Abstract
Background
Community based breastfeeding promotion programmes have been shown to be effective in increasing breastfeeding prevalence. However, there is limited data on the cost-effectiveness of these programmes in sub-Saharan Africa. This paper evaluates the cost-effectiveness of a breastfeeding promotion intervention targeting mothers and their 0 to 6 month old children.
Methods
Data were obtained from a community randomized trial conducted in Uganda between 2006–2008, and supplemented with evidence from several studies in sub-Saharan Africa. In the trial, peer counselling was offered to women in intervention clusters. In the control and intervention clusters, women could access standard health facility breastfeeding promotion services (HFP). Thus, two methods of breastfeeding promotion were compared: community based peer counselling (in addition to HFP) and standard HFP alone. A Markov model was used to calculate incremental cost-effectiveness ratios between the two strategies. The model estimated changes in breastfeeding prevalence and disability adjusted life years. Costs were estimated from a provider perspective. Uncertainty around the results was characterized using one-way sensitivity analyses and a probabilistic sensitivity analysis.
Findings
Peer counselling more than doubled the breastfeeding prevalence as reported by mothers, but there was no observable impact on diarrhoea prevalence. Estimated incremental cost-effectiveness ratios were US$68 per month of exclusive or predominant breastfeeding and U$11,353 per disability adjusted life year (DALY) averted. The findings were robust to parameter variations in the sensitivity analyses
Conclusions
Our strategy to promote community based peer counselling is unlikely to be cost-effective in reducing diarrhoea prevalence and mortality in Uganda, because its cost per DALY averted far exceeds the commonly assumed willingness-to-pay threshold of three times Uganda’s GDP per capita (US$1653). However, since the intervention significantly increases prevalence of exclusive or predominant breastfeeding, it could be adopted in Uganda if benefits other than reducing the occurrence of diarrhoea are believed to be important.
Introduction
Breastfeeding is vital to child survival, as it provides protection against diseases, including diarrhoea and acute respiratory infections [1–3]. Breastfeeding promotion is therefore central to infant health promotion in low and middle income countries (LMICS). The World Health Organisation (WHO) recommends exclusive breastfeeding (EBF) for the first six months [4]. Though breastfeeding is widespread in most LMICS, EBF is less common [5]. Globally, early initiation of breastfeeding averages about 43%, and EBF at six months is close to 39%; only a third of African children below six months are exclusively breastfed [6]. In Uganda, EBF at six months was 63% in 2011 [7].
Interventions to educate mothers on the importance of breastfeeding can increase EBF prevalence [8]. EBF promotion is mainly undertaken at health facilities, through initiatives such as the Baby Friendly Hospital Initiative and the Integrated Management of Childhood Illness [9, 10]. Community-based strategies are also reported to be efficacious [11–14]. We previously reported the results of a trial (PROMISE-EBF), where peer counselling substantially increased breastfeeding prevalence [11].
Community-based breastfeeding initiatives are rarely undertaken on a large scale in sub-Saharan Africa, and there is limited data on economic impact. In earlier publications, we presented the costs of breastfeeding promotion in Uganda [15] and South Africa [16]. The only other cost analysis of a breastfeeding intervention in sub-Saharan Africa [17] showed that community-based peer counselling could increase EBF prevalence at five months by over 70%; costs ranged from US$23 to US$126 per month of EBF [17].
In this paper, we estimate the cost-effectiveness of community-based peer counselling to promote EBF, for the reduction of diarrhoeal morbidity and mortality in Uganda, compared to facility-based breastfeeding promotion. We assess the additional benefits that can be gained from implementing a community-based breastfeeding strategy, and the associated incremental costs. This economic evaluation is based on the cluster randomised PROMISE-EBF trial, which was undertaken in Uganda between January 2006 and June 2008. The findings of this study can potentially influence investments to improve breastfeeding promotion interventions in Uganda.
The PROMISE-EBF Trial
PROMISE-EBF was a multi-site cluster-randomised trial conducted in Burkina Faso, South Africa, Uganda, and Zambia. The aim of the study was to assess the impact of individual home-based peer counselling to promote exclusive breastfeeding for 6 months after birth. The PROMISE-EBF trial is registered with ClinicalTrials.gov, number NCT00397150. The protocol for this is available as supporting information (see the trial protocol in S1 Protocol). The trial design and results have been published elsewhere [11], and are summarised in this paper.
Study setting and design
In Uganda, the study was undertaken in Mbale district, with a population of about 400,000 [18]. The unit of randomization were clusters of villages with an average of 1,000 inhabitants, with a birth rate of approximately 35 per cluster. Twenty-four clusters in Mbale district were selected and randomized 1:1 to either control or intervention groups, based on similarities in terms of location, urban-rural set ups and socio-economic status. Women in intervention clusters received home-based individual peer counselling from lay counsellors, to encourage EBF for six month. The women received five visits, once in the third trimester and during the first week of birth, and in weeks four, seven and ten. Extra visits were offered to mothers with breastfeeding problems or if the mother or counsellor for other reasons deemed it necessary. In the control group, women received standard care provided at public health facilities.
Selection of peer supporters
Sensitization workshops and meetings aimed at introducing the project to community leaders were held prior to commencement of the trial. The community leaders facilitated the mobilisation of local women to work as peer counsellors on the project. Twelve women were selected as peer supporters, one in each cluster.
Data collection
Study questionnaires were developed and adapted from previous work undertaken in the countries that participated in the trial [19–22], and were piloted in Uganda in 2005 [23]. Data were gathered between 2006 and 2008 in the mothers’ homes by trained data gatherers. The questionnaire was administered at 3, 6, 12 and 24 weeks postpartum, to determine their children’s well-being, including the breastfeeding and diarrhoea status.
Ethics considerations
Study approval was obtained from Faculty of Medicine, Makerere University; Research and Ethics Committee, Uganda National Council for Science and Technology; and regional committees for Medical and Health Research Ethics in Norway. Women provided verbal informed consent in the peer counselling programme and signed or thumb-printed informed consent during data gathering. The age eligibility was 15 years and older. Mothers younger than 18 years had witnessed informed consent.
Data analysis
The primary trial outcome trial was EBF prevalence and diarrhoea at 12 and 24 weeks, as reported by mothers. Current breastfeeding was assessed at all post-partum visits using a mother’s 24-hour and 7-day recall. These recall periods are widely used [7] and have been shown to provide reliable estimates [23, 24]. Children who did not receive any other food or liquids other than breastmilk were deemed to be exclusively breastfed, even if they had been administered drugs. Diarrhoea prevalence was also based on the mothers’ reports and was assessed using a 2-week recall [11], considering that shorter recall periods could provide underestimates when symptoms fluctuate from day-to-day [25]. Data analysis was done on an intention to treat basis and included all mother-infant pairs in the analysis, thus all missing, loss to follow-up and deaths were recoded as non-events (not having EBF or diarrhoea). Analyses excluding cases without valid results were also provided [11].
Study outcomes
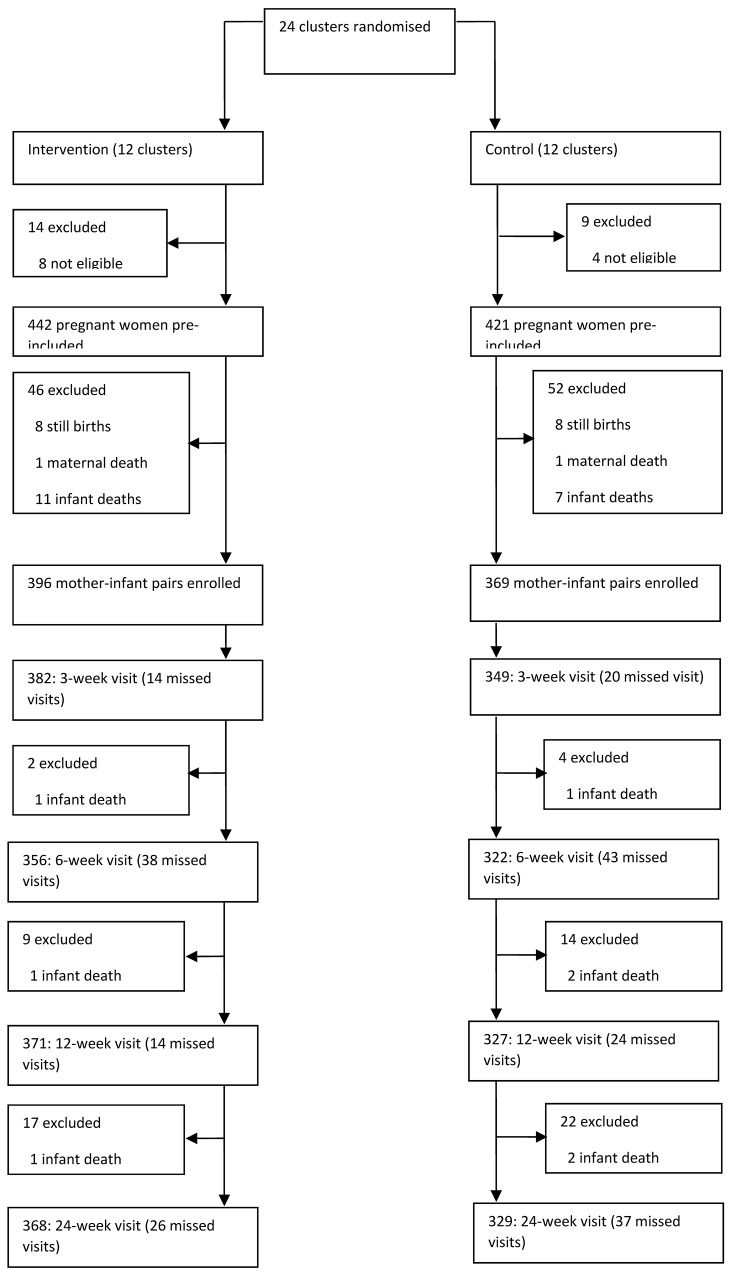
A total of 329 mother-infant pairs in the intervention and 368 in the control group were followed up until 24 weeks after birth (Fig 1). At 12 weeks, EBF prevalence (24 hour recall) in the intervention group was 82%, compared to 44% in the control group, with a prevalence ratio (confidence interval) of 1.89 (1.70–2.11) [11]. The 12 week diarrhoea prevalence was 10% in the intervention and 9% in the control group, prevalence ratio 1.13 (0.81–1.59). Other factors, including socio-economic status, maternal age, parity, mothers’ education and marital status were fairly evenly distributed between intervention and control groups, and were not deemed to affect breastfeeding and diarrhoea outcomes [11].
Fig 1. Uganda PROMISE-EBF trial profile.
Methods for the Economic Evaluation
An economic model based on the PROMISE-EBF trial was created in order to estimate the cost-effectiveness of using peer counselling to promote exclusive breastfeeding. Because some of the data needed to populate the model, such as hospitalisation rates and disease costs, were not captured during the trial, this was supplemented by evidence from the literature. The CHEERS checklist was used to guide reporting (see the checklist in S1 Checklist).
Comparators in the economic evaluation
The economic evaluation compares two breastfeeding promotion strategies, based on the control and intervention groups in the trial: 1) community-based peer counselling conducted alongside breastfeeding promotion in facility-based maternal and child health services, including antenatal and postnatal services (herein after referred to as peer counselling); and 2) breastfeeding promotion in facility-based maternal and child health services only (herein after referred to as health facility breastfeeding promotion—HFP). This followed the set-up in the PROMISE-EBF trial, where peer counselling was offered to pregnant women and mothers of newborns, while in both control and intervention clusters, women could access standard health facility services.
Facility-based breastfeeding promotion is often done at antenatal and postnatal services. Many facility-based maternal and child health packages provide health advice and other services for improved maternal and child health. Women are encouraged to attend antenatal clinics at least once during pregnancy and other under-five services after birth. Over 90% of pregnant women in Uganda access antenatal care (ANC) at least once before or after delivery, and over 50% attend postnatal services [7]. PROMISE-EBF did not assess the quantity or quality of facility-based services, but parallel research within our study group has described current practices in the study setting [26].
The economic model
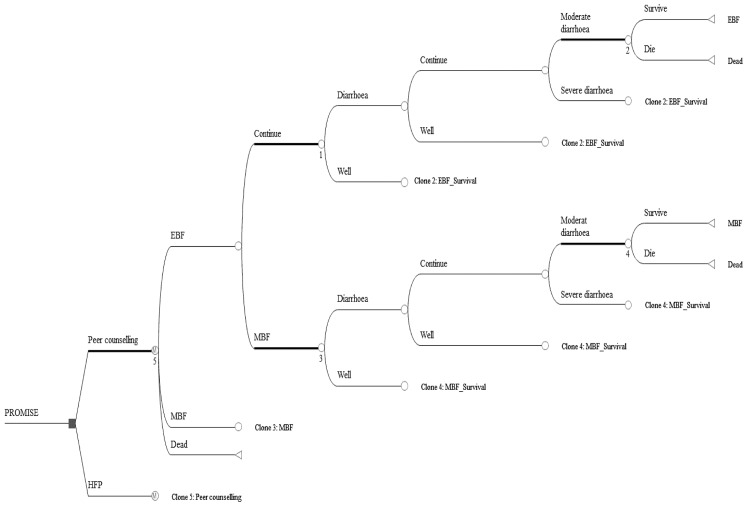
A decision tree with a state Markov model was used to depict breastfeeding promotion and the associated feeding patterns in the first six months of life (Fig 2). The model predicts feeding patterns and children can have one of two options: exclusive or predominant breastfeeding (EBF/PBF) and mixed or replacement feeding (MF/RF), see Box 1 for definitions. The merging of EBF and PBF was done because few studies indicate clear differences in health outcomes between EBF and PBF [11, 27]. MF and RF were merged because only 0.3% of the children in the PROMISE-EBF trial received replacement feeding. In the model, children were allowed to move both ways between feeding states. This was done to reflect the findings of the PROMISE-EBF trial, which showed that mothers tended to periodically switch between EBF/PBF and MF [28]. This might be the case, since the definition of EBF/PBF was based on shorter periods (24-hours and 1-week recall), which may allow for more frequent fluctuations and may under-represent usual practice [29].
Fig 2. Decision model of cost-effectiveness analysis for peer counselling vs HFP.
Box 1. Definitions of infant feeding patterns.
Exclusive breastfeeding is when only breast milk is given to the child, except for medicines, vitamins or mineral supplements.
Predominant breastfeeding is when breast milk is nutritionally dominant while given in addition to water-based fluids including fruit juices, tea without milk or oral rehydration salts.
Complementary feeding including breast milk (partial breastfeeding or mixed feeding): These terms are used to describe when non-human milk, semi-solids or other solids are given to the child in addition to breast milk. The term mixed feeding does normally refer to the feeding practice specified above during the first half of infancy (under 6 months old).
Replacement feeding is defined as the feeding strategy when breastfeeding has been stopped, or if the child never has been given any breast milk. Exclusive replacement feeding was defined as when never having given any breast milk.
At each feeding state a decision tree model depicts three health scenarios: sick (diarrhoea), well (no diarrhoea) and dead. The diarrhoea state could either be severe, in which case the child would be hospitalised, moderate–requiring outpatient treatment, or mild–requiring no treatment. Probabilities of diarrhoea and mortality were assumed to depend on the feeding practice, i.e. whether a child belonged to the EBF/PBF or MF/RF pattern.
The model had monthly cycles, terminating at six months, based on the PROMISE-EBF trial. It was based on the hypothesis that additional breastfeeding promotion increases EBF/PBF prevalence, which in turn could affect morbidity and mortality.
Model parameters
EBF and diarrhoea prevalence
In the PROMISE-EBF trial, mothers were followed up for six months, and a questionnaire was administered at 3, 6, 12 and 24 weeks postpartum. These data were used to estimate feeding and diarrhoea state transition probabilities.
Feeding state transition probabilities were estimated using parametric survival models [30] of EBF/PBF duration (Table 1) [28]. A multiple events model was used to estimate survival. The event was cessation of EBF/PBF, a time dependent variable indicating the age at which EBF/PBF was stopped. Cases were censored if respondents were lost to follow-up, missed the last valid interview, or continued EBF/PBF beyond six months.
Table 1. Transition probabilities between feeding states in the PROMISE trial.
| Months | Control | Intervention | ||||||
|---|---|---|---|---|---|---|---|---|
| α 1 | α 2 | α 3 | α 4 | α 1 | α 2 | α 3 | α 4 | |
| 1 | 0.67 | 0.33 | 0.82 | 0.18 | 0.81 | 0.19 | 0.93 | 0.07 |
| 2 | 0.73 | 0.27 | 0.76 | 0.24 | 0.89 | 0.11 | 0.85 | 0.15 |
| 3 | 0.74 | 0.26 | 0.72 | 0.28 | 0.91 | 0.09 | 0.79 | 0.21 |
| 4 | 0.75 | 0.25 | 0.70 | 0.30 | 0.92 | 0.08 | 0.75 | 0.25 |
| 5 | 0.76 | 0.24 | 0.68 | 0.32 | 0.92 | 0.08 | 0.71 | 0.29 |
| 6 | 0.77 | 0.23 | 0.66 | 0.34 | 0.93 | 0.07 | 0.68 | 0.32 |
Source: Chola et al. 2013. Infant feeding transitions among Ugandan children from the cluster-randomised trial PROMISE-EBF.
α 1 = Probability of remaining in EBF/PBF; α 2 = Probability of transitioning from EBF/PBF to MF/RF; α 3 = Probability of remaining in MF/RF; α 4 = Probability of transitioning from MF/RF to EBF/PBF.
Probabilities of diarrhoea events (sick or well) were estimated using longitudinal/panel methods in STATA. The aim was to assess diarrhoea morbidity in infants during the six month follow-up. We estimated four transition probabilities between two states (sick and well) as shown in Table 2. The events were dependent on a child’s feeding state.
Table 2. Transition probabilities between health states in the PROMISE-EBF trial.
| β 1 | β 2 | β 3 | β 4 | |
|---|---|---|---|---|
| Intervention group | ||||
| EBF/PBF | 0.08 | 0.88 | 0.12 | 0.92 |
| MF/RF | 0.07 | 0.86 | 0.14 | 0.93 |
| Control group | ||||
| EBF/PBF | 0.06 | 0.64 | 0.36 | 0.94 |
| MF/RF | 0.10 | 0.71 | 0.29 | 0.90 |
β 1 = Probability to be sick with diarrhoea in respective visit and continued diarrhoea in next visit; β 2 = Probability of diarrhoea in respective visit and changed to well (no diarrhoea) in next visit; β 3 = Probability of being well in respective visit, and continue in this state in next visit; β 4 = Probability of well in respective visit and sick in next visit.
Hospitalisation for diarrhoea
The PROMISE-EBF trial did not collect data on diarrhoea hospitalisation. We based our assumptions on diarrhoea hospitalisation on a systematic review of breastfeeding and the risk of diarrhoea and diarrheal death [31]. This study reviewed literature published from 1980 to 2009 on suboptimal breastfeeding and the associated diarrhoea morbidity and mortality. Random effects meta-analyses were conducted on 18 studies, to generate pooled relative risks of diarrhoea hospitalisation and death. The estimated relative risk (95% confidence interval) of diarrhoea hospitalisation was 2.3 (0.08–6.55) for predominantly, 4.4 (1.75–13.84) for partially and 19.5 (6.13–33.86) for not breastfed infants 0–5 months of age, compared to those exclusively breastfed (Table 3).
Table 3. Base parameters used in the decision model for exclusive breastfeeding promotion.
| Parameter | Estimate | Source | |
|---|---|---|---|
| Mortality rates | Prevalence | SA range | |
| Control | 3/1000 | 0.001–0.01 | PROMISE-EBF |
| Intervention | 8/1000 | 0.001–0.01 | PROMISE-EBF |
| Hospitalisation | Relative risk | 95%CI | |
| Predominantly breastfed | 2.28 | (0.08–6.55) | Lamberti et al. (2011) |
| Partially breastfed | 4.43 | (1.75–13.84) | Lamberti et al. (2011) |
| Not breastfed | 14.40 | (6.13–33.86) | Lamberti et al. (2011) |
| Costs (US$) | Mean | SA range | |
| Health facility promotion per child per year | 3.60 | 2.20–6.43 | Orach et al (2003)/Levin et al (2007) |
| Peer counselling per child per year | 139 | 74–233 | Chola et al (2011) |
| Hospitalised diarrhoea per case | 95 | 65–134 | Chola & Robberstad (2009), Aikins et al (2010), Tate et al (2009) |
| Non-hospitalised diarrhoea per case | 9 | 3.80–26 | Chola & Robberstad (2009), Aikins et al (2010), Tate et al (2009) |
| Other parameters | Estimate | SA range | |
| Discount rate (r) | 0.03 | 0–0.06 | |
| Life expectancy (years) | 53 | 38–77 | WHO (2014) |
| Diarrhoea disability weight | Murray et al (2012) | ||
| Mild | 0.061 | ||
| Moderate | 0.202 | ||
| Severe | 0.281 | ||
| Age at onset (years) | 0.1 | Own assumption | |
| Mean duration of diarrhoea (days) | 7 | Kirkwood et al (1991) | |
CI = Confidence interval; RR = Relative risk; SA = Sensitivity analysis.
Cost estimates
Costs were assessed from a provider perspective (Table 3), mainly because the cost analysis done on the PROMISE-EBF trial was retrospective and only costs from project accounts could be obtained [15].
Costs of peer counselling were estimated in the PROMISE-EBF trial. The structure of the trial could be broken in four basic functions: administration; peer supervision and coordination; peer support; and recruitment (done by recruiters, who recruited mothers into the trial. These were not peer supporters). We thus used an ingredients approach to costing, in order to capture the different elements of the trial. Costs included capital items such as motor vehicles, furniture and computers; and recurrent items such as salaries, fuel and rentals. Staff costs were based on the local rates paid to employees in the trial. Peer supporters and recruiters were not permanent members of staff. They were offered a token US$20 every month for their participation, a figure that was arrived at in a meeting with peer supporters, where they agreed on this as adequate compensation for their time. Intervention start-up costs, as well as costs of training and retraining of peer supporters were also included in the analysis. Detailed descriptions and analyses of the costs of the PROMISE-EBF trial in Uganda are given elsewhere [15]. Annual intervention costs were estimated to be US$56,308, and the cost per mother/infant pair (the output required in the economic model) was US$139 per year. The cost per visit was US$26 and the cost per week of EBF was estimated to be US$15 at 12 weeks post-partum.
Costs of HFP were not collected in the PROMISE-EBF trial, but were averaged from results of two studies assessing maternal health services. Orach et al.[32] estimated an average cost of antenatal and postnatal services ranging from US$2 to US$6 per child in three rural districts in Uganda. Levin et al. [33] estimated an average antenatal and postnatal services cost between US$2 to US$4 per child in Uganda. The average cost of US$3.6 was used in the model.
Diarrhoea treatment costs were also not collected in the trial. These were estimated form ad hoc and PubMed searches for articles in English undertaken in the last decade on treatment costs of diarrhoeal disease among infants in sub-Saharan Africa. The search words were costs, cost analysis and diarrhoea. The criteria for selection were that studies presented results from a provider perspective and provided costs for children less than five years. Studies were considered if costs were provided for outpatient, inpatient care or both. The search yielded 321 articles, whose abstracts were individually scrutinised. Fifteen articles were then selected for full text assessment, out of which 3 were finally included from Kenya [34], Ghana [35] and Zambia [36] (Table 3).
All costs were converted to 2007 prices using the local (specific country) consumer price index, and then converted to US$ at the estimated exchange rate in 2007.
Effect measures
Two measures of effectiveness were calculated as follows:
Person months of EBF/PBF extracted from PROMISE-EBF data–estimated as the cumulative months of EBF/PBF (MEBF). The mean duration of EBF/PBF estimated from the survival analysis was 1.5 in the control (HFP) and 3.5 months in the intervention (peer counselling) group [28].
Disability adjusted life years (DALYs) due to diarrhoea–calculated as years of life lived with disability (YLD), added to years of life lost (YLL) due to diarrhoea death. YLLs were calculated assuming a mean duration of seven days [37] average age at onset of 0.1 years, disability weights of 0.061 for mild, 0.202 for moderate and 0.281 for severe diarrhoea [38] and life expectancy at birth of 53 years [39]. Mortality estimates were made using PROMISE-EBF data. The estimated all-cause mortality rates were 3/1000 children in the control and 8/1000 children in the intervention group [11]. DALYs were not age-weighted, but discounted at a rate of 3% per annum.
Sensitivity analyses
Parameter uncertainty was assessed in one way sensitivity analyses. Life expectancy was varied from 38 years (males in Sierra Leone) to 77 years (females in Libya) [39]. Discount rates for both costs and outcomes were varied from 0% to 6% as recommended in the literature [40]. Disease costs were varied as follows: US$3.8 to US$26 for non-hospitalised and US$65 to US$134 for hospitalised diarrhoea cases, based on the maximum and minimum ranges of costs found in literature [34–36]. Diarrhoea hospitalisation rates were varied according to the upper and lower limits of the confidence intervals of the respective relative risk [31]. Costs of peer counselling were varied from US$74 to US$233. The low value was obtained from a scenario analysis performed in the costing study [15] and the high value from a similar study estimating the costs of peer counselling in Zambia (data available on request). A range of US$2.2 to US$6.4 was adopted for costs of HFP, representing the lowest and highest costs identified in the literature [32, 33].
Probabilistic sensitivity analyses were also undertaken. Beta distributions were fitted for probability parameters using point estimates and standard errors. Lognormal distributions were fitted for relative risks using point estimates and their confidence intervals. The gamma distribution was used for costs and DALYs, with mean costs also representing the standard error [30].
Statistical analyses
We used Microsoft Excel to summarise cost data, STATA version 11 for statistical analyses and TreeAge Pro 2012 Suite to model cost effectiveness.
Results
Base case cost-effectiveness
The base case results are presented in Table 4. The costs of breastfeeding promotion were US$112 for HFP and US$249 for peer counselling, with an incremental cost between the interventions of US$137.
Table 4. Base case results of the cost-effectiveness analysis.
| CEA of increasing EBF prevalence | ||||||
|---|---|---|---|---|---|---|
| Cost per MEBF | ||||||
| Strategy | Cost (US$/Child) | IC | MEBF/Child | IE | Cost/MEBF | ICER |
| HFP | 113 | 1.50 | 75 | |||
| Peer counselling | 250 | 137 | 3.50 | 2.00 | 71 | 68 |
| Cost per DALYs averted | ||||||
| Strategy | Cost (US$/Child) | IC | DALY/Child | DALYs | Cost/DALY | ICER |
| averted | ||||||
| HFP | 113 | 5.76 | 19 | |||
| Peer counselling | 250 | 137 | 5.78 | 0.01 | 43 | 11,353 |
IC = Incremental cost; IE = Incremental effect; DALYs = Disability adjusted life years; MEBF = Months of EBF; ICER = Incremental cost effectiveness ratio (IC/IE). US$ = United States Dollars.
The expected cumulative period of EBF was longer for mothers receiving peer counselling (3.5 months) than for mothers in the control group who relied on HFP alone (1.5 months) [28]. The associated incremental effectiveness was two months. The average cost per MEBF was US$75 for HFP and US$71 for peer counselling, with an incremental cost-effectiveness ratio of US$68, in favour of peer counselling.
The difference in DALYs averted for HFP and peer counselling was negligible. Irrespective of intervention, the health loss attributable to morbidity and mortality is about five DALYs per child, with negligible difference of 0.01 in favour of peer counselling. The incremental cost-effectiveness ratio of peer counselling is US$11,353 compared to HFP.
Sensitivity analysis
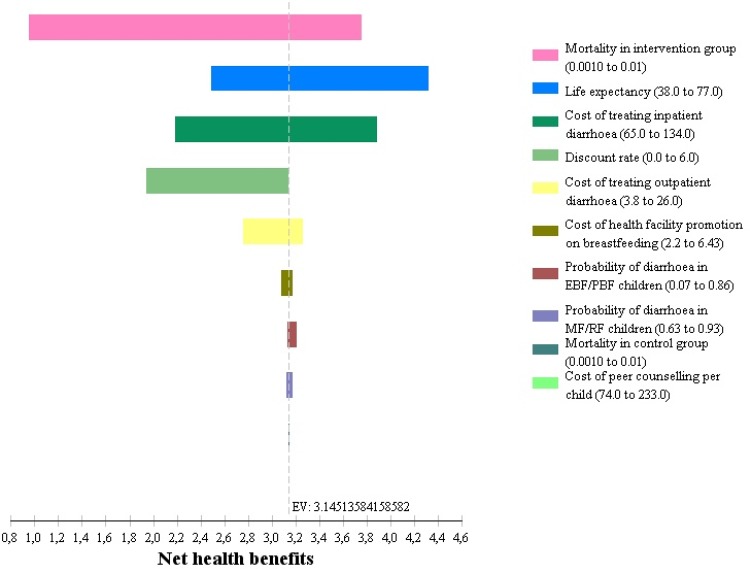
As shown in the tornado diagram in Fig 3, the mortality rate in the intervention group had the greatest impact, accounting for 49% of the uncertainty, followed by life expectancy (21%), and cost of treating severe diarrhoea (18%). A further 10% of the variation was accounted for by the discount rate (9%) and the cost of treating moderate diarrhoea (1%). Varying the other parameters had little impact on cost-effectiveness.
Fig 3. Tornado diagram of one-way sensitivity analyses results of uncertain model parameters.
EV = expected value. Net health benefits = ((Effectiveness–Costs)/Willingness to pay).
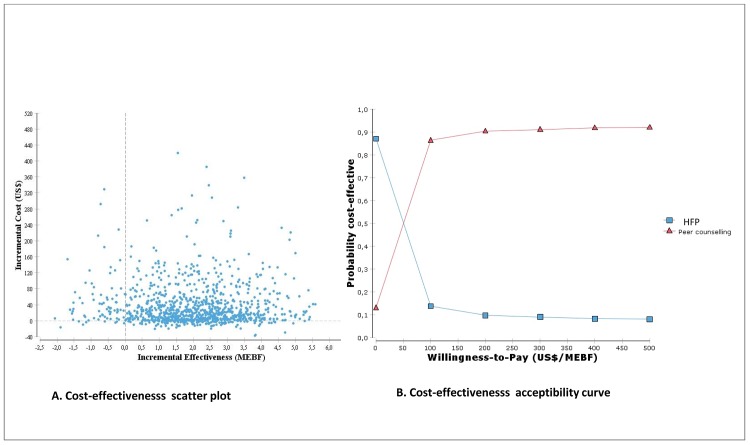
Results from probabilistic sensitivity analyses are illustrated in Figs 4 and 5. Fig 4 shows the scatter plot and incremental cost-effectiveness acceptability curve of peer counselling vs HFP for the MEBF outcome. Fig 4A illustrates how the uncertainty is distributed across both costs and effectiveness, while Fig 4B, shows that peer counselling has a higher likelihood of being cost-effective compared to HFP if willingness-to-pay exceeds US$68 per MEBF.
Fig 4. Cost-effectiveness scatterplot and acceptability curves for peer counselling vs HFP (Cost/MEBF).
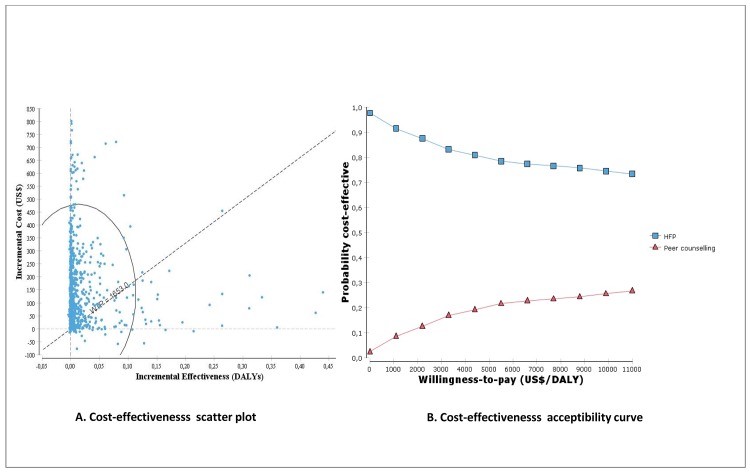
Fig 5. Cost-effectiveness scatterplot and acceptability curves for peer counselling vs HFP (Cost/DALY).
Fig 5 shows the incremental cost-effectiveness scatterplot and acceptability curves of peer counselling vs HFP for DALYs. The scatter plot (Fig 5A) shows that peer counselling is not likely to be a cost-effective strategy in averting DALYs. Only 9% of the ICERs in the scatter plot are cost-effective i.e. fall below a willingness-to-pay threshold of US$1653 per DALY (about 3 times Uganda’s per capita GDP of US$551 [41]). In Fig 5B, it is shown that HFP is more likely to be cost-effective than peer counselling.
Discussion
This study is the first economic evaluation of a breastfeeding intervention in Uganda and the second such study in sub-Saharan Africa [17]. Peer counselling is substantially more costly than standard facility-based services alone, and is only likely to be considered cost-effective if the willingness-to-pay to increase breastfeeding prevalence exceeds US$68 per MEBF, or US$11,353 per DALY averted. Uganda has a number of pending maternal and child health interventions with low coverage that are far more cost-effective.
Results from the PROMISE-EBF trial indicate that there was no association between increased breastfeeding prevalence and diarrhoea morbidity [11]. It should be noted that even though we calculated DALYs using trial data, the PROMISE-EBF trial was not powered to identify an effect of the intervention on mortality. A discussion on these issues is provided elsewhere [11].
Study strengths and limitations
A major strength of this study is that we primarily use data from a randomised trial, which minimises selection bias [42]. However, the trial only compared two options: an intervention and the standard of care. Our economic evaluation therefore only compares a subset of all available options for promotion of EBF. For example, we did not consider the option of introducing group counselling, which has also proved to be effective [13].
It was out of the scope of this study to make head-to-head economic comparisons of breastfeeding promotion and other cost-effective child health interventions such as rotavirus vaccinations, diarrhoea management, zinc and vitamin A supplementation [43–45]. Since results are presented using DALYs averted, comparison is possible across a broad range of disease groups. Cautious priority considerations are therefore possible on a broader basis if the perspectives and methods of the different studies are reasonably comparable.
Furthermore, this study only addresses diarrhoea despite evidence that breastfeeding influences several illnesses, among them acute respiratory infections (ARI), otitis media and juvenile diabetes [46, 47]. In addition, mortality is influenced by factors other than feeding practice, and this was not fully addressed in this study. Our estimates of the cost-effectiveness of EBF promotion are thus conservative. In addition, we only considered the impact of breastfeeding for infants up to six months, while some evidence indicate benefits of breastfeeding well into childhood [48, 49]. This again is likely to underestimate the full effect of breastfeeding promotion interventions, which should be taken into consideration in future evaluations as the empirical evidence improves.
Limited health system cost and disease data for breastfeeding infants meant that we had to make assumptions about some parameters, especially regarding the transferability of hospitalisation and cost data. Data on the costs of different feeding modes from the perspective of the mothers and families is missing altogether. Since we did not conduct a marginal costing analysis we cannot inform the impact of changing the number of health facility visits, personnel or other resources that will have to be increased when the intervention is scaled up. Data on costs of diarrhoea are taken from studies done in other countries, which may have different cost structures from Uganda. This could underestimate or overestimate the true diarrhoea costs in Uganda. Comprehensive sensitivity analyses are conducted on this parameter.
Comparison with other studies
Our result in terms of cost per MEBF is similar to that found in a South African study [17]. Desmond et al. estimate incremental costs of breastfeeding promotion ranging from US$23 to US$126 per MEBF, compared to US$68 in our study. The South African intervention was more intensive, with four home visits to mothers antenatally, compared to one in PROMISE-EBF, and 14 postnatal visits compared to four in our intervention. The mean duration of EBF in the intervention arm of the South African study was five months [17], compared to 3.5 months in ours [11]. While Desmond and colleagues conclude that EBF promotion through peer counselling is a cost-effective strategy, we are reluctant to draw this conclusion because the cost per DALY estimates were not favourable. In separate cost analyses of the PROMISE-EBF trial in South Africa and Uganda, we undertook scenario analyses to show the impact on unit costs, if the intervention was integrated with primary healthcare. The results showed that the cost per visit could US$32 in South Africa [16] and US$14 in Uganda [15].
Policy implications
The estimated incremental cost of US$68 per MEBF is more than Uganda’s per capita health expenditure of US$43 [50]. It is difficult to conclude on the basis of this evidence whether the intervention is justified, especially since it did not seem to carry any advantages in terms of infant growth [51]. This study shows that peer counselling is likely to be the cost-effective strategy for all willingness-to-pay thresholds above US$68 per MEBF.
PROMISE-EBF was a community randomised trial, with more supervision and intensity than a comparable facility-based intervention. We are uncertain about the impact of scaling up the intervention to district or even national level over a lengthy period. However, it has been shown that community strategies may achieve better health outcomes than facility-based strategies, especially in areas with limited health facilities [52].
In the sensitivity analyses, we tested a range of values that were most likely to capture the extreme changes in parameter estimates. The most sensitive parameter was the mortality rate in the intervention group, which accounted for 49% of the uncertainty. The other parameters used in the model were less influential on the cost-effectiveness estimates. This is an important result, particularly given the scarcity of information in resource poor settings. The model used in this study can thus be used to make fairly accurate projections on the most probable costs and consequences of undertaking similar interventions in Uganda and other sub-Saharan African countries.
Conclusions
Peer counselling to promote EBF is substantially more expensive than providing advice only through standard health facility based care. In our study, peer counselling increased both exclusive and predominant breastfeeding prevalence in the first six months. Based on our estimations, peer counselling is not likely to be cost-effective in averting DALYs associated with diarrhoea. This conclusion may be different in areas where increased EBF prevalence has been demonstrated to reduce diarrhoea prevalence more than in Uganda or if assessing other morbidity outcomes. We recommend analysis of cost-effectiveness when beneficial effects of the occurrence also of other illnesses are captured.
Supporting Information
(PDF)
(PDF)
Acknowledgments
The authors acknowledge the support of all those involved in the preparation and collection of data.
List of members for the PROMISE-EBF Study Group:
Steering Committee:
Thorkild Tylleskär, Philippe Van de Perre, Eva-Charlotte Ekström, Nicolas Meda, James K. Tumwine, Chipepo Kankasa, Debra Jackson.
Participating countries and investigators (country PI first, others in alphabetical order of surname):
Norway: Thorkild Tylleskär, Ingunn MS Engebretsen, Lars Thore Fadnes, Eli Fjeld, Knut Fylkesnes, Jørn Klungsøyr, Anne Nordrehaug-Åstrøm, Øystein Evjen Olsen, Bjarne Robberstad, Halvor Sommerfelt
France: Philippe Van de Perre
Sweden: Eva-Charlotte Ekström, Barni Nor
Burkina Faso: Nicolas Meda, A. Hama Diallo, Thomas Ouedrago, Jeremi Rouamba, Bernadette Traoré Germain Traoré, Emmanuel Zabsonré
Uganda: James K. Tumwine, Caleb Bwengye, Charles Karamagi, Victoria Nankabirwa, Jolly Nankunda, Grace Ndeezi, Margaret Wandera
Zambia: Chipepo Kankasa, Mary Katepa-Bwalya, Lumbwe Chola, Chafye Siuluta, Seter Siziya
South Africa: Debra Jackson, Carl Lombard, Mickey Chopra, Mark Colvin, Tanya Doherty, Ameena E Googa, Lyness Matizirofa, Lungiswa Nkonki, David Sanders, Rebecca Shanmugam, Wanga Zembe.
Data Availability
The dataset used for this manuscript has been archived in a repository available to the public through the Norwegian Social Science Data Servies (NSD), identification number NSD2224.
Funding Statement
The study was part of the EU-funded project PROMISE-EBF (contract no INCO-CT 2004-003660). It was also financially supported by the Research Council of Norway’s GlobVac Programme, grant No. 172226 “Focus on nutrition and child health: Intervention studies in low-income countries”, the NUFU-funded project Strengthening HIV-related interventions in Uganda: cooperation in research and institution capacity building, and the University of Bergen. No funding bodies had any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bhandari N, Kabir AK, Salam MA. Mainstreaming nutrition into maternal and child health programmes: scaling up of exclusive breastfeeding. Matern Child Nutr. 2008;4 Suppl 1:5–23. Epub 2008/03/20. doi: MCN126 [pii] 10.1111/j.1740-8709.2007.00126.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arifeen S, Black RE, Antelman G, Baqui A, Caulfield L, Becker S. Exclusive breastfeeding reduces acute respiratory infection and diarrhea deaths among infants in Dhaka slums. Pediatrics. 2001;108(4):E67 Epub 2001/10/03. . [DOI] [PubMed] [Google Scholar]
- 3. Kramer MS, Chalmers B, Hodnett ED, Sevkovskaya Z, Dzikovich I, Shapiro S, et al. Promotion of Breastfeeding Intervention Trial (PROBIT): a randomized trial in the Republic of Belarus. JAMA. 2001;285(4):413–20. Epub 2001/03/10. doi: joc00933 [pii]. . [DOI] [PubMed] [Google Scholar]
- 4. WHO. Global Strategy for Infant and Young Child Feeding. Geneva: WHO, 2003. [PubMed] [Google Scholar]
- 5. Mukuria AG, Kothari MT, Abderrahim N. Infant and Young Child Feeding Updates. Calverton, Maryland, USA: ORC Macro, 2006. [Google Scholar]
- 6. UNICEF. State of the world's children 2013: children with disabilities New York: UNICEF, 2013. [Google Scholar]
- 7. Uganda Bureau of Statistics (UBOS), ICF International Inc. Uganda Demographic and Health Survey 2011 Kampala, Uganda: UBOS; and Calverton, Maryland: ICF International Inc.: 2012. [Google Scholar]
- 8. Haroon S, Das J, Salam R, Imdad A, Bhutta Z. Breastfeeding promotion interventions and breastfeeding practices: a systematic review. BMC Public Health. 2013;13(Suppl 3):S20 10.1186/1471-2458-13-S3-S20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Adam T, Manzi F, Kakundwa C, Schellenberg J, Mgalula L, de Savigny D, et al. Multi-Country Evaluation of the Integrated Management of Childhood Illness (IMCI): Analysis Report on the Costs of IMCI in Tanzania. Daresalaam: WHO, 2004. [Google Scholar]
- 10. Abrahams SW, Labbok MH. Exploring the impact of the Baby-Friendly Hospital Initiative on trends in exclusive breastfeeding. Int Breastfeed J. 2009;4:11. Epub 2009/10/31. doi: 1746-4358-4-11 [pii] 10.1186/1746-4358-4-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tylleskar T, Jackson D, Meda N, Engebretsen IM, Chopra M, Diallo AH, et al. Exclusive breastfeeding promotion by peer counsellors in sub-Saharan Africa (PROMISE-EBF): a cluster-randomised trial. Lancet. 2011;378(9789):420–7. Epub 2011/07/15. doi: S0140-6736(11)60738-1 [pii] 10.1016/S0140-6736(11)60738-1 . [DOI] [PubMed] [Google Scholar]
- 12. Britton C, McCormick FM, Renfrew MJ, Wade A, King SE. Support for breastfeeding mothers. Cochrane Database Syst Rev. 2007;(1):CD001141 Epub 2007/01/27. 10.1002/14651858.CD001141.pub3 . [DOI] [PubMed] [Google Scholar]
- 13. Bhandari N, Bahl R, Mazumdar S, Martines J, Black RE, Bhan MK. Effect of community-based promotion of exclusive breastfeeding on diarrhoeal illness and growth: a cluster randomised controlled trial. Lancet. 2003;361(9367):1418–23. Epub 2003/05/03. doi: S0140-6736(03)13134-0 [pii] 10.1016/S0140-6736(03)13134-0 . [DOI] [PubMed] [Google Scholar]
- 14. Morrow AL, Guerrero ML, Shults J, Calva JJ, Lutter C, Bravo J, et al. Efficacy of home-based peer counselling to promote exclusive breastfeeding: a randomised controlled trial. Lancet. 1999;353(9160):1226–31. Epub 1999/04/27. doi: S0140-6736(98)08037-4 [pii] 10.1016/S0140-6736(98)08037-4 . [DOI] [PubMed] [Google Scholar]
- 15. Chola L, Nkonki L, Kankasa C, Nankunda J, Tumwine J, Tylleskar T, et al. Cost of individual peer counselling for the promotion of exclusive breastfeeding in Uganda. Cost Eff Resour Alloc. 2011;9(1):11. Epub 2011/07/01. doi: 1478-7547-9-11 [pii] 10.1186/1478-7547-9-11 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nkonki LL, Daviaud E, Jackson D, Chola L, Doherty T, Chopra M, et al. Costs of promoting exclusive breastfeeding at community level in three sites in South Africa. PLoS One. 2014;9(1):e79784 10.1371/journal.pone.0079784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Desmond C, Bland RM, Boyce G, Coovadia HM, Coutsoudis A, Rollins N, et al. Scaling-up exclusive breastfeeding support programmes: the example of KwaZulu-Natal. PLoS One. 2008;3(6):e2454 Epub 2008/06/19. 10.1371/journal.pone.0002454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Uganda Bureau of Statistics. Uganda population and housing census UBOS, 2002. [Google Scholar]
- 19. Doherty T, Chopra M, Jackson D, Goga A, Colvin M, Persson LA. Effectiveness of the WHO/UNICEF guidelines on infant feeding for HIV-positive women: results from a prospective cohort study in South Africa. Aids. 2007;21(13):1791–7. 10.1097/QAD.0b013e32827b1462 . [DOI] [PubMed] [Google Scholar]
- 20. Engebretsen IM, Wamani H, Karamagi C, Semiyaga N, Tumwine J, Tylleskar T. Low adherence to exclusive breastfeeding in Eastern Uganda: a community-based cross-sectional study comparing dietary recall since birth with 24-hour recall. BMC Pediatr. 2007;7:10. Epub 2007/03/03. doi: 1471-2431-7-10 [pii] 10.1186/1471-2431-7-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fjeld E, Siziya S, Katepa-Bwalya M, Kankasa C, Moland KM, Tylleskar T. 'No sister, the breast alone is not enough for my baby' a qualitative assessment of potentials and barriers in the promotion of exclusive breastfeeding in southern Zambia. Int Breastfeed J. 2008;3:26. Epub 2008/11/07. doi: 1746-4358-3-26 [pii] 10.1186/1746-4358-3-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goga AE, Van Wyk B, Doherty T, Colvin M, Jackson DJ, Chopra M, et al. Operational effectiveness of guidelines on complete breast-feeding cessation to reduce mother-to-child transmission of HIV: results from a prospective observational cohort study at routine prevention of mother-to-child transmission sites, South Africa. Journal of acquired immune deficiency syndromes. 2009;50(5):521–8. . [DOI] [PubMed] [Google Scholar]
- 23. Engebretsen IM, Shanmugam R, Sommerfelt AE, Tumwine JK, Tylleskar T. Infant feeding modalities addressed in two different ways in Eastern Uganda. Int Breastfeed J. 2010;5(1):2 10.1186/1746-4358-5-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bland RM, Rollins NC, Solarsh G, Van den Broeck J, Coovadia HM, Child Health G. Maternal recall of exclusive breast feeding duration. Arch Dis Child. 2003;88(9):778–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Norquist JM, Girman C, Fehnel S, DeMuro-Mercon C, Santanello N. Choice of recall period for patient-reported outcome (PRO) measures: criteria for consideration. Quality of life research: an international journal of quality of life aspects of treatment, care and rehabilitation. 2012;21(6):1013–20. 10.1007/s11136-011-0003-8 . [DOI] [PubMed] [Google Scholar]
- 26. Fadnes LT, Engebretsen IM, Moland KM, Nankunda J, Tumwine JK, Tylleskar T. Infant feeding counselling in Uganda in a changing environment with focus on the general population and HIV-positive mothers—a mixed method approach. BMC Health Serv Res. 2010;10:260 10.1186/1472-6963-10-260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bahl R, Frost C, Kirkwood B, Edmond K, Martines J, Bhandari N, et al. Infant feeding patterns and risks of death and hospitalization in the first half of infancy: multicentre cohort study. Bulletin of the World Health Organization. 2005;83:418–26. [PMC free article] [PubMed] [Google Scholar]
- 28. Chola L, Fadnes LT, Engebretsen IM, Tumwine JK, Tylleskar T, Robberstad B, et al. Infant Feeding Survival and Markov Transition Probabilities Among Children Under Age 6 Months in Uganda. American journal of epidemiology. 2013;177(5):453–62. 10.1093/aje/kws254 . [DOI] [PubMed] [Google Scholar]
- 29. Ruel MT, Arimond M. Measuring child practices: Approaches, indicators and implications for programs Washington DC: International Food Policy Research Institute; 2003. [Google Scholar]
- 30. Briggs A, Claxton K, Sculpher M. Decision modelling for health economic evaluation New York: Oxford university press; 2008. [Google Scholar]
- 31. Lamberti LM, Fischer Walker CL, Noiman A, Victora C, Black RE. Breastfeeding and the risk for diarrhea morbidity and mortality. BMC Public Health. 2011;11 Suppl 3:S15 10.1186/1471-2458-11-S3-S15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Orach CG, Dubourg D, De Brouwere V. Costs and coverage of reproductive health interventions in three rural refugee-affected districts, Uganda. Trop Med Int Health. 2007;12(3):459–69. Epub 2007/02/23. doi: TMI1788 [pii] 10.1111/j.1365-3156.2006.01788.x . [DOI] [PubMed] [Google Scholar]
- 33. Levin A, Dmytraczenko T, McEuen M, Ssengooba F, Mangani R, Van Dyck G. Costs of maternal health care services in three anglophone African countries. Int J Health Plann Manage. 2003;18(1):3–22. Epub 2003/04/10. 10.1002/hpm.690 . [DOI] [PubMed] [Google Scholar]
- 34. Tate JE, Rheingans RD, O'Reilly CE, Obonyo B, Burton DC, Tornheim JA, et al. Rotavirus disease burden and impact and cost-effectiveness of a rotavirus vaccination program in kenya. J Infect Dis. 2009;200 Suppl 1:S76–84. Epub 2009/10/13. 10.1086/605058 . [DOI] [PubMed] [Google Scholar]
- 35. Aikins M, Armah G, Akazili J, Hodgson A. Hospital health care cost of diarrheal disease in Northern Ghana. J Infect Dis. 2010;202 Suppl:S126–30. Epub 2010/08/13. 10.1086/653573 . [DOI] [PubMed] [Google Scholar]
- 36. Chola L, Robberstad B. Estimating average inpatient and outpatient costs and childhood pneumonia and diarrhoea treatment costs in an urban health centre in Zambia. Cost Eff Resour Alloc. 2009;7:16. Epub 2009/10/23. doi: 1478-7547-7-16 [pii] 10.1186/1478-7547-7-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kirkwood B. Diarrhoea In: Feachem RG JD, editor. Disease and mortality in Sub-Saharan Africa. Washington: Oxford University Press; 1991. p. 134–57. [Google Scholar]
- 38. Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2197–223. 10.1016/S0140-6736(12)61689-4 . [DOI] [PubMed] [Google Scholar]
- 39. WHO. World Health Statistics. Geneva: WHO; 2014. [Google Scholar]
- 40. Walker D, Kumaranayake L. Allowing for differential timing in cost analyses: discounting and annualization. Health Policy Plan. 2002;17(1):112–8. Epub 2002/02/28. . [DOI] [PubMed] [Google Scholar]
- 41. World Bank. World development indicators Washington DC: World Bank, 2014. [Google Scholar]
- 42. Sibbald B, Roland M. Understanding controlled trials: Why are randomised controlled trials important? BMJ. 1998;316(7126):201 10.1136/bmj.316.7126.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Robberstad B, Strand T, Black RE, Sommerfelt H. Cost-effectiveness of zinc as adjunct therapy for acute childhood diarrhoea in developing countries. Bull World Health Organ. 2004;82(7):523–31. Epub 2004/10/27. [PMC free article] [PubMed] [Google Scholar]
- 44. Bhutta ZA, Ahmed T, Black RE, Cousens S, Dewey K, Giugliani E, et al. What works? Interventions for maternal and child undernutrition and survival. Lancet. 2008;371(9610):417–40. Epub 2008/01/22. doi: S0140-6736(07)61693-6 [pii] 10.1016/S0140-6736(07)61693-6 . [DOI] [PubMed] [Google Scholar]
- 45. Richardson V, Hernandez-Pichardo J, Quintanar-Solares M, Esparza-Aguilar M, Johnson B, Gomez-Altamirano CM, et al. Effect of rotavirus vaccination on death from childhood diarrhea in Mexico. N Engl J Med. 362(4):299–305. Epub 2010/01/29. doi: 362/4/299 [pii] 10.1056/NEJMoa0905211 . [DOI] [PubMed] [Google Scholar]
- 46. Gartner LM, Morton J, Lawrence RA, Naylor AJ, O'Hare D, Schanler RJ, et al. Breastfeeding and the use of human milk. Pediatrics. 2005;115(2):496–506. Epub 2005/02/03. doi: 115/2/496 [pii] 10.1542/peds.2004-2491 . [DOI] [PubMed] [Google Scholar]
- 47. Ziegler AG, Schmid S, Huber D, Hummel M, Bonifacio E. Early infant feeding and risk of developing type 1 diabetes-associated autoantibodies. JAMA. 2003;290(13):1721–8. Epub 2003/10/02. 10.1001/jama.290.13.1721 290/13/1721 [pii]. . [DOI] [PubMed] [Google Scholar]
- 48. Victora CG, Adair L, Fall C, Hallal PC, Martorell R, Richter L, et al. Maternal and child undernutrition: consequences for adult health and human capital. The Lancet. 2008;371(9609):340–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Daniels MC, Adair LS. Breast-feeding influences cognitive development in Filipino children. J Nutr. 2005;135(11):2589–95. Epub 2005/10/28. doi: 135/11/2589 [pii]. . [DOI] [PubMed] [Google Scholar]
- 50. The World Bank. Africa development indicators—2011 Washington DC: The World Bank, 2011. [Google Scholar]
- 51. Engebretsen IM, Jackson D, Fadnes LT, Nankabirwa V, Diallo AH, Doherty T, et al. Growth effects of exclusive breastfeeding promotion by peer counsellors in sub-Saharan Africa: the cluster-randomised PROMISE EBF trial. BMC Public Health. 2014;14:633 10.1186/1471-2458-14-633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bhutta ZA, Darmstadt GL, Hasan BS, Haws RA. Community-based interventions for improving perinatal and neonatal health outcomes in developing countries: a review of the evidence. Pediatrics. 2005;115(2 Suppl):519–617. Epub 2005/05/04. doi: 115/2/S1/519 [pii] 10.1542/peds.2004-1441 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
Data Availability Statement
The dataset used for this manuscript has been archived in a repository available to the public through the Norwegian Social Science Data Servies (NSD), identification number NSD2224.