Significance
Ectodomain shedding is a central event in a range of biological processes and pathways, however the underlying mechanisms are still not fully understood. Here we present evidence that site-specific O-glycosylation regulated by individual GalNAc-Transferase isoforms, serve to coregulate ectodomain shedding, predominantly through blocking of cleavage. Our general finding is exemplified by the specific role of a single GalNAc-T isoform (GalNAc-T2) in coregulating TNF-alpha release in vitro, ex vivo in isogenic cell models, and in vivo in mouse Galnt2 knockouts. Adding the large family of GalNAc-T isoforms to regulation of ectodomain shedding substantially increase the ability to fine-tune this important process on a substrate level.
Keywords: ACE, IL-6RA, ErbB4, ADAM10, TACE
Abstract
Regulated shedding of the ectodomain of cell membrane proteins by proteases is a common process that releases the extracellular domain from the cell and activates cell signaling. Ectodomain shedding occurs in the immediate extracellular juxtamembrane region, which is also where O-glycosylation is often found and examples of crosstalk between shedding and O-glycosylation have been reported. Here, we systematically investigated the potential of site-specific O-glycosylation mediated by distinct polypeptide GalNAc-transferase (GalNAc-T) isoforms to coregulate ectodomain shedding mediated by the A Disintegrin And Metalloproteinase (ADAM) subfamily of proteases and in particular ADAM17. We analyzed 25 membrane proteins that are known to undergo ADAM17 shedding and where the processing sites included Ser/Thr residues within ± 4 residues that could represent O-glycosites. We used in vitro GalNAc-T enzyme and ADAM cleavage assays to demonstrate that shedding of at least 12 of these proteins are potentially coregulated by O-glycosylation. Using TNF-α as an example, we confirmed that shedding mediated by ADAM17 is coregulated by O-glycosylation controlled by the GalNAc-T2 isoform both ex vivo in isogenic cell models and in vivo in mouse Galnt2 knockouts. The study provides compelling evidence for a wider role of site-specific O-glycosylation in ectodomain shedding.
Ectodomain shedding, i.e., the proteolytic release of the extracellular domain of membrane proteins is a very common and highly regulated process, in which specific proteases selectively cleave substrate proteins in juxtamembrane regions. Ectodomain shedding is usually regulated in response to appropriate stimuli and affects a large number of different classes of proteins including cell membrane receptors, growth factors, cytokines, and cell adhesion molecules (1). The shedding event may induce different effects including activation, inactivation, or modulation of the functional properties of proteins (2) and are important in regulating a number of biological processes such as signal transduction, adhesion, proliferation and differentiation (1, 3). Ectodomain shedding is predominantly mediated by Zinc-dependent metalloproteinases, and one of the key classes of proteases involved is the ADAM subfamily. Members of the ADAM family are type 1 transmembrane proteins that share conserved domains, including a prodomain that serves as an inhibitor of the proteolytic activity; a metalloprotease domain that may or may not contain an active site with the conserved motif (HEXGHXXGXXHD) common for Zinc-binding proteases; a disintegrin domain and a cysteine-rich region, which are both believed to be involved in substrate recognition; an EGF-like domain; a transmembrane domain; and a cytoplasmic domain important for intracellular signaling. Dysregulation of ectodomain shedding is involved in a variety of diseases including inflammation, cardiovascular diseases, diabetes, Alzheimer disease, and cancer (1, 4). However, the underlying mechanisms for dysregulation are poorly understood. Defined consensus motifs for ADAM cleavage sites have not been identified, but for a number of membrane proteins undergoing regulated ectodomain shedding the cleavage sites and the specific ADAM proteases involved have been identified (5). Interestingly, cleavage sites in the extracellular juxtamembrane region are often found in close proximity to O-glycosylation sites, hence glycosylation may affect susceptibility for proteolytic processing of proteins (6).
GalNAc-type O-glycosylation (hereafter simply O-glycosylation) is found on the majority of proteins passing through the secretory pathway and often in linker and stem regions of proteins (7). This type of protein glycosylation is unique in being differentially regulated by a large family of up to 20 distinct polypeptide GalNAc-transferases (GalNAc-Ts), which initiate O-glycosylation by catalyzing addition of the first GalNAc residue to Ser and Thr residues (and possibly Tyr residues) (8). These GalNAc-T isoforms have different yet partly overlapping peptide acceptor substrate specificities, and have different expression patterns in cells and tissues, which opens for a high degree of differential and possibly dynamic regulation of the O-glycoproteome and individual O-glycosites in proteins (8). Previously it has been shown that site-specific O-glycosylation specifically inhibits Furin proprotein processing ± 3 residues of cleavage sites (9), and in general O-glycosylation is known to confer structure and stability to stem regions of proteins and preventing proteolysis. Classic studies demonstrated the importance of O-glycosylation in the stem region of the low-density-lipoprotein (LDL) receptor using the CHO ldlD cell system that enables analysis of the general effect of O-glycosylation (10). A number of other examples have confirmed this, and extended it to related receptors (11–13). Recently, it was shown that mutational loss of O-glycosylation in the stem region of the Interleukin-2 receptor subunit alpha (IL2Rα) result in increased shedding of the ectodomain (14). However, it is currently unclear to what extent O-glycosylation in the stem region serves a protective role for proteolytic processing in general or more specific and regulated roles in ectodomain shedding by, e.g., ADAMs.
O-glycosylation has been proposed to specifically affect ADAM-mediated ectodomain shedding (15, 16). Tumor necrosis factor alpha (TNF-α), a type 2 transmembrane precursor protein that undergoes ectodomain shedding to produce a soluble truncated form, was shown to be O-glycosylated near a known ADAM cleavage site in lymphoblastic leukemia B cells (16), and Minond and colleagues showed that glycosylation of synthetic peptides covering the TNF-α cleavage site affected in vitro ADAM processing (15). Furthermore, it was recently found that also ADAM mediated cleavage of the extracellular N-terminal tail of the GPCR β1-adrenergic receptor may be affected by O-glycosylation (17). We hypothesized that important roles of site-specific O-glycosylation would have to be carried out by individual GalNAc-T isoforms to enable tuneable coregulation. This hypothesis leaves the substrate specificities of GalNAc-T isoforms and their unique functions as a central element in deciphering the potential for O-glycosylation to coregulate ectodomain shedding. This function was recently exemplified with the GalNAc-T11 isoform identified as a candidate disease gene in new-born heart heterotaxy (18). Thus, in vitro studies suggested that the GalNAc-T11 enzyme exhibited selective substrate specificity for the juxtamembrane region of Notch1 and coregulated ADAM17 processing of Notch1 (19).
In the present study, we have systematically investigated the potential of site-specific O-glycosylation mediated by distinct GalNAc-T isoforms to coregulate known ADAM processing. We chose ADAM17 (also known as TACE) as a representative example identified 25 membrane proteins with known ADAM17 processing sites in juxtamembrane regions and putative proximal O-glycosylation sites defined as Ser/Thr residues within ± 4 residues of the processing site. Synthetic peptides covering such regions from approximately half of the analyzed proteins were O-glycosylated by one or more GalNAc-T isoforms and in most cases glycosylation modulated in vitro ADAM cleavage. We used TNF-α as an example to demonstrate that shedding by ADAM17 is coregulated by site-specific O-glycosylation controlled by the GalNAc-T2 isoform ex vivo and in vivo. Our study provides evidence for a wider role of site-specific O-glycosylation in coregulation of ectodomain shedding.
Results
Screening for Site-Specific O-Glycosylation Adjacent to Known ADAM Cleavage Sites.
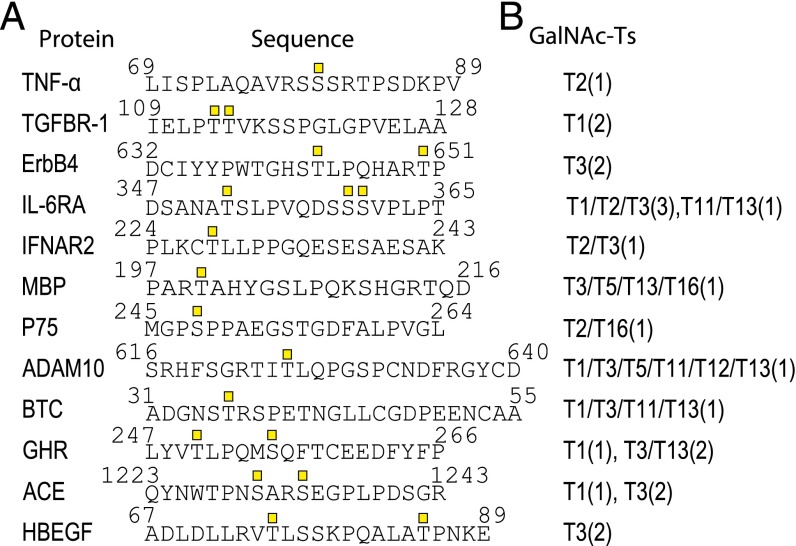
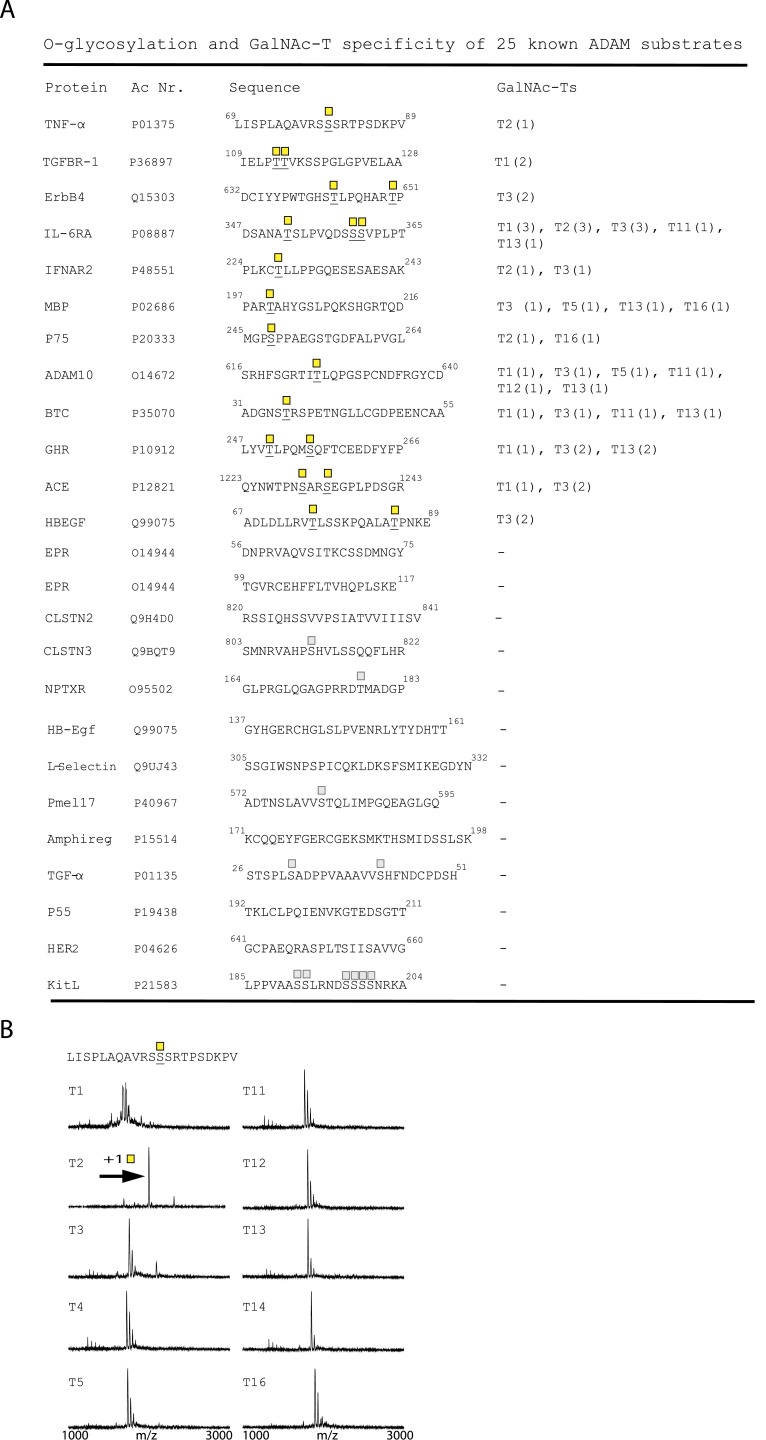
ADAM17 is the best-characterized member of the ADAM family with respect to membrane protein ectodomain cleavage sites, so we chose to focus on ADAM17 to investigate the potential role of site-specific O-glycosylation. We data mined the literature for experimentally validated ADAM17 cleavage sites with Ser/Thr residues ± 4 amino acid residues of the cleavage sites, and identified 25 cleavage sites from 23 different membrane proteins (Fig. 1 and Fig. S1). We then designed short (20mer) peptides covering the cleavage and putative adjacent glycosylation sites, and used in vitro enzyme time-course analyses monitored by MALDI-TOF mass spectrometry to evaluate potential for O-glycosylation with a panel of recombinant GalNAc-Ts. We tested 10 human recombinant GalNAc-Ts covering the major isoforms expressed in most cells and found that 12 of the 25 designed peptide substrates served as substrates for O-glycosylation by one or more of the enzymes (Fig. 1 and Fig. S1). Considerable data indicate that in vitro GalNAc-T enzyme analysis of short peptide substrates correlates well with in vivo glycosylation in cells (7, 20), and in a large scale analysis of peptides designed to cover known O-glycosites about 40% were not glycosylated (20). This finding suggests, if anything, that in vitro analysis appears to underestimate O-glycosylation capacity (20), and hence that additional candidates among the 25 identified in fact may be glycosylated in vivo.
Fig. 1.
In vitro O-glycosylation analysis of known ADAM substrates. (A) Peptides of 20–28 residues covering published ADAM cleavage sites. O-glycosylated residues are indicated by GalNAc (square). (B) In vitro O-glycosylation with GalNAc-T1, -T2, -T3, -T4, -T5, -T11, -T12, -T13, -T14, and -T16. The number of incorporated sites is shown in parenthesis.
Fig. S1.
In vitro O-glycosylation analysis of known ADAM substrates (A) Peptides of 20–28 residues covering published ADAM cleavage sites. O-glycosylated residues are underlined and indicated by GalNAc (yellow square). In vitro O-glycosylation with GalNAc-T1, -T2, -T3, -T4, -T5, -T11, -T12, -T13, -T14, and -T16. The number of incorporated sites is shown in parenthesis. Predicted (NetOGlyc 4.0) sites in peptides not found to be O-glycosylated are marked by a faded GalNAc. (B) In vitro glycosylation of the juxtamembrane part of TNF-α with 10 different GalNAc-Ts. Horizontal arrows indicate mass shift due to GalNAc glycosylation.
Among the 12 candidates for combined cleavage and glycosylation were six membrane receptors: TGF-beta receptor type-1 (TGFR-1), Receptor tyrosine-protein kinase erbB-4 (ErbB4), Interleukin-6 receptor subunit alpha (IL6-RA), Tumor necrosis factor receptor superfamily member 1B (TNF-R2), IFN alpha/beta receptor 2 (IFN-R-2) and Growth hormone receptor (GHR); two proteases: ADAM10 and Angiotensin-converting enzyme (ACE); two growth factors: Betacellulin (BTC) and Proheparin-binding EGF-like growth factor (HB-EGF); as well as the cytokine TNF-α, and Myelin basic protein (MBP). Interestingly, the peptide substrates designed for TNF-α, TGFR-1, ErbB4, and HB-EGF were only glycosylated at one or two sites by a single GalNAc-T isoform (Fig. 1). Several peptide substrates were glycosylated by two GalNAc-T isoforms (IFN-R2, p75, and ACE), whereas the peptides for BTC, MBP, ADAM10, IL6-RA, GHR, and ACE were glycosylated at one or more sites by multiple GalNAc-Ts (Fig. 1). Thus, the first group of proteins has potential for being differentially O-glycosylated by a single GalNAc-T isoform, but the second group may also be regulated in cells where subsets of GalNAc-T isoforms are expressed.
Site-Specific O-Glycosylation Modulates in Vitro ADAM Processing.
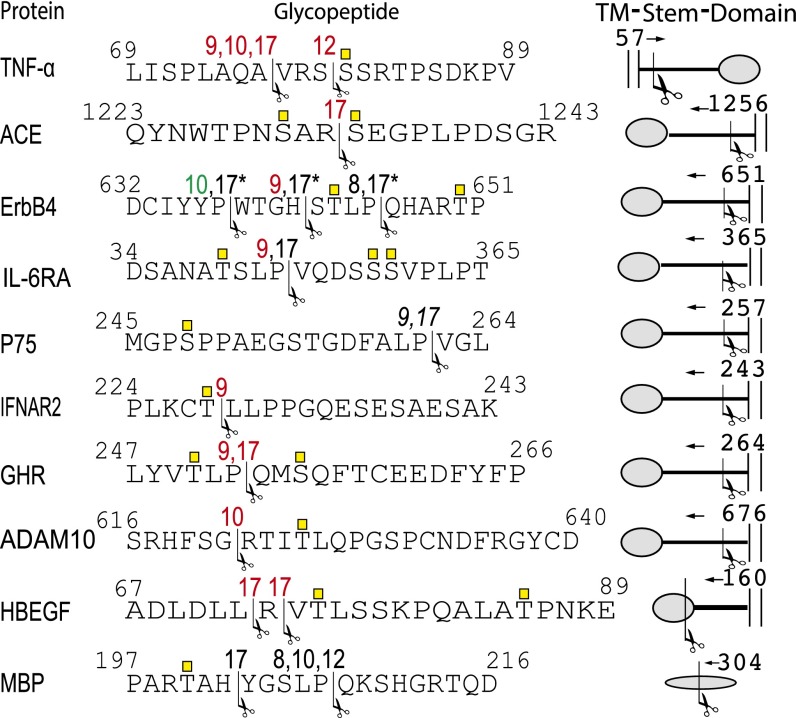
To evaluate the effect of GalNAc O-glycosylation on ADAM processing of the 12 candidate peptides we analyzed in vitro ADAM cleavage of peptides and glycopeptides produced by the relevant GalNAc-Ts. The O-glycosites of these glycopeptides were characterized by ESI-LIT-FT-MS, and five recombinant human ADAM proteases (ADAM8, -9, -10, -12, and -17) were tested in in vitro enzyme assays, where cleavage product formation was monitored by time-course MALDI-TOF MS as described (21). This assay provides a relative estimate of the efficiencies of ADAMs in cleaving the (GalNAc)-peptides in vitro, and more detailed analysis of kinetic properties of the ADAMs with these substrates is beyond this study. Ten out of 12 peptides were cleaved in vitro by one or more ADAM isoforms and, among the corresponding glycopeptides, 8 of 10 cleavages by one or more ADAMs were affected by glycosylation (Fig. 2). In most cases, glycosylation inhibited cleavage (TNF-α, ADAM10, GHR, IL-6R, ErbB4, HB-EGF, and ACE), and in one case glycosylation enhanced cleavage (ErbB4). For TNF-α glycosylation of S80 resulted in partial protection from ADAM17, -9, and -10 processing at 76A↓V77, whereas ADAM12 processing at 79S↓S80 was completely blocked (Figs. 2 and 3). For ADAM10 T625, glycosylation blocked cleavage at 621G↓R622 (Fig. 2). Glycosylation of T250 and S255 in GHR blocked ADAM9 and -17 cleavages at 252P↓Q253. Glycosylation of T352, S359, and S360 in IL6-RA blocked ADAM9 cleavage at 359P↓V360. IFN-R2 glycosylation at T228 blocked ADAM9 cleavage at 228T↓L229. Cleavage of ACE by ADAM17 at 1232R↓S1233 was blocked by glycosylation at S1230 and S12333, and glycosylation of HB-EGF at T75 and T85 blocked ADAM17 processing at 72L↓R72 and 72R↓V73; no difference in efficiency of the two cleavage sites was observed. In ErbB4, glycosylation at T643 and T650 led to increased cleavage by ADAM10 at 637P↓W638, and the ADAM17 cleavage sites shifted from 641H↓S642 and from 645P↓Q646 to 637P↓W638, no difference in cleavage efficiency of 645P↓Q646 and 637P↓W638 was observed. In addition, the ADAM9 cleavage at 641H↓S642 was blocked and ADAM8 cleavage at 645P↓Q646 was unaffected (Fig. 2). We did not observe any effect of glycosylation on cleavage of p75 (ADAM17 and -9 at 261P↓V262) or MBP (ADAM17 at 202H↓Y203, and ADAM8, -10, and -12 at 207P↓Q208) (Fig. 2).
Fig. 2.
Summary of in vitro ADAM cleavage of naked peptides and glycopeptides. Peptide sequences covering known ADAM processing sites (arrows and numbers indicate cleavage sites and ADAM isoforms, respectively). ADAM8, -9, -10, -12, and -17 were tested on all peptides that were in vitro O-glycosylated. Numbers in red indicate that we observed protection from cleavage after glycosylation, numbers in black indicate no effect, and numbers in green indicate that cleavage was increased. No cleavage was observed of BTC and TGFR1. *The ADAM17 cleavage of ERBb4 shifted from H642↓STLP↓Q of the naked peptide to 637P↓W of the glycopeptide, and ERBb4 glycosylation increased cleavage by ADAM10.
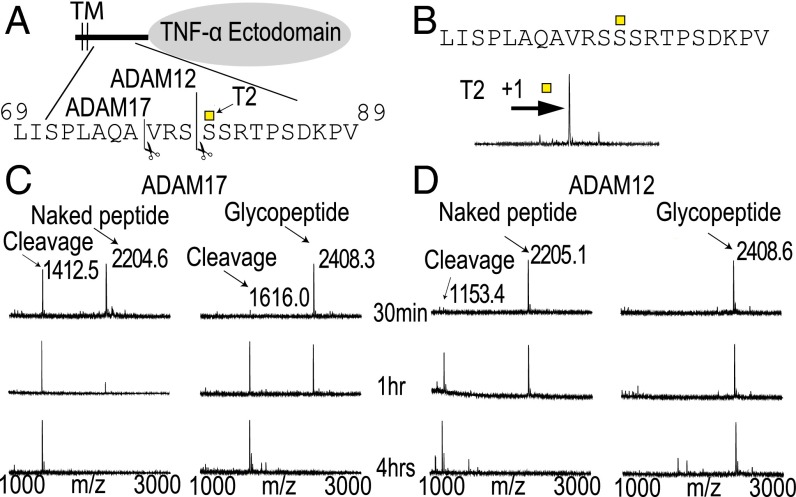
Fig. 3.
TNF-α is selectively O-glycosylated by GalNAc-T2, and this modulates in vitro ADAM cleavage. (A) Schematic drawing of TNF-ectodomain shedding zooming in on the juxtamembrane part that undergoes cleavage with indicated cleavage and glycosylation sites. (B) In vitro glycosylation of the juxtamembrane part of TNF-α with GalNAc-T2. Horizontal arrow indicates mass shift due to GalNAc glycosylation. (C) In vitro cleavage analysis of TNF-α peptide and glycopeptide by ADAM17 monitored by MALDI-TOF. Products formed after 30 min, 1 h, and 4 h are shown. ADAM17 cleaved at 76A↓V. Cleavage of the glycopeptide was less efficient. (D) ADAM12 cleaved at 79S↓S. Cleavage of the glycopeptide was almost completely abolished. ADAM9 and -10 had similar cleavage patterns as ADAM17 (Fig. 2). ADAM8 was not observed to cleave the peptide. Sequence, cleavage site (scissors), and site of glycosylation (square) are shown above each spectra.
Ectodomain Shedding of TNF-α Is Coregulated by GalNAc-T2.
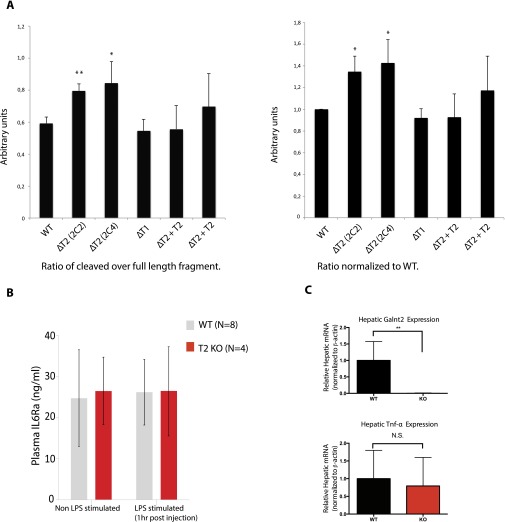
We selected TNF-α as a model for ex vivo validation in cells because O-glycosylation has been speculated to coregulate the shedding of TNF-α and because it served as an example of GalNAc-T isoform-specific (GalNAc-T2) glycosylation (Fig. 3 and Fig. S1B). TNF-α is produced as a type II transmembrane precursor (26 kDa) that undergoes regulated ectodomain shedding of a 17-kDa C-terminal form that assembles into functional homotrimers and enters the bloodstream (22). To probe the role of glycosylation for TNF-α shedding ex vivo we expressed an alkaline phosphatase-tagged juxtamembrane TNF-α reporter construct (Fig. 4A) in a panel of isogenic cell lines with and without specific GalNAc-Ts. Human liver HepG2 isogenic cells without GalNAc-T1 (HepG2ΔT1) or GalNAc-T2 (HepG2ΔT2), as well as the latter with GalNAc-T2 reintroduced (HepG2ΔT2+T2) were transiently transfected for 48 h using the TNF-α reporter construct, and total cell lysates were analyzed by Western blotting (Fig. 4B). Comparing HepG2ΔT2 to WT and ΔT1 isogenic cells, we observed specific increased shedding in T2 KO cells, which were abrogated when HepG2ΔT2 clones were rescued by site-directed AAVS1 insertion of GalNAc-T2 (Fig. 4B). The increased shedding was verified in four different HepG2ΔT2 clones and in two GalNAc-T2 rescue clones in three independent experiments (Fig. 4B and Fig. S2).
Fig. 4.
Ectodomain shedding of TNF-α is coregulated by GalNAc-T2 in Cell line models and in vivo. (A) Depiction of the TNF-α reporter construct containing GalNAc-T2 specific glycosylation and ADAM cleavage sites. (B) Western blot visualizing cleavage patterns in HepG2 WT, ΔT2, ΔT1, and ΔT2+T2 cells transiently transfected (48 h) with TNF-α reporter construct. (C) Visualization of AP activity in SDS-polyacrylamide gel in cell lysates and supernatants from HEK293 wild type and ΔT2 of full-length reporter and cleaved fragment. (D) Relative levels of shedding in HEK293 wild type, ΔT2, and ΔT3. Alkaline activity was measured in media and lysate. (E) Plasma levels (pg/mL) of TNF-α after LPS induction in WT and GalNAc-T2 KO mice. Statistical analyses were performed using the Student’s t test. Error bars show SDs.
Fig. S2.
Quantification of Western blot ratios of full length and cleaved fragments of the TNF-α reporter construct. (A) Three independent biological replicates were conducted. Each of these assaying the cleavage pattern of a TNF-α reporter construct in a panel of glycoengineered HepG2 cells. Westerns blots were performed as described, and bands were analyzed using image processing program “ImageJ”. We find that the two GalNAc-T2 KO clones show a significant increase in cleavage compared with wild type, GalNAc-T1 KO, and two individual GalNAc-T2 rescue clones. Statistical analysis was performed using Student’s t test. Error bars show the SD. (B) Plasma levels (pg/mL) of IL6Ra with or without LPS induction in WT and GalNAc-T2 KO mice. Statistical analyses were performed using the Student’s t test. Error bars show SDs. (C) Hepatic expression in arbitrary units of Galnt2 and TNF-α in WT and GalNAc-T2 KO mice. Statistical analyses were performed using the Student’s t test. Error bars show the SD.
To further confirm this finding and exclude cell-specific factors, we also tested the TNF-α reporter construct in a panel of HEK293 isogenic cell lines (HEK293 WT, HEK293ΔT2, and HEK293ΔT3), which express a different repertoire of GalNAc-Ts with the major difference being GalNAc-T3. Similar to HepG2 cells we found selective increased shedding of TNF-α (∼40%) in the HEK293ΔT2 clones, confirming that GalNAc-T2 may play a critical role for processing and shedding of the TNF-α reporter construct in agreement with the in vitro analysis (Fig. 4 C and D).
LPS-Induced Plasma Levels of TNF-α Is Elevated in T2 KO Mice Compared with WT.
We next tested the in vivo effect of complete GalNAc-T2 deficiency on levels of circulating TNF-α after lipopolysaccharide (LPS) stimulation. We compared TNF-α release in WT and Galnt2−/− KO mice. Animals were injected using 0.5 mg/kg body weight of LPS by IP injection and plasma levels were measured by ELISA at 0, 1-h, 3-h, and 6-h time points. Galnt2−/− mice showed a statistically significant increase in TNF-α plasma levels at all measured time points (1 h, 3 h, and 6 h) (Fig. 4E), confirming that GalNAc-T2 plays a role in TNF-α release and regulation. Furthermore, we observed no difference in the plasma levels of IL6-RA before or after LPS induction in these animals (Fig. S2). Finally, expression analysis showed that TNF-α expression levels were not changed between WT and KO animals (Fig. S2).
Congenital Gene Variants with Potential Dysregulated Ectodomain Shedding due to Loss of O-Glycosylation.
Several diseases or syndromes have been linked to deficiencies in GalNAc-T isoforms by various genetic association studies. The molecular mechanisms behind these associations are in most cases unresolved, but deficiencies in distinct GalNAc-T isoform functions have been shown to cause diseases due to lack of coregulation of proteolytic processing by site-specific O-glycosylation (19, 23, 24). In line with these observations, we considered whether any of the O-glycosites potentially involved in coregulation of ectodomain shedding identified in this study were found to be mutated in disease. We therefore searched for known missense SNVs associated with phenotypes or functional change of the variant protein that were located in the juxtamembrane regions in close proximity to our newly identified glycosylation sites presented here (±5 amino acids).
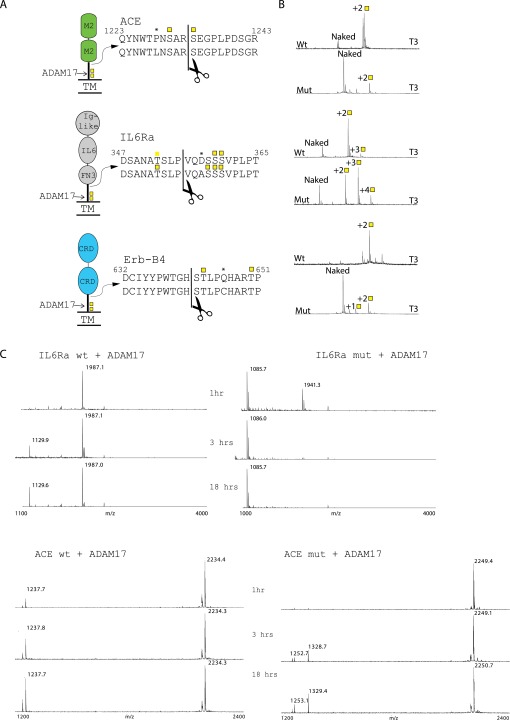
We identified two missense SNVs in 2 (ACE and IL6-RA) of the 10 proteins with putative O-glycosites affecting ADAM processing within a distance of ±5 residues of the processing site. Interestingly, these two variants of ACE and IL6-RA (P1228L and D358A, respectively) have been found to be associated with increased serum levels of their shed ectodomains (25, 26). To test whether these variations in amino acid sequence affected in vitro O-glycosylation and/or ADAM processing, we tested peptides with the substitutions. As shown in Fig. S3B, the glycosylation efficiency by GalNAc-T3 of the ACE P1228L variant peptide was dramatically decreased. However, the IL6-RA D358A variant was a better substrate for GalNAc-T1, GalNAc-T2, and GalNAc-T3, i.e., more peptide were glycosylated of the variant compared with the WT when comparing the glycosylation reactions at different time points (1 h, 2 h, 4 h) (Fig. S3B). We also observed an additional fourth site added by GalNAc-T3, which was not detected before (Fig. S3B). The amino acid substitution did not seem to affect the substrate efficiency for GalNAc-T11 and GalNAc-T13. To test if the amino acid substitutions alone affected ADAM activity, we analyzed in vitro cleavage of the nonglycosylated variant peptides. We found that the ACE variant peptide was cleaved with comparable efficiency as the WT peptide without glycosylation (Fig. S3C), indicating that it was not the amino acid substitution itself, but rather the loss of O-glycosylation, that is responsible for increased shed ACE levels in individuals with this gene variant. Conversely, the naked IL6-RA variant peptide was much more efficiently cleaved, which is likely to account for the increased serum levels in people carrying this genotype. Nevertheless, the glycosylation was still found to inhibit ADAM mediated cleavage of the IL6-RA peptide. Our results highlight the importance of considering the glycosylation status of the juxtamembrane part of a protein when investigating shedding and activation of membrane receptors.
Fig. S3.
Known variants associated with higher serum levels show change in in vitro O-glycosylation. (A) Depiction of receptors, their domains and peptides used in assaying the variants. Peptides from both WT and variants are shown, and cleavage sites and glycosylations sites are indicated. (B) MALDI-TOF time course showing incorporation of glycosylation sites after 18 h of incubation. Domains are as follows: CRD, cystein rich domain; FN3, Fibronectin 3 domain; M2, peptidase M2 domain. Variant amino acids are marked with an asterisk and cleavage sites with a scissor. (C) In vitro cleavage analysis of IL6Ra and ACE WT and mut peptides by ADAM17 monitored by MALDI-TOF. Products formed after 1 h, 3 h, and 18 h are shown.
Finally, we tested the artificially produced ErbB4 mutant Q646C, which has been reported to induce a constitutively active receptor predicted to undergo constitutive dimerization (27). ErbB4 is also known to undergo Regulated Intramembrane Proteolysis (RIP) leading to intracellular signaling (28), and this mechanism could also play a role in the increased receptor activity. We tested the mutant Q646C peptide by in vitro assays and observed a dramatic reduction in the glycosylation compared with the WT peptide (Fig. S3B).
Discussion
O-glycosylation in the juxtamembrane region of cell membrane proteins have for long been known to confer general stability and extended structure to the stem region that may be important for protrusion of the receptor domains (6). However, the extent to which isolated distinct sites of O-glycosylation play specific roles for regulated proteolytic processing events such as ectodomain shedding has remained unclear. Moreover, provided distinct O-glycosites serve such functions it seems conceivable that the glycosylation of these sites would be directed by distinct members of the large GalNAc-T family to enable reasonable regulation. Here, through systematic analysis of membrane proteins with known ADAM17 processing sites, we demonstrate that proximal site-specific O-glycosylation is likely to serve a common coregulatory role in ectodomain shedding. We further demonstrate that most of the identified O-glycosites were controlled by only one or a few GalNAc-T isoforms, which opens the possibility for selective regulation through tuning of the expression of specific GalNAc-T isoforms. We selectively studied ADAM17 processing sites, but it is likely that these findings can be extrapolated to other proteases involved in ectodomain shedding, stressing the need to include analysis of potential O-glycosylation adjacent to processing sites in future studies of ectodomain shedding. We also demonstrate the importance of considering O-glycosylation when analyzing the effects SNVs may exert.
GalNAc-type O-glycosylation is highly abundant with perhaps over 85% of all proteins trafficking the secretory pathway being O-glycosylated, and interestingly a majority of the identified O-glycoproteins only have very few O-glycosites in isolated positions (7). Although our knowledge of the O-glycoproteome has greatly expanded in recent years with the introduction of different O-glycoproteomics strategies (29, 30), there are still technical difficulties in identifying, for example, O-glycosites close to the juxtamembrane region due to lack of efficient proteolytic cleavage sites for bottom-up mass spectrometry sequencing strategies (31). Although prediction algorithms for O-glycosylation are available (7), these may not have sufficient examples from juxtamembrane regions to serve as good predictors for O-glycosites in this region. Another obstacle is our limited knowledge on the substrate specificities of individual GalNAc-T isoforms and in particular the specific O-glycosites they control nonredundantly in cells. Only with such information will it be possible to define the regulatable part of the O-glycoproteome. Progress has been made with differential O-glycoproteomes using isogenic SimpleCells with and without individual GalNAc-T isoforms (32) and systematic in vitro analysis of O-glycosylation (20), but we are still far from being able to identify O-glycosites that are being regulated by individual GalNAc-Ts. We therefore used in vitro GalNAc-T enzyme assays to identify putative O-glycosylation sites, which revealed potential O-glycosites within ±4 residues of almost 50% of the analyzed ADAM17 cleavage sites and glycosylation of most of these affected in vitro ADAM cleavage (Fig. 2). Interestingly, in most cases glycosylation affected cleavage negatively, but in one case cleavage was positively affected, similar to what we have previously reported (19). It is important to note that we only tested the simplest O-glycoform with just a GalNAc residue attached, whereas most cells will produce larger structures mainly of core1 structure (Galβ1–3GalNAcα1-O-Ser/Thr) with sialic acid capping. We have, however, previously shown that the mere presence of an O-glycan is sufficient to evaluate an effect on proprotein processing (21), but also that the complete sialylated core1 structure may exert more pronounced effects (23).
In agreement with our starting hypothesis, we also found that the majority of the glycosites identified that modulated ADAM17 processing in vitro were glycosylated in a GalNAc-T isoform-specific manner. The majority of the O-glycoproteome is covered by functional redundancy among GalNAc-T isoforms, and the widely expressed isoforms, GalNAc-T1, T2, and T3, appear to cover the majority of the O-glycosylation events in cells, whereas other GalNAc-T isoforms have more restricted and specialized functions (8, 20). It is therefore striking that most of the O-glycosites predicted to modulate ADAM17 ectodomain shedding events were controlled by nonredundant GalNAc-T isoform-specific functions, further supporting that they likely play coregulatory roles in ectodomain shedding. In four cases (IFNAR2, ADAM10, GHR, and BTC) the O-glycosites were potentially controlled by two or three GalNAc-T isoforms with overlapping functions (Fig. 1), however, because most cells express only a limited subset of GalNAc-T isoforms these glycosites may still be regulated by individual isoforms. In this respect, it is important to include the repertoire of GalNAc-Ts expressed in a given cell when considering redundancies of GalNAc-T isoform activities (8, 32). HB-EGF was specifically glycosylated by GalNAc-T3 at two residues, which blocked ADAM17 cleavage (Figs. 1 and 2). Given that GalNAc-T3 is not expressed in normal liver (24), the hepatic form of HB-EGF is likely not glycosylated in these sites, but GalNAc-T3 is widely expressed in other cells and can be strongly up-regulated in disease (33). HB-EGF has been shown to play a protective role in acute liver damage (34). Similarly GHR was glycosylated at one site by GalNAc-T1 and at two sites by GalNAc-T3 and GalNAc-T13, suggesting that there may be some degree of redundancy in control of O-glycosylation of these sites. However, again, GalNAc-T3 is not expressed in liver and GalNAc-T13 is predominantly expressed in the brain and a few other tissues (8), so it is plausible that GalNAc-T1 alone controls glycosylation of GHR in liver. These examples underline the complexity in deciphering the roles site-specific O-glycosylation may exert on biological processes such as ectodomain shedding.
Precise gene editing tools offer unprecedented options to dissect and validate nonredundant biological functions of glycosyltransferase isoenzymes (35). The knockout strategy used in this study overcome knockdown strategies issue with insufficient reduction in enzyme levels to produce substantial changes in glycosylation. We used a panel of ZFN edited isogenic cell lines to demonstrate that ectodomain shedding of TNF-α is specifically coregulated by GalNAc-T2 site-specific glycosylation at Ser80 and a ZFN edited mouse model Galnt2−/− to validate this in vivo. TNF-α is a central mediator in several diseases including rheumatoid arthritis and Crohn’s disease (36), and blockage of TNF-α is a well established treatment regime for these and other conditions (37). Several regulatory mechanisms of TNF-α release and ectodomain shedding have been proposed (38–40), but the regulation and the importance of the different mechanisms still remain poorly understood. The coregulatory role of GalNAc-T2 offers new insight to the molecular mechanisms behind TNF-α release and may be important to consider in diseases with elevated secreted TNF-α levels. GALNT2 play additional important roles in dyslipidaemia (24), and future studies need to address the extent to which these functions are regulated and fine-tuned.
A number of diseases are associated with altered levels of shed cell membrane receptor ectodomains in serum, and in some cases mutations in the juxtamembrane region of these receptors have been identified. More recently, somatic mutations in the juxtamembrane region of the colony-stimulating factor 3 receptor (CSF3R) that appear to eliminate O-glycosylation has been identified as driver mutations in chronic neutrophilic leukemia (41). In this case, the mechanism was proposed to involve enhanced homodimerization of the receptor due to loss of O-glycosylation and constitutive signaling. The data presented here provide an additional scenario where mutations may alter efficiency of site-specific O-glycosylation, which subsequently affect the proteolytic cleavage event and ectodomain shedding. One illuminating example may be ACE in which a SNV (P1228L) is associated with 2.5-fold higher levels of shed circulating ACE (25). Elevated plasma ACE activity have been suggested to be associated with increased risk of cardiovascular disease (42), although a later report has not been able to reproduce this effect (43). Similarly, an IL6-RA SNV (D358A) has also been linked to higher soluble levels of the receptor (44). We found that the D358A mutation enhanced O-glycosylation and also enhanced ADAM17 cleavage (Fig. S3C). Interestingly, site-specific O-glycosylation appears to play a role in coregulating both variants and the change in IL6-RA signaling caused by the D358A SNV has been suggested to have a pathogenic role in asthma and is associated with more severe disease (45). Finally, an experimental mutation in ErbB4 in close proximity to the glycosylation sites identified here has previously been shown to result in a marked increase in signaling (27). Again, this mutation affected efficiency of site-specific O-glycosylation (Fig. S3B). It is possible that the loss of O-glycosylation affects the signaling through modulating the ectodomain shedding and/or leads to increase in dimerization and ligand independent signaling as recently described for the CSF3R (41).
In summary, we present evidence that site-specific O-glycosylation regulated by distinct GalNAc-T isoforms may widely serve to coregulate ADAM mediated ectodomain shedding. Although, the role of glycosylation predominantly appears to reduce shedding, examples of enhancement of shedding are also found. Site-specific O-glycosylation has potential for complex modulation of ectodomain shedding to regulate efficiency and direction of processing site and protease selectivity. Adding the many GalNAc-T isoforms to regulation of ectodomain shedding substantially increase the ability to fine-tune the selectivity of this important process. Moreover, as demonstrated, disease-associated mutations in the juxtamembrane region of membrane proteins may indirectly affect ectodomain shedding by affecting glycosylation efficiency. Our study clearly underlines the importance of considering O-glycosylation and the GalNAc-T repertoire as coregulators of ectodomain shedding
Experimental Procedures
Glycosyltransferase Assays.
Recombinant glycosyltransferases were expressed as soluble secreted truncated proteins in insect cells (45). In vitro activity assays for GalNAc-T glycosylation of peptides (Schafer-N, NeoBioSci) were performed as described and monitored with MALDI-TOF (20).
Characterization of O-Glycosylation Sites by Electron Transfer Dissociation-MS2.
Products of O-glycosylation reactions were characterized by electrospray ionization-linear ion trap-Fourier transform mass spectrometry (ESI-LIT-FT-MS) in an LTQ-Orbitrap XL hybrid spectrometer (Thermo Scientific) equipped for electron transfer dissociation for peptide sequence analysis by MS/MS (MS2) with retention of glycan site-specific fragments (SI Experimental Procedures).
ADAM Cleavage of Peptides and Glycopeptides.
In vitro ADAM cleavage activity were assayed by adding 150–600 nM ADAM17, -10 (Enzo Life Sciences), -8, -9 (provided by Alexander Matthias Kotzsch, NNF Center for Protein Research, Faculty of Health, University of Copenhagen, Copenhagen), or -12 (a gift from the Kveiborg group, BRIC, Faculty of Health University of Copenhagen), using 10 μg of peptide or glycopeptide substrate in a total volume of 25 μL (SI Experimental Procedures).
Precise Gene Edited Cell Models.
To confirm the in vitro assay findings, we developed cell line models capable of probing both the effect on cleavage by site-specific glycosylation and the GalNAc-T isoform involved. Because GalNAc-Ts are differentially expressed we used two different cell lines, HepG2 and HEK293, representing different GalNAc-T repertoires. We used Zinc finger nuclease (ZFN) gene editing to knockout and/or knock in GALNT1 and GALNT2 (Sigma-Aldrich) in HepG2 as described (32) (SI Experimental Procedures).
TNF-α Shedding Assay.
HepG2 WT, ΔT1, ΔT2, and T2 rescue and HEK293 WT, ΔT1, and ΔT2 were plated in six-well plates and transiently transfected with the juxtamembrane-TNF-α-AP expression construct (generously provided by Carl Blobel, Cornell University, Ithaca, NY). The TNF-α reporter construct consists of a N-terminal intracellular FLAG tag fused to the first 87 amino acids of TNF-α, followed by alkaline phosphatase and a MYC tag. The full-length fusion protein has a size of ∼70.2 kDa, and the shed part is ∼60.4 kDa. The construct has been described in details by Zheng and colleagues (46) (SI Experimental Procedures).
T2 KO Mouse and Measurement of TNF-α Plasma Levels After LPS Induction.
T2 KO male mice (n = 4) and WT male controls (n = 8) were administered LPS reconstituted in PBS at a dose of 0.5 mg/kg body weight by IP injection in total volume of 250 μL. Blood was collected via retroorbital bleed before LPS administration and at 1, 3, and 6 h after LPS injection. Plasma TNF-α was measured from all timepoints by a mouse TNF-α ELISA (Quantikine ELISA, R&D Systems). All procedures were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania.
SI Experimental Procedures
Characterization of O-Glycosylation Sites by Electron Transfer Dissociation-MS2.
Samples were dissolved in methanol/water (1:1) containing 1% formic acid and introduced by direct infusion via a TriVersa NanoMate ESI-Chip interface (Advion BioSystems) at a flow rate of 100 nL/min and 1.4 kV spray voltage. Mass spectra were acquired in positive ion FT mode using parameters similar to previous studies (30), except at a nominal resolving power of either 30,000 or 60,000. Electron transfer dissociation-MS2 spectra were analyzed by comparison with theoretical c and z• fragment m/z values calculated for all positional combinations of one HexNAc residue distributed on the all potential S and T glycosylation sites in the sequence. Calculations were performed using the web-based Protein Prospector MS-Product software routine. In addition, samples were analyzed on a setup composed of an EASY-nanoLC 1000 (Thermo Scientific) connected via a nanoSpray Flex ion source to an LTQ-Orbitrap Velos Pro hybrid mass spectrometer. Glycopeptides were dissolved in 0.1% formic acid and separated on an in-house packed reverse phase column (1. Reprosil-Pure-AQ C18 particles, Dr. Maisch). MS1 precursor ion scan was performed in the orbitrap using a nominal resolution of 30,000 followed by two MS2 scan events (15,000 resolving power at m/z 400) using HCD and ETD fragmentation modes. Glycopeptide identification and glycosite assignment was accomplished by Proteome Discoverer 1.4 with final validation through manual inspection of the assigned peaks.
ADAM Cleavage of Peptides and Glycopeptides.
Reactions were performed in 25 mM Tris pH 9 (ADAM17), 20 mM Tris pH 8, 25 mM CaCl2, 0,0005% Brij-35, (ADAM9), 25 mM Tris pH 9, 2 mM CaCl2, 0.0005% Brij-35, (ADAM10), 20 mM Tris pH8, 0,0005% Brij-35 (ADAM12), or 20 mM Tris pH8, 25 mM CaCl2, and 0.0005% Brij-35 (ADAM8). Reactions were incubated at 37 °C (ADAM17, -9, -10, and -12) or 30 °C (ADAM8). Product development was evaluated after 0.5, 1, and 3 h by MALDI-TOF.
Precise Gene Edited Cell Models.
We further knocked out GALNT2 and GALNT3 in HEK293 using the same procedures. ZFN targeting constructs for GALNT3 and GALNT2 were custom produced (Sigma-Aldrich) with the following binding sites. Cutting sites are indicated in parentheses: GALNT3 5′-TTCAACAAACCTTCT(CCTTAT)GGAAGTAACCATAAC-3 and GALNT2 5′-GTCGGCCCTACTCAGGAC(CGTGGT)CAGGTGAGGCCAGGAGAT-3′. HEK293 cells (ATTC) were transfected with 2 μg of DNA (Sigma-Aldrich) using nucleofection on an Amaxa Nucleofector (Lonza). Cells were cloned by limited dilution, trypsinized and fixed in ice-cold acetone on teflon coated slides and stained with monoclonal antibodies (MAbs) to GalNAc-T2 (4C4 and 2E10) and GalNAc-T3 (2D10). Knockout clones were selected for loss of reactivity with MAbs to GalNAc-T2 and T3, and mutations confirmed by PCR using primers GALNT2PZFN F/R (5′-CCATCCCAGTTGGTCAGTCT-3′/5′- CTGTGCTGAGCAGTCAGGAG-3′) or GALNT3PZFN F/R (5′-TCCCTCCAGGTGAGTGTTTC-3′/5′-AAAGCAAACAGTGTGTACATATTCAA-3′) and sequencing. Fluorescence microscopy was performed using a Zeiss Axioskop 2 plus with an AxioCam MR3. Bit depth and pixel dimensions were 36 bit and 1,388 × 1,040 pixels.
TNF-α Shedding Assay.
Transfection was carried out using X-tremeGENE HP DNA Transfection Reagent (Roche Applied Science). Processing of the construct was evaluated by NuPage Western blot analysis and AP activity. WB analysis was performed 48 h posttransfection, and cells were washed with PBS, lyzed in RIPA buffer for 30 min, and centrifuged at 12,000 × g. The supernatant was removed and DTT (final concentration of 1.4 mM) and 4 × LDS sample buffer was added. Samples were run on NuPage bis-Tris gels 4–12% gradient in Mes buffer. Blotting to Nitrocellulose membrane was performed using Bio Rads blotting system at 320 mA for 60 min, and blots were blocked for 60 min with 5% (wt/vol) milk in PBS and the TNFα-AP myc tagged construct detected with anti-myc (9E10) at 4 °C ON. It is important to note that these experiments were done using transiently transfected clones, thus protein levels will vary between these and one should therefore compare the difference in cleavage patterns rather than the absolute cleavage levels. Measurement of alkaline phosphatase (AP) activity was performed 48 h after transfection and cell were washed in Opti-MEM reduced serum media followed by incubation with fresh Opti-MEM for 1 h. Media was harvested and cells lysed in 1% Triton X-100 in PBS with complete protease inhibitor mixture (Roche Applied Science). Fifty microliters of media or lysates were mixed with 150 μL of p-nitrophenylphosphate (Kem-En-Tec Diagnostics) and incubated at 37 °C. Alkaline phosphatase activity was quantified at 405 nm as a function of time, and basal shedding was determined in arbitrary units as the ratio between AP activity in the media and AP activity in the lysate. The basal shedding level found in WT cells was set as a value of 1. Statistical analysis was performed using Student’s t test (type 2, two tailed). Shedding was further visualized on SDS-polyacrylamide gels by adding 40 μL ConA beads to 900 μL of media or lysate, eluting and running the samples at 100 V for 1 h. Gels were incubated twice in 2.5% Triton X-100 for 30 min followed by an incubation in 100 mM Tris (pH 9.5), 100 mM NaCl and 20 mM MgCl2 for 10 min and stopped by the addition of 50% methanol/10% glacial acid.
T2 KO Mouse and Measurement of TNF-α and IL6Ra Plasma Levels After LPS Induction.
Animals were maintained on a normal chow diet for 14–18 wk of age. Mice plasma levels of IL6Ra were measured with a mouse IL6Ra ELISA (Sigma RAB0314). Statistical analyses were performed using the Student’s t test; error bars show SDs. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania.
Hepatic Expression of Galnt2 and TNF-α.
Total hepatic RNA was isolated from livers of Galnt2 WT and KO mice using a TRIzol-based extraction (Thermo Fisher). For each sample, 1 μg of total RNA was used to synthesize cDNA using the High Capacity cDNA Reverse Transcription kit (Thermo Fisher). Transcript levels of Galnt2, TNF-a, and Actb were measured using cDNA samples by quantitative real-time PCR using the following murine Taqman (Thermo Fisher) primer/probe sets: Galnt2 (Mm00519804_g1), TNF-a (Mm00443258_m1), Actb (Mm00607939_s1). Relative expression of Galnt2 and TNF-a was determined by normalizing to expression of Actb.
Acknowledgments
This work was supported by the Danish Research Councils (including Sapere Aude Research Talent Grant to K.T.-B.G.S.), a program of excellence from the University of Copenhagen, The Novo Nordisk Foundation, a program of excellence from the University of Copenhagen (CDO2016), and The Danish National Research Foundation (DNRF107).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1511175112/-/DCSupplemental.
References
- 1.Weber S, Saftig P. Ectodomain shedding and ADAMs in development. Development. 2012;139(20):3693–3709. doi: 10.1242/dev.076398. [DOI] [PubMed] [Google Scholar]
- 2.Schlöndorff J, Blobel CP. Metalloprotease-disintegrins: Modular proteins capable of promoting cell-cell interactions and triggering signals by protein-ectodomain shedding. J Cell Sci. 1999;112(Pt 21):3603–3617. doi: 10.1242/jcs.112.21.3603. [DOI] [PubMed] [Google Scholar]
- 3.Blobel CP. ADAMs: Key components in EGFR signalling and development. Nat Rev Mol Cell Biol. 2005;6(1):32–43. doi: 10.1038/nrm1548. [DOI] [PubMed] [Google Scholar]
- 4.Arribas J, Esselens C. ADAM17 as a therapeutic target in multiple diseases. Curr Pharm Des. 2009;15(20):2319–2335. doi: 10.2174/138161209788682398. [DOI] [PubMed] [Google Scholar]
- 5.Lange PF, Overall CM. TopFIND, a knowledgebase linking protein termini with function. Nat Methods. 2011;8(9):703–704. doi: 10.1038/nmeth.1669. [DOI] [PubMed] [Google Scholar]
- 6.Varki A. Biological roles of oligosaccharides: All of the theories are correct. Glycobiology. 1993;3(2):97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steentoft C, et al. Precision mapping of the human O-GalNAc glycoproteome through SimpleCell technology. EMBO J. 2013;32(10):1478–1488. doi: 10.1038/emboj.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bennett EP, et al. Control of mucin-type O-glycosylation: A classification of the polypeptide GalNAc-transferase gene family. Glycobiology. 2012;22(6):736–756. doi: 10.1093/glycob/cwr182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schjoldager KT, Clausen H. Site-specific protein O-glycosylation modulates proprotein processing - deciphering specific functions of the large polypeptide GalNAc-transferase gene family. Biochim Biophys Acta. 2012;1820(12):2079–2094. doi: 10.1016/j.bbagen.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 10.Kingsley DM, Kozarsky KF, Segal M, Krieger M. Three types of low density lipoprotein receptor-deficient mutant have pleiotropic defects in the synthesis of N-linked, O-linked, and lipid-linked carbohydrate chains. J Cell Biol. 1986;102(5):1576–1585. doi: 10.1083/jcb.102.5.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kozarsky K, Kingsley D, Krieger M. Use of a mutant cell line to study the kinetics and function of O-linked glycosylation of low density lipoprotein receptors. Proc Natl Acad Sci USA. 1988;85(12):4335–4339. doi: 10.1073/pnas.85.12.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.May P, Bock HH, Nimpf J, Herz J. Differential glycosylation regulates processing of lipoprotein receptors by gamma-secretase. J Biol Chem. 2003;278(39):37386–37392. doi: 10.1074/jbc.M305858200. [DOI] [PubMed] [Google Scholar]
- 13.Magrané J, Casaroli-Marano RP, Reina M, Gåfvels M, Vilaró S. The role of O-linked sugars in determining the very low density lipoprotein receptor stability or release from the cell. FEBS Lett. 1999;451(1):56–62. doi: 10.1016/s0014-5793(99)00494-9. [DOI] [PubMed] [Google Scholar]
- 14.Karabasheva D, Cole NB, Donaldson JG. Roles for trafficking and O-linked glycosylation in the turnover of model cell surface proteins. J Biol Chem. 2014;289(28):19477–19490. doi: 10.1074/jbc.M114.564666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minond D, et al. Discovery of novel inhibitors of a disintegrin and metalloprotease 17 (ADAM17) using glycosylated and non-glycosylated substrates. J Biol Chem. 2012;287(43):36473–36487. doi: 10.1074/jbc.M112.389114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takakura-Yamamoto R, Yamamoto S, Fukuda S, Kurimoto M. O-glycosylated species of natural human tumor-necrosis factor-alpha. Eur J Biochem. 1996;235(1-2):431–437. doi: 10.1111/j.1432-1033.1996.00431.x. [DOI] [PubMed] [Google Scholar]
- 17.Hakalahti AE, et al. Human beta1-adrenergic receptor is subject to constitutive and regulated N-terminal cleavage. J Biol Chem. 2010;285(37):28850–28861. doi: 10.1074/jbc.M110.149989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fakhro KA, et al. Rare copy number variations in congenital heart disease patients identify unique genes in left-right patterning. Proc Natl Acad Sci USA. 2011;108(7):2915–2920. doi: 10.1073/pnas.1019645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boskovski MT, et al. The heterotaxy gene GALNT11 glycosylates Notch to orchestrate cilia type and laterality. Nature. 2013;504(7480):456–459. doi: 10.1038/nature12723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kong Y, et al. Probing polypeptide GalNAc-transferase isoform substrate specificities by in vitro analysis. Glycobiology. 2015;25(1):55–65. doi: 10.1093/glycob/cwu089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schjoldager KT, et al. A systematic study of site-specific GalNAc-type O-glycosylation modulating proprotein convertase processing. J Biol Chem. 2011;286(46):40122–40132. doi: 10.1074/jbc.M111.287912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGeehan GM, et al. Regulation of tumour necrosis factor-alpha processing by a metalloproteinase inhibitor. Nature. 1994;370(6490):558–561. doi: 10.1038/370558a0. [DOI] [PubMed] [Google Scholar]
- 23.Kato K, et al. Polypeptide GalNAc-transferase T3 and familial tumoral calcinosis. Secretion of fibroblast growth factor 23 requires O-glycosylation. J Biol Chem. 2006;281(27):18370–18377. doi: 10.1074/jbc.M602469200. [DOI] [PubMed] [Google Scholar]
- 24.Schjoldager KT, et al. O-glycosylation modulates proprotein convertase activation of angiopoietin-like protein 3: Possible role of polypeptide GalNAc-transferase-2 in regulation of concentrations of plasma lipids. J Biol Chem. 2010;285(47):36293–36303. doi: 10.1074/jbc.M110.156950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kramers C, et al. Point mutation in the stalk of angiotensin-converting enzyme causes a dramatic increase in serum angiotensin-converting enzyme but no cardiovascular disease. Circulation. 2001;104(11):1236–1240. doi: 10.1161/hc3601.095932. [DOI] [PubMed] [Google Scholar]
- 26.Reich D, et al. Health, Aging and Body Composition (Health ABC) Study Admixture mapping of an allele affecting interleukin 6 soluble receptor and interleukin 6 levels. Am J Hum Genet. 2007;80(4):716–726. doi: 10.1086/513206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Penington DJ, Bryant I, Riese DJ., 2nd Constitutively active ErbB4 and ErbB2 mutants exhibit distinct biological activities. Cell Growth Differ. 2002;13(6):247–256. [PubMed] [Google Scholar]
- 28.Rio C, Buxbaum JD, Peschon JJ, Corfas G. Tumor necrosis factor-alpha-converting enzyme is required for cleavage of erbB4/HER4. J Biol Chem. 2000;275(14):10379–10387. doi: 10.1074/jbc.275.14.10379. [DOI] [PubMed] [Google Scholar]
- 29.Halim A, Nilsson J, Ruetschi U, Hesse C, Larson G. Human urinary glycoproteomics; attachment site specific analysis of N- and O-linked glycosylations by CID and ECD. Mol Cell Proteomics. 2012;11(4):M111 013649. doi: 10.1074/mcp.M111.013649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steentoft C, et al. Mining the O-glycoproteome using zinc-finger nuclease-glycoengineered SimpleCell lines. Nat Methods. 2011;8(11):977–982. doi: 10.1038/nmeth.1731. [DOI] [PubMed] [Google Scholar]
- 31.Levery SB, et al. Advances in mass spectrometry driven O-glycoproteomics. Biochim Biophys Acta. 2015;1850(1):33–42. doi: 10.1016/j.bbagen.2014.09.026. [DOI] [PubMed] [Google Scholar]
- 32.Schjoldager KT, et al. Probing isoform-specific functions of polypeptide GalNAc-transferases using zinc finger nuclease glycoengineered SimpleCells. Proc Natl Acad Sci USA. 2012;109(25):9893–9898. doi: 10.1073/pnas.1203563109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taniuchi K, et al. Overexpression of GalNAc-transferase GalNAc-T3 promotes pancreatic cancer cell growth. Oncogene. 2011;30(49):4843–4854. doi: 10.1038/onc.2011.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takemura T, et al. Conditional knockout of heparin-binding epidermal growth factor-like growth factor in the liver accelerates carbon tetrachloride-induced liver injury in mice. Hepatology Res. 2013;43(4):384–393. doi: 10.1111/j.1872-034X.2012.01074.x. [DOI] [PubMed] [Google Scholar]
- 35.Steentoft C, et al. Precision genome editing: A small revolution for glycobiology. Glycobiology. 2014;24(8):663–680. doi: 10.1093/glycob/cwu046. [DOI] [PubMed] [Google Scholar]
- 36.Vassalli P. The pathophysiology of tumor necrosis factors. Annu Rev Immunol. 1992;10:411–452. doi: 10.1146/annurev.iy.10.040192.002211. [DOI] [PubMed] [Google Scholar]
- 37.Tracey D, Klareskog L, Sasso EH, Salfeld JG, Tak PP. Tumor necrosis factor antagonist mechanisms of action: A comprehensive review. Pharmacol Ther. 2008;117(2):244–279. doi: 10.1016/j.pharmthera.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 38.Le Gall SM, et al. ADAM17 is regulated by a rapid and reversible mechanism that controls access to its catalytic site. J Cell Sci. 2010;123(Pt 22):3913–3922. doi: 10.1242/jcs.069997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soond SM, Everson B, Riches DW, Murphy G. ERK-mediated phosphorylation of Thr735 in TNFalpha-converting enzyme and its potential role in TACE protein trafficking. J Cell Sci. 2005;118(Pt 11):2371–2380. doi: 10.1242/jcs.02357. [DOI] [PubMed] [Google Scholar]
- 40.Xu P, Derynck R. Direct activation of TACE-mediated ectodomain shedding by p38 MAP kinase regulates EGF receptor-dependent cell proliferation. Mol Cell. 2010;37(4):551–566. doi: 10.1016/j.molcel.2010.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maxson JE, et al. Ligand independence of the T618I mutation in the colony-stimulating factor 3 receptor (CSF3R) protein results from loss of O-linked glycosylation and increased receptor dimerization. J Biol Chem. 2014;289(9):5820–5827. doi: 10.1074/jbc.M113.508440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cambien F, et al. Deletion polymorphism in the gene for angiotensin-converting enzyme is a potent risk factor for myocardial infarction. Nature. 1992;359(6396):641–644. doi: 10.1038/359641a0. [DOI] [PubMed] [Google Scholar]
- 43.Keavney B, et al. Large-scale test of hypothesised associations between the angiotensin-converting-enzyme insertion/deletion polymorphism and myocardial infarction in about 5000 cases and 6000 controls. International Studies of Infarct Survival (ISIS) Collaborators. Lancet. 2000;355(9202):434–442. doi: 10.1016/s0140-6736(00)82009-7. [DOI] [PubMed] [Google Scholar]
- 44.Ota T, et al. Complete sequencing and characterization of 21,243 full-length human cDNAs. Nat Genet. 2004;36(1):40–45. doi: 10.1038/ng1285. [DOI] [PubMed] [Google Scholar]
- 45.Hawkins GA, et al. The IL6R variation Asp(358)Ala is a potential modifier of lung function in subjects with asthma. J Allergy Clin Immunol. 2012;130(2):510–515. doi: 10.1016/j.jaci.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zheng Y, Saftig P, Hartmann D, Blobel C. Evaluation of the contribution of different ADAMs to tumor necrosis factor alpha (TNFalpha) shedding and of the function of the TNFalpha ectodomain in ensuring selective stimulated shedding by the TNFalpha convertase (TACE/ADAM17) J Biol Chem. 2004;279(41):42898–42906. doi: 10.1074/jbc.M403193200. [DOI] [PubMed] [Google Scholar]