Abstract
Chrysanthemums (Chrysanthemum morifolium Ramat.) exhibit a variety of flower colors due to their differing abilities to accumulate anthocyanins. One MYB member, CmMYB6, has been verified as a transcription regulator of chrysanthemum genes involved in anthocyanin biosynthesis; however, the co-regulators for CmMYB6 remain unclear in chrysanthemum. Here, the expression pattern of CmbHLH2, which is clustered in the IIIf bHLH subgroup, was shown to be positively correlated with the anthocyanin content of cultivars with red, pink and yellow flower colors, respectively. CmbHLH2 significantly upregulated the CmDFR promoter and triggered anthocyanin accumulation when co-expressed with CmMYB6. Yeast one-hybrid analyses indicated that CmbHLH2 was able to bind directly to the CmDFR promoter. Moreover, yeast two-hybrid assays indicated protein-protein interaction between CmbHLH2 and CmMYB6. These results suggest that CmbHLH2 is the essential partner for CmMYB6 in regulating anthocyanin biosynthesis in chrysanthemum.
Introduction
Chrysanthemum (Chrysanthemum morifolium Ramat.) is one of the most popular ornament plants in the world. Flower color is an important trait for its commercial value. In general, there are three classes of pigments that contribute to flower color: flavonoids, carotenoids and betalains [1]. Anthocyanins, which are derived from the phenylpropanoid pathway by a series of enzymes [2], are the most conspicuous class of flavonoids, which not only contribute to flower color but are also important in attracting pollinators, aiding seed dispersal and protecting plants from UV irradiation damage [3,4]. Studies on the molecular mechanism regulating anthocyanin biosynthesis have shown that the structural genes encoding the enzymes catalyzing anthocyanin biosynthesis are expressed synergistically, regulated by transcription factors, during anthocyanin accumulation [5–7]. Many results indicate MYBs play a crucial role in controlling the spatial and temporal expression of anthocyanin biosynthetic genes [8–10].
Recent developments have led to the isolation of chrysanthemum anthocyanin biosynthetic genes [11–16] and their use in the modification of flower colors [11, 17–18]. However, the transcriptional regulatory mechanisms have rarely studied. Hong et al. [16] identified three CmMYBs and one CmbHLH (basic helix-loop-helix) as the candidate transcription factors for anthocyanin biosynthesis mainly based on clustering analysis. In our previous studies, CmMYB6 was verified as being involved in the transcriptional regulation on CmDFR (Dihydroflavonol 4-reductase) and transient over-expression of CmMYB6 and MrbHLH1 (a known anthocyanin co-regulator in Myrica rubra) could trigger anthocyanin accumulation in tobacco leaves [10, 19]. This suggested that CmMYB6 controlled anthocyanin biosynthesis in chrysanthemum flowers in conjunction with endogenous bHLH member(s), which led the present research to find the putative anthocyanin related bHLH in chrysanthemum.
The basic helix-loop-helix (bHLH) proteins are a superfamily of transcription factors which contain a basic region consisting of 15–17 amino acids that are essential for DNA binding, and a helix-loop-helix domain that is important for formation of homodimers or heterodimers [20]. The bHLH transcription factors regulate numerous metabolic processes in plants such as photomorphogenesis [21], fate of epidermal cells [22], metal homeostasis [23, 24] and the flavonoid pathway [5] and their roles in flavonoid, especially anthocyanin, metabolism has been well illustrated in previous research [5–7]. The first bHLH transcription factors regulating the flavonoid pathway, R and B, were identified in maize in 1989 [25], then An1 was verified in petunia [26], and AtTT8 in Arabidopsis [27]. Subsequently, the bHLH member was identified and shown to participate in anthocyanin biosynthesis regulation in dahlia [28] and Asiatic hybrid lily [29]. Recently, Hsu et al. [9] identified three PeMYBs responsible for the floral pigmentation patterning in Phalaenopsis that interact with endogenous bHLH members PebHLH1-3. These results suggested that together with MYB, the bHLHs are essential transcription factors involved in anthocyanin biosynthesis in most plants.
In the present research, in order to investigate regulatory roles of CmbHLH in anthocyanin biosynthesis, CmbHLH2 and a previous reported stress related CmbHLH1 [30] were isolated, their expression patterns studied. The transcriptional regulatory role of CmbHLH was investigated and Yeast one- and two-hybrid assays were utilized to study interactions between CmbHLH2, CmMYB6 and target gene.
Materials and Methods
Plant materials
The ray florets of three chrysanthemum (Chrysanthemum morifolium Ramat.) cultivars, named ‘Zaoxiaoju no.1’ (‘Z1’), ‘Zaoxiaoju no.2’ (‘Z2’) and ‘Zaoxiaoju no.3’ (‘Z3’) with red, pink and yellow flower petals, respectively, were chosen as plant materials and picked at the full bloom from Hangzhou Administration Garden, China, during the 2014 season. Hangzhou Administration Garden is a national park and concerns with protection of genetic resources of ornamentals including chrysanthemums. It supports our study and offered the authority of sampling these plant materials used in this study.
Three biological replicates (10 g per replicate) of ray florets were frozen in liquid nitrogen immediately after being cut into small pieces and then stored at -80°C for subsequent measurement of anthocyanin content and RNA isolation.
Anthocyanin contents measurements
Total anthocyanin contents in the ray florets were measured by the pH difference method as described in our previous report [10]. Anthocyanins were extracted from 1 g frozen powdered samples in methanol/0.05% HCl and then absorbance measured in a UV-2550 spectrophotometer (Shimadzu) at 510 and 700 nm. For measurement of anthocyanin in tobacco leaves, frozen leaves were ground to a powder, extracted with methanol/1% HCl overnight at 4°C in the dark and the chlorophyll was then removed with an equal volume of chloroform before measurement of the absorbance at 530 and 657 nm. The relative anthocyanin content in tobacco leaves was calculated on a per g fresh weight basis by ((A530—A657)/mg FW tissue) ×1,000.
Gene cloning and sequence analysis
A partial CDS (Coding sequence) for one candidate bHLH gene related to anthocyanin biosynthesis regulation was isolated from the chrysanthemum ESTs (expressed sequence tags) database (http://www.ncbi.nlm.nih.gov/nucest/term=chrysanthemum). To obtain the full length ORF (Open reading frame), 3′ RACE (Rapid amplification of cDNA ends) and 5′ RACE were performed using the SMARTTM RACE cDNA Amplification Kit (Clontech, USA) with primers listed in Table 1. The ORF of this member was predicted by ORF Finder (http://www.ncbi.nlm.nih.gov/gorf/gorf.html) and then verified through PCR with FastStart High Fidelity (Roche, Switzerland) with the primers listed in Table 1.
Table 1. Primers used in this research.
| Genes | Assays | Primers | Sequences (5'-3') |
|---|---|---|---|
| CmbHLH1 | QPCR | Forward | CCTCCACCTGGAATCCAGGCTGC |
| Reverse | CAGATGGAAGCTCCTCGGCTCAG | ||
| SK | Forward | ATGGTTTCACCGGAGACTACTAC | |
| Reverse | TTAGGCAACAGGAGGGCGAAGCAC | ||
| AD/BD | Forward | ATCGATACCGAATTCATGGTTTCACCGGAGACTACTAC | |
| Reverse | ATTGGTACCGGATCCTTAGGCAACAGGAGGGCGAAGCAC | ||
| CmbHLH2 | 3'RACE | GSP1 | GTGACAGAGAACCAGCAAGCAGCAAC |
| GSP2 | TGGTGTCGGATTGGTTGGAGAGGCATAC | ||
| 5'RACE | GSP1 | GTCGCTGCTTCGTGTATGCCTCTCCAAC | |
| GSP2 | TAGTTGCTGCTTGCTGGTTCTCTGTCAC | ||
| QPCR | Forward | GTGAAGGTGAAGGGTATTAGGGGG | |
| Reverse | CTCTTCAAACGTCCTTCACATACC | ||
| SK | Forward | ATCGATACCGTCGACATGGCTGCCAGCGGACCACCTCG | |
| Reverse | ATTGGTACCGGGCCCCTAAGGAGATATTATTTGGTTGAT | ||
| AD/BD | Forward | ATGAATTCATGGCTGCCAGCGGACCACCTCG | |
| Reverse | ATGGATCCCTAAGGAGATATTATTTGGTTGAT | ||
| CmMYB6 | AD/BD | Forward | ATGAATTCATGGGGGAGTACAGAAAAATGAGAC |
| Reverse | ATGGATCCGGATTGCAATATCATAGTTGGTCCG | ||
| CmDFR-P | Y1H | Forward | ATGAGCTCGATGTGATTTTGGTGTTGACTTGG |
| AbAi | Reverse | ATGTCGACGTTGTTTAATCTTGTGGTTTTTGAAG |
The bHLH member was designated CmbHLH2 following the first identified member CmbHLH1 (GenBank KC686698.1) which was chosen as control in this study. The sequence alignment of two CmbHLHs and other bHLH members related to anthocyanin biosynthesis regulation in other species was performed by CLC Sequence Viewer 6 and Gendoc. Phylogenetic analysis was conducted by MEGA 6.06 [31].
QPCR (Real-time quantitative PCR) analysis
Total RNA extraction and cDNA synthesis were conducted according to our previous report [10]. RNA extractions were conducted with three biological replicates. The gene expression patterns were analyzed by QPCR analysis following the manufacturers’ instructions using Ssofast EvaGreen supermix (Bio-rad, USA). The QPCR primers for the two bHLH members are listed in Table 1, while those for anthocyanin biosynthetic genes were as referred to by Huang et al. [11]. All of the primers specificities were verified by both melting curves and QPCR products sequencing [32]. CmACT (GenBank AB770471) was used as a reference gene to evaluate the expression of genes of interest. No-template reactions were set as negative controls for each gene.
Dual luciferase assay
Dual luciferase assay, using pGreenII0029 62-SK and pGreenII 0800-LUC vectors, has been widely used to study the transcription regulating effects of transcription factors on their target promoters [10, 33–35]. The ORFs of CmbHLH1 and CmbHLH2 were amplified with the primers listed in Table 1 and recombined into pGreenII0029 62-SK vectors’ MCS (multiple cloning sites), respectively. The recombinant plasmids of CmMYB6-SK and CmDFR promoter-LUC in this study were constructed by Liu et al. [10]. Each of these recombinant plasmids was transferred into Agrobacterium tumefaciens GV3101 (MP90) individually.
GV3101 (MP90) containing CmbHLH-SK or/and CmMYB6-SK were mixed with CmDFR promoter-LUC at 10:1 ratio before infiltration into tobacco leaves (Nicotiana benthamiana). The ratio of enzyme activities of firefly luciferase (CmDFR::LUC) to renilla luciferase (35S::REN) was analyzed by Dual-Luciferase Reporter Assay System (Promega, USA) with Modulus Luminometer (Promega, USA) following the manufacturers’ instructions. Three independent experiments were carried out with at least four biological replicates for each.
Transient over-expression
Tobacco leaves (N. tabacum) were chosen for transient over-expression analysis of CmbHLH2. GV3101 (MP90) containing CmbHLH1-SK or CmbHLH2-SK was mixed with CmMYB6-SK or empty vector at 1:1 ratio before infiltration into tobacco leaves. The infiltrated patches of tobacco leaves were photographed and anthocyanin contents measured eight days after infiltration. Three independent experiments were carried out with three biological replicates for each combination.
Yeast hybridization
Yeast one-/two-hybrid (Y1H/Y2H) system were used to study protein-DNA or protein-protein interaction, respectively, according to the manufacturers’ instruction and as described in our previous publications [34, 35].
The CmDFR promoter was integrated into the genome of the Y1H Gold yeast strain by homologous recombination to generate the CmDFR-pAbAi bait strain after it was cloned into the pAbAi vector. The potential DNA-binding proteins, CmMYB6, CmbHLH1 or CmbHLH2, were expressed as fusion proteins containing the yeast GAL4 transcription activation domain (AD-CmMYB6, AD-CmbHLH1 or AD-CmbHLH2) recombined into pGADT7 vector, respectively, with the primers listed in Table 1. Then the minimal inhibitory concentration of Aureobasidin A for the bait strain was tested on SD/-Ura and SD/-Ura+AbAx media before testing the protein-DNA interactions which were screened on SD/-Leu media containing the minimal concentration of AbA antibiotic.
The ORFs of CmMYB6, CmbHLH1 and CmbHLH2 were recombined into pGBKT7 vector to express the bait proteins BD-CmMYB6, BD-CmbHLH1 or BD-CmbHLH2. Then all of these bait proteins were transformed into Y2H yeast stain, individually, to test their auto-activation on SD/-Trp media with X-α-Gal and AbA antibiotic background. Subsequently, the protein-protein interaction were detected by co-transforming the prey and bait plasmids into Y2H yeast strain and screening for growth of colonies on quadruple SD/-Ade/-His/-Leu/-Trp and SD/-Ade/-His/-Leu/-Trp+ X-α-Gal+ AbA media.
Results
CmbHLH2 predicted to regulate anthocyanin biosynthesis
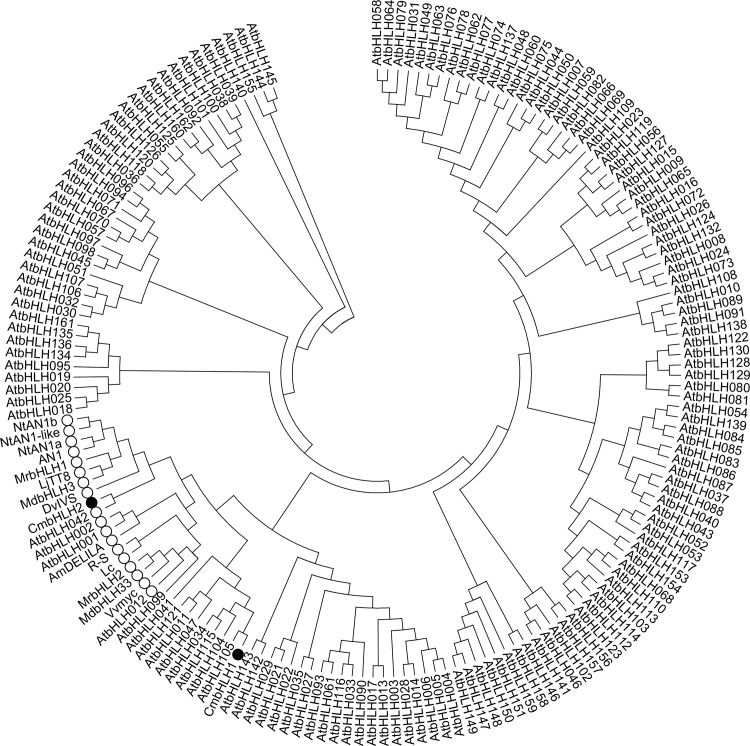
Based on the similarities with bHLHs verified as being related to anthocyanin biosynthesis in other species, one partial sequence bHLH member was mined from the chrysanthemum EST database. The full ORF, which was obtained by RACE and named as CmbHLH2 (GenBank KT724056), contained 1842 nucleotides and encoded 614 amino acids. A second bHLH member isolated from chrysanthemum, CmbHLH1, which was reported to be involved in the response to iron deficiency [30] was also selected. Phylogenetic analysis of these two members and 174 bHLHs including 158 from Arabidopsis and 14 from other plants, showed that CmbHLH2 was clustered in the subgroup containing sequences related to the regulation of anthocyanin biosynthesis, while CmbHLH1 was in a different subgroup (Fig 1).
Fig 1. Phylogenetic analysis of 178 bHLH members including 162 from Arabidopsis, 14 from other plants, and 2 from chrysanthemum.
The two bHLHs from chrysanthemum are marked with solid circles, while those related to anthocyanin biosynthesis regulation in Arabidopsis and other species are marked with open circles. Genes IDs are as follows: NtAn1b (Nicotiana tabacum, GenBank accession number AEE99258), NtAN1-like (N. tomentosiformis, AEE99260), NtAn1a (N. tabacum, AEE99257), AN1 (Petunia hybrid, AF260918), MrbHLH1 (Myrica rubra, JX629461), LjTT8 (Lotus japonicus, BAH28881), MdbHLH3 (Malus domestica, HM122458), DvIVS (Dahlia variabilis, AB601005), AmDELILA (Antirrhinum majus, AAA32663), R-S (Zea mays, X15806), L-c (Z. mays, NM001111869), MrbHLH2 (M. rubra, JX629462), MdbHLH33 (M. domestica, DQ266451), VvMYC (Vitis vinifera, EU447172).
The conserved basic helix-loop-helix (bHLH) domain containing approximately 60 basic amino acids was present in both CmbHLH1 and CmbHLH2 (S1 Fig). However, there were some differences between these two bHLH domains. The N-terminus of CmbHLH2 bHLH included a highly conserved HER motif (His5-Glu9-Arg13) which was also found in other bHLH members related to anthocyanin biosynthesis in other species and participated in binding the E-box (CANNTG) DNA motif (S1 Fig). Furthermore, the MYB interaction region (MIR) including box11, box13 and box18, which is able to bind the MYB to form a transcription complex, was located in the N-terminal region of the CmbHLH2 amino acid sequence (S1 Fig). However, these two characteristic features were not found in the CmbHLH1 sequence (S1 Fig).
Differences in ability to accumulate Anthocyanin among three chrysanthemum cultivars
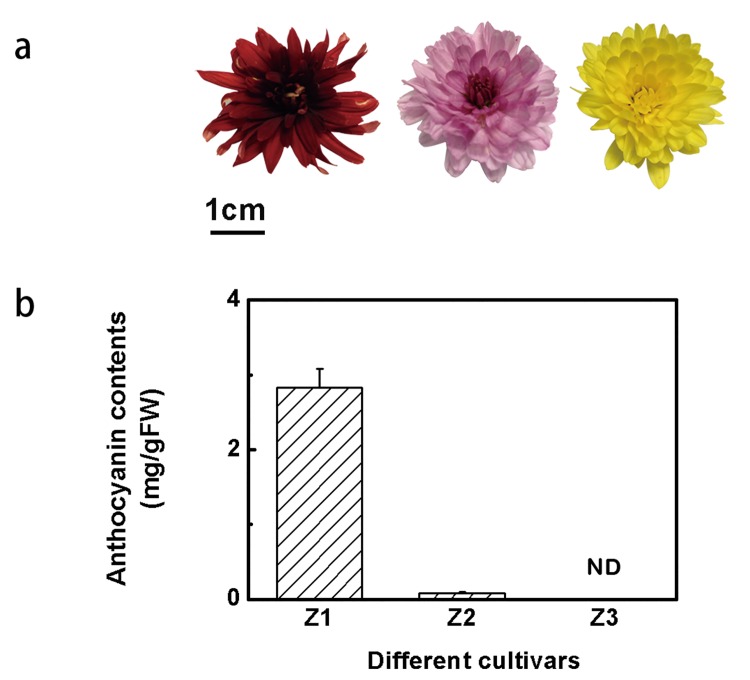
Three chrysanthemum cultivars used in this study had different flower colors (Fig 2A). Further measurement indicated that anthocyanin contents varied among them. ‘Z1’, with deep red ray florets, accumulated 2.83 mg/gFW anthocyanin compared with 0.08 mg/gFW in ‘Z2’ which had pink flower colors (Fig 2B). No anthocyanin was detected in ‘Z3’, which had yellow flowers (Fig 2B). This suggested that difference in anthocyanin contents was the main reason for the different colors of ray florets of these three chrysanthemum flower cultivars.
Fig 2. The anthocyanin contents of flowers of three different chrysanthemum cultivars.
(a) The photographs of three chrysanthemum cultivars, named ‘Z1’, ‘Z2’ and ‘Z3’, respectively, where the bar represents 1 cm. (b) Anthocyanin contents in flower petals from Z1, Z2 and Z3. The vertical bars represent S.E. of three biological replicates.
CmbHLH2 expression positively correlated with anthocyanin accumulation
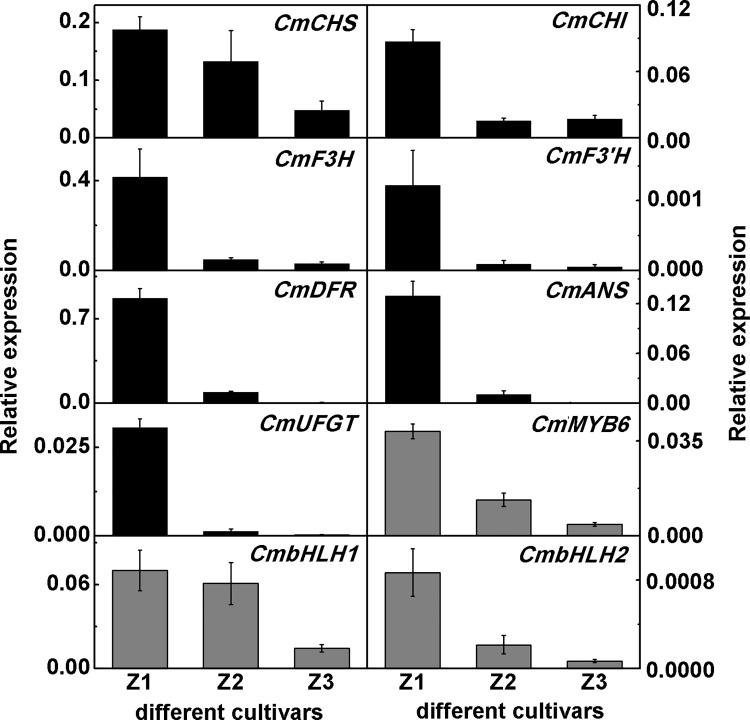
Accumulation of mRNAs for three TFs, CmMYB6, CmbHLH1 and CmbHLH2, and seven anthocyanin biosynthetic genes in the ray florets of three cultivars were analyzed using QPCR. All biosynthetic genes were expressed at the highest level in ‘Z1’ and were positively correlated with anthocyanin accumulation in these three cultivars, although the transcriptional level of CmCHI (Chalcone isomerase) was similar in ‘Z2’ and ‘Z3’ (Fig 3). The transcript levels of CmDFR and CmUFGT (UDP flavonoid glucosyl transferase) were very low in ‘Z3’ compared with those in ‘Z1’, and CmANS (Anthocyanidin synthase) was undetectable in ‘Z3’ (Fig 3). Accumulation of mRNA for each of the three TFs was highest in ‘Z1’ and lowest in ‘Z3’ (Fig 3) as found for the biosynthetic genes.
Fig 3. The transcript levels of seven anthocyanin biosynthetic genes and three TFs in the ray florets of three chrysanthemum cultivars used in this study.
The CmACT gene was used to normalize expression of the genes. Non-template reactions were set as the negative control for each gene. The vertical bars represent S.E. of three biological replicates.
CmbHLH2 stimulated CmDFR promoter activity when coupled with CmMYB6
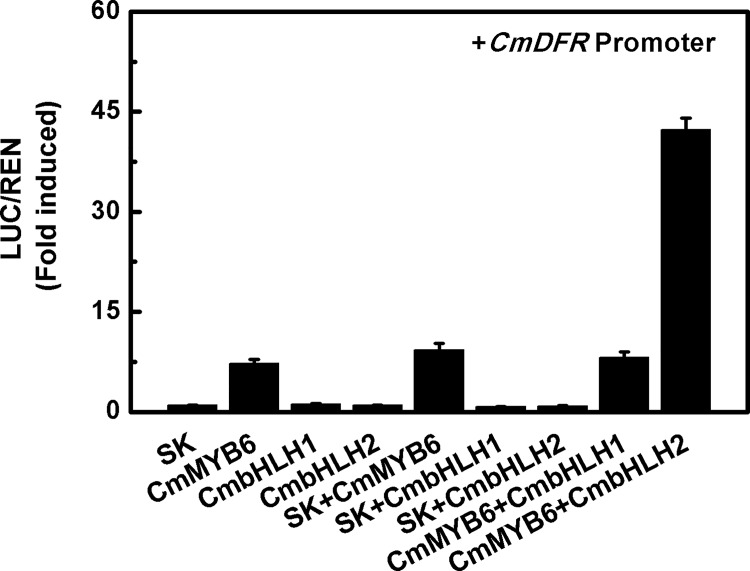
To predict the transcriptional regulating roles of CmbHLH1 and CmbHLH2, dual luciferase assay with the CmDFR promoter were used in this study. Based on the results, CmbHLH1 or CmbHLH2 singly had no effect on the activity of CmDFR promoter compared to the empty vector, activity of which was set as one, whereas CmMYB6 induced an approximately 8-fold increase in CmDFR promoter activity (Fig 4). However, when co-expressed with CmMYB6, CmbHLH2 showed a significant synergistic effect and CmDFR promoter activity was stimulated over 40-fold (Fig 4). CmbHLH1, on the other hand, was unable to up-regulate the activity of CmDFR promoter when co-expressed either with empty vector or with CmMYB6 (Fig 4). This indicated that CmbHLH2, but not CmbHLH1, has the ability to regulate anthocyanin biosynthesis.
Fig 4. In vivo interactions between CmMYB6, CmbHLHs and the CmDFR promoter were revealed by dual luciferase assays in tobacco leaves.
Error bars are the S.E. of three independent experiments with at least four replicates.
CmbHLH2 directly bound to CmDFR promoter
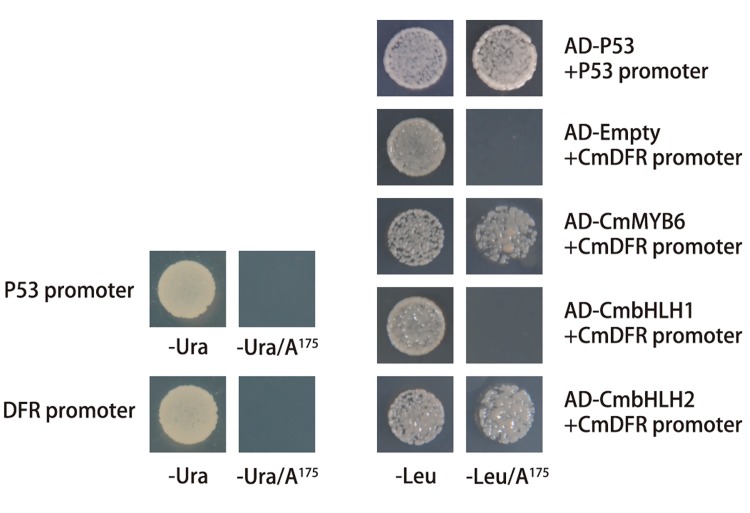
The relationship between CmbHLH2 and the CmDFR promoter was further analyzed by yeast one-hybrid assay. Successful generation of the CmDFR-pAbAi bait strain was confirmed by screening colony growth on SD/-Ura media and auto-activation was inhibited with 175 ng/ml AbA antibiotic (Fig 5). When the bait strains were transformed with the fusion protein AD-CmMYB6, AD-CmbHLH1 or AD-CmbHLH2, which has the activation domain, different colony growing abilities were detected on the testing media. Strains transformed with AD-CmMYB6 or AD-CmbHLH2 could grow on SD/-Ura+AbA175, while those with either empty vector or AD-CmbHLH1 could not (Fig 5).
Fig 5. Characterization of the interaction of three TFs with the CmDFR promoter by yeast one-hybrid assays.
P53 and its promoter supplied with the kit were used as a positive control to verify the stability of these assays. The auto-activity of CmDFR promoter bait stain was tested on SD media lacking Ura in presence of AbA. CmbHLH1, CmbHLH2 and CmMYB6 were fused into pGADT7 (AD) and transformed separately into the bait stain. The binding was screened on SD media lacking Leu in presence of AbA175.
CmbHLH2 interacted with CmMYB6 to form a transcriptional complex
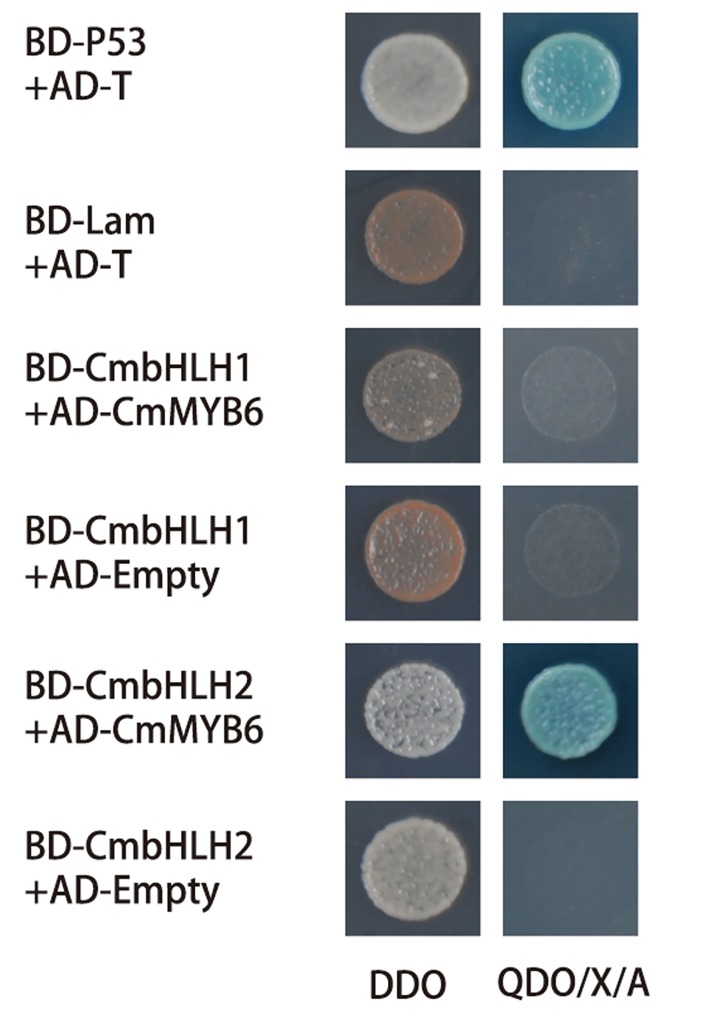
To study the mode of interaction between CmbHLH2 and CmMYB6 proteins during the transcriptional regulation of CmDFR promoter, Y2H assay were carried out. Based on the testing results, the auto-activation of BD-CmbHLH1 and BD-CmbHLH2 were inhibited with 100 ng/ml and 400 ng/ml AbA, respectively (S2 Fig). However, auto-activation of BD-CmMYB6 could not be inhibited even with 1000 ng/ml AbA (S2 Fig), thus CmMYB6 was used as the prey protein and CmbHLH1 or CmbHLH2 as bait proteins in subsequent experiments. Protein-protein interaction between CmMYB6 and CmbHLH2 was indicated by growth of colonies containing both AD-CmMYB6 and BD-CmbHLH2 proteins on SD/-Ade/-His/-Leu/-Trp/+X-α-Gal/+AbA screening media (Fig 6).
Fig 6. Protein-protein interactions between CmbHLHs and CmMYB6 studied by yeast two-hybrid assay.
The yeast strain was co-transformed with the indicated combinations of CmbHLH1 or CmbHLH2 fused into pGBKT7 (BD) and CmMYB6 fused into pGADT7 (AD). BD-p53 and AD-T were used as positive controls, while BD-Lam and AD-T were used as negative controls. Protein-protein interactions were detected on DDO (SD media lacking Leu and Trp) and QDO/X/A (SD media lacking Leu, Trp, His and Ade, with AbA and X-α-gal) media.
CmbHLH2 together with CmMYB6 triggered anthocyanin accumulation in tobacco leaves
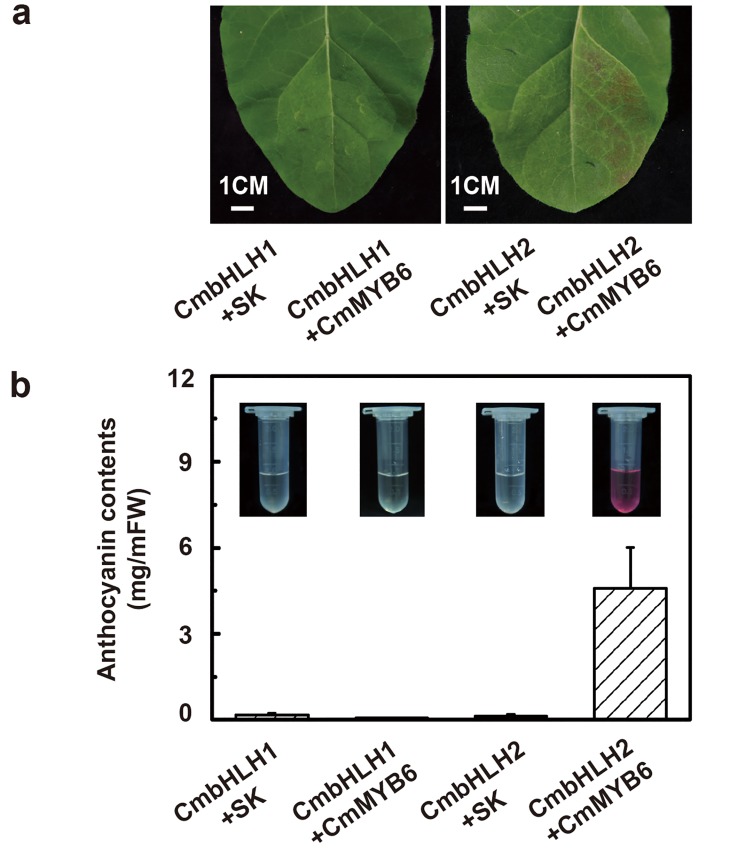
To confirm the role of CmbHLH2 in the regulation of anthocyanin biosynthesis, a transient expression assay was performed in tobacco leaves. Although CmMYB6 could stimulate the activity of the CmDFR promoter (Fig 4), anthocyanin accumulation could not be triggered by a single CmMYB6 member (S3 Fig). Similarly, CmbHLH1 or CmbHLH2 singly could not regulate anthocyanin biosynthesis in tobacco leaves (Fig 7). Only the patches infiltrated with both CmMYB6 and CmbHLH2 had the ability to produce a dramatic accumulation of anthocyanin, of up to 5.0 mg/gFW on the 8th day after infiltration (Fig 7). Based on these results, CmbHLH2 is the crucial and essential member of the MBW transcription complex regulating anthocyanin biosynthesis together with CmMYB6 in chrysanthemum.
Fig 7. Transient over-expression of CmMYB6 and CmbHLH1 or CmbHLH2 carried out in tobacco leaves.
(a) Photographs taken 8 days after the infiltration. (b) Analysis of anthocyanin contents of tobacco leaves 8 days after infiltration with the TFs. Each experiment was carried out with three biological repeats and the error bars represent the S.E. of these replicate reactions.
Discussion
Chrysanthemum is an important and popular ornamental crop worldwide. During the long breeding history, lots of cultivars have been generated and cultivated [36]. The agricultural traits vary significantly among these cultivars. The three different cultivars ‘Z1’, ‘Z2’ and ‘Z3’ were chosen as plant materials because of their high similarities except for the flower colors. Most of the agricultural traits of these three cultivars, such as plant height and type and size of, leaves and, flowers are similar. Furthermore, the full blooming dates of ‘Z1’, ‘Z2’ and ‘Z3’ are almost the same.
CmbHLH2 was the first confirmed bHLH member related to anthocyanin biosynthesis regulation in chrysanthemum. Its sequence alignment showed conserved among different cultivars of chrysanthemum, while suggested variable compared to members related to anthocyanin biosynthesis regulation in other species. The full length sequence of CmbHLH2 was obtained from ‘Z1’ cDNA by means of RACE using the reference sequence mined from the EST database of the ‘Zhongshanzigui’ cultivar [37]. Subsequently, comparison of CmbHLH2 ORFs in different cultivars, including ‘Z2’, ‘Z3’ and ‘Amadea’ which is a cut chrysanthemum cultivar with purple flower colors [10], were carried out through gene cloning and sequence alignments. However, no significant differences were found in these sequences (data not shown). This indicated the strong conservation of CmbHLH2 ORF in different chrysanthemum cultivars studied. Sequence comparisons with related genes from other species, however, gave low identities. For example, CmbHLH2 shared 64% identities with DvIVS (Dahlia pinnata, [28]), 48% with PhAN1 (Petunia×hybrida, [26]) and 59% with AtTT8 (AtbHLH042, Arabidopsis thaliana, [27]). Although all of these bHLH members play essential roles in the regulation of anthocyanin biosynthesis, the similarities in their sequences were restricted to the MIR (box 11, box 13 and box 18) and bHLH domain which are responsible for the interaction with MYB partners and binding to the target gene promoters, respectively [20]. Thus, these sequences could be used to predict the potential role of bHLH members in anthocyanin biosynthesis regulation.
TFs regulate the target genes through binding to the cis-elements located in the gene promoters. The bHLH encoded proteins are known to bind to the E/G-box (CANNTG/CACGTG), but the MRE (MYB recognizing elements) motifs vary greatly in different gene promoters [7]. For example, there were as many as nine different kinds of MRE motifs found in MrDFR1 and MrUFGT promoters involved in anthocyanin biosynthesis in Chinese bayberry [19]. Cis-elements recognized by MYB or bHLH have been found in the CmDFR promoter sequence, for example, MYBPZM (CCWACC), MYB1AT (WAACCA) and MYBATRD22 (CACATG), recognized by MYB, and E-box (CANNTG) and ACEs (ACGT containing elements), recognized by bHLH [10]. In this study, both CmMYB6 and CmbHLH2 could bind to the CmDFR promoter as tested by Y1H assay (Fig 5). However, whether they recognizing the exact cis-elements predicted in CmDFR promoter remains unclear and need further verification. Furthermore, the last 26 amino acids located before the CmbHLH2 termination codon played an important role in binding the CmDFR promoter during anthocyanin biosynthesis regulation. In our study, another bHLH member which showed 100% identities to CmbHLH2 sequence except for the last 26 amino acids, had no ability to bind the CmDFR promoter based on Y1H assay. No anthocyanin accumulation could be detected in tobacco leaves transient over-expressed both this bHLH member and CmMYB6, although it could interact with CmMYB6 in Y2H system (data not showed). However, the novel characteristic of this bHLH member in anthocyanin biosynthesis in chrysanthemum still needs further study.
Interactions between TFs are the central to life processes in bionts and the interaction among MYB, bHLH and WDR have been well characterized in all angiosperms analyzed so far [7]. Two MBW complexes related to flavonoid biosynthesis regulation, such as PhAN2-PhAN1-PhAN11 in petunia [26] and AtTT2-AtTT8-AtTTG1 in Arabidopsis [38] have been characterized. The interaction between CmMYB6 and CmbHLH2 indicated by Y2H test in this study (Fig 6) was in some respects similar to the AtTT2/AtTT8 physical interaction to form a TF complex active during the accumulation of PAs (proanthocyanins) in Arabidopsis [38]. Based on this evidence, it is suggested that CmMYB6 and CmbHLH2 form a binary complex through physically interacting with each other to regulate expression of CmDFR during anthocyanin biosynthesis in chrysanthemum.
Based on previous results, MYB proteins frequently determine the involvement of MBW complexes in specific pathways, although the specificity of the MBW complexes for different targets most probably relies on both MYB and bHLH, as well as on other regulators [7]. Our results show that anthocyanin biosynthesis was triggered only when CmMYB6 and CmbHLH2 were co-expressed in tobacco leaves (Fig 7). It is unclear, however, whether the endogenous WDR member(s) in tobacco leaves participated this process the possible role(s) of endogenous WDR member(s) in anthocyanin accumulation in chrysanthemum requires further study.
Conclusions
Differences in flower colors between ‘Z1’, ‘Z2’ and ‘Z3’ were due to their differing abilities to accumulate anthocyanins. Expression of all seven biosynthetic genes, as well as TFs CmbHLH2 and CmMYB6, was positively correlated with the anthocyanin content in different cultivars. The IIIf subgroup member, CmbHLH2, contained a conserved bHLH domain and MIR regions and stimulated CmDFR promoter activity up to 40-fold through physical interaction with CmMYB6, indicating it is a partner in the transcription complex controlling expression of anthocyanin biosynthesis genes in developing chrysanthemum flowers.
Supporting Information
Three conserved boxes, including box11, 18 and 13, found in these bHLHs play essential roles in the interaction with MYBs transcription factors leading to enhanced transcription of anthocyanin biosynthesis genes. Amino acid residues (H-E-R) at position 5, 9 and 13 of the bHLH domains are critical for DNA binding.
(TIF)
Three TFs, CmMYB6, CmbHLH1 and CmbHLH2, were cloned separately into pGBKT7 (BD). Auto-activation was screened on SD/-Trp media with X-α-Gal and AbA antibiotic background.
(TIF)
The full ORF of each gene was cloned into pGreenII0029 62-SK and electroporated into Agrobacterium tumefaciens GV3101 (MP90). Different combinations of these Agrobacterium tumefaciens were infiltrated into tobacco leaves. Photographs were taken 8 days later. Each experiment was carried out with three biological repeats.
(TIF)
Abbreviations
- AbA
Aureobasidin
- ANS
Anthocyanidin synthase
- bHLH
basic helix-loop-helix
- CDS
Coding sequence
- CHI
Chalcone isomerase
- CHS
Chalcone synthase
- DFR
Dihydroflavonol 4-reductase
- ESTs
Expressed sequence tags
- F3H
Flavanone 3-hydroxylase
- F3'H
Flavanone 3'-hydroxylase
- LUC
Firefly luciferase
- MBW
MYB-bHLH-WD40
- MCS
Multiple cloning sites
- MRE
MYB recognizing elements
- MIR
MYB interaction region
- ORF
Open reading frame
- QPCR
Real-time quantitative PCR
- RACE
Rapid amplification of cDNA ends
- REN
Renilla luciferase
- SD
Synthetic dropout
- TFs
Transcription factors
- UFGT
UDP flavonoid glucosyl transferase
- UTR
Untranslated region
- Y1H
Yeast one-hybrid
- Y2H
Yeast two-hybrid
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was supported by the National High Technology Research and Development Program of China (2013AA102700), the National Natural Science Foundation of China (31401909), and the China Postdoctoral Science Foundation (2014M551758).
References
- 1. Tanaka Y, Sasaki N, Ohmiya A. Biosynthesis of plant pigments: anthocyanins, betalains and carotenoids. Plant J. 2008; 54(4): 733–749. 10.1111/j.1365-313X.2008.03447.x [DOI] [PubMed] [Google Scholar]
- 2. Holton TA, Cornish EC. Genetics and biochemistry of anthocyanin biosynthesis. Plant Cell 1995; 7(7): 1071–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Winkel-Shirley B. Flavonoid Biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001; 126(2): 485–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lepiniec L, Debeaujon I, Routaboul JM, Baudry A, Pourcel L, Nesi N, et al. Genetics and biochemistry of seed flavonoids. Annu Rev Plant Biol. 2006; 57: 405–430. [DOI] [PubMed] [Google Scholar]
- 5. Hichri I, Barrieu F, Bogs J, Kappel C, Delrot S, Lauvergeat V. Recent advances in the transcriptional regulation of the flavonoid biosynthetic pathway. J Exp Bot. 2011; 62(8): 2465–2483. 10.1093/jxb/erq442 [DOI] [PubMed] [Google Scholar]
- 6. Albert NW, Davies KM, Lewis DH, Zhang H, Montefiori M, Brendolise C, et al. A conserved network of transcriptional activators and repressors regulates anthocyanin pigmentation in eudicots. Plant Cell 2014; 26(3): 962–980. 10.1105/tpc.113.122069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xu W, Dubos C, Lepiniec L. Transcriptional control of flavonoid biosynthesis by MYB-bHLH-WDR complexes. Trends Plant Sci. 2015; 20(3): 176–185. 10.1016/j.tplants.2014.12.001 [DOI] [PubMed] [Google Scholar]
- 8. Elomaa P, Uimari A, Mehto M, Albert VA, Laitinen RAE, Teeri TH. Activation of anthocyanin biosynthesis in Gerbera hybrid (Asteraceae) suggests conserved protein-protein and protein-promoter interactions between the anciently diverged monocots and eudicots. Plant Physiol. 2003; 133(4): 1831–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hsu CC, Chen YY, Tsai WC, Chen WH, Chen HH. Three R2R3-MYB transcription factors regulate distinct floral pigmentation patterning in Phalaenopsis orchids. Plant Physiol. 2015; 168(1): 175–191. 10.1104/pp.114.254599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu XF, Xiang LL, Yin XR, Grierson D, Li F, Chen KS. The identification of a MYB transcription factor controlling anthocyanin biosynthesis regulation in Chrysanthemum flowers. Sci Hortic. 2015; 194: 278–285. [Google Scholar]
- 11. Huang H, Hu K, Han KT, Xiang QY, Dai SL. Flower colour modification of chrysanthemum by suppression of f3'h and overexpression of the exogenous senecio cruentus f3'5'h gene. PLoS ONE. 2013; 8(11): e74395 10.1371/journal.pone.0074395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sung S, Kim S, Velusamy V, Lee Y-M, Ha B-K, Kim J-B, et al. Comparative gene expression analysis in a highly anthocyanin pigmented mutant of colorless chrysanthemum. Mol Biol Rep. 2013; 40(8): 5177–5189. 10.1007/s11033-013-2620-5 [DOI] [PubMed] [Google Scholar]
- 13. Zhu L, Shan H, Chen S, Jiang J, Gu C, Zhou G, et al. The heterologous expression of the chrysanthemum R2R3-MYB transcription factor CmMYB1 alters lignin composition and represses flavonoid synthesis in Arabidopsis thaliana . PLoS ONE. 2013; 8(6): e65680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang T, Guo QS, Mao PF. Flavonoid accumulation during florescence in three Chrysanthemum morifolium Ramat cv. ‘Hangju’ genotypes. Biochem Syst Ecol. 2014; 55(0): 79–83. [Google Scholar]
- 15. Hong Y, Dai SL. Selection of reference genes for real-time quantitative polymerase chain reaction analysis of light-dependent anthocyanin biosynthesis in chrysanthemum. J Am Soc Hortic Sci. 2015; 140(1): 68–77. [Google Scholar]
- 16. Hong Y, Tang X, Huang H, Zhang Y, Dai SL. Transcriptomic analyses reveal species-specific light-induced anthocyanin biosynthesis in chrysanthemum. BMC Genomics 2015; 16(1): 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brugliera F, Tao GQ, Tems U, Kalc G, Mouradova E, Price K, et al. Violet/blue chrysanthemums-metabolic engineering of the anthocyanin biosynthetic pathway results in novel petal colors. Plant Cell Physiol. 2013; 54(10): 1696–1710. 10.1093/pcp/pct110 [DOI] [PubMed] [Google Scholar]
- 18. Noda N, Aida R, Kishimoto S, Ishiguro K, Fukuchi-Mizutani M, Tanaka Y, et al. Genetic engineering of novel bluer-colored chrysanthemums produced by accumulation of delphinidin-based anthocyanins. Plant Cell Physiol. 2013; 54(10): 1684–1695. 10.1093/pcp/pct111 [DOI] [PubMed] [Google Scholar]
- 19. Liu XF, Yin XR, Allan A, Lin-Wang K, Shi YN, Huang YJ, et al. The role of MrbHLH1 and MrMYB1 in regulating anthocyanin biosynthetic genes in tobacco and Chinese bayberry (Myrica rubra) during anthocyanin biosynthesis. Plant Cell Tiss Org. 2013; 115(3): 285–298. [Google Scholar]
- 20. Heim MA, Jakoby M, Werber M, Martin C, Weisshaar B, Bailey PC. The basic Helix-Loop-Helix transcription factor family in plants: a genome-wide study of protein structure and functional diversity. Mol Biol Evol. 2003; 20(5): 735–747. [DOI] [PubMed] [Google Scholar]
- 21. Leivar P, Monte E, Oka Y, Liu T, Carle C, Castillon A, et al. Multiple phytochrome-interacting bHLH transcription factors repress premature seedling photomorphogenesis in darkness. Curr Biol. 2008; 18 (23): 1815–1823. 10.1016/j.cub.2008.10.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morohashi K, Zhao MZ, Yang ML, Read B, Lloyd A, Lamb R, et al. Participation of the Arabidopsis bHLH factor GL3 in trichome initiation regulatory events. Plant Physiol. 2007; 145 (3): 736–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Abe H, Urao T, Ito T, Seki M, Shinozaki K, Yamaguchi-Shnizaki K. Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell 2003; 15 (1): 63–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li HM, Sun JQ, Xu YX, Jiang HL, Wu XY, Li CY. The bHLH-type transcription factor AtAIB positively regulates ABA response in Arabidopsis . Plant Mol Biol. 2007; 65 (5): 655–665. [DOI] [PubMed] [Google Scholar]
- 25. Chandler VL, Radicella JP, Robbins TP, Chen J, Turks D. Two regulatory genes of the maize anthocyanin pathway are homologous: isolation of B utilizing R genomic sequences. Plant Cell 1989; 1(12): 1175–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Quattrocchio F, Wing JF, Leppen H, Mol J, Koes RE. Regulatory genes controlling anthocyanin pigmentation are functionally conserved among plant species and have distinct sets of target genes. Plant Cell 1993; 5(11): 1497–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nesi N, Debeaujon I, Jond C, Pelletier G, Caboche M, Lepiniec L. The TT8 gene encodes a basic Helix-Loop-Helix domain protein required for expression of DFR and BAN genes in Arabidopsis siliques. Plant Cell 2000; 12(10): 1863–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ohno S, Deguchi A, Hosokawa M, Tatsuzawa F, Doi M. A basic helix-loop-helix transcription factor DvIVS determines flower color intensity in cyanic dahlia cultivars. Planta 2013; 238(2): 331–343. 10.1007/s00425-013-1897-x [DOI] [PubMed] [Google Scholar]
- 29. Nakatsuka A, Yamagishi M, Nakano M, Tasaki K, Kobayashi N. Light-induced expression of basic helix-loop-helix genes involved in anthocyanin biosynthesis in flowers and leaves of Asiatic hybrid lily. Sci Hortic. 2009; 121 (1): 84–91. [Google Scholar]
- 30. Zhao M, Song AP, Li PL, Chen SM, Jiang JF, Chen FD. A bHLH transcription factor regulates iron intake under Fe deficiency in chrysanthemum. Sci Rep. 2014; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011; 28(10): 2731–2739. 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yin XR, Allan AC, Zhang B, Wu RM, Burdon J, Wang P, et al. Ethylene-related genes show a differential response to low temperature during ‘Hayward’ kiwifruit ripening. Postharvest Biol Technol. 2009; 52(1): 9–15. [Google Scholar]
- 33. Hellens R, Allan A, Friel E, Bolitho K, Grafton K, Templeton M, et al. Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant Methods 2005; 1(1): 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Xu Q, Yin XR, Zeng JK, Ge H, Song M, Xu CJ, et al. Activator- and repressor-type MYB transcription factors are involved in chilling injury induced flesh lignification in loquat via their interactions with the phenylpropanoid pathway. J Exp Bot. 2014; 65(15): 4349–4359. 10.1093/jxb/eru208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zeng JK, Li X, Xu Q, Chen JY, Yin XR, Ferguson IB, et al. EjAP2-1, an AP2/ERF gene, is a novel regulator of fruit lignification induced by chilling injury, via interaction with EjMYB transcription factors. Plant Biotechnol J. 2015; 13: 1325–1334 [DOI] [PubMed] [Google Scholar]
- 36. Anderson NO. Flower breeding and genetics Chapter 14: Chrysanthemum. Published by springer, The Netherland, 2007; Page 389–392. [Google Scholar]
- 37. Chen SM, Miao HB, Chen FD, Jiang BB, Lu JG, Fang WM. Analysis of expressed sequence tags (ESTs) collected from the inflorescence of Chrysanthemum. Plant Mol Biol Rep. 2009; 27: 503–510. [Google Scholar]
- 38. Baudry A, Heim MA, Dubreucq B, Caboche M, Weisshaar B, Lepiniec L. TT2, TT8, and TTG1 synergistically specify the expression of BANYULS and proanthocyanidin biosynthesis in Arabidopsis thaliana . Plant J. 2004; 39(3): 366–380. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Three conserved boxes, including box11, 18 and 13, found in these bHLHs play essential roles in the interaction with MYBs transcription factors leading to enhanced transcription of anthocyanin biosynthesis genes. Amino acid residues (H-E-R) at position 5, 9 and 13 of the bHLH domains are critical for DNA binding.
(TIF)
Three TFs, CmMYB6, CmbHLH1 and CmbHLH2, were cloned separately into pGBKT7 (BD). Auto-activation was screened on SD/-Trp media with X-α-Gal and AbA antibiotic background.
(TIF)
The full ORF of each gene was cloned into pGreenII0029 62-SK and electroporated into Agrobacterium tumefaciens GV3101 (MP90). Different combinations of these Agrobacterium tumefaciens were infiltrated into tobacco leaves. Photographs were taken 8 days later. Each experiment was carried out with three biological repeats.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.