fig. S1A). Basal TCR signaling on preselection DP thymocytes is required to up-regulate CD5 expression and down-regulate surface TCR-β expression in this subset (36). Consequently, CD45−/− and CD45L/− express low CD5 and high TCR-β surface levels (10, 31). CS Tg failed to alter or rescue either thymic selection or DP surface marker expression in the context of −/−, L/−, or +/− endogenous CD45 expression (Fig. 2 A–C).
To further assess the functional consequences of CS Tg expression upon positive selection directly, we studied thymic development in the context of a fixed TCR repertoire by breeding CS Tg onto either the class II-restricted OTII TCR transgenic or the class I-restricted OTI TCR transgenic in the context of CD45+/− endogenous expression (37, 38). CS Tg had no effect on either class II- or class I-restricted thymic development (Fig. 2 D–F and Fig. S1D).
To determine the effect of the CS transgene on CD45 phosphatase activity more directly, we assayed phosphorylation of the direct substrate of CD45, the inhibitory tyrosine of Lck (Y505) in thymocytes. We observed no effect of either CS or ΔC transgenes on Lck Y505 phosphorylation, irrespective of endogenous CD45 expression (Fig. 2G). We next assayed proximal biochemical responses to TCR stimulation, such as calcium increase and Erk phosphorylation. CS Tg had no effect on TCR signaling by these measures in the CD45+/− genetic background (Fig. S1 E and F). Together, we found no evidence of an extracatalytic CD45 function in thymocyte development or TCR signaling, or for a dominant negative function of the CS Tg in regulating endogenous CD45 phosphatase activity in thymocytes.
We went on to assess the effect of the CS Tg signal transduction and activation of peripheral T cells. We observed no effect of CS Tg on intracellular calcium increase in either naïve or memory CD4 LN T cells (Fig. S2A). We assessed T-cell activation in response to plate-bound anti-CD3ε stimulation and found no effect of the CS Tg in the context of either L/− or +/− endogenous CD45 alleles on CD69 up-regulation (Fig. S2 B and C). Because CD45 has been postulated to play a role in kinetic segregation, we also wanted to assess T-cell activation in the context of a bona fide APC–T-cell interaction. To do so, we stimulated either OTII CD4 or OTI CD8 T cells from CS Tg+ and Tg− mice with varying doses of ovalbumin (OVA)-derived peptide. We observed no effect of the CS Tg on CD69 up-regulation (Fig. S2 D and E). We went on to titrate a lower affinity peptide (T4) in the context of the OTI Tg (39), yet were unable to unmask an effect of the CS Tg (Fig. S2D).
Fig. S2.
CS transgene does not affect mature T-cell signaling or activation. (A) Lymphocytes from CD45+/− mice with (blue) or without (red) CS Tg were mixed together, loaded with indo-1 to detect intracellular calcium, surface stained to identify CD4+ T cells, genotype (CD45.1+ CS Tg and CD45.1− non Tg), as well as CD62Lhi naïve, and CD62Llo memory compartments. Cells were stimulated with varying doses of anti-CD3ε or with ionomycin and assessed via flow cytometry (experiments were also performed unmixed without CD45.1 staining with similar results). Data are representative of at least two independent experiments. (B–E) CD4 or CD8 T cells from CD45+/− (B), CD45 L/− (C), CD45+/− OT1 (D), or CD45+/− OT2 (E) with or without CS Tg expression were stimulated with varying doses of plate-bound anti-CD3ε (B and C), or OVA, T4 peptides (D and E), and assessed for CD69 expression after 20 h. Graphs depict percent of CD69+ cells within each sample and are representative of at least two independent experiments.
Csk Titration Rescues Basal Thymic but Not B-Cell Phenotypes in CD45 Allelic Series Mice.
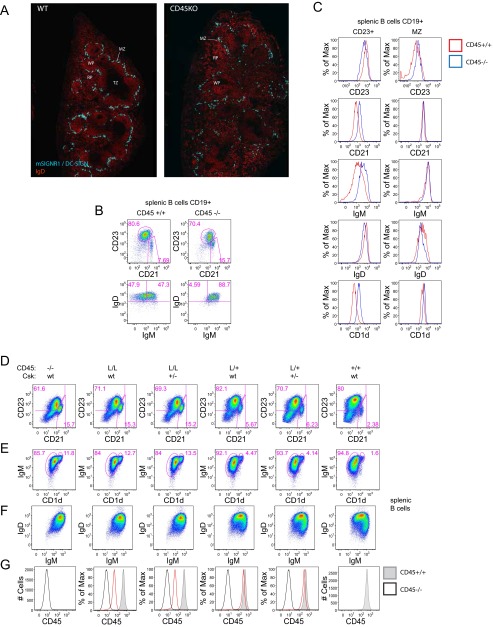
CD45-deficient B cells exhibit an intact physical MZ as marked by MZ macrophages (Fig. S3A), but an expanded MZ B-cell compartment with increased CD23 expression (Fig. S3 B and C). Importantly, other markers of the MZ B-cell compartment are intact, including CD21, IgM, and CD1d, confirming the identity of this cell population (Fig. S3C). Furthermore, CD45−/− B cells develop IgMhiIgDhi (referred to as FO-II) B cells in the spleen, but lack IgMloIgDhi (referred to as FO-I) B cells (Fig. S3B) (11, 40). FO-II and -I have both been described as long-lived (and therefore “mature”) splenic B-cell populations, but FO-I does not develop in the absence of antigen or in Btk-deficient mice with impaired BCR signaling (41, 42). Therefore, it has been suggested that strong BCR signaling is required for the development of FO-I B cells. Surface expression of coreceptors on IgDhi splenic CD45−/− B cells is also altered (Fig. S3C). Collectively, these phenotypes have been attributed to impaired BCR signal transduction during B-cell development in CD45−/− mice and vary continuously with titration of CD45 surface expression in allelic series mice (Fig. S3 D–G) (32).
Fig. S3.
Csk titration does not rescue B-cell phenotypes in CD45 allelic series mice. (A) Frozen spleen sections from CD45+/+ (WT; Left) and CD45−/− mice were stained by immunofluorescence to detect MZ macrophages with anti-mSIGNR1/DC-SIGN Ab and anti-IgD to detect B cells. MZ, MZ macrophages; TZ, T-cell zone; WP, white pulp; RP, red pulp. (B) Plots represent CD19+ splenocytes (Upper) from CD45+/+ (Left) and CD45−/− (Right) mice stained with CD23 and CD21 to detect MZ (CD21hi CD23lo) and T2/Follicular mature (CD23hi) subsets. Lower depicts CD23hiIgDhi subsets stained with IgD and IgM to detect FO-2 (IgMhi) and FO-I (IgMlo) subsets. (C) Histograms depict CD23+ (Left) and MZ (Right) splenic B-cell subsets from CD45+/+ (red) and CD45−/− (blue) mice as gated in B stained for CD23, CD21, IgM, IgD, and CD1d expression. (D and E) Plots represent CD19+ splenocytes from allelic series mice (−/−, L/L, L/+, and WT CD45 endogenous alleles) harboring one or two endogenous Csk alleles. (+/− or WT) stained with CD21 and CD23 to identify MZ (CD21hi CD23lo) and T2/Follicular mature (CD23hi) subsets (D) or IgM and CD1d to identify MZ B cells (IgMhi CD1dhi) (E). (F) Plots represent CD19+ splenocytes from mice above with MZ B cells excluded from gate and stained with IgM and IgD to identify receptor expression. (G) Histograms represent surface CD45 expression on CD19+ splenocytes from mice above (red line) overlaid with CD45−/− (black line) and CD45+/+ (shaded gray histogram) or reference. Data are representative of at least three independent experiments.
To assess whether CD45 phosphatase activity indeed mediates these phenotypes, we reduced Csk expression by half in CD45 allelic series mice to look for rescue of B-cell phenotypes. We reasoned that rebalancing CD45 phosphatase/Csk kinase activity at the level of the inhibitory SFK tyrosines might rescue those phenotypes that are directly driven by SFK activity. Csk+/− on the background of CD45L/L and L/+ mice with reduced surface CD45 expression (30% and 50%, respectively) was sufficient to rescue basal thymic phenotypes such as TCR-β expression on DP thymocytes as reported (31). However, to our initial surprise, we observed no rescue of B-cell phenotypes driven by reduced CD45 expression, such as expanded MZ compartment, upon titration of Csk expression, suggesting that these B-cell phenotypes may be driven by a nonenzymatic function of CD45 (Fig. S3 D–F).
CS and ΔC Transgenes Regulate B-Cell Development Independently of Endogenous CD45.
In contrast to the functional silence of CS and ΔC Tgs in thymocytes and naïve T cells, both Tgs similarly and partially rescued the B-cell phenotype of CD45-deficient mice (Fig. 3 A–C). CS and ΔC Tgs reduced the size of the MZ compartment, as well as CD23 and IgD expression on MZ B cells, and partially rescued development of IgDhiIgMlo FO-I B cells as indicated by IgM down-regulation. Similar effects were observed irrespective of endogenous CD45 expression and partially recapitulate the effect of titrating CD45 surface levels in the allelic series (Fig. S4 A–D). CD45 titration also alters production of B1a B cells in the peritoneum. However, this phenotype was not modified by CS Tg (Fig. S4E). Together with the failure of Csk titration to rescue B-cell phenotypes in allelic series mice, these complementary data argue that abnormal B-cell development in CD45-deficient mice is partly attributable to a nonenzymatic function of CD45.
Fig. 3.
CS and ΔC transgenes regulate B-cell development independently of endogenous CD45. (A) splenocytes from CD45−/− mice with or without CS or ΔC Tg expression were stained to detect CD21, CD23, IgM, and IgD. Upper depicts gating for CD21hi CD23lo MZ B cells. Lower depicts gating for FO-I (IgMloIgDhi) and FO-II (IgMhiIgDhi) subsets. (B and C) Histograms represent MZ B cells (B) as gated in A or CD23+ splenic B cells (C) stained to detect surface expression of CD23, IgD, and IgM from CD45−/− mice with (blue) or without (red) CS Tg (Upper) or ΔC Tg (Lower) expression. (D) Splenocytes from CD45+/− mice with or without CS Tg with an unrestricted repertoire (two left-hand columns) or with the IgHEL BCR Tg (MD4; two right-hand columns) were stained and gated as in A. (E and F) Histograms represent MZ B cells (E) as gated in D or CD23+ splenic B cells (F) stained to detect surface expression of CD23, IgD, and IgM from CD45+/− mice with (blue) or without (red) CS Tg with unrestricted repertoire (Upper) or IgHEL BCR Tg (Lower) expression. Data are representative of at least three independent experiments.
Fig. S4.
CS transgene regulates B-cell development independently of endogenous CD45. (A) Plots represent CD19+ splenocytes from allelic series mice (−/−, L/−, or +/− endogenous CD45 alleles) in the absence or presence of a single copy of the CS Tg stained with CD21 and CD23 to identify MZ B cells (CD21hi CD23lo). (B and D) histograms represent MZ B cells (B) as gated above or CD23+ splenic B cells (D) from allelic series mice with (blue) or without (red) CS Tg expression stained to detect surface expression of CD23, IgD, and IgM. (C) Plots represent CD19+ splenocytes excluding MZ B cells from mice in A stained for IgM and IgD expression. Gates identify FO-II and -I subsets. (E) Plots represent peritoneal CD19+ B cells from allelic series mice with or without CS Tg expression stained with CD5 and CD23 to identify B1a (CD5hiCD23neg) and B2 (CD23hiCD5neg) subsets. (F) Plots represent CD19+ splenocytes from IgHEL Tg mice with either +/− or +/+ endogenous CD45 expression in the presence or absence of a single copy of the CS Tg stained with CD21 and CD23 to identify MZ B cells (CD21hi CD23lo). Data are representative of at least three independent experiments.
CS and ΔC Transgenes Regulate B-Cell Development in an Antigen-Independent Manner.
To more directly test the contribution of antigenic stimulation and repertoire selection to the B-cell phenotypes driven by the CS Tg, we took advantage of the Ig hen egg lysozyme (IgHEL) BCR Tg (MD4 line) (43) to generate CD45+/− mice with a monoclonal BCR repertoire, but lacking endogenous antigen in the presence or absence of CS Tg. CS and ΔC developmental and surface marker phenotypes described above persisted with repertoire restriction in the absence of antigen (Fig. 3 D–F and Fig. S4F), suggesting that these phenotypes are antigen-independent and not driven by inducible BCR signaling. As previously described, development of IgMloIgDhi FO-I B cells, as indicated by IgM down-regulation, was completely blocked in IgHEL Tg mice lacking antigenic stimulation (42), but very slightly rescued by introduction of the CS Tg (Fig. 3D).
CS and ΔC Transgenes Decrease Basal B-Cell Calcium but Have Minimal Effects on Inducible BCR Signaling.
To determine the effect of CS and ΔC transgenes directly on the BCR signaling pathway, we assayed BCR-induced calcium entry and Erk phosphorylation in these mice. CS- and ΔC-expressing FO B-cell subsets displayed a marked reduction in basal calcium and a mildly dampened calcium response to IgM ligation that was largely attributable to the basal difference (Fig. 4A). This phenotype was similar in both Tg lines and was also independent of endogenous CD45 expression (i.e., apparent in either CD45−/− or CD45+/− genetic backgrounds). Repertoire restriction with the IgHEL BCR Tg eliminated this difference in basal calcium and revealed subtly dampened calcium signaling in response to either IgM ligation or stimulation with bona fide antigen (HEL protein) (Fig. 4 B and C). CS and ΔC had minimal effects on Erk phosphorylation in response to either IgM cross-linking or bona fide antigen (sHEL) in all splenic B-cell subsets (Fig. S5 A–D).
Fig. 4.
CS and ΔC transgenes decrease basal B-cell calcium but have minimal effects on inducible BCR signaling. (A) CD23+ splenocytes from either CD45−/− or CD45+/− mice with (blue) or without (red) CS or ΔC Tgs were loaded with indo-1 to detect intracellular calcium, stimulated with varying doses of anti-IgM or with ionomycin, and assessed via flow cytometry. (B and C) CD23+ splenocytes from CD45+/− IgHEL BCR Tg mice with (blue) or without (red) CS Tgs were treated as in A and stimulated either with anti-IgM (B) or HEL antigen (C). Data in A–C are representative of at least three independent experiments. (D) Blots represent B-cell lysates from CD45−/− and CD45+/− mice with or without CS Tg expression. B cells were treated with or without anti-IgM (10 μg/mL) for 3 min. Blots were probed to detect various total protein and phospho-specific epitopes. Data are representative of two independent experiments. (E and F) Serum was sampled before and 7 d after IV NP-Ficoll immunization of CD45+/− mice with (blue) or without (red) CS Tg. Graphs represent serum titers of NP-specific IgM (E) and IgG3 (F) antibodies from n = 5 mice (± SEM).
Fig. S5.
CS transgene has minimal effects on inducible BCR signaling. (A–D) CD45+/− B cells with or without IgHEL (MD4) Tg expression, in the presence or absence of CS Tg, were stimulated with varying doses of anti-IgM, sHEL cognate antigen, or phorbol 12-myristate 13-acetate (PMA), fixed, permeabilized with methanol, and stained to detect intracellular pErk as well as surface markers CD21 and CD23 to identify splenic B-cell subsets. Plots in A depict gating scheme to identify subsets. Histograms in B–D represent pErk expression in unstimulated or stimulated B-cell subsets as gated in A from mice with (blue line) or without (red line) CS Tg expression. Data in A–D are representative of at least three independent experiments. (E) LN B cells from CD45+/− mice with (blue line) or without CS Tg (red line) expression were stimulated overnight with anti-IgM and assessed for up-regulation of surface CD69 expression. Graph represents % CD69+ (± SEM) from three biological replicates.
As previously reported, we observe that CD45−/− B cells exhibit basal and inducible increases in phosphorylation of inhibitory Lyn Y507 and impaired phosphorylation of activation loop SFK tyrosines relative to CD45+/− B cells (Fig. 4D) (32). However, CS Tg had no effect on these biochemical phenotypes and, further, did not alter basal or inducible phosphorylation of either ITAM- or ITIM-dependent signaling proteins (Fig. 4D). In vitro activation of CS Tg B cells resulted in a very subtly impaired up-regulation of CD69 (Fig. S5E).
Finally, to assess the functional effect of catalytically inactive CD45 on B cells in vivo, we performed i.v. immunizations with the T-cell–independent antigen NP–Ficoll to specifically probe the MZ B-cell response. CS Tg in the context of CD45+/− background conferred a decreased NP-specific IgG3 and IgM serum antibody response at 7 d (Fig. 4 D and E). This result may in turn be due to a reduction in MZ B-cell number and/or impaired MZ B-cell function.
Extracatalytic Function of CD45 Tgs Is Mediated by CD22.
We reasoned that similar B-cell developmental, surface marker, and signaling phenotypes in CS and ΔC Tg mice implicate either the EC or transmembrane domains of CD45 in extracatalytic function. The pronounced Tg phenotypes in the absence of endogenous CD45 expression argue against a dominant-negative mechanism mediated by homodimerization. Furthermore, these phenotypes were largely independent of Ag, and the CS/ ΔC Tgs had little effect on directly assayed inducible BCR signaling. Together, these features led us to hypothesize that CS and ΔC proteins modulate tonic, but not inducible, BCR signaling and that they might do so via constitutive cis-interaction of their EC domains with a coreceptor on the surface of B cells. We reasoned that the inhibitory coreceptor CD22 was an attractive candidate because it contains an EC sia-binding domain, and the EC domain of CD45 has many glycosylation and sialylation sites (6, 44). Further, prior cross-linking, immunoprecipitation, and SPR studies of CD45 have identified CD22 as a putative interacting partner, albeit of unclear functional significance (21, 45–47). Finally, the CS and ΔC Tgs partially phenocopy CD22-mutant alleles with impaired sia-binding (R130E) (26).
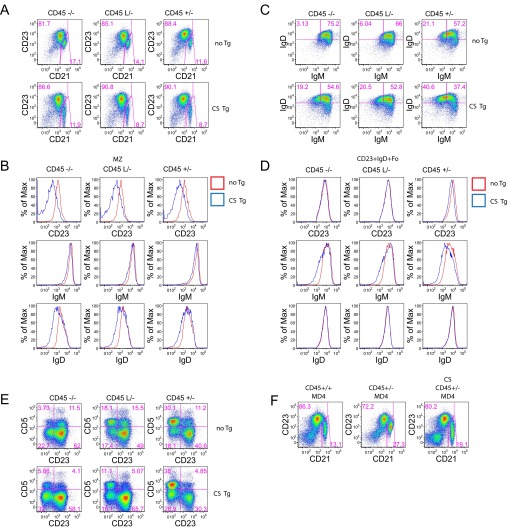
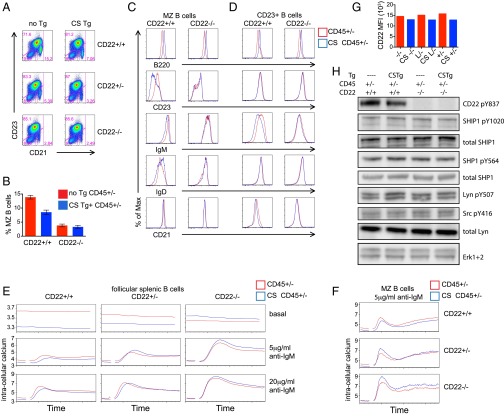
To determine whether the extracatalytic function of CD45 in B cells requires CD22 expression, we introduced CS Tg in the context of CD45+/− onto a CD22-deficient genetic background. Strikingly, the CS Tg had no impact on MZ development, FO-I follicular B-cell development as indicated by IgM down-regulation, or surface receptor phenotypes, in the absence of CD22 (Fig. 5 A–D). Additionally, the marked decrease in basal calcium in the presence of CS Tg was rescued by decreased CD22 expression in a dose-dependent manner (Fig. 5 E and F). Together, these data suggest that CD22 expression is essential to mediate the effects of the CS Tg. Interestingly, although reducing CD22 dose dramatically enhances inducible BCR signaling, CS Tg expression has minimal effect on inducible calcium on either a CD22-sufficient background or CD22 deficient background (Fig. 5 E and F). Importantly, we show that titration of endogenous CD45 expression or introduction of the CS Tg have minimal effects on CD22 surface expression levels, such that altered function of CD22 rather than altered expression must mediate the CS Tg phenotype (Fig. 5G). These data suggest that the CS Tg exerts its effect on B-cell development via modulation of CD22 function during basal, but not inducible, BCR signaling.
Fig. 5.
The extracatalytic function of CD45 Tgs on CD45+/− genetic background is mediated by CD22. (A) Splenocytes from CD45+/− mice with or without CS Tg in the context of CD22+/+, CD22+/−, or CD22−/− were stained to detect CD21 and CD23 expression. Gating represents T1 (CD21loCD23lo), MZ (CD21hiCD23lo), and T2/Fo (CD23hi). (B) Graph represents percent MZ B cells as gated in A (± SEM) from three biological replicates. (C and D) Histograms represent MZ B cells (C) as gated in A or CD23+ splenic B cells (D) stained to detect surface expression of B220, CD23, IgM, IgD, and CD21 from CD45+/− mice with (blue) or without (red) CS Tg in the context of either CD22+/+ or CD22−/−. (E and F) CD23+ splenocytes (E) or MZ B cells (F) from CD45+/− mice with (blue) or without (red) CS Tg expression in the context of either CD22+/+, CD22+/−, or CD22−/− were loaded with indo-1 to detect intracellular calcium, stimulated with varying doses of anti-IgM or with ionomycin, and assessed via flow cytometry. E, Top depicts unstimulated B cells. Data in A–F are representative of at least three independent experiments. (G) Graph represents MFI of surface CD22 expression on allelic series splenic B cells with (blue) or without (red) CS expression. Data in G are representative of at least five independent experiments. (H) Blots represent unstimulated B cells from CD45+/− mice with or without CS Tg expression in the context of either CD22+/+ or CD22−/− probed to detect various total protein and phospho-specific epitopes. Data are representative of two independent experiments.
To understand whether this effect was occurring at the level of SFK phosphorylation or CD22 ITIM phosphorylation, both critical components in the CD22-dependent inhibitory pathway, we assessed relevant regulatory tyrosine phosphorylation in resting B cells from either CD22-sufficient or deficient CD45+/− mice in the presence or absence of CS Tg. The presence of the CS Tg did not clearly affect SFK, CD22, SHIP-1, or SHP-1 regulatory tyrosine phosphorylation in the basal state (Fig. 5H). We take this finding to indicate that altered localization of CD22 on the cell surface in the presence of CS Tg may mediate CS phenotypes, rather than a direct effect on SFK, CD22, or inhibitory PTPase activity. Indeed, physical association of CD22 with the IgM BCR is important for its inhibitory function (48).
Finally, we wanted to confirm that the partial rescue of B-cell development and alteration in basal calcium by CS Tg that was observed in the CD45−/− background was also mediated by CD22. To address this question, we generated CD45−/− mice sufficient or deficient for CD22 in the presence or absence of CS Tg. We assessed B-cell development and calcium signaling in these mice. We observe that CD22 deficiency resulted in partial rescue of B-cell phenotypes in CD45−/− mice (Fig. 6 A–D). The CS Tg phenocopies this effect, and, in the absence of CD22, has virtually no superimposed effect on B-cell development, maturation, and surface phenotype. Additionally, the marked decrease in basal calcium in the presence of CS Tg was recapitulated by deletion of CD22 (Fig. 6E). Interestingly, CD22 deficiency enhanced inducible BCR signaling, even in the complete absence of CD45 expression, possibly because of the expression of the partially redundant phosphatase CD148 and residual Lyn kinase activity (12). Importantly, CS transgene had no effect on either basal or inducible calcium signaling by CD45−/− B cells in the absence of CD22. These data demonstrate that CD22 partially mediates the phenotype of CD45−/− B cells and is essential for rescue of this phenotype by phosphatase-inactive CS Tg.
Fig. 6.
Partial rescue of CD45−/− B-cell development by catalytically inactive CD45 CS Tg is mediated by CD22. (A and B) Splenocytes from either CD45+/+ or CD45−/− mice with or without CS Tg in the context of either CD22+/+ or CD22−/− were stained to detect CD21, CD23, IgM, and IgD expression on CD19+ B cells. Gating in A represents T1 (CD21loCD23lo), MZ (CD21hiCD23lo), and T2/Fo (CD23hi). (C and D) Histograms represent MZ B cells (C) as gated in A or CD23+ splenic B cells (D) stained to detect surface expression of CD23, IgD, CD21, and IgM from CD45−/− mice (first two columns) with (blue) or without (red) CS Tg in the context of either CD22+/+ (Left) or CD22−/− (Right) background. Analogous samples from CD45+/+ mice (third column) with either CD22+/+ (black) or CD22−/− (green) background are depicted for reference. (E) CD23+ splenocytes from CD45−/− mice (first two columns) with (blue) or without (red) CS Tg expression in the context of either CD22+/+ (Left) or CD22−/− (Right) were loaded with indo-1 to detect intracellular calcium, stimulated with varying doses of anti-IgM or with ionomycin, and assessed via flow cytometry. Analogous data from CD45+/+ mice (third column) with either CD22+/+ (black) or CD22−/− (green) background are depicted for reference. Top depicts unstimulated B cells. Data are representative of three independent experiments.
Discussion
The function of the ectodomain of CD45 has remained elusive even though the role of its PTPase domain in dephosphorylating the C-terminal inhibitory tyrosine of the SFKs has been very well established in cell lines and mice (6). Several features of CD45 structure and expression demand an explanation, including its remarkable abundance that far exceeds the theoretical requirements for enzyme function. Indeed, CD45 is at least 10 times more abundant than the partially redundant phosphatase CD148, yet each protein plays a relatively comparable role in regulating Lyn phosphorylation in myeloid cells (12). Buffering of basal PTPase activity by the kinase Csk results in a very broad dynamic range of SFK inhibitory tyrosine phosphorylation with extensive titration of CD45 expression in allelic series mice (31, 32). However, whether CD45 abundance drives the requirement for such buffering by Csk, or vice versa, is not clear. Finally, the large ectodomain of CD45 is both alternatively spliced and heavily glycosylated (6), yet the function of these features remains unknown.
Several groups have hypothesized that CD45 could form homodimers (6). It has been proposed that a cytosolic membrane-proximal “wedge”-like domain in CD45 could project into the PTPase domain of an adjacent CD45 molecule in the context of a homodimer and inhibit enzymatic activity (17). Indeed, a point mutation introduced into the wedge domain (E624R) was sufficient to abolish dimerization-induced inhibition of PTPase activity in human cell lines (49). However, mice harboring the analogous mutation (E613R) unexpectedly exhibit dysregulated BCR signaling attributable to impaired access of mutant CD45 to the SFK substrate Lyn (50–52). One prediction of this dimerization model is that full-length CD45 lacking PTPase activity (CS Tg) should exert an inhibitory effect on endogenous CD45 PTPase function, whereas ΔC Tg lacking the wedge domain entirely should not. We engineered mice in which CS Tg expression far exceeded endogenous CD45 expression in the L/− genetic background (estimated 5- to 10-fold relative abundance of Tg to endogenous CD45). Importantly, total surface CD45 expression in these Tg animals reached approximately wild-type levels. We failed to detect any effect of the CS Tg on Lck Y505 phosphorylation, TCR signaling, T-cell development, or T-cell activation—all phenotypes that are readily and broadly titratable across CD45 allelic series mice (31, 32). These data argue against physiologically relevant dimerization-induced inhibition of CD45 PTPase activity in thymocytes or naïve T cells. It has been shown that CD45 cross-linking more efficiently captures dimers of short-isoform CD45RO proteins than long-isoform CD45RABC proteins (53). This finding raised the possibility that dimerization may play a role selectively in CD45RO-expressing effector/memory T cells in vivo. However, our signaling studies of CD62Llo CD45RBlo memory CD4 T cells in the context of CS Tg reveal no evidence of a dominant negative effect.
CS and ΔC Tgs regulate B-cell phenotypes identically and independently of endogenous CD45 expression, arguing against a role for homodimerization, for the wedge domain, or for any unanticipated substrate trapping by the CS Tg in mediating these phenotypes. We were thus unable to detect a nonenzymatic role for the cytosolic domain of CD45. The presence of a robust phenotype on the CD45-null background also excludes the possibility that subtle reduction in endogenous CD45 expression by Tg in the CD45+/− B-cell background accounts for these phenotypes.
Interestingly, CS and ΔC Tgs partially rescue the B-cell phenotype of CD45−/− mice and do so at approximately wild-type surface CD45 concentrations, implying that the CD45-deficient phenotype is driven partly by loss of a nonenzymatic function of CD45. Indeed, this finding also implies that B-cell phenotypes that vary across the previously described CD45 allelic series of mice are also partly attributable to nonenzymatic functions of CD45 (32). This finding is consistent with failure to rescue such B-cell phenotypes by titrating Csk expression in allelic series mice. These findings provide a plausible explanation for a long-standing paradox in CD45 biology, in which inducible BCR signaling is minimally impaired in CD45−/− B cells, whereas B-cell development and cell surface phenotypes are quite aberrant, particularly in the context of a BCR Tg, where repertoire compensation cannot mask the phenotype (54). Relatively preserved inducible BCR signaling in CD45−/− B cells is attributable to expression of the partially redundant phosphatase CD148, but begs the question of why signaling and development appear so discordant in these animals. Here, we propose that this discordance is due to a unique nonenzymatic function of CD45 in restricting inhibitory function of CD22 in the basal state (Fig. S6). This model predicts, counterintuitively, that CD22 deficiency should partially rescue CD45 deficiency, even though the substrate of CD45 is required for CD22 function. Indeed, it has been previously reported that the “motheaten” Shp-1 hypomorph partially rescues CD45−/− B-cell development, consistent with this model (55). Our data indeed reveal a partial rescue of CD45−/− B-cell phenotypes by CD22 deletion, including MZ B-cell development, FO-I B-cell development, and basal intracellular calcium. Consistent with such rescue, CD22−/− mice exhibit a reduced MZ B-cell compartment and enhanced FO-I B-cell development (56). Interestingly, rescue of MZ B-cell development and phenotype by CS Tg was independent of BCR repertoire and cognate antigen, whereas rescue of FO-I B-cell development was not. Indeed, it has been shown that FO-I B-cell development requires cognate antigen, a diverse BCR repertoire, or exaggerated tonic BCR signaling (32, 42, 54). Similarly, CS Tg has no effect on basal intracellular calcium in the absence of a diverse BCR repertoire and cognate antigen, perhaps because chronic antigen stimulation rather than merely tonic BCR signaling is necessary to alter basal calcium.
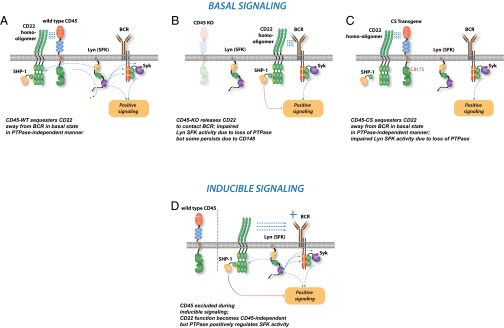
Fig. S6.
Model of CD45 and CD22 interaction during basal and inducible BCR signaling. In the basal state, the SFK Lyn is constitutively dephosphorylated by active CD45 (A) and in turn phosphorylates both ITIMs on CD22 and ITAMs on the CD79 chains of the BCR. CD22 exerts an inhibitory effect on basal BCR signaling. In addition, CD45 restricts the inhibitory function of CD22 in a noncatalytic manner that is independent of its cytosolic domain, perhaps via relocalization of CD22. In the absence of CD45 in the basal state (B), both effects of CD45, catalytic and noncatalytic, are curtailed. Lyn is less active, but some residual kinase activity remains due to expression of the partially redundant phosphatase CD148 in B cells. The unchecked residual inhibitory function of CD22 in the absence of CD45 exacerbates the developmental and basal phenotype of CD45−/− B cells. (C) Reintroducing the CS CD45 Tg on the CD45−/− background in the basal state does not alter Lyn phosphorylation relative to CD45−/− cells, but restricts access of CD22 to the BCR and presumably its associated pool of Lyn, thereby limiting the inhibitory function of CD22 and partially rescuing the phenotype of CD45−/− B cells. (D) Upon BCR ligation, CD22 is recruited to interact with the BCR in a CD45-independent manner. Therefore, in the context of inducible stimulation, CD45 CS Tg has no discernable phenotype.
Interestingly, a reduced TI-2 response has also been reported in CD22−/− mice correlating with impaired MZ B-cell development (57). We propose that impaired TI-2 responses in CS Tg mice on the CD45+/− background may also be mediated by CD22. Because BCR-induced MZ B-cell signal transduction as assayed by Erk phosphorylation and intracellular calcium increase are largely preserved in CS Tg mice, we propose that impaired MZ B-cell development, rather than MZ B-cell function, as mediated by CD22, may account for impaired TI-2 responses in CS Tg mice.
In contrast to MZ and follicular B-cell development, CS Tg is unable to rescue impaired B1a B-cell development in CD45 allelic series mice. This result may perhaps be explained by the fact that CD22−/− mice have minimal B1a phenotype, attributable to the more central role of Siglec-G in this cell population (18).
CD45 has been suggested to interact with CD22 in trans and/or in cis through cell line adhesion assays, biochemical assays with immunoprecipitation and cross-linking, and surface plasmon resonance (21, 45–47). There are contradictory data about whether this interaction involves and/or requires sialic acid binding by CD22. Furthermore, the functional role of a CD45–CD22 interaction has not been previously defined. We have previously shown that CD45 allelic series B-cell phenotypes are B-cell–intrinsic via mixed chimera studies, and CD22 has an expression pattern that is narrowly restricted to the B-cell lineage (www.immgen.org). Here we provide clear genetic evidence for a functional interaction between these proteins in vivo, and further suggest that the interaction occurs in cis on B cells.
CD22 R130E mice, in which sialic acid binding is lost, were recently described (26). B cells in these mice exhibit impaired BCR-induced calcium signaling, consistent with a gain-of-function for CD22 R130E allele. One model proposes that sialic acid binding by CD22 restricts access of CD22 to the BCR. However, the B-cell development phenotype of these mice is discordant with the inducible signaling phenotype. R130E mice have reduced MZ B-cell compartment, and follicular B cells express reduced levels of surface IgM, much like CS and ΔC Tg mice. The discordance between basal and inducible BCR signaling phenotypes is also reminiscent of the CD45 allelic series B-cell phenotypes and that of the CS Tg.
We propose a model whereby interaction with CD45 limits access of CD22 to the BCR in the resting state (Fig. S6 A–C). However, upon BCR ligation, CD22 access to the BCR becomes CD45 ectodomain-independent, perhaps because of cytoskeletal interaction (Fig. S6D). This relocalization of CD22 has functional importance because the association of CD22 and the BCR has been shown to be critical to inhibit inducible BCR signaling (48). CD45 also positively regulates Lyn function, which is in turn essential for CD22 ITIM phosphorylation and SHP-1/SHIP1 effector phosphatase recruitment (18). However, some Lyn function and CD22 ITIM phosphorylation can persist even in the absence of CD45, probably attributable to the partially redundant phosphatase CD148 (12). Consistent with this model, we see little difference in basal and inducible CD22 phosphorylation in the presence or absence of CD45 (Fig. 4D). Indeed, CD22 can continue to exert some basal and inducible inhibitory function in the CD45−/− genetic background (Fig. 6). Thus, dissecting the catalytic and noncatalytic functions of CD45 in B cells requires CD45 mutants that uncouple these functions, such as CS and ΔC Tgs.
Here, we provide evidence for the function of the ectodomain of CD45 in vivo, independent of catalytic function. We provide genetic evidence for a physiologic interaction between CD45 and CD22 that mediates this noncatalytic function. This interaction serves to restrain CD22 inhibition of tonic BCR signaling, while permitting CD22 to enforce clonal ignorance of weakly autoreactive B cells in the face of autoantigen encounter.
Experimental Procedures
Mice.
Lightning mice were generated directly on the C57BL/6 genetic background during N-ethyl-N-nitrosourea mutagenesis screen conducted at Australian National University and have been described (31). CD45−/− mice were described (9). CD22−/− mice were obtained from Edward Clark, University of Washington, Seattle, and have been described (23). CS Tg and ΔC Tg CD45 mice were generated in the W.C.R. laboratory, and CS mice have been described (33, 34). IgHEL (MD4), OT1, and OT2 mice were described (37, 38, 43). All knockout and transgenic strains were fully back-crossed to C57BL/6 genetic background. Mice were used at 5–9 wk of age for all functional and biochemical experiments. All mice were housed in a specific pathogen-free facility at University of California, San Francisco, according to the University Animal Care Committee and National Institutes of Health (NIH) guidelines.
Antibodies and Reagents.
Antibodies were obtained as follows: murine AA4.1, CD3, CD4, CD5, CD8, CD19, CD21, CD22, CD23, CD44, panCD45, CD45RB, B220, CD45.1, CD45.2, CD62L, CD69, IgM, IgD, TCR-β, and vα2 antibodies conjugated to FITC, PE, PerCP-Cy5.5, PE-Cy5.5, PE-Cy7, Pacific Blue, APC, or Alexa 647 (eBiosciences or BD Biosciences); pErk1/2 T202/Y204 (197G2) antibody for intracellular staining; pSrc Y416, pLyn Y507, pSHP-1 Y564, pSHIP1 Y1020, SHP-1, pPLCg2 Y1217, total pPLCg2, pCD79a, pSyk Y525/526, total Syk, and Lyn antibodies (Cell Signaling); SHIP1 and Erk 1 and 2 antibodies (Santa Cruz Biotechnology); pCD22 Y822 (mouse Y837) antibodies (Epitomics); pLck Y505 (BD Transduction); Lck (1F6) antibodies (prepared in our laboratory); unconjugated CD3ε (2C11) antibody (Harlan); goat anti-Armenian hamster IgG(H+L) antibody, goat anti-mouse IgM, and goat anti-rabbit IgG antibody conjugated to either PE or APC (Jackson Immunoresearch). HEL was obtained from Sigma. OVA, Q4R7, and T4 peptides (New England Peptide) have been described (39).
Flow Cytometry and Data Analysis.
Cells were stained with indicated antibodies and analyzed on FACSCalibur or Fortessa (Becton Dickson) as described (50). Data analysis was performed by using FlowJo software (Version 9.7.6; Treestar). Statistical analysis and graphs were generated by using Prism (Version 4c; GraphPad Software).
Lymph Node T- and B-Cell Stimulation and Intracellular Phospho-Erk Staining.
Lymph node T- and B-cell stimulation and intracellular phospho-Erk staining were performed as described (58), except that CS Tg+ and Tg− lymphocytes were mixed together for activation assays and were distinguished from one another by congenic marker staining at harvest. Because CD45.1 sequence was used to generate CS and ΔC Tgs, cells from Tg+ mice are identifiable by CD45.1 congenic marker staining. Activation of OT1 and OT2 lymph node T cells were assessed similarly, except with titration of peptide rather than of plate-bound anti-CD3ε.
Calcium Measurements.
Assays were performed as described (58), except that CS Tg+ and Tg− lymphocytes were mixed together, loaded with Indo-1 dye (Invitrogen), and a UV laser on the BD Fortessa was used for detection. Before stimulation and analysis, lymphocytes were surface-stained for expression of CD45.1, to distinguish Tg+ (CD45.1+) and Tg− (CD45.1−) cells. For B-cell studies, CD21, CD23, and AA4.1 staining was used to identify B-cell subsets. For T-cell studies, CD4, CD8, and CD62L surface staining were undertaken as indicated in the figure legends. Stimulation was carried out by using varying doses of anti-IgM Fab′2 and/or sHEL for B cells or soluble anti-CD3ε (2C11) followed by anti-Armenian hamster antibody cross-linking for T cells.
Immunoblotting.
Immunoblotting was performed as described (12). Purified cell populations of splenic B cells were obtained by sorting with MACS kit as per manufacturer’s instructions (Miltenyi).
Immunization and ELISA.
NP-Ficoll (Biosearch) was dissolved in PBS and injected i.v. by tail vein at 100 μg per mouse. Serum was harvested at baseline and 7 d after immunization. ELISA to detect NP-specific IgM and IgG3 antibodies was performed as described (58), except that plates were coated with NP-(23)-KLH (10 μg/mL), and secondary antibody conjugated to HRP was used to detect IgM and IgG3 (Southern Biotech).
Acknowledgments
We thank Al Roque for assistance with animal husbandry; Facundo Batista, Tony DeFranco, and Anna Bakardjiev for helpful discussions; Mehrdad Matloubian for assistance with immunofluorescence; and Edward Clark for generously providing CD22−/− mice. This work was supported by the Rheumatology Research Foundation (J.Z.); NIH Grant K08 AR059723 (to J.Z.); and the Arthritis National Research Foundation (J.Z.). S.C. was a Howard Hughes Medical Institute Medical Research Fellow. M.N. is supported by a National Science Foundation fellowship.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1519925112/-/DCSupplemental.
References
- 1.Wardemann H, et al. Predominant autoantibody production by early human B cell precursors. Science. 2003;301(5638):1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 2.Pillai S, Mattoo H, Cariappa A. B cells and autoimmunity. Curr Opin Immunol. 2011;23(6):721–731. doi: 10.1016/j.coi.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zouali M, Sarmay G. B lymphocyte signaling pathways in systemic autoimmunity: Implications for pathogenesis and treatment. Arthritis Rheum. 2004;50(9):2730–2741. doi: 10.1002/art.20487. [DOI] [PubMed] [Google Scholar]
- 4.Lam KP, Kühn R, Rajewsky K. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 1997;90(6):1073–1083. doi: 10.1016/s0092-8674(00)80373-6. [DOI] [PubMed] [Google Scholar]
- 5.Koretzky GA, Kohmetscher MA, Kadleck T, Weiss A. Restoration of T cell receptor-mediated signal transduction by transfection of CD45 cDNA into a CD45-deficient variant of the Jurkat T cell line. J Immunol. 1992;149(4):1138–1142. [PubMed] [Google Scholar]
- 6.Hermiston ML, Zikherman J, Zhu JW. CD45, CD148, and Lyp/Pep: Critical phosphatases regulating Src family kinase signaling networks in immune cells. Immunol Rev. 2009;228(1):288–311. doi: 10.1111/j.1600-065X.2008.00752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwashima M, Irving BA, van Oers NS, Chan AC, Weiss A. Sequential interactions of the TCR with two distinct cytoplasmic tyrosine kinases. Science. 1994;263(5150):1136–1139. doi: 10.1126/science.7509083. [DOI] [PubMed] [Google Scholar]
- 8.Stone JD, et al. Aberrant TCR-mediated signaling in CD45-null thymocytes involves dysfunctional regulation of Lck, Fyn, TCR-zeta, and ZAP-70. J Immunol. 1997;158(12):5773–5782. [PubMed] [Google Scholar]
- 9.Kishihara K, et al. Normal B lymphocyte development but impaired T cell maturation in CD45-exon6 protein tyrosine phosphatase-deficient mice. Cell. 1993;74(1):143–156. doi: 10.1016/0092-8674(93)90302-7. [DOI] [PubMed] [Google Scholar]
- 10.Mee PJ, et al. Greatly reduced efficiency of both positive and negative selection of thymocytes in CD45 tyrosine phosphatase-deficient mice. Eur J Immunol. 1999;29(9):2923–2933. doi: 10.1002/(SICI)1521-4141(199909)29:09<2923::AID-IMMU2923>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 11.Byth KF, et al. CD45-null transgenic mice reveal a positive regulatory role for CD45 in early thymocyte development, in the selection of CD4+CD8+ thymocytes, and B cell maturation. J Exp Med. 1996;183(4):1707–1718. doi: 10.1084/jem.183.4.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu JW, Brdicka T, Katsumoto TR, Lin J, Weiss A. Structurally distinct phosphatases CD45 and CD148 both regulate B cell and macrophage immunoreceptor signaling. Immunity. 2008;28(2):183–196. doi: 10.1016/j.immuni.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McNeill L, et al. CD45 isoforms in T cell signalling and development. Immunol Lett. 2004;92(1-2):125–134. doi: 10.1016/j.imlet.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 14.Tchilian EZ, Beverley PC. Altered CD45 expression and disease. Trends Immunol. 2006;27(3):146–153. doi: 10.1016/j.it.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Dornan S, et al. Differential association of CD45 isoforms with CD4 and CD8 regulates the actions of specific pools of p56lck tyrosine kinase in T cell antigen receptor signal transduction. J Biol Chem. 2002;277(3):1912–1918. doi: 10.1074/jbc.M108386200. [DOI] [PubMed] [Google Scholar]
- 16.Davis SJ, van der Merwe PA. The kinetic-segregation model: TCR triggering and beyond. Nat Immunol. 2006;7(8):803–809. doi: 10.1038/ni1369. [DOI] [PubMed] [Google Scholar]
- 17.Desai DM, Sap J, Schlessinger J, Weiss A. Ligand-mediated negative regulation of a chimeric transmembrane receptor tyrosine phosphatase. Cell. 1993;73(3):541–554. doi: 10.1016/0092-8674(93)90141-c. [DOI] [PubMed] [Google Scholar]
- 18.Nitschke L. CD22 and Siglec-G regulate inhibition of B-cell signaling by sialic acid ligand binding and control B-cell tolerance. Glycobiology. 2014;24(9):807–817. doi: 10.1093/glycob/cwu066. [DOI] [PubMed] [Google Scholar]
- 19.Doody GM, et al. A role in B cell activation for CD22 and the protein tyrosine phosphatase SHP. Science. 1995;269(5221):242–244. doi: 10.1126/science.7618087. [DOI] [PubMed] [Google Scholar]
- 20.Leprince C, Draves KE, Geahlen RL, Ledbetter JA, Clark EA. CD22 associates with the human surface IgM-B-cell antigen receptor complex. Proc Natl Acad Sci USA. 1993;90(8):3236–3240. doi: 10.1073/pnas.90.8.3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang M, Varki A. Cell surface sialic acids do not affect primary CD22 interactions with CD45 and surface IgM nor the rate of constitutive CD22 endocytosis. Glycobiology. 2004;14(11):939–949. doi: 10.1093/glycob/cwh126. [DOI] [PubMed] [Google Scholar]
- 22.Nitschke L, Carsetti R, Ocker B, Köhler G, Lamers MC. CD22 is a negative regulator of B-cell receptor signalling. Curr Biol. 1997;7(2):133–143. doi: 10.1016/s0960-9822(06)00057-1. [DOI] [PubMed] [Google Scholar]
- 23.Otipoby KL, et al. CD22 regulates thymus-independent responses and the lifespan of B cells. Nature. 1996;384(6610):634–637. doi: 10.1038/384634a0. [DOI] [PubMed] [Google Scholar]
- 24.Sato S, et al. CD22 is both a positive and negative regulator of B lymphocyte antigen receptor signal transduction: Altered signaling in CD22-deficient mice. Immunity. 1996;5(6):551–562. doi: 10.1016/s1074-7613(00)80270-8. [DOI] [PubMed] [Google Scholar]
- 25.O’Keefe TL, Williams GT, Davies SL, Neuberger MS. Hyperresponsive B cells in CD22-deficient mice. Science. 1996;274(5288):798–801. doi: 10.1126/science.274.5288.798. [DOI] [PubMed] [Google Scholar]
- 26.Müller J, et al. CD22 ligand-binding and signaling domains reciprocally regulate B-cell Ca2+ signaling. Proc Natl Acad Sci USA. 2013;110(30):12402–12407. doi: 10.1073/pnas.1304888110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hennet T, Chui D, Paulson JC, Marth JD. Immune regulation by the ST6Gal sialyltransferase. Proc Natl Acad Sci USA. 1998;95(8):4504–4509. doi: 10.1073/pnas.95.8.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han S, Collins BE, Bengtson P, Paulson JC. Homomultimeric complexes of CD22 in B cells revealed by protein-glycan cross-linking. Nat Chem Biol. 2005;1(2):93–97. doi: 10.1038/nchembio713. [DOI] [PubMed] [Google Scholar]
- 29.O’Keefe TL, Williams GT, Batista FD, Neuberger MS. Deficiency in CD22, a B cell-specific inhibitory receptor, is sufficient to predispose to development of high affinity autoantibodies. J Exp Med. 1999;189(8):1307–1313. doi: 10.1084/jem.189.8.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duong BH, et al. Decoration of T-independent antigen with ligands for CD22 and Siglec-G can suppress immunity and induce B cell tolerance in vivo. J Exp Med. 2010;207(1):173–187. doi: 10.1084/jem.20091873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zikherman J, et al. CD45-Csk phosphatase-kinase titration uncouples basal and inducible T cell receptor signaling during thymic development. Immunity. 2010;32(3):342–354. doi: 10.1016/j.immuni.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zikherman J, Doan K, Parameswaran R, Raschke W, Weiss A. Quantitative differences in CD45 expression unmask functions for CD45 in B-cell development, tolerance, and survival. Proc Natl Acad Sci USA. 2011 doi: 10.1073/pnas.1117374108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Panchal RG, et al. Reduced levels of protein tyrosine phosphatase CD45 protect mice from the lethal effects of Ebola virus infection. Cell Host Microbe. 2009;6(2):162–173. doi: 10.1016/j.chom.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Panchal RG, et al. Reduced expression of CD45 protein-tyrosine phosphatase provides protection against anthrax pathogenesis. J Biol Chem. 2009;284(19):12874–12885. doi: 10.1074/jbc.M809633200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Virts EL, Diago O, Raschke WC. A CD45 minigene restores regulated isoform expression and immune function in CD45-deficient mice: Therapeutic implications for human CD45-null severe combined immunodeficiency. Blood. 2003;101(3):849–855. doi: 10.1182/blood-2002-07-1969. [DOI] [PubMed] [Google Scholar]
- 36.Azzam HS, et al. CD5 expression is developmentally regulated by T cell receptor (TCR) signals and TCR avidity. J Exp Med. 1998;188(12):2301–2311. doi: 10.1084/jem.188.12.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76(1):34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- 38.Hogquist KA, et al. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76(1):17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 39.Daniels MA, et al. Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature. 2006;444(7120):724–729. doi: 10.1038/nature05269. [DOI] [PubMed] [Google Scholar]
- 40.Benatar T, et al. Immunoglobulin-mediated signal transduction in B cells from CD45-deficient mice. J Exp Med. 1996;183(1):329–334. doi: 10.1084/jem.183.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Allman D, Pillai S. Peripheral B cell subsets. Curr Opin Immunol. 2008;20(2):149–157. doi: 10.1016/j.coi.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cariappa A, et al. The recirculating B cell pool contains two functionally distinct, long-lived, posttransitional, follicular B cell populations. J Immunol. 2007;179(4):2270–2281. doi: 10.4049/jimmunol.179.4.2270. [DOI] [PubMed] [Google Scholar]
- 43.Goodnow CC, et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988;334(6184):676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- 44.van der Merwe PA, et al. Localization of the putative sialic acid-binding site on the immunoglobulin superfamily cell-surface molecule CD22. J Biol Chem. 1996;271(16):9273–9280. [PubMed] [Google Scholar]
- 45.Bakker TR, Piperi C, Davies EA, Merwe PA. Comparison of CD22 binding to native CD45 and synthetic oligosaccharide. Eur J Immunol. 2002;32(7):1924–1932. doi: 10.1002/1521-4141(200207)32:7<1924::AID-IMMU1924>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 46.Sgroi D, Koretzky GA, Stamenkovic I. Regulation of CD45 engagement by the B-cell receptor CD22. Proc Natl Acad Sci USA. 1995;92(9):4026–4030. doi: 10.1073/pnas.92.9.4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stamenkovic I, Sgroi D, Aruffo A, Sy MS, Anderson T. The B lymphocyte adhesion molecule CD22 interacts with leukocyte common antigen CD45RO on T cells and alpha 2-6 sialyltransferase, CD75, on B cells. Cell. 1991;66(6):1133–1144. doi: 10.1016/0092-8674(91)90036-x. [DOI] [PubMed] [Google Scholar]
- 48.Tooze RM, Doody GM, Fearon DT. Counterregulation by the coreceptors CD19 and CD22 of MAP kinase activation by membrane immunoglobulin. Immunity. 1997;7(1):59–67. doi: 10.1016/s1074-7613(00)80510-5. [DOI] [PubMed] [Google Scholar]
- 49.Majeti R, Bilwes AM, Noel JP, Hunter T, Weiss A. Dimerization-induced inhibition of receptor protein tyrosine phosphatase function through an inhibitory wedge. Science. 1998;279(5347):88–91. doi: 10.1126/science.279.5347.88. [DOI] [PubMed] [Google Scholar]
- 50.Hermiston ML, Tan AL, Gupta VA, Majeti R, Weiss A. The juxtamembrane wedge negatively regulates CD45 function in B cells. Immunity. 2005;23(6):635–647. doi: 10.1016/j.immuni.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 51.Majeti R, et al. An inactivating point mutation in the inhibitory wedge of CD45 causes lymphoproliferation and autoimmunity. Cell. 2000;103(7):1059–1070. doi: 10.1016/s0092-8674(00)00209-9. [DOI] [PubMed] [Google Scholar]
- 52.Zikherman J, Parameswaran R, Hermiston M, Weiss A. The structural wedge domain of the receptor-like tyrosine phosphatase CD45 enforces B cell tolerance by regulating substrate specificity. J Immunol. 2013;190(6):2527–2535. doi: 10.4049/jimmunol.1202928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu Z, Weiss A. Negative regulation of CD45 by differential homodimerization of the alternatively spliced isoforms. Nat Immunol. 2002;3(8):764–771. doi: 10.1038/ni822. [DOI] [PubMed] [Google Scholar]
- 54.Cyster JG, et al. Regulation of B-lymphocyte negative and positive selection by tyrosine phosphatase CD45. Nature. 1996;381(6580):325–328. doi: 10.1038/381325a0. [DOI] [PubMed] [Google Scholar]
- 55.Pani G, Siminovitch KA, Paige CJ. The motheaten mutation rescues B cell signaling and development in CD45-deficient mice. J Exp Med. 1997;186(4):581–588. doi: 10.1084/jem.186.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gerlach J, et al. B cell defects in SLP65/BLNK-deficient mice can be partially corrected by the absence of CD22, an inhibitory coreceptor for BCR signaling. Eur J Immunol. 2003;33(12):3418–3426. doi: 10.1002/eji.200324290. [DOI] [PubMed] [Google Scholar]
- 57.Samardzic T, et al. Reduction of marginal zone B cells in CD22-deficient mice. Eur J Immunol. 2002;32(2):561–567. doi: 10.1002/1521-4141(200202)32:2<561::AID-IMMU561>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 58.Zikherman J, et al. PTPN22 deficiency cooperates with the CD45 E613R allele to break tolerance on a non-autoimmune background. J Immunol. 2009;182(7):4093–4106. doi: 10.4049/jimmunol.0803317. [DOI] [PMC free article] [PubMed] [Google Scholar]