Significance
The noncanonical NF-κB signaling pathway via the serine kinase NIK (NF-κB–inducing kinase) is essential for normal immune system development and has been implicated in tumor cell survival and growth. Because NIK is under investigation as a therapeutic target, it is important to understand NIK’s role in the context of a fully developed immune system, particularly in regard to mounting adaptive T-cell responses. We have generated and characterized transgenic mice with conditionally deleted NIK in CD11c+ dendritic cells and observe impaired antigen cross-priming of a naive CD8 T-cell response. This defect results from defective antigen cross-presentation by CD8+ dendritic cells and also is associated with their reduced ability to secrete IL-12p40, a cytokine known to promote cross-priming in vivo.
Keywords: NIK, dendritic cells, CD8 T cells, cross-priming, antigen cross-presentation
Abstract
Dendritic cells (DCs) link innate and adaptive immunity and use a host of innate immune and inflammatory receptors to respond to pathogens and inflammatory stimuli. Although DC maturation via canonical NF-κB signaling is critical for many of these functions, the role of noncanonical NF-κB signaling via the serine/threonine kinase NIK (NF-κB–inducing kinase) remains unclear. Because NIK-deficient mice lack secondary lymphoid organs, we generated transgenic mice with targeted NIK deletion in CD11c+ cells. Although these mice exhibited normal lymphoid organs, they were defective in cross-priming naive CD8+ T cells following vaccination, even in the presence of anti-CD40 or polyinosinic:polycytidylic acid to induce DC maturation. This impairment reflected two intrinsic defects observed in splenic CD8+ DCs in vitro, namely antigen cross-presentation to CD8+ T cells and secretion of IL-12p40, a cytokine known to promote cross-priming in vivo. In contrast, antigen presentation to CD4+ T cells was not affected. These findings reveal that NIK, and thus probably the noncanonical NF-κB pathway, is critical to allow DCs to acquire the capacity to cross-present antigen and prime CD8 T cells after exposure to licensing stimuli, such as an agonistic anti-CD40 antibody or Toll-like receptor 3 ligand.
Dendritic cells (DCs) play a strategic role in immune surveillance, initiating and directing antigen-specific adaptive immunity through their unique ability to stimulate naive T cells after antigen presentation on MHC-I and MHC-II molecules. First, however, this marked immunostimulatory function must be triggered by a variety of microbial or proinflammatory stimuli, a process known as “DC maturation” (1, 2). DCs and other immune cells recognize maturation signals through a wide variety of TNF receptor and Toll-like receptor (TLR)–IL-1 receptor superfamily members, which typically require signaling through the canonical NF-κB pathway to elicit inflammatory effector responses (3). Although the canonical NF-κB pathway is well established as a critical component of DC inflammatory stimulus–response coupling (4), the role of the noncanonical NF-κB pathway remains unclear.
NF-κB–inducing kinase (NIK) is the central component of noncanonical NF-κB signaling, transducing signals from a small subset of activated TNF receptor family members, such as BAFFR, CD40, lymphotoxin β receptor (LTβR), and RANK (5). NIK binds to and activates the downstream kinase IKKα, leading to p100 phosphorylation and the nuclear translocation of active RelB/p52 transcription factor complexes. Mice in which NIK is functionally impaired (Aly/Aly) or deleted (NIK-KO) have profound immune system defects, including alymphoplasia, splenic and thymic architectural disorganization, abnormal B-cell maturation, and abnormal Th17 cell development (6–10). The presence of severe developmental immune defects in these mice complicates the evaluation of NIK’s role in immune responses and in intrinsic functions of individual immune cell populations, such as T cells and DCs (9, 10). Perhaps because of this obstacle, there is no clear consensus regarding the role of NIK in DC responses (7, 10–15). To evaluate the role of NIK in DCs in an immune system free of these developmental defects, we generated transgenic mice with targeted NIK deletion in CD11c+ DCs. We observe that NIK is required for antigen cross-priming of naive CD8+ T cells in vivo, a finding attributable to impaired antigen cross-presentation and IL-12p40 secretion by CD8+ DCs, the dominant antigen cross-presenting cell in the mouse (16).
Results and Discussion
Impaired Antigen Cross-Priming in CD11c-Cre-NIKflox Mice in Vivo.
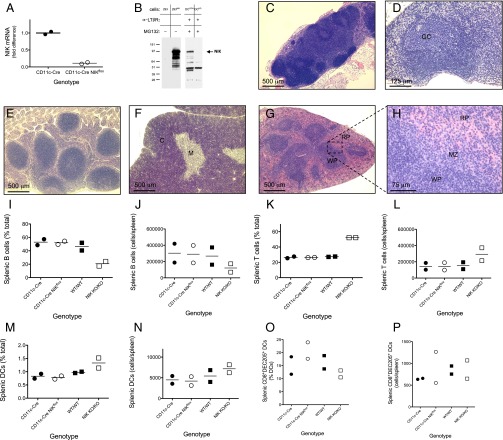
To evaluate the role of NIK in DC function in vivo, we generated CD11c-Cre-NIKflox transgenic mice conditionally NIK deficient in CD11c+ DCs by crossing a transgenic mouse line expressing Cre recombinase behind the CD11c+ promoter with another transgenic line homozygous for a floxed NIK allele (Fig. 1 A and B). In contrast to observations in Aly/Aly and NIK-KO mice (6–8), lymphoid tissues were normal in the CD11c-Cre-NIKflox mice (Fig. 1 C–H), demonstrating that NIK deficiency in DCs did not account for defective lymphoid tissue development in Aly/Aly and NIK-KO mice. Indeed, no perturbation in the frequency of B cells, T cells, or DCs was observed in spleens of untreated CD11c-Cre-NIKflox mice, whereas NIK KO/KO mice showed an expected decrease in splenic B cells compared with controls (Fig. 1 I–P).
Fig. 1.
Transgenic mice with conditional NIK deficiency in CD11c+ DCs have normally developed lymphoid tissues. (A and B) qRT-PCR (A) and Western blotting (B) confirm the absence of NIK mRNA (results normalized to reference RPL19 mRNA) (A) and protein (B), respectively, in CD11c+ bone marrow-derived cells from CD11c-Cre-NIKflox transgenic mice but not CD11c-Cre control mice. For Western blotting, bone marrow-derived cells were pretreated for 16 h with 4 μg/mL activating α-lLRβR antibody to induce NIK protein. To improve the sensitivity for NIK Western detection, proteasomal degradation was blocked for 5 h at the end of the incubation with 10 μM MG132. NIK expression controls include 293 cells with or without transfected NIK. Results are representative of at least two similar experiments. (C–H) Lymphoid tissues were harvested from three CD11c-Cre-NIKflox transgenic mice for routine histological evaluation, including periaortic, hilar, mesenteric, inguinal, cervical, brachial, axillary, and sacral lymph nodes (representative histology is shown in C and D), Peyer’s patches (E), thymus (F), and spleen (G and H). All tissues were present and were histologically normal. C, thymic cortex; GC, germinal center; M, thymic medulla; MZ, marginal zones; RP, splenic red pulp; WP, splenic white pulp. (I–P) Single-cell suspensions from spleens of untreated mice of each indicated genotype were evaluated by flow cytometry for B220+ B cells (I and J), CD3+ T cells (K and L), CD11c+ MHC-IIhi DCs (M and N), and CD8+MHC-IIhiDEC205+ DCs (O and P). Numerical results are representatives or combinations of at least two independent experiments.
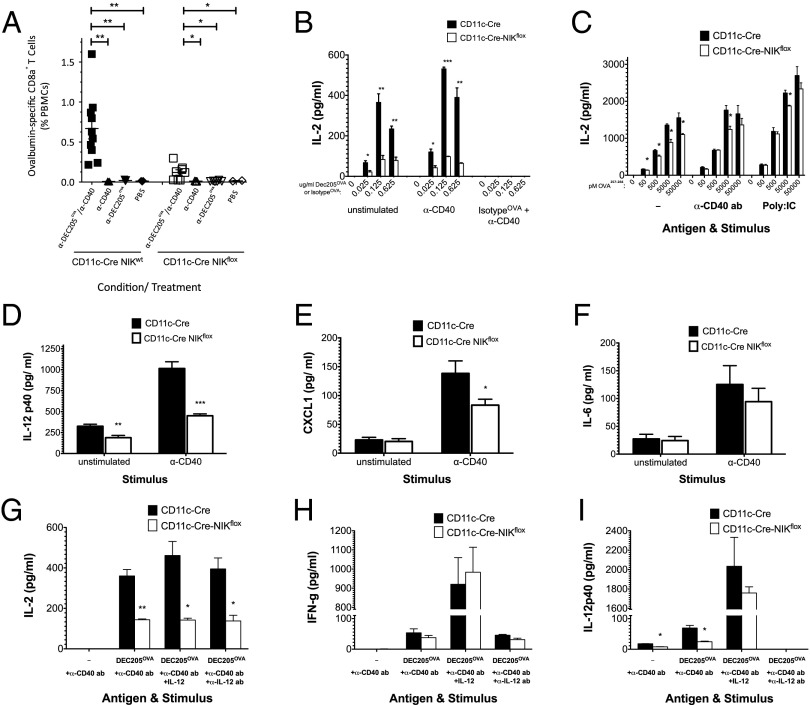
We next examined the capacity of the CD11c-Cre-NIKflox mice to mount CD8 T-cell responses upon vaccination. Mice were immunized with ovalbumin (OVA) fused to a monoclonal α-DEC205 antibody (α-DEC205OVA), which preferentially targets the immunogen to CD8+ DCs (17), which appear to be specialized for cross-priming. α-DEC205OVA was injected together with a stimulatory α-CD40 antibody as a DC maturation stimulus that facilitates antigen cross-presentation (18). As expected, in vaccinated CD11c-Cre control mice we observed a robust OVA-specific α-CD40–dependent CD8+ T-cell response (Fig. 2A, closed squares). In contrast, CD11c-Cre-NIKflox mice displayed a greatly (more than fivefold) diminished CD8+ T-cell response (Fig. 2A, open squares). This diminished response was not attributed to insufficient numbers of CD8+ DEC205+ DCs, because this population was preserved in CD11c-Cre-NIKflox mice (Fig. 1 O and P, open circles). These findings indicate that antigen cross-priming by CD8+ DCs requires DC expression of NIK and suggest that NIK deficiency impacts DC function rather than frequency.
Fig. 2.
CD11c-Cre-NIKflox mice exhibit impaired antigen cross-priming in vivo and defective antigen cross-presentation and IL-12p40 secretion by splenic-derived CD8+ DCs in vitro. CD11c-Cre NIKflox mice and CD11c-Cre NIKWT controls were immunized with or without 0.5 μg α-DEC205OVA in the presence or absence of 30 μg α-CD40 mAb. (A) After 8 d, peripheral blood mononuclear cells were stained, gated on CD3+B220−CD8+MHC-PentamerH-2b/SIINFEK+ cells, and analyzed for OVA-specific CD8+ T cells. (B–I) CD8+ DCs (B, C, G–I) or CD11c+ DCs (D–F) isolated from spleens of CD11c-Cre NIKflox mice and CD11c-Cre NIKWT controls were cultured with (B and C and G–I) or without (D–F) CD8+ T cells from OT-I TCR transgenic mice as described (Materials and Methods), in the presence or absence of the indicated concentrations (B) or 0.125 μg/mL (D–I) α-DEC205OVA, 10 μg/mL α-CD40 mAb stimulus (B–I), indicated concentrations of OVA257–264 SIINFEKL peptide (C), 10 μg/mL poly I:C stimulus (C), 10 ng/mL exogenous IL-12 (G–I), and/or 10 μg/mL blocking anti–IL-12 IgG (G–I), as indicated. After 1–2 d in culture, media were analyzed for secretion of IL-2 (B, C, and G), IL-12p40 (D and I), CXCL1 (E), IL-6 (F), or IFN-γ (H). Results are representative of at least two similar experiments. *P < 0.05; **P < 0.005; ***P < 0.0005.
CD11c-Cre-NIKflox CD8+ DCs Exhibit a Cross-Presentation Defect and Secrete Reduced IL-12p40 upon Stimulation.
To confirm a direct role for DCs in the impaired CD8+ T-cell response, CD11c-Cre-NIKflox CD8+ DCs were cultured with OVA-specific OT-I transgenic CD8+ T cells in the presence of the α-DEC205OVA and stimulatory α-CD40 antibody in vitro. CD11c-Cre-NIKflox CD8+ DCs showed greatly diminished capacity to stimulate OT-I T cells in comparison with CD11c-Cre controls (Fig. 2B, open versus closed bars). This phenotype could be attributed to multiple factors, given that DC stimulation by α-CD40 triggers a series of events, including increased cell-surface expression of MHC and costimulatory molecules (especially CD70, the ligand for CD27 on T cells, which helps drive CD8+ T-cell function) (19), increased antigen processing and antigenic peptide loading onto MHC molecules, and induction of specific cytokines required to optimize DC stimulation of T cells (1, 2, 20, 21). To circumvent the need for the intracellular antigen-handling functions of the DCs, we used as antigen preprocessed OVA257–264 peptide that is loaded directly on surface-expressed MHC-I. Under these assay conditions, the cross-presentation defect observed in CD11c-Cre-NIKflox CD8+ DCs was largely (although not completely) rescued, suggesting that NIK signaling controls the expression or function of intracellular events in the cross-presentation pathway (Fig. 2C).
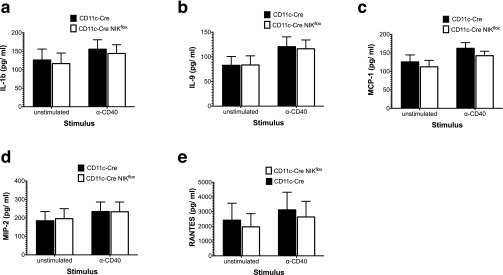
Because IL-12p40 is critical for efficient antigen cross-priming in vivo (16), we examined IL-12p40 secretion by splenic CD11c+ DCs after in vitro stimulation with α-CD40 antibody, as one of a panel of 32 cytokines/chemokines tested in all. As shown in Fig. 2D, IL-12p40 secretion was reduced in the NIK-deficient DCs in comparison with the CD11c-Cre controls (open versus closed bars). NIK-deficient DCs also secreted modestly reduced amounts of the chemokine CXCL1/KC (Fig. 2E). In contrast with the remaining consistently detectable factors evaluated, secretion of IL-6 (Fig. 2F, open versus closed bars) and several others not shown, including IL-1b, IL-9, RANTES, MCP-1, and MIP-2 (Fig. S1), were not significantly affected by NIK deficiency. To determine the contribution of IL-12p40 to CD8+ T-cell stimulation in vitro, we supplemented the coculture with exogenous IL-12p40 and observed no rescue of the antigen cross-presentation defect in CD11c-Cre-NIKflox CD8+ DCs (Fig. 2G), although it did increase IFN-γ secretion by CD8+ T cells, as expected (Fig. 2H). Conversely, IL-12 neutralization had no inhibitory effect on cross-presentation (Fig. 2G) although it eliminated detectable secreted IL-12p40 in the assay (Fig. 2I). Curiously, neither IL-12 neutralization nor coculture with CD11c-Cre-NIKflox CD8+ DCs significantly inhibited IFN-γ secretion in cocultures with OT-I T cells. Taken together, these findings demonstrate that NIK is essential to the antigen cross-presentation pathway per se in CD8+ DCs, whereas IL-12p40 is not essential for CD8 T-cell stimulation, at least in vitro.
Fig. S1.
Factors secreted in vitro by splenic-derived CD11c+ DCs that are not impaired by NIK deletion. CD11c+ DCs isolated from spleens of CD11c-Cre NIKflox mice and CD11c-Cre NIKwt controls were cultured with or without 10 µg/mL α-CD40 mAb. After 1–2 d in culture, media were analyzed for secretion of IL-1b (A), IL-9 (B), MCP1 (C), MIP-2 (D), or RANTES (E). Results are of three similar experiments. *P < 0.05, **P < 0.005, ***P < 0.0005.
The MHC-II Presentation Pathway Is Independent of NIK.
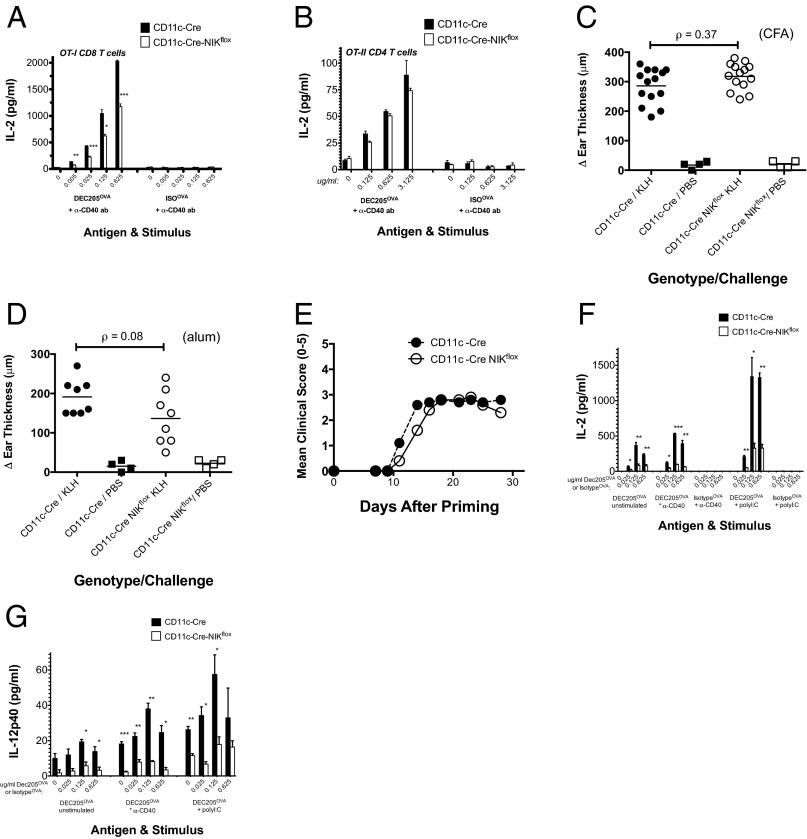
We next examined if NIK is required in antigen presentation by MHC-II. Splenocytes were cultured with either OVA-specific OT-II transgenic CD4+ T cells or OT-I transgenic CD8+ T cells in the presence of the α-DEC205OVA and stimulatory α-CD40 antibody. As expected CD11c-Cre-NIKflox splenocytes showed less efficient antigen cross-presentation than CD11c-Cre controls (Fig. 3A, open versus closed bars). In contrast, CD11c-Cre-NIKflox splenocytes presented antigen to OT-II transgenic CD4+ T cells as efficiently as the CD11c-Cre controls (Fig. 3B, open versus closed bars), demonstrating that NIK is not required for MHCII-restricted antigen presentation.
Fig. 3.
Antigen presentation via MHC-II in CD11c-Cre-NIKflox splenocytes is preserved, and the cross-presentation defect via MHC-I is not specific to CD40 stimulation. (A and B) Splenocytes from CD11c-Cre NIKflox mice and CD11c-Cre NIKWT controls were cultured as described (Materials and Methods) with CD8+ T cells from OT-I (A) or CD4+ T cells from OT-II (B) TCR transgenic mice in the presence of the indicated concentrations of anti-DEC205OVA or an isotype IgOVA control, with 10 μg/mL anti-CD40 mAb. After 1–2 d in culture, media were analyzed for secretion of IL-2. (C and D) In separate in vivo experiments, CD11c-Cre NIKflox mice and CD11c-Cre NIKwt controls were sensitized with 100 μg KLH SQ using as an adjuvant either CFA (C) or alum (D) and after 6 d were challenged with 50 μg KLH SQ in the ear. Swelling was evaluated 1 d later by measurement of ear thickness. (E) Another paired cohort of CD11c-Cre NIKflox mice and CD11c-Cre NIKWT controls (n = 11–15 each) were primed on day 0 with 300 μg MOG/CFA SQ plus 200 ng PTX i.p. on days 0 and 2 and were graded clinically for encephalitic symptoms as described in Materials and Methods. Mean scores for each genotype at each time point are plotted. Results are representative of three separate experiments. (F and G) CD8+ DCs from CD11c-Cre NIKflox mice and CD11c-Cre NIKWT controls were cultured in vitro with CD8+ T cells from OT-I TCR transgenic mice in the presence of indicated concentrations of α-DEC205OVA or isotype IgOVA, with or without 10 μg/mL a-CD40 mAb or 10 μg/mL poly I:C. After 1–2 d in culture, media were analyzed for secretion of IL-2 (F) and IL-12p40 (G). To facilitate comparisons between stimuli, data from Fig. 2B, which were collected in the same experiments, are included in F. Results are representative of at least two similar experiments. *P < 0.05; **P < 0.005; ***P < 0.0005.
Because this in vitro observation conflicted with a published report that effector CD4 T-cell function absolutely requires NIK in DCs in vivo (10), we next investigated CD4 T-cell–dependent immunity in the CD11c-Cre-NIKflox mice using two different model systems: a delayed-type hypersensitivity model and an experimental autoimmune encephalitis model. The delayed-type hypersensitivity response in these mice was not significantly different from that of the CD11c-Cre NIKWT controls, regardless of the type of adjuvant used (Fig. 3 C and D, open versus closed circles). Similarly, development of experimental autoimmune encephalitis in the CD11c-Cre-NIKflox mice was only somewhat delayed in comparison with the CD11c-Cre NIKWT controls (Fig. 3E, open versus closed circles), in contrast to the profound defect in this response observed in globally NIK-deficient NIKaly/aly mice (10, 22) and NIKKO/KO mice (9). These findings conflict with Hoffman and colleagues’ assertion that CD4 T-cell–mediated immunity depends extrinsically upon NIK in DCs (10) but are consistent with a collection of other findings implicating NIK intrinsically in CD4 effector T-cell function and immunity (9, 23–25).
The Antigen Cross-Presentation Defect in CD11c-Cre-NIKflox CD8+ DCs Is Not Specific to CD40 Stimulation.
NIK-dependent activation of the noncanonical NF-κB pathway is a known component of CD40 signaling (26). To determine whether the antigen cross-presentation defect observed in CD11c-Cre-NIKflox CD8+ DCs is specific to CD40 stimulation, we next evaluated antigen cross-presentation in the context of a DC stimulus not previously associated with the noncanonical NF-κB pathway, namely the TLR3 ligand polyinosinic:polycytidylic acid (poly I:C). Interestingly, antigen cross-presentation also was defective in poly I:C-stimulated CD11c-Cre-NIKflox CD8+ DCs (Fig. 3F, open versus closed bars). Notably, even the variably observed background cross-presentation activity observed in the absence of any added stimulus [presumably attributed to spontaneous DC activation during purification (27)] was defective in CD11c-Cre-NIKflox CD8+ DCs. As seen with α-CD40 stimulation, an IL-12p40 secretion defect was observed in poly I:C-stimulated CD11c-Cre-NIKflox CD8+ DCs (Fig. 3G, open versus closed bars), and incubation with preprocessed OVA257–264 peptide restored presentation via MHC-I (Fig. 2C, open versus closed bars). These findings reveal a generalized antigen cross-presentation defect in CD11c-Cre-NIKflox CD8+ DCs not limited to the context of CD40 signaling.
The generation of transgenic CD11c-Cre-NIKflox mice has allowed us to evaluate NIK’s role under conditions that were free from the severe immune system developmental defects observed in mice globally deficient in NIK function (6–10). Using an α-DEC205 antibody to target immunogen to cross-priming specialized CD8+ DCs, we have demonstrated that CD11c-Cre-NIKflox mice mount a poor CD8+ T-cell response to exogenous antigen in vivo. This finding can be explained by an intrinsic CD8+ DC defect in antigen cross-presentation, which we have demonstrated in vitro. We also observed a substantial defect in IL12p40 secretion by CD8+ DCs. Although diminished IL-12p40 secretion did not account for the lack of CD8+ T-cell stimulation by CD11c-Cre-NIKflox CD8+ DCs in vitro, it is likely to play an essential role in cross-priming in vivo (16). Indeed, we observed that CD8 T cells produced much higher levels of IFN-γ when stimulated in presence of IL-12p40. We did not observe diminished IFN-γ production in CD8 T-cells stimulated in vitro by CD11c-Cre-NIKflox CD8+ DCs despite the significantly reduced IL-2 production, raising the possibility that NIK-deficient DCs may have an altered ability to prime antigen-specific multifunctional T cells that produce several effector cytokines, such as IFN-γ, IL-2, and TNF-α. Although we cannot exclude the possibility that an additional unidentified defect such as altered expression of a surface molecule and/or secretion of a soluble factor indirectly impairs antigen cross-presentation in CD11c-Cre-NIKflox CD8+ DCs, our finding that preprocessed peptide rescues this impairment nevertheless implicates intracellular antigen processing and presentation as a defective function in these cells, whether it is caused directly or indirectly by NIK deficiency. Our findings confirm and extend a previously published observation of impaired cross-priming in Aly/Aly mice (15) but demonstrate that the defect is cell autonomous to CD8+ DCs and eliminate the possibility that either deranged lymphoid organ development or a gain-of-function of NIKAly could account for the defect. NIK is required in CD8+ DCs for proper effector responses to licensing stimuli during cross-priming of naive CD8+ T cells. Although it now seems likely that NIK plays an important role in one or more signaling pathways required to license cross-presentation activity by CD8+ DCs, the precise mechanisms are unclear. The function of NIK is not limited to its involvement in CD40 signaling, because DCs stimulated through TLR3 (by poly-I:C) show the same defect. Also, although the canonical NF-κB subunit c-Rel has been linked to regulation of IL-12p40 in macrophages (28), DCs appear not to share this mode of IL-12p40 regulation (29, 30). The link between NIK and these functions will require considerable further study.
Our findings provide a straightforward explanation for the impaired cell-mediated immunity observed in Aly/Aly and NIK-KO mice (9, 10) and support a prior finding that antigen cross-presentation via MHC-I and presentation via MHC-II are differentially regulated (18). NIK has been considered clinically as a potential oncology target, because some cancers appear to depend on constitutive NIK activation for survival and growth (31, 32). Our findings raise the possibility that targeting NIK in an oncology setting also might alter the induction or maintenance of cell-mediated immunity, conceivably with negative or positive consequences on disease course, depending on how this alteration affects the balance of cancer immunity to tolerance. Our findings also provide a potential therapeutic rationale for targeting NIK in disease involving cell-mediated immunity, such as transplant rejection.
Materials and Methods
Mice.
CD11c-Cre transgenic mice were obtained from Boris Reizis (Columbia University, New York) and were bred with NIKflox transgenic mice (33). NIK and Cre allele status was positively confirmed in all progeny by exon-specific genomic PCR as described elsewhere (33). All mice were housed under specific pathogen-free conditions, and all experiments were conducted in accordance with NIH Guide for the Care and Use of Laboratory Animals (34) using protocols approved by the Genentech Institutional Animal Care and Use Committee.
Quantitative RT-PCR.
Bone marrow-derived DCs were prepared as previously described (35) and were enriched using α-CD11c+– based sorting on a FACSAria cytometer (Becton Dickson) and total RNA isolated with an RNeasy Plus Mini kit according to the manufacturer’s protocol (Qiagen). Quantitative RT-PCR (qRT-PCR) was performed using the TaqMan Gene Expression Assay (Mm00444154_m1; Applied Biosystems) with the NIK expression level normalized to a reference, RPL19 (Mm02601633_g1).
Western Blotting.
CD11c+ bone marrow-derived DCs were stimulated for 16 h with 4 μg/mL stimulatory α-LTβR polyclonal antibody (AF1008; R&D), and proteosomal degradation was blocked for an additional 5 h with 10 μM MG132 (Cayman Chemicals). Cells were lysed in 2× Laemmli buffer containing β-mercaptoethanol (Bio-Rad). Proteins were separated on 4–12% Bis-Tris gels under denaturing conditions using a NuPAGE electrophoresis system (Invitrogen), transferred to PVDF membrane, probed with a rabbit α-NIK IgG (4994; Cell Signaling Technology), and detected with HRP-α rabbit IgG (7074; Cell Signaling Technology). Blots were visualized using Super Signal West Pico (Pierce Chemical Co.). HEK293 cells with or without transiently transfected human NIK (pCMV-NIK) were used as expression controls for detection.
Histology.
Lymphoid tissues from 15 mice of each genotype were harvested and immersed in 10% neutral buffered formalin at ambient temperature for 24 h and then were paraffin-embedded. Histologic sections (4 μm) were stained with H&E and were evaluated visually in a blinded fashion by a board-certified pathologist.
Evaluation of Homeostatic Splenic Lymphocyte and DC Populations.
Single-cell suspensions were prepared from freshly harvested spleens from untreated mice of each genotype (kit 130-095-926; Miltenyi), were stained with α NuPAGE electrophoresis system B220 clone RA3-6b2 AF-700 (eBioscience), α-CD3 clone 145-2c11 PerCP-Cy5.5 (BioLegend), α-CD11c clone N418 Pacific Blue (BioLegend), α-MHC-II (I-A/I-E) clone M5/114.15.2 (BioLegend), and α-CD8a clone (53-6.7 APC-Cy7 (BioLegend), and then were analyzed by flow cytometry using an LSRII instrument (Becton Dickinson) and were data processed with FlowJo software (Tree Star).
Cross-Priming Experiments.
α-DEC205 antibody conjugated to OVA was obtained from Ralph Steinman (Rockefeller University, New York). CD11c-Cre NIKflox and CD11c-Cre NIKWT mice (6 to 16 wk old) were primed i.p. with or without 0.5 μg α-DEC205OVA in the presence or absence of 30 μg of the α-CD40 clone FGK45 (Genentech antibody facility) in 100 μL of PBS. Peripheral blood was collected in heparinized tubes 8 d later by terminal cardiac puncture, followed by red blood lysis (kit #20110; Stem Cell Technologies). Mononuclear cells were isolated and stained with MHC pentamer H-2Kb/SIINFEKL-R-PE (ProImmune Limited), combined with α-CD3 clone 145-2c11 APC-Cy7 (BD Biosciences), α-CD4 clone RM4-5 PerCP-Cy5.5 (BD Biosciences), α-B220 clone RA3-6b2 V500 (BD Biosciences), and α-CD8 clone 53-6.7 APC (eBioscience), and then were fixed in 1% paraformaldehyde, analyzed by flow cytometry using an LSRII instrument (Becton Dickinson), and data processed with FlowJo software (Tree Star).
Models of Delayed-Type Hypersensitivity and Experimental Autoimmune Encephalitis.
For delayed hypersensitivity experiments, CD11c-Cre NIKflox mice and CD11c-Cre NIKWT controls were sensitized with 100 μg keyhole limpet hemocyanin (KLH) subcutaneously (SQ) using as an adjuvant either complete Freund’s adjuvant (CFA) (Fig. 3C) or alum (Fig. 3D) and after 6 d were challenged with 50 μg KLK SQ in the ear. Ear swelling was evaluated 1 d later by measurement of ear thickness, and data were plotted individually for each mouse. For experimental autoimmune encephalitis experiments, 11–15 CD11c-Cre NIKflox mice and CD11c-Cre NIKWT controls were primed SQ on day 0 with 300 μg myelin-oligodendrocyte glycoprotein peptide (MOG) corresponding to amino acid residues 35–55 with CFA as an adjuvant, plus 200 ng pertussis toxin (PTX) i.p. on days 0 and 2 as a coadjuvant. Starting on day 8, mice were scored clinically three times per week for encephalitic symptoms as follows: 0, no overt signs of disease; 1, limp tail or hind limb weakness but not both; 2, both limp tail and hind limb weakness; 3, partial hind limb paralysis; 4, complete hind limb paralysis; 5, moribund or complete hindlimb paralysis with moderate to severe forelimb paresis requiring euthanasia. Mean scores for each genotype at each time point were calculated and plotted as a function of days after priming.
Antigen Cross-Presentation.
Splenocytes were harvested aseptically from CD11c-Cre NIKflox and CD11c-Cre NIKWT mice by enzymatic digestion followed by red blood cell lysis to obtain single-cell suspensions. For the splenocyte-based assay, 4 × 105 splenocytes per well in a 96-well plate were pulsed for 3 h at 37 °C with α-DEC205OVA or isotype IgOVA (Genentech antibody facility) in growth medium (RPMI-1640 GlutaMAX supplemented with 10% FBS/10 mM Hepes/1 mM sodium pyruvate). Splenocytes then were washed and chased for 1–2 d at 37 °C in growth medium in the presence of 2 × 105 OT-I TCR transgenic CD8+ or OT-II TCR transgenic CD4+ T cells isolated from spleens by negative selection (Miltenyi kits 130-095-236 and 130-095-248, respectively), with or without 10 μg/mL α-CD40 mAb FGK45 or isotype mAb GP120 (Genentech antibody facility). For some antigen-presentation assays, CD11c+CD8+ DCs were purified to ∼70% purity from single-cell splenic suspensions using a CD8+ DC isolation kit (130-091-169; Miltenyi); 1 × 104 CD8+ DCs per well were pulsed for 3 h at 37 °C as indicated with α-DEC205OVA, isotype IgOVA, or 0–5*104 pM OVA257–264 SIINFEKL peptide (Invivogen), washed, and chased for 1–2 d at 37 °C in the presence of 1.2 × 105 OT-I TCR transgenic CD8+ cells with or without 10 μg/mL α-CD40 mAb FGK45, isotype mAb GP120, or poly I:C (Invivogen),10 ng/mL exogenous IL-12 (419-ML-010-CF; R&D), or 10 μg/mL neutralizing α-IL-12 IgG (AF-419-NA; R&D). Supernatants in all assays were analyzed for cytokines by Luminex (Bio-Plex; Bio-Rad).
Stimulated Cytokines in CD11c+ DCs.
Splenic DCs were isolated from CD11c-Cre NIKflox and CD11c-Cre NIKWT mice to 50–75% purity using a cell isolation kit (130-052-001; Miltenyi) with or without subsequent further DC enrichment to >95% purity by sorting on a FACSAria flow cytometer (Becton Dickinson). DCs (1 × 105 per well) were incubated for 24 h in a flat-bottomed 96-well plate with or without α-CD40 mAb FGK (Enzo) (10 μg/mL) or an isotype control IgG (ALX-804-836; Enzo), and supernatants were analyzed for cytokines by Luminex.
Acknowledgments
We thank Ryan Pabalate, Gregg Sy, James Cupp, Andres Paler Martinez, Vida Asghari, David Davis, Stephanie Bumbaca, Linda Wang, and James Lee for technical assistance and Ron Firestein for NIK-transfected 293 cells.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1520627112/-/DCSupplemental.
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Cohn L, Delamarre L. (2014) Dendritic cell-targeted vaccines Front Immunol 5:255:1–11. [DOI] [PMC free article] [PubMed]
- 3.Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25(6):280–288. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Hayden MS, Ghosh S. NF-κB in immunobiology. Cell Res. 2011;21(2):223–244. doi: 10.1038/cr.2011.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun SC. Non-canonical NF-κB signaling pathway. Cell Res. 2011;21(1):71–85. doi: 10.1038/cr.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miyawaki S, et al. A new mutation, aly, that induces a generalized lack of lymph nodes accompanied by immunodeficiency in mice. Eur J Immunol. 1994;24(2):429–434. doi: 10.1002/eji.1830240224. [DOI] [PubMed] [Google Scholar]
- 7.Yamada T, et al. Abnormal immune function of hemopoietic cells from alymphoplasia (aly) mice, a natural strain with mutant NF-kappa B-inducing kinase. J Immunol. 2000;165(2):804–812. doi: 10.4049/jimmunol.165.2.804. [DOI] [PubMed] [Google Scholar]
- 8.Yin L, et al. Defective lymphotoxin-beta receptor-induced NF-kappaB transcriptional activity in NIK-deficient mice. Science. 2001;291(5511):2162–2165. doi: 10.1126/science.1058453. [DOI] [PubMed] [Google Scholar]
- 9.Jin W, Zhou XF, Yu J, Cheng X, Sun SC. Regulation of Th17 cell differentiation and EAE induction by MAP3K NIK. Blood. 2009;113(26):6603–6610. doi: 10.1182/blood-2008-12-192914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hofmann J, Mair F, Greter M, Schmidt-Supprian M, Becher B. NIK signaling in dendritic cells but not in T cells is required for the development of effector T cells and cell-mediated immune responses. J Exp Med. 2011;208(9):1917–1929. doi: 10.1084/jem.20110128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garceau N, et al. Lineage-restricted function of nuclear factor kappaB-inducing kinase (NIK) in transducing signals via CD40. J Exp Med. 2000;191(2):381–386. doi: 10.1084/jem.191.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andreakos E, et al. Ikappa B kinase 2 but not NF-kappa B-inducing kinase is essential for effective DC antigen presentation in the allogeneic mixed lymphocyte reaction. Blood. 2003;101(3):983–991. doi: 10.1182/blood-2002-06-1835. [DOI] [PubMed] [Google Scholar]
- 13.Yanagawa Y, Onoé K. Distinct regulation of CD40-mediated interleukin-6 and interleukin-12 productions via mitogen-activated protein kinase and nuclear factor kappaB-inducing kinase in mature dendritic cells. Immunology. 2006;117(4):526–535. doi: 10.1111/j.1365-2567.2006.02329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tamura C, et al. Impaired function of dendritic cells in alymphoplasia (aly/aly) mice for expansion of CD25+CD4+ regulatory T cells. Autoimmunity. 2006;39(6):445–453. doi: 10.1080/08916930600833390. [DOI] [PubMed] [Google Scholar]
- 15.Lind EF, et al. Dendritic cells require the NF-kappaB2 pathway for cross-presentation of soluble antigens. J Immunol. 2008;181(1):354–363. doi: 10.4049/jimmunol.181.1.354. [DOI] [PubMed] [Google Scholar]
- 16.Shortman K, Heath WR. The CD8+ dendritic cell subset. Immunol Rev. 2010;234(1):18–31. doi: 10.1111/j.0105-2896.2009.00870.x. [DOI] [PubMed] [Google Scholar]
- 17.Bonifaz L, et al. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196(12):1627–1638. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delamarre L, Holcombe H, Mellman I. Presentation of exogenous antigens on major histocompatibility complex (MHC) class I and MHC class II molecules is differentially regulated during dendritic cell maturation. J Exp Med. 2003;198(1):111–122. doi: 10.1084/jem.20021542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feau S, et al. (2012) The CD4⁺ T-cell help signal is transmitted from APC to CD8⁺ T-cells via CD27-CD70 interactions Nat Commun 3:948:1–9. [DOI] [PMC free article] [PubMed]
- 20.Delamarre L, Mellman I. Harnessing dendritic cells for immunotherapy. Semin Immunol. 2011;23(1):2–11. doi: 10.1016/j.smim.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Joffre OP, Segura E, Savina A, Amigorena S. Cross-presentation by dendritic cells. Nat Rev Immunol. 2012;12(8):557–569. doi: 10.1038/nri3254. [DOI] [PubMed] [Google Scholar]
- 22.Greter M, Hofmann J, Becher B. Neo-lymphoid aggregates in the adult liver can initiate potent cell-mediated immunity. PLoS Biol. 2009;7(5):e1000109. doi: 10.1371/journal.pbio.1000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aya K, et al. NF-(kappa)B-inducing kinase controls lymphocyte and osteoclast activities in inflammatory arthritis. J Clin Invest. 2005;115(7):1848–1854. doi: 10.1172/JCI23763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murray SE, et al. NF-κB–inducing kinase plays an essential T cell–intrinsic role in graft-versus-host disease and lethal autoimmunity in mice. J Clin Invest. 2011;121(12):4775–4786. doi: 10.1172/JCI44943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao X, et al. OX40 signaling favors the induction of T(H)9 cells and airway inflammation. Nat Immunol. 2012;13(10):981–990. doi: 10.1038/ni.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Razani B, Reichardt AD, Cheng G. Non-canonical NF-κB signaling activation and regulation: Principles and perspectives. Immunol Rev. 2011;244(1):44–54. doi: 10.1111/j.1600-065X.2011.01059.x. [DOI] [PubMed] [Google Scholar]
- 27.Jiang A, et al. Disruption of E-cadherin-mediated adhesion induces a functionally distinct pathway of dendritic cell maturation. Immunity. 2007;27(4):610–624. doi: 10.1016/j.immuni.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanjabi S, Hoffmann A, Liou HC, Baltimore D, Smale ST. Selective requirement for c-Rel during IL-12 P40 gene induction in macrophages. Proc Natl Acad Sci USA. 2000;97(23):12705–12710. doi: 10.1073/pnas.230436397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grumont R, et al. c-Rel regulates interleukin 12 p70 expression in CD8(+) dendritic cells by specifically inducing p35 gene transcription. J Exp Med. 2001;194(8):1021–1032. doi: 10.1084/jem.194.8.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanjabi S, et al. A c-Rel subdomain responsible for enhanced DNA-binding affinity and selective gene activation. Genes Dev. 2005;19(18):2138–2151. doi: 10.1101/gad.1329805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gardam S, Beyaert R. The kinase NIK as a therapeutic target in multiple myeloma. Expert Opin Ther Targets. 2011;15(2):207–218. doi: 10.1517/14728222.2011.548861. [DOI] [PubMed] [Google Scholar]
- 32.Uno M, et al. NF-κB inducing kinase, a central signaling component of the non-canonical pathway of NF-κB, contributes to ovarian cancer progression. PLoS One. 2014;9(2):e88347. doi: 10.1371/journal.pone.0088347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brightbill HD, et al. Conditional deletion of NF-kappaB-inducing kinase (NIK) in adult mice disrupts mature B-Cell survival and activation. J Immunol. 2015;195(3):953–964. doi: 10.4049/jimmunol.1401514. [DOI] [PubMed] [Google Scholar]
- 34. Committee on Care and Use of Laboratory Animals (1996) Guide for the Care and Use of Laboratory Animals (Natl Inst Health, Bethesda), DHHS Publ No (NIH) 85-23.
- 35.Pierre P, et al. Developmental regulation of MHC class II transport in mouse dendritic cells. Nature. 1997;388(6644):787–792. doi: 10.1038/42039. [DOI] [PubMed] [Google Scholar]