Metastatic prostate cancer (PCa) requires immediate attention in order to identify driving molecular mechanisms and therapeutic targets that will achieve sustainable regression of disease. Despite advances with novel therapies, patients either do not respond or develop rapid resistance to these agents (1). An emerging resistant phenotype is small-cell neuroendocrine prostate cancer or prostate adenocarcinoma with neuroendocrine features (NEPC) (2). The diagnosis of NEPC has clinical implications because these patients are often treated with platinum chemotherapy (3). Neuroendocrine differentiation in advanced PCa represents a significant therapeutic dilemma, and its prevalence may be underestimated at the present time because bone biopsies of metastatic sites are infrequent. In addition, the very definition of NEPC is subject to intense debate, in part because of the lack of objective criteria, ascribing an unequivocal molecular phenotype to this entity. Finally, the molecular mechanisms leading to and maintaining NEPC are not well understood.
Benign and neoplastic stem cells share similar molecular programs and are functionally similar. Using lineage-tracing studies, Pignon et al. have previously shown that all cell types in the adult prostate, including neuroendocrine cells, derive from a common multipotent p63-expressing basal cell (4). These developmental studies and the current finding that metastatic small-cell neuroendocrine carcinoma is molecularly more stem-like than the more canonical adenocarcinoma, suggest either a de-differentiation of prostate cancer cells toward a more pluripotent (and neuroendocrine) phenotype or a selective therapeutic pressure for the expansion of existing rare tumor cells that are basal/stem/neuroendocrine from the start (4). The cell of origin of prostate cancer is still subject to debate. Although tissue regeneration experiments point to basal cells, genetically engineered mouse models, on the other hand, show that both basal and luminal cells can be precursors. De novo development of NEPC occurs in mice with deletion of Tp53 and Rb in either basal or luminal prostate cells (5, 6). Furthermore, mice expressing luminal cell Myc and Pim1 kinase develop adenocarcinoma with neuroendoricine features (7, 8). These data illustrate that despite the cell of origin, NEPC share stemness and neuronal signatures that can be therapeutically exploited. In fact, the microenvironment, including drug treatment, may influence the type of cell that is transformed (9). As in other cancers, the understanding of cell lineage in development is of critical importance in classifying and treating malignancies arising from a given organ, and for inferring changes in differentiation under selective pressures.
In PNAS, Smith et al. (10) present an advance in our understanding of molecular traits underlying aggressive PCa. FACS-purified basal (CD49f Hi) and luminal epithelial (CD49f low) populations from benign and cancerous regions of primary human PCa were used to perform RNA-sequencing. With this approach, the authors describe a molecular signature that accompanies neuroendocrine differentiation and demonstrate that it recapitulates the basal and stem cell phenotype.
Genes overexpressed in the basal/stem/neuroendocrine cells compared with CD49f Hi include pathways found in development, such as WNT, as well as those regulating epithelial to mesenchymal transitions, thus influencing cell invasion and migration. Interestingly, circulating tumor cells (CTCs) from patients progressing under treatment with an androgen receptor inhibitor, compared with untreated cases, were previously found to activate WNT signaling (11). This again suggests that selective pressure under androgen treatment my induce a stem cell phenotype and, perhaps more importantly, now that single-cell RNA-seq can be accomplished in CTCs, the signature defined by Smith et al. (10) may be used in monitoring the insurgence of a neuroendocrine phenotype and help guide treatment options.
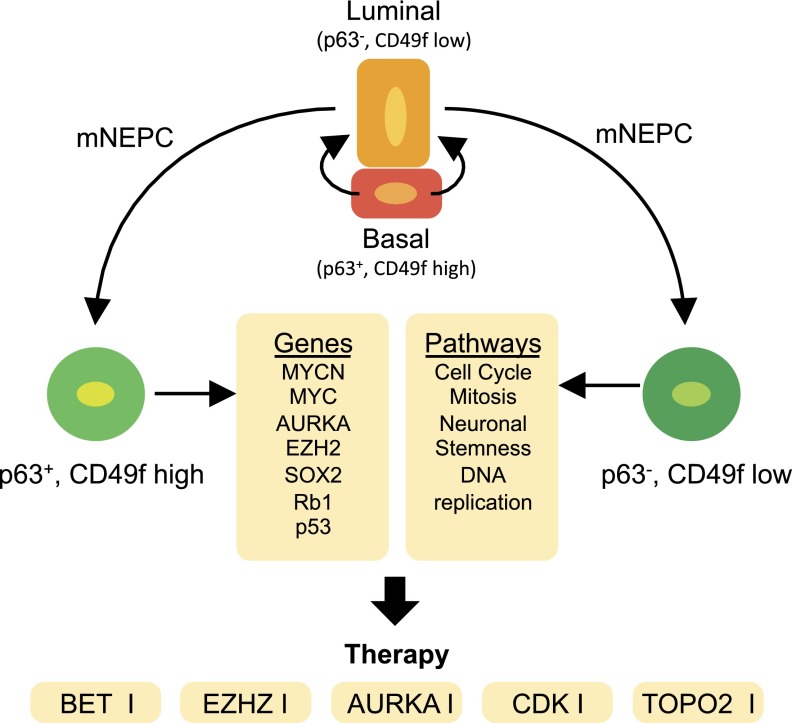
Most noticeable in the molecular signature of basal/stem cells by Smith et al. (10) was a shared gene network associated with E2F regulated genes. Overall, it was demonstrated that this prostate basal stem cell signature and NEPC clinical samples shared a 34-gene signature that was associated with E2F target genes and enriched for biological processes, including cell cycle, mitosis, DNA replication, and DNA repair in recently published human metastatic datasets including small-cell and neuroendocrine PCa samples (12, 13). This finding may represent novel therapeutic targets for treating advanced PCa (Fig. 1). E2F hyperactivity can be exploited therapeutically by cyclin-dependent kinase 4 (CDK4) and CDK6 inhibitors. CDK4/6 inhibitors show promise in preclinical PCa models (14), and currently a phase Ib/II clinical trial is ongoing in patients with metastatic castration resistant prostate cancer (ClinicalTrials.gov identifier: NCT02494921). In addition, Kirk et al. (15), recently demonstrated that concurrent targeting of DNA replication and mitotic-related proteins topoisomerase IIα (TOP2A) and enhancer of zeste homologue 2 (EZH2) provided significant therapeutic efficacy in preclinical models of aggressive PCa, including NEPC.
Fig. 1.
Potential drivers and therapeutic targets for the treatment of NEPC. The origin of metastatic NEPC (mNEPC) is still not well understood. mNEPC may emerge from a p63/CD49f Hi-positive cell or a p63/CD49f low cell. The progression to metastatic disease, however, can involve overlapping gene signatures and signaling pathways, providing novel targeted therapies to be tested and validated for the treatment of mNEPC.
The loss of Rb expression and function is highly apparent in human NEPC (13). Rb loss is critical for pluripotency networks driving reprogramming and tumorigenesis (16). Shared observations between the Rb-E2F axis in stem cell biology and small-cell cancers, including NEPC, identifies the potential for novel therapeutic targets to treat these aggressive tumors. Importantly, deregulation of the Rb1-E2F axis leads to increased expression of EZH2 (17). EZH2 is critical in regulating pluripotency and recently EZH2 activation was identified in small-cell lung cancer and NEPC (18, 19). Although little is known about EZH2 underlying mechanisms in driving small-cell phenotypes, targeting EZH2, especially by combination therapeutic strategies, is extremely attractive (15).
Beltran et al. (13) identified NEPC-specific overexpression and amplification of MYCN and AURKA (aurora kinase A). Although there was an overlay of E2F signaling with data from Beltran et al., Smith et al. (10) do not highlight MYCN or AURKA as significantly expressed in their CD49f Hi population. The enrichment of c-Myc signaling with maintained p63 expression in the CD49f stemness gene signature may explain the absence of MYCN and AURKA expression, because of overlapping functions of MYC family members restricting concurrent expression. These data raise an interesting question regarding the origin of NEPC, and whether the retention of p63 is more associated with small-cell phenotypes rather than adenocarcinomas with neuroendocrine differentiation, as suggested (7). Nevertheless, both aggressive phenotypes of prostate cancer share associated pathways that can be exploited therapeutically. These data also highlight the potential importance of mitotic and neural development pathways as underlying mechanisms driving progression to NEPC. Beltran et al. (13) indicated that targeting AURKA therapeutically would benefit patients with NEPC, and an ongoing phase II study is evaluating the efficacy of the AURKA inhibitor MLN8237 in patients with metastatic NEPC (ClinicalTrials.gov identifier: NCT01799278).
Because of MYCN involvement in NEPC, another therapeutic direction that must be considered is BET bromodomain inhibitors. MYCN is amplified in neuroblastoma and a significant correlation has been observed between MYCN amplification and sensitivity to BET inhibitors (20). If this correlation holds true, NEPC should be highly responsive to inhibition of BET bromodomains. Recently, BET inhibitors were demonstrated to be potent inhibitors of MYC activity in PCa preclinical models (21). Additional studies are required to evaluate the full potential of BET inhibitors and their potential impact for the clinical management of NEPC.
Overall, the study by Smith et al. (10) provides an innovative advancement in our understanding of molecular mechanisms underlying aggressive PCa. This study describes a gene signature that is most associated with stem cell signaling and invasiveness, which reveals that metastatic small-cell neuroendocrine carcinoma is molecularly more basal and stem-like than the adenocarcinoma phenotypes. Importantly, identification of E2F signaling as a common transcriptional program between small-cell neuroendocrine carcinoma and the basal prostate stem cells highlights a novel therapeutic direction with inhibitors of CDKs or targets involved in DNA replication, like topoisomerases and EZH2. Additional integrative analysis by Smith et al. (10) with already published gene signatures also reveals that small-cell prostate cancer and adenocarcinoma with neuroendocrine can share targetable actions for immediate evaluation, including inhibitors of AURKA, BET, and EZH2. As research into NEPC moves forward, clinical testing of such targeted therapies can be verified in real time and patients with NEPC will be provided much-needed treatment options for this lethal phenotype.
Footnotes
The authors declare no conflict of interest.
See companion article on page E6544.
References
- 1.Antonarakis ES, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371(11):1028–1038. doi: 10.1056/NEJMoa1315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aggarwal R, Zhang T, Small EJ, Armstrong AJ. Neuroendocrine prostate cancer: Subtypes, biology, and clinical outcomes. J Natl Compr Canc Netw. 2014;12(5):719–726. doi: 10.6004/jnccn.2014.0073. [DOI] [PubMed] [Google Scholar]
- 3.Beltran H, et al. Aggressive variants of castration-resistant prostate cancer. Clin Cancer Res. 2014;20(11):2846–2850. doi: 10.1158/1078-0432.CCR-13-3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pignon JC, et al. p63-expressing cells are the stem cells of developing prostate, bladder, and colorectal epithelia. Proc Natl Acad Sci USA. 2013;110(20):8105–8110. doi: 10.1073/pnas.1221216110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaplan-Lefko PJ, et al. Pathobiology of autochthonous prostate cancer in a pre-clinical transgenic mouse model. Prostate. 2003;55(3):219–237. doi: 10.1002/pros.10215. [DOI] [PubMed] [Google Scholar]
- 6.Zhou Z, et al. Synergy of p53 and Rb deficiency in a conditional mouse model for metastatic prostate cancer. Cancer Res. 2006;66(16):7889–7898. doi: 10.1158/0008-5472.CAN-06-0486. [DOI] [PubMed] [Google Scholar]
- 7.Vias M, et al. Pro-neural transcription factors as cancer markers. BMC Med Genomics. 2008;1:17. doi: 10.1186/1755-8794-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang J, et al. Pim1 kinase synergizes with c-MYC to induce advanced prostate carcinoma. Oncogene. 2010;29(17):2477–2487. doi: 10.1038/onc.2010.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldstein AS, Witte ON. Does the microenvironment influence the cell types of origin for prostate cancer? Genes Dev. 2013;27(14):1539–1544. doi: 10.1101/gad.222380.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith BA, et al. A basal stem cell signature identifies aggressive prostate cancer phenotypes. Proc Natl Acad Sci USA. 2015;112:E6544–E6552. doi: 10.1073/pnas.1518007112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyamoto DT, et al. Androgen receptor signaling in circulating tumor cells as a marker of hormonally responsive prostate cancer. Cancer Discov. 2012;2(11):995–1003. doi: 10.1158/2159-8290.CD-12-0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robinson D, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161(5):1215–1228. doi: 10.1016/j.cell.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beltran H, et al. Molecular characterization of neuroendocrine prostate cancer and identification of new drug targets. Cancer Discov. 2011;1(6):487–495. doi: 10.1158/2159-8290.CD-11-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Comstock CE, et al. Targeting cell cycle and hormone receptor pathways in cancer. Oncogene. 2013;32(48):5481–5491. doi: 10.1038/onc.2013.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirk JS, et al. Top2a identifies and provides epigenetic rationale for novel combination therapeutic strategies for aggressive prostate cancer. Oncotarget. 2015;6(5):3136–3146. doi: 10.18632/oncotarget.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kareta MS, et al. Inhibition of pluripotency networks by the Rb tumor suppressor restricts reprogramming and tumorigenesis. Cell Stem Cell. 2015;16(1):39–50. doi: 10.1016/j.stem.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bracken AP, et al. EZH2 is downstream of the pRB-E2F pathway, essential for proliferation and amplified in cancer. EMBO J. 2003;22(20):5323–5335. doi: 10.1093/emboj/cdg542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coe BP, et al. Genomic deregulation of the E2F/Rb pathway leads to activation of the oncogene EZH2 in small cell lung cancer. PLoS One. 2013;8(8):e71670. doi: 10.1371/journal.pone.0071670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clermont PL, et al. Polycomb-mediated silencing in neuroendocrine prostate cancer. Clin Epigenetics. 2015;7(1):40. doi: 10.1186/s13148-015-0074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Puissant A, et al. Targeting MYCN in neuroblastoma by BET bromodomain inhibition. Cancer Discov. 2013;3(3):308–323. doi: 10.1158/2159-8290.CD-12-0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wyce A, et al. Inhibition of BET bromodomain proteins as a therapeutic approach in prostate cancer. Oncotarget. 2013;4(12):2419–2429. doi: 10.18632/oncotarget.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]